-
PDF
- Split View
-
Views
-
Cite
Cite
Shane P. McGrath, Matt K. Broadhurst, Paul A. Butcher, Stuart C. Cairns, Fate of three Australian teleosts after ingesting conventional and modified stainless- and carbon-steel hooks, ICES Journal of Marine Science, Volume 68, Issue 10, November 2011, Pages 2114–2122, https://doi.org/10.1093/icesjms/fsr151
Close - Share Icon Share
Abstract
In response to concerns over the fate of three Australian teleosts (mulloway, Argyrosomus japonicus, yellowfin bream, Acanthopagrus australis, and snapper, Pagrus auratus) released with ingested recreational hooks, experiments were carried out to determine whether ejection could be promoted via different wire materials and/or their modification. Between 108 and 114 fish of each species were angled and allowed to ingest conventional or modified (with notches to reduce wire diameter by ∼20%) J-hooks (∼250 mm2) made from three materials (stainless steel and nickel-plated and red-lacquer carbon steel), before being released into tanks and monitored with control fish for up to 61 d. Total mortalities were 35, 24, and 25% for mulloway (over 61 d), yellowfin bream (over 35 d), and snapper (over 41 d), respectively. Of the survivors, 30, 61, and 77%, respectively, ejected their hooks (and only one hook-ejected fish died). For yellowfin bream, hook ejection (and hence survival) was positively correlated with total length, and hook oxidation was the key predictor of ejection from mulloway and snapper, which could be promoted by selecting carbon-steel designs with narrow wire diameters and minimal protective coating. The choice of coating might also be important, with relatively greater mortality among mulloway and yellowfin bream that ingested nickel-plated hooks.McGrath, S. P., Broadhurst, M. K., Butcher, P. A., and Cairns, S. C. 2011. Fate of three Australian teleosts after ingesting conventional and modified stainless- and carbon-steel hooks. – ICES Journal of Marine Science, 68: 2114–2122.
Introduction
With a participation rate of >19% of the population, recreational fishing is one of the most popular sports in Australia (Henry and Lyle, 2003). There are no current definitive estimates of catches, but a 12-month survey carried out late last century estimated that >260 species of elasmobranchs, teleosts, crustaceans, and cephalopods delivered a total harvest of 135 million fish (Henry and Lyle, 2003). Most of the teleosts were caught by anglers (85%), mainly inshore.
As in other developed nations, large numbers of angled teleosts are released throughout Australia; traditionally in response to mandated legal sizes and personal quotas but also, more recently, to the growth in popularity of voluntary non-consumptive angling (Henry and Lyle, 2003; Arlinghaus et al., 2007). Three of the more popular coastal species are mulloway (Argyrosomus japonicus), yellowfin bream (Acanthopagrus australis), and snapper (Pagrus auratus), which have a combined total recreational catch annually of >17 million fish and are released at approximate rates of 46, 62, and 66%, respectively (Henry and Lyle, 2003).
Concerns over the potential for at least some mortality and/or negative sublethal impact on angled-and-released mulloway, yellowfin bream, and snapper have led to several quantitative studies being embarked upon (Broadhurst et al., 1999, 2005, 2007; Broadhurst and Barker, 2000; Butcher et al., 2007, 2008, 2010; Grixti et al., 2010). Such work estimated short-term (<10 d) total fatalities of <23% for mulloway (Butcher et al., 2007), <38% for yellowfin bream (Broadhurst et al., 2005; Butcher et al., 2007), and <33% for snapper (Broadhurst et al., 2005). Many fatalities were attributed to the cumulative impacts of several biological, technical, and environmental factors, but anatomical hook location had a dominant effect, manifesting as proportionally more deaths among hook-ingested fish than those caught in the mouth (e.g. Butcher et al., 2006, 2007; Grixti et al., 2010). Moreover, short-term hook-ingested fatalities were fewer when all three species were released with their lines cut than when their hooks were removed with force (Butcher et al., 2007; Grixti et al., 2010).
Based on the above association between hook removal and mortality, and in accord with the results of other studies (e.g. Jordan and Woodward, 1992; Aalbers et al., 2004), a proposal was made in New South Wales (NSW) for hook-ingested fish to be released with their lines cut, rather than attempting to remove the hooks (Broadhurst et al., 2007; Butcher et al., 2007). The long-term utility of this approach for minimizing negative impacts has been validated for yellowfin bream (Broadhurst et al., 2007; Butcher et al., 2010). In particular, Broadhurst et al. (2007) observed a non-significant mortality of 15% for line-cut, hook-ingested fish over more than three months, with 76% of survivors ejecting their hooks after an average of ∼20 d while maintaining their overall condition. No similar data are available for snapper or mulloway, but during 3 d of monitoring, Grixti et al. (2010) recorded 13% hook ejection by similarly treated snapper (n= 59).
It is apparent that many line-cut, hook-ingested teleosts can survive and eventually eject their hooks (reviewed by Hall et al., 2009), but little is known of the mechanisms underlying this process. For some species, their morphology and feeding strategies are probably important (Aalbers et al., 2004; Broadhurst et al., 2007; McGrath et al., 2009). However, irrespective of biological traits, it is likely that both the mortality of fish and their hook ejection are strongly influenced by hook decay, mainly because this dictates how long the point and barb remain sharp (and the potential for associated internal damage), and overall structural integrity is maintained (Aalbers et al., 2004; Broadhurst et al., 2007). Recognition of the importance of hook decay precipitated a recent study by McGrath et al. (in press) to isolate some of the key contributory technical factors. This work revealed that following submersion in seawater for up to 28 d, the material and diameter of the wire used to manufacture hooks strongly influenced the percentage weight and point remaining (as a result of oxidation), and the subsequent compression and tensile strengths.
More specifically, after a month of submersion, carbon-steel hooks were 19% weaker than stainless-steel hooks (McGrath et al., in press). The relationship between the diameter of the wire and hook decay was less clear, being further influenced by several other interacting variables. In particular, the presence of bait-holder barbs and other similar modifications (notches) along the wire length negated a consistent, significantly positive relationship between wire diameter and tensile and compression strengths for a range of hooks. It was hypothesized that, irrespective of wire diameter, the bait-holders and notches provided weak points and, for carbon-steel hooks, facilitated deterioration of the protective coating and exposed the metal (McGrath et al., in press). Broadhurst et al. (2007) argued that such modifications would encourage corrosion and increase the chance of hooks breaking into smaller pieces and being ejected.
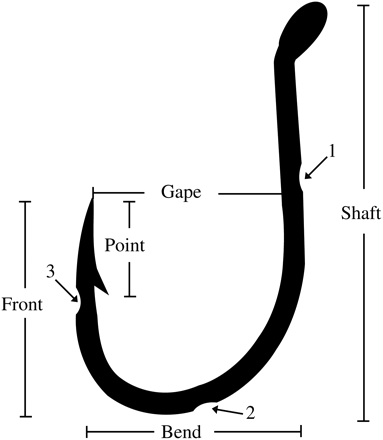
The strong influence of hook material on decay is intuitive, with many agencies advocating against the use of stainless steel. Such a strategy could be extended to include other hooks that have resilient anticorrosive coatings, although this would have obvious economic impacts and would be difficult to enforce. An alternative approach to this issue, supported by McGrath et al. (in press), might involve choosing inherently weaker hook designs, or modifying others (irrespective of their material) either to promote their decay and/or to reduce their strength to encourage breakage during ingestion. The present study sought to test the potential for such effects among hook-ingested yellowfin bream, snapper, and mulloway over a period of up to 9 weeks. In addition to providing the first information on the longer-term post-release fate of the last two species, the specific aims were to determine whether or not wire material (including stainless steel and carbon steel with different coatings) and/or modifications including notches along the shaft, bend, and under the point of the hook (McGrath et al., in press; Figure 1) influenced mortality and hook breakage and ejection.
Silhouette of the treatment hooks used in the study, and the location of the three small notches (1–3) cut into the shaft, bend, and point of the modified designs.
Material and methods
Three experiments were carried out at the National Marine Science Centre (NMSC), Coffs Harbour, Australia, during 2008. The facilities included five 3000-l covered holding tanks located in an open area, and 63 experimental tanks of 110 l arranged in an enclosed room with a regulated photoperiod (light-to-dark ratio 12 h:12 h). The tanks and aquaria were supplied with seawater (at ambient temperature; 16.7–24.1°C) at a rate of 30 l min–1 and aerated using stone diffusers.
Fish collection
Up to 3 months before starting each experiment, ∼300 juveniles of the three species under study were collected and transferred to the five holding tanks (∼60 fish per tank). Snapper (12–33 cm total length, LT) and yellowfin bream (15–28 cm LT) were caught by commercial fishers (using traps) and researchers (using castnets and hook and line), respectively, and mulloway (24–30 cm LT; first-generation reared) were purchased from a local aquaculture facility. All fish were handled according to the methods described by Butcher et al. (2007) and fed commercially available 4-mm fish pellets and Australian sardine (Sardinops neopilchardus) at a rate of ∼1% biomass d–1 until 2 d before commencing the experiments.
Hooks
The hooks were chosen based on their almost identical design (J) and shape (absolute size ∼250 mm2; Figure 1; McGrath et al., in press), but different wire material: stainless steel, and red-lacquer-coated and nickel-plated carbon steel (Table 1). These materials have varying levels of corrosion resistance; estimated by the manufacturer as evidence of oxidation across 3, 30, and 52%, respectively, of the total surface area after several hours of exposure to salt spray. Using a rotary tool (disc 24 mm), half the hooks (termed here as modified) made from each material had three small notches (similar to bait-holder barbs; Broadhurst et al., 2007) cut into the shaft, bend, and point (to ∼80% of the wire diameter; Figure 1). The notches were designed to reduce hook strength and, for the carbon-steel designs, to increase the surface area and subsequent oxidation. The other 50% of hooks made from each material were left unmodified (and termed here conventional). All hooks were weighed to the nearest 0.0001 g using an electronic balance (Ohaus adventurer analytical, AR2140). Modified hooks were only used if they weighed at least 99% of their original mass.
Initial technical specifications of the six treatment hooks investigated for their rates of ejection and decay and influences on the mortality of mulloway, A. japonicus, yellowfin bream, A. australis, and snapper, P. auratus, after being ingested.
| Hook no. . | Steel type . | Manufacturer's coating . | Wire diameter . | Shaft . | Front . | Bend . | Gape . | Absolute size . | Weight . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | C | Nickel | 1.06 | 21.33 ± 0.01 | 11.21 ± 0.01 | 11.41 ± 0.01 | 9.96 ± 0.01 | 243.44 ± 0.16 | 279.41 ± 0.26 |
| 1aa | C | Nickel | 1.06 | 21.34 ± 0.01 | 11.21 ± 0.01 | 11.42 ± 0.01 | 9.96 ± 0.01 | 243.65 ± 0.16 | 276.38 ± 0.20 |
| 2 | C | Red lacquer | 1.06 | 21.00 ± 0.02 | 11.58 ± 0.01 | 11.82 ± 0.01 | 9.80 ± 0.01 | 248.24 ± 0.33 | 277.97 ± 0.22 |
| 2aa | C | Red lacquer | 1.06 | 21.00 ± 0.02 | 11.58 ± 0.01 | 11.82 ± 0.01 | 9.81 ± 0.01 | 248.23 ± 0.33 | 274.66 ± 0.20 |
| 3 | S | na | 0.98 | 21.43 ± 0.02 | 11.47 ± 0.01 | 11.91 ± 0.01 | 10.44 ± 0.01 | 255.16 ± 0.24 | 229.44 ± 0.29 |
| 3aa | S | na | 0.98 | 21.42 ± 0.02 | 11.45 ± 0.01 | 11.90 ± 0.01 | 10.30 ± 0.04 | 254.92 ± 0.28 | 226.30 ± 0.31 |
| Hook no. . | Steel type . | Manufacturer's coating . | Wire diameter . | Shaft . | Front . | Bend . | Gape . | Absolute size . | Weight . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | C | Nickel | 1.06 | 21.33 ± 0.01 | 11.21 ± 0.01 | 11.41 ± 0.01 | 9.96 ± 0.01 | 243.44 ± 0.16 | 279.41 ± 0.26 |
| 1aa | C | Nickel | 1.06 | 21.34 ± 0.01 | 11.21 ± 0.01 | 11.42 ± 0.01 | 9.96 ± 0.01 | 243.65 ± 0.16 | 276.38 ± 0.20 |
| 2 | C | Red lacquer | 1.06 | 21.00 ± 0.02 | 11.58 ± 0.01 | 11.82 ± 0.01 | 9.80 ± 0.01 | 248.24 ± 0.33 | 277.97 ± 0.22 |
| 2aa | C | Red lacquer | 1.06 | 21.00 ± 0.02 | 11.58 ± 0.01 | 11.82 ± 0.01 | 9.81 ± 0.01 | 248.23 ± 0.33 | 274.66 ± 0.20 |
| 3 | S | na | 0.98 | 21.43 ± 0.02 | 11.47 ± 0.01 | 11.91 ± 0.01 | 10.44 ± 0.01 | 255.16 ± 0.24 | 229.44 ± 0.29 |
| 3aa | S | na | 0.98 | 21.42 ± 0.02 | 11.45 ± 0.01 | 11.90 ± 0.01 | 10.30 ± 0.04 | 254.92 ± 0.28 | 226.30 ± 0.31 |
Lengths (n= 12), weight (n= 54–56), and absolute size are in mm, mg, and mm2, respectively. Hook lengths and absolute size were derived from McGrath et al. (in press), but the weights were recorded here. C, carbon steel; S, stainless steel; na, not applicable.
aModified with three notches on the shaft, bend, and front (Figure 1).
Initial technical specifications of the six treatment hooks investigated for their rates of ejection and decay and influences on the mortality of mulloway, A. japonicus, yellowfin bream, A. australis, and snapper, P. auratus, after being ingested.
| Hook no. . | Steel type . | Manufacturer's coating . | Wire diameter . | Shaft . | Front . | Bend . | Gape . | Absolute size . | Weight . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | C | Nickel | 1.06 | 21.33 ± 0.01 | 11.21 ± 0.01 | 11.41 ± 0.01 | 9.96 ± 0.01 | 243.44 ± 0.16 | 279.41 ± 0.26 |
| 1aa | C | Nickel | 1.06 | 21.34 ± 0.01 | 11.21 ± 0.01 | 11.42 ± 0.01 | 9.96 ± 0.01 | 243.65 ± 0.16 | 276.38 ± 0.20 |
| 2 | C | Red lacquer | 1.06 | 21.00 ± 0.02 | 11.58 ± 0.01 | 11.82 ± 0.01 | 9.80 ± 0.01 | 248.24 ± 0.33 | 277.97 ± 0.22 |
| 2aa | C | Red lacquer | 1.06 | 21.00 ± 0.02 | 11.58 ± 0.01 | 11.82 ± 0.01 | 9.81 ± 0.01 | 248.23 ± 0.33 | 274.66 ± 0.20 |
| 3 | S | na | 0.98 | 21.43 ± 0.02 | 11.47 ± 0.01 | 11.91 ± 0.01 | 10.44 ± 0.01 | 255.16 ± 0.24 | 229.44 ± 0.29 |
| 3aa | S | na | 0.98 | 21.42 ± 0.02 | 11.45 ± 0.01 | 11.90 ± 0.01 | 10.30 ± 0.04 | 254.92 ± 0.28 | 226.30 ± 0.31 |
| Hook no. . | Steel type . | Manufacturer's coating . | Wire diameter . | Shaft . | Front . | Bend . | Gape . | Absolute size . | Weight . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | C | Nickel | 1.06 | 21.33 ± 0.01 | 11.21 ± 0.01 | 11.41 ± 0.01 | 9.96 ± 0.01 | 243.44 ± 0.16 | 279.41 ± 0.26 |
| 1aa | C | Nickel | 1.06 | 21.34 ± 0.01 | 11.21 ± 0.01 | 11.42 ± 0.01 | 9.96 ± 0.01 | 243.65 ± 0.16 | 276.38 ± 0.20 |
| 2 | C | Red lacquer | 1.06 | 21.00 ± 0.02 | 11.58 ± 0.01 | 11.82 ± 0.01 | 9.80 ± 0.01 | 248.24 ± 0.33 | 277.97 ± 0.22 |
| 2aa | C | Red lacquer | 1.06 | 21.00 ± 0.02 | 11.58 ± 0.01 | 11.82 ± 0.01 | 9.81 ± 0.01 | 248.23 ± 0.33 | 274.66 ± 0.20 |
| 3 | S | na | 0.98 | 21.43 ± 0.02 | 11.47 ± 0.01 | 11.91 ± 0.01 | 10.44 ± 0.01 | 255.16 ± 0.24 | 229.44 ± 0.29 |
| 3aa | S | na | 0.98 | 21.42 ± 0.02 | 11.45 ± 0.01 | 11.90 ± 0.01 | 10.30 ± 0.04 | 254.92 ± 0.28 | 226.30 ± 0.31 |
Lengths (n= 12), weight (n= 54–56), and absolute size are in mm, mg, and mm2, respectively. Hook lengths and absolute size were derived from McGrath et al. (in press), but the weights were recorded here. C, carbon steel; S, stainless steel; na, not applicable.
aModified with three notches on the shaft, bend, and front (Figure 1).
Replicates of each of the three conventional hook types were analysed for their elemental constituents by Spectrometer Services (Coburg, Australia). Two replicate analyses were carried out, each involving ten hooks (∼2 g of steel). Approximately 0.5 g of each sample was ground into particles (<10 µm in diameter), and the percentage of carbon and sulphur was determined using infrared absorption after combustion in an induction furnace (LECO combustion analyser, CS230), following Australian standards AS1050.16 and AS1050.32. The remaining sample was then dissolved using an acid-digestion process, and an inductively coupled plasma atomic emission spectroscopy scan was applied to determine the composition of all other elements.
Experimental procedure
On the first day of each experiment, 24 fish (termed controls) were selected randomly from two of the holding tanks, removed using knotless nets, then measured and weighed. Within 2 min of capture, six of these fish were secured in a foam block sample (1 ml) collected using heparinized syringes (22-gauge needles; see Broadhurst et al., 2005, for detail). The other 18 fish were placed in pairs into nine, randomly allocated, experimental tanks. Where two fish of similar size were placed in the same tank, one had the top caudal fin clipped to facilitate subsequent identification. Six of each species were concurrently angled from the wild near the NMSC and also immediately sampled for blood as above, to provide baseline estimates of plasma glucose as an index of stress (see below).
Following distribution of the controls, hooks within each of the six categories (Table 1) were attached to either clear or yellow 8-kg polyvinylidene fluoride line. The different line colours were used to facilitate the eventual identification of replicate hooks between fish within tanks (see below). Each category (n= 6) of hook was used to angle at least 18 fish (i.e. a minimum total number of 108 fish) from the three remaining holding tanks. All fish were allowed to ingest the hook before being removed from their holding tank, held in one hand while the line was cut ∼5 cm from their mouth, measured (LT), weighed, then released in pairs (caught with yellow and clear line) into 54 of the experimental tanks. Because there were some short-term mortalities (i.e. within 120 min of release) among angled mulloway, the dead fish were replaced in the experimental tanks with additional hook-ingested fish (up to two fish per treatment). All experimental fish were offered food (as above) and monitored daily for 5–9 weeks. To maintain stocking densities in the experimental tanks and, except for the short-term mortalities to mulloway (see above), dead fish were removed and replaced with fish from the holding tanks (caudal fin clipped for identification).
Data collection and analyses
The times of capture and release into the experimental tanks, type of treatment or control, LT (cm), monitoring period, daily mortality, and information on the resumption of feeding were recorded for all fish. The presence or the absence of blood at the mouth during initial capture and the daily ejection of hooks were also noted for treatment fish. Any completely (whole) ejected hooks were cleaned and re-weighed to determine the percentage loss of mass attributable to corrosion. Any hook breakages were noted. Water temperature (°C) and dissolved oxygen (DO, mg l–1) were monitored daily using a water-quality sensor (U-10 Horiba).
At the end of each monitoring period, appropriate numbers (see below) of the surviving treatment and control fish were sampled for blood as above. Any remaining hook-ingested treatment fish were euthanized in a solution of benzocaine (100 mg l–1 in seawater) before the ingested hooks were removed and weighed as above. All other fish were released.
The data were analysed separately within each experiment using several approaches. Generalized linear mixed models (GLMMs) were used to test for the independence of various continuous and categorical fixed effects and the random effect of experimental tanks on the survival of individual fish at the end of the monitoring periods. The fixed factors included in the analyses were: the continuous factors of LT (cm), hook weight (g), and wire diameter (mm); the binary factors of hook breakage, hook ejection, hook steel type, hook modification, nickel plating, red-lacquer coating, and the presence of blood; and the nominal factor of researcher. All categorical effects were treated in a qualitative manner by converting them to dummy variables using reference cell coding (Quinn and Keough, 2002). A second group of GLMMs were run for the surviving angled fish using the same factors as above, but with hook ejection as the response variable.
GLMMs were fitted using the lmer function in the lme4 package of the R statistical package, version 2. 12.0 (Bates et al., 2011), following a procedure similar to that outlined by Zuur et al. (2007). This involved starting with what might be a “just beyond optimal” model that included all fixed components and the single random effect. Derivation of the optimal, most parsimonious model involved a stepwise selection process in which redundant explanatory variables (fixed effects) were progressively deleted from the model. The most parsimonious model was identified by the lowest value for a penalized log-likelihood in the form of Akaike's information criterion [AIC = −2 × log-likelihood + 2(p + 1), where p is the number of parameters in the model; Burnham and Anderson, 2002; Johnson and Omland, 2004]. Where the difference between any particular model and the top-ranked model (i.e. the one that resulted in the smallest AIC) was <2, model adequacy was assessed using a likelihood ratio test (Zuur et al., 2007), and the significance of individual model coefficients was assessed with the Wald statistic (Agresti, 1996; Crawley, 2005).
Variability in the rate of oxidation (defined as the arcsine-root-transformed proportion of hook weight lost per day) of the various ejected (whole) and dissected hooks for each species was assessed using an LMM. The fixed factors in that analysis were hook type (i.e. the six treatments), their location at the end of the experiment (i.e. ejected or still in fish, i.e. “dissected”) and the interaction between these two parameters. The LT of fish was included as a covariate, and tanks were included as a random effect. Predicted means of interest were back-transformed and depicted graphically.
Blood samples from the three experiments were analysed for concentrations of glucose (mmol l–1) derived by colorimetric clinical kits (Roche Diagnostics, USA), using an enzymatic spectrophotometric assay according to the manufacturer's instructions. These data were collected to assess whether fish were unusually stressed as a consequence of their treatment and/or confinement during the experiments. Specifically, the hypothesis of no difference in blood glucose among (i) immediately sampled wild-caught fish (i.e. the baseline), (ii) confined fish in the holding tanks at the start of the experiment, and (iii) angled fish with both hooks ejected and still ingested, and (iv) control fish in tanks at the end of the experiments, was tested using an appropriate LMM, in which tanks were treated as random. All LMMs were fitted in ASReml-R (Butler et al., 2009).
Results
All treatment hooks had an almost identical shape and profile, with comparable shaft, front, and bend lengths (Table 1). The gape lengths, absolute sizes, weights, and wire diameters were slightly more divergent (Table 1). Of the four parameters, wire diameter and hook weight were considered the most important in terms of oxidation, so were included in the subsequent GLMMs (see below, and McGrath et al., in press).
Much greater differences were observed in the elemental constituents among hooks, especially between the stainless-steel and two carbon-steel designs (Table 2). In particular, the stainless-steel hooks had proportionally less iron, carbon, manganese, and nickel, but more silicon, cobalt, copper, chromium, vanadium, and molybdenum (Table 2). The main difference between the nickel-plated and red-lacquer-coated hooks was in the quantity of nickel. Among all hooks, 15 other elements were present in trace quantities (Table 2).
Elemental composition of the three hook types (% composition), with n= 2.
| Element . | Nickel-plated carbon steel . | Red-lacquer carbon steel . | Stainless steel . |
|---|---|---|---|
| Iron | 96.016 ± 0.280 | 96.976 ± 0.180 | 84.532 ± 0.045 |
| Chromium | 0.010 ± 0.000 | 0.025 ± 0.005 | 13.115 ± 0.050 |
| Nickel | 2.520 ± 0.280 | 1.475 ± 0.235 | 0.170 ± 0.010 |
| Carbon | 0.735 ± 0.005 | 0.740 ± 0.010 | 0.400 ± 0.000 |
| Manganese | 0.490 ± 0.000 | 0.615 ± 0.035 | 0.280 ± 0.020 |
| Silicon | 0.195 ± 0.005 | 0.075 ± 0.015 | 0.390 ± 0.030 |
| Copper | 0.020 ± 0.000 | 0.060 ± 0.010 | 0.110 ± 0.030 |
| Cobalt | 0.004 ± 0.000 | 0.004 ± 0.000 | 0.014 ± 0.001 |
| Phosphorus | 0.010 ± 0.000 | 0.010 ± 0.000 | 0.010 ± 0.000 |
| Molybdenum | <0.010 ± 0.000 | <0.010 ± 0.000 | 0.880 ± 0.000 |
| Vanadium | <0.010 ± 0.000 | <0.010 ± 0.000 | 0.065 ± 0.015 |
| Sulphur | <0.010 ± 0.000 | 0.020 ± 0.000 | <0.010 ± 0.000 |
| Aluminium | <0.010 ± 0.000 | <0.010 ± 0.000 | <0.010 ± 0.000 |
| Niobium | <0.010 ± 0.000 | <0.010 ± 0.000 | <0.010 ± 0.000 |
| Titanium | <0.010 ± 0.000 | <0.010 ± 0.000 | <0.010 ± 0.000 |
| Arsenic | <0.005 ± 0.000 | <0.005 ± 0.000 | <0.005 ± 0.000 |
| Barium | <0.005 ± 0.000 | <0.005 ± 0.000 | <0.005 ± 0.000 |
| Beryllium | <0.005 ± 0.000 | <0.005 ± 0.000 | <0.005 ± 0.000 |
| Bismuth | <0.005 ± 0.000 | <0.005 ± 0.000 | <0.005 ± 0.000 |
| Cadmium | <0.005 ± 0.000 | <0.005 ± 0.000 | <0.005 ± 0.000 |
| Lead | <0.005 ± 0.000 | <0.005 ± 0.000 | <0.005 ± 0.000 |
| Mercury | <0.005 ± 0.000 | <0.005 ± 0.000 | <0.005 ± 0.000 |
| Selenium | <0.005 ± 0.000 | <0.005 ± 0.000 | <0.005 ± 0.000 |
| Silver | <0.005 ± 0.000 | <0.005 ± 0.000 | <0.005 ± 0.000 |
| Tin | <0.005 ± 0.000 | <0.005 ± 0.000 | <0.005 ± 0.000 |
| Element . | Nickel-plated carbon steel . | Red-lacquer carbon steel . | Stainless steel . |
|---|---|---|---|
| Iron | 96.016 ± 0.280 | 96.976 ± 0.180 | 84.532 ± 0.045 |
| Chromium | 0.010 ± 0.000 | 0.025 ± 0.005 | 13.115 ± 0.050 |
| Nickel | 2.520 ± 0.280 | 1.475 ± 0.235 | 0.170 ± 0.010 |
| Carbon | 0.735 ± 0.005 | 0.740 ± 0.010 | 0.400 ± 0.000 |
| Manganese | 0.490 ± 0.000 | 0.615 ± 0.035 | 0.280 ± 0.020 |
| Silicon | 0.195 ± 0.005 | 0.075 ± 0.015 | 0.390 ± 0.030 |
| Copper | 0.020 ± 0.000 | 0.060 ± 0.010 | 0.110 ± 0.030 |
| Cobalt | 0.004 ± 0.000 | 0.004 ± 0.000 | 0.014 ± 0.001 |
| Phosphorus | 0.010 ± 0.000 | 0.010 ± 0.000 | 0.010 ± 0.000 |
| Molybdenum | <0.010 ± 0.000 | <0.010 ± 0.000 | 0.880 ± 0.000 |
| Vanadium | <0.010 ± 0.000 | <0.010 ± 0.000 | 0.065 ± 0.015 |
| Sulphur | <0.010 ± 0.000 | 0.020 ± 0.000 | <0.010 ± 0.000 |
| Aluminium | <0.010 ± 0.000 | <0.010 ± 0.000 | <0.010 ± 0.000 |
| Niobium | <0.010 ± 0.000 | <0.010 ± 0.000 | <0.010 ± 0.000 |
| Titanium | <0.010 ± 0.000 | <0.010 ± 0.000 | <0.010 ± 0.000 |
| Arsenic | <0.005 ± 0.000 | <0.005 ± 0.000 | <0.005 ± 0.000 |
| Barium | <0.005 ± 0.000 | <0.005 ± 0.000 | <0.005 ± 0.000 |
| Beryllium | <0.005 ± 0.000 | <0.005 ± 0.000 | <0.005 ± 0.000 |
| Bismuth | <0.005 ± 0.000 | <0.005 ± 0.000 | <0.005 ± 0.000 |
| Cadmium | <0.005 ± 0.000 | <0.005 ± 0.000 | <0.005 ± 0.000 |
| Lead | <0.005 ± 0.000 | <0.005 ± 0.000 | <0.005 ± 0.000 |
| Mercury | <0.005 ± 0.000 | <0.005 ± 0.000 | <0.005 ± 0.000 |
| Selenium | <0.005 ± 0.000 | <0.005 ± 0.000 | <0.005 ± 0.000 |
| Silver | <0.005 ± 0.000 | <0.005 ± 0.000 | <0.005 ± 0.000 |
| Tin | <0.005 ± 0.000 | <0.005 ± 0.000 | <0.005 ± 0.000 |
Elemental composition of the three hook types (% composition), with n= 2.
| Element . | Nickel-plated carbon steel . | Red-lacquer carbon steel . | Stainless steel . |
|---|---|---|---|
| Iron | 96.016 ± 0.280 | 96.976 ± 0.180 | 84.532 ± 0.045 |
| Chromium | 0.010 ± 0.000 | 0.025 ± 0.005 | 13.115 ± 0.050 |
| Nickel | 2.520 ± 0.280 | 1.475 ± 0.235 | 0.170 ± 0.010 |
| Carbon | 0.735 ± 0.005 | 0.740 ± 0.010 | 0.400 ± 0.000 |
| Manganese | 0.490 ± 0.000 | 0.615 ± 0.035 | 0.280 ± 0.020 |
| Silicon | 0.195 ± 0.005 | 0.075 ± 0.015 | 0.390 ± 0.030 |
| Copper | 0.020 ± 0.000 | 0.060 ± 0.010 | 0.110 ± 0.030 |
| Cobalt | 0.004 ± 0.000 | 0.004 ± 0.000 | 0.014 ± 0.001 |
| Phosphorus | 0.010 ± 0.000 | 0.010 ± 0.000 | 0.010 ± 0.000 |
| Molybdenum | <0.010 ± 0.000 | <0.010 ± 0.000 | 0.880 ± 0.000 |
| Vanadium | <0.010 ± 0.000 | <0.010 ± 0.000 | 0.065 ± 0.015 |
| Sulphur | <0.010 ± 0.000 | 0.020 ± 0.000 | <0.010 ± 0.000 |
| Aluminium | <0.010 ± 0.000 | <0.010 ± 0.000 | <0.010 ± 0.000 |
| Niobium | <0.010 ± 0.000 | <0.010 ± 0.000 | <0.010 ± 0.000 |
| Titanium | <0.010 ± 0.000 | <0.010 ± 0.000 | <0.010 ± 0.000 |
| Arsenic | <0.005 ± 0.000 | <0.005 ± 0.000 | <0.005 ± 0.000 |
| Barium | <0.005 ± 0.000 | <0.005 ± 0.000 | <0.005 ± 0.000 |
| Beryllium | <0.005 ± 0.000 | <0.005 ± 0.000 | <0.005 ± 0.000 |
| Bismuth | <0.005 ± 0.000 | <0.005 ± 0.000 | <0.005 ± 0.000 |
| Cadmium | <0.005 ± 0.000 | <0.005 ± 0.000 | <0.005 ± 0.000 |
| Lead | <0.005 ± 0.000 | <0.005 ± 0.000 | <0.005 ± 0.000 |
| Mercury | <0.005 ± 0.000 | <0.005 ± 0.000 | <0.005 ± 0.000 |
| Selenium | <0.005 ± 0.000 | <0.005 ± 0.000 | <0.005 ± 0.000 |
| Silver | <0.005 ± 0.000 | <0.005 ± 0.000 | <0.005 ± 0.000 |
| Tin | <0.005 ± 0.000 | <0.005 ± 0.000 | <0.005 ± 0.000 |
| Element . | Nickel-plated carbon steel . | Red-lacquer carbon steel . | Stainless steel . |
|---|---|---|---|
| Iron | 96.016 ± 0.280 | 96.976 ± 0.180 | 84.532 ± 0.045 |
| Chromium | 0.010 ± 0.000 | 0.025 ± 0.005 | 13.115 ± 0.050 |
| Nickel | 2.520 ± 0.280 | 1.475 ± 0.235 | 0.170 ± 0.010 |
| Carbon | 0.735 ± 0.005 | 0.740 ± 0.010 | 0.400 ± 0.000 |
| Manganese | 0.490 ± 0.000 | 0.615 ± 0.035 | 0.280 ± 0.020 |
| Silicon | 0.195 ± 0.005 | 0.075 ± 0.015 | 0.390 ± 0.030 |
| Copper | 0.020 ± 0.000 | 0.060 ± 0.010 | 0.110 ± 0.030 |
| Cobalt | 0.004 ± 0.000 | 0.004 ± 0.000 | 0.014 ± 0.001 |
| Phosphorus | 0.010 ± 0.000 | 0.010 ± 0.000 | 0.010 ± 0.000 |
| Molybdenum | <0.010 ± 0.000 | <0.010 ± 0.000 | 0.880 ± 0.000 |
| Vanadium | <0.010 ± 0.000 | <0.010 ± 0.000 | 0.065 ± 0.015 |
| Sulphur | <0.010 ± 0.000 | 0.020 ± 0.000 | <0.010 ± 0.000 |
| Aluminium | <0.010 ± 0.000 | <0.010 ± 0.000 | <0.010 ± 0.000 |
| Niobium | <0.010 ± 0.000 | <0.010 ± 0.000 | <0.010 ± 0.000 |
| Titanium | <0.010 ± 0.000 | <0.010 ± 0.000 | <0.010 ± 0.000 |
| Arsenic | <0.005 ± 0.000 | <0.005 ± 0.000 | <0.005 ± 0.000 |
| Barium | <0.005 ± 0.000 | <0.005 ± 0.000 | <0.005 ± 0.000 |
| Beryllium | <0.005 ± 0.000 | <0.005 ± 0.000 | <0.005 ± 0.000 |
| Bismuth | <0.005 ± 0.000 | <0.005 ± 0.000 | <0.005 ± 0.000 |
| Cadmium | <0.005 ± 0.000 | <0.005 ± 0.000 | <0.005 ± 0.000 |
| Lead | <0.005 ± 0.000 | <0.005 ± 0.000 | <0.005 ± 0.000 |
| Mercury | <0.005 ± 0.000 | <0.005 ± 0.000 | <0.005 ± 0.000 |
| Selenium | <0.005 ± 0.000 | <0.005 ± 0.000 | <0.005 ± 0.000 |
| Silver | <0.005 ± 0.000 | <0.005 ± 0.000 | <0.005 ± 0.000 |
| Tin | <0.005 ± 0.000 | <0.005 ± 0.000 | <0.005 ± 0.000 |
The results for mulloway, yellowfin bream, and snapper are presented separately below. However, all three species were assessed across comparable water quality, with mean DO ± s.e. of 6.34 ± 0.07, 6.48 ± 0.04, and 6.66 ± 0.03 mg l–1, respectively, and temperatures of 20.48 ± 0.21, 21.38 ± 0.19, and 17.79 ± 0.11°C, respectively. Further, all fish resumed normal feeding within a week of being placed into the 110-l tanks and remained active throughout their monitoring. For those fish that ejected hooks, it was not possible to determine whether the ejection was from the mouth or the anus. None of the control fish died.
Fate of mulloway
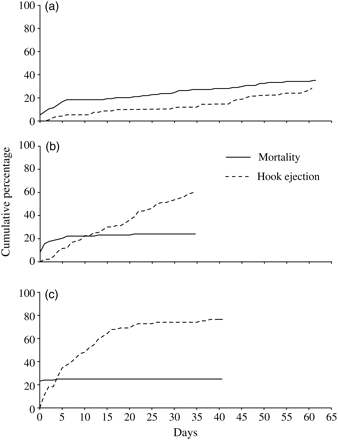
In addition to the 18 control fish (mean size ± s.d. of 26.4 ± 1.5 cm LT), 114 mulloway (18–20 fish for each of the six hook-ingestion treatments; 26.6 ± 1.3 cm LT) were monitored for up to 61 d. The additional replicates for some treatments were attributable to deaths occurring within 4 h of hooking (Figure 2a). Mortalities and hook-ejection reached cumulative totals of 35 and 30%, the latter over an average (±s.e.) of 29.2 ± 4.4 d (Figure 2a).
Cumulative percentage of total hook ejection and mortality of hook-ingested (a) mulloway, A. japonicus, (b) yellowfin bream, A. australis, and (c) snapper, P. auratus.
The most parsimonious GLMM explaining the variation among mortality to mulloway was reduced to three significant fixed variables: hook modification, nickel plating, and hook ejection (p <0.05; Tables 3 and 4). Specifically, more fish died after ingesting modified than unmodified hooks (42 vs. 27%) and those that had nickel plating, rather than those that did not (43 vs. 31%; p< 0.05; Tables 3 and 4). None of the fish (n= 22) that ejected their hooks died, compared with 43% of those that remained hook-ingested (p< 0.001; Tables 3 and 4).
Summary of fixed variables tested in parsimonious generalized linear mixed models for their independence on the mortality and hook ejection of hook-ingested mulloway, A. japonicus, yellowfin bream, A. australis, and snapper, P. auratus, after 61, 35, and 41 d, respectively.
| Variable . | Mulloway . | Yellowfin bream . | Snapper . | |||
|---|---|---|---|---|---|---|
| . | Mortality . | Hook ejection . | Mortality . | Hook ejection . | Mortality . | Hook ejection . |
| Hook ejection | *** | na | *** | na | *** | na |
| Hook modification | * | ○ | – | – | * | * |
| Nickel plating | ** | * | * | ○ | – | – |
| Hook broken | – | * | – | – | – | ○ |
| Steel type | – | – | ○ | – | – | ** |
| LT | – | – | – | * | * | – |
| Bleeding | – | – | – | – | • | – |
| Variable . | Mulloway . | Yellowfin bream . | Snapper . | |||
|---|---|---|---|---|---|---|
| . | Mortality . | Hook ejection . | Mortality . | Hook ejection . | Mortality . | Hook ejection . |
| Hook ejection | *** | na | *** | na | *** | na |
| Hook modification | * | ○ | – | – | * | * |
| Nickel plating | ** | * | * | ○ | – | – |
| Hook broken | – | * | – | – | – | ○ |
| Steel type | – | – | ○ | – | – | ** |
| LT | – | – | – | * | * | – |
| Bleeding | – | – | – | – | • | – |
–, term not included in the final parsimonious model; na, not applicable.
***p< 0.001.
**p< 0.01.
*p< 0.05.
•p< 0.1.
○p> 0.1.
Summary of fixed variables tested in parsimonious generalized linear mixed models for their independence on the mortality and hook ejection of hook-ingested mulloway, A. japonicus, yellowfin bream, A. australis, and snapper, P. auratus, after 61, 35, and 41 d, respectively.
| Variable . | Mulloway . | Yellowfin bream . | Snapper . | |||
|---|---|---|---|---|---|---|
| . | Mortality . | Hook ejection . | Mortality . | Hook ejection . | Mortality . | Hook ejection . |
| Hook ejection | *** | na | *** | na | *** | na |
| Hook modification | * | ○ | – | – | * | * |
| Nickel plating | ** | * | * | ○ | – | – |
| Hook broken | – | * | – | – | – | ○ |
| Steel type | – | – | ○ | – | – | ** |
| LT | – | – | – | * | * | – |
| Bleeding | – | – | – | – | • | – |
| Variable . | Mulloway . | Yellowfin bream . | Snapper . | |||
|---|---|---|---|---|---|---|
| . | Mortality . | Hook ejection . | Mortality . | Hook ejection . | Mortality . | Hook ejection . |
| Hook ejection | *** | na | *** | na | *** | na |
| Hook modification | * | ○ | – | – | * | * |
| Nickel plating | ** | * | * | ○ | – | – |
| Hook broken | – | * | – | – | – | ○ |
| Steel type | – | – | ○ | – | – | ** |
| LT | – | – | – | * | * | – |
| Bleeding | – | – | – | – | • | – |
–, term not included in the final parsimonious model; na, not applicable.
***p< 0.001.
**p< 0.01.
*p< 0.05.
•p< 0.1.
○p> 0.1.
Significant categorical (counts) and continuous (mean ± s.d.) fixed effects (p< 0.05) identified in GLMMs affecting the mortality and hook ejection of mulloway, A. japonicus, yellowfin bream, A. australis, and snapper, P. auratus, over 61, 35, and 41 d, respectively.
| Variable . | Mulloway . | Yellowfin bream . | Snapper . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Mortality . | Hook ejection . | Mortality . | Hook ejection . | Mortality . | Hook ejection . | ||||||
| Hook ejection | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes |
| Yes | 22 | 0 | na | na | 50 | 0 | na | na | 62 | 1 | na | na |
| No | 52 | 40 | na | na | 32 | 26 | na | na | 19 | 26 | na | na |
| Hook modification | ||||||||||||
| Yes | 34 | 25 | – | – | – | – | – | – | 48 | 6 | 17 | 35 |
| No | 40 | 15 | – | – | – | – | – | – | 33 | 21 | 28 | 26 |
| Nickel plating | ||||||||||||
| Yes | 21 | 16 | 22 | 15 | 22 | 14 | – | – | – | – | – | – |
| No | 53 | 24 | 70 | 7 | 60 | 12 | – | – | – | – | – | – |
| Hook broken | ||||||||||||
| Yes | – | – | 7 | 9 | – | – | – | – | – | – | – | – |
| No | – | – | 85 | 13 | – | – | – | – | – | – | – | – |
| Steel type | ||||||||||||
| Carbon | – | – | – | – | – | – | – | – | – | – | 22 | 50 |
| Stainless | – | – | – | – | – | – | – | – | – | – | 23 | 13 |
| LT (cm) | – | – | – | – | – | – | 21.8 (2.1) | 23.0 (1.8) | 24.4 (4.1) | 26.0 (3.5) | – | – |
| Variable . | Mulloway . | Yellowfin bream . | Snapper . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Mortality . | Hook ejection . | Mortality . | Hook ejection . | Mortality . | Hook ejection . | ||||||
| Hook ejection | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes |
| Yes | 22 | 0 | na | na | 50 | 0 | na | na | 62 | 1 | na | na |
| No | 52 | 40 | na | na | 32 | 26 | na | na | 19 | 26 | na | na |
| Hook modification | ||||||||||||
| Yes | 34 | 25 | – | – | – | – | – | – | 48 | 6 | 17 | 35 |
| No | 40 | 15 | – | – | – | – | – | – | 33 | 21 | 28 | 26 |
| Nickel plating | ||||||||||||
| Yes | 21 | 16 | 22 | 15 | 22 | 14 | – | – | – | – | – | – |
| No | 53 | 24 | 70 | 7 | 60 | 12 | – | – | – | – | – | – |
| Hook broken | ||||||||||||
| Yes | – | – | 7 | 9 | – | – | – | – | – | – | – | – |
| No | – | – | 85 | 13 | – | – | – | – | – | – | – | – |
| Steel type | ||||||||||||
| Carbon | – | – | – | – | – | – | – | – | – | – | 22 | 50 |
| Stainless | – | – | – | – | – | – | – | – | – | – | 23 | 13 |
| LT (cm) | – | – | – | – | – | – | 21.8 (2.1) | 23.0 (1.8) | 24.4 (4.1) | 26.0 (3.5) | – | – |
–, Term not significant in the parsimonious model (p< 0.05); na, not applicable.
Significant categorical (counts) and continuous (mean ± s.d.) fixed effects (p< 0.05) identified in GLMMs affecting the mortality and hook ejection of mulloway, A. japonicus, yellowfin bream, A. australis, and snapper, P. auratus, over 61, 35, and 41 d, respectively.
| Variable . | Mulloway . | Yellowfin bream . | Snapper . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Mortality . | Hook ejection . | Mortality . | Hook ejection . | Mortality . | Hook ejection . | ||||||
| Hook ejection | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes |
| Yes | 22 | 0 | na | na | 50 | 0 | na | na | 62 | 1 | na | na |
| No | 52 | 40 | na | na | 32 | 26 | na | na | 19 | 26 | na | na |
| Hook modification | ||||||||||||
| Yes | 34 | 25 | – | – | – | – | – | – | 48 | 6 | 17 | 35 |
| No | 40 | 15 | – | – | – | – | – | – | 33 | 21 | 28 | 26 |
| Nickel plating | ||||||||||||
| Yes | 21 | 16 | 22 | 15 | 22 | 14 | – | – | – | – | – | – |
| No | 53 | 24 | 70 | 7 | 60 | 12 | – | – | – | – | – | – |
| Hook broken | ||||||||||||
| Yes | – | – | 7 | 9 | – | – | – | – | – | – | – | – |
| No | – | – | 85 | 13 | – | – | – | – | – | – | – | – |
| Steel type | ||||||||||||
| Carbon | – | – | – | – | – | – | – | – | – | – | 22 | 50 |
| Stainless | – | – | – | – | – | – | – | – | – | – | 23 | 13 |
| LT (cm) | – | – | – | – | – | – | 21.8 (2.1) | 23.0 (1.8) | 24.4 (4.1) | 26.0 (3.5) | – | – |
| Variable . | Mulloway . | Yellowfin bream . | Snapper . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Mortality . | Hook ejection . | Mortality . | Hook ejection . | Mortality . | Hook ejection . | ||||||
| Hook ejection | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes |
| Yes | 22 | 0 | na | na | 50 | 0 | na | na | 62 | 1 | na | na |
| No | 52 | 40 | na | na | 32 | 26 | na | na | 19 | 26 | na | na |
| Hook modification | ||||||||||||
| Yes | 34 | 25 | – | – | – | – | – | – | 48 | 6 | 17 | 35 |
| No | 40 | 15 | – | – | – | – | – | – | 33 | 21 | 28 | 26 |
| Nickel plating | ||||||||||||
| Yes | 21 | 16 | 22 | 15 | 22 | 14 | – | – | – | – | – | – |
| No | 53 | 24 | 70 | 7 | 60 | 12 | – | – | – | – | – | – |
| Hook broken | ||||||||||||
| Yes | – | – | 7 | 9 | – | – | – | – | – | – | – | – |
| No | – | – | 85 | 13 | – | – | – | – | – | – | – | – |
| Steel type | ||||||||||||
| Carbon | – | – | – | – | – | – | – | – | – | – | 22 | 50 |
| Stainless | – | – | – | – | – | – | – | – | – | – | 23 | 13 |
| LT (cm) | – | – | – | – | – | – | 21.8 (2.1) | 23.0 (1.8) | 24.4 (4.1) | 26.0 (3.5) | – | – |
–, Term not significant in the parsimonious model (p< 0.05); na, not applicable.
For the surviving mulloway, the most parsimonious GLMM explaining their hook ejection was reduced to a model consisting of hook modification, hook breakage, and nickel plating (p< 0.05; Tables 3 and 4). Of these three factors, only the last two were significant, with 41% of nickel-plated hooks ejected, compared with just 9% of the other two steel types (p< 0.05; Tables 3 and 4). Further, significantly more ejected hooks were broken than remained whole (56 vs. 13%; p< 0.05; Tables 3 and 4).
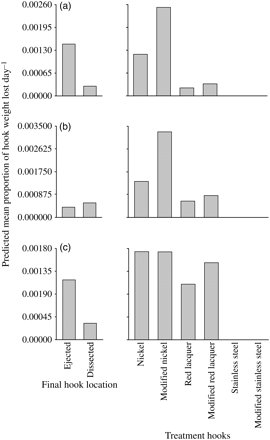
There were significant differences in the mean predicted proportions of hook weight lost per day (i.e. the rate of oxidation) between all ejected and dissected hooks (LMM; Wald F= 28.45, p< 0.01), and among the different treatments, irrespective of their location (LMM; Wald F= 30.70, p< 0.01; Figure 3a). Specifically, ejected whole hooks had oxidized at a mean predicted rate that was almost five times greater than those that remained ingested (Figure 3a). Also, nickel-plated hooks, especially those that were modified, oxidized more than ten times faster than red-lacquer hooks, whereas stainless-steel hooks had virtually no corrosion (Figure 3a). There was no significant interaction between the location of hooks and treatment type, nor any effect of the LT of fish on the rate of hook oxidation (LMM; p> 0.05).
Back-transformed predicted mean proportions of weights lost per day (through oxidation) for all ejected and dissected hooks, and each of the treatment hooks (irrespective of their final location) in (a) mulloway, A. japonicus, (b) yellowfin bream, A. australis, and (c) snapper, P. auratus.
The LMM detected significant differences in the concentrations of ln(x+ 1)-transformed plasma glucose among the groups of sampled fish (Wald F= 4.68, p< 0.01). In particular, fish that were immediately sampled from the wild had slightly lower glucose levels (back-transformed predicted mean of 1.00 mmol l–1) than those in the holding tanks (2.03 mmol l–1) before the experiment, and the control (2.37 mmol l–1), hook-ingested (1.88 mmol l–1), and hook-ejected (2.60 mmol l–1) fish at the end of the experiment. All plasma glucose levels were within previously assessed baseline limits.
Fate of yellowfin bream
Totals of 18 control (mean LT± s.d. of 19.7 ± 2.1 cm) and 108 treatment (22.3 ± 2.0 cm LT) yellowfin bream were monitored for 35 d. Total mortality was 24% (mainly in the first 24 h; Figure 2b). The most parsimonious GLMM explaining these deaths was reduced to the significant fixed effects of nickel plating and hook ejection, with 39% mortality among fish that ingested nickel-plated hooks compared with just 17% mortality for the other steel types combined (p< 0.05; Tables 3 and 4). There were no deaths among any fish (n= 50) that ejected their hooks, but 45% of the remaining fish (n= 58) that retained their hooks died (p< 0.05; Tables 3 and 4).
For the surviving fish, cumulative hook ejection reached a total of 61% over a mean (± s.e.) of 17.1 ± 1.4 d (Figure 2b). The most parsimonious GLMM included LT and nickel plating, but only the former was significant (p< 0.01; Tables 3 and 4). Irrespective of hook type, there was a clear positive relationship between their ejection and the size of fish (Table 4).
The mean predicted rate of oxidation was not significantly different between hooks that were ejected or dissected from yellowfin bream (LMM; Wald F= 0.014, p> 0.05), but it did vary significantly among treatment designs, irrespective of their location (LMM; Wald F= 45.39, p< 0.01). Specifically, modified nickel-plated hooks oxidized up to 500 times faster than the red-lacquer and stainless-steel designs (Figure 3b). There was no significant interaction between hook location and treatment type, nor a significant effect of LT on the rate of hook oxidation (LMM; p> 0.05).
There were significant differences in the concentrations of ln(x+ 1)-transformed plasma glucose of yellowfin bream that were immediately sampled from the wild (back-transformed mean of 2.25 mmol l–1) and those in the holding tanks (2.18 mmol l–1) before the experiment and the control (1.24 mmol l–1), and hook-ingested (1.41 mmol l–1) and hook-ejected (1.33 mmol l–1) fish at the end of the experiment (LMM; Wald F= 7.74, p< 0.01). However, all glucose levels were within the range of previously assessed baseline estimates.
Fate of snapper
In all, 18 control (mean LT ± s.d. 23.7 ± 3.5 cm) and 108 hook-ingested (24.8 ± 4.0 cm LT) fish were monitored for 41 d. Mortalities among the treatment groups reached a total of 25% (Figure 2c). Like yellowfin bream, most deaths were soon after the treatment, many on the first day (Figure 2c). The most parsimonious GLMM explaining these deaths included hook ejection, LT, hook modification, and bleeding, but only the first three of these were significant (p< 0.05; Tables 3 and 4). Like the other species, hook ejection had a strong impact, with a single fatality among 63 fish that ejected their hook, but 26 deaths (58%) from the remaining 45 hook-ingested fish (p <0.001; Tables 3 and 4). Mortalities were also significantly biased towards fish that ingested conventional rather than modified hooks (39 vs. 11%) and towards those that were larger (Table 4).
Hook ejection reached a total of 77%, with a mean (±s.e.) of 9.3 ± 1.0 d and was mostly explained by steel type and modification (parsimonious GLMM, p< 0.05; Tables 3 and 4). Carbon-steel hooks were ejected faster than those made from stainless steel (69 vs. 36%; p< 0.001; Tables 3 and 4). Similarly, modified designs were ejected more frequently than conventional designs (67 vs. 48%; p< 0.05; Tables 3 and 4).
LMM detected significant effects of the location (LMM; Wald F= 18.7, p< 0.01) and type of hook (LMM; Wald F= 11.36, p< 0.01) on their rates of oxidation (Figure 3c). Ejected hooks had a predicted mean rate of oxidation that was ∼3.5 times faster than those that were dissected, and nickel-plated hooks oxidized slightly faster than red-lacquer designs (Figure 3c). Stainless-steel hooks remained mostly corrosion-free (Figure 3c). There was no significant interaction between hook location and treatment type, nor an effect of LT on the rate of oxidation (LMM; p> 0.05).
The concentrations of ln(x + 1)-transformed plasma glucose were significantly different among wild fish (back-transformed mean of 1.02 mmol l–1) and those in the holding tanks (2.90 mmol l–1) at the start of the experiment, and the control (2.50 mmol l–1), and hook-ingested (2.77 mmol l–1) and hook-ejected (2.37 mmol l–1) fish at the end of the experiments (LMM, Wald F= 6.55, p <0.05). However, as for the other two species, all levels of glucose were within baseline estimates.
Discussion
The total mortalities to mulloway, yellowfin bream, and snapper were comparable when considered across the same times (e.g. 27, 24, and 25%, respectively, at the end of 5 weeks) and well within the range (15–52%) for other line-cut, hook-ingested teleosts assessed for similar durations (reviewed by Hall et al., 2009). Moreover, all fatalities were much lower than those observed for the three species after being released with their ingested hooks removed (e.g. 58–88%; Broadhurst et al., 2007; Butcher et al., 2007; Grixti et al., 2010). These results, combined with the rapid resumption of normal feeding (within 1 week), along with the conformity among mean plasma glucose between controls and treatment groups at the end of sampling, provide strong support for cutting the line as a means of minimizing negative impacts associated with hook ingestion (Aalbers et al., 2004; Hall et al., 2009; Butcher et al., 2010).
Notwithstanding these positive results, the observed mortalities and the potential for at least some sublethal welfare impacts to fish released with ingested hooks warrant consideration of ancillary mitigation strategies, the prioritization of which requires comprehension of the key deleterious factors. Irrespective of monitoring period, the strongest predictor of fatalities for all three species was the presence of the hook (manifesting as a consistent significant effect of hook ejection). In particular, except for a single snapper, all fatalities were limited to those fish with hooks still lodged in their digestive tract, with many (especially for the sparids) deaths occurring soon after hooking, e.g. within 24 h. These rapid deaths were attributed to either perforated vital organs or the diffusion of water into the digestive tract (through torn tissue) and associated osmoregulatory dysfunction. For snapper, such impacts were significantly aggravated by fish size, probably because larger fish exerted relatively greater force during hooking and therefore suffered more damage.
The trauma caused by ingested hooks was also exacerbated by subtle differences in their physical properties. For example, some of the variability among fatalities to mulloway and yellowfin bream was attributed to the nickel-plated hooks. One explanation for this effect on mulloway was that, as a consequence of rapid oxidation and the reduced strength associated with a narrower wire diameter at the notches (McGrath et al., in press), the modified nickel-plated hooks broke more frequently (e.g. 13 broken hooks with five associated mortalities) than all other treatments (a total of three breakages). Broken hooks were more readily ejected, but any associated sharp hook-ends could have further damaged the digestive tract.
Although highly speculative, another possible cumulative negative impact to both mulloway and yellowfin bream associated with nickel-plated hooks was the separation of elemental compounds during their decay. According to the composition analyses, these hooks had ∼2.5% or ∼7 mg of nickel, some component of which would have been released during their relatively rapid oxidation. Few studies are available on the physiological impacts of excessive nickel in fish, but this element is considered harmful at >0.02 mg l–1 in drinking water for humans. The potential for the accumulation of this and other elements in fish during hook oxidation warrants further consideration.
Another significant hook-related factor influencing mortality was their modification, the presence of which had divergent impacts on mulloway and snapper. For mulloway, there was significantly more mortality among fish caught on modified hooks, but these hooks were also the only designs that broke, so some fatalities at least might be explained by the associated trauma discussed above. A converse, and more immediate, relationship between hook modification and mortality, i.e. most deaths within 24 h, was noted for snapper. This result implicates the initial angling response as a key factor, because insufficient time had passed for any protracted impacts associated with hooks oxidizing or their progression along the digestive tract. Although entirely speculative, perhaps, owing to their relatively lesser strength and therefore greater flexibility (McGrath et al., in press), the modified hooks may have absorbed some of the lateral force imposed during ingestion, which could have reduced the initial damage to the digestive tract.
Irrespective of the negative physical and physiological factors associated with the presence of hooks in the digestive tract in all three species, it is clear that promoting their rapid ejection is by far the most appropriate strategy for reducing deaths. Although there were clear interspecific differences in the amounts and times of hook ejection, many of the significant contributory technical factors were related to their oxidation and decay, with relatively greater shedding of nickel-plated and broken hooks from mulloway and modified and carbon-steel hooks from snapper than the other treatments.
There are at least two key mechanisms underlying this broad positive relationship between the oxidation and ejection of hooks for these species, including (i) a reduction in their relative size and/or (ii) their blunter point and barb (Broadhurst et al., 2007). Notwithstanding possible trauma attributable to broken ends, e.g. for mulloway, it is conceivable that owing to their smaller sizes, hook pieces would be more easily ejected than hooks that remained whole. For whole hooks, the amount of decay was insufficient to decrease their overall area by any perceivable quantity. Rather, it is more likely that because their sharp point and barb were oxidized (McGrath et al., in press), these hooks were more easily regurgitated or progressed along the digestive tract, especially from snapper and mulloway.
Although hook ejection by mulloway and snapper was largely explained by oxidation, for yellowfin bream LT was more important, probably reflecting relative digestive-tract volume and associated hook displacement. For example, Broadhurst et al. (2007) observed that similar hooks ingested by yellowfin bream maintained the same lateral plane as the fish, but rotated into various positions within the stomach over 42 d, which may have better facilitated their ejection. Conceivably, such movement would be easier in larger fish.
Similar impacts of relative size were used to explain poor ejection (25%) of long-shank hooks by sand whiting (McGrath et al., 2009) and may also account for the broad differences in overall ejection and timings between mulloway (30% over an average of ∼30 d) and the two sparids (61 and 77% over means of 17 and 9 d for yellowfin bream and snapper, respectively) observed here. Specifically, all three species had comparable LTs, but mulloway are more fusiform with a relatively lower dorsal profile, which could translate to a lower vertical height in their digestive tract and less room for hooks to be re-orientated into positions more suitable for ejection. Also, both sparids typically consume molluscs and crustaceans and are presumably accustomed to ejecting hard structures (Kailola et al., 1993). Like many teleosts, yellowfin bream and snapper have pharyngeal teeth that help to digest hard and sharp materials by breaking the indigestible parts into smaller pieces.
Despite possible interspecific variability among the importance of fish size/morphology, it is clear that for snapper and mulloway, promoting hook oxidation would facilitate ejection and, usually, reduce mortality. In addition to the technical factors assessed here, there are other important predictors of hook oxidation and decay. In particular, McGrath et al. (in press) showed that the oxidation of carbon-steel hooks can be promoted simply by increasing the front length and reducing the bend, gape, and especially the wire diameter. The latter would also reduce hook strength, but even a 10–20% reduction in the wire diameter of the hooks assessed in this study would be strong enough to catch all three species at the sizes examined (McGrath et al., in press).
The above and other modifications to hooks designed to increase their decay after ingestion need to be assessed in future work, along with the potential for other sources of indirect mortality, such as predation and a reduced ability to acquire food, neither of which could be investigated here. Butcher et al. (2010) showed few negative impacts to line-cut, hook-ingested yellowfin bream released into the wild with bio-telemetry tags, but the long-term fate of the other two species remains unknown. The conformity in results for the two sparids, combined with the plateau in low mortalities and rapid, high rate of ejection by snapper, may support few subsequent impacts. However, hook retention by mulloway and the consistent increase in fatalities over the monitored 61 d indicates a protracted response, which needs to be investigated further. In the meantime, the results from this work support a strategy of targeting all three species with carbon-steel hooks made from narrow wire diameters (≤0.9 mm proposed by McGrath et al., in press) and with minimal protective coating. Any fish that ingest hooks should be released with the line cut; doing so would reduce unaccounted fishing mortality.
Acknowledgements
The study was funded by the NSW Department of Primary Industries, the NSW Recreational Fishing Trusts, and the University of New England, Armidale. The research was approved by the NSW DPI Animal Care and Ethics Authority. Craig Brand and Alex Hulme are thanked for technical assistance.