-
PDF
- Split View
-
Views
-
Cite
Cite
Hendrik Jan T. Hoving, Marek R. Lipiński, Lammertjan Dam, The male reproductive strategy of a deep-sea squid: sperm allocation, continuous production, and long-term storage of spermatophores in Histioteuthis miranda, ICES Journal of Marine Science, Volume 67, Issue 7, October 2010, Pages 1478–1486, https://doi.org/10.1093/icesjms/fsq041
Close - Share Icon Share
Abstract
Squid are semelparous organisms. Much of what we know about squid reproduction relates to females, because few studies have addressed males and, although males are similarly challenged by semelparity, it remains virtually unknown what tactics squid have evolved to allocate sperm to spermatophores. The male reproductive strategy of the deep-sea squid Histioteuthis miranda was examined by describing the male reproductive anatomy, which appears unique among cephalopods, and by quantifying spermatophore production and sperm allocation. The species produces and stores spermatophores over a considerable period of continuous somatic growth. Body size and spermatophore length (SpL) are positively correlated, and the size difference between spermatophores stored by a single individual was up to 270%. Individuals had between 136 and 2276 spermatophores inside their reproductive system, and spermatophores may be stored for as long as 4.5 months. The relationship between SpL and the mass of sperm per spermatophore was polynomial, demonstrating that larger spermatophores did not necessarily contain more sperm. The unique male morphology and the continuous production and long-term storage of spermatophores in H. miranda seem to allow the species to increase the window in which reproduction can take place, a strategy that may be valuable in the deep-sea environment.Hoving, H. J. T., Lipiński, M. R., and Dam, L. 2010. The male reproductive strategy of a deep-sea squid: sperm allocation, continuous production, and long-term storage of spermatophores in Histioteuthis miranda. – ICES Journal of Marine Science, 67: 1478–1486.
Introduction
Squid are nektonic, carnivorous, and coleoid cephalopods that are successful in marine ecosystems, from the coast to the deepest parts of the ocean. Although there continue to be challenges with classifying the reproductive mode of squid, in part because of the general paucity of information for many (especially deep-sea) species (Rocha et al., 2001), there is consensus that all squid are semelparous, i.e. there is no regeneration of the gonads (Nesis, 1987). Despite their shared life-history strategy, there is accumulating evidence to suggest that there are differences between deep-sea squid and neritic squid in such reproductive tactics as mating, maturation, and spawning (Nesis, 1995; Seibel et al., 2005; Laptikhovsky et al., 2007; Hoving, 2008). For example, post-spawning egg care in female gonatid squid has been observed recently by remotely operated vehicle (Seibel et al., 2005). These deep-sea squid spawn all their eggs at once (Laptikhovsky et al., 2007) and “brood” them for an estimated 8–9 months, which is as long as the complete life cycle of small neritic loliginids (Jackson and O'Dor, 2001).
Reproductive strategies in cephalopods are mostly studied for females. However, strategies underlying male reproduction in squid may be expected because males are similarly challenged by semelparity: reproduction, maturation, and mating can only take place in a limited time. Additionally, males produce spermatophores, complex structures that hold millions of spermatozoa. During mating, spermatophores discharge and spermatangia are formed (during the spermatophoric reaction). Spermatangia are attached to the female and enclose the sperm until fertilization and spawning takes place.
Spermatophores are produced by a complex series of glands, then stored in the male reproductive system until mating (Drew, 1919). Sperm production is associated with significant physiological cost (Dewsbury, 1982). In cephalopods, the production of spermatophores may be a significant additional physiological cost, given the complexity of cephalopod spermatophores compared with those of other molluscs (Mann, 1984). The combination of the potential physiological costs associated with spermatophore production and the limited time for reproduction as a result of semelparity strongly indicates that squid have evolved species-specific strategies in sperm allocation to spermatophores. This is confirmed in the few studies providing quantitative information on spermatophore production in squid (Hess, 1987; Laptikhovsky and Nigmatullin, 1987; Lipiński, 1993; Lordan et al., 1998; Nigmatullin et al., 2003; Hoving et al., 2004, 2008a).
Among species and families, there is variation in spermatophore number, size, and sperm content. The strategies underlying spermatophore production in cephalopod species probably follow a continuum between the production of few large and many small spermatophores, where the adopted strategy is shaped by the species-specific aspects of the reproductive system, such as risks and intensity of sperm competition, mechanisms of sperm reception and storage, and encounter rates between the sexes. As these aspects are also influenced by the environment, we expect that sperm allocation tactics differ between deep-sea and neritic and epipelagic squid.
We examined how male deep-sea squid cope with semelparity by studying the reproductive strategy of male Histioteuthis miranda, one of the most abundant mesopelagic squid on the continental slope of southern Africa (Roeleveld et al., 1992). Histioteuthid squid are very important in the oceanic foodweb, as shown by their abundance in the diets of sperm whales and other cetaceans (Clarke, 1996). Despite their abundance and ecological importance worldwide, the biology of histioteuthids is poorly known.
For this research, spermatophore production was quantified and compared between individual male H. miranda, lifetime individual spermatophore production was reconstructed, individual sperm allocation to spermatophores quantified, and the time that males may store their spermatophores estimated. Additionally, the unique morphology of the male reproductive system of H. miranda, which appears structurally different from any other squid species reported to date, was investigated and the morphology linked to our quantitative observations.
Material and methods
All specimens were caught by bottom trawl. In March 1988, among other squid, 20 mature male H. miranda were caught by the South African FRS “Africana” in South African waters at 700–894 m, between 32 and 36°S and 17 and 19°E. Body mass (BM) and the masses of the testis (TM) and spermatophoric organ (SOM) were measured to the nearest 0.1 g and mantle length (ML) to the nearest 0.1 mm on board the vessel. The reproductive systems were then preserved in 10% formalin. Five of the males were used in the current study to correlate maximum spermatophore length (maxSpL) with body size. In February 2005, the Norwegian RV “Dr Fridtjof Nansen” collected another 12 males at 753–852 m, between 31 and 33°S and 16 and 17°E. The ML, BM, and maturity stage of each specimen was determined on board, after which the whole animal was preserved in 10% formalin. Those reproductive systems were dissected out and measured after preservation.
In Table 1, information is presented on the number of specimens collected in each of 1988 and 2005 and the number used in each part of this study. Because of the large number of spermatophores present in the male system, it was decided to measure spermatophore components for just four males, because this sample size resulted in sufficient data for our purposes. One reproductive system from a male collected in 2005 was used for dissection, without collecting data except for total mass. Another 11 reproductive systems were used to determine maxSpL, to count spermatophores, and to determine SpLs in the eight Needham's sac components. As in two reproductive systems of the 2005 collection the spermatophores were ruptured, sperm content of spermatophores could only be determined from nine males. It was not possible to weigh the sperm inside the spermatophores of animals from the 1988 collection because they were preserved in ethanol, making separation of the sperm masses from the spermatophores impossible. Many of the reproductive systems from the males collected in 1988 were already dissected, so only a few male systems could be used for obtaining the total number of spermatophores and the maximum spermatophore size.
Overview of the measurements taken and the number (n) of H. miranda used per measurement type.
| Type of sampling . | n in 1988 . | n in 2005 . |
|---|---|---|
| ML | 20 | 12 |
| BM | 20 | 12 |
| TM | 20 | 12 |
| SOM | 20 | 12 |
| Total spermatophore number | 4 | 11 |
| SpL in eight Needham's sac sections | 0 | 11 |
| MaxSpL | 5 | 11 |
| Sperm content of spermatophores | 0 | 9 |
| Size of spermatophore components | 0 | 4 |
| Type of sampling . | n in 1988 . | n in 2005 . |
|---|---|---|
| ML | 20 | 12 |
| BM | 20 | 12 |
| TM | 20 | 12 |
| SOM | 20 | 12 |
| Total spermatophore number | 4 | 11 |
| SpL in eight Needham's sac sections | 0 | 11 |
| MaxSpL | 5 | 11 |
| Sperm content of spermatophores | 0 | 9 |
| Size of spermatophore components | 0 | 4 |
Overview of the measurements taken and the number (n) of H. miranda used per measurement type.
| Type of sampling . | n in 1988 . | n in 2005 . |
|---|---|---|
| ML | 20 | 12 |
| BM | 20 | 12 |
| TM | 20 | 12 |
| SOM | 20 | 12 |
| Total spermatophore number | 4 | 11 |
| SpL in eight Needham's sac sections | 0 | 11 |
| MaxSpL | 5 | 11 |
| Sperm content of spermatophores | 0 | 9 |
| Size of spermatophore components | 0 | 4 |
| Type of sampling . | n in 1988 . | n in 2005 . |
|---|---|---|
| ML | 20 | 12 |
| BM | 20 | 12 |
| TM | 20 | 12 |
| SOM | 20 | 12 |
| Total spermatophore number | 4 | 11 |
| SpL in eight Needham's sac sections | 0 | 11 |
| MaxSpL | 5 | 11 |
| Sperm content of spermatophores | 0 | 9 |
| Size of spermatophore components | 0 | 4 |
For 11 males (ML 171–290 mm) collected in 2005, the Needham's sac and terminal organ were divided into eight parts of approximately equal length/volume, from distal to proximal. The proximal part of the Needham's sac and terminal organ (1 in Figure 1) contained the most recently produced spermatophores, and the distal part (8 in Figure 1) contained the earliest-produced spermatophores. In each part, the number of spermatophores was counted and the SpL determined for 2–25 (usually 20) spermatophores using a calibrated stereomicroscope ocular micrometer. In nine of these males, the sperm masses of 36 spermatophores from each part of the Needham's sac and the terminal organ were removed and weighed collectively to the nearest 0.1 mg (the sperm mass of a single spermatophore weighed too little to measure). Although the sperm mass is semi-liquid in fresh spermatophores, after preservation in formalin it is solid, easily removed from the spermatophore, and can be handled without loss of sperm. Using a pair of scissors, pieces of plastic foil were cut and weighed. The sperm masses were dried on a tissue for 10 s, wrapped in the weighed plastic foil to prevent evaporation, then weighed on a calibrated microscale.
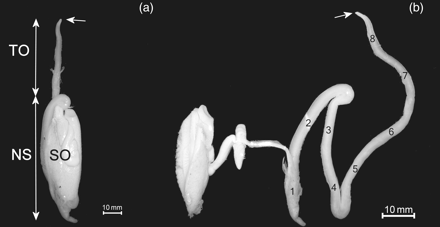
(a) Spermatophoric organ (SO), Needham's sac (NS), and terminal organ (TO) removed from the mantle cavity of a mature male H. miranda. (b) The glands of the spermatophoric organ and loops of the Needham's sac unravelled. Numbers 1–6 indicate the sections of the Needham's sac, and 7 and 8 are the sections of the terminal organ. The opening of the terminal organ is indicated by a white arrow.
In one animal, fewer than 36 spermatophores were present in one of the parts of the Needham's sac and terminal organ. Therefore, 18 sperm masses were taken from spermatophores from each of the two adjacent parts, and the mean SpL was taken and used for determining the relationship between SpL and sperm mass.
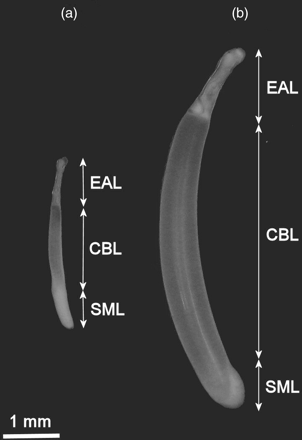
In four of the specimens, the lengths of the ejaculatory apparatus (EAL), cement body (CBL), and sperm mass (SML) of the spermatophores from the eight parts of the Needham's sac were also measured (Figure 2), using a calibrated ocular micrometer in a stereomicroscope. The index of these measurements, calculated as %SpL, was also determined and abbreviated as EALI, CBLI, and SMLI.
Two spermatophores from a single H. miranda (ML 286 mm and BM 1530 g), illustrating the large size difference. (a) Spermatophore removed from section 7 and (b) removed from section 1. Measured spermatophore components are labelled: EAL, ejaculatory apparatus length; CBL, cement body length; SML, sperm mass length.
To correlate the length of the most recently produced spermatophores (maxSpL) with body size, the mean SpL of the spermatophores from the proximal part of the Needham's sac was determined for each of the 16 specimens (5 from the 1988 sample and 11 from 2005 sampling).
The squid investigated were in different stages of reproductive activity, and this work aimed partly to gauge the spermatophore production of one animal during its lifetime. Because analysing one animal gives only a snapshot of its total spermatophore production, we pooled the data from all nine animals to reconstruct lifetime spermatophore production. There was a significant correlation between the length of the latest-produced spermatophores and ML, demonstrating that larger squid produce larger spermatophores.
The SpL frequency results and the relationship between sperm content and SpL of all nine animals were initially determined. By estimating first a non-linear relationship between SpL and sperm content (in g) and second a non-linear relationship for SpL frequency in time, it is possible to reconstruct how much sperm (in g) is invested into spermatophores of different lengths. The estimate of the length frequency over time is made possible by the positive correlation between body and spermatophore sizes, so larger spermatophores can be assumed to be more recently produced (in other words by older animals). Consequently, the curve created by combining the estimated curves represents the productivity of the testis in time.
Statistical analyses were performed in STATA 9.2 (Stata 2006, Statistical Software, Release 9.2, Stata Corporation StataCorp, College Station, TX, USA).
Results
Male reproductive morphology, and TM and SOM
In H. miranda, a very long Needham's sac is curled up inside the genital sac and terminates in a short terminal organ, which is not used for spermatophore transfer because it does not extend from the mantle cavity (Figure 3). The terminal organ was attached to the intestine for all but the distal portion of its length, which lay along the digestive gland. The terminal organ opened approximately at the base of the funnel and could not extend much. When dissected out, the Needham's sac formed two loops inside the genital sac (Figure 1). The loops were connected to each other and had to be separated using a scalpel. The total length of the organ when extended was 114–140% ML (n = 5). The terminal organ opening was simple and narrow. The basal parts of the dorsal arms are also modified in males (Voss et al., 1998), and they presumably act as hectocotyli.
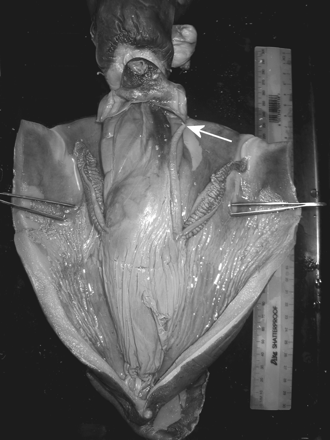
Opened mantle of H. miranda, in ventral view. The opening of the terminal organ is indicated by a white arrow. The ruler on the right side of the animal is 300 mm long.
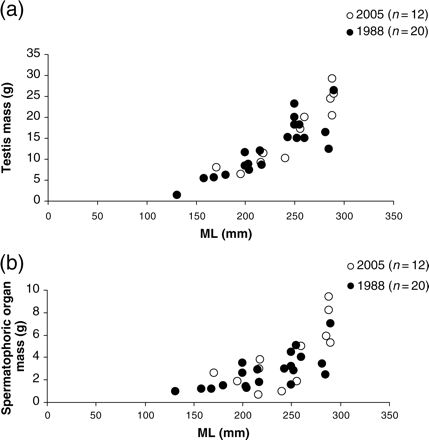
Despite different treatments of the measured reproductive systems, animals of similar size from the two collections seemed not to vary in their testis and SOM, which increased with increasing ML (Figure 4). In fresh animals (1988; n = 20), testis mass was 1–7 g (0.15–0.58% BM) for animals between 131 and 290 mm ML, and SOM was 1.4–26.4 g (0.40–2.88% BM). Testes from formalin-preserved reproductive systems (2005; n = 12) weighed 0.7–9.4 g (0.06–0.53% BM) in animals between 171 and 290 mm ML, and SOM was 6.5–25.6 g (0.83–1.75% BM).
Male H. miranda reproductive system parameters in relation to size: (a) testis mass in relation to ML; (b) mass of the spermatophoric organ in relation to ML.
Spermatophore production
The spermatophores most recently produced are stored in the proximal part of the male system, and the least recently produced in the distal part. A detailed reconstruction of the history of sperm and the spermatophore production of an individual squid is therefore possible.
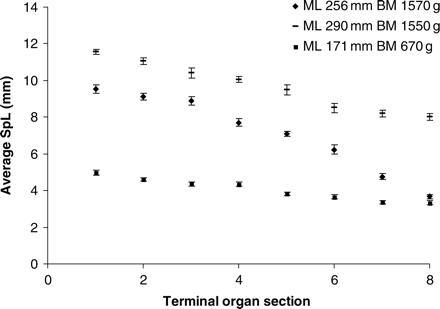
The total number of spermatophores ranged from 136 to 2276 (n = 15), suggesting that some males had already used spermatophores during mating. In all animals, SpL increased greatly from the distal part of the terminal organ, near the opening (8 in Figure 1), to the proximal part (1 in Figure 1). Figures 2 and 5 illustrate the large size difference between spermatophores removed from the reproductive system of a single animal. Within one individual, the length of the proximal spermatophores was 107–265% that of the oldest, distal spermatophores, and no dysfunctional or tentative spermatophores were found. All males had passed the tentative maturity stage and had expelled the tentative spermatophores.
Spermatophores in the reproductive system of H. miranda. Mean SpL, with standard deviations, from each of eight sections of the Needham's sac and terminal organ (measured for 2–25 spermatophores, mostly 20 spermatophores), in three males; note that SpL decreases markedly from proximally stored to distally stored spermatophores.
MaxSpL in the proximal part of the Needham's sac was positively correlated with body size (ML and BM) and ranged from 5 mm (ML 171 mm, BM 670 g) to 11.6 mm (ML 290 mm, BM 1550 g). Simple correlations of maxSpL with BM and maxSpL with ML were significant (respectively, r = 0.84, n = 16, p < 0.05, and r = 0.85, n = 16, p < 0.05), showing that maxSpL increases with body size. The relationship between body size and minimum SpL (minSpL) was less profound (the correlations of minSpL with ML and with BM were, respectively, r = 0.5148, n = 16, p < 0.05, and r = 0.3211, n = 16, p > 0.05), suggesting that some animals had expelled spermatophores (perhaps during mating) whereas others still had all spermatophores stored. This suggestion was supported by the absence of a relationship between total spermatophore number and body size.
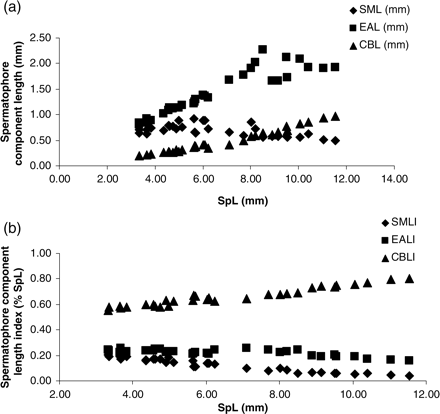
The lengths of the functional components of the spermatophores were also measured. The absolute sizes of cement bodies (CBL), ejaculatory apparatus (EAL), and sperm masses in relation to SpL are depicted in Figure 6. Both CBL and EAL increased continuously with increasing SpL, whereas SML increased up to SpL ∼6 mm, after which it seemed to decrease. The SML was not a good measure for sperm content because the former became more compressed aborally in larger spermatophores; it was considered therefore as indicative only. The relative lengths of the spermatophore components showed that SMLI and EALI decreased with increasing SpL, whereas CBLI increased with increasing SpL (Figure 6b).
Size of the functional components of the spermatophores of H. miranda. (a) Absolute mean EAL, CBL, and SML in relation to mean total SpL. (b) Mean relative EAL, DBL, and SML as proportions of mean total SpL. The relationship is pooled data from four animals, and each point is the mean of 2–25 spermatophores (mostly 20), taken from each of the eight sections of the Needham's sac.
Sperm allocation to spermatophores
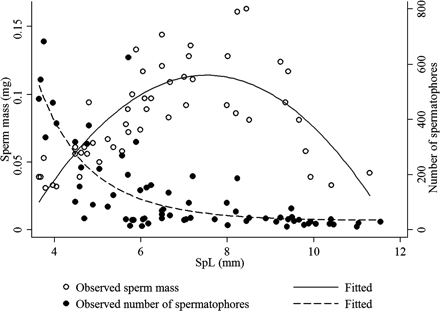
The mass of sperm in each spermatophore (hereafter referred to as sperm content) increased gradually with increasing SpL, peaking at SpL ∼8 mm (Figures 7 and 8). The longest spermatophores contained approximately as much (in g) sperm as the shortest ones. In addition, the Ramsey RESET test rejected a linear relationship between SpL and sperm content [F(3,47) = 26.96, p < 0.001], and the best fit was a parabola [sperm content =−0.006 SpL2 + 0.093 SpL − 0.237: r2 = 0.6153, F(2,49) = 9.18, p < 0.001], which was significant (Figure 8).
Sperm content and number of spermatophores in relation to SpL in H. miranda. The sperm mass is the mean of the sperm mass of 36 spermatophores, and the SpL the mean of 2–25 (mostly 20) spermatophores from each Needham's sac section.
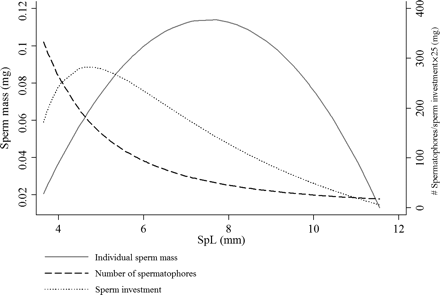
Fitted curves of sperm mass and number of spermatophores against SpL in H. miranda and the product of these curves, which gives the total investment of sperm in relation to SpL.
The distal part of the terminal organ contained more smaller spermatophores than the proximal part of the Needham's sac (Figure 8). For example, in the distal (8 in Figure 1) and proximal parts (1 in Figure 1) of the Needham's sac and terminal organ of one animal (ML 288 mm and BM 1670 g), there were 624 spermatophores (SpL 5.7 ± 0.1 mm) and 22 spermatophores (SpL 10.4 ± 0.6 mm), respectively, and there was a clear inverse relationship between the number of spermatophores and SpL. Again, a linear regression was refuted, both on the account of non-linearity and heteroscedasticity [the Breusch–Pagan/Cook–Weisberg test for heteroscedasticity yielded a χ2(1) statistic of 22.18, p < 0.001, indicating heteroscedasticity]. The relationship is best described by a power function [number of spermatophores = 8793 SpL−2.5: r2 = 0.5369, F(1,67) = 77.67, p < 0.001], so we implicitly assumed lognormally distributed error terms (Figure 7). We could not reject the hypothesis that the error terms were lognormally distributed [p (skewness) = 0.185, p (kurtosis) = 0.472, p (jointly) = 0.3066], and the fact that SpL and number of spermatophores were inversely related meant that all terminal organ parts were filled with spermatophores to their maximum capacity (i.e. full).
By combining the formulae for the SpL–sperm content and SpL–frequency relationships (Figure 7), i.e. by multiplying the number of spermatophores of a certain size by the mass (in g) of sperm found in that particular size, an estimate of the total investment of sperm into a given SpL was obtained (Figure 8). As mentioned above, the spermatophores present in an animal only represent a portion of the individual's total spermatophore production. By combining the results from all nine animals investigated here, we indirectly reconstructed lifetime spermatophore production. The greatest mass of sperm was invested in spermatophores of SpL ∼5 mm; strikingly, this size is neither the most frequent SpL (<5 mm) nor the one containing the most sperm per spermatophore (SpL ∼8 mm), nor even the longest observed SpL. According to the model, at ML 175 mm, some 75% of the estimated total mass of produced sperm is invested in spermatophores of SpL 3–7.5 mm.
Discussion
To understand fully the functioning of an ecosystem, it is important to have sound knowledge of the reproductive strategies of its components. Here, we investigated the male reproductive strategy of a deep-sea squid, an organism with a semelparous life-history strategy that plays a key role in marine ecosystems. Reproductive strategies in squid have mainly been studied for females, but we show that males also optimize gamete production. Our observations on the male anatomy and the spermatophore production and storage of H. miranda indicate a strategy that increases the time window in which reproduction can take place, a strategy that fits well in the deep-sea environment where mating opportunities may be limited.
Reproductive morphology and maturation
The elongate and permanently folded Needham's sac of H. miranda is unique among known cephalopods. The Needham's sac of ommastrephids attains a maxL of ∼60% ML (Nigmatullin et al., 2003), and in Thysanoteuthis rhombus it is 40–60% ML (Nigmatullin et al., 1991). Such squid use their Needham's sacs purely for spermatophore storage; transfer is accomplished by the hectocotylus. The Needham's sac and terminal organ are longer in squid species that use those structures for spermatophore transfer, e.g. Architeuthis sp. maximum terminal organ length, maxTOL = 92% ML (Hoving et al., 2004), and Octopoteuthis sicula maxTOL = 92% ML (Hoving et al., 2008b). Histioteuthis miranda, which has a long Needham's sac and a short terminal organ (together 114–140% ML) that are, however, not used for transfer of spermatophores, adds a third type to the two previously known male reproductive system types known for squid (Nesis, 1995).
The absence of a positive correlation between spermatophore number and body size suggests that spermatophores in some animals had been expelled from the Needham's sac and terminal organ, most likely during mating. In mature males, the basal parts of the dorsal arms (arms I) are modified (Voss et al., 1998), and those arms may be used as hectocotyli. Although the Needham's sac and terminal organ of H. miranda are long when extended, they are attached for nearly their full length (unlike the terminal organs in unhectocotylized species, which are mostly free within the mantle cavity). In none of the males we examined did the terminal organs extend from the mantle cavity. Long terminal organs were also not observed in mature Histioteuthis eltaninae, H. reversa, H. heteropsis (HJTH, pers. obs.), or H. atlantica (Lipiński, 1993). Our observations are not in line with reports that histioteuthids (including H. miranda) have terminal organs that may extend well out of the mantle cavity, so are sufficiently long to use for spermatophore transfer (Nesis, 1995; Voss et al., 1998).
All the males we examined were mature. The increase in the mass of the spermatophoric complex meant that spermatophores accumulated there, the glands of the spermatophoric organ grew, or both. Interestingly, a dramatic decrease in TM after attaining full maturity, as observed for some other oceanic squid (Jackson and Mladenov, 1994; Hoving et al., 2007), was not observed in H. miranda, for which the TM continued to increase with increasing body size while sperm production seemed to decrease. This was an unexpected finding that currently defies explanation.
Continuous spermatophore production
The large range in SpL within individuals (greatest SpL up to 265% of the smallest), the positive correlation between body size and maxSpL, and the absence of a correlation between body size and spermatophore number all suggest that H. miranda starts producing spermatophores at a small size, stores spermatophores over a considerable period, and continues to grow and mate while producing them. The large size range of mature males (ML 131–290 mm), and the fact that larger males are older (Hoving and Lipiński, 2009), support this conclusion.
The reproductive strategy of male H. miranda may be defined as continuous reproduction, sensuRocha et al. (2001). In cephalopods, continuous spermatophore production has also been found in male cirrate octopods (Villanueva, 1992) and in male giant squid Architeuthis. In the latter species differences in SpL in a single male were up to 300% (Lordan et al., 1998; Hoving et al., 2004). Male ommastrephids appear to follow a different strategy because the SpL within one observed animal varied by just 110% (Nigmatullin et al., 2003). Spermatophore production in ommastrephids may be limited by growth of the spermatophoric organ, so may take place over a shorter time, or perhaps greater numbers of spermatophores may be expelled at each mating than in histioteuthids (in proportion to the total number present in the Needham's sac).
Prolonged production and storage of spermatophores, as observed in H. miranda and Architeuthis, may be an adaptation to reproduction in the deep sea. Males start producing spermatophores as soon as and for as long as possible, permitted by continuous growth of both the spermatophoric glands (and therefore the spermatophores) and body size. Early and continuous production of spermatophores allows a maximum window of time for mating. High mortality as a result of high predation pressure (H. miranda is consumed in large quantities by sperm whales and sharks; Clarke, 1980, 1996; Smale and Cliff, 1998), low density of mates, and absence of spawning aggregations may all confer advantages on such a strategy.
A constraint on continuous spermatophore production may be a limited viability of inactively stored sperm inside the spermatophores. In Sepia apama, the spermatozoa inside spermatophores are viable for up to 2 months when retained at 4°C (Naud and Havenhand, 2006). Unfortunately, however, S. apama is the only cephalopod for which sperm longevity has been measured directly. As SpL is positively correlated with ML (SpL = 0.04 ML − 2.62) and ML positively correlated with age (ML = 0.57 age + 1.89; Hoving and Lipiński, 2009, assuming that increments are deposited daily in statoliths), it is possible to estimate indirectly the age at which spermatophores within an individual H. miranda were produced. One male examined (ML 288 mm, BM 1770 g) carried spermatophores with SpL in the range 3.1–8.7 mm. According to the relationships above, spermatophores with an SpL of 3.1 were produced at an age of ∼130 d and those with an SpL of 8.7 mm at an age of ∼260 d, suggesting that spermatophores may be stored and remain viable for a minimum of 4.5 months.
Quality of spermatophores and reproductive phases
The positive correlations we observed between body size and SpL for H. miranda were also found for loliginids and ommastrephids (Boyle et al., 1995; González and Guerra, 1996). In ommastrephids, the correlation coincided with an increase in sperm with increasing SpL, a relationship not found in H. miranda, for which a polynomial relationship exists between SpL and sperm content, i.e. large spermatophores do not necessarily contain more sperm than small ones. In octopods, the relation between sperm reservoir length and SpL is fixed, even among different species (Voight, 2001).
The contributions of the testis and the spermatophoric organ to the spermatophore are not synchronized immediately the animal matures. Presumably to reduce waste of sperm, functional spermatophore production is preceded by a trial period referred to by Laptikhovsky and Nigmatullin (1987) as “tentative maturity”, wherein spermatozoa are produced in the testis but the spermatophoric organ produces spermatophore-like structures that contain no sperm. Hence, the spermatophoric organ is already productive before sperm enter the spermatophoric glands (Nigmatullin et al., 2003). Following tentative maturity, functional spermatophores with sperm start to accumulate in the male reproductive system.
Although all the spermatophores observed in male H. miranda were presumably functional because all functional spermatophore components were present, the species probably also has a tentative maturity phase. Tentative spermatophores have been observed in the congener H. atlantica by Lipiński (1993). The phenomenon of tentative maturity in cephalopods, and the wide variety in spermatophore size in H. miranda, suggests that the quality of spermatophores also varies. Tentative/dysfunctional spermatophores have no quality because either they do not contain sperm or they are not able to attach to the female because of underdevelopment of functional spermatophore components (cement body, ejaculatory apparatus, etc.). After tentative maturity, functional spermatophores are produced until the testis is depleted. Senescent males may produce spermatophore-like structures that contain no sperm, again of no value for reproduction. The key to understanding male reproductive strategies in squid is in knowing how species allocate sperm over functional spermatophores between tentative maturity and senescence.
Functional spermatophore production in H. miranda is not uniform, but it can be divided into phases using the total sperm investment and spermatophore size (and therefore the body size). The first phase involves the production of small spermatophores (SpL <4 mm), which were most frequently produced but contained a low mass of sperm per spermatophore; therefore, total sperm investment in these spermatophores was low. The second phase corresponds to spermatophores of intermediate size (SpL ∼4–8 mm), in which most sperm is invested. The testis attains maximum sperm production at that phase (although this is not reflected in the mass of the testis); the now-more-experienced male may have better chances of successful mating than during the previous phase. In the final phase, the mass of sperm per spermatophore decreased with increasing size for spermatophores of SpL >8 mm, corresponding to decreased testis activity (but not with decreased TM). The total sperm investment was low compared with the preceding phase, which may be related to the fact that these spermatophores are less likely to be used, because they are situated in the posterior portion of the Needham's sac, and perhaps because mating opportunities probably decrease with age (after reaching an optimum).
Although the sperm content of the largest spermatophores was low, males did invest a lot of cement in these spermatophores. The increase in overall SpL with increasing body size was attributable to increasing CBL, perhaps related to the glands producing the cement body growing as body size increases, although males may also strategically invest cement into spermatophores. Histological sections of implanted spermatangia from Rossia moelleri show that the cement body probably facilitates the implantation of the spermatangium into female tissue, the cement body being secreted orally from the everting spermatophore (Hoving et al., 2009). Although mated female H. miranda have not been examined, other histioteuthids implant spermatangia deeply into female tissue (Nesis, 1995), similar to R. moelleri. In H. miranda, a spermatophore with a large cement body may be better able to attach to or implant into female tissue. Spermatophore quality may therefore not only be determined by the quantity of sperm it holds but also by the size of the cement body, which may influence attachment success.
Conclusions
Spermatophore production in H. miranda takes place over an extended period of significant somatic growth. The combination of increased cement-gland production and the polynomial production of the testis results in the longest spermatophores containing the fewest sperm. Variations in spermatophore dimension between individual squid have been attributed to geographical differences (Voss et al., 1998). However, as shown here, spermatophores and the dimensions of spermatophore components may vary greatly within and between animals and are not suitable for differentiating populations.
Histioteuthis miranda stores spermatophores within a unique looped Needham's sac and a short terminal organ that is attached along the intestine for almost its full length. The function of the looped Needham's sac may be to prevent the mixing of the produced spermatophores of different quality, where quality is measured in both the amounts of sperm and cement. The continuation of somatic growth after reaching maturity may facilitate continued mating. Growth, however, takes time and increases predation risk. By producing spermatophores early in life, the chances of reproduction are maximized. Spermatophores may be stored for as long as 4.5 months. The long-looped Needham's sac may enable male H. miranda to continue their somatic growth but to make use of (smaller) spermatophores produced earlier, which contain most of the sperm. The reproductive strategy of male H. miranda illustrates how spermatophore production is optimized to suit an environment where encounters between mates may be low and predation heavy.
We present qualitative and quantitative data on males of the abundant but scarcely studied deep-sea squid H. miranda. Besides describing a unique reproductive anatomy in squid, the results also show how dynamic the male reproductive strategy of squid may be in terms of spermatophore production. Similar studies for other species may in future allow us to classify male reproductive strategies in a similar way as Rocha et al. (2001) did for female cephalopods. Such a classification will enable us to compare spermatophore production strategies between species and to identify how different environments affect male squid reproduction.
Acknowledgements
We thank Dick Young, Kat Bolstad, Vladimir Laptkhovsky, and Deniz Haydar for valued feedback on earlier versions of the manuscript, and editors Gretta Pecl and Andrew Payne for grammatical, stylistic, and content support. We also acknowledge Schure-Beijerinck-Popping Fonds for financial support to HJTH.