-
PDF
- Split View
-
Views
-
Cite
Cite
Steven X. Cadrin, Matthias Bernreuther, Anna Kristín Daníelsdóttir, Einar Hjörleifsson, Torild Johansen, Lisa Kerr, Kristjan Kristinsson, Stefano Mariani, Kjell Nedreaas, Christophe Pampoulie, Benjamin Planque, Jákup Reinert, Fran Saborido-Rey, Thorsteinn Sigurðsson, Christoph Stransky, Population structure of beaked redfish, Sebastes mentella: evidence of divergence associated with different habitats, ICES Journal of Marine Science, Volume 67, Issue 8, November 2010, Pages 1617–1630, https://doi.org/10.1093/icesjms/fsq046
Close - Share Icon Share
Abstract
Throughout their range, Sebastes spp. are adapted to a diversity of ecological niches, with overlapping spatial distributions of different species that have little or no morphological differences. Divergence of behavioural groups into depth-defined adult habitats has led to reproductive isolation, adaptive radiation, and speciation in the genus Sebastes. Recent genetic research, supported by life-history information, indicates four biological stocks of Sebastes mentella in the Irminger Sea and adjacent waters: a western stock, a deep-pelagic stock, a shallow-pelagic stock, and an Iceland slope stock. Congruent differences in fatty acids and parasites suggest that these genetically distinct populations are adapted to disparate trophic habitats in pelagic waters (shallower and deeper than the deep-scattering layer) and in demersal habitats on the continental slope. Morphology of pelagic forms is also more streamlined than demersal forms. Although genetic differences and evidence for reproductive isolation are clear, these populations appear to share common nursery habitats on the Greenland shelf. We propose a redefinition of practical management units near the Irminger Sea based on geographic proxies for biological stocks and minimizing mixed-stock catches according to the spatial patterns of the recent fishery.Cadrin, S. X., Bernreuther, M., Daníelsdóttir, A. K., Hjörleifsson, E., Johansen, T., Kerr, L., Kristinsson, K., Mariani, S., Nedreaas, K., Pampoulie, C., Planque, B., Reinert, J., Saborido-Rey, F., Sigurðsson, T., and Stransky, C. 2010. Population structure of beaked redfish, Sebastes mentella: evidence of divergence associated with different habitats. – ICES Journal of Marine Science, 67: 1617–1630.
Introduction
Stock structure of beaked redfish, Sebastes mentella, and appropriate spatial units for fisheries management have been debated for decades (e.g. Magnússon and Magnússon, 1995; ICES, 1998, 2005). Analysis of new genetic markers has indicated reproductive isolation among groups of adults in different, yet overlapping, habitats (Johansen et al., 2000; Johansen, 2003; Daníelsdóttir et al., 2008; Stefánsson et al., 2009a, b). In 2009, the International Council for the Exploration of the Sea (ICES) organized a workshop to reconcile the new genetic results with all previous information on stock structure to identify the most likely definition of biological stocks and to recommend practical management units in the Irminger Sea and adjacent waters (ICES, 2009b). The objective of this work is to demonstrate how multidisciplinary information on population structure can be synthesized to form a holistic view and provide clear advice for fishery science and management. The population structure of S. mentella offers an instructive case study of depth-associated reproductive isolation and its practical consequences for resource management. The genus Sebastes has unique life-history characteristics that influence population structure.
Throughout their wide geographic range in the North Pacific, North Atlantic, and southern hemisphere, Sebastes spp. are adapted to a diversity of ecological niches, with overlapping spatial distributions of different species that have little or no morphological differences (Johns and Avise, 1998; Alesandrini and Bernardi, 1999). For some species of Sebastes, divergence of behavioural groups into depth-defined habitats has led to reproductive isolation (Hyde et al., 2008). Sympatric diversity of the genus Sebastes is a commonly used example of adaptive radiation, a relatively rapid evolutionary diversification characterized by an increase in the morphological and ecological diversity of a single, rapidly diversifying lineage (Schluter, 2000).
The combination of reproductive biology, early life history, and longevity of Sebastes spp. is unique in that they are viviparous with wide dispersal and long lifespans (Love et al., 2002). Ovoviviparity involves internal fertilization and development of ova until they are ready to hatch. Mate recognition, courtship behaviour, and mate choice are prerequisites of fertilization, providing an additional mechanism of reproductive isolation (Johns and Avise, 1998). However, unlike most live-bearers, Sebastes spp. produce many larvae that are extruded soon after they hatch from eggs (Rocha-Olivares, 2004). The small larvae have a longer planktonic period than most viviparous species, and they can disperse far from the location of extrusion to many habitats. The typically long lifespan of Sebastes spp. also tends to promote adaptation to diverse habitats (Mangel et al., 2007). Most importantly, the relatively strict reproductive constraints allow adaptive radiation into a diversity of ecological niches (Rocha-Olivares, 2004). Sebastes mentella exhibits all these typical Sebastes traits that tend to facilitate adaptive divergence.
Research on S. mentella population structure in the Irminger Sea and adjacent waters reflects the historical development of fisheries. The fishery traditionally targeted mixed redfish species on the continental slopes of Iceland, Greenland, Norway, Canada, and the Faroe Islands, and a pelagic fishery developed in the Irminger Sea in the early 1980s (Sigurðsson et al., 2006a). Genetic research confirmed that shallow shelf catches were of S. marinus, with some S. viviparus off the Faroe Islands, Iceland, and Norway (Johansen, 2003), and deeper catches on the continental slope were of S. mentella (Nævdal, 1978; Nedreaas and Nævdal, 1991); pelagic catches in the Irminger Sea were also S. mentella (Johansen, 2003). Icelandic researchers considered that the pelagic fishery targeted a separate stock of S. mentella from the traditional demersal fishery and that the pelagic stock (referred to as oceanic redfish) could be discriminated from the deep-sea stock based on darker, patchy skin colour, heavy parasite infestation, and associated muscle spots, as well as a smaller size-at-maturity (Magnússon and Magnússon, 1995). In the mid-1990s, the Irminger Sea fishery expanded geographically and vertically to depths >500 m (Sigurðsson et al., 2006a), and the relationships between the traditional demersal resource, the shallow-pelagic resource, and the newly developed pelagic, deep-sea fishery were unknown.
Stock identification became a critical issue in the science and management of S. mentella. In 1998, the ICES Study Group on Redfish Stocks (SGRS) met to coordinate future research on redfish stocks, including an acoustic survey in the Irminger Sea and adjacent areas, but recognized that stock identification was a critical issue for surveying S. mentella resources and managing the redfish fisheries (ICES, 1998). Information on morphology, parasites, and early genetic analyses was reviewed, but the Study Group concluded that the evidence for one or two pelagic stocks was not conclusive.
In response to the Study Group's recommendation for research on stock identification of S. mentella, the EU Redfish project was established to study population structure, reproductive strategies, and demography of redfish in the Irminger Sea and adjacent waters (Anon., 2004; Figure 1). The project involved collaborative sampling efforts and analyses of genetics, morphometrics, reproduction and maturation, otolith shape, otolith chemistry, and growth. A related initiative was funded by the Faroe Islands, which included analysis of genetics, morphometrics, otolith shape, and fatty acids (Joensen, 2002). Morphometric and otolith analyses did not indicate stock structure in the Irminger Sea and adjacent areas, but genetics revealed significant differences between demersal, oceanic (<500 m), and deep-sea pelagic components (Anon., 2004). However, there was disagreement on the cause of genetic differences, from either reproductive isolation among three distinct subpopulations in the Irminger Sea or an artefact of age-related effects, misrepresentative sampling, or natural selection.
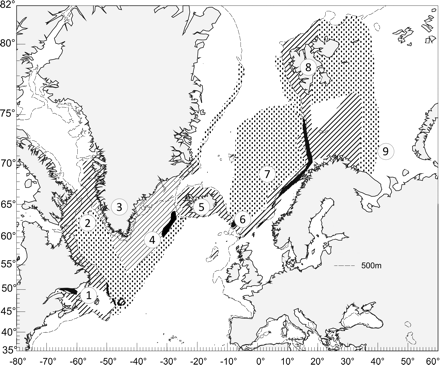
Geographic range of Sebastes mentella. The hatched area shows the centre of abundance. The dotted area is the outer sector of the distribution range, the black area along the slope shows the main area of larva release, and the dashed line indicates the 500-m depth contour. Numbers indicate locations referred to in text (1, Newfoundland; 2, Davis Strait; 3, Greenland; 4, Irminger Sea; 5, Iceland; 6, Faroe Islands; 7, Norwegian Sea; 8, Svalbard; 9, Barents Sea).
A one-stock hypothesis was proposed by Saborido-Rey et al. (2004), who reviewed the ecology of S. mentella near the Irminger Sea. Their conclusion was based on deductive inference supported by information that suggested a semi-continuous distribution of larvae in the Irminger Sea, a common nursery area on the Greenland shelf, and ontogenetic movement of fish from nursery areas to shallow areas, then to deep-pelagic habitats. As the EU Redfish Project was in the final stages of documenting results, the ICES Study Group on Stock Identity and Management Units of Redfishes (SGSIMUR) concluded that there was population structure of S. mentella, but that the nature of the structure, i.e. reproductively isolated or demographic groups, was not clear (ICES, 2005). A subsequent expert group, the ICES Stock Identification Methods Working Group (SIMWG), concluded that recent genetic information was compelling and that the new results should be reviewed and considered for a re-evaluation of ICES management units (ICES, 2007). Several experts on redfish biology and stock-identification methods were therefore invited to the Workshop on Redfish Stock Structure (WKREDS) to consider new information on genetic stock structure in the context of existing biological information (ICES, 2009b). The work here demonstrates the approach, summarizes results, and discusses broader scientific implications of that 2009 workshop.
Methods
Available information on the stock structure of S. mentella was reviewed in stages, by discipline. At each stage, a consensus summary statement within each discipline was developed. All information available on each topic was reviewed, and all perspectives were considered. Some sources of information could be interpreted in alternative ways, so the consensus statement on some issues was simply that there was no single unambiguous interpretation. Final conclusions were based on information that was objective and unequivocal.
The procedural first step was to define all a priori hypotheses. Hypotheses were formed according to previous reviews and advisory decisions. Hypotheses were posed as one-, two-, three-, and four-stock scenarios, with some variations within each scenario. Once a priori stock-structure hypotheses had been defined, case studies relating to population structure of S. mentella in the Irminger Sea and adjacent areas were reviewed individually. Studies published in the primary literature since 1995 (when the deep-sea resource was discovered) received priority. Five criteria were used to form a consensus interpretation of the results from each case study: All case studies in a discipline were considered for developing a summary of the available information within each discipline and a general conclusion about stock structure from the perspective of that discipline. After the multidisciplinary review was complete, each perception of stock structure was considered in an interdisciplinary evaluation. Conclusions on geographic distribution, geographic variation, and connectivity were integrated to obtain a holistic perspective on biological stocks. The unique perspective offered from each discipline along with the sensitivity of specific characters for detecting population structure was considered to identify the congruent results and to reconcile apparent differences. The final stage of evaluating biological stock structure involved (i) consideration of each a priori hypothesis, (ii) identification of information that rigorously tested the hypotheses, and (iii) evaluation of whether the information could be used to reject hypotheses. The testing of hypotheses was based on the most objective information available, i.e. information not subject to alternative or equivocal interpretation. Conclusions on biological stocks were based on the most robust and parsimonious view of stock structure that was consistent with the best scientific information available.
Was stock identification an explicit objective of the study?
Did the samples accurately represent hypothetical stocks (e.g. from a scientific sampling design)?
Was sample size adequate to detect a meaningful difference between groups?
Were differences between hypothetical stocks tested statistically?
Was the analytical methodology sound, i.e. adequate for the task of determining population structure? The critiques and protocols described in Cadrin et al. (2005) served as a guide.
Proposals for practical management units considered geographic delineations that most accurately reflect the consensus on biological stock structure. The definition of proposed management units accounted for the practical aspects and the limitations of monitoring fisheries and the resource, so the management units proposed are geographic proxies for biological stocks that were partly defined by depth.
Results
Geographic distribution
Spatial patterns of abundance offer a basic indication of stock structure, contribute to our understanding of isolating mechanisms or connectivity in a population, and should be the first consideration for exploring stock structure (Begg, 2005). The geographic range of S. mentella extends across the North Atlantic, from the Grand Bank (south of Newfoundland) to the Barents Sea (Figure 1). Its distribution is essentially continuous throughout its range on continental shelves and in pelagic waters near continental shelves to 1000 m deep (Garabana, 2005; Bakay and Melnikov, 2008).
Spatial analysis of developing females, those with fertilized eggs, in fishery catches suggests three different, but overlapping, distributions: (i) on the Iceland slope, (ii) in the deep layer of the northeast Irminger Sea, and (iii) the shallow layer of the southwest Irminger Sea (Anon., 2004). Although areas with developing females can indicate separate or continuous spawning groups, the viviparous reproductive strategy of S. mentella complicates any inference of reproductive mixing. Locations with developing females may indicate where larvae are extruded, but the seasonality of gonad development is indicative of copulation some 6 months earlier (Anon., 2004). Moreover, males and females have different distributions during larval extrusion (Magnússon and Magnússon, 1995), suggesting that copulation takes place in an area different from that at extrusion.
Distribution of early life-history stages can reflect separate spawning groups, larval dispersal, and connectivity among spawning groups (Hare, 2005). Sebastes mentella release their larvae in April or May (Magnússon and Magnússon, 1995; Anon., 2004; Saborido-Rey et al., 2004). Distribution of the larvae in the Irminger Sea varies among years, with a relatively continuous distribution in some years, but discontinuous concentrations in the northeast and southwest in others (ICES, 2009b). Larval extrusion areas of S. mentella are also in the Gulf of St Lawrence (west of Newfoundland), on the Grand Bank, off the coast of Norway, and along the western slope of the Barents Sea (Figure 1; Saborido-Rey and Nedreaas, 2000).
Fishing patterns reflect geographic and depth distribution of the resource. Sigurðsson et al. (2006a) provide an overview of the development of the pelagic S. mentella fishery, including locations, depth, season, and size composition of the catches. In 1982, after exploratory surveys the previous year, a commercial fishery began on prespawning and spawning schools west of the Reykjanes Ridge from early April to mid-May at depths of 80–150 m at night and 150–250 m by day. In the 1990s, two distinct pelagic fisheries developed. From 1992 to 1994, one fishery expanded to the north and into deeper waters (500–600 m) of the northeast Irminger Sea. In 1994, a shallower fishery (150–350 m) expanded to the southwest Irminger Sea, and the fishery has extended even farther southwest since 1996. The northeast fishery in deep water is typically from late spring to summer, and the southwest fishery in relatively shallow water is usually later in the year. Spatial analysis of survey data show that the shift in the fishery to the southwest since 1996 (Sigurðsson et al., 2006a) reflects a similar change in the distribution of the resource coincident with environmental changes (ICES, 2005).
A synthesis of geographic distributions of S. mentella in the Irminger Sea and adjacent waters for successive ontogenetic stages is provided by Saborido-Rey et al. (2004) and Melnikov et al. (2007). Sebastes mentella with developing gonads are caught in separate, but overlapping, areas of the Iceland shelf and the Irminger Sea. Larvae are distributed in the Irminger Sea, in more or less continuous concentrations. Juveniles and adults are caught on the Greenland shelf. Adults are distributed across continental shelves and in the Irminger Sea, where size distributions are larger in deeper water (>500 m) than in shallow. When the fishery expanded to deep water of the Irminger Sea (>500 m), the average size of fish in deep water was 7 cm larger than in earlier catches (Sigurðsson et al., 2006a). Size distributions of fish caught in the deep, northeast fishery are generally larger than those in the shallow, southwest fishery. Size distributions in the deep Irminger Sea are also bimodal in some years, suggesting recruitment from other areas. Bakay and Melnikov (2002) determined age from scale circuli from 1995–1999 samples and found a broader age range deeper than 500 m (6–25 years) than in much shallower habitats (6–17 years), which possibly reflects an ontogenetic movement to deep habitats or a more-intense fishery shallower than 500 m (Johansen, 2003). Rikther (1996) also found a broad age distribution deeper than 500 m (9–23 years). Moreover, age determination of S. mentella in the Irminger Sea using scale circuli is not reliable (ICES, 2006), and published data are inconsistent (Stransky et al., 2005b).
Reviews by Saborido-Rey et al. (2004) and Melnikov et al. (2007) concluded that there is one stock of S. mentella in the Irminger Sea and adjacent areas, based on the spatial distribution of larvae, juveniles, and adults. However, several alternative inferences of movement and connectivity between ontogenetic stages can be deduced from spatial distributions. For example, Rikther (1996) examined the same distributional data of larvae, juveniles, and adults and concluded that there was strong evidence of two S. mentella populations in the Irminger Sea. Although distributional data offer valuable exploratory information for developing stock-structure hypotheses, they cannot be used to test alternative hypotheses rigorously.
Geographic variation
Genetic characters
Among the suite of approaches for stock identification, genetic analyses are the most rigorous to test for reproductive isolation among population components (Begg and Waldman, 1999). Technological development of new genetic markers improves the ability to detect genetic differences and identify discrete stocks, but reconciling new results with information from traditional approaches, including previous genetic research, life-history patterns, phenotypic variation, and connectivity can be challenging. Early research on genetic variation in S. mentella provided weak or equivocal evidence for genetic structure or weak differences among locations (e.g. Nedreaas and Nævdal, 1991; Nedreaas et al., 1994). However, a common scenario in the investigation of stock structure of marine resources is that early studies reveal little variation among areas; as more sensitive molecular markers are investigated and sampling designs are improved, new and stronger differences are revealed among groups that were previously perceived to be genetically similar (Ward and Grewe, 1994; Wirgin and Waldman, 2005). Although different genetic methods can provide different results with respect to group differences, precise interpretation of each type of genetic marker can support a deeper understanding of population structure (Sala-Bozano et al., 2009), and demographic consequences of dispersal cannot be determined by genetics alone (Grant and Waples, 2000).
Over the past decade, several molecular genetic markers have been used in studies of S. mentella and other redfish species. These markers vary widely in terms of function, response to natural selection, mutational features, mode of inheritance, and statistical properties. Therefore, the unique perspective of each type of genetic marker should be considered in the synthesis of information from different studies. Furthermore, the sampling design and the type of statistical analyses conducted also play a significant role in determining and interpreting the results.
Allozymes
Protein expressions of a genetic locus (or gene) were the first genetic characters used to study population structure (Koljonen and Wilmot, 2005). Before the discovery of the deep-sea resource, allozymes failed to indicate geographic variation in S. mentella sampled in shallow water (<500 m). The first attempt to differentiate between Sebastes spp. with molecular markers was performed by Nævdal (1978), who identified S. mentella and S. marinus in the Barents Sea. Dushchenko (1986) found polymorphism in the malic enzyme (MEP), but allelic frequencies were not different among six shallow locations in the Irminger Sea. Similarly, in their study of genetic differences among Sebastes spp., Nedreaas and Nævdal (1991) and Nedreaas et al. (1994) found genetic uniformity among shallow samples from off West Greenland and East Greenland, in the Irminger Sea, around the Faroe Islands, and off Norway and Svalbard. However, soon after the deep-sea fishery began, preliminary information indicated a difference in allozyme frequencies between the oceanic and deep-sea phenotypes, with some alleles that were unique to deep-sea specimens, and large-scale regional differences between samples from Canadian waters, the Irminger Sea, and off Norway (ICES, 1998).
Johansen et al. (2000) and Johansen (2003) found significantly different allozyme frequencies among S. mentella sampled in the Irminger Sea, on the Flemish Cap (east of Newfoundland), and in the Gulf of St Lawrence. Johansen and Sevigny (2003) determined that the source of regional variation was hybridization of S. mentella and S. fasciatus where their distributions overlap, i.e. on the Flemish Cap and in Canadian waters. Johansen et al. (2000) and Johansen (2003) also found significant differences between oceanic and deep-sea phenotypes in the Irminger Sea, with only minor differences between the deep-sea type and those from the Iceland slope (note that individual fish were morphologically classified as oceanic and deep-sea types). However, these studies compared each locus separately, which provided limited power for detecting significant differences. Allelic differences were identified at the polymorphic MEP (a malic enzyme involved in the reduction of NADP+ in the respiration) and IDHP (isocitrate dehydrogenase) loci, and were supported by haemoglobin analyses (Johansen et al., 2000; Johansen, 2003). They concluded that the S. mentella in the Irminger Sea were two different stocks. However, these comparisons were not based on depth-structured samples, and many of the deep-sea fish were sampled above the deep-scattering layer (270–500 m).
Subsequent investigations by Novikov et al. (2006) and Melnikov et al. (2007) again found differences in frequency at the MEP locus between oceanic and deep-sea phenotypes. The Faroese redfish project found differences in allozyme frequencies between shallow samples (the southwest Irminger Sea, the western Iceland shelf, north and east of the Faroes, and off Norway) and deep samples (the northeast Irminger Sea, the eastern Iceland slope, and southwest of the Faroe Islands; Joensen, 2002; ICES, 2005).
The most recent study of S. mentella allozymes was by Daníelsdóttir et al. (2008), who sampled nearly 2000 fish, tested a large number of allozymic loci (33, 13 of which were polymorphic), and analysed all loci simultaneously to test for differences between oceanic and deep-sea phenotypes. Although nearly all the deep-sea samples (95%) were collected deeper than 500 m, and nearly all oceanic samples (93%) were collected from shallower water (<500 m), comparisons were not depth-based, and some sample locations included a mix of both phenotypes. In addition, fish within a sample were classified as belonging to either the deep-sea or oceanic phenotype based on external morphology. Allozyme differences were persistent over the 3-year sampling period, suggesting the existence of two pelagic stocks on the southwest Iceland slope and the central Irminger Sea. This conclusion was supported by significant heterozygote deficiency at all loci in pooled samples, i.e. the Wahlund effect, significant differences in allele frequencies between samples classified as deep-sea and oceanic phenotypes, and clustering of the samples from different phenotypes.
Given that substantial differences in allelic frequencies were initially only observed in a minority of loci (most notably MEP), an alternative interpretation for the pattern observed between oceanic and deep-sea phenotypes is that the allelic frequencies may be influenced by different selective pressures linked to different metabolic efficiencies above and below the deep-scattering layer. Such a scenario would not rule out exchange of genes between the shallow and deep-pelagic populations, but it would indicate the existence of some degree of local adaptation. Moreover, the most recent analysis by Daníelsdóttir et al. (2008) found differences in allelic frequency at many loci. Adaptive differences between shallow and deep populations could affect fitness and demographic dynamics of these populations, which should be considered in the fishery management process. Saborido-Rey et al. (2004) and Melnikov et al. (2007) contend that the pattern of divergence at the MEP enzyme locus reflects a shift of allelic frequencies resulting from selective forces that act after larger, older fish move deeper. Until recently, age determination of S. mentella in the Irminger Sea was unreliable, because ages determined from fish scales tended to yield underestimates of older ages (Stransky et al., 2005b, c; ICES, 2006). Therefore, the hypothesis of ontogenetic movement cannot be tested rigorously from the available data. Assuming that most spawning would be achieved by the larger, older fish in the deep layer, there is no reasonable explanation for the maintenance of high frequencies in the juveniles of the alleles that are selected against after the movement to the deeper layer. Therefore, variation at the MEP locus between oceanic and deep-sea phenotypes is more parsimoniously explained as the result of adaptation to different environments by two diverging populations.
Mitochondrial DNA
In contrast to allozymes, which are the protein expressions of coding genes, DNA structure and polymorphisms can be analysed directly. Mitochondria contain a single circular molecule of DNA (mtDNA) that is maternally inherited and simpler in form than nuclear DNA (nDNA). Alternative sequences of mtDNA, i.e. haplotypes, are easier to analyse than nDNA (Magoulas, 2005). One corollary of the simplicity of mtDNA is that its mutation rates are assumed to be relatively constant, and mtDNA divergence can be used as a molecular clock to indicate the duration of reproductive isolation between two populations. However, a recent review by Galtier et al. (2009) argues that the mutation rates of mtDNA are not constant and that mtDNA characters are subject to selection.
Sundt and Johansen (1998) found a low level of mtDNA variation among Sebastes spp. in the North Atlantic, suggesting recent evolutionary divergence. As a component of the EU Redfish Project, Schmidt (2005) also found a low level of genetic differentiation in mtDNA among North Atlantic Sebastes spp. Phylogenetic analysis revealed genetic distances and patterns of divergence similar to those normally observed within the same species, suggesting that speciation in this group was evolutionarily recent and rapid. Analysis of molecular variance indicated that most of the genetic variation was between species, but that there was also significant variation among samples within species. Haplotype frequencies differed between samples of the deep-sea phenotype and other samples of S. mentella, because one haplotype was frequent in deep-sea samples but occurred in just two other S. mentella samples. Ingimarsdóttir (2008) also found differences in mtDNA haplotype frequencies among oceanic, deep-sea, and demersal samples of S. mentella and estimated that the subgroups in the Irminger Sea diverged ∼4000 years ago. One limit to the application of mtDNA as the only method for stock discrimination in Sebastes spp. may be the relatively frequent occurrence of hybridization between different species (Pampoulie and Daníelsdóttir, 2008).
Nuclear DNA
Several aspects of nDNA are commonly used to study population structure, and each has different sensitivities and interpretations. When little is known about the genome of a species, i.e. DNA sequences have not been indentified, random amplified polymorphic DNA (RAPD) can be used to explore the patterns of variability, because RAPD primers recognize simple nucleotide sequences that should arise frequently in any DNA (Smith, 2005). Johansen et al. (1997) and Johansen and Dahle (2004) found significant differences in allele frequencies of four RAPD primers among all samples of S. mentella from the Gulf of St Lawrence, Norway, and the Irminger Sea (oceanic and deep-sea). However, RAPDs produce results that may not be repeatable and are no longer considered to be a reliable approach for testing population-structure hypotheses.
Amplified fragment length polymorphism (AFLP) is another type of nDNA character that can be used for stock identification. Similar to RAPD, AFLP can be applied to species without prior genomic information, but it uses fragment lengths between arbitrary restriction sites to measure genetic variation (Liu, 2005). Schmidt (2005) found genetic patterns among S. mentella sampled from the Irminger Sea, Greenland, and Iceland, but the significant differences between all samples indicated that AFLP markers may be too variable to detect biologically meaningful patterns of genetic structure among subpopulations of S. mentella. Similar to RAPD, AFLP results are not always repeatable among laboratories, and AFLP characters are inherited as dominant markers (Liu, 2005). Therefore, both RAPD and AFLP are considered to be more exploratory than confirmatory for stock-identification studies.
Microsatellites are segments of nDNA consisting of tandem nucleotide repeats. Microsatellites are non-coding, so they are generally not subject to selection and have a rapid mutation rate. Both these characteristics make them the most effective character for studying population structure (Wirgin and Waldman, 2005). A series of increasingly rigorous analyses of microsatellite characters indicates a general pattern of population structure of S. mentella that involves three distinct genetic groups located in (i) the deep Irminger Sea, (ii) shallow-pelagic habitats, and (iii) demersal habitats.
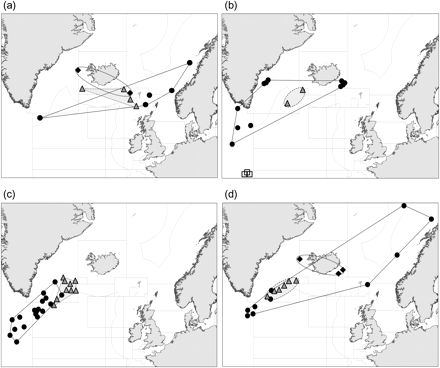
The Faroese redfish project found differences in microsatellite allelic frequencies between three geographically overlapping, but genetically distinct groups: (i) shallow (<500 m) samples from the southwest Irminger Sea, the northern and eastern Faroese shelf, and the Norwegian coast, (ii) deep (>500 m) samples from the northeast Irminger Sea, the eastern Iceland slope, and the southwest Faroese slope, and (iii) the western Iceland shelf and southwest Faroese shelf (Figure 2a; Joensen, 2002; ICES, 2005).
Genetically distinct clusters of S. mentella samples detected by analysis of microsatellites from the Faroese Redfish Project (Joensen, 2002; panel a), Schmidt (2005; panel b), Stefánsson et al. (2009a; panel c), and Bayesian-based cluster analysis (Stefánsson et al. 2009b; panel d). Symbols indicate sample locations by genotype: western (squares), shallow pelagic (black dots and solid-line polygons), deep pelagic (grey triangles and dashed-line polygons), and Icelandic slope (black diamonds with dotted-line polygons).
Roques et al. (2002) used microsatellite loci to demonstrate the presence of hybridization between S. mentella and S. fasciatus in the Gulf of St Lawrence, which appears to represent a unique evolutionarily significant unit. They concluded that there are three distinct populations of S. mentella: (i) in the area of hybridization with S. fasciatus off southern Canada, (ii) in a panoceanic area from Labrador to the Faroe Islands, and (iii) on the Norwegian shelf. However, all the panoceanic samples in the Irminger Sea and adjacent areas were from shallow water (<500 m).
Using eight highly variable microsatellite loci, Schmidt (2005) found weak but significant genetic structure in S. mentella. Significant genetic differences were found between three groups of samples: (i) on the Flemish Cap, (ii) in the deep (>500 m), central Irminger Sea, and (iii) in shallow (<500 m) samples off Greenland and Iceland and in the southern Irminger Sea (Figure 2b).
Pampoulie and Daníelsdóttir (2008) used nine microsatellite loci to distinguish all the Atlantic species of Sebastes, but the analyses also indicate that the oceanic and deep-sea phenotypes of S. mentella are genetically distinct, with considerable misclassification of genotype using phenotyping. Although the comparisons tested by Pampoulie and Daníelsdóttir (2008) were primarily based on phenotypic identification, i.e. size-at-maturity, morphometry, and colour, Stefánsson et al. (2009b) regrouped the data by depth. The revised and expanded analysis of nearly 2000 fish shows that populations below and above the 550-m depth boundary are well differentiated based on microsatellite variation (Figure 2c). The analyses also suggest that the shallow and deep-pelagic subpopulations may represent incipient species that were allopatric, i.e. geographically separate, during the Pleistocene glaciations, but secondarily came in contact to form their current sympatric, i.e. overlapping, distribution (Stefánsson et al., 2009a, b).
A spatially expanded analysis rigorously tested for genetic differences between shallow (<550 m) and deep (>550 m) S. mentella in the Irminger Sea (Stefánsson et al., 2009b). The analyses revealed temporally stable differences between deep and shallow-pelagic samples, providing evidence that fish inhabiting waters deeper than 550 m are genetically distinct from shallower ones (Stefánsson et al., 2009b). Analyses of shallow samples are similar to those reported by Roques et al. (2002), with the addition of genetically distinct deep samples and samples on the Iceland slope. Geographic distributions of the three genetically distinct clusters are shown on Figure 2d.
Synthesis of all genetic information suggests that S. mentella from south of Newfoundland and the Gulf of St Lawrence are genetically distinct from S. mentella in the rest of the North Atlantic, because of adaptive local hybridization with S. fasciatus. A panoceanic shallow (<500 m) subpopulation of S. mentella extends from Labrador to at least the coast of Norway, perhaps to the Barents Sea. Roques et al. (2002) found one Norwegian sample that was significantly differentiated from the panoceanic group, but Stefánsson et al. (2009b) did not. S. mentella in the deep Irminger Sea (>500 m) and S. mentella on the Iceland slope are also distinct subpopulations.
This new perception of genetic structure in the Irminger Sea and adjacent waters contrasts with the previously posed single-stock hypothesis (Saborido-Rey et al., 2004; Melnikov et al., 2007). The revised view of stock structure is a result of more extensive genetic testing, the use of neutral and powerful markers, refinement of analyses by depth (rather than by phenotype), and robust statistical approaches. The hypothesis that fish move to deeper water as they grow older is refuted by the substantial differences between deep and shallow-pelagic samples in microsatellite allelic frequencies (which are not under selection and have been tested for temporal stability). Saborido-Rey et al. (2004) suggested that genetic differences may result from genetic drift or a sweepstakes effect, i.e. each year class being genetically distinct because it is produced from a small, randomly selected portion of the adult population, both of which imply some genetic population structure and reproductive isolation, so refuting the assumption of panmixia. The revised perception of genetic structure explains some of the previously observed patterns in genetic analyses. For example, the lack of correlation between genetic distance and geographic distance among samples from the Faroe Islands to the Grand Banks (e.g. Roques et al., 2002) probably results from depth-based differences among locations that are geographically close.
Based primarily on microsatellite information, but also supported by results from analyses of allozyme, AFLP, and RAPD characters, there are four genetic stocks of S. mentella in the Irminger Sea and adjacent waters: Note that juveniles on the Greenland shelf may be from the shallow-pelagic, deep-pelagic, and Iceland slope stocks (Johansen, 2003; Anon., 2004; Schmidt, 2005). The relationships between the deep-pelagic stock, S. mentella in the Norwegian Sea, and demersal S. mentella in the Barents Sea, require further investigation.
A western stock extending south of Newfoundland and west to the Gulf of St Lawrence.
A shallow-pelagic stock extending from the Grand Bank to the Faroe Islands, perhaps farther east. The adults of this stock are primarily in pelagic habitats, but are also in some demersal habitats east of the Faroe Islands.
A deep-pelagic stock also primarily consisting of S. mentella in pelagic habitats, but including demersal habitats west of the Faroe Islands. Note that this genetic stock does not necessarily equate to the deep-sea phenotype.
An Iceland slope stock inhabiting demersal habitats of the continental slope; the northwest Faroese slope may be part of this stock.
Phenotypic variation
Geographic variation in phenotypic characters (measurable traits that are influenced by both genetics and environmental factors) is valuable for stock identification, because maintenance of phenotypic differences among groups indicates limited mixing, and adaptive differences have a genetic basis. Investigation of phenotypic variation can be used to define putative genetic stocks (for confirmation using genetic techniques). If phenotypic differences are indicative of distinct biological stocks and if they are temporally stable, they can offer a practical measure for stock discrimination or stock composition analysis.
The study of phenotypic variation has played a large role in the investigation of S. mentella population structure. Soon after the discovery of the deep-sea resource, phenotypic differences between oceanic and deep-sea forms were recognized (Magnússon and Magnússon, 1995). Several phenotypic characters have been used to study the stock structure of S. mentella, including life-history traits (e.g. size distributions, size-at-maturity), morphology (e.g. body form, meristics, otolith shape), and fatty acid composition.
Phenotypic variation can be particularly valuable for stock identification when it is associated with life-history characteristics and vital rates (e.g. growth, reproduction, mortality) that are also critically important for population dynamics, stock assessment, and fishery management (Begg, 2005). There are some indications of different growth rates among the genetic stocks of S. mentella identified above. Size distributions are relatively smaller in the shallow, southwest Irminger Sea than in the deep, northeast area, and this relative difference has persisted over time. However, age determination is not reliable for S. mentella in this area (Stransky et al., 2005b, 2005c), so the cause of different size distributions, i.e. growth differences, mortality differences, movement patterns, is difficult to interpret. Pelagic phenotypes have been partially identified based on size-at-maturity, with the deep-sea types having larger size-at-maturity than the oceanic type (Magnússon and Magnússon, 1995; ICES, 1998). Melnikov et al. (2007) used length distributions, maturity stages, and the distribution of various life stages to infer that fast-growing, early-maturing fish of each year class recruit to the pelagic areas of the Irminger Sea, whereas slow-growing, late-maturing fish recruit to the deep-water habitat along the slopes of East Greenland, and along the Iceland–Greenland Ridge to the slopes west and south of Iceland (the Iceland slope S. mentella stock).
Geographic differences in morphology can also indicate subpopulations that have limited mixing. Morphological patterns can also reflect life-history differences and possibly adaptations to different environments (Cadrin, 2000). Several studies have investigated patterns of morphology for S. mentella. Nagel et al. (1991) found that S. mentella on the Reykjanes Ridge had more vertebrae than those collected in other areas (off east and west Greenland, the Irminger Sea, off Norway, and in the Barents Sea). Rikther (1996) also found significantly more vertebrae, and also more anal fin and pectoral fin rays in S. mentella sampled from the Iceland slope than from those from the Irminger Sea. Saborido-Rey and Nedreaas (2000) found morphometric differences between three regions off Norway: Svalbard, the Barents Sea, and the Norwegian Sea, and that these morphs appeared to mix in the area of larval extrusion in spring.
Significant differences in meristic features usually indicate environmental differences experienced during early life stages (Waldman, 2005). Reinert and Lastein (1992) found morphometric differences between samples from the Irminger Sea, the Faroe Islands, and off Norway, with some morphometric heterogeneity within Faroese samples, but not among Irminger Sea samples (which were all collected from shallow water).
After the discovery of the deep Irminger Sea resources of S. mentella, pelagic phenotypes were defined primarily based on colour (deep-sea types are redder, and oceanic types more greyish red), body shape (deep-sea fish are stouter), as well as size-at-maturity and parasites (Magnússon and Magnússon, 1995; ICES, 1998). Pelagic phenotypes were secondarily identified based on general appearance (deep-sea are brighter, and oceanic less clean), colour pattern (oceanic have black and red spots), fillets (oceanic fillets have dark spots), and morphometry (oceanic types have a narrower head; ICES, 1998).
Garabana (2005) found morphometric differences between deep-sea and oceanic phenotypes, but morphometric variation could not accurately classify individuals to type. Fish from the Irminger Sea were more fusiform than those from other areas, and the orientation of third and fifth preopercular spines was more forward-pointing in deep-sea types. Morphology was compared among samples from the Flemish Cap, Faroe Islands, Greenland shelf, Iceland slope, Irminger Sea, and Norwegian shelf, but all were morphometrically too similar to support accurate classification of fish to location.
Stransky (2005) measured outline shape of otoliths collected from S. mentella throughout the North Atlantic. Otolith shape could not accurately classify fish to specific areas (Flemish Cap, Davis Strait, West Greenland shelf, East Greenland shelf, Irminger Sea, Iceland slope, Faroe Islands, and Barents Sea), but it could accurately classify specimens to three broad regions: (i) Flemish Cap and Davis Strait, (ii) Greenland shelf, Irminger Sea, Iceland slope, and Faroe Islands, and (iii) Barents Sea. Stransky (2002) compared otolith shape among samples in the Irminger Sea by depth, but found no clear differences between depth groups.
Johansen (2003) and Daníelsdóttir et al. (2008) found significant differences in allelic frequency of allozyme loci between fish classified to oceanic or deep-sea phenotypes based on external morphology. However, there was considerable misclassification between phenotypes and genotypes (Daníelsdóttir et al., 2008). Stefánsson et al. (2009a) found significant differences in meristics and morphometry between specimens of oceanic and deep-sea genotypic groups within the Irminger Sea, but statistical analyses suggest that these differences could not be used alone for stock identification (individual classification was not possible). Phenotyping has had limited utility for stock identification, because the two types have overlapping geographic and depth distributions (Kristinsson and Sigurðsson, 2007), and possibly because of recent divergence.
A relatively new approach to stock identification is the investigation of fatty acid composition. Fatty acids are phenotypic characters in that they reflect both genetic and environmental factors, with some specific fatty acid concentrations more heritable than others (Grahl-Nielsen, 2005). Joensen and Grahl-Nielsen (2004) measured fatty acid profiles in the heart tissue of S. mentella from 11 areas in the North Atlantic from Norway to the Irminger Sea. Significant differences were found among four stocks: (i) the shallow Irminger Sea, (ii) the deep Irminger Sea, southeast Iceland slope and southwest Faroese slope, (iii) the western Iceland shelf, and (iv) north and northeast Faroe Islands and Norway. Stocks identified through fatty acid profiles are similar to those identified from allozyme and microsatellite DNA analysis, supporting the conclusions drawn from genetic analysis. Fatty acid profiles, however, must be viewed cautiously in the context of stock identification, because they may be influenced by environmental factors such as diet or temperature (Joensen, 2002).
In summary, geographic variation in life history is apparent from size distributions and size-at-maturity, but precise evaluation of growth or maturity is difficult without reliable age determination. Subtle morphological differences exist between oceanic and deep-sea forms, and geographic patterns of fatty acid profiles are similar to those from genetic analysis. Although interpretation of phenotypic traits is somewhat subjective, i.e. can be validly interpreted in several ways, all information on phenotypic variability is consistent with our perception of genetic stocks.
Connectivity
The extent of isolation or mixing of subpopulations is an important aspect of defining the stock structure. Resource management strategies should consider population dispersal and connectivity among populations in the definition of the appropriate spatial scale of stock assessment and fishery management (Fogarty and Botsford, 2007). Connectivity can be evaluated by modelling the dispersal of early life stages, mark-recapture analysis of artificial tags, or examination of natural tags (e.g. otolith chemistry, parasite infestation). Mixing of groups can involve two distinct patterns that have different influence on population structure and reproductive dynamics: (i) overlap is a pattern in which individuals from reproductively isolated subpopulations share the same habitats in some seasons, but have isolating mechanisms, e.g. separate areas or seasons for reproduction, and (ii) diffusion, or reproductive mixing, allows gene flow and correspondence in reproductive dynamics among groups (Cadrin and Secor, 2009).
Connectivity among geographic groups of S. mentella has been studied using several approaches. Rikther (1996) inferred larval drift from distribution of larvae, surface currents, and the distribution of age-0 demersal stages. He concluded that there are two different stocks in the Irminger Sea area: (i) a coastal stock, inhabiting the Iceland and eastern Greenland shelf slopes, and (ii) a pelagic stock, which occurs in the open sea. The significant differences in the number of vertebrae and fin rays he found may also indicate different larval environments, because those features are typically determined during early life stages (Waldman, 2005). Saborido-Rey et al. (2004) reviewed previous investigations of S. mentella larval drift in the Irminger Sea and adjacent areas and concluded that the larvae drift from the central and eastern Irminger Sea towards the Greenland shelf.
A traditional approach to stock identification and connectivity is the use of parasites as biological tags. The approach has been revived through advancements in methodology (Mackenzie and Abaunza, 2005), but parasites used for stock identification should be selected and applied cautiously (Lester and Mackenzie, 2009). Patterns of parasite infestation have played a large role in the study of S. mentella stock structure. Parasite fauna are distinctly different for pelagic and demersal S. mentella in the Reykjanes Ridge area (Melnikov et al., 2005; Melnikov and Bakay, 2006). Pelagic S. mentella are frequently infested by Sphyrion lumpi, but those on the Iceland slope are not. Conversely, pelagic S. mentella do not have some parasites that are found on the slope, such as Microcotyle sebastis (Monogenea), or the rarer parasites Spinitectus oviflagellis (Nematoda), Echinorhynchus gadi, Corynosoma strumosum, and Acanthocephalus sp. (Acanthocephala). The infestation rate of the nematod Anisakis simplex is also significantly different between pelagic and demersal S. mentella.
Similarity in the parasite fauna of S. mentella from different areas of the southeastern slope of Greenland and the pelagic Irminger Sea suggests that redfish concentrations in those regions are closely related. A shift in composition of parasite fauna suggests that maturing redfish migrate from shallow habitats on the slope to the Irminger Sea and to deep waters of the Greenland slope. The parasite fauna of fish in the Irminger Sea changes ontogentically as a result of fish moving from the continental shelf, which is the indigenous area of myxosporidians, to a pelagic environment, where they feed on copepods, meso- and bathypelagic fish, and young squid. The decrease in the composition of trematodes, nematodes, and acanthocephalans is accounted for by a shift in diet away from near-bottom crustaceans. The presence of the copepod S. lumpi indirectly suggests a movement of maturing S. mentella from the slope to pelagic waters during summer (Melnikov et al., 2005).
Pelagic phenotypes are partially defined by the prevalence of the parasitic copepod Sphyrion lumpi. Unfortunately, there are conflicting patterns of S. lumpi infestation (ICES, 1998). Some researchers noted increasing infestation rates with depth (Magnússon et al., 1995; Magnússon and Magnússon, 1995), but others found decreasing infestation with depth (Del Rio et al., 1996; Sarralde et al., 1997).
Parasites and pigmented patches have been used as indicators of population structure of S. mentella in the Irminger Sea and adjacent waters by a long series of Russian investigations (1983–2008). The infestation rate of S. lumpi and the entire parasite fauna were similar in all the areas of the pelagic Irminger Sea and adjacent waters (Bakay, 1988, 2000, 2001), and the parasite fauna shallower and deeper than 500 m was also similar (Bakay and Melnikov, 2002, 2008). Moreover, in terms of pigment patches on the skin, there was no geographic variability, but considerable annual differences (Bogovski and Bakay, 1989). Bakay and Melnikov (2008) interpreted the predominance of pigment patches in S. mentella taken deeper than 500 m, and the progressively fewer pigment patches on the skin and muscular melanosis of fish >40 cm long to be a consequence of age-dependent changes and ontogenetic movement to deeper pelagic habitats, and concluded that the resource at all depths of the Irminger Sea and adjacent Labrador waters is a single stock. However, the interpretation of movement from shallow to deep-pelagic environments is refuted by recent genetic evidence of groups being reproductively isolated (Pampoulie and Daníelsdóttir, 2008; Stefánsson et al., 2009b).
A recently developed natural tag for stock identification lies in the otolith chemistry. As otoliths grow, they incorporate the chemical signature of the fish's environment, so that each growth zone is an archive of the fish's environmental history (Campana, 2005). Stransky et al. (2005a) used otolith microchemistry to investigate connectivity between redfish habitats on the East Greenland shelf and in the Irminger Sea. They confirmed that elemental signatures in cores of otoliths collected from East Greenland were temporally stable, and that similar elemental concentrations (Li, Sr, Mg, Ba, and Cu) were found between redfish otoliths collected in the Irminger Sea and from East Greenland. A lack of clear spatial differences in otolith chemistry could indicate either a common natal origin of the adults or a lack of variation in elemental chemistry across large expanses of the ocean in the region.
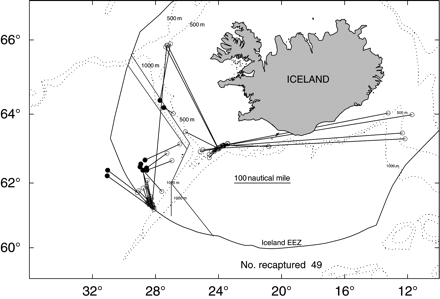
Tagging has a long history in fishery research and the study of movement patterns (Thorsteinsson, 2002). A particular challenge in tagging redfish is the barotrauma associated with bringing fish to the surface. Sigurðsson et al. (2006b) solved this dilemma for S. mentella using an innovative in situ tagging device. Although the objective of his tagging project was to demonstrate the effectiveness of in situ tagging technology, some tag releases and a few long-term recaptures offered valuable insights into movement patterns. Sample sizes were low and not designed to represent a management unit or biological stock, but the 49 tags recovered included several movements from deep, pelagic environments to demersal habitats on the Iceland slope (Figure 3). Those movements document distributional overlap between the two groups, and perhaps connectivity.
Results of tagging experiments, updated from Sigurðsson et al. (2006b). Dots indicate the tagging site, and open circles the recapture site. The line differentiating management units is also shown.
Information from larval drift, parasites, otolith chemistry, and tagging suggest that subpopulations of S. mentella mix during early life stages, as larvae and juveniles, then the adults recruit to different habitat groups with overlapping distributions. Synthesis of information on connectivity and genetic composition indicates that overlapping adult distributions do not involve reproductive mixing.
Interdisciplinary analysis
A synthesis of all available information was used to test each of the a priori stock-structure hypotheses. The single-population hypothesis was rejected based on significant differences in microsatellite allelic frequencies among deep Irminger Sea, shallow Irminger Sea, and Iceland slope samples, as well as significant differences in allozymes and fatty acid profiles among the same three groups, along with the distinct parasitological differences between Iceland slope and pelagic fish.
Several two-stock hypotheses were also considered. The current management unit hypothesis (one pelagic stock and one demersal stock; ICES, 2008a) was rejected based on significant differences in microsatellite allelic frequencies between deep and shallow Irminger Sea samples, as well as significant differences in allozymes and fatty acid profiles. The hypothesis of two depth-defined stocks (one stock <500 m and one >500 m) was rejected based on significant differences in microsatellite allelic frequencies between continental slope and pelagic samples, as well as significant differences in allozymes, fatty acid profiles, and parasite fauna. The two-phenotype hypothesis (oceanic and deep sea) was similarly rejected based on significant differences in microsatellite allelic frequencies between continental slope and pelagic fish, along with the significant differences in allozymes, fatty acid profiles, and parasite fauna.
An a priori three-stock hypothesis (slope and shallow- and deep-pelagic stocks) was not entirely consistent with all data, because heterogeneity within continental slope samples from Iceland and the Faroe Islands was indicated by microsatellites and fatty acids. However, such heterogeneity was recognized in the a priori evaluation as a possible alternative hypothesis.
A four-stock hypothesis was consistent with all information on stock structure. Based primarily on microsatellite information, supported by analyses of allozymes, fatty acids, as well as some patterns in parasite infestation and general morphology, the conclusion was that there are four biological stocks of S. mentella in the Irminger Sea and adjacent waters:
a western stock (from south and west of Newfoundland);
a shallow-pelagic stock (from the Grand Bank to the Faroe Islands, perhaps farther east and shallower than 500 m), the adults of which stock are primarily in pelagic habitats, but also in some demersal habitats east of the Faroe Islands;
a deep-pelagic stock, found from Labrador to the Faroe Islands deeper than 500 m, whose adults are primarily in pelagic habitats, but also in some demersal habitats west of the Faroe Islands (note that this stock is not equivalent to the deep-sea phenotype);
an Iceland slope stock.
Juveniles on the Greenland shelf may be from the shallow-pelagic, deep-pelagic, and Iceland slope stocks. Although the Greenland shelf appears to be a nursery area for the three stocks near the Irminger Sea, there are also nursery areas off Canada and Norway (Saborido-Rey and Nedreaas, 2000).
Stock identity of S. mentella in the Norwegian and Barents Seas has not been examined rigorously, but significant differences from the above four stocks have been found in genetics (Roques et al., 2002), fatty acids (Joensen, 2002), morphometrics, and otolith morphology (Anon., 2004; Stransky 2005), suggesting that S. mentella east of the Faroe Islands and in the Norwegian and Barents Seas may be a distinct phenotypic stock. This conclusion is also supported by the separate area of extrusion of larvae along the continental slope off Norway (Figure 1; Nedreaas 1995).
This perception of biological stock structure is based primarily on genetic patterns among adult fish. Other stock-identification information, e.g. overlapping distributions of life stages, growth and maturity patterns, generally similar morphometry, and parasite patterns, cannot be used to reject any hypothesis, because several alternative interpretations of those data are equally valid. This view of biological stock structure cannot be rejected by any of the information available. On the contrary, areas of larva extrusion and many of the phenotypic patterns can be interpreted as a reflection of this biological stock structure, e.g. subtle morphological differences among areas, different size distributions by depth, different size-at-maturity, and phenotypes.
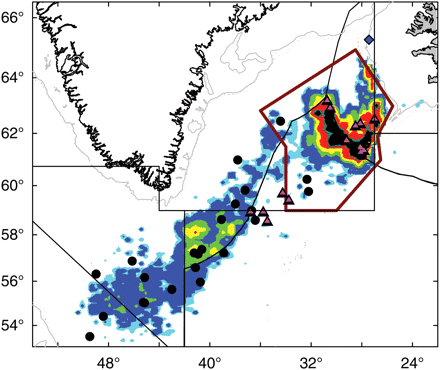
Although biological stocks of S. mentella are partially defined by depth, we recognize that the definition of management units by depth and the associated fishery monitoring by depth would be impractical. Depth-based differences in genetic stocks can be viewed as geographically separated units. Based on the above view of biological stock structure, ICES has revised its advice for management of S. mentella fisheries as three management units based on geographic proxies for biological stocks that minimize mixed-stock catches (ICES, 2009a). Spatial and seasonal patterns in the pelagic fishery have been relatively stable since 1996 (Sigurðsson et al., 2006a; ICES, 2008b). Spatial analysis of pelagic fishery catch and effort by depth, inside and outside the recommended deep-pelagic management unit boundaries indicate that those boundaries effectively delineate the deep, pelagic fishery from the shallow, pelagic fishery, but with a small portion of mixed-stock catches (Figure 4). Given the overlapping distributions of the associated biological stocks, mixed-stock catches in the Irminger Sea clearly need to be monitored for stock composition.
Revised management unit boundaries (red polygon), with fishing distribution and genetic sample locations. Fishery data are from Iceland, Russia, Germany, Norway, the Faroe Islands, and Greenland (1996–2007). Black dots, shallow-pelagic genotype; pink triangles, deep-pelagic genotype; blue diamonds, Icelandic slope genotype.
Discussion
Despite the increasing application of genetic techniques for identifying population structure, the application of genetic information for fishery management has been slow to develop (Waples et al., 2008). After a decade of researching the stock structure of S. mentella near the Irminger Sea, ICES revised the form of its advice to fishery managers to account for genetic differences between the deep-pelagic and shallow-pelagic stocks (ICES, 2009c). Advice for the shallow-pelagic stock was “given the very low state of the stock, the directed fishery should be closed”, and for the deep-pelagic stock “given the reduced abundance of this stock in recent years, a total catch limit of no greater than 20 000 tonnes should be implemented in 2010”. The difference in advice for the two stocks illustrates the importance of stock identification for fishery management.
Synthesis of recent genetic results with information from previous stock-identification research demonstrates the interdisciplinary analysis required for a holistic perception of population structure. Spatial complexities, sampling difficulties, unstandardized methods, and technological subtleties present challenges for determining stock structure, but all sources of information need to be considered in formulating a conclusion that is both comprehensive and consistent. Although the results from various studies of S. mentella population structure appear to be contradictory, many of the apparent contradictions result from differences in sampling design (particularly with respect to depth). Precise interpretations of what aspect of stock structure each methodological approach represents, e.g. the different perspectives gained from genetic vs. phenotypic approaches, or the different sensitivities of genetic markers, as developed by Cadrin et al. (2005), helped to resolve incongruent results. The synthesis did, however, require the collaboration of a multidisciplinary group of scientists with complementary expertise.
The unique life history of Sebastes spp. offers an illustrative example of the mechanisms of population structure. Reproductive isolation of oviparous fish species is usually associated with discrete spawning areas or seasons, forming spatial or temporal barriers to gene flow among groups. The viviparous nature of Sebastes spp. and the associated mate recognition, courtship behaviour, and mate choice act as additional mechanisms of reproductive isolation (Johns and Avise, 1998). These isolating mechanisms, together with high fecundity and wide dispersal of larvae, allow relatively rapid adaptive radiation (Rocha-Olivares, 2004).
Sebastes spp. in the North Pacific are considered to be ancient species flocks because adaptive radiation was relatively rapid, but present-day species are completely isolated (Johns and Avise, 1998; Alesandrini and Bernardi, 1999). In contrast, Atlantic Sebastes spp. are relatively young, formed from North Pacific ancestors that moved to the North Atlantic during the “great transarctic biotic interchange”, when the Bering land bridge opened and Arctic waters warmed, allowing the movement of sub-Boreal species through the Arctic Ocean (Love et al., 2002). Patterns of variation in genetics and otolith shape between Pacific and Atlantic Sebastes spp. are consistent with the movement of a common ancestor species (Stransky and MacLellan, 2005; Hyde and Vetter, 2007).
Recent and rapid evolutionary divergence of North Atlantic Sebastes spp. is evidenced by the little variation in mtDNA among Sebastes spp. in the North Atlantic (Sundt and Johansen, 1998; Schmidt, 2005) and the low variation in allozymes between/across species (Johansen, 2003). Ingimarsdóttir (2008) estimated that the subgroups in the Irminger Sea diverged some 4000 years ago. Hybridization between S. mentella and S. fasciatus and the high frequency of back-crossed genotypes demonstrate that reproductive isolation between species is not complete, so North Atlantic Sebastes spp. must represent a more recent species flock (Hyde and Vetter, 2007), and the formation of distinct genotypes within S. mentella represents even more recent divergence (4000–27 000 years ago; Ingimarsdóttir, 2008; Stefánsson et al., 2009a).
The population structure of S. mentella illustrates how divergent behavioural groups (i.e. adults from the same nursery grounds exploiting different habitats) can lead to demographic independence, reproductive isolation, and adaptive differences. Moreover, the phylogeny of Sebastes spp. demonstrates how intraspecific variation, i.e. “stock concept”, and interspecific variation, i.e. “species concept”, are a continuum of divergence over ecological and evolutionary time-scales.
Acknowledgements
We thank the many scientists who have contributed to research on redfish stock structure, particularly our colleagues in ICES working, study, review, and advisory groups, as well as the ICES Secretariat. Much of this work was completed for the Workshop on Redfish Stock Structure, and the review presented at an ICES Annual Science Conference theme session on “Life cycle diversity within populations, mechanisms and consequences”; we are grateful to the conference hosts and session conveners for the invitation to publish, and the reviewers for their valued comments.