-
PDF
- Split View
-
Views
-
Cite
Cite
Richard D. Hedger, Ingebrigt Uglem, Eva B. Thorstad, Bengt Finstad, Cedar M. Chittenden, Pablo Arechavala-Lopez, Arne J. Jensen, Rune Nilsen, Finn Økland, Behaviour of Atlantic cod, a marine fish predator, during Atlantic salmon post-smolt migration, ICES Journal of Marine Science, Volume 68, Issue 10, November 2011, Pages 2152–2162, https://doi.org/10.1093/icesjms/fsr143
Close - Share Icon Share
Abstract
Acoustic telemetry was used to determine the behavioural strategies of Atlantic cod (Gadus morhua) during Atlantic salmon (Salmo salar) post-smolt migration within a Norwegian fjord (Eresfjord). In all, 38 adult cod captured in the inner fjord were tagged with acoustic transmitters in 2008 and 2009, and their behaviour was determined using a fixed hydrophone array. Cod tended to aggregate in the innermost part of the fjord, occupying distinct demersal home territories, showing horizontal movements consistent with foraging. Tidal influences were not observed; cod spent more time near the surface at night. Cod behaviour during peak post-smolt migration differed from that before and after migration. First, cod tended to have more-focused spatial distributions during peak post-smolt migration, consistent with them not having to forage so far when prey were available in abundance. Second, some half the cod were detected nocturnally (but rarely during daylight) near the river mouth during peak post-smolt migration, consistent with them feeding on nocturnally migrating post-smolts. Third, cod were more common near the surface, consistent with them feeding on post-smolts migrating through near-surface waters. These patterns, however, were not shown by all the cod, suggesting that this opportunistic feeder was also preying on other species.Hedger, R. D., Uglem, I., Thorstad, E. B., Finstad, B., Chittenden, C. M., Arechavala-Lopez, P., Jensen, A. J., Nilsen, R., and Økland, F. 2011. Behaviour of Atlantic cod, a marine fish predator, during Atlantic salmon post-smolt migration. – ICES Journal of Marine Science, 68: 2152–2162.
Introduction
Predator and prey interactions may shape the behaviour and habitat utilization of both prey and predators (Lima, 2002). In general, a mobile predator should feed preferentially in patches with the greatest density of mobile prey, whereas the prey should prefer patches with the least risk of being eaten. However, in specific situations, the prey has little or no choice of selecting patches with low predation risk. This may happen during life-history-related prey migrations through spatially and temporally limited migratory passages. One example of such a situation is when juvenile anadromous salmonids migrate from their natal rivers to their feeding areas in the ocean. In that life history phase, smoltified salmonids experience a transition from freshwater to seawater, which involves demanding physiological changes and radical habitat alteration (Thorstad et al., 2011b). In addition, the post-smolts encounter a range of new predators, perhaps especially in the estuary phase, where their spatial movements are often restricted in the narrow and shallow river mouths and fjord bottoms. The transition period from freshwater to coastal waters is therefore considered to be a period during which the natural mortality of Atlantic salmon (Salmo salar) is high (Hansen and Quinn, 1998; Jepsen et al., 2000; Klemetsen et al., 2003). Recent research has shown that mortalities may exceed 60% during the first 40 km of the migration in coastal waters (Thorstad et al., 2007).
The Atlantic cod (Gadus morhua) is an opportunistic feeder (Link and Garrison, 2002) that may prey on salmonid post-smolts during post-smolt runs, which are typically during May and June in Norway. Previous studies over shorter stretches estimated that predation from Atlantic cod and saithe (Pollachius virens) was up to 25% in a single estuary (Hvidsten and Møkkelgjerd, 1987) and 20% by Atlantic cod alone in another estuary (Hvidsten and Lund, 1988). Rates of predation in estuaries are high for both wild and hatchery-reared post-smolts (Hvidsten and Lund, 1988; Jepsen et al., 2006), but the presence of other fish prey for marine predators, such as lesser sandeel (Ammodytes marinus), may prevent notable predation on Atlantic salmon post-smolts (Svenning et al., 2005). Atlantic cod are noted for their behavioural plasticity, with cod diving behaviour dependent on whether the stock is migratory or resident (Hobson et al., 2007), foraging strategies dependent on fjord location (Espeland et al., 2010), and diel activity patterns dependent on water temperature (Clark and Green, 1990). However, there has not been much research on behavioural plasticity in fjords in relation to salmonid post-smolt runs, so a study of this is necessary to show whether this opportunistic predator is aware of and able to exploit rich but spatially and temporally restricted resources.
The Atlantic cod has several relatively independent stocks with differing life history characteristics and migration patterns (Brander, 1994; Robichaud and Rose, 2004). In western and northern Norwegian waters, Atlantic cod can be divided into two major groups, northeast Arctic cod (NEAC) and Norwegian coastal cod (NCC). The NEAC migrate from feeding areas in the Barents Sea to spawning areas close to the coast (Hylen, 1964; Bergstad et al., 1987; Brander, 1994), whereas the NCC migrate over shorter distances and spawn in fjords and coastal areas (Rollefsen, 1954; Jakobsen, 1987; Godø, 1995; Maurstad and Sundet, 1998). In Norway, the NCC has declined in southern areas, although the stocks in northern Norway have remained stable. Detailed information on the life history of NCC is limited (Berg and Pedersen, 2001), but recent research indicates that it might consist of genetically divergent inner and outer subpopulations (Sarvas and Fevolden, 2005a, b). In the current study, the cod studied were caught in the innermost part of a large Norwegian fjord, and it is reasonable to assume that most or all of them were NCC.
The main aim of the study was to examine if and how the habitat utilization of Atlantic cod changes during a restricted period when specific prey species, such as Atlantic salmon post-smolts, are in large numbers. This was achieved by (i) quantifying the movement patterns (both horizontal and vertical) of Atlantic cod within a fjord, and (ii) comparing those patterns during salmonid post-smolt runs with the patterns before and after these runs. The influence of abiotic environmental influences on cod movement was also analysed.
Methods
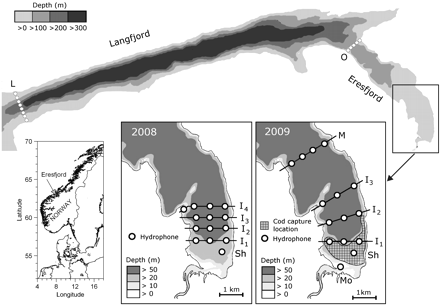
Eresfjord (62.702°N 8.128°E) is situated in central Norway and discharges into Langfjord (Figure 1). Together, these two fjords make up the innermost part of the Romsdalfjord system. Eresfjord is fed by the River Eira (mean annual discharge 17 m3 s–1), which contains both Atlantic salmon and anadromous brown trout (Salmo trutta). The river is regulated for hydropower production, so hatchery-reared salmon and trout juveniles are released into the river to augment the wild salmonid populations. The survival and migration routes of salmon are well studied in the inner parts of the fjord in a range of studies on both wild- and hatchery-reared post-smolts (e.g. Finstad et al., 2005; Thorstad et al., 2007, 2011a), and the predation of out-migrating post-smolts by marine predators has also been documented (Jepsen et al., 2006). The river inflow is on the southeastern side of the fjord.
The study area, with hydrophone positions shown by white circles. The cod capture location in 2008 and 2009 is shown on the panel for 2009.
Atlantic cod acoustic telemetry
Acoustic telemetry was used to monitor spatio-temporal distributions and movement patterns of individual Atlantic cod during May and June of 2008 and 2009. In 2008, 25 hydrophones were deployed in three groups: (i) a hydrophone on the shelf (Sh) and four hydrophone rows within inner Eresfjord (I1, I2, I3, and I4); (ii) a hydrophone row crossing the mouth of outer Eresfjord (O); and (iii) a hydrophone row crossing the mouth of Langfjord (L; Figure 1). In 2009, 28 hydrophones were deployed. The configuration was similar to that of 2008, except that an additional hydrophone was deployed near the mouth of the river, only three hydrophone rows were deployed in inner Eresfjord, and an extra hydrophone row was deployed in middle Eresfjord (M). The receiver range of the hydrophones was typically 200–450 m. Hydrophones were deployed before the tagging and release of the first individual fish. Data were downloaded on 26 June 2008 and 15 June 2009. In 2009, hydrophones positioned near the river mouth (Mo) and on the shelf (Sh) malfunctioned during the study, though after the peak post-smolt run. Signals from fish transmitters were recorded automatically when a tagged fish was within the range of a hydrophone. Range tests indicated that under most conditions, a tagged fish would not pass through any of the hydrophone rows without being recorded. However, the range may have varied with environmental conditions and wave action.
In all, 38 Atlantic cod were captured, tagged, and released (2008, 11 cod; 2009, 27 cod; Table 1). Fish were captured up to ∼1 km from the river mouth, relatively close to the coast on the southeastern side of the fjord. The cod were captured with hook and line fishing gear in shallow water (<15 m), to reduce the probability of their swimbladders bursting on being raised to the surface. The cod were then anaesthetized by immersion in an aqueous solution of metacaine (Norsk medisinaldepot, 0.5 g l–1), then placed ventral side up onto a V-shaped surgical table. An incision (∼1.5 cm) was made with a scalpel on the ventral surface posterior to the pelvic girdle. The coded transmitter (V9P-1L-69KHz-S256, including a depth sensor with a maximum registered depth of 50 m, VEMCO Ltd, Canada, 9 × 39 mm, weight in air/water 5.2/2.7 g) was inserted through the incision and pushed into the body cavity in front of the pelvic girdle. The incision was closed with two or three independent silk sutures (2/0, Ethicon). The fish were regularly sprayed with water during the surgery. Before each incision, the surgical equipment was rinsed with 70% ethanol and allowed to dry. After surgery, the tagged cod were left to recover in a tank with a continuous flow of seawater. Individuals spent an average of 2:14, 2:26, and 6:38 min in anaesthesia, surgery, and recovery, respectively. The tagged cod were released 0–350 m from the site of their capture between 00:00 and 17:50. No abnormal swimming patterns were observed immediately after release, and it is believed that tagging effects will have been minor. All handling and tagging was conducted according to the Norwegian regulations on treatment and welfare of animals. Data from each individual cod were analysed to determine subsequent mortality. Death was indicated by a pattern of depth occupancy that would have resulted from a fish resting on the fjord bottom (i.e. no horizontal movement and a pattern of recorded depth that coincided with the tidal cycle). Three cod died soon after release in 2008 and were excluded from further analysis. Two cod died or expelled their transmitter towards the end of the study in 2009, as indicated by a switch in the detection pattern from one of migration to one of stationary position, and detections from this switch in the detection pattern on were removed from the dataset. In all, therefore, data from 35 cod were available for analysis.
Characteristics of tagged Atlantic cod.
| Parameter . | 2008 . | 2009 . |
|---|---|---|
| Number released | 11 | 27 |
| Release dates | 4, 6, 9, 10, 14 May | 25, 26, 28, 29 April; 1, 2, 3, 4 May |
| Number surviving throughout study period | 8 | 27 |
| Mean length and range (mm) | 661 (450–1 090) | 574 (308–750) |
| Mean body mass and range (g) | 3 340 (1 070–14 620) | 1 990 (290–4 470) |
| Parameter . | 2008 . | 2009 . |
|---|---|---|
| Number released | 11 | 27 |
| Release dates | 4, 6, 9, 10, 14 May | 25, 26, 28, 29 April; 1, 2, 3, 4 May |
| Number surviving throughout study period | 8 | 27 |
| Mean length and range (mm) | 661 (450–1 090) | 574 (308–750) |
| Mean body mass and range (g) | 3 340 (1 070–14 620) | 1 990 (290–4 470) |
Characteristics of tagged Atlantic cod.
| Parameter . | 2008 . | 2009 . |
|---|---|---|
| Number released | 11 | 27 |
| Release dates | 4, 6, 9, 10, 14 May | 25, 26, 28, 29 April; 1, 2, 3, 4 May |
| Number surviving throughout study period | 8 | 27 |
| Mean length and range (mm) | 661 (450–1 090) | 574 (308–750) |
| Mean body mass and range (g) | 3 340 (1 070–14 620) | 1 990 (290–4 470) |
| Parameter . | 2008 . | 2009 . |
|---|---|---|
| Number released | 11 | 27 |
| Release dates | 4, 6, 9, 10, 14 May | 25, 26, 28, 29 April; 1, 2, 3, 4 May |
| Number surviving throughout study period | 8 | 27 |
| Mean length and range (mm) | 661 (450–1 090) | 574 (308–750) |
| Mean body mass and range (g) | 3 340 (1 070–14 620) | 1 990 (290–4 470) |
Abiotic environmental characteristics
Local times of sunrise and sunset were calculated using the NOAA procedure (srrb.noaa.gov/highlights/sunrise/sunrise.html). Temperature and depth on the seabed near the river mouth were measured by a TD (temperature, depth, Sensus ultra, ReefNet Inc., Ontario, Canada) logger from 7 May to 10 August 2009. Point measurements of the vertical profiles of salinity and temperature were made using a CTD (conductivity, temperature, depth) logger (Valeport miniCTD, Valeport Limited, Devon, UK) at selected hydrophones throughout Eresfjord in May of both 2008 and 2009. Surface temperature increased from ∼10°C at the beginning of May to peak at ∼20°C at the end of July, before decreasing to ∼10°C by mid-August. Salinity was highly dependent on tidal phase. Eresfjord had strong thermal and salinity stratification, with a thermocline and pycnocline at a depth of ∼10 m. Tidal elevation was predicted using the WTides package (wtides.com). Verification of the accuracy of this prediction was made by comparison with the depth measured by the TD logger.
Salmonid post-smolt runs
A smolt trap situated 1.5 km upstream in the river was used to determine the timing of the smolt run of both wild- and hatchery-reared Atlantic salmon and brown trout smolts. Most of the migrants were Atlantic salmon; 425 wild and 51 500 hatchery salmon, and just 81 wild and 9000 hatchery trout in 2008; 536 wild and 63 000 hatchery salmon, and just 326 wild and 8000 hatchery trout in 2009. The travel time from release at the trap to the river mouth may take a smolt several hours. The greatest temporal variation in smolt runs resulted from the aperiodic release of hatchery salmon. Peak smolt runs were arbitrarily defined as any period when the number of smolts passing the trap exceeded 5000 d–1, plus the day following this, given that it took some time for the post-smolts to leave the fjord. The post-smolt runs peaked from 7 to 8 May 2008 and from 9 to 18 May 2009.
To understand how post-smolt movement patterns influence cod foraging behaviour, including residence time in the fjord, depth use, and migratory pattern relative to the coast, acoustic telemetry was also used to track outmigrating Atlantic salmon post-smolts. In 2008 and 2009, 99 and 74 hatchery-reared smolts, respectively, were tagged with acoustic transmitters and released (transmitter model V9P-1L-69KHz-S256 with depth sensor, VEMCO Ltd, Canada, 9 × 39 mm, weight in air/water 5.2/2.7 g, tagging method as described in Finstad et al., 2005). To quantify the migratory patterns of salmon post-smolts, detections from those that died in the fjord were removed. Criteria for identifying post-smolts that may have died were (i) a lack of detections in hydrophone row O in outer Eresfjord, and (ii) a depth-use pattern that was consistent with them having been eaten by a predator. In all, 52 salmon post-smolts were gauged to have survived until the outermost hydrophone row. Most tagged smolts were released at the river mouth, but six were released upstream in the river.
Statistical analysis
All data processing and statistical analysis was conducted in R (R Development Core Team, 2010). Migration patterns of the tagged salmon post-smolts were analysed with respect to how long it took them to migrate through the inner fjord, their vertical distribution, the changes in their vertical distribution as they migrated through the fjord (using the Kruskal–Wallis tests), and the time of day of migration for the six smolts released upstream in the river.
Cod loci (principal positions of residence) and daily areal ranges (area covered during a day) were estimated for each individual cod within the inner part of Eresfjord. These metrics require abundant spatial data; the density of hydrophones was too low for these to provide such data beyond inner Eresfjord, and one cod was not detected enough times for a locus to be determined. The locus occupied by each individual was identified using kernel smoothing [R function kde2d(MASS), bandwidth 1000 m]. The daily areal range was estimated using a two-stage procedure. First, individual cod positions were interpolated as a function of time from the hydrophone detections using kernel smoothing [Hedger et al., 2008a; R function ksmooth(), kernel distribution normal, bandwidth 15 min, estimation interval 15 min]. Second, from the individual cod positions, the daily areal range was determined using the minimum convex polygon (MCP) method [R function calc_mcp(aspace)]. This method calculates the smallest polygon that will encompass a set of coordinates (Burgman and Fox, 2003), and has been used frequently to define the areas occupied by fish (see Collins et al., 2007; Thibault et al., 2007; Abecasis and Erzini, 2008). The MCP for each cod and each day was estimated using all positions for the respective cod and day. A minimum of five observations was required for estimating the MCP.
The effect of tidal phase and diel period on seaward or inland longitudinal movements of cod within inner Eresfjord was determined using Pearson's χ2 test. The effect of time of day and tidal phase on depth was determined using a Kruskal–Wallis test and a Mann–Whitney U-test, respectively. Cod behaviour relative to peak post-smolt run timing was only examined for 2009, because the period (2 d) during which the post-smolt run peaked in 2008 was too short to obtain sufficient observations of cod behaviour to compare with cod behaviour before and after the post-smolt run. This problem was compounded by the relatively few cod tagged in 2008. The across-fjord spatial distribution of cod in 2009 (numbers detected in the column of hydrophones on the western coast, in the column of hydrophones on the eastern coast, and in the column of hydrophones mid-fjord away from either coast) was compared with the detected across-fjord spatial distribution of tagged post-smolts using Pearson's χ2 test.
Cod in 2009 were classified into two groups: (i) upper inner fjord cod (fish that were detected at the river mouth hydrophone; n = 14), and (ii) lower inner fjord cod (fish that were not detected at the river hydrophone; n = 13). For each group, a Kruskal–Wallis test and a Mann–Whitney U-test were used to compare daily areal ranges and the proportion of nocturnal detections in near-surface layers (depth ≤2 m) before, during, and after the post-smolt run in 2009.
Results
Salmonid post-smolt runs
Migration of the 52 tagged Atlantic salmon post-smolts through Eresfjord was rapid, but varied greatly according to individual (time from the first detection in the inner fjord to the first detection in row O: 1st quartile 0.32 d; median 0.64 d; 3rd quartile 1.60 d; n = 52). One post-smolt remained within Eresfjord for 10.5 d. Salmon post-smolts were detected at all hydrophones. However, there was an across-fjord trend in post-smolt detection at the innermost two hydrophone rows, with greater numbers of post-smolts detected on the eastern coast than the western. This trend was not apparent outward from the third hydrophone row. The tagged post-smolts were detected with a similar frequency at the hydrophones positioned near the coast as those in the middle of the fjord.
Post-smolts migrated through the surface waters of Eresfjord (2008, mean depth 0.85 m, n = 25; 2009, mean depth 0.64 m, n = 27). The individual-specific mean depth of occupancy did not vary significantly according to hydrophone row in 2008 (Kruskal–Wallis test, p = 0.66), but it did vary in 2009 (Kruskal–Wallis test, p < 0.001), showing a slight trend of decreasing depth with increasing distance into the fjord. For operational reasons, most post-smolts were released at the river mouth during daylight, so the diel pattern of behaviour through the fjord will have been affected by the time and place of release: these post-smolts migrated through the fjord mainly by day. However, for the six smolts released in the river (which presumably would have shown a pattern more consistent with a natural smolt run), migration through Eresfjord was mainly nocturnal: five migrated through the fjord at night, and one during the evening. Post-smolt depth within the fjord did not depend on release location (Mann–Whitney U-test, p = 0.07, n = 27).
Spatio-temporal distribution and patterns of cod movements
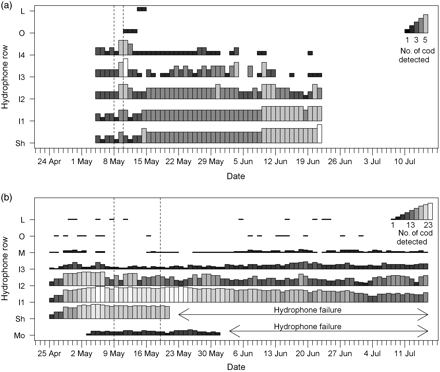
In both years, >90% of cod detections were in inner Eresfjord (Table 2). The highest rates of detection in 2008 were at the hydrophone positioned on the shelf in inner Eresfjord (Sh), and in 2009 were at hydrophone row I1, but the relative percentage of detections would have been biased by the premature failure of the hydrophones positioned on the shelf and at the river mouth (Table 1). Moreover, if there had been an equivalent number of hydrophones on the shelf as in row I1, it could be inferred that the detection rates at the shelf would have been even higher. In 2008, there was a slight temporal trend of increasing numbers of cod detected in the inner fjord (Sh–I2; Figure 2). This contrasted with the situation in 2009, where there was a temporal trend of decreasing numbers of cod detected in rows I1–I2.
Percentage of detections of Atlantic cod in each hydrophone row.
| Site . | Hydrophone row . | Distance from river mouth (km) . | Number of hydrophones in row . | Percentage of total detections in hydrophone row . | ||
|---|---|---|---|---|---|---|
| 2008 . | 2009 . | 2008 . | 2009 . | |||
| Inner Eresfjord | Mo | – | 0.00 | 1 | – | 0.61 |
| Inner Eresfjord | Sh | 0.48 | 0.57 | 1 | 43.37 | 12.32 |
| Inner Eresfjord | I1 | 0.59 | 0.59 | 3 | 36.69 | 57.28 |
| Inner Eresfjord | I2 | 1.00 | 1.31 | 3 | 8.62 | 17.97 |
| Inner Eresfjord | I3 | 1.40 | 2.03 | 3 | 4.96 | 8.49 |
| Inner Eresfjord | I4 | 2.90 | – | 4 | 6.02 | – |
| Middle Eresfjord | M | – | 3.52 | 4 | – | 3.17 |
| Outer Eresfjord | O | 10.94 | 9.91 | 5 | 0.24 | 0.07 |
| Langfjord | L | 37.91 | 36.72 | 8 | 0.09 | 0.08 |
| Site . | Hydrophone row . | Distance from river mouth (km) . | Number of hydrophones in row . | Percentage of total detections in hydrophone row . | ||
|---|---|---|---|---|---|---|
| 2008 . | 2009 . | 2008 . | 2009 . | |||
| Inner Eresfjord | Mo | – | 0.00 | 1 | – | 0.61 |
| Inner Eresfjord | Sh | 0.48 | 0.57 | 1 | 43.37 | 12.32 |
| Inner Eresfjord | I1 | 0.59 | 0.59 | 3 | 36.69 | 57.28 |
| Inner Eresfjord | I2 | 1.00 | 1.31 | 3 | 8.62 | 17.97 |
| Inner Eresfjord | I3 | 1.40 | 2.03 | 3 | 4.96 | 8.49 |
| Inner Eresfjord | I4 | 2.90 | – | 4 | 6.02 | – |
| Middle Eresfjord | M | – | 3.52 | 4 | – | 3.17 |
| Outer Eresfjord | O | 10.94 | 9.91 | 5 | 0.24 | 0.07 |
| Langfjord | L | 37.91 | 36.72 | 8 | 0.09 | 0.08 |
Hydrophones Mo and Sh failed during the study, resulting in their low percentage of total detections.
Percentage of detections of Atlantic cod in each hydrophone row.
| Site . | Hydrophone row . | Distance from river mouth (km) . | Number of hydrophones in row . | Percentage of total detections in hydrophone row . | ||
|---|---|---|---|---|---|---|
| 2008 . | 2009 . | 2008 . | 2009 . | |||
| Inner Eresfjord | Mo | – | 0.00 | 1 | – | 0.61 |
| Inner Eresfjord | Sh | 0.48 | 0.57 | 1 | 43.37 | 12.32 |
| Inner Eresfjord | I1 | 0.59 | 0.59 | 3 | 36.69 | 57.28 |
| Inner Eresfjord | I2 | 1.00 | 1.31 | 3 | 8.62 | 17.97 |
| Inner Eresfjord | I3 | 1.40 | 2.03 | 3 | 4.96 | 8.49 |
| Inner Eresfjord | I4 | 2.90 | – | 4 | 6.02 | – |
| Middle Eresfjord | M | – | 3.52 | 4 | – | 3.17 |
| Outer Eresfjord | O | 10.94 | 9.91 | 5 | 0.24 | 0.07 |
| Langfjord | L | 37.91 | 36.72 | 8 | 0.09 | 0.08 |
| Site . | Hydrophone row . | Distance from river mouth (km) . | Number of hydrophones in row . | Percentage of total detections in hydrophone row . | ||
|---|---|---|---|---|---|---|
| 2008 . | 2009 . | 2008 . | 2009 . | |||
| Inner Eresfjord | Mo | – | 0.00 | 1 | – | 0.61 |
| Inner Eresfjord | Sh | 0.48 | 0.57 | 1 | 43.37 | 12.32 |
| Inner Eresfjord | I1 | 0.59 | 0.59 | 3 | 36.69 | 57.28 |
| Inner Eresfjord | I2 | 1.00 | 1.31 | 3 | 8.62 | 17.97 |
| Inner Eresfjord | I3 | 1.40 | 2.03 | 3 | 4.96 | 8.49 |
| Inner Eresfjord | I4 | 2.90 | – | 4 | 6.02 | – |
| Middle Eresfjord | M | – | 3.52 | 4 | – | 3.17 |
| Outer Eresfjord | O | 10.94 | 9.91 | 5 | 0.24 | 0.07 |
| Langfjord | L | 37.91 | 36.72 | 8 | 0.09 | 0.08 |
Hydrophones Mo and Sh failed during the study, resulting in their low percentage of total detections.
The number of cod detected as a function of hydrophone row and date: (a) 2008, and (b) 2009. Dashed vertical lines show the start and end of the peak post-smolt run.
Five of the 35 surviving tagged cod (14%) had final detections at the hydrophone row at the mouth of Langfjord (L). This pattern may have resulted either from the cod migrating permanently out of the fjord or from individual cod temporarily moving out of the fjord and not returning until after the hydrophones were removed. Another five cod were detected at this row, but were later detected in Eresfjord, indicating that there were occasional longitudinal movements by some fish seawards then inland along the length of Langfjord. Ground speeds between inner Eresfjord (rows I1–I4) and the mouth of Eresfjord (row O) were similar (16 cm s–1) to those between the mouth of Eresfjord and the mouth of Langfjord (17 cm s–1). Note, however, that these groundspeeds show the straight-line distance moved over time, and that the actual swimming speed of the cod will have been greater because the cod would have moved in multiple directions (Coté et al., 2002; Fernö et al., 2011).
Within inner Eresfjord, individual cod had distinct home loci. Of the 35 fish for which individual loci were determined, 26 had single loci and 9 had multiple loci (Figure 3). Of the 26 with single loci, 16 were in the uppermost part of the inner fjord around hydrophone row I1, and 10 of these were at the eastern hydrophone of this row, where the main current of the river crossed the sill (including cod with TagIDs 154, 156, and 163). Other loci were farther out in the inner fjord on both eastern and western sides (e.g. the cod with TagID 142). Cod with multiple loci often covered a larger area of the inner fjord (e.g. the cod with TagIDs 164 and 165). Cod loci tended to be distinct from the release sites (mean distance of separation 867 m, minimum 282 m, maximum 1933 m; n = 34).
Detection densities of six selected cod within the inner and middle Eresfjord from 2009. Images have been made using the density estimation [kde2(MASS) function of R, bandwidth 1000 m], and are density-sliced in 10 layers from minimum (black) to maximum (white) density. Hydrophone positions are shown by the white circles.
The mean daily areal range increased with body length in 2009 (Spearman rank correlation, r = 0.9, p < 0.001). In 2008, just five cod were detected frequently enough to determine a daily areal range, so it was not possible to test for changes in daily areal range with body length. However, daily areal ranges of the two largest cod were more than double those of the three smallest ones.
In terms of depth, tagged cod were observed mainly at depths >10 m, but all were also detected near the surface. Densities of detections were high when cod were at depths extending down to ∼40–50 m. Cod may also have occupied deeper water in a seaward direction, but the depth sensors could not register depths >50 m. There was an increase in median depth the farther out into the fjord the cod were detected. Always, the cod showed wide ranges in depth over short time-scales (Figure 4; e.g. ∼10 m depth change over several minutes). This analysis of cod depth relative to fjord depth was complicated by the imprecision of the cod positions, but the swimming depths of some fish were consistent with the depth profile of the fjord bottom (e.g. Tag ID 164 in Figure 4); i.e. cod were demersal. The remaining cod were not detected for a sufficiently long period to confirm the existence or absence of a depth trend. For cod that showed long-term trends in depth occupancy, changes were sometimes associated with changes in horizontal location within the fjord, such as an increase in depth occupancy as the fish moved longitudinally seawards within the fjord, or changes while the individual cod remained relatively stationary in horizontal space.
Depth plots of six selected cod within the inner and middle Eresfjord from 2009. Grey lines link recorded depths, and show short-term diving and surfacing behaviour. Solid and dashed black lines are fitted from kernel smoothing of the tag depth recordings and water column depths of the receivers [R function ksmooth(), kernel normal, bandwidth 1 d], and show long-term trends in depth occupancy. Dashed vertical lines show the start and end of the peak post-smolt run. Note that cod may be detected within ∼500 m of a receiver, so the water column bottom depth at the receiver may differ from that where the cod was located.
Cod behaviour and abiotic environmental characteristics
On the days when vertical temperature and salinity profiles were measured, cod were found in a wide range of temperature and salinity (mean temperature 8.7°C, range 5.3–10.6°C; mean salinity 30.5, range 23.5–32.7). Some two-thirds of detections were registered in warmer, less saline waters above the thermocline/pycnocline.
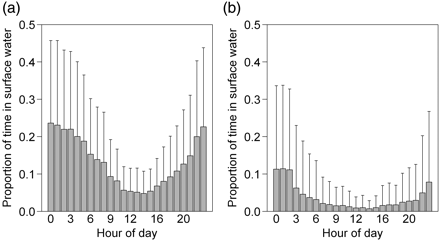
No effect of tidal phase or diel period on the seaward or inland longitudinal movements of individual cod within Eresfjord was apparent (χ2 test, p = 0.24). Approximately half the movements were seawards and the other half landwards, regardless of tidal phase and diel period. There was clear evidence of some cod changing their position according to time of day, tending to remain deeper within the water column during day, then spending more time near the surface at night in both the upper inner fjord (Kruskal–Wallis, p < 0.001) and the lower inner fjord (Kruskal–Wallis test, p = 0.019; Figure 5). Upper inner fjord cod occupied near-surface waters more than lower fjord cod by more than a factor of two. There was no significant difference between registered cod depths according to tidal phase (Mann–Whitney U-test, p = 0.27).
Proportion of cod detections from 2009 during occupancy of near-surface water (≤2 m) as a function of hour of day: (a) upper inner fjord cod (individuals detected at the river mouth hydrophone), and (b) lower inner fjord cod (individuals not detected at the river-mouth hydrophone). Barplots show the mean, with whiskers of 1 s.d.
Cod behaviour and post-smolt run
In the innermost part of the inner fjord (rows I1 and I2), cod were more often present at the central and eastern hydrophones than at the western hydrophone, showing a similar pattern to the detections of salmon post-smolts. Within inner and middle Eresfjord, across-fjord cod distributions were only significantly different from across-fjord post-smolt distributions in row M (χ2 test, p = 0.020). However, across-fjord cod distributions during the post-smolt run were not significantly different from those before or after the run in any row: I1 (χ2 test, p = 0.90), I2 (χ2 test, p = 0.52), I3 (χ2 test, p = 0.94), and M (χ2 test, p = 0.55).
Daily areal ranges of upper inner fjord cod (cod detected at the hydrophone at the river mouth) had smaller daily area ranges than lower inner fjord cod (Figure 6). The ranges depended on time relative to the peak post-smolt run (before, during, or after; Kruskal–Wallis, p = 0.019 and p = 0.001 for upper and lower inner fjord cod, respectively). For lower inner fjord cod, mean daily areal ranges during the post-smolt run were significantly smaller than those before the run (Mann–Whitney U-test, p < 0.001) and those after the run (Mann–Whitney U-test, p = 0.027). For upper inner fjord cod, mean daily areal ranges during the post-smolt run were significantly smaller than those before the run (Mann–Whitney U-test, p = 0.011), but not smaller than those after the run (Mann–Whitney U-test, p = 0.81). The mean proportion of nocturnal detections near the surface (depth ≤2 m) during post-smolt runs was 25.1%, greater than the 15.2% before and the 11.9% after; however, this difference was not significant (Kruskal–Wallis, p = 0.44).
Daily areal range of cod in 2009 as a function of date: (a) upper inner fjord cod (individuals detected at the river mouth hydrophone), and (b) lower inner fjord cod (individuals not detected at the river-mouth hydrophone). The line running through the data was produced using Friedman's supersmoother [R function supsmu()]. Dashed vertical lines show the start and end of the peak post-smolt run.
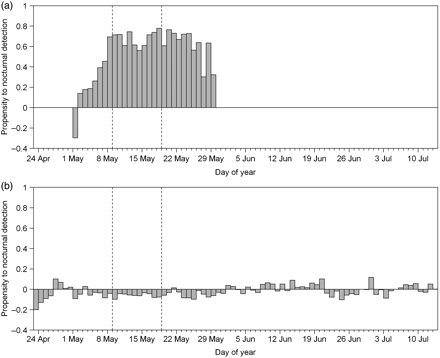
Most detections at the hydrophone located at the river mouth were at night (median 76%, n = 14); 11 cod had >62% of their detections at night, and the other 3 were only detected a few times, so their lack of a bias towards nocturnal detection could have been caused by the small sample size. The diel pattern of detections at the hydrophone located at the river mouth was markedly different from that found at the other hydrophones (Figure 7). Cod were seemingly at the river mouth more at night than by day, the nocturnal presence peaking by 9 May 2009 then declining from 26 May 2009 (Figure 7a). At most other hydrophones, cod showed a much smaller propensity to daylight or night-time detection (Figure 7b). The increase in nocturnal detection at the hydrophone located at the river mouth was concurrent with the increase in estimated abundance of migrating salmonid post-smolts.
Propensity to nocturnal detections of cod in 2009 at (a) the river-mouth hydrophone, and (b) the westernmost hydrophone in row I1. Dashed vertical lines show the start and end of the peak post-smolt run.
Discussion
The tagged cod exhibited little migratory behaviour throughout the period of the study. Most remained within Eresfjord, i.e. <9 km from the release site. However, some individual cod moved more extensively, some >35 km out from Eresfjord and either returning later or migrating out of the Langfjord system, indicating differences in movement strategies between individual fish. This pattern concurs with that identified by Brooking et al. (2006) in North America, which showed most cod remaining close to their point of release, but several making more extensive movements before returning. The observed movement patterns in the current study support the paradigm that a large proportion of the NCC reside in inshore coastal areas and are relatively non-migratory in the inner parts of fjords (Godø, 1995; Robichaud and Rose, 2004; Bergstad et al., 2008; Bjørn et al., 2009). Cod had distinct loci, i.e. separate areas of activity of home ranges, similar spatially to that identified for adult NCC in other coastal areas (Coté et al., 2004; Espeland et al., 2007). The finding that cod loci were distinct from the areas where the fish were released may indicate that cod were actively moving to selected locations or habitats. The daily areal range increased with increasing body size, perhaps linked to different feeding behavioural strategies of cod of different size, or perhaps that larger cod had fewer enemies or a greater food demand so required a bigger foraging area. In general, home ranges of fish tend to increase with body size (Kramer and Chapman, 1999).
Cod depth data indicated demersal residence with frequent changes in depth over short time-scales. The short time-scale variation in depth was consistent with previous tagging studies of adult Atlantic cod (Berg and Albert, 2003; Neat et al., 2006). The frequent changes in depth could be caused by (i) cod diving and surfacing vertically within the water column, or (ii) cod swimming along the fjord bottom to deeper or shallower areas than the position where the detecting hydrophone was located, or both. It was not possible to position the cod with sufficient accuracy to determine the relative importance of vertical and horizontal movements, but regardless of relative importance, the pattern of movement is indicative of foraging. Diel effects were apparent for cod swimming depth, but not for horizontal movement. The latter is likely a result of the hydrophone detection ranges being too large for short-range vertical movements across sills to be detected. The finding that cod tend to be deeper during daylight indicates that they may stay in shallower waters close to the shore at night, because cod are generally thought to stay close to the seabed (Fahay et al., 1999). This concurs with the results of a previous field study of NCC (Espeland et al., 2010), and also with tank studies (Claireaux et al., 1995). Strong diel behavioural effects, with great individual variation, have also been demonstrated in other studies (e.g. Neat et al., 2006).
Except a diel effect on depth, there was no environmental influence and no evidence of a tidal effect on longitudinal movement. The limited information that exists on tidal influences within fjords suggests an absence of consistent relationships (Svendsen, 1995). There was no evidence of tidal effects on cod swimming depth, suggesting that there was no selective tidal stream transport (STST). There has been relatively little research on STST, and conclusions have varied on an individual and regional basis (Arnold et al., 1994; Righton et al., 2007). Utilization of tidal currents is associated with migration, and the lack of tidal effects is consistent with a non-migratory group of cod.
Cod behaviour in the fjord was consistent with cod feeding on migrating salmonid post-smolts. First, the daily areal range of lower inner fjord cod (those not detected at the hydrophone at the river mouth) contracted during the peak post-smolt run. The change in daily areal range suggests that cod behaviour involved more wide-ranging foraging outside the post-smolt run when food was less abundant, whereas cod could remain in relatively stationary positions to prey on the abundant migrating post-smolts during the run itself. The daily areal range of upper inner fjord cod was less during the peak post-smolt run than before it, but the range did not rise until a week later. Smolts were still entering the fjord after the peak post-smolt run until early June, so perhaps these cod were still able to prey on post-smolts. The large difference in overall daily areal range between lower and upper inner fjord cod suggests plasticity in foraging behaviour, with lower inner fjord cod foraging over wide areas and upper inner fjord cod exhibiting more localized feeding. Second, the detection of 14 cod at the hydrophone positioned at the mouth of the Eira was mainly nocturnal, in marked contrast to the other hydrophones, where there was no clear bias to daytime or night-time detection. Salmon smolts released in the river tended to migrate through the fjord at night, consistent with other findings that have shown nocturnal migration (Hedger et al., 2008b; Davidsen et al., 2009; Martin et al., 2009). Hence, perhaps individual cod were moving into shallower water close to the river mouth to prey on the migrating smolts at night, consistent with previous visual observations of cod predation on salmonid post-smolts in the area (Jepsen et al., 2006). The hydrophone at the river mouth unfortunately failed after the post-smolt run, so it was not possible to determine how diel patterns of cod presence at this area changed after the run. Finally, the depth-use pattern suggests that some cod were feeding on post-smolts. Upper inner fjord cod spent more than twice the time in surface waters that lower inner fjord cod did, suggesting that upper inner fjord cod spent relatively more time foraging for pelagic fish, e.g. Atlantic salmon post-smolts, and that lower inner fjord cod were spending more time foraging for demersal species. Some cod showed a marked increase in the proportion of nocturnal detections near the surface (depth ≤2 m) during peak post-smolt runs, from which we infer that some cod were surfacing to feed on the post-smolts migrating nocturnally through the fjord. When the behaviour of all cod was considered, however, there was no significant difference in the propensity to migration towards the surface, demonstrating that not all cod move to near the surface at night. Given that not all cod moved to the river mouth during feeding and not all cod showed a depth-use change during peak post-smolt runs, it is likely therefore that some cod were feeding on other species.
Cod are important predators on salmonid post-smolts (see Introduction), and the results from this study indicate that some cod adjusted their behaviour to prey on post-smolts at the river mouth during the main post-smolt run. Post-smolt densities were greater at the river mouth than in the wider parts of the fjord, so preferentially preying on post-smolts at the river mouth is likely an effective foraging strategy. However, cod may also prey on salmon post-smolts farther out in the fjord, although to a lesser extent than at the river mouth (Thorstad et al., 2011a), possibly as a result of a clear vertical segregation between cod and salmonid post-smolts in those areas. Similar behavioural adjustments related to post-smolt migration have also been recorded for the freshwater fish predator pike-perch (Stizostedion lucioperca), which spent more time near a sluice outlet during the post-smolt run, apparently actively hunting post-smolts delayed in the area (Jepsen et al., 2000). Given that cod were captured in the innermost part of the fjord in shallow water, their subsequent behaviour can only be deemed to be representative of individuals inhabiting these locations, and not necessarily of fish farther out in the fjord system. Although cod had undergone surgery and tagging, it is believed that tagging effects on behaviour will have been minimal: Coté et al. (1999) found no significant effect on cod swimming performance after tagging.
In conclusion, the tagged cod tended to remain within the innermost part of the fjord for most of the study, although there were some instances of them making longitudinal traverses of the complete Eresfjord/Langfjord system. Tidal influences on cod depth were not observed, but cod tended to occupy near-surface waters at night. There was some indication of cod feeding on migrating salmonid post-smolts in terms of a contraction in daily areal range and a greater presence in shallow nearshore waters during the post-smolt run. Cod presence at the river mouth was mainly nocturnal, concurring with the belief that they were feeding on migrating post-smolts. However, not all cod showed this behaviour, indicating that some were preying on other species.
Acknowledgements
Funding for the project was supplied by the Norwegian Research Council (project 183992/S30) and the Norwegian Institute for Nature (NINA). We thank the staff at the Statkraft hatchery in Eresfjord for their help and cooperation throughout, Mareno Nauste, John Kofoed, Ole Kristian Bjølstad, and Ronny Jakobsen (NOFIMA) for support, and David Coté and an anonymous reviewer for their constructive comments on an earlier version of the manuscript.



![Detection densities of six selected cod within the inner and middle Eresfjord from 2009. Images have been made using the density estimation [kde2(MASS) function of R, bandwidth 1000 m], and are density-sliced in 10 layers from minimum (black) to maximum (white) density. Hydrophone positions are shown by the white circles.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/icesjms/68/10/10.1093_icesjms_fsr143/1/m_fsr14303.gif?Expires=1716325167&Signature=0OIQBM--z8QgArc44XPZEYvYNGyKf0Uv~F2ouCz-Sz27gJW-HBgJ8f3MxX6wP3syUjrweuhZh9~CyKL0Eggp2I4Fa7paLXHQZZTsebY1oAehYzPN5R2ZQIVhHeWwOwbUiV4Vegm0MFK5OtvC1rM-d7h~NAO3yF4ASKwfUcUKFBf4xcw9rJ559UT1sEA2kZlz87Oat0rp-MW3BRbtUPb0BCWFBVczZgVlQAF2UPnzmSEwCEBAbxnb5ZGopjJ3haz7Dbr1Pmfi3wkK09~ghokEQoqL3YHAvKCCpAPeleLLlp832YZpfBOHSpVQBqeIRfDfJO1TdKbsMRaHnMJvWH1KvA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Depth plots of six selected cod within the inner and middle Eresfjord from 2009. Grey lines link recorded depths, and show short-term diving and surfacing behaviour. Solid and dashed black lines are fitted from kernel smoothing of the tag depth recordings and water column depths of the receivers [R function ksmooth(), kernel normal, bandwidth 1 d], and show long-term trends in depth occupancy. Dashed vertical lines show the start and end of the peak post-smolt run. Note that cod may be detected within ∼500 m of a receiver, so the water column bottom depth at the receiver may differ from that where the cod was located.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/icesjms/68/10/10.1093_icesjms_fsr143/1/m_fsr14304.gif?Expires=1716325167&Signature=AMB~idm2pkASTMwkFD6-ym4PNtBGAzeNac4z30m8uf8iNF-RTWfRPOBds4cuJdxn-H4Nf-EY8OywJMs88iASAYDGbjA9LSBTgMLRXe-ij1sgDcyhHxQnvbIIY2APUMCKZWl4n2tNTDteI99dXJ5sKyU~lnJGNfaOKN83pk6zCw-jEiQ-mN0BZ~2x~GInDu7egNOHjNdJGuTXEYiPH~0d2eED8QwkBvLP98V6WEg32Ayf9c0RwZKfnfIItg0gyQcz5ZtDBNUIwQWNw7-DPquGrrC9s0x9dcG6toXRr~N4vCgE0IBia6Cp~yNYXGO7LjKtwH3vuI4OCGWlm9RP2MBRYA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Daily areal range of cod in 2009 as a function of date: (a) upper inner fjord cod (individuals detected at the river mouth hydrophone), and (b) lower inner fjord cod (individuals not detected at the river-mouth hydrophone). The line running through the data was produced using Friedman's supersmoother [R function supsmu()]. Dashed vertical lines show the start and end of the peak post-smolt run.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/icesjms/68/10/10.1093_icesjms_fsr143/1/m_fsr14306.gif?Expires=1716325167&Signature=iY7S94TOf1vANrYAoJNA4zxinWsmdqy093~EBxoaCQ9ksfK5~Dotgkk-k82SxCSPvcpwgyIok6mzm7yskwJoJ4XGYJu1~B57yohcn8GafzqI0oXQu6QrWxeKs9n55toZ3RPu~n0oRn6TTxFqzaeYlX2eRXIbvsokRlZvkOlQwidhPr9dHKVpMPtTtuFw84uiAE7oGXBU57utu41MAdiQUp-YOAkrhA7hhM2DpVrDtTj6PBgp23h09Z4HZIENI8Hk3JaUq9li0w5Yw3yHBBXmCr90FFegvBfhU-aYGJ69mpExImFG7jW~BS3fGHa2i3EN1lUzL0QT-CL4BRapyJuJ5Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)