-
PDF
- Split View
-
Views
-
Cite
Cite
Markus Vetemaa, Redik Eschbaum, Anu Albert, Lauri Saks, Aare Verliin, Kristiina Jürgens, Martin Kesler, Kalvi Hubel, Rögnvaldur Hannesson, Toomas Saat, Changes in fish stocks in an Estonian estuary: overfishing by cormorants?, ICES Journal of Marine Science, Volume 67, Issue 9, December 2010, Pages 1972–1979, https://doi.org/10.1093/icesjms/fsq113
Close - Share Icon Share
Abstract
In Estonia, the cormorant Phalacrocorax carbo sinensis is a newcomer, and its numbers have increased rapidly since 1985. In the shallow protected (no fishery) Käina Bay in Väinameri (West Estonia), the colony was established in 1995. Gillnet sampling indicated that roach was the most abundant spawning fish species in 1995. Ten years later, when the study was repeated, the catch per unit effort was already more than 100 times lower than in 1995. The number of spawning perch decreased tenfold from 1995 to 2005. During the same period, commercial fishing effort in the entire Väinameri area decreased several times. The change in fish abundance in the Käina Bay and in the coastal fish-monitoring areas in the archipelago sea nearby, together with an analysis of food of cormorants, indicates that the decline in fish abundance might be related to the increased numbers of cormorants. The conclusion is drawn that the establishment of a cormorant colony could have seriously damaged or even prevented normal functioning of historically important spawning grounds and affected fish recruitment to adjacent areas. Therefore, expanding bird colonies might play a role similar to an expanding fishing fleet, by overexploiting the resource.Vetemaa, M., Eschbaum, R., Albert, A., Saks, L., Verliin, A., Jürgens, K., Kesler, M., Hubel, K., Hannesson, R., and Saat, T. 2010. Changes in fish stocks in an Estonian estuary: overfishing by cormorants? – ICES Journal of Marine Science, 67: 1972–1979.
Introduction
The ecosystem approach to fisheries management involves an evaluation of the wider role that fish play in the ecosystem in which they are embedded, for instance as food resource for species that might be valued for reasons other than their yield. Small fish represent an important food source for many bird species. To the extent that thriving bird colonies are seen as desirable, fish might have to be reserved to provide food for these colonies and to be protected against fishing. However, if the same fish would otherwise recruit to commercial stocks, a conflict could arise between two use values: does the forfeited commercial catch exceed the benefits for nature conservation?
There is perhaps a general conjecture that such conflicts tend to be resolved to the disadvantage of the birds: exploitation results in a decline in bird populations (Frederiksen et al., 2004; Okes et al., 2009). However, conceptually at least, the shoe might well be on the other foot: increasing bird colonies might outcompete the fisheries. We argue that expanding cormorant colonies in Estonia might play a role similar to an expanding fishing fleet, by overexploiting the resource.
The first colony of great cormorants (Phalacrocorax carbo) in Estonia was established in 1984, and by 2005, 20 colonies had been counted, with ∼10 000 nesting pairs (Eschbaum, 2008). In central Europe, the number of cormorants had already increased rapidly by the end of the 1970s (Van Eerden and Gregersen, 1995).
Evidence that double-crested cormorants (Phalacrocorax auritus) might cause declines in fish stocks is available for North America. In Lake Oneida, nesting of these cormorants was first observed in 1984 and the colony had increased to more than 360 pairs by 2000 (Rudstam et al., 2004). Concomitant with the increase, adult walleye (Stizostedion vitreum) and yellow perch (Perca flavescens) populations declined. The estimated number of these species consumed clearly indicated that the increase in subadult mortality could be explained by predation by cormorants only. Burnett et al. (2002) demonstrated that cormorant predation potentially played a role in the decline in abundance of yellow perch in Lake Ontario from 1976 to 1999, whereas Lantry et al. (2002) found that survival of juvenile smallmouth bass (Micropterus dolomieu) had declined in the same area after the increase in the cormorant population. A detailed analysis demonstrated again that much of the increased mortality could be explained by cormorant predation. In contrast, Diana et al. (2006) found no such relationship in Lake Huron and concluded that the predation effect could vary annually, possibly in relation to the timing of cormorant migration relative to perch spawning.
The EU Concerted Action REDCAFE (Reducing the conflict between cormorants and fisheries on a pan-European scale) concluded that “demonstrating the impact of Cormorants in large rivers and other waterbodies is difficult, because of ecological complexities” (Carss, 2003). European studies mostly failed in finding clear correlations between abundance of cormorants and fish stock dynamics in larger ecosystems. Nevertheless, there are clear cases of damage to fishing gear and ensnared fish by cormorants, as well as documented cases of considerable impact at fish farms and small waterbodies (Van Dam and Asbirk, 1997; Stewart et al., 2005). Engström (2001a) monitored fish species composition and abundance before and after a great cormorant colony became established in a highly productive lake in Sweden. Despite considerable consumption of fish by the cormorants, changes in fish numbers or biomass were not detected. In addition, because of a lack of overlap in diet of cormorants and catches of commercial fishers, little effect on the fishery was found in eutrophic freshwater lakes in the Netherlands (Marteijn and Dirksen, 1991). Still, one confirmed case of impact emanated from the North Sea, where cormorant predation during summer accounted for 49.5 and 27.3% of the total mortality of 0-group plaice (Pleuronectes platessa) in 1992 and 1993, respectively (Leopold et al., 1998).
We present data on the abundance of some freshwater fish species in an important estuarine spawning area in western Estonia and relate the observed changes to a rapidly growing local colony of great cormorants. In 1995, the local fish fauna was sampled as part of a larger basic research project. In 2005, the sampling was repeated to investigate whether the cormorants had affected the fish community. The bay where the colony is situated is closed to fishing, but fisheries in the potential feeding area during the breeding season have been declining.
Study area
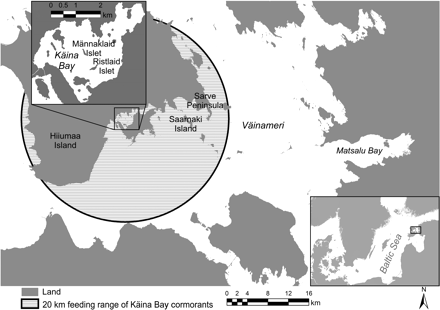
Väinameri is a shallow (average depth ca. 5 m), semi-closed archipelago area in western Estonia, bordered by some big islands and the Estonian mainland (Figure 1). Because of its shallowness, ice is formed early during winter and it usually lasts from December to April. However, the water warms up quickly in spring and can exceed 25°C during midsummer. The smaller bays and sheltered areas are covered with large reed beds. The water is brackish; with average salinity ranging between 2 and 5 psu, and freshwater fish species are most abundant (Saat and Kikas, 2002). Dominating species belong to the percids and cyprinids (perch Perca fluviatilis, ruffe Gymnocephalus cernuus, roach Rutilus rutilus, ide Leuciscus idus), whereas only two commercially important marine species—herring Clupea harengus membras, and garpike Belone belone—enter the area in large numbers during spring for spawning (Vetemaa et al., 2006b).
The Väinameri with the location of the regular fish-monitoring surveys (Sarve, Saarnaki, and Matsalu Bay) and the study area in Käina Bay (inset). The cormorant colonies are located on Männaklaid and Ristlaid islets and their approximate feeding range is indicated by the circle.
Käina Bay (ca. 9 km2) is a very shallow bay area (average depth <1 m) with several islets situated in the northern Väinameri (Figure 1) and almost totally enclosed by the Hiiumaa and Kassari islands. The first colony of cormorants was established in 1995 on one of these islets (Männaklaid), and a few years later Ristlaid was also colonized. The number of breeding pairs, determined annually by counting occupied nests at the colonies (according to the methodology described by Engström, 2001b) increased rapidly to 1401 in 2005 (Table 1). Käina Bay is a nature protection area (bird sanctuary) and both commercial and recreational fishing are prohibited. Fish migrate into this rapidly warming sea area in early spring and most adults leave after the spawning period (Saat and Kikas, 2002). It represents an important spawning area for freshwater species from a much larger area of Väinameri.
Number of nesting pairs of cormorants (occupied nests) in Käina Bay, 1994–2005.
| Year . | Nesting pairs . |
|---|---|
| 1994 | 0 |
| 1995 | 18 |
| 1996 | 0 |
| 1997 | 18 |
| 1998 | 150 |
| 1999 | 335 |
| 2000 | 500 |
| 2001 | 390 |
| 2002 | 611 |
| 2003 | No data |
| 2004 | 724 |
| 2005 | 1 401 |
| Year . | Nesting pairs . |
|---|---|
| 1994 | 0 |
| 1995 | 18 |
| 1996 | 0 |
| 1997 | 18 |
| 1998 | 150 |
| 1999 | 335 |
| 2000 | 500 |
| 2001 | 390 |
| 2002 | 611 |
| 2003 | No data |
| 2004 | 724 |
| 2005 | 1 401 |
Number of nesting pairs of cormorants (occupied nests) in Käina Bay, 1994–2005.
| Year . | Nesting pairs . |
|---|---|
| 1994 | 0 |
| 1995 | 18 |
| 1996 | 0 |
| 1997 | 18 |
| 1998 | 150 |
| 1999 | 335 |
| 2000 | 500 |
| 2001 | 390 |
| 2002 | 611 |
| 2003 | No data |
| 2004 | 724 |
| 2005 | 1 401 |
| Year . | Nesting pairs . |
|---|---|
| 1994 | 0 |
| 1995 | 18 |
| 1996 | 0 |
| 1997 | 18 |
| 1998 | 150 |
| 1999 | 335 |
| 2000 | 500 |
| 2001 | 390 |
| 2002 | 611 |
| 2003 | No data |
| 2004 | 724 |
| 2005 | 1 401 |
Material and methods
Fish were sampled with gillnets in the Käina Bay in 1995 and 2005, following standardized monitoring methodology developed for coastal areas of the northern Baltic Sea (Thoresson, 1996; Saat et al., 2003a). Six nets 30 m long with different mesh size (stretched mesh: 34, 43, 50, 60, 76, and 90 mm) were used in line (from now on referred to as a “station”). In both years, a station was set before sunset in the deepest area (1.0–1.5 m) of the bay, between Ristlaid and Männaklaid islets (Figure 1), covering the entire water column from bottom to surface, and lifted after sunrise on the following day. Fish were sampled during 19 station nights in both 1995 and 2005, although the number of nights fished was halved in 2005 (mostly two stations per night; Table 2). Because water temperature is one of the most important factors determining the timing of spawning migrations and of spawning in the Baltic area (Sandström et al., 1997; Gillet and Quetin, 2006), sampling dates in 2005 were chosen to be comparable with 1995 for water temperature (Table 2; Mann–Whitney U-test: U = 169; p = 0.75; n = 19). All fish caught were measured (to the nearest millimetre) and weighed (to the nearest 0.1 g) and the catch per unit effort (cpue) was calculated as the total number by species caught per station. Perch were aged based on ring formation in the opercula (Thoresson, 1996). The temporal variability of species composition was determined by PERMANOVA analysis (permutational MANOVA, with 999 permutations). The model included year (1995 and 2005) as a factor, temperature and date (number of days elapsed from 1 April each year) as covariates, and the interactions between the factor and covariates. The contributions of different species to this variability were assessed using SIMPER analysis from the PRIMER-E v6 statistical package (Clarke and Gorley, 2006).
Comparative cpue of fish in Käina Bay in 1995 and 2005 (each row represents one station, i.e. one series of gillnets set for one night; in 2005, two stations were sampled per night, indicated by the numbers 1 and 2 in parenthesis).
| Date . | T (°C) . | Perch . | Giebel carp . | Crucian carp . | Ruffe . | Rudd . | Ide . | Roach . | Bleak . |
|---|---|---|---|---|---|---|---|---|---|
| 1995 | |||||||||
| 3 April | 3.1 | 15 | 0 | 0 | 41 | 0 | 12 | 4 | 0 |
| 5 April | 2.6 | 39 | 0 | 0 | 82 | 0 | 31 | 15 | 0 |
| 8 April | 3.3 | 25 | 0 | 0 | 64 | 0 | 21 | 2 | 0 |
| 11 April | 5.5 | 34 | 0 | 0 | 0 | 0 | 30 | 1 | 0 |
| 14 April | 4.9 | 37 | 0 | 0 | 251 | 0 | 30 | 79 | 1 |
| 18 April | 7.3 | 75 | 0 | 0 | 129 | 0 | 24 | 230 | 1 |
| 22 April | 9.5 | 63 | 0 | 0 | 194 | 0 | 18 | 463 | 1 |
| 28 April | 8.8 | 30 | 0 | 0 | 250 | 0 | 3 | 353 | 1 |
| 5 May | 12.9 | 37 | 0 | 2 | 221 | 0 | 0 | 636 | 2 |
| 12 May | 12.2 | 24 | 0 | 5 | 162 | 0 | 4 | 574 | 2 |
| 17 May | 10.1 | 10 | 0 | 1 | 12 | 0 | 0 | 17 | 0 |
| 28 May | 17.4 | 17 | 0 | 3 | 60 | 0 | 0 | 464 | 2 |
| 3 June | 26.1 | 104 | 0 | 1 | 42 | 0 | 0 | 387 | 2 |
| 8 June | 20.9 | 43 | 0 | 0 | 26 | 0 | 0 | 41 | 2 |
| 18 June | 22.1 | 92 | 0 | 0 | 8 | 0 | 4 | 42 | 0 |
| 1 July | 18.0 | 58 | 0 | 0 | 0 | 4 | 1 | 46 | 0 |
| 14 July | 23.2 | 76 | 0 | 0 | 0 | 21 | 0 | 34 | 0 |
| 8 August | 19.5 | 170 | 0 | 2 | 4 | 35 | 4 | 122 | 0 |
| 24 August | 21.5 | 81 | 0 | 10 | 0 | 14 | 0 | 44 | 0 |
| 2005 | |||||||||
| 13 April (1) | 8.4 | 8 | 4 | 0 | 1 379 | 3 | 1 | 0 | 0 |
| 13 April (2) | 8.4 | 11 | 4 | 0 | 1 020 | 0 | 1 | 3 | 0 |
| 14 April (1) | 9.8 | 18 | 2 | 0 | 1 881 | 3 | 0 | 2 | 0 |
| 25 April (1) | 8.9 | 13 | 1 | 0 | 440 | 0 | 4 | 1 | 0 |
| 25 April (2) | 8.9 | 5 | 3 | 0 | 363 | 0 | 0 | 3 | 0 |
| 26 April (1) | 10.6 | 4 | 1 | 0 | 598 | 0 | 3 | 0 | 0 |
| 26 April (2) | 10.6 | 17 | 3 | 0 | 474 | 0 | 1 | 6 | 0 |
| 6 May (1) | 11.5 | 8 | 0 | 0 | 327 | 3 | 2 | 20 | 0 |
| 6 May (2) | 11.5 | 8 | 0 | 0 | 336 | 9 | 0 | 18 | 0 |
| 7 May (1) | 13.7 | 8 | 3 | 0 | 427 | 3 | 1 | 0 | 0 |
| 7 May (2) | 13.7 | 3 | 0 | 0 | 370 | 0 | 1 | 0 | 0 |
| 27 May (1) | 16.6 | 0 | 0 | 0 | 28 | 0 | 2 | 0 | 0 |
| 27 May (2) | 16.6 | 2 | 2 | 0 | 51 | 5 | 4 | 1 | 2 |
| 28 May (1) | 15.6 | 1 | 0 | 0 | 39 | 0 | 1 | 0 | 1 |
| 28 May (2) | 15.6 | 0 | 1 | 0 | 17 | 0 | 1 | 0 | 0 |
| 31 July (1) | 19.7 | 0 | 14 | 0 | 8 | 7 | 3 | 1 | 3 |
| 31 July (2) | 19.7 | 1 | 8 | 0 | 1 | 3 | 3 | 0 | 0 |
| 2 August (1) | 20.2 | 0 | 2 | 0 | 0 | 1 | 1 | 0 | 0 |
| 2 August (2) | 20.2 | 0 | 10 | 0 | 1 | 1 | 2 | 0 | 1 |
| Date . | T (°C) . | Perch . | Giebel carp . | Crucian carp . | Ruffe . | Rudd . | Ide . | Roach . | Bleak . |
|---|---|---|---|---|---|---|---|---|---|
| 1995 | |||||||||
| 3 April | 3.1 | 15 | 0 | 0 | 41 | 0 | 12 | 4 | 0 |
| 5 April | 2.6 | 39 | 0 | 0 | 82 | 0 | 31 | 15 | 0 |
| 8 April | 3.3 | 25 | 0 | 0 | 64 | 0 | 21 | 2 | 0 |
| 11 April | 5.5 | 34 | 0 | 0 | 0 | 0 | 30 | 1 | 0 |
| 14 April | 4.9 | 37 | 0 | 0 | 251 | 0 | 30 | 79 | 1 |
| 18 April | 7.3 | 75 | 0 | 0 | 129 | 0 | 24 | 230 | 1 |
| 22 April | 9.5 | 63 | 0 | 0 | 194 | 0 | 18 | 463 | 1 |
| 28 April | 8.8 | 30 | 0 | 0 | 250 | 0 | 3 | 353 | 1 |
| 5 May | 12.9 | 37 | 0 | 2 | 221 | 0 | 0 | 636 | 2 |
| 12 May | 12.2 | 24 | 0 | 5 | 162 | 0 | 4 | 574 | 2 |
| 17 May | 10.1 | 10 | 0 | 1 | 12 | 0 | 0 | 17 | 0 |
| 28 May | 17.4 | 17 | 0 | 3 | 60 | 0 | 0 | 464 | 2 |
| 3 June | 26.1 | 104 | 0 | 1 | 42 | 0 | 0 | 387 | 2 |
| 8 June | 20.9 | 43 | 0 | 0 | 26 | 0 | 0 | 41 | 2 |
| 18 June | 22.1 | 92 | 0 | 0 | 8 | 0 | 4 | 42 | 0 |
| 1 July | 18.0 | 58 | 0 | 0 | 0 | 4 | 1 | 46 | 0 |
| 14 July | 23.2 | 76 | 0 | 0 | 0 | 21 | 0 | 34 | 0 |
| 8 August | 19.5 | 170 | 0 | 2 | 4 | 35 | 4 | 122 | 0 |
| 24 August | 21.5 | 81 | 0 | 10 | 0 | 14 | 0 | 44 | 0 |
| 2005 | |||||||||
| 13 April (1) | 8.4 | 8 | 4 | 0 | 1 379 | 3 | 1 | 0 | 0 |
| 13 April (2) | 8.4 | 11 | 4 | 0 | 1 020 | 0 | 1 | 3 | 0 |
| 14 April (1) | 9.8 | 18 | 2 | 0 | 1 881 | 3 | 0 | 2 | 0 |
| 25 April (1) | 8.9 | 13 | 1 | 0 | 440 | 0 | 4 | 1 | 0 |
| 25 April (2) | 8.9 | 5 | 3 | 0 | 363 | 0 | 0 | 3 | 0 |
| 26 April (1) | 10.6 | 4 | 1 | 0 | 598 | 0 | 3 | 0 | 0 |
| 26 April (2) | 10.6 | 17 | 3 | 0 | 474 | 0 | 1 | 6 | 0 |
| 6 May (1) | 11.5 | 8 | 0 | 0 | 327 | 3 | 2 | 20 | 0 |
| 6 May (2) | 11.5 | 8 | 0 | 0 | 336 | 9 | 0 | 18 | 0 |
| 7 May (1) | 13.7 | 8 | 3 | 0 | 427 | 3 | 1 | 0 | 0 |
| 7 May (2) | 13.7 | 3 | 0 | 0 | 370 | 0 | 1 | 0 | 0 |
| 27 May (1) | 16.6 | 0 | 0 | 0 | 28 | 0 | 2 | 0 | 0 |
| 27 May (2) | 16.6 | 2 | 2 | 0 | 51 | 5 | 4 | 1 | 2 |
| 28 May (1) | 15.6 | 1 | 0 | 0 | 39 | 0 | 1 | 0 | 1 |
| 28 May (2) | 15.6 | 0 | 1 | 0 | 17 | 0 | 1 | 0 | 0 |
| 31 July (1) | 19.7 | 0 | 14 | 0 | 8 | 7 | 3 | 1 | 3 |
| 31 July (2) | 19.7 | 1 | 8 | 0 | 1 | 3 | 3 | 0 | 0 |
| 2 August (1) | 20.2 | 0 | 2 | 0 | 0 | 1 | 1 | 0 | 0 |
| 2 August (2) | 20.2 | 0 | 10 | 0 | 1 | 1 | 2 | 0 | 1 |
Comparative cpue of fish in Käina Bay in 1995 and 2005 (each row represents one station, i.e. one series of gillnets set for one night; in 2005, two stations were sampled per night, indicated by the numbers 1 and 2 in parenthesis).
| Date . | T (°C) . | Perch . | Giebel carp . | Crucian carp . | Ruffe . | Rudd . | Ide . | Roach . | Bleak . |
|---|---|---|---|---|---|---|---|---|---|
| 1995 | |||||||||
| 3 April | 3.1 | 15 | 0 | 0 | 41 | 0 | 12 | 4 | 0 |
| 5 April | 2.6 | 39 | 0 | 0 | 82 | 0 | 31 | 15 | 0 |
| 8 April | 3.3 | 25 | 0 | 0 | 64 | 0 | 21 | 2 | 0 |
| 11 April | 5.5 | 34 | 0 | 0 | 0 | 0 | 30 | 1 | 0 |
| 14 April | 4.9 | 37 | 0 | 0 | 251 | 0 | 30 | 79 | 1 |
| 18 April | 7.3 | 75 | 0 | 0 | 129 | 0 | 24 | 230 | 1 |
| 22 April | 9.5 | 63 | 0 | 0 | 194 | 0 | 18 | 463 | 1 |
| 28 April | 8.8 | 30 | 0 | 0 | 250 | 0 | 3 | 353 | 1 |
| 5 May | 12.9 | 37 | 0 | 2 | 221 | 0 | 0 | 636 | 2 |
| 12 May | 12.2 | 24 | 0 | 5 | 162 | 0 | 4 | 574 | 2 |
| 17 May | 10.1 | 10 | 0 | 1 | 12 | 0 | 0 | 17 | 0 |
| 28 May | 17.4 | 17 | 0 | 3 | 60 | 0 | 0 | 464 | 2 |
| 3 June | 26.1 | 104 | 0 | 1 | 42 | 0 | 0 | 387 | 2 |
| 8 June | 20.9 | 43 | 0 | 0 | 26 | 0 | 0 | 41 | 2 |
| 18 June | 22.1 | 92 | 0 | 0 | 8 | 0 | 4 | 42 | 0 |
| 1 July | 18.0 | 58 | 0 | 0 | 0 | 4 | 1 | 46 | 0 |
| 14 July | 23.2 | 76 | 0 | 0 | 0 | 21 | 0 | 34 | 0 |
| 8 August | 19.5 | 170 | 0 | 2 | 4 | 35 | 4 | 122 | 0 |
| 24 August | 21.5 | 81 | 0 | 10 | 0 | 14 | 0 | 44 | 0 |
| 2005 | |||||||||
| 13 April (1) | 8.4 | 8 | 4 | 0 | 1 379 | 3 | 1 | 0 | 0 |
| 13 April (2) | 8.4 | 11 | 4 | 0 | 1 020 | 0 | 1 | 3 | 0 |
| 14 April (1) | 9.8 | 18 | 2 | 0 | 1 881 | 3 | 0 | 2 | 0 |
| 25 April (1) | 8.9 | 13 | 1 | 0 | 440 | 0 | 4 | 1 | 0 |
| 25 April (2) | 8.9 | 5 | 3 | 0 | 363 | 0 | 0 | 3 | 0 |
| 26 April (1) | 10.6 | 4 | 1 | 0 | 598 | 0 | 3 | 0 | 0 |
| 26 April (2) | 10.6 | 17 | 3 | 0 | 474 | 0 | 1 | 6 | 0 |
| 6 May (1) | 11.5 | 8 | 0 | 0 | 327 | 3 | 2 | 20 | 0 |
| 6 May (2) | 11.5 | 8 | 0 | 0 | 336 | 9 | 0 | 18 | 0 |
| 7 May (1) | 13.7 | 8 | 3 | 0 | 427 | 3 | 1 | 0 | 0 |
| 7 May (2) | 13.7 | 3 | 0 | 0 | 370 | 0 | 1 | 0 | 0 |
| 27 May (1) | 16.6 | 0 | 0 | 0 | 28 | 0 | 2 | 0 | 0 |
| 27 May (2) | 16.6 | 2 | 2 | 0 | 51 | 5 | 4 | 1 | 2 |
| 28 May (1) | 15.6 | 1 | 0 | 0 | 39 | 0 | 1 | 0 | 1 |
| 28 May (2) | 15.6 | 0 | 1 | 0 | 17 | 0 | 1 | 0 | 0 |
| 31 July (1) | 19.7 | 0 | 14 | 0 | 8 | 7 | 3 | 1 | 3 |
| 31 July (2) | 19.7 | 1 | 8 | 0 | 1 | 3 | 3 | 0 | 0 |
| 2 August (1) | 20.2 | 0 | 2 | 0 | 0 | 1 | 1 | 0 | 0 |
| 2 August (2) | 20.2 | 0 | 10 | 0 | 1 | 1 | 2 | 0 | 1 |
| Date . | T (°C) . | Perch . | Giebel carp . | Crucian carp . | Ruffe . | Rudd . | Ide . | Roach . | Bleak . |
|---|---|---|---|---|---|---|---|---|---|
| 1995 | |||||||||
| 3 April | 3.1 | 15 | 0 | 0 | 41 | 0 | 12 | 4 | 0 |
| 5 April | 2.6 | 39 | 0 | 0 | 82 | 0 | 31 | 15 | 0 |
| 8 April | 3.3 | 25 | 0 | 0 | 64 | 0 | 21 | 2 | 0 |
| 11 April | 5.5 | 34 | 0 | 0 | 0 | 0 | 30 | 1 | 0 |
| 14 April | 4.9 | 37 | 0 | 0 | 251 | 0 | 30 | 79 | 1 |
| 18 April | 7.3 | 75 | 0 | 0 | 129 | 0 | 24 | 230 | 1 |
| 22 April | 9.5 | 63 | 0 | 0 | 194 | 0 | 18 | 463 | 1 |
| 28 April | 8.8 | 30 | 0 | 0 | 250 | 0 | 3 | 353 | 1 |
| 5 May | 12.9 | 37 | 0 | 2 | 221 | 0 | 0 | 636 | 2 |
| 12 May | 12.2 | 24 | 0 | 5 | 162 | 0 | 4 | 574 | 2 |
| 17 May | 10.1 | 10 | 0 | 1 | 12 | 0 | 0 | 17 | 0 |
| 28 May | 17.4 | 17 | 0 | 3 | 60 | 0 | 0 | 464 | 2 |
| 3 June | 26.1 | 104 | 0 | 1 | 42 | 0 | 0 | 387 | 2 |
| 8 June | 20.9 | 43 | 0 | 0 | 26 | 0 | 0 | 41 | 2 |
| 18 June | 22.1 | 92 | 0 | 0 | 8 | 0 | 4 | 42 | 0 |
| 1 July | 18.0 | 58 | 0 | 0 | 0 | 4 | 1 | 46 | 0 |
| 14 July | 23.2 | 76 | 0 | 0 | 0 | 21 | 0 | 34 | 0 |
| 8 August | 19.5 | 170 | 0 | 2 | 4 | 35 | 4 | 122 | 0 |
| 24 August | 21.5 | 81 | 0 | 10 | 0 | 14 | 0 | 44 | 0 |
| 2005 | |||||||||
| 13 April (1) | 8.4 | 8 | 4 | 0 | 1 379 | 3 | 1 | 0 | 0 |
| 13 April (2) | 8.4 | 11 | 4 | 0 | 1 020 | 0 | 1 | 3 | 0 |
| 14 April (1) | 9.8 | 18 | 2 | 0 | 1 881 | 3 | 0 | 2 | 0 |
| 25 April (1) | 8.9 | 13 | 1 | 0 | 440 | 0 | 4 | 1 | 0 |
| 25 April (2) | 8.9 | 5 | 3 | 0 | 363 | 0 | 0 | 3 | 0 |
| 26 April (1) | 10.6 | 4 | 1 | 0 | 598 | 0 | 3 | 0 | 0 |
| 26 April (2) | 10.6 | 17 | 3 | 0 | 474 | 0 | 1 | 6 | 0 |
| 6 May (1) | 11.5 | 8 | 0 | 0 | 327 | 3 | 2 | 20 | 0 |
| 6 May (2) | 11.5 | 8 | 0 | 0 | 336 | 9 | 0 | 18 | 0 |
| 7 May (1) | 13.7 | 8 | 3 | 0 | 427 | 3 | 1 | 0 | 0 |
| 7 May (2) | 13.7 | 3 | 0 | 0 | 370 | 0 | 1 | 0 | 0 |
| 27 May (1) | 16.6 | 0 | 0 | 0 | 28 | 0 | 2 | 0 | 0 |
| 27 May (2) | 16.6 | 2 | 2 | 0 | 51 | 5 | 4 | 1 | 2 |
| 28 May (1) | 15.6 | 1 | 0 | 0 | 39 | 0 | 1 | 0 | 1 |
| 28 May (2) | 15.6 | 0 | 1 | 0 | 17 | 0 | 1 | 0 | 0 |
| 31 July (1) | 19.7 | 0 | 14 | 0 | 8 | 7 | 3 | 1 | 3 |
| 31 July (2) | 19.7 | 1 | 8 | 0 | 1 | 3 | 3 | 0 | 0 |
| 2 August (1) | 20.2 | 0 | 2 | 0 | 0 | 1 | 1 | 0 | 0 |
| 2 August (2) | 20.2 | 0 | 10 | 0 | 1 | 1 | 2 | 0 | 1 |
In addition, cpue data (1993–2009, Table 3) from regular gillnet surveys in the Väinameri (Figure 1) were available for Sarve (15 km northeast from the Käina Bay), Saarnaki (12 km east), and Matsalu Bay (40 km southeast). These surveys used essentially the same technique, but only the four smaller mesh sizes (stretched mesh: 34, 43, 50, and 60) were used. Only data for the three main species are presented (ide is excluded, because it is mainly caught using larger mesh sizes than used in these surveys).
Comparative cpue and the results from long-term trend (Spearman's rank) analysis of perch, roach, and ruffe in two monitoring areas close to Hiiumaa island (Sarve and Saarnaki) and in Matsalu Bay in 1993–2009.
| . | Sarve . | Saarnaki . | Matsalu . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Year . | Perch . | Roach . | Ruffe . | Perch . | Roach . | Ruffe . | Perch . | Roach . | Ruffe . |
| 1993 | 16.3 | 1 | 0.3 | 18.8 | 45.9 | 0.8 | 19.3 | 2 | 0 |
| 1994 | 23.3 | 5.4 | 1.3 | 35.5 | 11.1 | 0.2 | 25.7 | 19.9 | 1.2 |
| 1995 | 6.2 | 6.1 | 2.0 | 13.8 | 19.8 | 0.6 | 19.7 | 53 | 1.6 |
| 1996 | 4.5 | 9.0 | 4.2 | 3.2 | 18.2 | 1.0 | 6.7 | 35.3 | 0.3 |
| 1997 | 1.0 | 4.3 | 1.2 | 1.1 | 33.1 | 1.8 | 8.7 | 24.4 | 0.2 |
| 1998 | 11.7 | 23.7 | 1.5 | 4.4 | 36.5 | 2.4 | 1.1 | 11.2 | 0.1 |
| 1999 | 1.3 | 12.1 | 5.8 | 1.2 | 7.7 | 0.8 | 3.5 | 50.1 | 0.3 |
| 2000 | 28.1 | 4.3 | 0.4 | 26.6 | 11.3 | 0.5 | 9.4 | 34.7 | 0.3 |
| 2001 | 29.2 | 7.5 | 2.3 | 31.3 | 8.8 | 1.3 | 13.9 | 11.7 | 0.1 |
| 2002 | 5.6 | 2.3 | 1.9 | 11.9 | 4.4 | 2.6 | 8.9 | 13.6 | 0 |
| 2003 | 11.6 | 4.4 | 3.3 | 15.7 | 3.6 | 6.1 | 23.5 | 23.4 | 0.1 |
| 2004 | 8.5 | 3.7 | 2.7 | 6.2 | 0.2 | 3.8 | 8.2 | 21.2 | 0.3 |
| 2005 | 2.2 | 1.2 | 1.1 | 8.9 | 4.1 | 2.2 | 14.9 | 55.7 | 0.2 |
| 2006 | 3.2 | 0.7 | 0.3 | 22.6 | 0.3 | 1.5 | 11.0 | 33.1 | 0.4 |
| 2007 | 6.6 | 1.8 | 0.8 | 17.4 | 2.1 | 2.2 | 13.4 | 34.7 | 0.1 |
| 2008 | 6.4 | 0.5 | 0.5 | 5.7 | 0.1 | 1.3 | 12.2 | 76.6 | 0.2 |
| 2009 | 3.4 | 0.9 | 0.2 | 9.8 | 0.6 | 0.4 | 11.6 | 43.4 | 0.2 |
| Spearman R | −0.24 | −0.61 | −0.33 | −0.04 | −0.89 | 0.34 | −0.05 | 0.40 | −0.16 |
| p | n.s. | <0.05 | n.s. | n.s. | <0.001 | n.s. | n.s. | n.s. | n.s. |
| . | Sarve . | Saarnaki . | Matsalu . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Year . | Perch . | Roach . | Ruffe . | Perch . | Roach . | Ruffe . | Perch . | Roach . | Ruffe . |
| 1993 | 16.3 | 1 | 0.3 | 18.8 | 45.9 | 0.8 | 19.3 | 2 | 0 |
| 1994 | 23.3 | 5.4 | 1.3 | 35.5 | 11.1 | 0.2 | 25.7 | 19.9 | 1.2 |
| 1995 | 6.2 | 6.1 | 2.0 | 13.8 | 19.8 | 0.6 | 19.7 | 53 | 1.6 |
| 1996 | 4.5 | 9.0 | 4.2 | 3.2 | 18.2 | 1.0 | 6.7 | 35.3 | 0.3 |
| 1997 | 1.0 | 4.3 | 1.2 | 1.1 | 33.1 | 1.8 | 8.7 | 24.4 | 0.2 |
| 1998 | 11.7 | 23.7 | 1.5 | 4.4 | 36.5 | 2.4 | 1.1 | 11.2 | 0.1 |
| 1999 | 1.3 | 12.1 | 5.8 | 1.2 | 7.7 | 0.8 | 3.5 | 50.1 | 0.3 |
| 2000 | 28.1 | 4.3 | 0.4 | 26.6 | 11.3 | 0.5 | 9.4 | 34.7 | 0.3 |
| 2001 | 29.2 | 7.5 | 2.3 | 31.3 | 8.8 | 1.3 | 13.9 | 11.7 | 0.1 |
| 2002 | 5.6 | 2.3 | 1.9 | 11.9 | 4.4 | 2.6 | 8.9 | 13.6 | 0 |
| 2003 | 11.6 | 4.4 | 3.3 | 15.7 | 3.6 | 6.1 | 23.5 | 23.4 | 0.1 |
| 2004 | 8.5 | 3.7 | 2.7 | 6.2 | 0.2 | 3.8 | 8.2 | 21.2 | 0.3 |
| 2005 | 2.2 | 1.2 | 1.1 | 8.9 | 4.1 | 2.2 | 14.9 | 55.7 | 0.2 |
| 2006 | 3.2 | 0.7 | 0.3 | 22.6 | 0.3 | 1.5 | 11.0 | 33.1 | 0.4 |
| 2007 | 6.6 | 1.8 | 0.8 | 17.4 | 2.1 | 2.2 | 13.4 | 34.7 | 0.1 |
| 2008 | 6.4 | 0.5 | 0.5 | 5.7 | 0.1 | 1.3 | 12.2 | 76.6 | 0.2 |
| 2009 | 3.4 | 0.9 | 0.2 | 9.8 | 0.6 | 0.4 | 11.6 | 43.4 | 0.2 |
| Spearman R | −0.24 | −0.61 | −0.33 | −0.04 | −0.89 | 0.34 | −0.05 | 0.40 | −0.16 |
| p | n.s. | <0.05 | n.s. | n.s. | <0.001 | n.s. | n.s. | n.s. | n.s. |
n.s., not significant.
Emboldened values reflect the results for years when sampling was carried out concurrently in Käina Bay.
Comparative cpue and the results from long-term trend (Spearman's rank) analysis of perch, roach, and ruffe in two monitoring areas close to Hiiumaa island (Sarve and Saarnaki) and in Matsalu Bay in 1993–2009.
| . | Sarve . | Saarnaki . | Matsalu . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Year . | Perch . | Roach . | Ruffe . | Perch . | Roach . | Ruffe . | Perch . | Roach . | Ruffe . |
| 1993 | 16.3 | 1 | 0.3 | 18.8 | 45.9 | 0.8 | 19.3 | 2 | 0 |
| 1994 | 23.3 | 5.4 | 1.3 | 35.5 | 11.1 | 0.2 | 25.7 | 19.9 | 1.2 |
| 1995 | 6.2 | 6.1 | 2.0 | 13.8 | 19.8 | 0.6 | 19.7 | 53 | 1.6 |
| 1996 | 4.5 | 9.0 | 4.2 | 3.2 | 18.2 | 1.0 | 6.7 | 35.3 | 0.3 |
| 1997 | 1.0 | 4.3 | 1.2 | 1.1 | 33.1 | 1.8 | 8.7 | 24.4 | 0.2 |
| 1998 | 11.7 | 23.7 | 1.5 | 4.4 | 36.5 | 2.4 | 1.1 | 11.2 | 0.1 |
| 1999 | 1.3 | 12.1 | 5.8 | 1.2 | 7.7 | 0.8 | 3.5 | 50.1 | 0.3 |
| 2000 | 28.1 | 4.3 | 0.4 | 26.6 | 11.3 | 0.5 | 9.4 | 34.7 | 0.3 |
| 2001 | 29.2 | 7.5 | 2.3 | 31.3 | 8.8 | 1.3 | 13.9 | 11.7 | 0.1 |
| 2002 | 5.6 | 2.3 | 1.9 | 11.9 | 4.4 | 2.6 | 8.9 | 13.6 | 0 |
| 2003 | 11.6 | 4.4 | 3.3 | 15.7 | 3.6 | 6.1 | 23.5 | 23.4 | 0.1 |
| 2004 | 8.5 | 3.7 | 2.7 | 6.2 | 0.2 | 3.8 | 8.2 | 21.2 | 0.3 |
| 2005 | 2.2 | 1.2 | 1.1 | 8.9 | 4.1 | 2.2 | 14.9 | 55.7 | 0.2 |
| 2006 | 3.2 | 0.7 | 0.3 | 22.6 | 0.3 | 1.5 | 11.0 | 33.1 | 0.4 |
| 2007 | 6.6 | 1.8 | 0.8 | 17.4 | 2.1 | 2.2 | 13.4 | 34.7 | 0.1 |
| 2008 | 6.4 | 0.5 | 0.5 | 5.7 | 0.1 | 1.3 | 12.2 | 76.6 | 0.2 |
| 2009 | 3.4 | 0.9 | 0.2 | 9.8 | 0.6 | 0.4 | 11.6 | 43.4 | 0.2 |
| Spearman R | −0.24 | −0.61 | −0.33 | −0.04 | −0.89 | 0.34 | −0.05 | 0.40 | −0.16 |
| p | n.s. | <0.05 | n.s. | n.s. | <0.001 | n.s. | n.s. | n.s. | n.s. |
| . | Sarve . | Saarnaki . | Matsalu . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Year . | Perch . | Roach . | Ruffe . | Perch . | Roach . | Ruffe . | Perch . | Roach . | Ruffe . |
| 1993 | 16.3 | 1 | 0.3 | 18.8 | 45.9 | 0.8 | 19.3 | 2 | 0 |
| 1994 | 23.3 | 5.4 | 1.3 | 35.5 | 11.1 | 0.2 | 25.7 | 19.9 | 1.2 |
| 1995 | 6.2 | 6.1 | 2.0 | 13.8 | 19.8 | 0.6 | 19.7 | 53 | 1.6 |
| 1996 | 4.5 | 9.0 | 4.2 | 3.2 | 18.2 | 1.0 | 6.7 | 35.3 | 0.3 |
| 1997 | 1.0 | 4.3 | 1.2 | 1.1 | 33.1 | 1.8 | 8.7 | 24.4 | 0.2 |
| 1998 | 11.7 | 23.7 | 1.5 | 4.4 | 36.5 | 2.4 | 1.1 | 11.2 | 0.1 |
| 1999 | 1.3 | 12.1 | 5.8 | 1.2 | 7.7 | 0.8 | 3.5 | 50.1 | 0.3 |
| 2000 | 28.1 | 4.3 | 0.4 | 26.6 | 11.3 | 0.5 | 9.4 | 34.7 | 0.3 |
| 2001 | 29.2 | 7.5 | 2.3 | 31.3 | 8.8 | 1.3 | 13.9 | 11.7 | 0.1 |
| 2002 | 5.6 | 2.3 | 1.9 | 11.9 | 4.4 | 2.6 | 8.9 | 13.6 | 0 |
| 2003 | 11.6 | 4.4 | 3.3 | 15.7 | 3.6 | 6.1 | 23.5 | 23.4 | 0.1 |
| 2004 | 8.5 | 3.7 | 2.7 | 6.2 | 0.2 | 3.8 | 8.2 | 21.2 | 0.3 |
| 2005 | 2.2 | 1.2 | 1.1 | 8.9 | 4.1 | 2.2 | 14.9 | 55.7 | 0.2 |
| 2006 | 3.2 | 0.7 | 0.3 | 22.6 | 0.3 | 1.5 | 11.0 | 33.1 | 0.4 |
| 2007 | 6.6 | 1.8 | 0.8 | 17.4 | 2.1 | 2.2 | 13.4 | 34.7 | 0.1 |
| 2008 | 6.4 | 0.5 | 0.5 | 5.7 | 0.1 | 1.3 | 12.2 | 76.6 | 0.2 |
| 2009 | 3.4 | 0.9 | 0.2 | 9.8 | 0.6 | 0.4 | 11.6 | 43.4 | 0.2 |
| Spearman R | −0.24 | −0.61 | −0.33 | −0.04 | −0.89 | 0.34 | −0.05 | 0.40 | −0.16 |
| p | n.s. | <0.05 | n.s. | n.s. | <0.001 | n.s. | n.s. | n.s. | n.s. |
n.s., not significant.
Emboldened values reflect the results for years when sampling was carried out concurrently in Käina Bay.
The fishery
The Väinameri fishery employs only passive gears (gillnets and trapnets) and trawling is forbidden. Because the number of fishers in Estonia is registered by county and this sea area is shared by three coastal counties that also cover additional sea areas, there are no reliable statistics for the total number of fishers operating in Väinameri. Moreover, this number is not stable, because fishers might spend part (or most) of their effort elsewhere or fish only part-time. At the beginning of the 1990s (by the end of the Soviet period), and despite a high fishing effort, fish stocks in the Väinameri were considered rather healthy (Vetemaa et al., 2006a). The number of fishers (full-time equivalent) at the time has been estimated to lie in the order of several hundreds (Vetemaa et al., 2002, 2006a), which is 3–4 times more than during the past few years (Kangur, 2006). Effort in the coastal fisheries was not registered in the 1990s, and the only restriction was on a maximum number of gillnets allowed. Although the number used per boat has not decreased much, interviews with fishers indicate that the total effort in soaking time in the area was 5–10 times higher in the middle of the 1990s than in the middle of 2000s. Commercial catch statistics of perch, ide, and roach and white bream combined are available (Table 4), but their reliability is questionable and their interpretation hampered by the very unstable effort.
Available commercial catch statistics (t) in the Väinameri (R + WB: roach and white bream combined).
| Year . | Perch . | Ide . | R + WB . |
|---|---|---|---|
| 1993 | 453.9 | 85.7 | 75.3 |
| 1994 | 239.6 | 45.1 | 72.3 |
| 1995 | 170.5 | 40.0 | 136.5 |
| 1996 | 140.4 | 54.6 | 137.0 |
| 1997 | 39.2 | 60.2 | 121.0 |
| 1998 | 9.9 | 44.6 | 81.5 |
| 1999 | 5.3 | 28.0 | 35.4 |
| 2000 | 3.2 | 28.4 | 36.3 |
| 2001 | 14.5 | 16.8 | 48.2 |
| 2002 | 24.8 | 10.8 | 35.2 |
| 2003 | 44.4 | 15.8 | 37.1 |
| 2004 | 51.5 | 9.8 | 34.2 |
| 2005 | 15.1 | 4.3 | 21.6 |
| 2006 | 12.5 | 5.0 | 16.8 |
| 2007 | 20.7 | 6.9 | 24.0 |
| 2008 | 11.6 | 6.7 | 22.7 |
| 2009 | 14.6 | 5.4 | 21.8 |
| Year . | Perch . | Ide . | R + WB . |
|---|---|---|---|
| 1993 | 453.9 | 85.7 | 75.3 |
| 1994 | 239.6 | 45.1 | 72.3 |
| 1995 | 170.5 | 40.0 | 136.5 |
| 1996 | 140.4 | 54.6 | 137.0 |
| 1997 | 39.2 | 60.2 | 121.0 |
| 1998 | 9.9 | 44.6 | 81.5 |
| 1999 | 5.3 | 28.0 | 35.4 |
| 2000 | 3.2 | 28.4 | 36.3 |
| 2001 | 14.5 | 16.8 | 48.2 |
| 2002 | 24.8 | 10.8 | 35.2 |
| 2003 | 44.4 | 15.8 | 37.1 |
| 2004 | 51.5 | 9.8 | 34.2 |
| 2005 | 15.1 | 4.3 | 21.6 |
| 2006 | 12.5 | 5.0 | 16.8 |
| 2007 | 20.7 | 6.9 | 24.0 |
| 2008 | 11.6 | 6.7 | 22.7 |
| 2009 | 14.6 | 5.4 | 21.8 |
Available commercial catch statistics (t) in the Väinameri (R + WB: roach and white bream combined).
| Year . | Perch . | Ide . | R + WB . |
|---|---|---|---|
| 1993 | 453.9 | 85.7 | 75.3 |
| 1994 | 239.6 | 45.1 | 72.3 |
| 1995 | 170.5 | 40.0 | 136.5 |
| 1996 | 140.4 | 54.6 | 137.0 |
| 1997 | 39.2 | 60.2 | 121.0 |
| 1998 | 9.9 | 44.6 | 81.5 |
| 1999 | 5.3 | 28.0 | 35.4 |
| 2000 | 3.2 | 28.4 | 36.3 |
| 2001 | 14.5 | 16.8 | 48.2 |
| 2002 | 24.8 | 10.8 | 35.2 |
| 2003 | 44.4 | 15.8 | 37.1 |
| 2004 | 51.5 | 9.8 | 34.2 |
| 2005 | 15.1 | 4.3 | 21.6 |
| 2006 | 12.5 | 5.0 | 16.8 |
| 2007 | 20.7 | 6.9 | 24.0 |
| 2008 | 11.6 | 6.7 | 22.7 |
| 2009 | 14.6 | 5.4 | 21.8 |
| Year . | Perch . | Ide . | R + WB . |
|---|---|---|---|
| 1993 | 453.9 | 85.7 | 75.3 |
| 1994 | 239.6 | 45.1 | 72.3 |
| 1995 | 170.5 | 40.0 | 136.5 |
| 1996 | 140.4 | 54.6 | 137.0 |
| 1997 | 39.2 | 60.2 | 121.0 |
| 1998 | 9.9 | 44.6 | 81.5 |
| 1999 | 5.3 | 28.0 | 35.4 |
| 2000 | 3.2 | 28.4 | 36.3 |
| 2001 | 14.5 | 16.8 | 48.2 |
| 2002 | 24.8 | 10.8 | 35.2 |
| 2003 | 44.4 | 15.8 | 37.1 |
| 2004 | 51.5 | 9.8 | 34.2 |
| 2005 | 15.1 | 4.3 | 21.6 |
| 2006 | 12.5 | 5.0 | 16.8 |
| 2007 | 20.7 | 6.9 | 24.0 |
| 2008 | 11.6 | 6.7 | 22.7 |
| 2009 | 14.6 | 5.4 | 21.8 |
Results
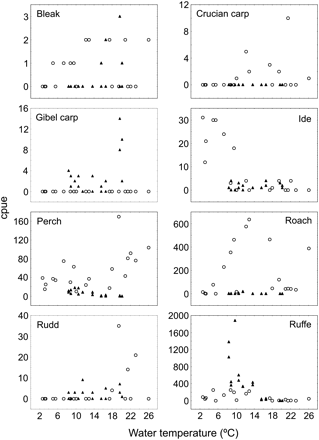
The structure of the fish community in Käina Bay appears to have changed substantially from 1995 to 2005. The effect of sampling year was statistically significant when the variations in the structure of the local fish community caused by variation in water temperature and sampling date, as well as their interactions with sampling year, were accounted for (PERMANOVA: main effect of sampling year: pseudo-F1,30 = 6.43; p = 0.003; main effect of covariate temperature: pseudo-F1,30 = 3.34; p = 0.022; main effect of covariate date: pseudo-F1,30 = 3.56; p = 0.011; year × temperature interaction term: pseudo-F1,30 = 3.95; p = 0.012; year × date interaction term: pseudo-F1,30 = 4.97; p = 0.002; year × temperature × date interaction term: pseudo-F1,30 = 6.0; p = 0.001). Figure 2 shows the relation between cpue and water temperature. Ruffe (50%), roach (28%), perch (15%), and ide (3%) together contributed 96% of the total dissimilarity (SIMPER) in community structure between the 2 years. Of these four species, only ruffe had increased in abundance compared with 1995 (by a factor of 5; Mann–Whitney U-test: U = 103.5; p = 0.02; n = 19), whereas roach and perch decreased (Table 2): roach by a factor of 65 (U = 20; p < 0.001) and perch by a factor of 10 (U = 7.5; p < 0.001). The cpue of ide decreased by a factor of 6, but the difference was not statistically significant (U = 141; p = 0.26). Bleak and rudd appeared in the catches only in relatively small numbers and differences were not significant. Crucian carp had disappeared completely from the catches in 2005, whereas the giebel carp was a newcomer (Table 2). These last four species are of little commercial interest.
Comparative cpue of fish in the Käina Bay in 1995 (open circles) and 2005 (filled triangles).
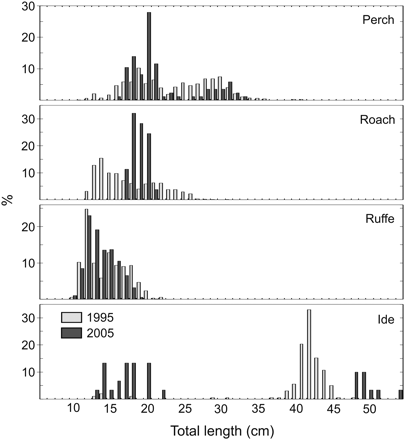
The length frequency distributions (Figure 3) revealed some marked differences between years, but these were not consistent among species. Small perch <20 cm and large perch of ∼30 cm were relatively more abundant in 2005, but perch of intermediate size were relatively more abundant in 1995. This is the only species for which age data are available, and catches were dominated by 5–6-year-old fish in both years (altogether nine age classes were represented). In contrast, all roach caught in 2005 were in the midrange and both small and large roach were absent. The size composition of ruffe did not change markedly, whereas the ide caught in 2005 were either larger or smaller than those caught in 1995.
Size distributions of the total catches of perch, roach, ruffe, and ide in Käina Bay, in 1995 and 2005.
The time-series of cpue in the three areas of the Väinameri (Table 3) reveal that the annual variations in the catches of most species were substantial. According to the Spearman rank-order correlation, the cpue of roach in the two areas off the Hiiumaa coast (Sarve and Saarnaki) displayed a significant downward (p < 0.05 and p < 0.001, respectively) trend, to reach the lowest observed values in 2008. The dynamics of perch and ruffe in these two areas did not reveal a significant trend, and none of the three species exhibited a trend in Matsalu Bay, ca. 40 km away. The perch cpue was generally high in the early 1990s, followed by a rapid decline after 1995 and an increase again since 2000 (resulting from the incoming strong 1998 year class) in all three areas. After 2000, the abundance was more stable in Matsalu Bay than in the areas off Hiiumaa. Ruffe cpue remained low in all areas, but especially so in Matsalu Bay.
Discussion
The use of gillnets has proven to work well for estimating the abundance of perch and roach (Saat et al., 2003a; Adjers et al., 2006). The samples taken with this method from Käina Bay revealed large and significant differences in the abundance of three of the four main fish species between 1995 and 2005. Catches of the three important commercial species displayed marked declines, whereas one species of no commercial interest (ruffe) had increased substantially. We are aware that the comparison had to be restricted to catches in two single years and that annual variation in catch rates in monitoring surveys outside the bay using comparable equipment would be large (Table 3). These large annual fluctuations are usually caused by recruitment variability induced by high productivity and variable environmental conditions.
Water temperature was also related to the variation in the structure of fish population in our dataset on Käina Bay. Indeed, water temperature is one of the most important factors determining the timing of spawning migrations and of spawning in the Baltic area (Sandström et al., 1997; Gillet and Quetin, 2006). However, the effect of sampling year was statistically significant when the effects of variation in water temperature and sampling date, as well as their interactions with sampling year on the structure of local fish community were accounted for. Moreover, the size and age distribution of perch did not indicate a major effect of a recruiting year class, whereas the size distribution of roach was truncated on both sides of what might be considered an average size. For ide, recruitment might have been higher in 2005 than in 1995 (Figure 3). Therefore, the temporal differences in abundances of these fish species appear not to have been caused by interannual water temperature differences during sampling or unusually strong year classes of perch and roach during 1995. Rather, high abundance in 1995 reflected the typical situation on this important spawning ground of Väinameri fish before the cormorant colony expanded (Saat and Kikas, 2002).
The coastal monitoring data collected since 1992–1993 reflect the dynamics of the important fish stocks in a broader area and serve as a suitable background with which the observed changes in Käina Bay may be compared. Only the cpue of roach in the two reference areas lying within the supposed feeding area of the cormorant colonies declined significantly, although less severely so than in Käina Bay. Perch and ruffe appear to have remained fairly stable in the rest of the Väinameri.
Fish abundance in the northeastern Baltic Sea is determined by many factors, the most important ones being fishing mortality and natural mortality (through predation, unfavourable environmental conditions, and pollution). Käina Bay was a protected area during recent decades, when fishing was allowed. Although relatively small, this bay serves as an important spawning ground for fish from a much wider area. Saat and Kikas (2002) related the good state of the fish populations in 1995 (relative to most other areas of the Väinameri) to the protected status of the bay. Because the bay does not contain isolated populations, fishing in other parts of the Väinameri affects the spawning aggregations. However, the available information (Vetemaa et al., 2006a) indicates that, coinciding with the sharp decline in the profitability of the small-scale fishery, the fishing pressure generally decreased markedly during the period 1995–2005, both in terms of total number of fishers and total effort. Therefore, fishing mortality can be excluded as a possible cause of the observed declines.
Unfavourable environmental conditions often play an important role in the dynamics of fish populations. However, stocks in other parts of the Väinameri have not undergone such drastic reductions as observed in Käina Bay (Vetemaa et al., 2006a), and those in other Estonian coastal waters have been even more stable (Saat et al., 2003a; Adjers et al., 2006). In addition, there are no local point sources of pollution in the area.
Is it possible then that the consumption of fish by cormorants in the Käina Bay area has had an impact on these stocks? To estimate the total number of birds present in an area, Engström (2001b) suggests that for an increasing population, the number of nests has to be multiplied by a factor of 4.7–5.2. Using a modest correction factor of 4, the total number of cormorants in 2005 in the area is estimated at ∼5600. The daily food intake of a cormorant has been estimated at around 500 g (Bauer and Glutz von Blotzheim, 1966; Müller, 1986; Zimmermann, 1989). Gremillet et al. (1995) found a somewhat lower value of 423 g for breeders and 250 g for non-breeders. If we base the calculations on a conservative daily intake of 300 g, the total daily food intake of all cormorants in the area during spring and summer in 2005 must have been at least 1.7 t. Cormorants are generalists, feeding on the most abundant species. Based on the analysis of pellets collected during multiple sampling events in April, Eschbaum et al. (2003) established that the share of roach in the diet of cormorants in the Väinameri area was 47–68%, whereas the share of perch was 6–10%.
To calculate the impact of cormorants on the main fish species of Käina Bay, their total biomass should be known. However, no stock-biomass estimates are available from Käina Bay or the Väinameri. Therefore, the only option was to relate the food intake to the commercial catch. The average daily catch of Väinameri fishers during the past decades has been estimated at ca. 0.1–0.2 kg ha−1, which was supposed to be close to the maximum sustainable yield (Vetemaa et al., 2002). A daily food intake of 1.7 t, if taken entirely from Käina Bay, would be ca. 1.9 kg ha−1, 10–20 times more than the average commercial catch. This would clearly be unsustainable. Although cormorants are for energetic reasons likely to feed as close to their colonies as possible, the most important feeding areas might be in the open Väinameri situated just a few kilometres away, because of the low fish density in the bay (except for ruffe). Platteeuw and Van Eerden (1995) suggest that feeding trips up to 20 km still could have an energetic value. Therefore, the fish fauna at the Sarve and Saarnaki monitoring stations might be directly affected by cormorant predation. However, an impact on the entire roach stock through reduced recruitment could also be expected, because Käina Bay used to be the most important spawning ground in the northern Väinameri. This might explain why roach has not only been declining severely in the bay itself, but also over a large area of the Väinameri.
Compared with roach, a perch tolerates a larger range of salinities during its embryonic development (Klinkhardt and Winkler, 1989). Therefore, even if the quickly warming bay might be ideal, perch could spawn also in other reedy places scattered over the Väinameri and, therefore, be less vulnerable to increased predation in one specific spawning area. The abundance of ide seems to have also decreased (although the difference was not statistically significant), but less so than roach and perch. In addition, the temperature could have played an important role in the observed differences in abundance of ide between 1995 and 2005, because fishing started at higher temperatures in 2005 (Figure 2). According to Keller (1995) and Dieperink (1995), the maximum prey size of cormorants is ∼1 kg, whereas fish in the range 10–20 cm dominate the diet (Martyniak et al., 1997). Adult ide weigh typically more than 1 kg and, therefore, might escape predation by cormorants. Therefore, it is quite likely that the cormorants do not affect the ide stock as much as they do roach and perch.
In contrast to 1995, the fish community in 2005 was dominated by just one abundant species: ruffe. Because ruffe is often found in the diet of cormorants (Engström, 2001b; Eschbaum et al., 2003), the question arises why this species was not affected? Because ruffe matures at a size well below the optimum prey size of cormorants (Martyniak et al., 1997) and has a high fecundity (Saat et al., 2003b), it is plausible that this species could withstand a high predation rate better than the larger species. In addition, ruffe is a bottom-dwelling fish, able to feed in much lower light intensity than perch, it is less active by day (Bergman, 1988; Dieterich et al., 2004; Okun et al., 2005), and its colouration provides some protection against predation by pikeperch (Swenson, 1977). Therefore, it seems quite possible that it is less vulnerable to predation by cormorants. Finally, a collapse of the perch and roach stocks probably meant less food competitors for young ruffe, probably resulting in good survival.
Ruffe often increase in abundance in response to eutrophication (Bergman, 1988; Tammi et al., 1999), but there is no evidence of a recent change in the local trophic status. Moreover, eutrophication also favours roach (Tammi et al., 1999), although this species has virtually vanished from the area. Therefore, less competition with other species seems a plausible explanation.
Homing of salmonids to the spawning grounds where they were born is well known, but recent data suggest that other fish species, such as the percid Micropterus salmoides (Waters and Noble, 2004) and the cyprinids Barbus barbus (Lucas and Batley, 1996) and Abramis brama (Tambets et al., 2002), also exhibit such natal homing behaviour. Therefore, it seems plausible that adult roach and perch still caught not far from Käina Bay during summer actually spawn elsewhere, which could explain their almost total absence in the bay.
Finally, Engström (2001a) hypothesized that cormorants could affect populations in other ways than simply removing individuals from the population. A permanent exposure to high predation pressure might elicit escape behaviour. Put simply, part of the population might just be scared away. However, even if this were the case, it still means that fish could not have used one of the traditionally important spawning areas.
Nowadays, a large effort is being made in establishing marine protected areas (MPAs). It has been argued that no-take zones help to sustain fisheries through spillover of adults and enhanced recruitment (Russ et al., 2004). However, our data indicate that such positive effects could be nullified—and worse—by an invasion of cormorants, which in itself could be brought about by the MPA (Debout et al., 1995), especially in a spatially restricted spawning ground with a high density of fish during the reproductive period.
During the past decade, the Estonian fisheries administration has followed a strong-hand policy: fishing restrictions are just based on scientific advice and they are hardly negotiated with fishers. This policy has been quite successful in curbing overfishing. However, the cormorants in the Väinameri area now take much higher catches than the fishers, and if there is a culprit responsible for overexploitation, it is not the fishery. Consequently, imposing further restrictions on fishers does not make sense. The idea that an invading species might affect coastal fish stocks in a way comparable with overfishing is not easily acceptable to environmental administrations, and this might have been the major reason why relatively slow steps are being taken to curb the rapid growth of cormorant populations in Estonia and Europe in general.
Acknowledgements
The study was financed by the Estonian state-funded research grant SF0180005s10 and Estonian Science Foundation Grants 7190 and 8281. We thank the two reviewers (Chantal van Dam and an anonymous one), and editors Niels Daan and Sarah B. M. Kraak, for valuable suggestions on the manuscript.