-
PDF
- Split View
-
Views
-
Cite
Cite
Henrik Jensen, Anna Rindorf, Peter J. Wright, Henrik Mosegaard, Inferring the location and scale of mixing between habitat areas of lesser sandeel through information from the fishery, ICES Journal of Marine Science, Volume 68, Issue 1, January 2011, Pages 43–51, https://doi.org/10.1093/icesjms/fsq154
Close - Share Icon Share
Abstract
Sandeels are small pelagic fish that play an important role in the diet of a range of natural predators. Because of their limited capture by traditional survey gear, little is known about their large-scale distribution or the degree of mixing between habitat areas. Detailed information collected directly from the fishery was used to map fishing grounds, which were then assumed to reflect the foraging habitat of the species. Length distributions from individual hauls were used to assess differences in the distributions as a function of distance between samples. Sandeel foraging habitat covered some 5% of the total area of the North Sea. Mixing between neighbouring fishing grounds was too low to eliminate differences in length distributions at distances between grounds down to 5 km. Within fishing grounds, mixing was sufficient to eliminate differences in length distributions at scales <28 km but insufficient at greater distances. The lack of mixing between grounds may result in large differences in sandeel abundance among adjacent fishing grounds. Further, notable abundance at one end of an extensive fishing ground is not necessarily indicative of similar abundance at its other end.Jensen, H., Rindorf, A., Wright, P. J., and Mosegaard, H. 2011. Inferring the location and scale of mixing between habitat areas of lesser sandeel through information from the fishery. – ICES Journal of Marine Science, 68: 43–51.
Introduction
Sandeels (Ammodytes spp.) are small semi-pelagic fish with a worldwide distribution (Smith and Heemstra, 1986). They usually constitute a large proportion of the fish biomass in those regions in which they are found (Reay, 1970) and are an important prey species for many fish, seabirds, and mammals (Daan, 1989; Furness, 1990; Wanless et al., 1998). In addition, they are the target of a large-scale industrial fishery in the North Sea (ICES, 2008a). This has led to concerns whether fisheries pose a threat to top predators through their reduction in the food supply (Macer, 1966; Monaghan, 1992; Wright, 1996; Wanless et al., 1998; Engelhard et al., 2008). Most sandeel species inhabit shallow, turbulent sandy areas, located at depths of 20–70 m where the content of the finest particles of silt and clay is low (Macer, 1966; Reay, 1970; Wright et al., 2000). Because of the limited availability of such substratum (Wright et al., 1998), the distribution of post-settled sandeels is very patchy (Macer, 1966; Wright et al., 2000; Freeman et al., 2004; Holland et al., 2005). Post-settled sandeels are rarely found >15 km from known habitat (Wright, 1996; Engelhard et al., 2008), and the maximum distance travelled by tagged fish displaced from grounds was 64 km (Gauld, 1990). This lack of large-scale dispersal combined with a limited larval exchange between areas (Proctor et al., 1998; Christensen et al., 2009) means that local aggregations may be vulnerable to depletion by the fishery and increases the risk of adverse effects on local predators even if the North Sea stock is inside biologically safe limits. Unfortunately, a lack of knowledge on the location of most of these aggregations in the North Sea and the population dynamics within them has hindered studies of local depletion, except in a few local areas (Wright, 1996; Rindorf et al., 2000; Daunt et al., 2008; Engelhard et al., 2008).
There are several reasons for the limited knowledge of sandeel distribution and population dynamics. First, sandeels bury into the sediment when not feeding in the water column or when approached by predators foraging near the seabed (Winslade, 1974a, b, c; Girsa and Danilov, 1976; Pearson et al., 1984; Pinto et al., 1984). This burying behaviour makes their accessibility to sampling in the water column highly variable, and because of this variability and the patchy distribution of habitat, none of the regular North Sea acoustic or trawl surveys provide reliable means of mapping sandeel distribution, although both approaches have been used to investigate density in a few areas of the North Sea (Greenstreet et al., 2006; ICES, 2008b; Johnsen et al., 2009). Second, although their requirement for specific habitat is likely to limit sandeel movement, little direct information exists on the extent of horizontal movements of post-settled sandeels within or between habitat areas. The few mark–recapture experiments that have been conducted were restricted to small regions because of the difficulty of marking a representative number of this abundant species. Moreover, the recapture location could not be determined because the species is not sorted on board fishing vessels (Gauld, 1990). Consequently, the distribution of habitat areas must be derived from non-conventional methods, and movements of sandeels throughout a large region such as the North Sea can only be evaluated indirectly.
Movements between habitat areas may be detected by differences between length distributions. Local differences in recruitment, growth, or mortality will result in differences between areas unless fish mix between the different areas. If such differences arise from variation in recruitment between areas, subsequent mixing would lead to a decrease in the difference between length distributions over the season. Alternatively, increasing differences in length distributions over the season could result from variation in growth or mortality and the lack of mixing between adjacent areas during the season. This increase should be greater the longer the distance between habitat areas if growth and mortality differences increase with distance. Alternatively, if mixing is only at small scales while growth and mortality varies independently of distance, differences between length distributions should increase at small distances, but vary independently of distance at large scales. Based on the extent of identified sandeel aggregations (Freeman et al., 2004; Holland et al., 2005) and the apparent limited movements of settled sandeels, it is entirely possible that mixing could be limited both within as well as among habitat areas.
The determination of length distributions in local aggregations repeatedly over a longer period is a costly exercise if scientific vessels are required to sample the fish. However, the sandeel fishery could provide crucial information at comparatively low cost. The fishery targets foraging sandeels near areas where they bury, so the fishing grounds should provide information on the distribution of sandeel habitat areas (Jensen, 2001). Recognizing the fact that information on sandeel habitat areas was crucial to the validity of the scientific advice given, collaboration between the Danish Fishermen's Association and the Danish Institute for Fisheries Research, now the Danish Technical University, was started in 1999 to provide the information needed to improve the understanding of North Sea sandeel population dynamics. Under this collaboration, data were collected by skippers of Danish sandeel vessels directly.
The objective of this study was to use the detailed data collected in the fishery to produce a map of the foraging habitat of sandeels, then to examine the extent of mixing between and within foraging habitats. Foraging habitats are defined here as areas with potential large densities of non-buried sandeels, because sandeels bury when not feeding (Winslade, 1974a). Four predictions were made of the relationship between the difference in length distributions and distance between samples in time and space, and the extent of mixing: In both (i) and (ii), the distance at which length distributions become significantly different indicates the distance at which the rate of mixing becomes too low to compensate for differences in recruitment, growth, and mortality.
If the difference between length distributions increases with distance within a fishing ground, the extent of mixing between subareas of the fishing ground must be limited.
If the difference between length distributions increases with distance between fishing grounds, the extent of mixing between fishing grounds must be limited.
If the difference between length distributions decreases over the season, initial differences caused by differences in, e.g., recruitment slowly disappear through mixing of fish.
If the difference between length distributions increases over the season, initial differences are enhanced through changes in growth, mortality, or emergence behaviour, and a lack of mixing.
Methods
Sandeel foraging habitat
Three types of information were combined to derive the spatial distribution of foraging habitat: global positioning system (GPS) records from individual ships, vessel monitoring system (VMS) data, and maps provided by fishers. GPS-based computer systems are used by the fishers together with information from charts and past experience to map and log fishing grounds and fishing activities. The maps contain data on longitude and latitude, and, in some cases, names of the fishing grounds. This detailed information was used to map some 10% of the grounds. To supplement these data, information on catches of sandeel in individual trawls for which the latitude and the longitude of hauling were known were used to locate another 50% of the grounds. The balance of 40% of the fishing grounds was obtained from the VMS data from Danish vessels catching sandeels. These data are logged continually while the vessel is at sea, meaning that the time spent not fishing for sandeel is included and must be excluded by filtering. These filtering routines were based on distance between successive sample points/positions and additional information collected from Danish fishers on the physical characteristics (i.e. depth and seabed type) of each fishing ground and the time of the year that the ground is generally fished.
From the data collected, the final map of the sandeel fishing grounds (Supplementary Figure S2) was produced using ArcMap and the Spatial Analyst extension (ESRI ArcGIS). Each fishing ground is defined as a polygon, adjusted manually considering raw data, information about individual grounds, the location of fishing tracks, and information on topography. During this process, the greatest weight was given to the information in the raw data. Fishers from different ports evaluated the map of the fishing grounds, after which it was modified according to guidelines they gave. Such evaluation resulted in the inclusion of additional grounds (from more navigation data) and the deletion of non-sandeel grounds. The map was subjected to several such evaluations before being finalized.
Length distributions of sandeels
From 1999 to 2008, the skippers of 55 sandeel fishing vessels collected detailed haul information on 2774 trawls. Each fishing vessel recorded information about the exact location and time of shooting and hauling of the trawl, the name of the fishing ground, and an estimate of the total weight of the catch in each trawl. Further, a sample of between 0.5 and 1 kg of fish was collected from each haul and frozen on board. In the laboratory, the sampled fish were thawed, the sandeels sorted by species, and the total length of all fish measured to the nearest 0.5 cm below. Samples where fewer than 50 fish were measured were excluded because of the low accuracy of length distributions based on such small samples. Further, as the objective was to compare length distributions between sites, hauls with >50 km between start and end positions and hauls with a distance >25% of the length of the fishing ground were excluded.
To derive an estimate of the variation in length distributions within and between fishing grounds while accounting for the unbalanced sampling of weeks and vessels, length distributions were analysed using generalized linear models of continuation-ratio logits (Rindorf and Lewy, 2001; Marques et al., 2005). This method allows statistical testing of the effect of both continuous and discrete variables. Further, by utilizing the smoothness of length distributions as a function of length, the method provides more accurate estimates of length distributions than traditional methods (Rindorf and Lewy, 2001). The observations analysed were distributions of observed lengths in 0.5 cm intervals for each haul sampled. They were modelled by fitting fifth-degree polynomials to the continuation-ratio logits (Rindorf and Lewy, 2001).
All models were fitted using the SAS® GENMOD procedure (SAS® version 9.1 for Windows; SAS® Institute Inc., 2004). The dispersion parameter was estimated by Pearson's χ2 statistic divided by the degrees of freedom. Only length groups with at least one sandeel on average in a sample were included in tests.
Within-fishing-ground variation
To examine whether length distributions differed within fishing grounds, fishing grounds where more than 20 samples were taken in a 2-week period in a given year were selected. This resulted in seven combinations of fishing ground and time. These fishing grounds were then subdivided into four parts. North–south orientated fishing grounds were divided according to latitude, and east–west orientated grounds according to longitude. Individual trawls were then attributed to each of these subareas based on the midpoints between shooting and hauling positions. Of the fishing grounds selected, Scooter Plads had hauls all originating in the same quarter of the fishing ground, so for that fishing ground, that quarter of the fishing ground was subdivided into two.
The statistical difference between subareas of a fishing ground was estimated using F-tests, to compare the deviance of a common length distribution with that of separate length distributions in different subareas (Rindorf and Lewy, 2001), still using fifth-degree polynomials to model length distributions. Fishing grounds were analysed separately to ensure that the values obtained were independent. From the F-test, a value of F and a probability of this value at the given number of degrees of freedom were obtained. If the number of degrees of freedom was unaltered, a larger value of F was taken to signify a greater difference between samples.
Between-fishing-ground variation
To examine whether length distributions differed between fishing grounds, grounds where more than five samples were taken in a 2-week period were selected. The distances between all hauls were computed, and for each pair of fishing grounds in a 2-week period, the hauls were grouped in bins of 10 km distance between hauls. Haul pairs from the same fishing ground or hauls >75 km apart were excluded. Two models were then fitted for each combination of fishing grounds and distance using the method described above: one modelled a common length distribution and the other modelled two length distributions, one for each fishing ground. From the residual deviances, the F-value of assuming a common length distribution was estimated along with the probability of this value. This resulted in a list of unique records containing the name of fishing ground 1, the name of fishing ground 2, distance-group (10 km groups), F-values, and the probability of all samples being derived from a common length distribution.
Trends over time
To examine whether the difference between length distributions generally decreased or increased over time, the correlations between F-values of the between-fishing-ground comparison and week of the year were estimated for each distance-group. Only groups where three or more 2-week periods were sampled in the main fishing season (weeks 14–22) were included.
Results
Sandeel foraging habitat
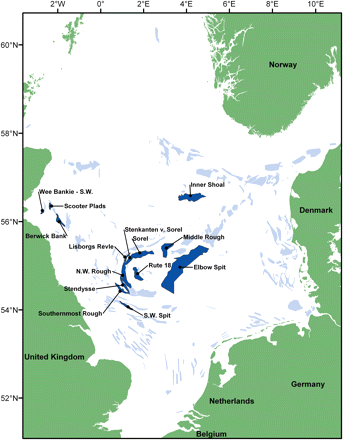
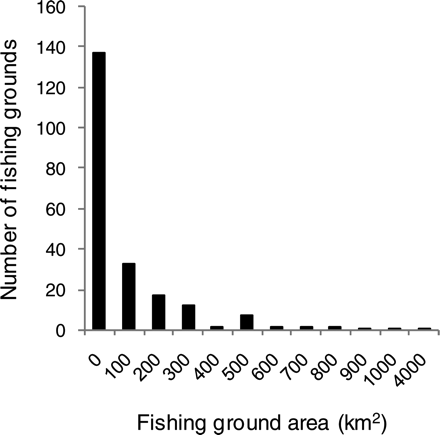
In all, 217 individual habitat areas were identified, a total area of 33 566 km2 or ∼5% of the combined area of the North Sea and Skagerrak (Figure 1). The habitat areas vary greatly in size, from 1 to 4023 km2 (Figure 2). Given the average estimated biomass of sandeel in the North Sea (ICES, 2008a), the density within habitat areas averaged 58 t km–2. With an average weight of sandeel of 7 g (ICES, 2008a), this corresponds to 8 sandeels m−2 of habitat.
Sandeel habitat areas (areas with potentially high density of non-buried sandeel) and the locations of the fishing grounds mentioned in text.
Frequency distribution of area of individual sandeel habitat areas, the x-axis indicating the lower limit of 100-km2 intervals.
Within-fishing-ground variation
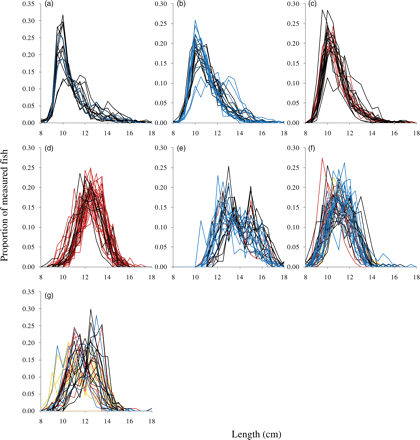
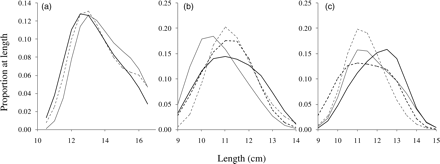
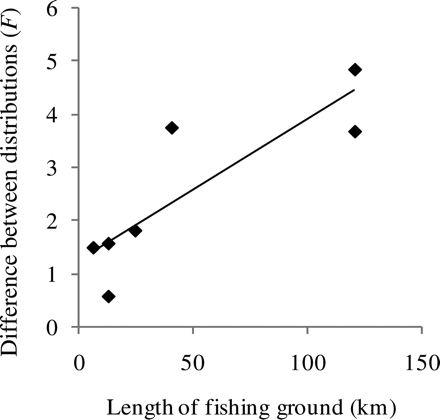
The length distributions of sandeels from 187 hauls taken on five fishing grounds were used. Of these grounds, the number of samples taken exceeded 20 in two consecutive 2-week periods on two of the grounds. It is clear from the visual inspection of the length distributions that the variation between fishing grounds was much larger than the within-fishing-ground variation (Figure 3). The length distributions differed significantly between subareas of the fishing ground on two fishing grounds (a total of three cases; Table 1, Figure 4) and the difference increased significantly with length of the fishing ground (correlation between F and distance between outer subarea midpoints = 0.86, p = 0.0135). The regression line exceeded an F-value of 2 (roughly equal to a difference significant at the 5% level when the number of degrees of freedom is large) at a minimum distance between subareas of 28 km (Figure 5). At lesser distances, the length distributions were not significantly different, so there was probably mixing at those scales. As the distance refers to the distance between fishing ground subarea centres and subareas are defined as quarters of fishing grounds, this corresponds to a total mixing of fish at fishing grounds of lengths <112 km.
Comparison of length distributions between subareas within fishing grounds.
| . | . | Total area . | Subarea . | . | . | ||
|---|---|---|---|---|---|---|---|
| Fishing ground . | Length (km) . | Deviance . | d.f. . | Deviance . | d.f. . | F-value . | p(F) . |
| Scooter Plads | 6 | 830.8 | 329 | 22.7 | 6 | 1.498 | 0.1780 |
| Berwick Bank | 13 | 1 191.7 | 281 | 46.8 | 7 | 1.576 | 0.1422 |
| Berwick Bank | 13 | 805.3 | 257 | 10.9 | 6 | 0.580 | 0.7464 |
| Inner Shoal | 25 | 1 166.3 | 468 | 27.2 | 6 | 1.819 | 0.0936 |
| Sorel | 41 | 806.4 | 307 | 98.7 | 10 | 3.758 | 0.0001 |
| Elbow Spit | 121 | 1 205.7 | 337 | 260.5 | 15 | 4.854 | <0.0001 |
| Elbow Spit | 121 | 1 635.8 | 341 | 336 | 19 | 3.686 | <0.0001 |
| . | . | Total area . | Subarea . | . | . | ||
|---|---|---|---|---|---|---|---|
| Fishing ground . | Length (km) . | Deviance . | d.f. . | Deviance . | d.f. . | F-value . | p(F) . |
| Scooter Plads | 6 | 830.8 | 329 | 22.7 | 6 | 1.498 | 0.1780 |
| Berwick Bank | 13 | 1 191.7 | 281 | 46.8 | 7 | 1.576 | 0.1422 |
| Berwick Bank | 13 | 805.3 | 257 | 10.9 | 6 | 0.580 | 0.7464 |
| Inner Shoal | 25 | 1 166.3 | 468 | 27.2 | 6 | 1.819 | 0.0936 |
| Sorel | 41 | 806.4 | 307 | 98.7 | 10 | 3.758 | 0.0001 |
| Elbow Spit | 121 | 1 205.7 | 337 | 260.5 | 15 | 4.854 | <0.0001 |
| Elbow Spit | 121 | 1 635.8 | 341 | 336 | 19 | 3.686 | <0.0001 |
Length is the maximum distance between midpoints of subareas sampled. Total area deviance and d.f. indicate the deviance from a common length distribution and the degrees of freedom of this common length distribution. Subarea deviance and d.f. indicate the reduction in deviance obtained by modelling the length distribution in each subarea separately along with the associated loss of degrees of freedom.
Comparison of length distributions between subareas within fishing grounds.
| . | . | Total area . | Subarea . | . | . | ||
|---|---|---|---|---|---|---|---|
| Fishing ground . | Length (km) . | Deviance . | d.f. . | Deviance . | d.f. . | F-value . | p(F) . |
| Scooter Plads | 6 | 830.8 | 329 | 22.7 | 6 | 1.498 | 0.1780 |
| Berwick Bank | 13 | 1 191.7 | 281 | 46.8 | 7 | 1.576 | 0.1422 |
| Berwick Bank | 13 | 805.3 | 257 | 10.9 | 6 | 0.580 | 0.7464 |
| Inner Shoal | 25 | 1 166.3 | 468 | 27.2 | 6 | 1.819 | 0.0936 |
| Sorel | 41 | 806.4 | 307 | 98.7 | 10 | 3.758 | 0.0001 |
| Elbow Spit | 121 | 1 205.7 | 337 | 260.5 | 15 | 4.854 | <0.0001 |
| Elbow Spit | 121 | 1 635.8 | 341 | 336 | 19 | 3.686 | <0.0001 |
| . | . | Total area . | Subarea . | . | . | ||
|---|---|---|---|---|---|---|---|
| Fishing ground . | Length (km) . | Deviance . | d.f. . | Deviance . | d.f. . | F-value . | p(F) . |
| Scooter Plads | 6 | 830.8 | 329 | 22.7 | 6 | 1.498 | 0.1780 |
| Berwick Bank | 13 | 1 191.7 | 281 | 46.8 | 7 | 1.576 | 0.1422 |
| Berwick Bank | 13 | 805.3 | 257 | 10.9 | 6 | 0.580 | 0.7464 |
| Inner Shoal | 25 | 1 166.3 | 468 | 27.2 | 6 | 1.819 | 0.0936 |
| Sorel | 41 | 806.4 | 307 | 98.7 | 10 | 3.758 | 0.0001 |
| Elbow Spit | 121 | 1 205.7 | 337 | 260.5 | 15 | 4.854 | <0.0001 |
| Elbow Spit | 121 | 1 635.8 | 341 | 336 | 19 | 3.686 | <0.0001 |
Length is the maximum distance between midpoints of subareas sampled. Total area deviance and d.f. indicate the deviance from a common length distribution and the degrees of freedom of this common length distribution. Subarea deviance and d.f. indicate the reduction in deviance obtained by modelling the length distribution in each subarea separately along with the associated loss of degrees of freedom.
Length distributions of sandeels at fishing grounds where >20 hauls were taken in a 2-week period. (a) Scooter Plads (maximum distance between subarea midpoints 6 km); (b and c) Berwick Bank (maximum distance between subarea midpoints 13 km) in weeks 22 and 24, respectively; (d) Inner Shoal (maximum distance between subarea midpoints 25 km); (e) Sorel (maximum distance between subarea midpoints 41 km); (f and g) Elbow Spit (maximum distance between subarea midpoints 121 km) in weeks 16 and 18, respectively. Colours indicate subareas of the fishing grounds.
Length distributions of sandeels in subareas (a) Sorel, (b) Elbow Spit in week 16, and (c) Elbow Spit in week 18. Solid black lines depict the subarea immediately west of centre, dashed black lines the subarea close to the western end of the fishing ground, solid grey lines the subarea immediately east of centre, and dashed grey lines the subarea close to the eastern end of the fishing ground.
F-value of the comparison of common and subarea-specific length distributions as a function of the distance between sampled fishing ground subareas. The line is a regression line.
Between-fishing-ground variation
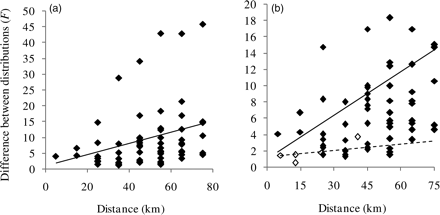
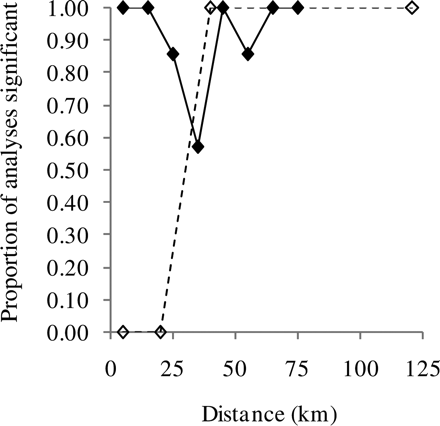
The length distributions differed significantly between fishing grounds in a total of 55 cases (90%). The length distributions of sandeels from 1038 hauls taken at 11 fishing grounds were used, resulting in a total of 61 combinations of fishing-ground pair and 10-km distance-group (Supplementary Table S1). The F-value of the difference between length distributions increased significantly with distance between fishing grounds (correlation between F and distance-group = 0.30, p = 0.0184; Figure 6a) and the regression line exceeded an F-value of 2 at a minimum distance between fishing grounds of 5 km. The difference between length distributions was substantially greater between fishing grounds than within fishing grounds even at the same geographic distance (Figure 6b). Whereas within-fishing ground comparisons did not reveal significant differences at distances of <40 km (Figure 5), all the between-fishing ground comparisons made at distances <20 km (three in all), plus all but one made at distances of <30 km (ten in all), were significantly different (Figure 7).
F-value of (a) the comparison of common and fishing-ground-specific length distributions as a function of the distance between sampled fishing grounds and (b) the comparison of within- and between-fishing-ground results. The line is a regression line. Filled symbols and solid line, between-fishing-ground comparisons; open symbols and dashed line, within-fishing-ground comparisons. Note that the values on the y-axis differ between panels because (b) shows only the lower half of (a).
Proportion of analyses showing significant differences as a function of distance. Filled symbols and solid line, between fishing grounds; open symbols and dashed line, within fishing grounds.
Trends over time
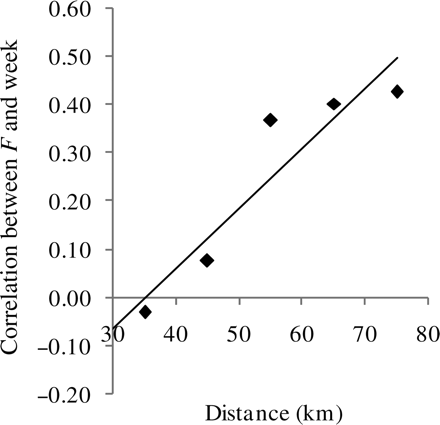
None of the correlations between F and week were significant at the 5% level when performed separately for each distance-group, so the differences did not increase or decrease significantly over time at a given distance. However, the correlation between F and week within a distance-group increased significantly with distance (Pearson correlation coefficient = 0.93, p= 0.0211; Figure 8). This indicates that there was no tendency for nearby grounds to become more similar over time (correlation between F and time was zero), whereas distant grounds became more different over time (F between distant grounds increased over time).
Correlation between week and F for a given distance. Positive values indicate a general rise in F over the season, negative values a decrease. The line is a regression line.
Discussion
This study utilized the first whole North Sea map of lesser sandeel distribution to evaluate dynamics over a complex mosaic of grounds differing widely in size and proximity to each other. The mixing of sandeels between these grounds was insufficient to eliminate significant differences in length distributions at spatial scales down to 5 km. Within grounds, mixing is greater, and at fishing grounds with a length <112 km, no difference in length distributions was found. However, beyond this distance, the mixing was again insufficient to eliminate differences in length distributions. This led to increased differences between fishing grounds late in the season because differences in growth and mortality enhanced early-season differences. A lack of mixing between fishing grounds potentially increases the risk of adverse effects of a fishery on local predators even if the population at a larger scale is inside biologically safe limits.
The validity of the sandeel map as an indicator of foraging habitat depends on whether all habitat areas are known and fished by the fishery and whether fishing is restricted to foraging areas. Comparison with smaller-scale topographic and benthic mapping suggests that the fishery tends to concentrate in areas where sandeels forage, which often coincide with the edge of fishing grounds (Jensen, 2001; Holland et al., 2005; Mackinson and van der Kooij, 2006; Engelhard et al., 2008). Given the proximity between foraging habitat and preferred substratum, fishing distribution should provide a good proxy for areas of sandeel habitat (Wright et al., 2000; van der Kooij et al., 2008). However, Danish vessels fish only in areas with clear tows and a high catch rate and avoid shallow water and areas within the territorial limits of countries other than Denmark. Hence, rugged and coastal habitat may be underrepresented in the map. Most of the catch from coastal habitats is, however, likely to consist of the more coastal sandeel species, Ammodytes tobianus (Jensen et al., 2004).
We are not aware of any similar investigations where mixing has been deduced from comparisons of length distributions. There may be several reasons for this. First, comparison of length distributions is not straightforward when the number of fish measured is limited (Rindorf and Lewy, 2001). Second, a difference in length distributions is not equivocal because it may be caused either by lack of mixing and highly variable growth and mortality rates or by size-dependent migration, where fish migrate to specific areas when they reach a certain size. Similarly, identical length distributions may be a result of identical recruitment, growth, and mortality patterns combined with a lack of mixing. The lack of differences in this study in length distribution seen within small fishing grounds is therefore not necessarily a result of mixing, but could be a result of homogeneous recruitment patterns combined with small differences in growth and mortality rates. However, as one subarea of the fishing ground did not consistently hold larger fish in the fishing ground sampled in two consecutive 2-week periods (Figure 4), it seems unlikely that size-dependent migration was the cause of the difference in length distributions between fishing ground subareas. Moreover, it seems unlikely that such size-dependent migration should be greater between fishing grounds than within fishing grounds, which would be required to explain the larger differences between grounds. Although there may size-dependent migration in sandeels, it is insufficient to explain the large differences between fishing grounds. This is consistent with the very high rates of recapture of tagged sandeels recorded on fished grounds (Kunzlik et al., 1986). The recorded differences between length distributions reveal not only lack of extensive movement, but also large differences in local recruitment, growth, or mortality, and possibly also in burying behaviour. Burying behaviour influences the length distribution if the proportion of time spent buried varies between different lengths of sandeel. If this behaviour also varies between fishing grounds, the effect can be increased differences over the season. Significant regional differences in length distribution, growth, and maturity have also been found in other studies (Wright, 1996; Bergstad et al., 2001; Boulcott et al., 2007; Johnsen et al., 2009). The present study provides further explanation for this regional variability in dynamics.
At the extreme, local depletion may occur at one end of a large ground whereas sandeel densities are great at the other end or on one of two neighbouring fishing grounds. This could be caused by fishing if the fishery remains at the low-abundance end of the ground even when density is higher at the other end, a behaviour which would, though, not appear to maximize profit. The limited exchange between nearby areas potentially increases the effectiveness of closing local areas to fishing as the sandeels inside the area will not simply spill over into adjacent fishing grounds and be caught there. However, the relationship between exchange and differences in local densities were not investigated, so a spill-over effect could take place if there were large density differences between two adjacent areas. In this case, less competition for food in the low-density area may give sandeels energetic reasons to switch area. Moreover, the analysis here deals only with mixing in the feeding season. Mature sandeels emerge from the sandbanks in December and January to spawn (Gauld and Hutcheon, 1990; Boulcott et al., 2007), and whether mixing takes place during that event is unknown. However, the distributions of eggs and early larvae closely match known overwintering habitat, so mixing during the spawning season appears to be unlikely (Wright and Bailey, 1996; Munk et al., 2002). After hatching, larvae may drift considerable distances (Proctor et al., 1998; Christensen et al., 2009), and the horizontal movement of pre-settled sandeels (Wright, 1996) most likely leads to exchange of recruits between fishing grounds. Hence, closing a fishing ground will protect sandeels during the fishing season and may contribute to recruitment on other fishing grounds, but is unlikely to ensure the presence of sandeels in the closed fishing ground in future years.
To conclude, the distribution of sandeels in the North Sea is very patchy and there is limited exchange between even close fishing grounds during the fishing season. There is some mixing within fishing grounds, but the mixing between grounds appears to be minimal. Management by closing fishing grounds or fishing ground subareas therefore has the potential to protect local aggregations of sandeels during the fishing season and may result in increased recruitment to nearby fishing grounds, but such protection is unlikely to assure continuous recruitment to a particular fishing ground.
Supplementary material
Supplementary material is available at ICESJMS online. Figure S1 shows the raw data used in developing the sandeel map, Figure S2 is the final habitat map derived, and Table S1 lists the results of the comparison of length distributions between fishing grounds.
Acknowledgements
We thank the fishing skippers who collected the data and placed them at our disposal, to the Danish Fishing association that supported the work, to Palle Brogaard, who improved the cooperation between the industry and scientists, and to Stina B. S. Hansen and the laboratory technicians who worked up the thousands of length samples. The work of AR and HM was supported by the Danish national project “ETOMTOBIS”. Georg Engelhard and an anonymous reviewer provided valuable comments on an earlier version of the manuscript.