-
PDF
- Split View
-
Views
-
Cite
Cite
Oleg N. Katugin, Gennadyi A. Shevtsov, Mikhail A. Zuev, The morphology and biology of Gonatus tinro and Gonatopsis okutanii (Teuthida: Gonatidae) indicate that they are conspecific, ICES Journal of Marine Science, Volume 67, Issue 7, October 2010, Pages 1464–1477, https://doi.org/10.1093/icesjms/fsq100
Close - Share Icon Share
Abstract
Distribution, size and maturity patterns, and ontogenetic changes in morphological characters of the squid species Gonatus tinro and Gonatopsis okutanii were examined. The database includes information collected during research surveys to the Sea of Okhotsk and the adjacent Northwest Pacific Ocean from 1972 through 2005. Both species are distributed within the same areas beyond the shelf: G. tinro within a wide range of depths and an active vertical migrant, G. okutanii mostly demersal, characteristic of many adult gonatids. Seasonal changes in size and maturity of G. tinro and G. okutanii are congruent in many respects: G. tinro are usually small and young with hookless tentacle clubs, and squid identified as G. okutanii tend to be larger adults with truncated tentacles. The comparative morphology of the two species and the discovery of individuals bearing external features of both indicate that G. okutanii is an adult stage and G. tinro a young stage of the same species. It is concluded that G. okutanii is a junior synonym of G. tinro, which becomes the valid name by precedence.Katugin, O. N., Shevtsov, G. A., and Zuev, M. A. 2010. The morphology and biology of Gonatus tinro and Gonatopsis okutanii (Teuthida: Gonatidae) indicate that they are conspecific. – ICES Journal of Marine Science, 67: 1464–1477.
Introduction
Squid of the family Gonatidae are abundant, and with their large numbers and biomass, they occupy key positions in pelagic communities in the boreal North Pacific Ocean (Okutani et al., 1988; Nesis, 1997). Despite their ecological importance and a series of publications on their intrafamilial relationships, the systematics of this evolutionarily distinct group has long been in a state of flux (Jefferts, 1983). Difficulties in identifying species are particularly acute when the animals under consideration are at the earliest or later stages of life, seriously damaged by trawlnets or partly mutilated (e.g. after being removed from the stomachs of whales). Such problems of identification frequently challenge the reliability of databases on species distribution and biology compiled from field studies, especially when animals are analysed and discarded at sea and not available for closer inspection by taxonomists. Having checked extensive data on cephalopods from research surveys of TINRO in the North Pacific Ocean and adjacent waters, problems associated with the assignment of squid, particularly those with truncated tentacles and fins, to the nominal species Gonatus tinroNesis, 1972, and Gonatopsis okutaniiNesis, 1972, were discovered.
These two species were described in a single publication, with the description of G. tinro preceding that of G. okutanii (Nesis, 1972). One year earlier, Canadian scientists provided a detailed description of an “unusual gonatid squid”, but they did not suggest a species name for that animal (Fields and Gauley, 1971), and it was later identified as G. tinro. Gonatus tinro was described by Nesis as the subgenus Gonatus (Eogonatus) tinro. Several authors considered G. tinro distinctive enough to warrant elevation of the subgenus to genus (e.g. Okutani et al., 1988; Sweeney and Roper, 1998; Kubodera et al., 2006a), but there was no really compelling phylogenetic reason for doing this: allozymes (Katugin, 2004) and mtDNA (Lindgren et al., 2005) have shown that the species is within the Gonatus lineage. Following Nesis (1972, 1982, 1997), Bublitz (1981), and Jefferts (1983), and taking into account gonatid phylogenies, we prefer to use the name G. tinro.
The holotype of G. tinro is of fairly good quality; in particular, it has intact tentacles with clubs, the morphology of which is crucial for correct species identification within the genus Gonatus. One notable feature is the total absence of hooks from the club, which is unique to the genus Gonatus and clearly separates G. tinro from all other congeners. In addition, the club of G. tinro is narrow, suckers on the manus are approximately of equal size, and large portions of the club, in particular the locking zone, are weakly defined compared with the highly structured clubs of other congeners. However, the type series of G. tinro consists of small, immature squid with dorsal mantle lengths (DMLs) ranging from 9 to 89 mm. Taking into account the significant morphological changes associated with growth and maturation in gonatid squid, e.g. the changes in arm and tentacle armament and allometric changes in various body parts during growth and especially at maturation, adult characters may differ markedly from those of early ontogenetic stages.
The purported second species, G. okutanii, was described based on three animals in relatively poor condition: the holotype was captured in the Pacific Ocean off the Kuril Islands, and two paratypes were captured in the Bering Sea (Nesis, 1972). Those specimens were comparatively large, with DMLs ranging from 180 to 230 mm. However, the living squid must have been somewhat larger, because the fixed specimens lack the posterior portions of their mantles and fins. This particular species appears to have been described earlier (Okutani, 1967), but it was not named, having been based on a badly mutilated individual from the stomach of a sperm whale. It was identified by Okutani as a species of the genus Gonatopsis (the major generic character of which is the absence of tentacles in adult animals), despite specimens possessing remnants of tentacles, which appeared as short broken stubs between arms III and IV. In neither of these cases was the specimen sufficiently well preserved to allow unequivocal, clear taxonomic characters to be observed, leading to the description of a new species. Subsequently, this ambiguity led to serious problems and errors associated with the identification of G. okutanii, and even to taxonomic decisions associated with its validity. For example, it was later suggested that G. okutanii should be considered as a junior synonym of the species Gonatus madokai (Nesis, 1997), yet others considered G. okutanii to be a valid species (Jefferts, 1983; Okutani et al., 1988; Okutani, 1995, 2005).
The aim of this study was to analyse carefully the existing data on G. tinro and G. okutanii and to clarify their taxonomic relationships. The study is based on an extensive database of the distribution and biology of G. tinro and G. okutanii, accumulated from many TINRO and other surveys, along with a re-examination of the holotypes, and data from the morphological measurements and from the well-preserved almost undamaged specimens of G. okutanii with intact tentacles and fins.
Material and methods
Data on the occurrence of G. tinro and G. okutanii were collected in the Sea of Okhotsk and the adjacent Northwest Pacific during 34 research surveys on Russian and Korean research vessels (RVs) from 1972 through 2005 (Table 1). Tows were made using pelagic (midwater) and bottom trawls at depths from the surface to a maximum of 3400 m, but usually no deeper than 1000 m. Tow speeds varied from 3 to 5 knots depending on the trawl type. Pelagic tows generally lasted 1 h, and bottom trawls 30 min of fishing time. Each net had a codend liner of 10-mm mesh. The horizontal mouth openings were ∼30–80 m for pelagic trawls and 16–25 m for bottom trawls, depending on the trawl type, modification, and trawling speed. To standardize the squid catches made by different types of trawls and modification, the catch per unit effort (cpue) was calculated for each capture. The standard cpue was calculated as the number of squid captured per unit area (km2) using the formula cpue = N/(0.001852 × horizontal opening × trawling speed × trawling time), where N is the number of squid captured.
The database used for G. tinro and G. okutanii collected in the Sea of Okhotsk (OS) and the adjacent Northwest Pacific Ocean (NWPO).
| . | . | . | . | . | . | . | G. tinro . | G. okutanii . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number . | Research vessel/source . | Dates . | Gear . | Depth (m) . | Region . | Hauls . | A . | B . | C . | A . | B . | C . |
| 1 | SRTM 8-452a | 20 September 1972 and 25 December 1972 | IKMT | 50 | NWPO | 36 | 1 | 1 | 1 | – | – | – |
| 2 | Shantar | 14 March 1975 and 26 July 1975 | BT | 3 400 | OS | 464 | – | – | – | 2 | 2 | 2 |
| 3 | SRTM 8-452 | 16 July 1977 and 3 September 1977 | Dredge | 50 | OS | 25 | 1 | 2 | 2 | – | – | – |
| 4 | Novoulyanovsk | 15 September 1984 and 28 October 1984 | MT, BT | 500–1 200 | OS | 156 | 28 | 91 | – | 4 | 4 | – |
| 5 | Novokotovsk | 29 September and 19 November 1984 | MT | 5–200 | NWPO | 127 | – | – | – | 1 | 7 | 2 |
| 6 | Gissar | 8 November and 2 December 1987 | MT | 300–600 | OS | 45 | 3 | 14 | 14 | 2 | 11 | 11 |
| 7 | Darwin | 27 April 1989 and 4 September 1989 | BT | 492–1 310 | OS | 370 | 6 | 14 | 14 | 2 | 2 | 2 |
| 8 | Professor Soldatov | 10 August 1989 and 2 September 1989 | MT | 750 | OS | 38 | 24 | 380 | 380 | 5 | 12 | 12 |
| 9 | Professor Soldatov | 28 November 1990 and 18 December 1990 | MT | 500–1 000 | OS | 23 | 16 | 55 | 27 | 4 | 7 | 7 |
| 10 | Novokotovsk | 24 September 1990 and 31 December 1990 | MT | 5–450 | OS | 309 | 2 | 18 | 4 | – | – | – |
| 11 | Mlechnyi Put | 1 and 30 January 1991 | MT | 100–475 | OS, NWPO | 193 | 45 | 4 296 | 118 | 2 | 3 | 3 |
| 12 | Professor Kizevetter | 12 and 24 November 1991 | MT | 240–859 | NWPO | 38 | 2 | 32 | 32 | – | – | – |
| 13 | Darwin | 21 October 1991 and 3 January 1992 | MT | 0–270 | OS, NWPO | 243 | 6 | 17 | 15 | 14 | 139 | 29 |
| 14 | Novoulyanovsk | 14 July and 17 August 1992 | MT | 0–50 | OS | 130 | 8 | 73 | 33 | – | – | – |
| 15 | Sunflower | 3 December 1992 and 7 April 1993 | BT | 440–995 | OS | 94 | – | – | – | 31 | 269 | 167 |
| 16 | Salvia Ho | 12 January and 2 April 1993 | BT | 250–585 | OS | 96 | 1 | 3 | 3 | 33 | 596 | 231 |
| 17 | TINRO | 6 July and 17 August 1993 | MT | 0 | OS | 131 | 3 | 83 | – | – | – | – |
| 18 | TINRO | 6 August and 13 October 1994 | MT | 0 | OS | 194 | 3 | 86 | 6 | – | – | – |
| 19 | Professor Kizevetter | 31 July and 15 September 1996 | MT | 0 | OS | 85 | 1 | 15 | 7 | – | – | – |
| 20 | Borodino | 1 January and 29 April 1997 | MT | 580 | OS | 287 | – | – | – | 1 | 11 | 11 |
| 21 | Professor Levanidov | 10 July and 31 August 1997 | MT | 20 | NWPO | 185 | 1 | 1 | 1 | – | – | – |
| 22 | TINRO | 8 February and 25 August 1998 | MT | 235–485 | OS | 373 | 19 | 312 | 34 | 2 | 12 | 4 |
| 23 | TINRO | 26 August and 11 December 1998 | MT | 275 | OS | 309 | – | – | – | 1 | 1 | 1 |
| 24 | TINRO | 25 February 13 June 2000 | MT | 155–450 | OS | 320 | 17 | 136 | 39 | – | – | – |
| 25 | Professor Levanidov | 10 September and 14 December 2000 | MT | 409–996 | OS, NWPO | 297 | 18 | 30 | 30 | 5 | 7 | 7 |
| 26 | Borodinoa | 1 March and 13 May 2001 | MT | 235–485 | OS | 210 | – | – | – | 56 | 215 | 194 |
| 27 | Kavrai | 27 June and 22 October 2001 | BT | 413–420 | OS | 403 | – | – | – | 1 | 1 | 1 |
| 28 | Professor Levanidova | 4 and 22 November 2001 | MT | 75–500 | NWPO | 45 | 10 | 49 | 45 | 3 | 13 | 13 |
| 29 | Borodino | 15 January and 15 April 2002 | MT | 285–430 | OS | 180 | – | – | – | 1 | 1 | 1 |
| 30 | Borodino | 10 and 20 May 2002 | MT | 300–450 | OS | 20 | 1 | 1 | 1 | – | – | – |
| 31 | Professor Levanidov | 21 July and 23 August 2002 | MT | 0–365 | NWPO | 109 | 3 | 11 | 10 | – | – | – |
| 32 | Дальокеан | 30 July and 22 October 2002 | BGN | 535 | OS | 104 | 1 | 1 | 1 | – | – | – |
| 33 | Professor Levanidov | 10 August and 31 December 2004 | BT | 420–675 | OS | 314 | 16 | 45 | 25 | 20 | 35 | 30 |
| 34 | Professor Kaganovskyi | 24 October and 30 November 2005 | MT | 50–275 | NWPO | 98 | 4 | 22 | 21 | – | – | – |
| Total | 240 | 5 788 | 863 | 190 | 1 348 | 726 | ||||||
| . | . | . | . | . | . | . | G. tinro . | G. okutanii . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number . | Research vessel/source . | Dates . | Gear . | Depth (m) . | Region . | Hauls . | A . | B . | C . | A . | B . | C . |
| 1 | SRTM 8-452a | 20 September 1972 and 25 December 1972 | IKMT | 50 | NWPO | 36 | 1 | 1 | 1 | – | – | – |
| 2 | Shantar | 14 March 1975 and 26 July 1975 | BT | 3 400 | OS | 464 | – | – | – | 2 | 2 | 2 |
| 3 | SRTM 8-452 | 16 July 1977 and 3 September 1977 | Dredge | 50 | OS | 25 | 1 | 2 | 2 | – | – | – |
| 4 | Novoulyanovsk | 15 September 1984 and 28 October 1984 | MT, BT | 500–1 200 | OS | 156 | 28 | 91 | – | 4 | 4 | – |
| 5 | Novokotovsk | 29 September and 19 November 1984 | MT | 5–200 | NWPO | 127 | – | – | – | 1 | 7 | 2 |
| 6 | Gissar | 8 November and 2 December 1987 | MT | 300–600 | OS | 45 | 3 | 14 | 14 | 2 | 11 | 11 |
| 7 | Darwin | 27 April 1989 and 4 September 1989 | BT | 492–1 310 | OS | 370 | 6 | 14 | 14 | 2 | 2 | 2 |
| 8 | Professor Soldatov | 10 August 1989 and 2 September 1989 | MT | 750 | OS | 38 | 24 | 380 | 380 | 5 | 12 | 12 |
| 9 | Professor Soldatov | 28 November 1990 and 18 December 1990 | MT | 500–1 000 | OS | 23 | 16 | 55 | 27 | 4 | 7 | 7 |
| 10 | Novokotovsk | 24 September 1990 and 31 December 1990 | MT | 5–450 | OS | 309 | 2 | 18 | 4 | – | – | – |
| 11 | Mlechnyi Put | 1 and 30 January 1991 | MT | 100–475 | OS, NWPO | 193 | 45 | 4 296 | 118 | 2 | 3 | 3 |
| 12 | Professor Kizevetter | 12 and 24 November 1991 | MT | 240–859 | NWPO | 38 | 2 | 32 | 32 | – | – | – |
| 13 | Darwin | 21 October 1991 and 3 January 1992 | MT | 0–270 | OS, NWPO | 243 | 6 | 17 | 15 | 14 | 139 | 29 |
| 14 | Novoulyanovsk | 14 July and 17 August 1992 | MT | 0–50 | OS | 130 | 8 | 73 | 33 | – | – | – |
| 15 | Sunflower | 3 December 1992 and 7 April 1993 | BT | 440–995 | OS | 94 | – | – | – | 31 | 269 | 167 |
| 16 | Salvia Ho | 12 January and 2 April 1993 | BT | 250–585 | OS | 96 | 1 | 3 | 3 | 33 | 596 | 231 |
| 17 | TINRO | 6 July and 17 August 1993 | MT | 0 | OS | 131 | 3 | 83 | – | – | – | – |
| 18 | TINRO | 6 August and 13 October 1994 | MT | 0 | OS | 194 | 3 | 86 | 6 | – | – | – |
| 19 | Professor Kizevetter | 31 July and 15 September 1996 | MT | 0 | OS | 85 | 1 | 15 | 7 | – | – | – |
| 20 | Borodino | 1 January and 29 April 1997 | MT | 580 | OS | 287 | – | – | – | 1 | 11 | 11 |
| 21 | Professor Levanidov | 10 July and 31 August 1997 | MT | 20 | NWPO | 185 | 1 | 1 | 1 | – | – | – |
| 22 | TINRO | 8 February and 25 August 1998 | MT | 235–485 | OS | 373 | 19 | 312 | 34 | 2 | 12 | 4 |
| 23 | TINRO | 26 August and 11 December 1998 | MT | 275 | OS | 309 | – | – | – | 1 | 1 | 1 |
| 24 | TINRO | 25 February 13 June 2000 | MT | 155–450 | OS | 320 | 17 | 136 | 39 | – | – | – |
| 25 | Professor Levanidov | 10 September and 14 December 2000 | MT | 409–996 | OS, NWPO | 297 | 18 | 30 | 30 | 5 | 7 | 7 |
| 26 | Borodinoa | 1 March and 13 May 2001 | MT | 235–485 | OS | 210 | – | – | – | 56 | 215 | 194 |
| 27 | Kavrai | 27 June and 22 October 2001 | BT | 413–420 | OS | 403 | – | – | – | 1 | 1 | 1 |
| 28 | Professor Levanidova | 4 and 22 November 2001 | MT | 75–500 | NWPO | 45 | 10 | 49 | 45 | 3 | 13 | 13 |
| 29 | Borodino | 15 January and 15 April 2002 | MT | 285–430 | OS | 180 | – | – | – | 1 | 1 | 1 |
| 30 | Borodino | 10 and 20 May 2002 | MT | 300–450 | OS | 20 | 1 | 1 | 1 | – | – | – |
| 31 | Professor Levanidov | 21 July and 23 August 2002 | MT | 0–365 | NWPO | 109 | 3 | 11 | 10 | – | – | – |
| 32 | Дальокеан | 30 July and 22 October 2002 | BGN | 535 | OS | 104 | 1 | 1 | 1 | – | – | – |
| 33 | Professor Levanidov | 10 August and 31 December 2004 | BT | 420–675 | OS | 314 | 16 | 45 | 25 | 20 | 35 | 30 |
| 34 | Professor Kaganovskyi | 24 October and 30 November 2005 | MT | 50–275 | NWPO | 98 | 4 | 22 | 21 | – | – | – |
| Total | 240 | 5 788 | 863 | 190 | 1 348 | 726 | ||||||
Gears: MT, midwater trawl; BT, bottom trawl; IKMT, Isaacs–Kidd midwater trawl; BGN, bottom gillnet. A, number of hauls with squid; B, number of squid captured; C, number of squid analysed.
aData used in the morphometric analysis.
The database used for G. tinro and G. okutanii collected in the Sea of Okhotsk (OS) and the adjacent Northwest Pacific Ocean (NWPO).
| . | . | . | . | . | . | . | G. tinro . | G. okutanii . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number . | Research vessel/source . | Dates . | Gear . | Depth (m) . | Region . | Hauls . | A . | B . | C . | A . | B . | C . |
| 1 | SRTM 8-452a | 20 September 1972 and 25 December 1972 | IKMT | 50 | NWPO | 36 | 1 | 1 | 1 | – | – | – |
| 2 | Shantar | 14 March 1975 and 26 July 1975 | BT | 3 400 | OS | 464 | – | – | – | 2 | 2 | 2 |
| 3 | SRTM 8-452 | 16 July 1977 and 3 September 1977 | Dredge | 50 | OS | 25 | 1 | 2 | 2 | – | – | – |
| 4 | Novoulyanovsk | 15 September 1984 and 28 October 1984 | MT, BT | 500–1 200 | OS | 156 | 28 | 91 | – | 4 | 4 | – |
| 5 | Novokotovsk | 29 September and 19 November 1984 | MT | 5–200 | NWPO | 127 | – | – | – | 1 | 7 | 2 |
| 6 | Gissar | 8 November and 2 December 1987 | MT | 300–600 | OS | 45 | 3 | 14 | 14 | 2 | 11 | 11 |
| 7 | Darwin | 27 April 1989 and 4 September 1989 | BT | 492–1 310 | OS | 370 | 6 | 14 | 14 | 2 | 2 | 2 |
| 8 | Professor Soldatov | 10 August 1989 and 2 September 1989 | MT | 750 | OS | 38 | 24 | 380 | 380 | 5 | 12 | 12 |
| 9 | Professor Soldatov | 28 November 1990 and 18 December 1990 | MT | 500–1 000 | OS | 23 | 16 | 55 | 27 | 4 | 7 | 7 |
| 10 | Novokotovsk | 24 September 1990 and 31 December 1990 | MT | 5–450 | OS | 309 | 2 | 18 | 4 | – | – | – |
| 11 | Mlechnyi Put | 1 and 30 January 1991 | MT | 100–475 | OS, NWPO | 193 | 45 | 4 296 | 118 | 2 | 3 | 3 |
| 12 | Professor Kizevetter | 12 and 24 November 1991 | MT | 240–859 | NWPO | 38 | 2 | 32 | 32 | – | – | – |
| 13 | Darwin | 21 October 1991 and 3 January 1992 | MT | 0–270 | OS, NWPO | 243 | 6 | 17 | 15 | 14 | 139 | 29 |
| 14 | Novoulyanovsk | 14 July and 17 August 1992 | MT | 0–50 | OS | 130 | 8 | 73 | 33 | – | – | – |
| 15 | Sunflower | 3 December 1992 and 7 April 1993 | BT | 440–995 | OS | 94 | – | – | – | 31 | 269 | 167 |
| 16 | Salvia Ho | 12 January and 2 April 1993 | BT | 250–585 | OS | 96 | 1 | 3 | 3 | 33 | 596 | 231 |
| 17 | TINRO | 6 July and 17 August 1993 | MT | 0 | OS | 131 | 3 | 83 | – | – | – | – |
| 18 | TINRO | 6 August and 13 October 1994 | MT | 0 | OS | 194 | 3 | 86 | 6 | – | – | – |
| 19 | Professor Kizevetter | 31 July and 15 September 1996 | MT | 0 | OS | 85 | 1 | 15 | 7 | – | – | – |
| 20 | Borodino | 1 January and 29 April 1997 | MT | 580 | OS | 287 | – | – | – | 1 | 11 | 11 |
| 21 | Professor Levanidov | 10 July and 31 August 1997 | MT | 20 | NWPO | 185 | 1 | 1 | 1 | – | – | – |
| 22 | TINRO | 8 February and 25 August 1998 | MT | 235–485 | OS | 373 | 19 | 312 | 34 | 2 | 12 | 4 |
| 23 | TINRO | 26 August and 11 December 1998 | MT | 275 | OS | 309 | – | – | – | 1 | 1 | 1 |
| 24 | TINRO | 25 February 13 June 2000 | MT | 155–450 | OS | 320 | 17 | 136 | 39 | – | – | – |
| 25 | Professor Levanidov | 10 September and 14 December 2000 | MT | 409–996 | OS, NWPO | 297 | 18 | 30 | 30 | 5 | 7 | 7 |
| 26 | Borodinoa | 1 March and 13 May 2001 | MT | 235–485 | OS | 210 | – | – | – | 56 | 215 | 194 |
| 27 | Kavrai | 27 June and 22 October 2001 | BT | 413–420 | OS | 403 | – | – | – | 1 | 1 | 1 |
| 28 | Professor Levanidova | 4 and 22 November 2001 | MT | 75–500 | NWPO | 45 | 10 | 49 | 45 | 3 | 13 | 13 |
| 29 | Borodino | 15 January and 15 April 2002 | MT | 285–430 | OS | 180 | – | – | – | 1 | 1 | 1 |
| 30 | Borodino | 10 and 20 May 2002 | MT | 300–450 | OS | 20 | 1 | 1 | 1 | – | – | – |
| 31 | Professor Levanidov | 21 July and 23 August 2002 | MT | 0–365 | NWPO | 109 | 3 | 11 | 10 | – | – | – |
| 32 | Дальокеан | 30 July and 22 October 2002 | BGN | 535 | OS | 104 | 1 | 1 | 1 | – | – | – |
| 33 | Professor Levanidov | 10 August and 31 December 2004 | BT | 420–675 | OS | 314 | 16 | 45 | 25 | 20 | 35 | 30 |
| 34 | Professor Kaganovskyi | 24 October and 30 November 2005 | MT | 50–275 | NWPO | 98 | 4 | 22 | 21 | – | – | – |
| Total | 240 | 5 788 | 863 | 190 | 1 348 | 726 | ||||||
| . | . | . | . | . | . | . | G. tinro . | G. okutanii . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number . | Research vessel/source . | Dates . | Gear . | Depth (m) . | Region . | Hauls . | A . | B . | C . | A . | B . | C . |
| 1 | SRTM 8-452a | 20 September 1972 and 25 December 1972 | IKMT | 50 | NWPO | 36 | 1 | 1 | 1 | – | – | – |
| 2 | Shantar | 14 March 1975 and 26 July 1975 | BT | 3 400 | OS | 464 | – | – | – | 2 | 2 | 2 |
| 3 | SRTM 8-452 | 16 July 1977 and 3 September 1977 | Dredge | 50 | OS | 25 | 1 | 2 | 2 | – | – | – |
| 4 | Novoulyanovsk | 15 September 1984 and 28 October 1984 | MT, BT | 500–1 200 | OS | 156 | 28 | 91 | – | 4 | 4 | – |
| 5 | Novokotovsk | 29 September and 19 November 1984 | MT | 5–200 | NWPO | 127 | – | – | – | 1 | 7 | 2 |
| 6 | Gissar | 8 November and 2 December 1987 | MT | 300–600 | OS | 45 | 3 | 14 | 14 | 2 | 11 | 11 |
| 7 | Darwin | 27 April 1989 and 4 September 1989 | BT | 492–1 310 | OS | 370 | 6 | 14 | 14 | 2 | 2 | 2 |
| 8 | Professor Soldatov | 10 August 1989 and 2 September 1989 | MT | 750 | OS | 38 | 24 | 380 | 380 | 5 | 12 | 12 |
| 9 | Professor Soldatov | 28 November 1990 and 18 December 1990 | MT | 500–1 000 | OS | 23 | 16 | 55 | 27 | 4 | 7 | 7 |
| 10 | Novokotovsk | 24 September 1990 and 31 December 1990 | MT | 5–450 | OS | 309 | 2 | 18 | 4 | – | – | – |
| 11 | Mlechnyi Put | 1 and 30 January 1991 | MT | 100–475 | OS, NWPO | 193 | 45 | 4 296 | 118 | 2 | 3 | 3 |
| 12 | Professor Kizevetter | 12 and 24 November 1991 | MT | 240–859 | NWPO | 38 | 2 | 32 | 32 | – | – | – |
| 13 | Darwin | 21 October 1991 and 3 January 1992 | MT | 0–270 | OS, NWPO | 243 | 6 | 17 | 15 | 14 | 139 | 29 |
| 14 | Novoulyanovsk | 14 July and 17 August 1992 | MT | 0–50 | OS | 130 | 8 | 73 | 33 | – | – | – |
| 15 | Sunflower | 3 December 1992 and 7 April 1993 | BT | 440–995 | OS | 94 | – | – | – | 31 | 269 | 167 |
| 16 | Salvia Ho | 12 January and 2 April 1993 | BT | 250–585 | OS | 96 | 1 | 3 | 3 | 33 | 596 | 231 |
| 17 | TINRO | 6 July and 17 August 1993 | MT | 0 | OS | 131 | 3 | 83 | – | – | – | – |
| 18 | TINRO | 6 August and 13 October 1994 | MT | 0 | OS | 194 | 3 | 86 | 6 | – | – | – |
| 19 | Professor Kizevetter | 31 July and 15 September 1996 | MT | 0 | OS | 85 | 1 | 15 | 7 | – | – | – |
| 20 | Borodino | 1 January and 29 April 1997 | MT | 580 | OS | 287 | – | – | – | 1 | 11 | 11 |
| 21 | Professor Levanidov | 10 July and 31 August 1997 | MT | 20 | NWPO | 185 | 1 | 1 | 1 | – | – | – |
| 22 | TINRO | 8 February and 25 August 1998 | MT | 235–485 | OS | 373 | 19 | 312 | 34 | 2 | 12 | 4 |
| 23 | TINRO | 26 August and 11 December 1998 | MT | 275 | OS | 309 | – | – | – | 1 | 1 | 1 |
| 24 | TINRO | 25 February 13 June 2000 | MT | 155–450 | OS | 320 | 17 | 136 | 39 | – | – | – |
| 25 | Professor Levanidov | 10 September and 14 December 2000 | MT | 409–996 | OS, NWPO | 297 | 18 | 30 | 30 | 5 | 7 | 7 |
| 26 | Borodinoa | 1 March and 13 May 2001 | MT | 235–485 | OS | 210 | – | – | – | 56 | 215 | 194 |
| 27 | Kavrai | 27 June and 22 October 2001 | BT | 413–420 | OS | 403 | – | – | – | 1 | 1 | 1 |
| 28 | Professor Levanidova | 4 and 22 November 2001 | MT | 75–500 | NWPO | 45 | 10 | 49 | 45 | 3 | 13 | 13 |
| 29 | Borodino | 15 January and 15 April 2002 | MT | 285–430 | OS | 180 | – | – | – | 1 | 1 | 1 |
| 30 | Borodino | 10 and 20 May 2002 | MT | 300–450 | OS | 20 | 1 | 1 | 1 | – | – | – |
| 31 | Professor Levanidov | 21 July and 23 August 2002 | MT | 0–365 | NWPO | 109 | 3 | 11 | 10 | – | – | – |
| 32 | Дальокеан | 30 July and 22 October 2002 | BGN | 535 | OS | 104 | 1 | 1 | 1 | – | – | – |
| 33 | Professor Levanidov | 10 August and 31 December 2004 | BT | 420–675 | OS | 314 | 16 | 45 | 25 | 20 | 35 | 30 |
| 34 | Professor Kaganovskyi | 24 October and 30 November 2005 | MT | 50–275 | NWPO | 98 | 4 | 22 | 21 | – | – | – |
| Total | 240 | 5 788 | 863 | 190 | 1 348 | 726 | ||||||
Gears: MT, midwater trawl; BT, bottom trawl; IKMT, Isaacs–Kidd midwater trawl; BGN, bottom gillnet. A, number of hauls with squid; B, number of squid captured; C, number of squid analysed.
aData used in the morphometric analysis.
Identification of G. tinro was based on the following distinct characters: mantle weak and conical posteriorly; thin skin; fins heart-shaped, moderately short, <50% DML, tapering into a cartilaginous “tail”; tentacles long and slender; club hookless, narrow, with the locking zone at the dorsal base consisting of a series of 3–4 alternating grooves and ridges (each ridge with a large smooth-ringed sucker and each groove with a large fleshy knob); dorsal series of alternating suckers and pads continuing along the distal half of the tentacle stalk; arms muscular, ∼50% of DML or longer, ventral arms usually shortest and always thinnest (Nesis, 1972, 1982; Bublitz, 1981). Identification of G. okutanii was based on the following characters: mantle weak, elongated, and conical posteriorly; thin skin; fins frequently shabby, fairly short, <50% DML, tapering into a cartilaginous “tail”; tentacles absent (autotomized or broken), remnants weak, narrow and short; arms strong and fairly long, about half DML or longer, lateral arms longest, ventral arms shortest and thinnest (Nesis, 1972, 1982).
It is relatively easy to identify G. tinro, but correct identification of G. okutanii can be difficult. The latter is clearly distinguished from other species of Gonatopsis (Kubodera et al., 2006b). Gonatopsis borealis is placed in a separate subgenus Boreoteuthis (Nesis, 1973) or even genus (Katugin, 2004), because it has seven rather than five teeth in a transverse row of the radula, no cartilaginous rostrum on the gladius conus, no tentacle stub after the paralarval stage, and different morphometrics. Gonatopsis octopedatus and Gonatopsis sp. A have distinctive arm tips with more than four series of suckers. Only Gonatopsis makko and Gonatopsis japonicus can be confused with G. okutanii, and those two lack stubs of the tentacles and have relatively longer arms. Other gonatid squid lose tentacles at sexual maturity and could be confused with mature G. okutanii, but they are not known to lose tentacles well before maturity. As a result, the presence of tentacular stubs and relatively short and thin ventral arms were used to identify G. okutanii.
DML was used as the standard measure of squid body size. Maturity was assessed using the scale described in Nesis (1982) and Zuev et al. (1985). For comparative morphology, the following external characters were measured: FL and FW (fin length and width), AI and AIw (length and width of the base of the first or dorsal arm), AII and AIIw (length and width of the base of the second or dorso-lateral arm), AIII and AIIIw (length and width of the base of the third or ventro-lateral arm), and AIV and AIVw (length and width of the base of the fourth or ventral arm). Comparisons were made using character measurements and indices, the latter calculated as the character measurement divided by DML.
A digital database was constructed for G. tinro and G. okutanii, including trawl station parameters and catch details (species name and biological characters, including size, weight, sex, and maturity). To obtain the patterns of distribution and information on the biology, the data were pooled, where necessary, by season, depth stratum, etc. The database was created using the computer program “Ichthyologist” (unpublished; developed by N. E. Kravchenko, TINRO), Microsoft Excel, and Microsoft Access. Data were analysed statistically using two programs: Microsoft Excel to construct the patterns of size structure and Surfer v.8.0 to produce spatial distribution patterns.
Results
Squid identified as G. tinro were caught during 26 cruises of the database used here, and those identified as G. okutanii during 20 cruises. In 23 of the 34 cruises (68%), only one of the two species was recorded, and in the other 11 cruises, both species were taken. In 18 of the 34 cruises (52%), these squid were taken in fewer than four hauls per cruise, and always in small numbers. Gonatus tinro distribution was the most biased: 74% of the total catch (4296 of 5791) was made during a single cruise in one winter month of 1991, when the most reliable estimate of the biomass of G. tinro was obtained. In the epipelagic and the upper mesopelagic (0–500 m) zones of the Sea of Okhotsk, species biomass was then assessed at ∼80 000 t, almost 8% of the 17 squid species found in the survey area in winter (Didenko, 1991). As for G. okutanii, 80% of all the squid (1077 of 1345) were captured during three research cruises (two Korean and one Russian) over the slope of the northern Sea of Okhotsk in winter and spring.
Distribution
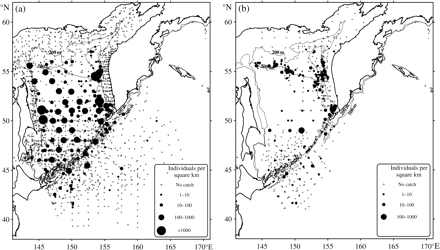
There are many similar traits in the spatial distribution patterns of G. tinro and G. okutanii (Figure 1). Both were found primarily off the shelf, were more common in the Sea of Okhotsk than in the Pacific Ocean off the Kuril Islands, and at greatest concentration in deep water in the southern Sea of Okhotsk and along the western Kamchatka slope. Also, there were differences in the distribution patterns: G. tinro was more common in the central Sea of Okhotsk and off the eastern Sakhalin Shelf, whereas G. okutanii was more local and found mainly over the slope along the 500-m isobath in the northern Sea of Okhotsk, and off western and southwestern Kamchatka. The mean density of G. tinro was 87 ± 15 km−2 (for 214 positive hauls) and of G. okutanii just 7 ± 1 km−2 (for 197 positive hauls). The differences in spatial distribution can be attributed to differences in the depth distributions of the two species: G. tinro were taken more frequently during pelagic surveys, particularly in the central Sea of Okhotsk, and G. okutanii mainly during the relatively few demersal surveys, most of which were carried out on the upper continental slope along the western Kamchatka and in the northern Sea of Okhotsk.
Spatial distribution of (a) G. tinro and (b) G. okutanii in the Sea of Okhotsk and Northwest Pacific Ocean (cpue is shown as the number of squid per square kilometre).
Both species were distributed over a wide range of depths, from the surface down to the bathypelagic zone, and were generally more common in deep water. However, G. tinro were relatively more common than G. okutanii in the upper layers. In the upper mesopelagic zone, densities were estimated at 1739 and 4 squid km−2 for G. tinro and G. okutanii, respectively. In the lower mesopelagic zone, G. tinro was caught relatively uniformly at an average density of ∼44 km−2 in the southern deep basin, and G. okutanii unevenly at greater density, up to 120 km−2, in the Alaid Trench (752 m) and in the deep-sea basin (540 m).
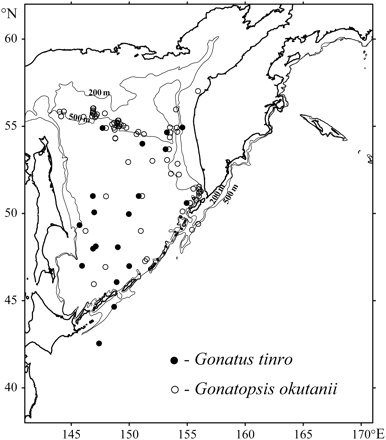
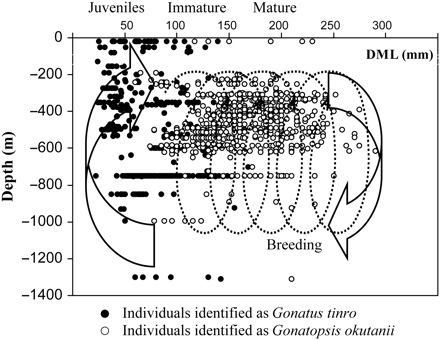
Notwithstanding the apparent differences between G. tinro and G. okutanii in their overall patterns of spatial and bathymetric distribution, late maturity stages (IV and later) were caught in the same geographic areas: over the continental slope and in the deep southern basin of the Sea of Okhotsk (Figure 2). Mature G. tinro and G. okutanii were captured in the upper layers only incidentally and were found almost exclusively at about the same depths in the lower mesopelagic zone and over the slope: 676 ± 72 and 569 ± 78 m, respectively. There was no statistical difference in the depth distributions of mature individuals of the two species (t = 1.01; p = 0.16).
Mature G. tinro and G. okutanii in the Sea of Okhotsk and Northwest Pacific Ocean. The circles indicate trawls where at least one mature (stage IV, V, or VI) squid was captured.
Patterns of squid distribution varied with season. In spring, both species were distributed locally along the western Kamchatka Peninsula and over the northern slopes of the Sea of Okhotsk, where densities of G. tinro and G. okutanii peaked at 1273 and 31 km−2, respectively. There was a notable difference in spring vertical distribution: G. tinro was found only deeper than 500 m, whereas G. okutanii was captured from the epipelagic zone down to the lower mesopelagic. In summer, both species were less frequent in catches than in spring, then mostly in the deep-sea basin, where the densities of G. tinro and G. okutanii reached 123 and 17 km−2, respectively. The vertical distribution in summer was opposite to that observed in spring: G. tinro was present from the surface down to 1310 m, and G. okutanii only at depths of 568–3400 m. In autumn, both species were caught over a wide depth range and in about the same areas, including in the southern basin and over the western Kamchatka slope, where the density of G. tinro and G. okutanii peaked at 90 and 30 km−2, respectively. In winter, the distribution of the two species appeared to be complementary: G. tinro occupied mainly southern deep water, was not registered >500 m, and was most abundant in the upper mesopelagic zone at a maximum density of 1740 km−2; G. okutanii was not found in the pelagic layers and instead was concentrated over the slope of the northern Sea of Okhotsk and off western Kamchatka, where density peaked at 1065 km−2.
Size structure
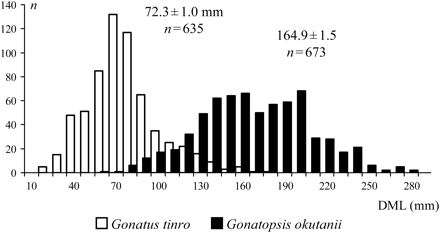
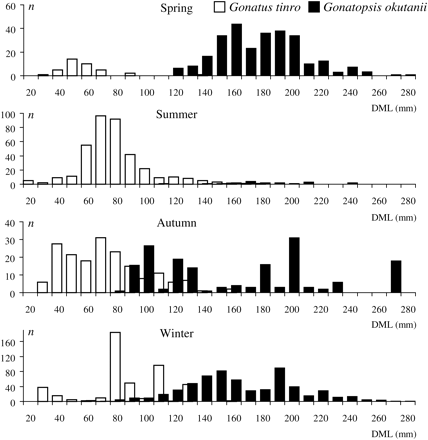
There were significant differences in the size of the G. tinro and G. okutanii caught: the former was generally very much smaller than the latter; the DMLs ranged between 14 and 180 mm and between 60 and 275 mm, respectively, with means of 72.3 ± 1.0 and 164.9 ± 1.5 mm (Figure 3). The size structure of G. tinro and G. okutanii followed varying bathymetric patterns (Figure 4).
Size of G. tinro and G. okutanii in the Sea of Okhotsk and Northwest Pacific Ocean (DML, dorsal mantle length; n, number of squid captured).
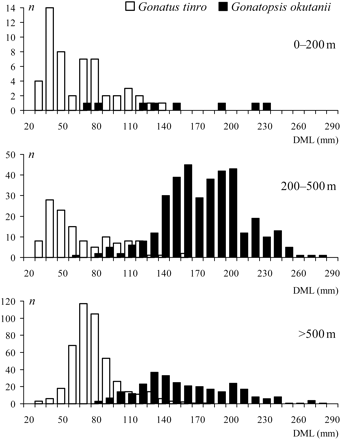
Size of G. tinro and G. okutanii in different depths (DML, dorsal mantle length; n, number of squid captured).
One modal class of 40 mm DML dominated the G. tinro catches in the upper 500 m, and larger squid with a modal class of 70 mm DML were more common deeper than 500 m. The size ranges of G. tinro were similar throughout the water column, mean DML being 62.1 ± 3.8, 67.9 ± 3.1, and 76.8 ± 1.1 mm in the depth ranges 0–200, 200–500, and >500 m, respectively. Size was not significantly different in the epipelagic and upper mesopelagic layers (t = 1.18; p = 0.12). There was a trend towards increasing G. tinro size with depth, and the mean DMLs at 0–200 and >500 m were significantly different (t = 2.73; p < 0.01). The sizes of G. okutanii were also similar throughout the depth range investigated, though so few squid were captured in the epipelagic zone that modal classes could not be discerned, but at least two modal classes were present in each of two deep-sea strata: 160 and 200 mm DML at 200–500 m, and 130 and 200 mm DML >500 m. The mean DMLs for G. okutanii were 153.8 ± 16.0, 170.2 ± 1.8, and 159.3 ± 2.7 mm at depths of 0–200, 200–500, and >500 m, respectively. The difference in sizes between 200–500 m and >500 m was significant (t = 3.34; p < 0.01).
Seasonal patterns in the size structure were apparent for both species (Figure 5). In spring, the G. tinro were small, with a single clear modal class at 40–50 mm DML, but at least two size groups could be discerned for G. okutanii, the main ones being 150–160 and 180–190 mm. In summer, the main modal class of G. tinro was 60–70 mm DML, suggesting growth of the spring mode; G. okutanii were too few to obtain a size structure. By autumn, the size of the two species was more variable, with a number of weakly defined modal classes likely present, probably a reflection of data scarcity. In winter, G. tinro retained polymodality, with a single strong modal class at 70–80 mm DML; the size structure of G. okutanii resembled the spring pattern, with two modal groups, although with somewhat smaller modal classes, at 140–150 and 180–190 mm DML. The last observation suggests that a winter–spring shift in modal classes of G. okutanii could be attributed to growth.
Size of G. tinro and G. okutanii in different seasons (DML, dorsal mantle length; n, number of squid captured).
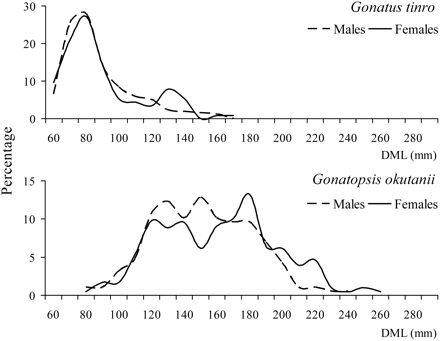
There were certain sex-associated differences in the sizes of both species (Figure 6). In G. tinro, there was no sexual dimorphism in size, the DML of males and females ranging from 52 to 156 and from 52 to 168 mm, respectively, with the same major modal classes of 70–80 mm and means of 83 and 86 mm, respectively. There was no significant difference between the means (t = 1, p = 0.32). Differences in size between males and females were observed in G. okutanii, however, DML ranging from 58 to 240 and from 95 to 275 mm, with means of 150 and 178 mm, respectively. The difference between the means was significant (t = 10.6, p < 0.01).
Sizes of male and female G. tinro and G. okutanii (DML, dorsal mantle length).
Maturity
Almost all the G. tinro were either juvenile or immature, whereas G. okutanii was represented by males and females at various maturity stages, from immature to spent. In both species, maturity increased with increasing DML (Tables 2 and 3). The maturity patterns displayed by G. tinro and G. okutanii were generally congruent. Differences in size with sex became more apparent with maturation in both species. No differences in size between sexes were observed in G. tinro at stage I, but at stages II and III, males appeared to be somewhat smaller than females. No stage IV female G. tinro were found to allow comparison of their size with stage IV males, and at stage V, only two females were caught to afford comparison with stage V males. Differences in size between the sexes appeared clearer at each maturity stage in G. okutanii: females were larger than males and, starting from stage III, females were ∼30% longer than males. On average, males matured at 164.2 ± 4.5 mm DML, and females at 206.0 ± 5.8; mean DML values of prespawning males and spent females were only slightly larger, at 176.8 ± 3.6 and 214.4 ± 2.7 mm, respectively. The average sizes at maturity for G. tinro were not really conclusive, because very few mature squid were present in the database. However, maturation starts at about the same size in both species: males at 110 mm DML and females at ∼140 mm DML in G. tinro and 150 mm in G. okutanii.
Size at maturity of G. tinro.
| . | DML (mm) . | . | . | . | . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maturity stage . | 60 . | 70 . | 80 . | 90 . | 100 . | 110 . | 120 . | 130 . | 140 . | 150 . | 160 . | 170 . | n . | Mean (mm) ± s.e. . | Min (mm) . | Max (mm) . |
| Males | ||||||||||||||||
| I | 17 | 66 | 71 | 34 | 13 | 5 | 3 | 1 | – | – | – | – | 210 | 75.2 ± 0.9 | 52 | 125 |
| II | – | – | 1 | 2 | 9 | 8 | 5 | 2 | – | – | – | – | 27 | 104.3 ± 2.2 | 77 | 128 |
| III | – | – | – | – | – | 1 | 1 | 1 | 2 | – | – | – | 5 | 125.8 ± 6.4 | 104 | 140 |
| IV | – | – | – | – | – | 1 | 2 | 1 | 2 | 4 | 1 | – | 11 | 135.2 ± 4.7 | 108 | 155 |
| V | – | – | – | – | – | – | 2 | 1 | 1 | – | 2 | – | 6 | 134.2 ± 7.6 | 111 | 146 |
| Females | ||||||||||||||||
| I | 11 | 24 | 31 | 16 | 3 | 2 | 1 | 3 | 1 | – | – | – | 92 | 77.0 ± 1.7 | 52 | 137 |
| II | – | – | – | – | 3 | 2 | 3 | 4 | 1 | – | – | – | 13 | 112.9 ± 3.3 | 95 | 132 |
| III | – | – | – | – | – | 1 | – | 2 | 2 | – | 1 | 1 | 7 | 135.9 ± 8.1 | 102 | 168 |
| IV | – | – | – | – | – | – | – | – | – | – | – | – | 0 | – | – | – |
| V | – | – | – | – | – | – | – | – | 2 | – | – | – | 2 | 133.5 ± 0.5 | 133 | 134 |
| . | DML (mm) . | . | . | . | . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maturity stage . | 60 . | 70 . | 80 . | 90 . | 100 . | 110 . | 120 . | 130 . | 140 . | 150 . | 160 . | 170 . | n . | Mean (mm) ± s.e. . | Min (mm) . | Max (mm) . |
| Males | ||||||||||||||||
| I | 17 | 66 | 71 | 34 | 13 | 5 | 3 | 1 | – | – | – | – | 210 | 75.2 ± 0.9 | 52 | 125 |
| II | – | – | 1 | 2 | 9 | 8 | 5 | 2 | – | – | – | – | 27 | 104.3 ± 2.2 | 77 | 128 |
| III | – | – | – | – | – | 1 | 1 | 1 | 2 | – | – | – | 5 | 125.8 ± 6.4 | 104 | 140 |
| IV | – | – | – | – | – | 1 | 2 | 1 | 2 | 4 | 1 | – | 11 | 135.2 ± 4.7 | 108 | 155 |
| V | – | – | – | – | – | – | 2 | 1 | 1 | – | 2 | – | 6 | 134.2 ± 7.6 | 111 | 146 |
| Females | ||||||||||||||||
| I | 11 | 24 | 31 | 16 | 3 | 2 | 1 | 3 | 1 | – | – | – | 92 | 77.0 ± 1.7 | 52 | 137 |
| II | – | – | – | – | 3 | 2 | 3 | 4 | 1 | – | – | – | 13 | 112.9 ± 3.3 | 95 | 132 |
| III | – | – | – | – | – | 1 | – | 2 | 2 | – | 1 | 1 | 7 | 135.9 ± 8.1 | 102 | 168 |
| IV | – | – | – | – | – | – | – | – | – | – | – | – | 0 | – | – | – |
| V | – | – | – | – | – | – | – | – | 2 | – | – | – | 2 | 133.5 ± 0.5 | 133 | 134 |
Size at maturity of G. tinro.
| . | DML (mm) . | . | . | . | . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maturity stage . | 60 . | 70 . | 80 . | 90 . | 100 . | 110 . | 120 . | 130 . | 140 . | 150 . | 160 . | 170 . | n . | Mean (mm) ± s.e. . | Min (mm) . | Max (mm) . |
| Males | ||||||||||||||||
| I | 17 | 66 | 71 | 34 | 13 | 5 | 3 | 1 | – | – | – | – | 210 | 75.2 ± 0.9 | 52 | 125 |
| II | – | – | 1 | 2 | 9 | 8 | 5 | 2 | – | – | – | – | 27 | 104.3 ± 2.2 | 77 | 128 |
| III | – | – | – | – | – | 1 | 1 | 1 | 2 | – | – | – | 5 | 125.8 ± 6.4 | 104 | 140 |
| IV | – | – | – | – | – | 1 | 2 | 1 | 2 | 4 | 1 | – | 11 | 135.2 ± 4.7 | 108 | 155 |
| V | – | – | – | – | – | – | 2 | 1 | 1 | – | 2 | – | 6 | 134.2 ± 7.6 | 111 | 146 |
| Females | ||||||||||||||||
| I | 11 | 24 | 31 | 16 | 3 | 2 | 1 | 3 | 1 | – | – | – | 92 | 77.0 ± 1.7 | 52 | 137 |
| II | – | – | – | – | 3 | 2 | 3 | 4 | 1 | – | – | – | 13 | 112.9 ± 3.3 | 95 | 132 |
| III | – | – | – | – | – | 1 | – | 2 | 2 | – | 1 | 1 | 7 | 135.9 ± 8.1 | 102 | 168 |
| IV | – | – | – | – | – | – | – | – | – | – | – | – | 0 | – | – | – |
| V | – | – | – | – | – | – | – | – | 2 | – | – | – | 2 | 133.5 ± 0.5 | 133 | 134 |
| . | DML (mm) . | . | . | . | . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maturity stage . | 60 . | 70 . | 80 . | 90 . | 100 . | 110 . | 120 . | 130 . | 140 . | 150 . | 160 . | 170 . | n . | Mean (mm) ± s.e. . | Min (mm) . | Max (mm) . |
| Males | ||||||||||||||||
| I | 17 | 66 | 71 | 34 | 13 | 5 | 3 | 1 | – | – | – | – | 210 | 75.2 ± 0.9 | 52 | 125 |
| II | – | – | 1 | 2 | 9 | 8 | 5 | 2 | – | – | – | – | 27 | 104.3 ± 2.2 | 77 | 128 |
| III | – | – | – | – | – | 1 | 1 | 1 | 2 | – | – | – | 5 | 125.8 ± 6.4 | 104 | 140 |
| IV | – | – | – | – | – | 1 | 2 | 1 | 2 | 4 | 1 | – | 11 | 135.2 ± 4.7 | 108 | 155 |
| V | – | – | – | – | – | – | 2 | 1 | 1 | – | 2 | – | 6 | 134.2 ± 7.6 | 111 | 146 |
| Females | ||||||||||||||||
| I | 11 | 24 | 31 | 16 | 3 | 2 | 1 | 3 | 1 | – | – | – | 92 | 77.0 ± 1.7 | 52 | 137 |
| II | – | – | – | – | 3 | 2 | 3 | 4 | 1 | – | – | – | 13 | 112.9 ± 3.3 | 95 | 132 |
| III | – | – | – | – | – | 1 | – | 2 | 2 | – | 1 | 1 | 7 | 135.9 ± 8.1 | 102 | 168 |
| IV | – | – | – | – | – | – | – | – | – | – | – | – | 0 | – | – | – |
| V | – | – | – | – | – | – | – | – | 2 | – | – | – | 2 | 133.5 ± 0.5 | 133 | 134 |
Size at maturity of G. okutanii.
| . | DML (mm) . | . | . | . | . | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maturity stage . | 80 . | 90 . | 100 . | 110 . | 120 . | 130 . | 140 . | 150 . | 160 . | 170 . | 180 . | 190 . | 200 . | 210 . | 220 . | 230 . | 240 . | 250 . | 260 . | 270 . | 280 . | n . | Mean (mm) ± s.e. . | Min (mm) . | Max (mm) . |
| Males | |||||||||||||||||||||||||
| I | – | – | 1 | 1 | 3 | 4 | 2 | 1 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | 13 | 127.3 ± 5.4 | 95 | 175 |
| II | 2 | 2 | 3 | 7 | 8 | 13 | 11 | 5 | 8 | 5 | 1 | 2 | 1 | – | – | 1 | – | – | – | – | – | 69 | 133.5 ± 3.3 | 78 | 235 |
| III | – | – | 2 | – | 7 | 5 | 5 | 8 | 7 | 2 | 5 | 1 | 2 | 1 | – | – | – | – | – | – | – | 45 | 147.5 ± 3.9 | 95 | 210 |
| IV | – | – | – | 1 | 2 | 1 | 1 | 8 | 3 | 6 | 5 | 5 | 3 | – | 1 | – | 1 | – | – | – | – | 37 | 164.2 ± 4.5 | 108 | 240 |
| V | – | – | – | – | – | – | – | 2 | 1 | 5 | 6 | 5 | 2 | 1 | 1 | – | – | – | – | – | – | 23 | 176.8 ± 3.6 | 145 | 211 |
| Females | |||||||||||||||||||||||||
| I | – | – | 2 | 4 | 2 | 4 | 1 | 7 | 2 | – | 1 | – | – | – | – | – | – | – | – | – | – | 26 | 137.1 ± 4.8 | 95 | 188 |
| II | – | – | – | 3 | 5 | 18 | 34 | 31 | 29 | 20 | 25 | 21 | 22 | 6 | 3 | 1 | 2 | – | – | – | – | 220 | 161.1 ± 1.8 | 105 | 232 |
| III | – | – | – | – | – | – | 3 | 3 | 2 | 1 | 5 | 5 | 14 | 8 | 11 | 2 | 3 | 2 | 1 | – | – | 60 | 196.9 ± 3.5 | 131 | 252 |
| IV | – | – | – | – | – | – | – | 1 | 2 | 2 | 3 | 6 | 2 | 1 | 1 | 6 | – | – | – | 1 | 25 | 206.0 ± 5.8 | 145 | 275 | |
| V | – | – | – | – | – | – | – | – | – | – | 5 | 8 | 12 | 10 | 9 | 12 | 7 | 3 | 1 | 4 | – | 71 | 214.4 ± 2.7 | 175 | 270 |
| VI | – | – | – | – | – | – | – | – | – | – | – | 2 | – | – | 1 | – | 1 | – | – | – | 1 | 5 | 222.0 ± 17 | 185 | 275 |
| . | DML (mm) . | . | . | . | . | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maturity stage . | 80 . | 90 . | 100 . | 110 . | 120 . | 130 . | 140 . | 150 . | 160 . | 170 . | 180 . | 190 . | 200 . | 210 . | 220 . | 230 . | 240 . | 250 . | 260 . | 270 . | 280 . | n . | Mean (mm) ± s.e. . | Min (mm) . | Max (mm) . |
| Males | |||||||||||||||||||||||||
| I | – | – | 1 | 1 | 3 | 4 | 2 | 1 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | 13 | 127.3 ± 5.4 | 95 | 175 |
| II | 2 | 2 | 3 | 7 | 8 | 13 | 11 | 5 | 8 | 5 | 1 | 2 | 1 | – | – | 1 | – | – | – | – | – | 69 | 133.5 ± 3.3 | 78 | 235 |
| III | – | – | 2 | – | 7 | 5 | 5 | 8 | 7 | 2 | 5 | 1 | 2 | 1 | – | – | – | – | – | – | – | 45 | 147.5 ± 3.9 | 95 | 210 |
| IV | – | – | – | 1 | 2 | 1 | 1 | 8 | 3 | 6 | 5 | 5 | 3 | – | 1 | – | 1 | – | – | – | – | 37 | 164.2 ± 4.5 | 108 | 240 |
| V | – | – | – | – | – | – | – | 2 | 1 | 5 | 6 | 5 | 2 | 1 | 1 | – | – | – | – | – | – | 23 | 176.8 ± 3.6 | 145 | 211 |
| Females | |||||||||||||||||||||||||
| I | – | – | 2 | 4 | 2 | 4 | 1 | 7 | 2 | – | 1 | – | – | – | – | – | – | – | – | – | – | 26 | 137.1 ± 4.8 | 95 | 188 |
| II | – | – | – | 3 | 5 | 18 | 34 | 31 | 29 | 20 | 25 | 21 | 22 | 6 | 3 | 1 | 2 | – | – | – | – | 220 | 161.1 ± 1.8 | 105 | 232 |
| III | – | – | – | – | – | – | 3 | 3 | 2 | 1 | 5 | 5 | 14 | 8 | 11 | 2 | 3 | 2 | 1 | – | – | 60 | 196.9 ± 3.5 | 131 | 252 |
| IV | – | – | – | – | – | – | – | 1 | 2 | 2 | 3 | 6 | 2 | 1 | 1 | 6 | – | – | – | 1 | 25 | 206.0 ± 5.8 | 145 | 275 | |
| V | – | – | – | – | – | – | – | – | – | – | 5 | 8 | 12 | 10 | 9 | 12 | 7 | 3 | 1 | 4 | – | 71 | 214.4 ± 2.7 | 175 | 270 |
| VI | – | – | – | – | – | – | – | – | – | – | – | 2 | – | – | 1 | – | 1 | – | – | – | 1 | 5 | 222.0 ± 17 | 185 | 275 |
Size at maturity of G. okutanii.
| . | DML (mm) . | . | . | . | . | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maturity stage . | 80 . | 90 . | 100 . | 110 . | 120 . | 130 . | 140 . | 150 . | 160 . | 170 . | 180 . | 190 . | 200 . | 210 . | 220 . | 230 . | 240 . | 250 . | 260 . | 270 . | 280 . | n . | Mean (mm) ± s.e. . | Min (mm) . | Max (mm) . |
| Males | |||||||||||||||||||||||||
| I | – | – | 1 | 1 | 3 | 4 | 2 | 1 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | 13 | 127.3 ± 5.4 | 95 | 175 |
| II | 2 | 2 | 3 | 7 | 8 | 13 | 11 | 5 | 8 | 5 | 1 | 2 | 1 | – | – | 1 | – | – | – | – | – | 69 | 133.5 ± 3.3 | 78 | 235 |
| III | – | – | 2 | – | 7 | 5 | 5 | 8 | 7 | 2 | 5 | 1 | 2 | 1 | – | – | – | – | – | – | – | 45 | 147.5 ± 3.9 | 95 | 210 |
| IV | – | – | – | 1 | 2 | 1 | 1 | 8 | 3 | 6 | 5 | 5 | 3 | – | 1 | – | 1 | – | – | – | – | 37 | 164.2 ± 4.5 | 108 | 240 |
| V | – | – | – | – | – | – | – | 2 | 1 | 5 | 6 | 5 | 2 | 1 | 1 | – | – | – | – | – | – | 23 | 176.8 ± 3.6 | 145 | 211 |
| Females | |||||||||||||||||||||||||
| I | – | – | 2 | 4 | 2 | 4 | 1 | 7 | 2 | – | 1 | – | – | – | – | – | – | – | – | – | – | 26 | 137.1 ± 4.8 | 95 | 188 |
| II | – | – | – | 3 | 5 | 18 | 34 | 31 | 29 | 20 | 25 | 21 | 22 | 6 | 3 | 1 | 2 | – | – | – | – | 220 | 161.1 ± 1.8 | 105 | 232 |
| III | – | – | – | – | – | – | 3 | 3 | 2 | 1 | 5 | 5 | 14 | 8 | 11 | 2 | 3 | 2 | 1 | – | – | 60 | 196.9 ± 3.5 | 131 | 252 |
| IV | – | – | – | – | – | – | – | 1 | 2 | 2 | 3 | 6 | 2 | 1 | 1 | 6 | – | – | – | 1 | 25 | 206.0 ± 5.8 | 145 | 275 | |
| V | – | – | – | – | – | – | – | – | – | – | 5 | 8 | 12 | 10 | 9 | 12 | 7 | 3 | 1 | 4 | – | 71 | 214.4 ± 2.7 | 175 | 270 |
| VI | – | – | – | – | – | – | – | – | – | – | – | 2 | – | – | 1 | – | 1 | – | – | – | 1 | 5 | 222.0 ± 17 | 185 | 275 |
| . | DML (mm) . | . | . | . | . | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maturity stage . | 80 . | 90 . | 100 . | 110 . | 120 . | 130 . | 140 . | 150 . | 160 . | 170 . | 180 . | 190 . | 200 . | 210 . | 220 . | 230 . | 240 . | 250 . | 260 . | 270 . | 280 . | n . | Mean (mm) ± s.e. . | Min (mm) . | Max (mm) . |
| Males | |||||||||||||||||||||||||
| I | – | – | 1 | 1 | 3 | 4 | 2 | 1 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | 13 | 127.3 ± 5.4 | 95 | 175 |
| II | 2 | 2 | 3 | 7 | 8 | 13 | 11 | 5 | 8 | 5 | 1 | 2 | 1 | – | – | 1 | – | – | – | – | – | 69 | 133.5 ± 3.3 | 78 | 235 |
| III | – | – | 2 | – | 7 | 5 | 5 | 8 | 7 | 2 | 5 | 1 | 2 | 1 | – | – | – | – | – | – | – | 45 | 147.5 ± 3.9 | 95 | 210 |
| IV | – | – | – | 1 | 2 | 1 | 1 | 8 | 3 | 6 | 5 | 5 | 3 | – | 1 | – | 1 | – | – | – | – | 37 | 164.2 ± 4.5 | 108 | 240 |
| V | – | – | – | – | – | – | – | 2 | 1 | 5 | 6 | 5 | 2 | 1 | 1 | – | – | – | – | – | – | 23 | 176.8 ± 3.6 | 145 | 211 |
| Females | |||||||||||||||||||||||||
| I | – | – | 2 | 4 | 2 | 4 | 1 | 7 | 2 | – | 1 | – | – | – | – | – | – | – | – | – | – | 26 | 137.1 ± 4.8 | 95 | 188 |
| II | – | – | – | 3 | 5 | 18 | 34 | 31 | 29 | 20 | 25 | 21 | 22 | 6 | 3 | 1 | 2 | – | – | – | – | 220 | 161.1 ± 1.8 | 105 | 232 |
| III | – | – | – | – | – | – | 3 | 3 | 2 | 1 | 5 | 5 | 14 | 8 | 11 | 2 | 3 | 2 | 1 | – | – | 60 | 196.9 ± 3.5 | 131 | 252 |
| IV | – | – | – | – | – | – | – | 1 | 2 | 2 | 3 | 6 | 2 | 1 | 1 | 6 | – | – | – | 1 | 25 | 206.0 ± 5.8 | 145 | 275 | |
| V | – | – | – | – | – | – | – | – | – | – | 5 | 8 | 12 | 10 | 9 | 12 | 7 | 3 | 1 | 4 | – | 71 | 214.4 ± 2.7 | 175 | 270 |
| VI | – | – | – | – | – | – | – | – | – | – | – | 2 | – | – | 1 | – | 1 | – | – | – | 1 | 5 | 222.0 ± 17 | 185 | 275 |
Monthly maturity patterns of the two species differed, but there were similar trends in the monthly patterns. Immature G. tinro dominated by number during late summer, autumn, and winter, and few maturing squid, mainly males, were caught in February or March and from summer through autumn (Table 4). Most of the immature G. okutanii were captured during winter and spring, but mature squid were present virtually year-round, and spent females were occasionally taken in March and April (Table 5). Mature males of both species were caught during all seasons.
Monthly maturity of G. tinro.
| . | DML (mm) . | Maturity stage (%) . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Month . | Min . | Max . | Mean . | n . | I . | II . | III . | IV . | V . |
| Males | |||||||||
| January | 85 | 135 | 108 | 11 | 63.6 | 27.3 | 9.1 | – | – |
| February | 120 | 157 | 146 | 4 | – | – | 50.0 | 25.0 | 25.0 |
| March | 130 | 155 | 142 | 5 | – | – | – | 40.0 | 60.0 |
| April | – | – | – | 0 | – | – | – | – | – |
| May | – | – | – | 0 | – | – | – | – | – |
| June | 142 | 142 | 142 | 1 | – | – | – | 100 | – |
| July | 61 | 130 | 85 | 6 | 83.3 | – | – | – | 16.7 |
| August | 52 | 145 | 77 | 182 | 85.7 | 10.0 | 0.5 | 3.3 | 0.5 |
| September | 58 | 135 | 75 | 25 | 92.0 | 4.0 | – | – | 4.0 |
| October | 88 | 155 | 113 | 8 | 75.0 | 12.5 | – | – | 12.5 |
| November | 62 | 125 | 91 | 14 | 78.6 | 14.3 | – | – | 7.1 |
| December | 80 | 115 | 103 | 4 | 75.0 | 25.0 | – | – | – |
| Females | |||||||||
| January | 90 | 135 | 108 | 11 | 63.6 | 27.3 | 9.1 | – | – |
| February | – | – | – | 0 | – | – | – | – | – |
| March | 135 | 135 | 135 | 1 | – | – | 100 | – | – |
| April | – | – | – | 0 | – | – | – | – | – |
| May | – | – | – | 0 | – | – | – | – | – |
| June | 142 | 142 | 142 | 1 | – | – | – | 100 | – |
| July | 60 | 168 | 128 | 3 | 33.3 | – | 66.7 | – | – |
| August | 52 | 137 | 82 | 92 | 83.6 | 10.9 | 3.3 | – | 2.2 |
| September | 59 | 124 | 78 | 6 | 83.3 | – | 16.7 | – | – |
| October | 129 | 129 | 129 | 1 | 100 | – | – | – | – |
| November | 63 | 137 | 102 | 8 | 75.0 | 25.0 | – | – | – |
| December | – | – | – | 0 | – | – | – | – | – |
| . | DML (mm) . | Maturity stage (%) . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Month . | Min . | Max . | Mean . | n . | I . | II . | III . | IV . | V . |
| Males | |||||||||
| January | 85 | 135 | 108 | 11 | 63.6 | 27.3 | 9.1 | – | – |
| February | 120 | 157 | 146 | 4 | – | – | 50.0 | 25.0 | 25.0 |
| March | 130 | 155 | 142 | 5 | – | – | – | 40.0 | 60.0 |
| April | – | – | – | 0 | – | – | – | – | – |
| May | – | – | – | 0 | – | – | – | – | – |
| June | 142 | 142 | 142 | 1 | – | – | – | 100 | – |
| July | 61 | 130 | 85 | 6 | 83.3 | – | – | – | 16.7 |
| August | 52 | 145 | 77 | 182 | 85.7 | 10.0 | 0.5 | 3.3 | 0.5 |
| September | 58 | 135 | 75 | 25 | 92.0 | 4.0 | – | – | 4.0 |
| October | 88 | 155 | 113 | 8 | 75.0 | 12.5 | – | – | 12.5 |
| November | 62 | 125 | 91 | 14 | 78.6 | 14.3 | – | – | 7.1 |
| December | 80 | 115 | 103 | 4 | 75.0 | 25.0 | – | – | – |
| Females | |||||||||
| January | 90 | 135 | 108 | 11 | 63.6 | 27.3 | 9.1 | – | – |
| February | – | – | – | 0 | – | – | – | – | – |
| March | 135 | 135 | 135 | 1 | – | – | 100 | – | – |
| April | – | – | – | 0 | – | – | – | – | – |
| May | – | – | – | 0 | – | – | – | – | – |
| June | 142 | 142 | 142 | 1 | – | – | – | 100 | – |
| July | 60 | 168 | 128 | 3 | 33.3 | – | 66.7 | – | – |
| August | 52 | 137 | 82 | 92 | 83.6 | 10.9 | 3.3 | – | 2.2 |
| September | 59 | 124 | 78 | 6 | 83.3 | – | 16.7 | – | – |
| October | 129 | 129 | 129 | 1 | 100 | – | – | – | – |
| November | 63 | 137 | 102 | 8 | 75.0 | 25.0 | – | – | – |
| December | – | – | – | 0 | – | – | – | – | – |
Monthly maturity of G. tinro.
| . | DML (mm) . | Maturity stage (%) . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Month . | Min . | Max . | Mean . | n . | I . | II . | III . | IV . | V . |
| Males | |||||||||
| January | 85 | 135 | 108 | 11 | 63.6 | 27.3 | 9.1 | – | – |
| February | 120 | 157 | 146 | 4 | – | – | 50.0 | 25.0 | 25.0 |
| March | 130 | 155 | 142 | 5 | – | – | – | 40.0 | 60.0 |
| April | – | – | – | 0 | – | – | – | – | – |
| May | – | – | – | 0 | – | – | – | – | – |
| June | 142 | 142 | 142 | 1 | – | – | – | 100 | – |
| July | 61 | 130 | 85 | 6 | 83.3 | – | – | – | 16.7 |
| August | 52 | 145 | 77 | 182 | 85.7 | 10.0 | 0.5 | 3.3 | 0.5 |
| September | 58 | 135 | 75 | 25 | 92.0 | 4.0 | – | – | 4.0 |
| October | 88 | 155 | 113 | 8 | 75.0 | 12.5 | – | – | 12.5 |
| November | 62 | 125 | 91 | 14 | 78.6 | 14.3 | – | – | 7.1 |
| December | 80 | 115 | 103 | 4 | 75.0 | 25.0 | – | – | – |
| Females | |||||||||
| January | 90 | 135 | 108 | 11 | 63.6 | 27.3 | 9.1 | – | – |
| February | – | – | – | 0 | – | – | – | – | – |
| March | 135 | 135 | 135 | 1 | – | – | 100 | – | – |
| April | – | – | – | 0 | – | – | – | – | – |
| May | – | – | – | 0 | – | – | – | – | – |
| June | 142 | 142 | 142 | 1 | – | – | – | 100 | – |
| July | 60 | 168 | 128 | 3 | 33.3 | – | 66.7 | – | – |
| August | 52 | 137 | 82 | 92 | 83.6 | 10.9 | 3.3 | – | 2.2 |
| September | 59 | 124 | 78 | 6 | 83.3 | – | 16.7 | – | – |
| October | 129 | 129 | 129 | 1 | 100 | – | – | – | – |
| November | 63 | 137 | 102 | 8 | 75.0 | 25.0 | – | – | – |
| December | – | – | – | 0 | – | – | – | – | – |
| . | DML (mm) . | Maturity stage (%) . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Month . | Min . | Max . | Mean . | n . | I . | II . | III . | IV . | V . |
| Males | |||||||||
| January | 85 | 135 | 108 | 11 | 63.6 | 27.3 | 9.1 | – | – |
| February | 120 | 157 | 146 | 4 | – | – | 50.0 | 25.0 | 25.0 |
| March | 130 | 155 | 142 | 5 | – | – | – | 40.0 | 60.0 |
| April | – | – | – | 0 | – | – | – | – | – |
| May | – | – | – | 0 | – | – | – | – | – |
| June | 142 | 142 | 142 | 1 | – | – | – | 100 | – |
| July | 61 | 130 | 85 | 6 | 83.3 | – | – | – | 16.7 |
| August | 52 | 145 | 77 | 182 | 85.7 | 10.0 | 0.5 | 3.3 | 0.5 |
| September | 58 | 135 | 75 | 25 | 92.0 | 4.0 | – | – | 4.0 |
| October | 88 | 155 | 113 | 8 | 75.0 | 12.5 | – | – | 12.5 |
| November | 62 | 125 | 91 | 14 | 78.6 | 14.3 | – | – | 7.1 |
| December | 80 | 115 | 103 | 4 | 75.0 | 25.0 | – | – | – |
| Females | |||||||||
| January | 90 | 135 | 108 | 11 | 63.6 | 27.3 | 9.1 | – | – |
| February | – | – | – | 0 | – | – | – | – | – |
| March | 135 | 135 | 135 | 1 | – | – | 100 | – | – |
| April | – | – | – | 0 | – | – | – | – | – |
| May | – | – | – | 0 | – | – | – | – | – |
| June | 142 | 142 | 142 | 1 | – | – | – | 100 | – |
| July | 60 | 168 | 128 | 3 | 33.3 | – | 66.7 | – | – |
| August | 52 | 137 | 82 | 92 | 83.6 | 10.9 | 3.3 | – | 2.2 |
| September | 59 | 124 | 78 | 6 | 83.3 | – | 16.7 | – | – |
| October | 129 | 129 | 129 | 1 | 100 | – | – | – | – |
| November | 63 | 137 | 102 | 8 | 75.0 | 25.0 | – | – | – |
| December | – | – | – | 0 | – | – | – | – | – |
Monthly maturity of G. okutanii.
| . | DML (mm) . | Maturity stage (%) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Month . | Min . | Max . | Mean . | n . | I . | II . | III . | IV . | V . | VI . |
| Males | ||||||||||
| January | 78 | 240 | 140 | 76 | 7.9 | 51.3 | 32.9 | 7.9 | – | – |
| February | 78 | 215 | 145 | 28 | 7.2 | 32.1 | 39.3 | 21.4 | – | – |
| March | 115 | 190 | 154 | 19 | 15.8 | 36.8 | 10.5 | 21.1 | 15.8 | – |
| April | 120 | 199 | 164 | 23 | – | – | 21.8 | 39.1 | 39.1 | – |
| May | 161 | 211 | 177 | 7 | – | – | – | 28.6 | 71.4 | – |
| June | – | – | – | 0 | – | – | – | – | – | – |
| July | 172 | 172 | 172 | 1 | – | – | – | – | 100 | – |
| August | 108 | 175 | 155 | 8 | – | – | 12.5 | 37.5 | 50.0 | – |
| September | – | – | – | 0 | – | – | – | – | – | – |
| October | 95 | 152 | 127 | 6 | 16.7 | 83.3 | – | – | – | – |
| November | 137 | 290 | 220 | 6 | 16.7 | 66.6 | – | – | 16.7 | – |
| December | 109 | 145 | 128 | 6 | – | – | 83.3 | 16.7 | – | – |
| Females | ||||||||||
| January | 95 | 275 | 171 | 157 | 11.5 | 51.5 | 4.5 | 4.5 | 28.0 | – |
| February | 108 | 240 | 171 | 49 | 4.1 | 69.3 | 12.3 | 4.1 | 10.2 | – |
| March | 115 | 270 | 177 | 44 | 2.3 | 65.8 | 11.4 | 11.4 | 8.6 | 0.5 |
| April | 120 | 275 | 185 | 72 | 2.8 | 61.0 | 30.6 | 1.4 | 3.8 | 0.4 |
| May | 125 | 242 | 187 | 40 | – | 55.0 | 32.5 | 10 | 2.5 | – |
| June | 190 | 238 | 207 | 4 | – | – | – | 75.0 | 25.0 | – |
| July | – | – | – | 0 | – | – | – | – | – | – |
| August | 170 | 232 | 202 | 5 | – | – | 20.0 | 20.0 | 60.0 | – |
| September | 198 | 200 | 199 | 2 | – | – | 100 | – | ||
| October | 107 | 265 | 179 | 8 | 25.0 | 25.0 | 25.0 | 12.5 | 12.5 | – |
| November | 190 | 270 | 220 | 9 | – | – | 11.1 | – | 88.9 | – |
| December | 125 | 240 | 175 | 15 | 6.7 | 40.0 | 20.0 | 6.7 | 26.6 | – |
| . | DML (mm) . | Maturity stage (%) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Month . | Min . | Max . | Mean . | n . | I . | II . | III . | IV . | V . | VI . |
| Males | ||||||||||
| January | 78 | 240 | 140 | 76 | 7.9 | 51.3 | 32.9 | 7.9 | – | – |
| February | 78 | 215 | 145 | 28 | 7.2 | 32.1 | 39.3 | 21.4 | – | – |
| March | 115 | 190 | 154 | 19 | 15.8 | 36.8 | 10.5 | 21.1 | 15.8 | – |
| April | 120 | 199 | 164 | 23 | – | – | 21.8 | 39.1 | 39.1 | – |
| May | 161 | 211 | 177 | 7 | – | – | – | 28.6 | 71.4 | – |
| June | – | – | – | 0 | – | – | – | – | – | – |
| July | 172 | 172 | 172 | 1 | – | – | – | – | 100 | – |
| August | 108 | 175 | 155 | 8 | – | – | 12.5 | 37.5 | 50.0 | – |
| September | – | – | – | 0 | – | – | – | – | – | – |
| October | 95 | 152 | 127 | 6 | 16.7 | 83.3 | – | – | – | – |
| November | 137 | 290 | 220 | 6 | 16.7 | 66.6 | – | – | 16.7 | – |
| December | 109 | 145 | 128 | 6 | – | – | 83.3 | 16.7 | – | – |
| Females | ||||||||||
| January | 95 | 275 | 171 | 157 | 11.5 | 51.5 | 4.5 | 4.5 | 28.0 | – |
| February | 108 | 240 | 171 | 49 | 4.1 | 69.3 | 12.3 | 4.1 | 10.2 | – |
| March | 115 | 270 | 177 | 44 | 2.3 | 65.8 | 11.4 | 11.4 | 8.6 | 0.5 |
| April | 120 | 275 | 185 | 72 | 2.8 | 61.0 | 30.6 | 1.4 | 3.8 | 0.4 |
| May | 125 | 242 | 187 | 40 | – | 55.0 | 32.5 | 10 | 2.5 | – |
| June | 190 | 238 | 207 | 4 | – | – | – | 75.0 | 25.0 | – |
| July | – | – | – | 0 | – | – | – | – | – | – |
| August | 170 | 232 | 202 | 5 | – | – | 20.0 | 20.0 | 60.0 | – |
| September | 198 | 200 | 199 | 2 | – | – | 100 | – | ||
| October | 107 | 265 | 179 | 8 | 25.0 | 25.0 | 25.0 | 12.5 | 12.5 | – |
| November | 190 | 270 | 220 | 9 | – | – | 11.1 | – | 88.9 | – |
| December | 125 | 240 | 175 | 15 | 6.7 | 40.0 | 20.0 | 6.7 | 26.6 | – |
Monthly maturity of G. okutanii.
| . | DML (mm) . | Maturity stage (%) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Month . | Min . | Max . | Mean . | n . | I . | II . | III . | IV . | V . | VI . |
| Males | ||||||||||
| January | 78 | 240 | 140 | 76 | 7.9 | 51.3 | 32.9 | 7.9 | – | – |
| February | 78 | 215 | 145 | 28 | 7.2 | 32.1 | 39.3 | 21.4 | – | – |
| March | 115 | 190 | 154 | 19 | 15.8 | 36.8 | 10.5 | 21.1 | 15.8 | – |
| April | 120 | 199 | 164 | 23 | – | – | 21.8 | 39.1 | 39.1 | – |
| May | 161 | 211 | 177 | 7 | – | – | – | 28.6 | 71.4 | – |
| June | – | – | – | 0 | – | – | – | – | – | – |
| July | 172 | 172 | 172 | 1 | – | – | – | – | 100 | – |
| August | 108 | 175 | 155 | 8 | – | – | 12.5 | 37.5 | 50.0 | – |
| September | – | – | – | 0 | – | – | – | – | – | – |
| October | 95 | 152 | 127 | 6 | 16.7 | 83.3 | – | – | – | – |
| November | 137 | 290 | 220 | 6 | 16.7 | 66.6 | – | – | 16.7 | – |
| December | 109 | 145 | 128 | 6 | – | – | 83.3 | 16.7 | – | – |
| Females | ||||||||||
| January | 95 | 275 | 171 | 157 | 11.5 | 51.5 | 4.5 | 4.5 | 28.0 | – |
| February | 108 | 240 | 171 | 49 | 4.1 | 69.3 | 12.3 | 4.1 | 10.2 | – |
| March | 115 | 270 | 177 | 44 | 2.3 | 65.8 | 11.4 | 11.4 | 8.6 | 0.5 |
| April | 120 | 275 | 185 | 72 | 2.8 | 61.0 | 30.6 | 1.4 | 3.8 | 0.4 |
| May | 125 | 242 | 187 | 40 | – | 55.0 | 32.5 | 10 | 2.5 | – |
| June | 190 | 238 | 207 | 4 | – | – | – | 75.0 | 25.0 | – |
| July | – | – | – | 0 | – | – | – | – | – | – |
| August | 170 | 232 | 202 | 5 | – | – | 20.0 | 20.0 | 60.0 | – |
| September | 198 | 200 | 199 | 2 | – | – | 100 | – | ||
| October | 107 | 265 | 179 | 8 | 25.0 | 25.0 | 25.0 | 12.5 | 12.5 | – |
| November | 190 | 270 | 220 | 9 | – | – | 11.1 | – | 88.9 | – |
| December | 125 | 240 | 175 | 15 | 6.7 | 40.0 | 20.0 | 6.7 | 26.6 | – |
| . | DML (mm) . | Maturity stage (%) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Month . | Min . | Max . | Mean . | n . | I . | II . | III . | IV . | V . | VI . |
| Males | ||||||||||
| January | 78 | 240 | 140 | 76 | 7.9 | 51.3 | 32.9 | 7.9 | – | – |
| February | 78 | 215 | 145 | 28 | 7.2 | 32.1 | 39.3 | 21.4 | – | – |
| March | 115 | 190 | 154 | 19 | 15.8 | 36.8 | 10.5 | 21.1 | 15.8 | – |
| April | 120 | 199 | 164 | 23 | – | – | 21.8 | 39.1 | 39.1 | – |
| May | 161 | 211 | 177 | 7 | – | – | – | 28.6 | 71.4 | – |
| June | – | – | – | 0 | – | – | – | – | – | – |
| July | 172 | 172 | 172 | 1 | – | – | – | – | 100 | – |
| August | 108 | 175 | 155 | 8 | – | – | 12.5 | 37.5 | 50.0 | – |
| September | – | – | – | 0 | – | – | – | – | – | – |
| October | 95 | 152 | 127 | 6 | 16.7 | 83.3 | – | – | – | – |
| November | 137 | 290 | 220 | 6 | 16.7 | 66.6 | – | – | 16.7 | – |
| December | 109 | 145 | 128 | 6 | – | – | 83.3 | 16.7 | – | – |
| Females | ||||||||||
| January | 95 | 275 | 171 | 157 | 11.5 | 51.5 | 4.5 | 4.5 | 28.0 | – |
| February | 108 | 240 | 171 | 49 | 4.1 | 69.3 | 12.3 | 4.1 | 10.2 | – |
| March | 115 | 270 | 177 | 44 | 2.3 | 65.8 | 11.4 | 11.4 | 8.6 | 0.5 |
| April | 120 | 275 | 185 | 72 | 2.8 | 61.0 | 30.6 | 1.4 | 3.8 | 0.4 |
| May | 125 | 242 | 187 | 40 | – | 55.0 | 32.5 | 10 | 2.5 | – |
| June | 190 | 238 | 207 | 4 | – | – | – | 75.0 | 25.0 | – |
| July | – | – | – | 0 | – | – | – | – | – | – |
| August | 170 | 232 | 202 | 5 | – | – | 20.0 | 20.0 | 60.0 | – |
| September | 198 | 200 | 199 | 2 | – | – | 100 | – | ||
| October | 107 | 265 | 179 | 8 | 25.0 | 25.0 | 25.0 | 12.5 | 12.5 | – |
| November | 190 | 270 | 220 | 9 | – | – | 11.1 | – | 88.9 | – |
| December | 125 | 240 | 175 | 15 | 6.7 | 40.0 | 20.0 | 6.7 | 26.6 | – |
Morphology
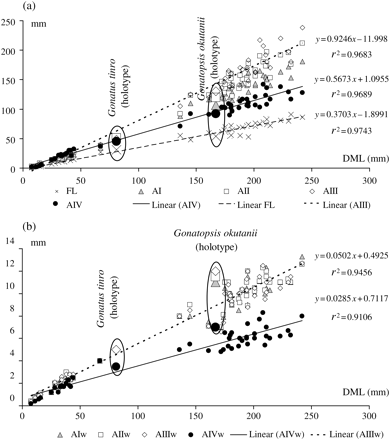
The two species formed two distinct size-related groups when their morphometric characters were plotted against DML: one group consisted of small animals (DML < 90 mm) identified as G. tinro, including the holotype, and the other consisted of much larger animals (DML > 130 mm) identified as G. okutanii, including the holotype (Figure 7). Regression lines were plotted for the three most characteristic length features (Figure 7a): the relative length of AIII (which was longest in the largest squid), the relative length of AIV (which seemed to become shorter in larger squid), and relative FL. Two other regression lines show the relative width of the longest (AIII) and the shortest and thinnest (AIV) arms (Figure 7b). The plots indicate that, in large squid (G. okutanii), differences in arm length and width are more pronounced than in small ones (G. tinro). However, the relationships between each character and DML can be explained by simple regressions with high correlation coefficients (r2 > 0.9), suggesting that both groups could have been sampled from a single “assemblage”. Pairwise comparisons of intercepts and slopes of regression lines for each character of G. tinro and G. okutanii yielded no significant differences for intercept and three out of ten significant differences for slope (Table 6). Statistical differences were attributable to the relatively longer arms II and III, and the relatively thinner arms IV in G. okutanii compared with those of G. tinro.
Regression coefficients for the morphometric characters of G. tinro and G. okutanii.
| . | Intercept . | Slope . | ||
|---|---|---|---|---|
| Comparison . | p-value . | Significance . | p-value . | Significance . |
| FL vs. DML | 0.57 | n.s. | 0.20 | n.s. |
| FW vs. DML | 0.71 | n.s. | 0.08 | n.s. |
| AI vs. DML | 0.49 | n.s. | 0.09 | n.s. |
| AII vs. DML | 0.56 | n.s. | 0.03 | ** |
| AIII vs. DML | 0.28 | n.s. | 0.015 | ** |
| AIV vs. DML | 0.91 | n.s. | 0.53 | n.s. |
| AIw vs. DML | 0.75 | n.s. | 0.22 | n.s. |
| AIIw vs. DML | 0.43 | n.s. | 0.16 | n.s. |
| AIIIw vs. DML | 0.53 | n.s. | 0.17 | n.s. |
| AIVw vs. DML | 0.92 | n.s. | 0.005 | *** |
| . | Intercept . | Slope . | ||
|---|---|---|---|---|
| Comparison . | p-value . | Significance . | p-value . | Significance . |
| FL vs. DML | 0.57 | n.s. | 0.20 | n.s. |
| FW vs. DML | 0.71 | n.s. | 0.08 | n.s. |
| AI vs. DML | 0.49 | n.s. | 0.09 | n.s. |
| AII vs. DML | 0.56 | n.s. | 0.03 | ** |
| AIII vs. DML | 0.28 | n.s. | 0.015 | ** |
| AIV vs. DML | 0.91 | n.s. | 0.53 | n.s. |
| AIw vs. DML | 0.75 | n.s. | 0.22 | n.s. |
| AIIw vs. DML | 0.43 | n.s. | 0.16 | n.s. |
| AIIIw vs. DML | 0.53 | n.s. | 0.17 | n.s. |
| AIVw vs. DML | 0.92 | n.s. | 0.005 | *** |
DML, dorsal mantle length; FL, fin length; FW, fin width; AI–AIV, length of arms 1–4; AIw–AIVw, width of the base of arms 1–4; n.s., not significant.
**95% significance level.
***99% significance level.
Regression coefficients for the morphometric characters of G. tinro and G. okutanii.
| . | Intercept . | Slope . | ||
|---|---|---|---|---|
| Comparison . | p-value . | Significance . | p-value . | Significance . |
| FL vs. DML | 0.57 | n.s. | 0.20 | n.s. |
| FW vs. DML | 0.71 | n.s. | 0.08 | n.s. |
| AI vs. DML | 0.49 | n.s. | 0.09 | n.s. |
| AII vs. DML | 0.56 | n.s. | 0.03 | ** |
| AIII vs. DML | 0.28 | n.s. | 0.015 | ** |
| AIV vs. DML | 0.91 | n.s. | 0.53 | n.s. |
| AIw vs. DML | 0.75 | n.s. | 0.22 | n.s. |
| AIIw vs. DML | 0.43 | n.s. | 0.16 | n.s. |
| AIIIw vs. DML | 0.53 | n.s. | 0.17 | n.s. |
| AIVw vs. DML | 0.92 | n.s. | 0.005 | *** |
| . | Intercept . | Slope . | ||
|---|---|---|---|---|
| Comparison . | p-value . | Significance . | p-value . | Significance . |
| FL vs. DML | 0.57 | n.s. | 0.20 | n.s. |
| FW vs. DML | 0.71 | n.s. | 0.08 | n.s. |
| AI vs. DML | 0.49 | n.s. | 0.09 | n.s. |
| AII vs. DML | 0.56 | n.s. | 0.03 | ** |
| AIII vs. DML | 0.28 | n.s. | 0.015 | ** |
| AIV vs. DML | 0.91 | n.s. | 0.53 | n.s. |
| AIw vs. DML | 0.75 | n.s. | 0.22 | n.s. |
| AIIw vs. DML | 0.43 | n.s. | 0.16 | n.s. |
| AIIIw vs. DML | 0.53 | n.s. | 0.17 | n.s. |
| AIVw vs. DML | 0.92 | n.s. | 0.005 | *** |
DML, dorsal mantle length; FL, fin length; FW, fin width; AI–AIV, length of arms 1–4; AIw–AIVw, width of the base of arms 1–4; n.s., not significant.
**95% significance level.
***99% significance level.
Metric characters of G. tinro and G. okutanii. (a) Arm and FL; (b) arm width. DML, dorsal mantle length; FL, fin length, AI–AIV, lengths of arms 1–4; AIw–AIVw, width of the base of arms 1–4. The regression lines are shown for most of the characters AIII, AIV, and FL.
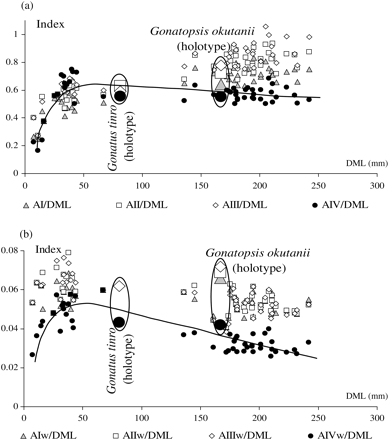
The differences in morphology observed could be related to allometric changes in the selected characters with growth, and this is evident from the plots of character indices against DML (Figure 8). Variations in the relative length of arms III and IV (Figure 8a) and the relative width of arms III and IV (Figure 8b) with growth (=size) suggest ontogenetic trends in most indices. In the early ontogenetic stages (G. tinro), arms IV are shortest and thinnest, an observation made also for the early stages of other gonatid squid (Katugin and Shevtsov, 2006). At ∼30–40 mm DML (G. tinro), arms IV become longer than the other arms, and in larger animals (G. okutanii), arms I, II, and III seemingly grow faster and become longer than arms IV, with arms I remaining generally shorter than arms II and III throughout ontogenesis. Arms III are the longest and almost as long as the mantle in squid >150 mm DML (G. okutanii). At ∼130 mm DML, arms IV are shorter than the other arms, a difference in arm length that is even more pronounced in larger squid. Arms IV are evidently the thinnest in both G. tinro and G. okutanii over the whole size range. In large squid, arms I, II, and III are about the same width, but arms IV are relatively thin, almost 1.5–1.8 times thinner than the other arms.
Indices of metric characters of G. tinro and G. okutanii. (a) Arm length; (b) arm width. DML, dorsal mantle length; characters as in Figure 7.
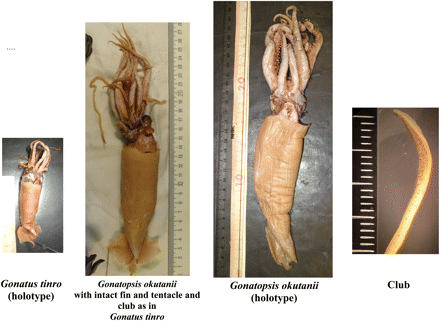
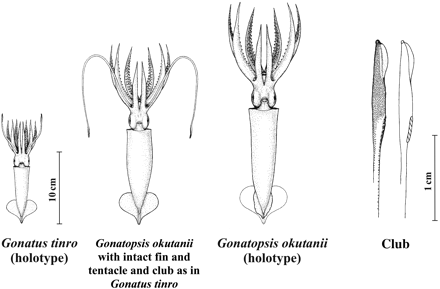
Until recently, the general absence of advanced stages of G. tinro and young stages of G. okutanii remained unexplained, notwithstanding the extensive research and surveying of cephalopods in the northwestern Pacific. The “missing link” was found in 2001 in the Sea of Okhotsk, when during sampling of G. okutanii, many examples of that species with intact fins and, more importantly, intact tentacles were captured. Those findings not only allowed a fit with existing data on the biological features of G. tinro and G. okutanii, but also provided a solution to the issue of the rather vague taxonomic status of G. okutanii. The ambiguity in taxonomic position and validity of G. okutanii had arisen because all the type specimens had broken tentacles, and the remaining stubs (tentacle remnants between arms III and IV) appeared as very thin, small appendages, which led to placement of the squid in the genus Gonatopsis (Nesis, 1972). Moreover, the type specimens of G. okutanii had damaged fins and a partly broken cartilaginous “tail”, so making it impossible to describe the fin morphology, particularly its shape and size (length and width), both of which are crucial in determining the systematics of gonatid squid.
One G. okutanii with intact tentacles was selected as the reference animal and deposited under the name G. tinroNesis, 1972, number XII29277/cph-170, in the Zoological Museum of the Far Eastern State University (Vladivostok, Russia). In intact G. okutanii, the general appearance, main body proportions, and tentacle morphology coincide with those of G. tinro (Figures 9 and 10; Table 7). In particular, the fins of G. okutanii are heart-shaped, as in G. tinro, and are approximately of the same proportions. Arm formulae of the well-preserved G. okutanii, with tentacles and clubs as in G. tinro, the G. okutanii holotype, and G. tinro from the type series are almost identical; in all these squid, the lateral arms are the longest and the ventral arms the shortest and thinnest. However, the most striking morphological evidence indicating synonymy is the presence of tentacles with distinct clubs (G. tinro) in the squid previously identified as G. okutanii.
Morphometric character measurements (mm) for G. tinro and G. okutanii types (current measurements) and G. okutanii with intact tentacles as in G. tinro (“missing link” individuals from the northern Sea of Okhotsk, RV “Borodino”, spring 2001).
| . | Gonatus tinro . | Gonatopsis okutanii . | Gonatopsis okutanii with intact tentacles, as in G. tinro . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Character . | Holotype . | Paratype . | Holotype . | Trawl 256 . | Trawl 283 . | Trawl 297 . | Trawl 290a . | Trawl 317 . | Trawl 362 . |
| DML [GL] | 81 | 67 [66] | 160+ | 136 [118] | 158 | 166 | 151 | 180 [159] | 194 [160] |
| FL (FL I) | 33 (0.41) | 29 (0.43) | 47+ (0.29+) | 59 (0.43) | 62 (0.39) | 61 (0.37) | 52 (0.34) | 64 (0.36) | 77 (0.40) |
| FW (FW I) | 41 (0.51) | 34 (0.51) | 30+ (0.19+) | – (–) | 75 (0.47) | 76 (0.46) | 63 (0.42) | 72 (0.40) | 76 (0.39) |
| MW (MW I) | 22 (0.27) | 23 (0.34) | 42 (0.26–) | 35 (0.26) | 45 (0.28) | 50 (0.30) | 37 (0.25) | 53 (0.29) | 43 (0.22) |
| AI (AI I) | 46 (0.57) | 34 (0.51) | 107 (0.67–) | 88 (0.65) | 98 (0.62) | 116 (0.70) | 92 (0.61) | 120 (0.67) | 140 (0.72) |
| AII (AII I) | 51 (0.63) | 38 (0.57) | 120 (0.75–) | 92 (0.68) | 108 (0.68) | 137 (0.83) | 105 (0.70) | 133 (0.74) | 150 (0.77) |
| AIII (AIII I) | 48 (0.59) | 40 (0.60) | 130 (0.81–) | 105 (0.77) | 113 (0.72) | 134 (0.81) | 110 (0.73) | 142 (0.79) | 167 (0.86) |
| AIIIw (AIIIw I) | 5 (0.062) | 4 (0.060) | 12 (0.075–) | 8 (0.058) | 8 (0.051) | 9 (0.054) | 9 (0.060) | 10 (0.056) | 10 (0.052) |
| AIV (AIV I) | 45 (0.56) | 37 (0.55) | 92 (0.58–) | 71 (0.52) | 77 (0.48) | 91 (0.55) | 76 (0.50) | 102 (0.57) | 106 (0.55) |
| AIVw (AIVw I) | 3.5 (0.043) | 3.5 (0.052) | 7 (0.044–) | 5 (0.037) | 5 (0.032) | 6 (0.036) | 5 (0.033) | 5 (0.029) | 6 (0.031) |
| Arm formula | II > III > I > IV | III > II > IV > I | III > II > I > IV | III > II > I > IV | III > II > I > IV | II > III > I > IV | III > II > I > IV | III > II > I > IV | III > II > I > IV |
| TL (TL I) | 54 (0.67) | 57 (0.85) | 5+ (0.03 + ) | 170 (1.25) | 160 (1.01) | 205 (1.23) | 158 (1.05) | 190 (1.06) | 280 (1.44) |
| CL (CL I) | 13 (0.16) | 9 (0.13) | – (–) | 19 (0.14) | 17 (0.11) | 19 (0.11) | 16 (0.11) | 22 (0.12) | 30 (0.15) |
| Sex | Juvenile | Juvenile | Immature female (stage II) | Immature female (stage II) | Unsexed | Mature male (stage V) | Unsexed | Mature male (stage V) | Immature female (stage II) |
| . | Gonatus tinro . | Gonatopsis okutanii . | Gonatopsis okutanii with intact tentacles, as in G. tinro . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Character . | Holotype . | Paratype . | Holotype . | Trawl 256 . | Trawl 283 . | Trawl 297 . | Trawl 290a . | Trawl 317 . | Trawl 362 . |
| DML [GL] | 81 | 67 [66] | 160+ | 136 [118] | 158 | 166 | 151 | 180 [159] | 194 [160] |
| FL (FL I) | 33 (0.41) | 29 (0.43) | 47+ (0.29+) | 59 (0.43) | 62 (0.39) | 61 (0.37) | 52 (0.34) | 64 (0.36) | 77 (0.40) |
| FW (FW I) | 41 (0.51) | 34 (0.51) | 30+ (0.19+) | – (–) | 75 (0.47) | 76 (0.46) | 63 (0.42) | 72 (0.40) | 76 (0.39) |
| MW (MW I) | 22 (0.27) | 23 (0.34) | 42 (0.26–) | 35 (0.26) | 45 (0.28) | 50 (0.30) | 37 (0.25) | 53 (0.29) | 43 (0.22) |
| AI (AI I) | 46 (0.57) | 34 (0.51) | 107 (0.67–) | 88 (0.65) | 98 (0.62) | 116 (0.70) | 92 (0.61) | 120 (0.67) | 140 (0.72) |
| AII (AII I) | 51 (0.63) | 38 (0.57) | 120 (0.75–) | 92 (0.68) | 108 (0.68) | 137 (0.83) | 105 (0.70) | 133 (0.74) | 150 (0.77) |
| AIII (AIII I) | 48 (0.59) | 40 (0.60) | 130 (0.81–) | 105 (0.77) | 113 (0.72) | 134 (0.81) | 110 (0.73) | 142 (0.79) | 167 (0.86) |
| AIIIw (AIIIw I) | 5 (0.062) | 4 (0.060) | 12 (0.075–) | 8 (0.058) | 8 (0.051) | 9 (0.054) | 9 (0.060) | 10 (0.056) | 10 (0.052) |
| AIV (AIV I) | 45 (0.56) | 37 (0.55) | 92 (0.58–) | 71 (0.52) | 77 (0.48) | 91 (0.55) | 76 (0.50) | 102 (0.57) | 106 (0.55) |
| AIVw (AIVw I) | 3.5 (0.043) | 3.5 (0.052) | 7 (0.044–) | 5 (0.037) | 5 (0.032) | 6 (0.036) | 5 (0.033) | 5 (0.029) | 6 (0.031) |
| Arm formula | II > III > I > IV | III > II > IV > I | III > II > I > IV | III > II > I > IV | III > II > I > IV | II > III > I > IV | III > II > I > IV | III > II > I > IV | III > II > I > IV |
| TL (TL I) | 54 (0.67) | 57 (0.85) | 5+ (0.03 + ) | 170 (1.25) | 160 (1.01) | 205 (1.23) | 158 (1.05) | 190 (1.06) | 280 (1.44) |
| CL (CL I) | 13 (0.16) | 9 (0.13) | – (–) | 19 (0.14) | 17 (0.11) | 19 (0.11) | 16 (0.11) | 22 (0.12) | 30 (0.15) |
| Sex | Juvenile | Juvenile | Immature female (stage II) | Immature female (stage II) | Unsexed | Mature male (stage V) | Unsexed | Mature male (stage V) | Immature female (stage II) |
DML, dorsal mantle length; GL, gladius length (in parenthesis); FL, fin length; FW, fin width; MW, mantle width; AI–AIV, length of arms 1–4; AIIIw–AIVw, width of the base of arms 3 and 4; TL, tentacle length; CL, club length; I, character index (in parenthesis).
aReference animal.
Morphometric character measurements (mm) for G. tinro and G. okutanii types (current measurements) and G. okutanii with intact tentacles as in G. tinro (“missing link” individuals from the northern Sea of Okhotsk, RV “Borodino”, spring 2001).
| . | Gonatus tinro . | Gonatopsis okutanii . | Gonatopsis okutanii with intact tentacles, as in G. tinro . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Character . | Holotype . | Paratype . | Holotype . | Trawl 256 . | Trawl 283 . | Trawl 297 . | Trawl 290a . | Trawl 317 . | Trawl 362 . |
| DML [GL] | 81 | 67 [66] | 160+ | 136 [118] | 158 | 166 | 151 | 180 [159] | 194 [160] |
| FL (FL I) | 33 (0.41) | 29 (0.43) | 47+ (0.29+) | 59 (0.43) | 62 (0.39) | 61 (0.37) | 52 (0.34) | 64 (0.36) | 77 (0.40) |
| FW (FW I) | 41 (0.51) | 34 (0.51) | 30+ (0.19+) | – (–) | 75 (0.47) | 76 (0.46) | 63 (0.42) | 72 (0.40) | 76 (0.39) |
| MW (MW I) | 22 (0.27) | 23 (0.34) | 42 (0.26–) | 35 (0.26) | 45 (0.28) | 50 (0.30) | 37 (0.25) | 53 (0.29) | 43 (0.22) |
| AI (AI I) | 46 (0.57) | 34 (0.51) | 107 (0.67–) | 88 (0.65) | 98 (0.62) | 116 (0.70) | 92 (0.61) | 120 (0.67) | 140 (0.72) |
| AII (AII I) | 51 (0.63) | 38 (0.57) | 120 (0.75–) | 92 (0.68) | 108 (0.68) | 137 (0.83) | 105 (0.70) | 133 (0.74) | 150 (0.77) |
| AIII (AIII I) | 48 (0.59) | 40 (0.60) | 130 (0.81–) | 105 (0.77) | 113 (0.72) | 134 (0.81) | 110 (0.73) | 142 (0.79) | 167 (0.86) |
| AIIIw (AIIIw I) | 5 (0.062) | 4 (0.060) | 12 (0.075–) | 8 (0.058) | 8 (0.051) | 9 (0.054) | 9 (0.060) | 10 (0.056) | 10 (0.052) |
| AIV (AIV I) | 45 (0.56) | 37 (0.55) | 92 (0.58–) | 71 (0.52) | 77 (0.48) | 91 (0.55) | 76 (0.50) | 102 (0.57) | 106 (0.55) |
| AIVw (AIVw I) | 3.5 (0.043) | 3.5 (0.052) | 7 (0.044–) | 5 (0.037) | 5 (0.032) | 6 (0.036) | 5 (0.033) | 5 (0.029) | 6 (0.031) |
| Arm formula | II > III > I > IV | III > II > IV > I | III > II > I > IV | III > II > I > IV | III > II > I > IV | II > III > I > IV | III > II > I > IV | III > II > I > IV | III > II > I > IV |
| TL (TL I) | 54 (0.67) | 57 (0.85) | 5+ (0.03 + ) | 170 (1.25) | 160 (1.01) | 205 (1.23) | 158 (1.05) | 190 (1.06) | 280 (1.44) |
| CL (CL I) | 13 (0.16) | 9 (0.13) | – (–) | 19 (0.14) | 17 (0.11) | 19 (0.11) | 16 (0.11) | 22 (0.12) | 30 (0.15) |
| Sex | Juvenile | Juvenile | Immature female (stage II) | Immature female (stage II) | Unsexed | Mature male (stage V) | Unsexed | Mature male (stage V) | Immature female (stage II) |
| . | Gonatus tinro . | Gonatopsis okutanii . | Gonatopsis okutanii with intact tentacles, as in G. tinro . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Character . | Holotype . | Paratype . | Holotype . | Trawl 256 . | Trawl 283 . | Trawl 297 . | Trawl 290a . | Trawl 317 . | Trawl 362 . |
| DML [GL] | 81 | 67 [66] | 160+ | 136 [118] | 158 | 166 | 151 | 180 [159] | 194 [160] |
| FL (FL I) | 33 (0.41) | 29 (0.43) | 47+ (0.29+) | 59 (0.43) | 62 (0.39) | 61 (0.37) | 52 (0.34) | 64 (0.36) | 77 (0.40) |
| FW (FW I) | 41 (0.51) | 34 (0.51) | 30+ (0.19+) | – (–) | 75 (0.47) | 76 (0.46) | 63 (0.42) | 72 (0.40) | 76 (0.39) |
| MW (MW I) | 22 (0.27) | 23 (0.34) | 42 (0.26–) | 35 (0.26) | 45 (0.28) | 50 (0.30) | 37 (0.25) | 53 (0.29) | 43 (0.22) |
| AI (AI I) | 46 (0.57) | 34 (0.51) | 107 (0.67–) | 88 (0.65) | 98 (0.62) | 116 (0.70) | 92 (0.61) | 120 (0.67) | 140 (0.72) |
| AII (AII I) | 51 (0.63) | 38 (0.57) | 120 (0.75–) | 92 (0.68) | 108 (0.68) | 137 (0.83) | 105 (0.70) | 133 (0.74) | 150 (0.77) |
| AIII (AIII I) | 48 (0.59) | 40 (0.60) | 130 (0.81–) | 105 (0.77) | 113 (0.72) | 134 (0.81) | 110 (0.73) | 142 (0.79) | 167 (0.86) |
| AIIIw (AIIIw I) | 5 (0.062) | 4 (0.060) | 12 (0.075–) | 8 (0.058) | 8 (0.051) | 9 (0.054) | 9 (0.060) | 10 (0.056) | 10 (0.052) |
| AIV (AIV I) | 45 (0.56) | 37 (0.55) | 92 (0.58–) | 71 (0.52) | 77 (0.48) | 91 (0.55) | 76 (0.50) | 102 (0.57) | 106 (0.55) |
| AIVw (AIVw I) | 3.5 (0.043) | 3.5 (0.052) | 7 (0.044–) | 5 (0.037) | 5 (0.032) | 6 (0.036) | 5 (0.033) | 5 (0.029) | 6 (0.031) |
| Arm formula | II > III > I > IV | III > II > IV > I | III > II > I > IV | III > II > I > IV | III > II > I > IV | II > III > I > IV | III > II > I > IV | III > II > I > IV | III > II > I > IV |
| TL (TL I) | 54 (0.67) | 57 (0.85) | 5+ (0.03 + ) | 170 (1.25) | 160 (1.01) | 205 (1.23) | 158 (1.05) | 190 (1.06) | 280 (1.44) |
| CL (CL I) | 13 (0.16) | 9 (0.13) | – (–) | 19 (0.14) | 17 (0.11) | 19 (0.11) | 16 (0.11) | 22 (0.12) | 30 (0.15) |
| Sex | Juvenile | Juvenile | Immature female (stage II) | Immature female (stage II) | Unsexed | Mature male (stage V) | Unsexed | Mature male (stage V) | Immature female (stage II) |
DML, dorsal mantle length; GL, gladius length (in parenthesis); FL, fin length; FW, fin width; MW, mantle width; AI–AIV, length of arms 1–4; AIIIw–AIVw, width of the base of arms 3 and 4; TL, tentacle length; CL, club length; I, character index (in parenthesis).
aReference animal.
Photographs of G. tinro and G. okutanii holotypes and of the reference individual of G. okutanii with intact fin and one tentacle, and a tentacle club of that individual.
Drawings of G. tinro and G. okutanii holotypes (fin restored) and of the reference individual of G. okutanii with intact fin and tentacles (one tentacle restored) and a tentacle club of that individual (the characteristic locking zone at the club's dorsal base is depicted hatched).
Discussion
Comparison of the various distributional, biological, and morphological patterns of G. tinro and G. okutanii suggests that despite the differences observed, there are indeed similarities between the two species, and frequently the “specific” features documented appear to be complementary rather than contradictory. In general terms, G. tinro is represented mainly by small juveniles and adolescents (mature examples have been reported only rarely); on the other hand, G. okutanii usually appears as much larger squid mainly at an advanced stage of sexual maturity. The patterns of spatial and vertical distribution, although seemingly different, are in fact compatible. Both squid live beyond the shelf in deep water and are more common below the epipelagic zone in deep water. Smaller young squid (G. tinro) are widely distributed across vast offshore areas and occasionally in upper layers, the latter perhaps the result of diel vertical migration, which is typical of the young stages of other gonatid squid (Nesis, 1997). On the other hand, the distribution of larger squid (G. okutanii) appears to be patchy, mainly because that squid is frequently associated with the slope, which is also typical for the advanced stages of many gonatid species which, when ontogenetically descending, mature and spawn in deep water (Nesis, 1997). Maturing and mature individuals of both G. tinro and G. okutanii are almost exclusively demersal, and their vertical and spatial distribution patterns coincide. There is no sexual dimorphism in the size of immature G. tinro, which is typical of young squid, but female G. okutanii are notably larger than the males, showing the pattern characteristic of other adult gonatids. Minimum sizes at the onset of maturation are similar in G. tinro and G. okutanii. Seasonal changes in the size and maturity of G. tinro and G. okutanii also appear to be congruent, yielding an explicable pattern of seasonal progression in size structure if the two squid species are treated as a single “assemblage”: a spring peak of early juveniles (G. tinro) can be traced through summer and autumn to winter, and in the following spring, a bimodal pattern of mature squid (G. okutanii) arises.
There exists a clear continuity between G. tinro and G. okutanii in morphometric features. In both G. tinro and G. okutanii holotypes, the ventral arms are the shortest and thinnest. In large squid, the difference in arm size increases: ventro-lateral arms become longest and ventral arms shortest and thinnest of all the arms. The changes in arm proportions can be explained by allometric growth.
The conclusion that G. tinro and G. okutanii represent a single taxonomic entity (=species) has received molecular genetic support. Nucleotide sequences of three mitochondrial loci (12S RNA, 16S rRNA, and COI) of two animals (shown on the graphs as G. tinro 08 and G. tinro 09; Lindgren et al., 2005; Figures 1–4) were virtually indistinguishable. One was a spent female, identified as G. okutanii, based on the general morphology, and the second had intact fins and one tentacle with a club and possessed the characteristic features of G. tinro.
In view of the observations on squid comparative morphology above, as well as the patterns of their distribution, size structure, sex, and maturity, there is now, in our opinion, strong evidence for considering G. okutanii as the adult phase of G. tinro. Also, recognizing that G. okutanii does in fact belong to the genus Gonatus and that the description of G. tinro preceded the description of G. okutanii in the text of Nesis (1972), G. okutanii is a junior synonym of the valid name G. tinro. The synonymy of G. tinro is therefore:
Gonatus tinro Nesis, 1972
Gonatopsis sp. Okutani, 1967: 65–68. Pl. 5. Figures 1–11
Gonatus sp. Fields et Gauley, 1971: 1796–1801. Figures 1 and 2
Gonatopsis okutanii Nesis, 1972: 1304–1307. Figure 2.
Depth of capture and schematic drawing of the life cycle of G. tinro.
Based on the data above and what is known about the life cycle of other gonatids, we suspect that the life cycle of G. tinro is as follows (Figure 11). Squid spawn at depth, presumably deeper than 400 m. After spawning, the males die and the females start brooding their egg masses between their arms, like other Gonatidae with five-rowed radulae, and their bodies acquire a jelly-like consistency at the most advanced stages of development (Okutani et al., 1995; Seibel et al., 2000; Katugin et al., 2004). Taking into account the large size of ripe eggs in G. tinro, ∼5.2 mm maximum diameter (unpublished data), and the low ambient water temperature, the period of egg care may last from several months to 1 year (Seibel et al., 2000). After hatching, the paralarvae are pelagic, and they make diel vertical migrations. There are no hatchlings in the collections available for this study, but the smallest squid were found in summer, and early juveniles of DML 20–30 mm are regularly taken in the pelagic zone in August and from October to December, proving an extended spawning season. Juveniles are found over a wide depth range, from bathy- to epipelagic. At ∼100–150 mm DML, the species becomes scarcer in the upper layers and is found mainly in the mesopelagic zone. Having reached sexual maturity at DML >140 mm (most males) and DML >170 mm (most females), they copulate and spawn at depth and the cycle starts again.
To conclude, the comparative morphology and the biological information now available has made it possible to resolve this taxonomic dilemma, which was associated with the fact that some species of the family Gonatidae undergo significant ontogenetic changes in general morphology, and notably become hardly recognizable when it comes to sexual maturation and spawning. The evidence presented here confirms, however, that despite a certain dissimilarity between the two nominal species (G. tinro and G. okutanii), they are actually different life-cycle phases of the single species G. tinro.
Acknowledgements
We thank Ian G. Gleadall and Richard E. Young for their sage comments during the preparation of the manuscript and for polishing the language, and the referees whose recommendations were both practical and helpful in allowing us to improve the final version of the manuscript.