-
PDF
- Split View
-
Views
-
Cite
Cite
Peter Davison, The specific gravity of mesopelagic fish from the northeastern Pacific Ocean and its implications for acoustic backscatter, ICES Journal of Marine Science, Volume 68, Issue 10, November 2011, Pages 2064–2074, https://doi.org/10.1093/icesjms/fsr140
Close - Share Icon Share
Abstract
Knowledge of the species present, their morphology, and their size distribution is required to infer biomass from acoustic surveys of fish. The gas content and specific gravity of the body (with gas removed), ρf, was measured for 71 species of mesopelagic fish in the NE Pacific Ocean. Those species that have functional swimbladders when large maintain constant ρf with increasing body size. Species without functional swimbladders as adults show decreased ρf with increasing body size. The acoustic-backscattering cross-section, σbs, was modelled for all individuals collected from three fish species that differed in the presence of a gas-filled swimbladder. The change in σbs with increasing body size was markedly different between the three. The low body density of those mesopelagic fish without gas-filled swimbladders greatly reduces their σbs. In species of fish that possess a functional swimbladder as juveniles and in which the swimbladder regresses with growth, the σbs first decreases, then increases with increased body size. Knowledge of the ontogenetic changes in swimbladder inflation and body density in mesopelagic fish is critical for the construction of the backscattering models used to interpret acoustic surveys.Davison, P. 2011. The specific gravity of mesopelagic fish from the northeastern Pacific Ocean and its implications for acoustic backscatter. – ICES Journal of Marine Science, 68: .
Introduction
Mesopelagic fish are ubiquitous in the world's oceans and are the most abundant vertebrates on earth (Mann, 1984), with an estimated biomass of a billion tonnes (Gjosæter and Kawaguchi, 1980). They are a major component of the acoustic deep-scattering layer (Hersey et al., 1962; Mann, 1984). The sheer numbers of mesopelagic fish make them ecologically important predators and prey, occupying mid-trophic levels (Mann, 1984; Beamish et al., 1999). Mesopelagic fish inhabit depths between 200 and 1000 m by day, and many migrate vertically to the euphotic zone at night to feed (Gjosæter and Kawaguchi, 1980). In mesopelagic fish, diel vertical migration (DVM) is a common behaviour that develops in response to the collocation of food supply and visual predation pressure near the sea surface (Marshall, 1960; Robison, 2003). Their daily vertical movement is often several hundred metres (Pearcy et al., 1977; Karnella, 1987), and it is physiologically difficult for any fish to maintain a gas-filled swimbladder over the pressure changes associated with DVM (Marshall, 1960; D'Aoust, 1971; Alexander, 1972). As the presence and size of a gas inclusion affects the acoustic properties of a fish, the means by which mesopelagic fish maintain buoyancy over their full vertical range are of consequence to fisheries acousticians and acoustic oceanographers.
Acoustic surveys are commonly used to estimate the abundance of fish (Simmonds and MacLennan, 2005). Estimates so derived are critically dependent on the assumed or measured target-strength (TS) distribution of the surveyed population or community (Simmonds and MacLennan, 2005). The TS (dB re 1 m2) is the logarithmic form of the acoustic-backscattering cross-section (σbs, m2), and the two variables are related by the equation TS = 10 log10(σbs) (Simmonds and MacLennan, 2005). Models of the form TS = mlog10(LS) + b are often used to describe the expected backscatter from a fish, where LS is the standard length of a fish, and m and b are species-specific constants (Simmonds and MacLennan, 2005). The appropriate TS selection is complicated by the diversity of species present, the size distribution of animals, and the orientation of the fish relative to the transducer (Simmonds and MacLennan, 2005). In particular, the presence or the absence of gas in the swimbladder is important, because the gas inclusion is responsible for some 90–95% of the σbs of a fish (Foote, 1980). Partitioning of measured acoustic backscattering within and between the species that constitute the deep scattering layer requires the use of nets to assess the species present and their size distribution for comparison with acoustic data collected simultaneously (McClatchie et al., 2000; Simmonds and MacLennan, 2005). This remains one of the most common methods of interpreting acoustic data from mixed aggregations such as the deep scattering layer, although the nets used to sample and quantify the targeted assemblage are subject to significant escapement and avoidance biases (Koslow et al., 1997; McClatchie et al., 2000).
Mesopelagic fish may have an extreme departure from length-based TS models because of ontogenetic changes in swimbladder morphology and body density. They reduce visual predation risk through occupation of a low-light environment (Mann, 1984). The release from visual predation allows them to optimize buoyancy and metabolic costs through the reduction in dense muscle tissue (Childress et al., 1980). Many fish species accumulate low-density fluids or lipids as they grow, decreasing their overall body density (Butler and Pearcy, 1972). Other mesopelagic species have negatively buoyant gelatinous tissue (Yancey et al., 1989). The reduction in body density decreases the density contrast with surrounding seawater and hence decreases the acoustic reflectivity of the fish. Some species that have gas in their swimbladder as juveniles do not possess it as adults, and other species vary individually in the presence of a gas-filled swimbladder (Butler and Pearcy, 1972; Neighbors, 1992; Yasuma et al., 2010). Some fish may allow the volume of gas to change over the course of DVM in accordance with Boyle's law, whereas others may maintain a constant gas volume (Hersey et al., 1962; Kalish et al., 1986). Knowledge of variations in body density and gas content within and between species is required before the construction of accurate acoustic models is possible.
As a consequence of the lack of data on the body density of midwater fish, assumptions need to be made to construct acoustic models. The typical body density of an epipelagic fish is 1.076 g ml−1 (Taylor, 1921). Several investigations of the buoyancy of mesopelagic fish have found body density to be considerably lower than that of epipelagic fish (Capen, 1967; Butler and Pearcy, 1972; Johnson, 1979; Neighbors and Nafpaktitis, 1982; Yasuma et al., 2006). Unfortunately, just a few species have been subject to such study from the speciose mesopelagic community.
To address some of the issues listed above, this study looked at the specific gravity and swimbladder inflation of some of the species making up the mesopelagic fish communities of two biogeographic provinces in the North Pacific (the California Current and the North Pacific Subtropical Gyre). Three hypotheses were tested: first, that mesopelagic fish have a lower ρf than epipelagic fish; second, that fish with functional swimbladders exhibit a constant ρf with growth; and third, that the ρf of fish species without functional swimbladders decreases with increased size. The implications of these results for the acoustic backscattering from mesopelagic fish were then investigated.
Material and methods
Mesopelagic fish were collected in 2009 and 2010 on three cruises of the RV “New Horizon” and one cruise of the National Oceanic and Atmospheric Administration (NOAA) FSV “Bell Shimada” in the North Pacific (Supplementary Figure S1). All four cruises sampled the California Current off southern California, and one cruise sampled the North Pacific Subtropical Gyre. Fish were captured using midwater trawls, bongo nets, manta nets, and dipnets. Midwater trawls were the main collection method, and 31 were made (Supplementary Table S1, Figure S1). The other nets were used in conjunction with them. Fish were separated from zooplankton within an hour of capture. A subset of the fish catch was then set aside in an ice bath for analysis, which took place within 8 h of capture.
Laboratory measurements
The specific gravity (density relative to freshwater, dimensionless) of fish, ρf, was measured by immersion in dense fluids after removal of any gas from the swimbladder. Seawater and glycerine solutions were prepared in 0.0025 increments of specific gravity over the range 1.025–1.090, although most measurements were only made to a precision of 0.005. The specific gravity of each solution was measured periodically with a hydrometer to 0.0005 precision and accuracy, and adjusted when necessary. Variation in specific gravity from dilution or evaporation was never more than ±0.001. The fish selected for analysis were immersed in room temperature seawater for at least 5 min to equilibrate the temperatures of the fish and glycerine solutions. Room temperature varied between 17 and 24°C over all four cruises. The seawater density change resulting from a 7°C room temperature range is <0.002 g ml−1 (Pilson, 1998). The effect of temperature on the density of fish tissue is unknown, but was presumed to be similar to that of seawater. Measurements of ρf were neither corrected for temperature variation nor converted to density.
The LS of each fish was measured to the nearest millimetre. Fish were dissected in seawater under a dissecting microscope to remove any gas from the swimbladder before measurement of ρf. The diameter of gas bubbles released from the body cavity during dissection was measured with the ocular micrometer of the microscope. If the swimbladder was not ruptured, the lengths and widths of visible gas bubbles were measured before puncturing the swimbladder. Gas volume was then calculated using the formula for a prolate spheroid: V = (4/3)πab2, where a and b are the major and the minor axis radii, respectively (Capen, 1967). In cases where the gas was released too quickly for measurement, it was simply recorded as present. Volume was then transformed to an equivalent spherical radius (ESR). Transparent fish with no visible gas bubble and opaque fish from taxa with no functional swimbladder at the family level (Stomiidae, Bathylagidae, Alepocephalidae, Platytroctidae, and Notosudidae) were not dissected. Fish with visible damage to the body wall that could have resulted from the escape of gas were recorded as ruptured. Once gas was released from the body cavity, the fish were placed progressively in graduated cylinders containing solutions of different specific gravity to find the highest specific gravity of sinking and the lowest specific gravity of floating. These were recorded as the same if the fish was neutrally buoyant in a cylinder. ρf was calculated as the mean of the two measurements. Care was taken to exclude all gas bubbles from the interior and exterior of the fish. After ρf measurement, each fish was blotted, then frozen in preweighed plastic bags. Ashore, the bags were weighed and the wet weight of the fish determined by subtracting the weight of the empty bag.
Data analysis
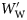 and
and 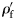 using the following equations to allow grouping of data from species of differing size and specific gravity:
using the following equations to allow grouping of data from species of differing size and specific gravity: 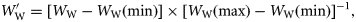
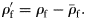
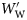 is the wet weight standardized to a range 0–1. WW(min) and WW(max) are, respectively, the wet weights of the smallest and largest fish within a species. The standardized specific gravity
is the wet weight standardized to a range 0–1. WW(min) and WW(max) are, respectively, the wet weights of the smallest and largest fish within a species. The standardized specific gravity 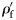 is simply the difference from the species mean specific gravity
is simply the difference from the species mean specific gravity 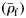 . Species were assigned to groups based on the presence of gas in the swimbladders of small and large fish. Large fish are defined for this purpose as those with a
. Species were assigned to groups based on the presence of gas in the swimbladders of small and large fish. Large fish are defined for this purpose as those with a 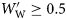 , and small fish as those with
, and small fish as those with 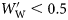 . Group I species had at least some small and large individuals with inflated swimbladders. The swimbladders of Group II species contained gas in at least some small fish, but not in large ones. Group III species never had inflated swimbladders. Changes in ρf with
. Group I species had at least some small and large individuals with inflated swimbladders. The swimbladders of Group II species contained gas in at least some small fish, but not in large ones. Group III species never had inflated swimbladders. Changes in ρf with 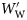 were tested statistically at the group level using Spearman's rank order correlation (rs). The maximum LS for each species (Table 1) was taken from Scripps Institution of Oceanography (SIO) Marine Vertebrate Collection (MVC) records to assess whether or not the sampled fish were representative of the species size range.
were tested statistically at the group level using Spearman's rank order correlation (rs). The maximum LS for each species (Table 1) was taken from Scripps Institution of Oceanography (SIO) Marine Vertebrate Collection (MVC) records to assess whether or not the sampled fish were representative of the species size range. Assignment of species to groups based on the presence or the absence of gas in their swimbladders.
| Species . | DVMa . | n . | LS range (mm) . | Max. LS (mm) . | 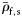 .
. | 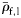 .
. | τ-value . | p-value . |
|---|---|---|---|---|---|---|---|---|
| Group I | ||||||||
| Tarletonbeania crenularis | y | 20 | 22–57 | 78 | 1.080 | 1.085 | 0.27 | 0.939 |
| Hygophum reinhardtii | y | 4 | 19–43 | 64 | 1.066 | 1.076 | 0.66 | 0.958 |
| Notoscopelus resplendens | y | 5 | 27–42b | 98 | 1.073 | 1.076 | 0.84 | 1.000 |
| Diplospinus multistriatus | y | 6 | 18–55b | 225 | 1.073 | 1.074 | 0.00 | 0.572 |
| Diogenichthys atlanticus | y | 25 | 16–23 | 30 | 1.064 | 1.073 | 0.55 | 1.000 |
| Myctophum nitidulum | y | 17 | 18–78 | 105 | 1.074 | 1.073 | −0.16 | 0.215 |
| Electrona risso | y | 3 | 11–32b | 81 | 1.071 | 1.073 | 0.82 | – |
| Hygophum proximum | y | 5 | 16–32 | 61 | 1.068 | 1.073 | 0.44 | 0.900 |
| Protomyctophum crockeri | n | 26 | 15–37 | 55 | 1.070 | 1.073 | 0.21 | 0.920 |
| Nannobrachium fernae | y | 5 | 32–63 | 81 | 1.069 | 1.073 | −0.20 | 0.408 |
| Lampadena urophaos | y | 12 | 19–26b | 115 | 1.060 | 1.073 | 0.34 | 0.925c |
| Bolinichthys longipes | y | 4 | 38–42d | 59 | 1.071 | 1.070 | −0.40 | 0.333c |
| Diaphus anderseni | y | 13 | 25–30 | 55 | 1.070 | 1·070 | 0.20 | 0.819c |
| Ceratoscopelus warmingii | y | 18 | 19–48 | 81 | 1.064 | 1·070 | 0.51 | 0.997 |
| Vinciguerria nimbaria | y | 9 | 17–32 | 53 | 1.067 | 1.069 | −0.21 | 0.269 |
| Vinciguerria poweriae | y | 15 | 18–38 | 37 | 1.069 | 1.069 | 0.04 | 0.601 |
| Diaphus fulgens | y | 7 | 36–52d | 58 | 1.073 | 1.068 | −0.69 | 0.029 |
| Lampanyctus tenuiformis | y | 13 | 28–43b | 153 | 1.067 | 1.067 | 0.13 | 0.737 |
| Microstoma microstoma | y | 6 | 20–55b | 210e | 1.067 | 1.067 | 0.17 | 0.700 |
| Danaphos oculatus | n | 23 | 22–41 | 46 | 1.062 | 1.066 | 0.49 | 0.999 |
| Symbolophorus californiensis | y | 20 | 25–88 | 116 | 1.072 | 1.064 | −0.26 | 0.071 |
| Chilara taylori | n | 3 | 35–55b | 366 | 1·060 | 1.064 | 0.00 | – |
| Taaningichthys bathyphilus | n | 3 | 42–66d | 85 | 1·060 | 1.063 | 0.82 | – |
| Argyropelecus lychnus | n | 3 | 13–37b | 78 | 1·068 | 1.063 | −0.82 | – |
| Argyropelecus sladeni | n | 26 | 12–41 | 60 | 1.062 | 1.062 | 0.09 | 0.727 |
| Argyropelecus hemigymnus | n | 17 | 15–30 | 38 | 1.064 | 1.062 | −0.25 | 0.105 |
| Argyropelecus affinis | n | 17 | 14–76 | 88 | 1.055 | 1.059 | 0.04 | 0.604 |
| Bathysphyraenops simplex | y | 7 | 17–47 | 80e | 1.075 | 1.058 | −0.13 | 0.429c |
| Cyclothone pseudopallida | n | 12 | 24–43 | 49 | 1.055 | 1.056 | −0.08 | 0.387 |
| Melamphaes simus | y | 5 | 15–29 | 29 | 1.069 | 1.055 | −0.89 | 0.033 |
| Sternoptyx obscura | n | 7 | 12–41 | 48 | 1.054 | 1.053 | 0.28 | 0.810 |
| Sternoptyx diaphana | n | 6 | 14–35 | 60 | 1.053 | 1.053 | 0.00 | 0.572 |
| Cyclothone signata | n | 36 | 15–35 | 39 | 1.058 | 1.053 | −0.21 | 0.051 |
| Sternoptyx pseudobscura | n | 8 | 17–44 | 61 | 1.052 | 1.049 | 0.12 | 0.696 |
| Diaphus theta | y | 32 | 14–73 | 86 | 1.059 | 1.049 | −0.38 | 0.002 |
| Triphoturus nigrescens | y | 4 | 32–41d | 45 | 1.064 | 1.043 | −0.91 | 0.083c |
| Scopeloberyx opisthopterus | n | 3 | 22–33 | 39 | 1.053 | 1.041 | −1.00 | – |
| Notolychnus valdiviae | y | 8 | 13–25 | 29 | 1.049 | 1.040 | −0.22 | 0.287c |
| Melamphaes suborbitalis | n | 3 | 24–68 | 119 | 1.069 | 1.040 | −0.33 | – |
| Ichthyococcus irregularis | n | 3 | 24–36 | 63 | 1.048 | 1.038 | −1.00 | – |
| Group II | ||||||||
| Melamphaes parvus | y | 4 | 21–45 | 54 | 1.071 | 1.055 | −0.33 | 0.375c |
| Cyclothone atraria | n | 10 | 21–47 | 70 | 1.051 | 1.048 | −0.33 | 0.117 |
| Nannobrachium hawaiiensis | y | 22 | 24–92 | 111 | 1.052 | 1.046 | −0.35 | 0.017 |
| Ceratoscopelus townsendi | y | 43 | 21–60 | 77 | 1.063 | 1.045 | −0.49 | <0.001 |
| Scopelogadus mizolepis | y | 19 | 25–83 | 97 | 1.052 | 1.044 | −0.72 | <0.001 |
| Poromitra crassiceps | n | 14 | 20–60b | 204 | 1.051 | 1.044 | −0.54 | 0.006 |
| Nannobrachium ritteri | y | 56 | 19–94 | 124 | 1.044 | 1.032 | −0.80 | <0.001 |
| Triphoturus mexicanus | y | 52 | 17–68 | 75 | 1.040 | 1.031 | −0.75 | <0.001 |
| Stenobrachius leucopsarus | y | 57 | 20–83 | 105 | 1.035 | 1.029 | −0.74 | <0.001 |
| Nannobrachium regale | n | 19 | 23–134 | 171 | 1.053 | 1.029 | −0.59 | <0.001 |
| Melamphaes lugubris | y | 9 | 22–79 | 98 | 1.060 | 1.029 | −0.59 | 0.019 |
| Group III | ||||||||
| Scopelarchus stephensi | y | 3 | 25–55 | 62 | 1.076 | 1.080 | 0.33 | – |
| Scopelosaurus harryi | y | 5 | 43–52b | 266 | 1.063 | 1.060 | −0.24 | 0.333c |
| Arctozenus risso | n | 6 | 35–124b | 255 | 1.068 | 1.055 | −0.97 | 0.003 |
| Chauliodus macouni | n | 6 | 30–122 | 236 | 1.050 | 1.050 | −0.21 | 0.356 |
| Aristostomias xenostoma | n | 5 | 33–41b | 108 | 1.049 | 1.050 | 0.17 | 0.800c |
| Leuroglossus stilbius | y | 6 | 25–29b | 130 | 1.043 | 1.049 | 0.08 | 0. 600c |
| Photonectes parvimanus | y | 6 | 30–67b | 261f | 1.046 | 1.048 | 0.67 | 0.983c |
| Holtbyrnia latifrons | n | 6 | 20–52b | 200 | 1.051 | 1.048 | −0.26 | 0.350c |
| Sagamichthys abei | n | 5 | 27–67b | 239 | 1.050 | 1.048 | −0.27 | 0.400c |
| Alepocephalus tenebrosus | n | 4 | 27–50b | 448 | 1.045 | 1.046 | 0.18 | 0.750c |
| Bathophilus flemingi | y | 6 | 34–46b | 140 | 1.048 | 1.046 | 0.57 | 0.933c |
| Cyclothone acclinidens | n | 44 | 25–61 | 67 | 1.051 | 1.043 | −0.62 | <0.001 |
| Cyclothone pallida | n | 6 | 30–68 | 74 | 1.046 | 1.043 | 0.30 | 0.833c |
| Bathylagoides wesethi | y | 55 | 24–76 | 104 | 1.048 | 1.041 | −0.41 | <0.001 |
| Tactostoma macropus | y | 4 | 76–254 | 344 | 1.042 | 1.039 | −0.55 | 0.250c |
| Stomias atriventer | n | 4 | 139–187d | 243 | 1.041 | 1.038 | −0.55 | 0.250c |
| Parvilux ingens | n | 6 | 80–160d | 204 | 1.044 | 1.036 | −0.69 | 0.042 |
| Idiacanthus antrostomus | y | 21 | 58–385 | 372 | 1.049 | 1.036 | −0.68 | <0.001 |
| Lobianchia gemellarii | y | 3 | 55–65d | 77 | 1.043 | 1.035 | −1.00 | – |
| Lipolagus ochotensis | y | 12 | 25–110 | 119 | 1.047 | 1.034 | −0.29 | 0.123c |
| Species . | DVMa . | n . | LS range (mm) . | Max. LS (mm) . | 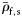 .
. | 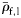 .
. | τ-value . | p-value . |
|---|---|---|---|---|---|---|---|---|
| Group I | ||||||||
| Tarletonbeania crenularis | y | 20 | 22–57 | 78 | 1.080 | 1.085 | 0.27 | 0.939 |
| Hygophum reinhardtii | y | 4 | 19–43 | 64 | 1.066 | 1.076 | 0.66 | 0.958 |
| Notoscopelus resplendens | y | 5 | 27–42b | 98 | 1.073 | 1.076 | 0.84 | 1.000 |
| Diplospinus multistriatus | y | 6 | 18–55b | 225 | 1.073 | 1.074 | 0.00 | 0.572 |
| Diogenichthys atlanticus | y | 25 | 16–23 | 30 | 1.064 | 1.073 | 0.55 | 1.000 |
| Myctophum nitidulum | y | 17 | 18–78 | 105 | 1.074 | 1.073 | −0.16 | 0.215 |
| Electrona risso | y | 3 | 11–32b | 81 | 1.071 | 1.073 | 0.82 | – |
| Hygophum proximum | y | 5 | 16–32 | 61 | 1.068 | 1.073 | 0.44 | 0.900 |
| Protomyctophum crockeri | n | 26 | 15–37 | 55 | 1.070 | 1.073 | 0.21 | 0.920 |
| Nannobrachium fernae | y | 5 | 32–63 | 81 | 1.069 | 1.073 | −0.20 | 0.408 |
| Lampadena urophaos | y | 12 | 19–26b | 115 | 1.060 | 1.073 | 0.34 | 0.925c |
| Bolinichthys longipes | y | 4 | 38–42d | 59 | 1.071 | 1.070 | −0.40 | 0.333c |
| Diaphus anderseni | y | 13 | 25–30 | 55 | 1.070 | 1·070 | 0.20 | 0.819c |
| Ceratoscopelus warmingii | y | 18 | 19–48 | 81 | 1.064 | 1·070 | 0.51 | 0.997 |
| Vinciguerria nimbaria | y | 9 | 17–32 | 53 | 1.067 | 1.069 | −0.21 | 0.269 |
| Vinciguerria poweriae | y | 15 | 18–38 | 37 | 1.069 | 1.069 | 0.04 | 0.601 |
| Diaphus fulgens | y | 7 | 36–52d | 58 | 1.073 | 1.068 | −0.69 | 0.029 |
| Lampanyctus tenuiformis | y | 13 | 28–43b | 153 | 1.067 | 1.067 | 0.13 | 0.737 |
| Microstoma microstoma | y | 6 | 20–55b | 210e | 1.067 | 1.067 | 0.17 | 0.700 |
| Danaphos oculatus | n | 23 | 22–41 | 46 | 1.062 | 1.066 | 0.49 | 0.999 |
| Symbolophorus californiensis | y | 20 | 25–88 | 116 | 1.072 | 1.064 | −0.26 | 0.071 |
| Chilara taylori | n | 3 | 35–55b | 366 | 1·060 | 1.064 | 0.00 | – |
| Taaningichthys bathyphilus | n | 3 | 42–66d | 85 | 1·060 | 1.063 | 0.82 | – |
| Argyropelecus lychnus | n | 3 | 13–37b | 78 | 1·068 | 1.063 | −0.82 | – |
| Argyropelecus sladeni | n | 26 | 12–41 | 60 | 1.062 | 1.062 | 0.09 | 0.727 |
| Argyropelecus hemigymnus | n | 17 | 15–30 | 38 | 1.064 | 1.062 | −0.25 | 0.105 |
| Argyropelecus affinis | n | 17 | 14–76 | 88 | 1.055 | 1.059 | 0.04 | 0.604 |
| Bathysphyraenops simplex | y | 7 | 17–47 | 80e | 1.075 | 1.058 | −0.13 | 0.429c |
| Cyclothone pseudopallida | n | 12 | 24–43 | 49 | 1.055 | 1.056 | −0.08 | 0.387 |
| Melamphaes simus | y | 5 | 15–29 | 29 | 1.069 | 1.055 | −0.89 | 0.033 |
| Sternoptyx obscura | n | 7 | 12–41 | 48 | 1.054 | 1.053 | 0.28 | 0.810 |
| Sternoptyx diaphana | n | 6 | 14–35 | 60 | 1.053 | 1.053 | 0.00 | 0.572 |
| Cyclothone signata | n | 36 | 15–35 | 39 | 1.058 | 1.053 | −0.21 | 0.051 |
| Sternoptyx pseudobscura | n | 8 | 17–44 | 61 | 1.052 | 1.049 | 0.12 | 0.696 |
| Diaphus theta | y | 32 | 14–73 | 86 | 1.059 | 1.049 | −0.38 | 0.002 |
| Triphoturus nigrescens | y | 4 | 32–41d | 45 | 1.064 | 1.043 | −0.91 | 0.083c |
| Scopeloberyx opisthopterus | n | 3 | 22–33 | 39 | 1.053 | 1.041 | −1.00 | – |
| Notolychnus valdiviae | y | 8 | 13–25 | 29 | 1.049 | 1.040 | −0.22 | 0.287c |
| Melamphaes suborbitalis | n | 3 | 24–68 | 119 | 1.069 | 1.040 | −0.33 | – |
| Ichthyococcus irregularis | n | 3 | 24–36 | 63 | 1.048 | 1.038 | −1.00 | – |
| Group II | ||||||||
| Melamphaes parvus | y | 4 | 21–45 | 54 | 1.071 | 1.055 | −0.33 | 0.375c |
| Cyclothone atraria | n | 10 | 21–47 | 70 | 1.051 | 1.048 | −0.33 | 0.117 |
| Nannobrachium hawaiiensis | y | 22 | 24–92 | 111 | 1.052 | 1.046 | −0.35 | 0.017 |
| Ceratoscopelus townsendi | y | 43 | 21–60 | 77 | 1.063 | 1.045 | −0.49 | <0.001 |
| Scopelogadus mizolepis | y | 19 | 25–83 | 97 | 1.052 | 1.044 | −0.72 | <0.001 |
| Poromitra crassiceps | n | 14 | 20–60b | 204 | 1.051 | 1.044 | −0.54 | 0.006 |
| Nannobrachium ritteri | y | 56 | 19–94 | 124 | 1.044 | 1.032 | −0.80 | <0.001 |
| Triphoturus mexicanus | y | 52 | 17–68 | 75 | 1.040 | 1.031 | −0.75 | <0.001 |
| Stenobrachius leucopsarus | y | 57 | 20–83 | 105 | 1.035 | 1.029 | −0.74 | <0.001 |
| Nannobrachium regale | n | 19 | 23–134 | 171 | 1.053 | 1.029 | −0.59 | <0.001 |
| Melamphaes lugubris | y | 9 | 22–79 | 98 | 1.060 | 1.029 | −0.59 | 0.019 |
| Group III | ||||||||
| Scopelarchus stephensi | y | 3 | 25–55 | 62 | 1.076 | 1.080 | 0.33 | – |
| Scopelosaurus harryi | y | 5 | 43–52b | 266 | 1.063 | 1.060 | −0.24 | 0.333c |
| Arctozenus risso | n | 6 | 35–124b | 255 | 1.068 | 1.055 | −0.97 | 0.003 |
| Chauliodus macouni | n | 6 | 30–122 | 236 | 1.050 | 1.050 | −0.21 | 0.356 |
| Aristostomias xenostoma | n | 5 | 33–41b | 108 | 1.049 | 1.050 | 0.17 | 0.800c |
| Leuroglossus stilbius | y | 6 | 25–29b | 130 | 1.043 | 1.049 | 0.08 | 0. 600c |
| Photonectes parvimanus | y | 6 | 30–67b | 261f | 1.046 | 1.048 | 0.67 | 0.983c |
| Holtbyrnia latifrons | n | 6 | 20–52b | 200 | 1.051 | 1.048 | −0.26 | 0.350c |
| Sagamichthys abei | n | 5 | 27–67b | 239 | 1.050 | 1.048 | −0.27 | 0.400c |
| Alepocephalus tenebrosus | n | 4 | 27–50b | 448 | 1.045 | 1.046 | 0.18 | 0.750c |
| Bathophilus flemingi | y | 6 | 34–46b | 140 | 1.048 | 1.046 | 0.57 | 0.933c |
| Cyclothone acclinidens | n | 44 | 25–61 | 67 | 1.051 | 1.043 | −0.62 | <0.001 |
| Cyclothone pallida | n | 6 | 30–68 | 74 | 1.046 | 1.043 | 0.30 | 0.833c |
| Bathylagoides wesethi | y | 55 | 24–76 | 104 | 1.048 | 1.041 | −0.41 | <0.001 |
| Tactostoma macropus | y | 4 | 76–254 | 344 | 1.042 | 1.039 | −0.55 | 0.250c |
| Stomias atriventer | n | 4 | 139–187d | 243 | 1.041 | 1.038 | −0.55 | 0.250c |
| Parvilux ingens | n | 6 | 80–160d | 204 | 1.044 | 1.036 | −0.69 | 0.042 |
| Idiacanthus antrostomus | y | 21 | 58–385 | 372 | 1.049 | 1.036 | −0.68 | <0.001 |
| Lobianchia gemellarii | y | 3 | 55–65d | 77 | 1.043 | 1.035 | −1.00 | – |
| Lipolagus ochotensis | y | 12 | 25–110 | 119 | 1.047 | 1.034 | −0.29 | 0.123c |
Group I species have at least some small and large individuals with inflated swimbladders. Group II species contain gas in at least some small fish, but not in large individuals. Group III fish never contain gas. Maximum standard length, LS, is taken from the SIO MVC. 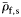 and
and 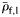 are the mean specific gravities of fish with normalized wet weight,
are the mean specific gravities of fish with normalized wet weight, 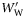 , <0.5 and
, <0.5 and 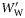 ≥0.5, respectively. Species are ordered within groups by decreasing
≥0.5, respectively. Species are ordered within groups by decreasing 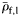 . Kendall's coefficient (τ) tests for association between LS and decreasing ρf (one-tailed).
. Kendall's coefficient (τ) tests for association between LS and decreasing ρf (one-tailed).
ay, yes; n, no.
bNo full-sized (>50% of maximum LS) fish were captured.
cSmall n and clusters of fish in a limited size range may have influenced the result.
dNo small individuals were captured.
eMaximum LS was taken from sources other than the SIO MVC (Cohen, 1986; Kubota et al., 1991).
fMaximum LS from material examined: Australian Museum AMS I.20315023.
Assignment of species to groups based on the presence or the absence of gas in their swimbladders.
| Species . | DVMa . | n . | LS range (mm) . | Max. LS (mm) . | 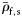 .
. | 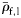 .
. | τ-value . | p-value . |
|---|---|---|---|---|---|---|---|---|
| Group I | ||||||||
| Tarletonbeania crenularis | y | 20 | 22–57 | 78 | 1.080 | 1.085 | 0.27 | 0.939 |
| Hygophum reinhardtii | y | 4 | 19–43 | 64 | 1.066 | 1.076 | 0.66 | 0.958 |
| Notoscopelus resplendens | y | 5 | 27–42b | 98 | 1.073 | 1.076 | 0.84 | 1.000 |
| Diplospinus multistriatus | y | 6 | 18–55b | 225 | 1.073 | 1.074 | 0.00 | 0.572 |
| Diogenichthys atlanticus | y | 25 | 16–23 | 30 | 1.064 | 1.073 | 0.55 | 1.000 |
| Myctophum nitidulum | y | 17 | 18–78 | 105 | 1.074 | 1.073 | −0.16 | 0.215 |
| Electrona risso | y | 3 | 11–32b | 81 | 1.071 | 1.073 | 0.82 | – |
| Hygophum proximum | y | 5 | 16–32 | 61 | 1.068 | 1.073 | 0.44 | 0.900 |
| Protomyctophum crockeri | n | 26 | 15–37 | 55 | 1.070 | 1.073 | 0.21 | 0.920 |
| Nannobrachium fernae | y | 5 | 32–63 | 81 | 1.069 | 1.073 | −0.20 | 0.408 |
| Lampadena urophaos | y | 12 | 19–26b | 115 | 1.060 | 1.073 | 0.34 | 0.925c |
| Bolinichthys longipes | y | 4 | 38–42d | 59 | 1.071 | 1.070 | −0.40 | 0.333c |
| Diaphus anderseni | y | 13 | 25–30 | 55 | 1.070 | 1·070 | 0.20 | 0.819c |
| Ceratoscopelus warmingii | y | 18 | 19–48 | 81 | 1.064 | 1·070 | 0.51 | 0.997 |
| Vinciguerria nimbaria | y | 9 | 17–32 | 53 | 1.067 | 1.069 | −0.21 | 0.269 |
| Vinciguerria poweriae | y | 15 | 18–38 | 37 | 1.069 | 1.069 | 0.04 | 0.601 |
| Diaphus fulgens | y | 7 | 36–52d | 58 | 1.073 | 1.068 | −0.69 | 0.029 |
| Lampanyctus tenuiformis | y | 13 | 28–43b | 153 | 1.067 | 1.067 | 0.13 | 0.737 |
| Microstoma microstoma | y | 6 | 20–55b | 210e | 1.067 | 1.067 | 0.17 | 0.700 |
| Danaphos oculatus | n | 23 | 22–41 | 46 | 1.062 | 1.066 | 0.49 | 0.999 |
| Symbolophorus californiensis | y | 20 | 25–88 | 116 | 1.072 | 1.064 | −0.26 | 0.071 |
| Chilara taylori | n | 3 | 35–55b | 366 | 1·060 | 1.064 | 0.00 | – |
| Taaningichthys bathyphilus | n | 3 | 42–66d | 85 | 1·060 | 1.063 | 0.82 | – |
| Argyropelecus lychnus | n | 3 | 13–37b | 78 | 1·068 | 1.063 | −0.82 | – |
| Argyropelecus sladeni | n | 26 | 12–41 | 60 | 1.062 | 1.062 | 0.09 | 0.727 |
| Argyropelecus hemigymnus | n | 17 | 15–30 | 38 | 1.064 | 1.062 | −0.25 | 0.105 |
| Argyropelecus affinis | n | 17 | 14–76 | 88 | 1.055 | 1.059 | 0.04 | 0.604 |
| Bathysphyraenops simplex | y | 7 | 17–47 | 80e | 1.075 | 1.058 | −0.13 | 0.429c |
| Cyclothone pseudopallida | n | 12 | 24–43 | 49 | 1.055 | 1.056 | −0.08 | 0.387 |
| Melamphaes simus | y | 5 | 15–29 | 29 | 1.069 | 1.055 | −0.89 | 0.033 |
| Sternoptyx obscura | n | 7 | 12–41 | 48 | 1.054 | 1.053 | 0.28 | 0.810 |
| Sternoptyx diaphana | n | 6 | 14–35 | 60 | 1.053 | 1.053 | 0.00 | 0.572 |
| Cyclothone signata | n | 36 | 15–35 | 39 | 1.058 | 1.053 | −0.21 | 0.051 |
| Sternoptyx pseudobscura | n | 8 | 17–44 | 61 | 1.052 | 1.049 | 0.12 | 0.696 |
| Diaphus theta | y | 32 | 14–73 | 86 | 1.059 | 1.049 | −0.38 | 0.002 |
| Triphoturus nigrescens | y | 4 | 32–41d | 45 | 1.064 | 1.043 | −0.91 | 0.083c |
| Scopeloberyx opisthopterus | n | 3 | 22–33 | 39 | 1.053 | 1.041 | −1.00 | – |
| Notolychnus valdiviae | y | 8 | 13–25 | 29 | 1.049 | 1.040 | −0.22 | 0.287c |
| Melamphaes suborbitalis | n | 3 | 24–68 | 119 | 1.069 | 1.040 | −0.33 | – |
| Ichthyococcus irregularis | n | 3 | 24–36 | 63 | 1.048 | 1.038 | −1.00 | – |
| Group II | ||||||||
| Melamphaes parvus | y | 4 | 21–45 | 54 | 1.071 | 1.055 | −0.33 | 0.375c |
| Cyclothone atraria | n | 10 | 21–47 | 70 | 1.051 | 1.048 | −0.33 | 0.117 |
| Nannobrachium hawaiiensis | y | 22 | 24–92 | 111 | 1.052 | 1.046 | −0.35 | 0.017 |
| Ceratoscopelus townsendi | y | 43 | 21–60 | 77 | 1.063 | 1.045 | −0.49 | <0.001 |
| Scopelogadus mizolepis | y | 19 | 25–83 | 97 | 1.052 | 1.044 | −0.72 | <0.001 |
| Poromitra crassiceps | n | 14 | 20–60b | 204 | 1.051 | 1.044 | −0.54 | 0.006 |
| Nannobrachium ritteri | y | 56 | 19–94 | 124 | 1.044 | 1.032 | −0.80 | <0.001 |
| Triphoturus mexicanus | y | 52 | 17–68 | 75 | 1.040 | 1.031 | −0.75 | <0.001 |
| Stenobrachius leucopsarus | y | 57 | 20–83 | 105 | 1.035 | 1.029 | −0.74 | <0.001 |
| Nannobrachium regale | n | 19 | 23–134 | 171 | 1.053 | 1.029 | −0.59 | <0.001 |
| Melamphaes lugubris | y | 9 | 22–79 | 98 | 1.060 | 1.029 | −0.59 | 0.019 |
| Group III | ||||||||
| Scopelarchus stephensi | y | 3 | 25–55 | 62 | 1.076 | 1.080 | 0.33 | – |
| Scopelosaurus harryi | y | 5 | 43–52b | 266 | 1.063 | 1.060 | −0.24 | 0.333c |
| Arctozenus risso | n | 6 | 35–124b | 255 | 1.068 | 1.055 | −0.97 | 0.003 |
| Chauliodus macouni | n | 6 | 30–122 | 236 | 1.050 | 1.050 | −0.21 | 0.356 |
| Aristostomias xenostoma | n | 5 | 33–41b | 108 | 1.049 | 1.050 | 0.17 | 0.800c |
| Leuroglossus stilbius | y | 6 | 25–29b | 130 | 1.043 | 1.049 | 0.08 | 0. 600c |
| Photonectes parvimanus | y | 6 | 30–67b | 261f | 1.046 | 1.048 | 0.67 | 0.983c |
| Holtbyrnia latifrons | n | 6 | 20–52b | 200 | 1.051 | 1.048 | −0.26 | 0.350c |
| Sagamichthys abei | n | 5 | 27–67b | 239 | 1.050 | 1.048 | −0.27 | 0.400c |
| Alepocephalus tenebrosus | n | 4 | 27–50b | 448 | 1.045 | 1.046 | 0.18 | 0.750c |
| Bathophilus flemingi | y | 6 | 34–46b | 140 | 1.048 | 1.046 | 0.57 | 0.933c |
| Cyclothone acclinidens | n | 44 | 25–61 | 67 | 1.051 | 1.043 | −0.62 | <0.001 |
| Cyclothone pallida | n | 6 | 30–68 | 74 | 1.046 | 1.043 | 0.30 | 0.833c |
| Bathylagoides wesethi | y | 55 | 24–76 | 104 | 1.048 | 1.041 | −0.41 | <0.001 |
| Tactostoma macropus | y | 4 | 76–254 | 344 | 1.042 | 1.039 | −0.55 | 0.250c |
| Stomias atriventer | n | 4 | 139–187d | 243 | 1.041 | 1.038 | −0.55 | 0.250c |
| Parvilux ingens | n | 6 | 80–160d | 204 | 1.044 | 1.036 | −0.69 | 0.042 |
| Idiacanthus antrostomus | y | 21 | 58–385 | 372 | 1.049 | 1.036 | −0.68 | <0.001 |
| Lobianchia gemellarii | y | 3 | 55–65d | 77 | 1.043 | 1.035 | −1.00 | – |
| Lipolagus ochotensis | y | 12 | 25–110 | 119 | 1.047 | 1.034 | −0.29 | 0.123c |
| Species . | DVMa . | n . | LS range (mm) . | Max. LS (mm) . | 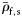 .
. | 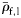 .
. | τ-value . | p-value . |
|---|---|---|---|---|---|---|---|---|
| Group I | ||||||||
| Tarletonbeania crenularis | y | 20 | 22–57 | 78 | 1.080 | 1.085 | 0.27 | 0.939 |
| Hygophum reinhardtii | y | 4 | 19–43 | 64 | 1.066 | 1.076 | 0.66 | 0.958 |
| Notoscopelus resplendens | y | 5 | 27–42b | 98 | 1.073 | 1.076 | 0.84 | 1.000 |
| Diplospinus multistriatus | y | 6 | 18–55b | 225 | 1.073 | 1.074 | 0.00 | 0.572 |
| Diogenichthys atlanticus | y | 25 | 16–23 | 30 | 1.064 | 1.073 | 0.55 | 1.000 |
| Myctophum nitidulum | y | 17 | 18–78 | 105 | 1.074 | 1.073 | −0.16 | 0.215 |
| Electrona risso | y | 3 | 11–32b | 81 | 1.071 | 1.073 | 0.82 | – |
| Hygophum proximum | y | 5 | 16–32 | 61 | 1.068 | 1.073 | 0.44 | 0.900 |
| Protomyctophum crockeri | n | 26 | 15–37 | 55 | 1.070 | 1.073 | 0.21 | 0.920 |
| Nannobrachium fernae | y | 5 | 32–63 | 81 | 1.069 | 1.073 | −0.20 | 0.408 |
| Lampadena urophaos | y | 12 | 19–26b | 115 | 1.060 | 1.073 | 0.34 | 0.925c |
| Bolinichthys longipes | y | 4 | 38–42d | 59 | 1.071 | 1.070 | −0.40 | 0.333c |
| Diaphus anderseni | y | 13 | 25–30 | 55 | 1.070 | 1·070 | 0.20 | 0.819c |
| Ceratoscopelus warmingii | y | 18 | 19–48 | 81 | 1.064 | 1·070 | 0.51 | 0.997 |
| Vinciguerria nimbaria | y | 9 | 17–32 | 53 | 1.067 | 1.069 | −0.21 | 0.269 |
| Vinciguerria poweriae | y | 15 | 18–38 | 37 | 1.069 | 1.069 | 0.04 | 0.601 |
| Diaphus fulgens | y | 7 | 36–52d | 58 | 1.073 | 1.068 | −0.69 | 0.029 |
| Lampanyctus tenuiformis | y | 13 | 28–43b | 153 | 1.067 | 1.067 | 0.13 | 0.737 |
| Microstoma microstoma | y | 6 | 20–55b | 210e | 1.067 | 1.067 | 0.17 | 0.700 |
| Danaphos oculatus | n | 23 | 22–41 | 46 | 1.062 | 1.066 | 0.49 | 0.999 |
| Symbolophorus californiensis | y | 20 | 25–88 | 116 | 1.072 | 1.064 | −0.26 | 0.071 |
| Chilara taylori | n | 3 | 35–55b | 366 | 1·060 | 1.064 | 0.00 | – |
| Taaningichthys bathyphilus | n | 3 | 42–66d | 85 | 1·060 | 1.063 | 0.82 | – |
| Argyropelecus lychnus | n | 3 | 13–37b | 78 | 1·068 | 1.063 | −0.82 | – |
| Argyropelecus sladeni | n | 26 | 12–41 | 60 | 1.062 | 1.062 | 0.09 | 0.727 |
| Argyropelecus hemigymnus | n | 17 | 15–30 | 38 | 1.064 | 1.062 | −0.25 | 0.105 |
| Argyropelecus affinis | n | 17 | 14–76 | 88 | 1.055 | 1.059 | 0.04 | 0.604 |
| Bathysphyraenops simplex | y | 7 | 17–47 | 80e | 1.075 | 1.058 | −0.13 | 0.429c |
| Cyclothone pseudopallida | n | 12 | 24–43 | 49 | 1.055 | 1.056 | −0.08 | 0.387 |
| Melamphaes simus | y | 5 | 15–29 | 29 | 1.069 | 1.055 | −0.89 | 0.033 |
| Sternoptyx obscura | n | 7 | 12–41 | 48 | 1.054 | 1.053 | 0.28 | 0.810 |
| Sternoptyx diaphana | n | 6 | 14–35 | 60 | 1.053 | 1.053 | 0.00 | 0.572 |
| Cyclothone signata | n | 36 | 15–35 | 39 | 1.058 | 1.053 | −0.21 | 0.051 |
| Sternoptyx pseudobscura | n | 8 | 17–44 | 61 | 1.052 | 1.049 | 0.12 | 0.696 |
| Diaphus theta | y | 32 | 14–73 | 86 | 1.059 | 1.049 | −0.38 | 0.002 |
| Triphoturus nigrescens | y | 4 | 32–41d | 45 | 1.064 | 1.043 | −0.91 | 0.083c |
| Scopeloberyx opisthopterus | n | 3 | 22–33 | 39 | 1.053 | 1.041 | −1.00 | – |
| Notolychnus valdiviae | y | 8 | 13–25 | 29 | 1.049 | 1.040 | −0.22 | 0.287c |
| Melamphaes suborbitalis | n | 3 | 24–68 | 119 | 1.069 | 1.040 | −0.33 | – |
| Ichthyococcus irregularis | n | 3 | 24–36 | 63 | 1.048 | 1.038 | −1.00 | – |
| Group II | ||||||||
| Melamphaes parvus | y | 4 | 21–45 | 54 | 1.071 | 1.055 | −0.33 | 0.375c |
| Cyclothone atraria | n | 10 | 21–47 | 70 | 1.051 | 1.048 | −0.33 | 0.117 |
| Nannobrachium hawaiiensis | y | 22 | 24–92 | 111 | 1.052 | 1.046 | −0.35 | 0.017 |
| Ceratoscopelus townsendi | y | 43 | 21–60 | 77 | 1.063 | 1.045 | −0.49 | <0.001 |
| Scopelogadus mizolepis | y | 19 | 25–83 | 97 | 1.052 | 1.044 | −0.72 | <0.001 |
| Poromitra crassiceps | n | 14 | 20–60b | 204 | 1.051 | 1.044 | −0.54 | 0.006 |
| Nannobrachium ritteri | y | 56 | 19–94 | 124 | 1.044 | 1.032 | −0.80 | <0.001 |
| Triphoturus mexicanus | y | 52 | 17–68 | 75 | 1.040 | 1.031 | −0.75 | <0.001 |
| Stenobrachius leucopsarus | y | 57 | 20–83 | 105 | 1.035 | 1.029 | −0.74 | <0.001 |
| Nannobrachium regale | n | 19 | 23–134 | 171 | 1.053 | 1.029 | −0.59 | <0.001 |
| Melamphaes lugubris | y | 9 | 22–79 | 98 | 1.060 | 1.029 | −0.59 | 0.019 |
| Group III | ||||||||
| Scopelarchus stephensi | y | 3 | 25–55 | 62 | 1.076 | 1.080 | 0.33 | – |
| Scopelosaurus harryi | y | 5 | 43–52b | 266 | 1.063 | 1.060 | −0.24 | 0.333c |
| Arctozenus risso | n | 6 | 35–124b | 255 | 1.068 | 1.055 | −0.97 | 0.003 |
| Chauliodus macouni | n | 6 | 30–122 | 236 | 1.050 | 1.050 | −0.21 | 0.356 |
| Aristostomias xenostoma | n | 5 | 33–41b | 108 | 1.049 | 1.050 | 0.17 | 0.800c |
| Leuroglossus stilbius | y | 6 | 25–29b | 130 | 1.043 | 1.049 | 0.08 | 0. 600c |
| Photonectes parvimanus | y | 6 | 30–67b | 261f | 1.046 | 1.048 | 0.67 | 0.983c |
| Holtbyrnia latifrons | n | 6 | 20–52b | 200 | 1.051 | 1.048 | −0.26 | 0.350c |
| Sagamichthys abei | n | 5 | 27–67b | 239 | 1.050 | 1.048 | −0.27 | 0.400c |
| Alepocephalus tenebrosus | n | 4 | 27–50b | 448 | 1.045 | 1.046 | 0.18 | 0.750c |
| Bathophilus flemingi | y | 6 | 34–46b | 140 | 1.048 | 1.046 | 0.57 | 0.933c |
| Cyclothone acclinidens | n | 44 | 25–61 | 67 | 1.051 | 1.043 | −0.62 | <0.001 |
| Cyclothone pallida | n | 6 | 30–68 | 74 | 1.046 | 1.043 | 0.30 | 0.833c |
| Bathylagoides wesethi | y | 55 | 24–76 | 104 | 1.048 | 1.041 | −0.41 | <0.001 |
| Tactostoma macropus | y | 4 | 76–254 | 344 | 1.042 | 1.039 | −0.55 | 0.250c |
| Stomias atriventer | n | 4 | 139–187d | 243 | 1.041 | 1.038 | −0.55 | 0.250c |
| Parvilux ingens | n | 6 | 80–160d | 204 | 1.044 | 1.036 | −0.69 | 0.042 |
| Idiacanthus antrostomus | y | 21 | 58–385 | 372 | 1.049 | 1.036 | −0.68 | <0.001 |
| Lobianchia gemellarii | y | 3 | 55–65d | 77 | 1.043 | 1.035 | −1.00 | – |
| Lipolagus ochotensis | y | 12 | 25–110 | 119 | 1.047 | 1.034 | −0.29 | 0.123c |
Group I species have at least some small and large individuals with inflated swimbladders. Group II species contain gas in at least some small fish, but not in large individuals. Group III fish never contain gas. Maximum standard length, LS, is taken from the SIO MVC. 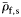 and
and 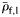 are the mean specific gravities of fish with normalized wet weight,
are the mean specific gravities of fish with normalized wet weight, 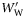 , <0.5 and
, <0.5 and 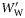 ≥0.5, respectively. Species are ordered within groups by decreasing
≥0.5, respectively. Species are ordered within groups by decreasing 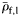 . Kendall's coefficient (τ) tests for association between LS and decreasing ρf (one-tailed).
. Kendall's coefficient (τ) tests for association between LS and decreasing ρf (one-tailed).
ay, yes; n, no.
bNo full-sized (>50% of maximum LS) fish were captured.
cSmall n and clusters of fish in a limited size range may have influenced the result.
dNo small individuals were captured.
eMaximum LS was taken from sources other than the SIO MVC (Cohen, 1986; Kubota et al., 1991).
fMaximum LS from material examined: Australian Museum AMS I.20315023.
Acoustic modelling
Neutral buoyancy
Results
Measurements and group assignments
In all, 71 species from 16 families were represented by three or more individuals (Supplementary Table S2). Family Myctophidae was by far the most speciose, being represented here by 28 species. Three epipelagic fish were incidentally captured from two species, Seriola lalandi and Cololabis saira. These fish (ρf = 1.078, 1.078, and 1.088; Supplementary Table S3) were not included in the analysis, except in comparison with Group I.
Group assignment, n, LS range, maximum LS, vertical migration behaviour, ρf, and τ for decreasing ρf with increasing LS are summarized in Table 1 for each of the species analysed. Measurements of ρf and the gas volume for all fish are listed in Supplementary Table S3. Fish belonging to each of the three groups were collected from both biogeographic provinces (Supplementary Table S2). Biogeographic province may be related to group assignment, although the relationship is not significant (Chi-squared test of contingency table, d.f. = 2, p = 0.07). Diel vertical migrators and non-migrators are found in each of the three groups. DVM is not significantly related to group assignment (Chi-squared test of contingency table, d.f. = 2, p = 0.46). No full-sized specimens were captured from several species, as determined by comparison with the maximum LS of fish in the SIO MVC. These species were allocated to group as described herewith. Species from the Stomiidae, Paralepididae, Alepocephalidae, Notosudidae, Platytroctidae, and Bathylagidae were assigned to Group III, because fish from those families do not have functional swimbladders as adults (Marshall, 1960). Argyropelecus lychnus was assigned to Group I because of the presence of a gas-filled swimbladder at the family level (Marshall, 1960). Notoscopelus resplendens, Lampadena urophaos, Electrona risso, Chilara taylori, and Microstoma microstoma were placed in Group I based on literature reports of the presence of gas in the swimbladders of adults (references in Supplementary Table S4). Diplospinus multistriatus and Lampanyctus tenuiformis were placed in Group I based on the presence of large, thin-walled swimbladders found in dissected specimens from the SIO MVC. No large Poromitra crassiceps were captured. This species was assigned to Group II based on published data indicating that gas is not used for buoyancy (references in Supplementary Table S4).
The specific gravities of species (means of large fish, 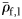 ) from each group were compared. Groups II and III did not have significantly different mean
) from each group were compared. Groups II and III did not have significantly different mean 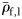 (Mann–Whitney rank-sum test, p = 0.094), so were combined for comparison of mean
(Mann–Whitney rank-sum test, p = 0.094), so were combined for comparison of mean 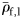 to Group I (Table 2). Group I has significantly higher mean
to Group I (Table 2). Group I has significantly higher mean 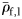 (t-test, 69 d.f., p < 0.001) than the combined Groups II and III. Group I has significantly lower mean
(t-test, 69 d.f., p < 0.001) than the combined Groups II and III. Group I has significantly lower mean 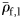 (Mann–Whitney rank-sum test, p = 0.027) than the mean
(Mann–Whitney rank-sum test, p = 0.027) than the mean 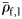 of epipelagic fish.
of epipelagic fish.
The specific gravity of species from each group (means of large fish, ρ̄f,1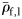 ).
).
| Fish group . | n . | Mean 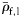 .
. | s.d. . |
|---|---|---|---|
| I | 40 | 1.062 | 0.012 |
| II | 11 | 1.039 | 0.010 |
| III | 20 | 1.046 | 0.011 |
| II + III | 31 | 1.044 | 0.011 |
| Fish group . | n . | Mean 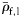 .
. | s.d. . |
|---|---|---|---|
| I | 40 | 1.062 | 0.012 |
| II | 11 | 1.039 | 0.010 |
| III | 20 | 1.046 | 0.011 |
| II + III | 31 | 1.044 | 0.011 |
The specific gravity of species from each group (means of large fish, ρ̄f,1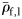 ).
).
| Fish group . | n . | Mean 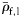 .
. | s.d. . |
|---|---|---|---|
| I | 40 | 1.062 | 0.012 |
| II | 11 | 1.039 | 0.010 |
| III | 20 | 1.046 | 0.011 |
| II + III | 31 | 1.044 | 0.011 |
| Fish group . | n . | Mean 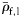 .
. | s.d. . |
|---|---|---|---|
| I | 40 | 1.062 | 0.012 |
| II | 11 | 1.039 | 0.010 |
| III | 20 | 1.046 | 0.011 |
| II + III | 31 | 1.044 | 0.011 |
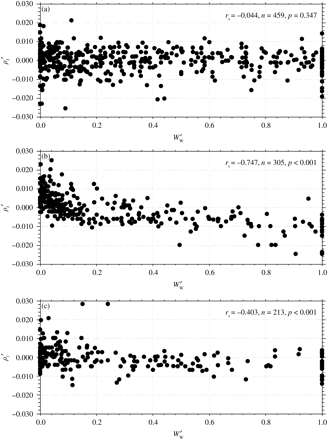
Group I fish do not change in ρf with increasing 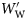 (Figure 1a; rs = −0.044, n = 459, p = 0.347). Group II fish decrease in ρf with increasing
(Figure 1a; rs = −0.044, n = 459, p = 0.347). Group II fish decrease in ρf with increasing 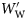 (Figure 1b; rs = −0.747, n = 305, p < 0.001), as do Group III fish (Figure 1c; rs = −0.403, n = 213, p < 0.001).
(Figure 1b; rs = −0.747, n = 305, p < 0.001), as do Group III fish (Figure 1c; rs = −0.403, n = 213, p < 0.001).
The relationship between standardized specific gravity, ρ′, and standardized wet weight, 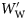 : (a) Group I, (b) Group II, and (c) Group III fish.
: (a) Group I, (b) Group II, and (c) Group III fish.
Group I was the most speciose category (40 species; Table 1) followed by Groups III and II (20 and 11 species, respectively; Table 1). Of the 33 species in Group I with n > 3, 30 species exhibited no significant relationship between ρf and LS. In Group II, 9 of the 11 species had a significant decline in ρf. The results from Group III were less clear, with just five of 18 species (with n > 3) exhibiting a significant decline in ρf. However, the declining species included all of those with n > 12, and those species with no significant decline included eight for which no large individuals were captured.
Acoustic modelling
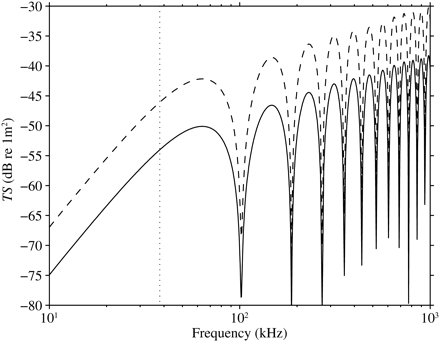
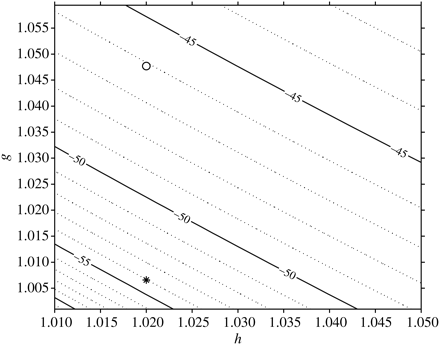
The fluid cylinder model was used to estimate σbs for an elongate dragonfish from Group III, I. antrostomus. The frequency response of σbs was modelled for both the body density of a typical epipelagic fish (1.076 g ml−1) and the measured ρf (1.034 ≈ 1.034 g ml−1) for this fish. The 38-kHz σbs of this fish differs by a factor of six between these two densities (Figure 2). At this frequency, the backscattering is in the Rayleigh region, because the fish is small compared with the acoustic wavelength, and there are no nearby resonant effects that may influence the result. The σbs (38 kHz) of this fish was modelled for reasonable variation in body density and sound-speed ratio, h (Figure 3). Body density varied between 1.028 and 1.088 g ml−1 and is expressed as the ratio, g, to the density of seawater (1.027 g ml−1). Over this range of g and h, σbs increases by a factor of 84 from the minimum (TS = −61.5 dB re 1 m2 at low g and h) to the maximum (TS = −42.3 dB re 1 m2 at high g and h).
Modelled TS as a function of acoustic frequency for a 385 mm, 25.89 g dragonfish (I. antrostomus) from Group III. Solid line, measured ρf of 1.034 (≈1.034 g ml−1); dashed line, body density 1.076 g ml−1, typical for an epipelagic fish; 38 kHz is marked with a vertical dotted line.
Contour plot of modelled 38 kHz TS as a function of the ratio of body density to seawater density, g, and the ratio of sound speed in the fish to sound speed in seawater, h, for a 385 mm, 25.89 g dragonfish (I. antrostomus) from Group III: asterisk, measured ρf of 1.034 (≈1.034 g ml−1); open circle, body density 1.076 g ml−1, typical for an epipelagic fish.
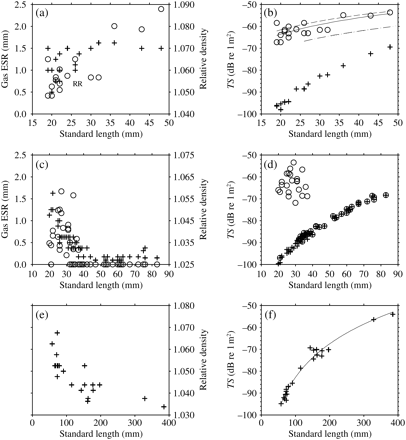
The 38-kHz σbs was modelled for each individual fish from the representative species C. warmingii, S. leucopsarus, and I. antrostomus (expressed as TS; Figure 4). Gas ESR and ρf for each of these three species are also shown in Figure 4. The Group I species, C. warmingii, exhibits increasing total σbs with growth. Total σbs varies by about an order of magnitude between the smallest and largest fish. The contribution of the body to the overall σbs is ∼0.1% for the smallest fish, increasing to 2.7% for the largest fish. When the x-axis of Figure 4b is log-transformed, the relationship between TS and LS becomes linear. A regression of TS against log10(LS) yields a slope of 24.90 and an intercept of −71.07 (LS in cm, d.f. = 15, p < 0.01, r2 = 0.61; Figure 4b). Use of VG (Figure 5), rather than the observed gas volume for the C. warmingii TS regression, results in a slope and intercept of 22.30 and −68.26, respectively, with an r2 of 0.97 (d.f. = 17, p < 0.01; Figure 4b). The Group II species, S. leucopsarus, shows dramatic changes in σbs with growth. σbs is high for small fish with gas in their swimbladder, drops more than two orders of magnitude for medium-sized fish, no gas present, and reapproaches the juvenile value in the longest fish. The Group III species, I. antrostomus, has a steep increase in σbs from small to moderate lengths, and a lower rate of increase from moderate to large lengths. A regression of TS against log10(LS) yields a slope of 50.95 and an intercept of −133.80 (LS in cm, d.f. = 18, p < 0.01, r2 = 0.97; Figure 4f). A 250-mm I. antrostomus has a σbs equivalent to that of a 20-mm myctophid with an inflated swimbladder (S. leucopsarus and C. warmingii in Figure 4).
The measured gas ESR (open circle, primary y-axis) and body specific gravity, ρf, (plus sign, secondary y-axis) for individual fish vs. standard length, LS: (a) C. warmingii, Group I; (c) S. leucopsarus, Group II; (e) I. antrostomus, Group III. The ESR of ruptured bladders is displayed as “R” at an arbitrary value. The modelled 38 kHz TS of individual fish for the body only (plus sign) and body summed with the swimbladder (open circle): (b) C. warmingii, Group I; (d) S. leucopsarus, Group II; (f) I. antrostomus, Group III. Transformed TS = mlog10(LS) + b regressions from the measured data here are shown as solid lines, with the assumption that swimbladder gas volume is that required for neutral buoyancy, VG, as a dashed line; as a dotted line for modelling by Yasuma et al. (2010); and as a dashed-dotted line for the cylinder model using gas-volume measurements from Yasuma et al. (2010).
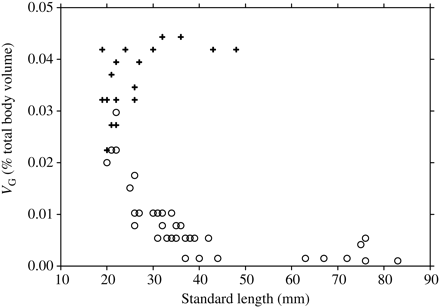
The calculated volume of gas required for neutral buoyancy, VG, expressed as a percentage of total body volume vs. standard length, LS: plus sign, C. warmingii (Group I); open circle, S. leucopsarus (Group II).
Gas required for neutral buoyancy
VG is ∼4% of the total body volume for large C. warmingii (Figure 5). For S. leucopsarus, VG starts at 2–3% of body volume for the smallest fish, then drops below 0.5% for individuals of LS > 40 mm (Figure 5). The longest S. leucopsarus in which gas was present was 37 mm LS. The similarity in VG as a percentage of body volume between small S. leucopsarus and C. warmingii is a result of the similarity of their relative densities. Intermediate values of ρf (∼1.050–1.060) were found in the smallest individuals of most myctophid species.
Discussion
Group comparison: relative densities and swimbladder inflation
The presence or the absence of a functional swimbladder in the mesopelagic fish examined in this study is significantly associated with whether or not the ρf is reduced by other means. In general, fish species in which some large individuals possess gas-filled swimbladders (Group I) have constant or increasing ρf with growth. Fish species without functional swimbladders in large individuals (Groups II and III) have a reduced ρf with growth.
The ρf values of individual species within Group I are high (Table 1), reflecting a reliance on gas for buoyancy or lift from swimming. Even so, most Group I fish species have a lower ρf than do epipelagic fish. In Melamphaes simus and Diaphus theta, ρf decreases significantly with an increasing LS. These species rely less on gas for buoyancy than other Group I species that maintain a constant ρf with increased size. Diaphus theta varies seasonally in lipid content (Neighbors and Nafpaktitis, 1982), so could benefit from the retention of swimbladder function. Diaphus fulgens also had significantly decreasing ρf with increasing LS, but because the overall ρf range is 0.007 and six of seven fish were within a LS range of 5 mm, this result may not be representative of this species. Ichthyococcus irregularis, Notolychnus valdiviae, and Triphoturus nigrescens are three other species combining gas with low and apparently decreasing ρf with increasing LS. The low n and narrow LS distribution of these species limits the power to detect a significant relationship between ρf and LS.
Except Scopelarchus stephensi, no Group II or Group III species has a 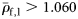 . Scopelarchus stephensi would need to be an active swimmer to maintain its place in the water column. Many large individuals of species from Groups II and III have ρf values approaching that of seawater. It is possible that
. Scopelarchus stephensi would need to be an active swimmer to maintain its place in the water column. Many large individuals of species from Groups II and III have ρf values approaching that of seawater. It is possible that 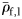 of some species was overestimated because of a lack of full-sized specimens in the sample. A bias of this nature is conservative and would lead to an overestimation of the slope (less negative) of
of some species was overestimated because of a lack of full-sized specimens in the sample. A bias of this nature is conservative and would lead to an overestimation of the slope (less negative) of 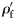 against
against 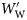 .
.
Small numbers of large individuals from some species may have resulted in the improper assignment of the species between Groups I and II. Gas was not found in the swimbladders of all fish of similar LS from many species that use gas for buoyancy. It is possible that gas-filled swimbladders are present in large individuals, but were not detected here. Similarly, the lack of small individuals that may contain gas could have resulted in the incorrect placement of some Group II species into Group III. Only confusion between Groups I and II could have affected the results presented here. The number of individuals from species potentially grouped in error is small compared with the group totals and unlikely to change the results significantly.
A low n also affects the power to detect significant relationships. A one-tailed τ-test cannot have a significant p-value for n < 4. An n value of 4 requires perfect rank order for significance. Body density is variable, both from measurement error and individual variation. A group of fish of similar size will show random rank ordering, obscuring the overall pattern if there are few or no points outside of the group. This bias chiefly affects the τ-tests for individual species (Table 1, see footnote c). At the group level, the bias is conservative for Groups II and III. The obfuscation of a true decline in ρf in some species with a low n is unlikely to affect the overall Group I result, given that it is from 460 fish.
The group assignments of L. urophaos (Group I) and Lobianchia gemellarii (Group III) are perhaps in error. The n for these two species was low, with restricted LS ranges, limiting the power to detect trends. These two species differ between Atlantic and Pacific populations. Those in the Atlantic Ocean have gas-filled swimbladders of increasing volume with growth consistent with Group I (Bone, 1973; Brooks, 1976; Saenger, 1989). Also consistent with Group I, L. gemellarii from the Gulf of Mexico have a low lipid content (Stickney and Torres, 1989). Fish from these species in the Pacific Ocean have high levels of lipid, low body densities, and non-inflated swimbladders when large, consistent with Group II (Neighbors and Nafpaktitis, 1982; Childress et al., 1990). Lampadena urophaos has been divided by some authors into Atlantic and Pacific subspecies based on differences in otolith shape and photophores (Wisner, 1976). The apparent developmental differences in buoyancy regulation between Atlantic and Pacific populations of these two species support taxonomic differentiation, so warrant additional investigation.
All three groups contain species that migrate vertically as well as species that are non-migratory. The presence or the absence of gas in large individuals is not significantly related to whether a species migrates vertically, although fish without inflated swimbladders have an energetic advantage and increased potential vertical range over those using gas for buoyancy (Marshall, 1960; Alexander, 1972).
The California Current and North Pacific Subtropical Gyre each contained species from all three groups. Group assignment is not significantly related to biogeographic province, although the significance test is marginal. More Group I and fewer Group II and Group III species are found in the North Pacific Subtropical Gyre than in the California Current. The abundant and lipid-rich Group II myctophid species from the California Current were not common in the North Pacific Subtropical Gyre and were not replaced by other lipid-rich species (Supplementary Tables S2 and S4). Low lipid levels in subtropical fish have been attributed to reduced food levels (Bailey and Robison, 1986) and reduced variability in food supply (Childress et al., 1990).
More than 30 studies have been published on the buoyancy of the fish species discussed here. Supplementary Table S4 summarizes past work for comparison. Only inconsistencies and generalities will be discussed, however, given the large number of species and reports. In general, Group I fish have low lipid content, high body density, and increasing gas volume with increasing LS. Group II species have low body density, high lipid content, and increasing lipid content with increasing LS. Group III species have low body density and low lipid content. Three of the Group II species (Scopelogadus mizolepis, P. crassiceps, and Cyclothone atraria) have low lipid content. All these species except C. atraria, for which water content is not reported, have water content >85% WW (Childress and Nygaard, 1973) and use dilute body fluids rather than lipids for buoyancy. Nannobrachium regale from the western Pacific Ocean are high in lipid (Seo et al., 1996; Saito and Murata, 1998), whereas those from the eastern Pacific have high water content (Butler and Pearcy, 1972; Neighbors and Nafpaktitis, 1982; Bailey and Robison, 1986).
There are four species from Group I (N. resplendens, Myctophum nitidulum, L. urophaos, and Symbolophorus californiensis) for which reported body-density measurements are much lower than those measured here (Neighbors and Nafpaktitis, 1982). High lipid levels (Seo et al., 1996; Saito and Murata, 1998) and seasonally high lipid content (Neighbors and Nafpaktitis, 1982) have been reported for S. californiensis, but paradoxically, the size class with the highest lipid content also had the highest body density (Neighbors and Nafpaktitis, 1982). Other researchers have found low lipid levels in N. resplendens, M. nitidulum, and S. californiensis (Nevenzel et al., 1969; Brooks, 1976; Neighbors and Nafpaktitis, 1982), and increasing gas volume with length for M. nitidulum and S. californiensis (Brooks, 1976; Neighbors, 1992). The measurement here of high and constant ρf with increasing LS is consistent with the latter findings.
Presence of gas in the swimbladder
Mesopelagic fish are generally thought to be negatively buoyant (Kanwisher and Ebeling, 1957; Capen, 1967; Bone, 1973; Brooks, 1976; Kalish et al., 1986, Saenger, 1989). A dense-bodied marine fish (i.e. ρf = 1.076 g ml−1) requires a swimbladder volume of ∼5% of the body volume for neutral buoyancy (Taylor, 1921; Marshall, 1960). The gas volume of mesopelagic fish has been measured to be in the range 0–5% of body volume for several species at surface temperature and pressure (Kanwisher and Ebeling, 1957; Capen, 1967; Kleckner and Gibbs, 1972; Kalish et al., 1986; Yasuma et al., 2010). Gas is present or absent on an individual or diel basis rather than uniformly within a species (Capen, 1967; Butler and Pearcy, 1972; Johnson, 1979; Neighbors, 1992; Yasuma et al., 2010). The low ρf measurements here demonstrate that many mesopelagic fish require gas volumes <5% of their body volume to be close to neutral buoyancy (Figure 5). This is especially true for species in Group II. The largest individual S. leucopsarus for which gas was present (37 mm) corresponded closely with the LS at which the VG drops to 0.5% of body volume.
Volume measurements of swimbladder gas here were variable, with many more than the requirement for neutral buoyancy (Supplementary Table S3). Gas was present or absent in individuals of similar size from the same species. There are several inherent problems with measurements of the volume of gas in the swimbladder of mesopelagic fish at surface temperature and pressure that make accurate quantification difficult. Elasticity of the swimbladder wall adds uncertainty to gas-volume calculations based on swimbladder dimensions. Except for fish collected at the surface, there is uncertainty about the depth of capture. The measured gas volume is subject to a pressure uncertainty of at least 15 atmospheres for midwater trawls and bongo nets as deployed here, plus uncertainty from temperature changes. Gas may be lost during capture, missed during processing, or compressed in life beyond the ambient pressure. Differences between capture methods in the fraction of fish with inflated swimbladders indicate either gas loss during capture or depth-related inflation (Neighbors, 1992). A fish that remains alive in the net for a period may actively remove gas from its swimbladder as the trawl ascends. Removal of swimbladder gas by an ascending fish is rapid compared with the addition of gas, and it can keep pace with the ascent rate of vertical migration (Marshall, 1960; D'Aoust, 1971). The time-frame of a vertical migration, ∼1 h, is comparable with the fishing time of a trawl. The absence of measured gas therefore does not mean that gas was not present at the time of capture, even when the fish appears to be undamaged. Given the biases described above with quantitative measurement of gas volume at the surface, it is difficult to know the true degree of swimbladder inflation from these data, except that it is likely to be less than VG. An assumption of neutral buoyancy is supported by observations of motionless fish from submersibles (Backus et al., 1968; Barham, 1971) and moored echosounders (Kaartvedt et al., 2009). Torpid, non-sinking fish must be close to neutral buoyancy. The buoyancy of fish swimming actively is unclear from visual observations, but there are large energetic advantages to neutral buoyancy. A fish of ρf = 1.077 swimming one body length per second expends 167% more energy if its swimbladder is not inflated (Alexander, 1966). The question of whether or not individuals of a species vary in their use of gas for buoyancy remains unresolved here. The final solution of the problem will require in situ measurements of swimbladder gas volume.
Acoustic model
The simple acoustic models used here are intended to illustrate the relative change in body σbs through variation in ρf. The effects of two of the simplifying assumptions for the swimbladder model are quantified here. The swimbladder of mesopelagic fish is spheroidal rather than spherical (Marshall, 1960; Yasuma et al., 2010). The TS of a prolate spheroid and a sphere are similar when the aspect ratio of the spheroid is <3, as is true for mesopelagic fish (Feuillade and Werby, 1994; Barr and Coombs, 2005). The sphere model was compared with a gas-filled prolate spheroid model (Ye, 1997; ESR = 2.4 mm, aspect ratio 3:1, broadside incidence, other parameters as described in “Material and methods” section) and σbs of the sphere was ∼37% less than that of the spheroid in the geometric region. A second simplifying assumption was made to model the swimbladder gas as a free bubble in seawater. Backscattering from the swimbladder occurs at the density interface between the gas and the surrounding medium. Increasing the density of the medium from 1.027 g ml–1 to a typical density of fish flesh (1.050 g ml−1) decreases σbs by <0.01%. Bias derived from the spherical shape and free-bubble assumptions for the swimbladder model is therefore judged to be minimal to exploring the relative change in σbs brought about by the variation in ρf.
A direct comparison can be made between the model used here, measurements, and other models. Yasuma et al. (2006) measured the TS of a S. leucopsarus to be −65.4 dB re 1 m2. The prolate-spheroid and deformed cylinder models used by Yasuma et al. (2006) estimate σbs to be −64 and −63.6 dB re 1 m2, respectively. The use of their parameters (64 mm LS, sound speed in fish cfish = 1518 m s−1, freshwater density, and body density = 1.035 g ml−1) with the fluid-cylinder model results in a TS of −62.5 dB re 1 m2. The simple cylindrical model used here yields results reasonably close to empirical results and more sophisticated models. Yasuma et al. (2010) reported a regression of modelled TS = 26.3 log10(LS) − 78.1 for C. warmingii (Figure 4b). The difference between that equation and the one reported here is predominantly a consequence of lower measurements of gas volume by Yasuma et al. (2010). When gas volume from Yasuma et al. (2010) is used with the spherical model here, the TS is almost identical between the two models (Figure 4b).
The tilt-angle of a mesopelagic fish relative to the acoustic beam is important for modelling the σbs of the body accurately, but less important for the gas inclusion (Yasuma et al., 2010). This important parameter is not addressed here, because dorsal incidence was assumed.
The sound-speed ratio, h, can also have a large impact on backscattering, and it varies with temperature (Yasuma et al., 2006). It seems likely that body density, ambient pressure, and h are not independent, analogous to the behaviour of sound in seawater, but these effects were not measured by Yasuma et al. (2006). The value of h used here (1.020 for c = 1490 m s−1; Yasuma et al., 2006) is smaller than the value of 1.050 typically assumed for dense-bodied epipelagic fish (Clay, 1991). Varying h across a reasonable range from 1.010 to 1.050 (Yasuma et al., 2006) in the acoustic-backscattering model for a 385 mm I. antrostomus (Figure 3) changes the 38-kHz σbs by a factor of 10. This parameter needs to be quantified better in future work.
Acoustic implications
Comparison of σbs from the three fish species in Figure 4 indicates that the σbs values of 20 mm C. warmingii and S. leucopsarus are similar to that of a 250 mm I. antrostomus. Small S. leucopsarus have a σbs ∼10× that of large individuals of the same species. Stenobrachius leucopsarus adults do not use gas for buoyancy, and large fish of this species are almost neutrally buoyant (Figures 4 and 5). The σbs of C. warmingii increases with growth because of increased gas volume. For small fish from Groups I and II, the acoustic backscatter from the swimbladder is a much greater proportion of the total than 90%. In myctophids of LS < 40 mm, the swimbladder contribution to σbs is 2–4 orders of magnitude greater than that of the body.
The disparity in σbs between large and small fish of Group II, in combination with larger numbers of small fish, will serve to obscure a direct relationship between acoustic backscattering and biomass. Four of the Group II myctophid species (S. leucopsarus, T. mexicanus, N. ritteri, and C. townsendi) are among the most abundant mesopelagic fish in the California Current. Juvenile fish often greatly outnumber adults, although they may not make up most of the biomass. Trawling is essential to establish the species present and their size distribution for the interpretation of acoustic surveys.
The TS for species from Groups I and III can be expressed in the form TS = mlog10(LS) + b (here, LS is in cm). The TS of Group II fish cannot be represented in equations of this form because of the non-allometric growth of the swimbladder, the major reflector of acoustic energy. The slope and the intercept of the TS equation for I. antrostomus (Group III) are dramatically different from those of C. warmingii (Group I), reflecting the absence of a swimbladder, elongate shape, and decrease in ρf with increased LS of that species.
Estimates for the body density of fish species that do not contain gas are critical for modelling the σbs of these fish, and hence for acoustic surveys of their abundance and distribution. A change in body density from a typical value for an epipelagic fish to the measured ρf of I. antrostomus reduced the σbs sixfold (Figure 2). For those species with functional swimbladders, VG is affected by body density. Overestimates of body density will result in biased calculations of σbs for both the body and gas inclusion of these fish.
Conclusions
Body density decreases with size in mesopelagic fish species in which large individuals do not have a functional swimbladder. Species with some large individuals having inflated swimbladders do not decrease in body density with increased weight. Mesopelagic fish in general have lower body density than fish living in shallower water.
The σbs of a fish is dominated by gas in the swimbladder, if present. The volume of gas in the swimbladder of a mesopelagic fish cannot be measured accurately at the surface as a result of the inherent unknown quantities of capture depth and loss of gas. For accuracy, calculation of the maximum volume of gas in the swimbladder, VG, requires knowledge of body density.
Information on ontogenetic changes in swimbladder inflation and the body density of fish is critical for the construction of the TS models used to interpret acoustic surveys. Knowledge of the fish species present, their relative abundance, their developmental morphology, and their size distribution is required for accurate acoustic surveys of mesopelagic fish. The measurements presented here of ρf and swimbladder inflation for 71 species of mesopelagic fish from the Northeast Pacific can be used to improve the accuracy of the backscattering models used to interpret acoustic surveys conducted there. However, those models will also require assumptions or new data regarding tilt-angle and swimbladder volume. Juveniles and adults from Group II species may need to be treated separately because of their disjunct TS distributions.
Supplementary material
Acknowledgements
I thank the RV “New Horizon” and FSV “Bell Shimada” crew, volunteers, and science parties for their help in deploying nets and processing samples. Equipment and chemicals were provided by the SIO MVC, the SIO Pelagic Invertebrates Collection, J. Koslow, and D. Checkley. Some fish were provided by M. Goldstein, J. Powell, and M. Decima. C. Klepadlo from the SIO MVC provided training, keys, and assistance with the identification of fish. P. Hastings of the SIO MVC allowed the dissection of some fish. D. Checkley, J. Koslow, J. Graham, M. Ohman, F. Powell, and P. Hastings provided valuable suggestions and comments on the manuscript. Wire time for a midwater trawl was provided by L. Levin (SIO 277). The study was supported by a NASA Earth and Space Science Fellowship. Ship time was funded by UC Ship Funds, NOAA, and the Kaisei Foundation/Ocean Voyages Institute.