-
PDF
- Split View
-
Views
-
Cite
Cite
Peter J. Auster, Kristina Gjerde, Eric Heupel, Les Watling, Anthony Grehan, Alex David Rogers, Definition and detection of vulnerable marine ecosystems on the high seas: problems with the “move-on” rule, ICES Journal of Marine Science, Volume 68, Issue 2, January 2011, Pages 254–264, https://doi.org/10.1093/icesjms/fsq074
Close - Share Icon Share
Abstract
Fishing in the deep sea in areas beyond national jurisdiction has produced multiple problems related to management for conservation and sustainable use. Based on a growing concern, the United Nations has called on States to prevent significant adverse impacts to vulnerable marine ecosystems (VMEs) in the deep sea. Although Food and Agriculture Organization (FAO) guidelines for management were produced through an international consultative process, implementing criteria for designation of VMEs and recognition of such areas when encountered by fishing gear have been problematic. Here we discuss assumptions used to identify VMEs and current requirements related to unforeseen encounters with fishing gear that do not meet technological or ecological realities. A more precautionary approach is needed, given the uncertainties about the location of VMEs and their resilience, such as greatly reducing the threshold for an encounter, implementation of large-scale permanent closed areas, and prohibition of bottom-contact fishing.Auster, P. J., Gjerde, K., Heupel, E., Watling, L., Grehan, A., and Rogers, A. D. 2011. Definition and detection of vulnerable marine ecosystems on the high seas: problems with the “move-on” rule. – ICES Journal of Marine Science, 68: 254–264.
Introduction
Exploitation of fish and other components of biological diversity in the deep sea and beyond areas of national jurisdiction have increased worldwide, especially over the past 20 years (Sissenwine and Mace, 2007; FAO, 2009). The sustainability of deep-sea fisheries to date has been very poor compared with shallow-water fisheries, especially for those operating deeper than 500 m (Gordon, 2003; Sissenwine and Mace, 2007). There are many deep-sea fisheries for which data are not available, with poor reporting of catch and limited information on fishing effort (Sissenwine and Mace, 2007; Arbuckle et al., 2008). There are also associated and largely unaccounted impacts to deep-sea communities and ecosystems resulting from bycatch (Devine et al., 2006) and fishing gear impacts, especially to seabed habitats (Freese et al., 1999; Koslow et al., 2001; Hall-Spencer et al., 2002; Freiwald et al., 2004; Grehan et al., 2004, 2005; Stone, 2006; Edinger et al., 2007; Clark and Koslow, 2007; Waller et al., 2007; Clark and Rowden, 2009).
Based on growing concern about the adverse ecosystem impacts of fishing on the high seas, the 2006 United Nations General Assembly Resolution 61/105 called “upon States to take action immediately, individually and through regional fisheries management organizations and arrangements, and consistent with the precautionary approach and ecosystem approaches, to sustainably manage fish stocks and protect vulnerable marine ecosystems [VMEs], including seamounts, hydrothermal vents and cold water corals, from destructive fishing practices, recognizing the immense importance and value of deep-sea ecosystems and the biodiversity they contain”. Most importantly, the resolution required that by 31 December 2008, States and Regional Fishery Management Organizations (RFMOs) manage fisheries to prevent significant adverse impacts (SAIs) to areas identified as vulnerable marine ecosystems.
To provide States and RFMOs with guidance for implementing the resolution, the United Nations Food and Agriculture Organization (FAO) sponsored an “Expert Consultation” in Bangkok, Thailand, in September 2007 which resulted in a draft of “International Guidelines for the Management of Deep-Sea Fisheries in the High Seas” (FAO, 2008). Two subsequent consultations involving delegations from 53 Nations, as well as inter- and non-governmental organizations, met during 2008 to negotiate, revise, and finalize the text of the guidelines, with the resultant text adopted in August 2008 (FAO, 2009).
The guidelines apply to “fisheries that occur in areas beyond the limits of national jurisdiction and have the following characteristics: (i) the total catch (everything brought up by the gear) includes species that can only sustain low exploitation rates, and (ii) the fishing gear is likely to contact the seabed during the normal course of fishing operations”. The overall objective of the guidelines is to ensure the long-term conservation and sustainable use of deep-sea resources and to prevent significant adverse impacts to VMEs from activities related to such exploitation.
Despite the concurrence by States and RFMOs to meet the overall objectives of the UN resolution by applying the guidelines, regional implementation has been problematic, partly because of a lack of specificity and partly because of ecological uncertainties. While there are criteria to identify VMEs, there have been no clear technical definitions proffered of what a VME is or is not (e.g. identifying spatially explicit reference points for density or abundance of indicator species or communities), although there have been efforts to identify the taxonomic groups and features that characterize VMEs. Although multiple RFMOs have closed areas based on the known presence of vulnerable species or communities using a variety of datasets and the application of varying degrees of precaution, the ability to define and identify new areas is hampered by a lack of knowledge and common approaches to define the thresholds for metrics indicative of VMEs. Such thresholds have been established within the context of establishing encounter and “move-on” rules. As applied, these move-on rules require a vessel to move a minimum distance when a particular level of catch of a particular taxon, indicating the presence of a VME, is encountered during fishing operations, thereby leaving the area where this “evidence” of a VME was encountered.
Although the implementation of any new management regime is expected to have growing pains, additional ecological and technical information may help to address some potential ambiguities in the guidelines and hence improve their application. Our objective herein is to identify specific problems that have emerged as RFMOs began to implement the guidelines and that we suggest require attention for identifying actual VMEs, as well as to make recommendations on setting thresholds for implementing encounter and move-on provisions.
The guidelines
A vulnerable marine ecosystem is based on a conceptual foundation described in the guidelines (FAO, 2009) as follows:
Vulnerability is related to the likelihood that a population, community, or habitat will experience substantial alteration from short-term or chronic disturbance, and the likelihood that it would recover and in what time-frame. These are, in turn, related to the characteristics of the ecosystems themselves, especially biological and structural aspects. VME features may be physically or functionally fragile. The most vulnerable ecosystems are those that are both easily disturbed and very slow to recover, or may never recover.
The vulnerability of populations, communities and habitats must be assessed relative to specific threats. Some features, particularly those that are physically fragile or inherently rare, may be vulnerable to most forms of disturbance, but the vulnerability of some populations, communities and habitats may vary greatly depending on the type of fishing gear used or the kind of disturbance experienced.
The risks to a marine ecosystem are determined by its vulnerability, the probability of a threat occurring and the mitigation means applied to the threat.
Criteria for identifying VMEs include uniqueness or rarity of species or habitats, their functional significance, fragility, and structural complexity as well as life histories that limit the probability of recovery. The guidelines also provide examples of taxa indicative of a VME: (i) cold-water corals of various types (e.g. reef builders and coral forest species) likely to be found on the edges and slopes of oceanic islands, continental shelves, seamounts, canyons, and trenches; (ii) sponge-dominated communities and structural biogenic habitats (e.g. those composed of large protozoans, hydrozoans or bryozoans) with a distribution similar to cold-water corals; (iii) endemic or rare types of hydrothermal vent and cold seep communities; and (iv) fish species that sustain low exploitation rates. Further, such taxa are generally associated with specific undersea landscape types, including topographically abrupt features, the summits and flanks of seamounts, submarine canyons, hydrothermal vents, and cold seeps.
Although this guidance provides advice on the types of criteria that RFMOs might use for designating VMEs, it does not provide explicit metrics, threshold values, or analytical approaches for identifying if one area contains a VME and another does not. By leaving all the decision criteria to each RFMO, there remained wide latitude for implementing the designation process. In a highly precautionary environment, this could be beneficial. For example, multiple data types and sources could be used in delineating boundaries for VMEs without an explicit set of thresholds to satisfy. Such is the case for evaluations of existing closed areas on seamounts and mid-ocean ridge areas in the Northeast Atlantic where data from seabed mapping, scientific faunal surveys, fishing effort, bycatch, and historical research results were evaluated on a case-by-case basis (ICES, 2009).
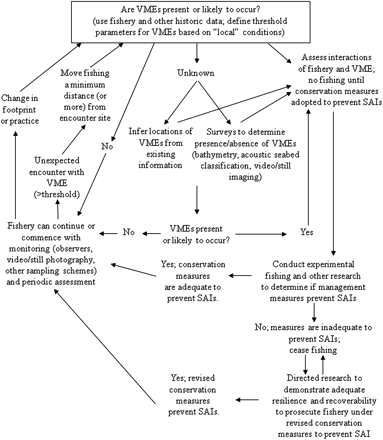
One overarching problem with designating such areas in the deep sea is the lack of geographically comprehensive data to be used for decision-making. Not unexpectedly, there will be surprises in terms of locations of unmapped VMEs. With the high cost and low research effort for dedicated high seas biodiversity research surveys, such information will most probably emerge during the course of fishing operations. How to integrate information for identifying VMEs was the topic of much discussion in the Expert Consultation, and three of us (PA, ADR, KG) developed a decision support diagram to aid managers in developing protocols to identify and minimize impacts to VMEs where information is limited and management decisions must be made in an adaptive fashion (FAO, 2008). Here we present a modified version that includes explicit steps regarding identification of VMEs and decisions criteria for encounters while fishing (Figure 1). The decision support diagram identifies the general types of questions, data, and alternative decision pathways available to managers of fisheries taking place in areas with different levels of prior knowledge. Here we will not propose threshold values for identifying VMEs explicitly, but identify the issues that may preclude establishing any such value. What follows from this is our view of the inevitable problems with implementation of encounter and move-on rules, given the uncertainties associated with criteria for determining evidence of VMEs. Finally, we discuss briefly a proposed route forward that is based on a precautionary approach, given the uncertainties regarding vulnerability and the poor ability to detect VME indicator species using commercial fishing gear as the sampling tool.
Decision-support diagram for managing vulnerable marine ecosystems based on FAO guidelines (modified from FAO, 2008).
The Northwest Atlantic Fisheries Organization (NAFO), Northeast Atlantic Fisheries Commission (NEAFC), and the Commission for the Conservation of Antarctic Living Marine Resources (CCAMLR) regions of the North Atlantic and Southern Ocean will be the focus of this discussion because these are regions with which we are most familiar, but the issues apply worldwide.
Problems identifying evidence of VMEs
Perhaps the biggest constraint in the process to protect VMEs is the uncertainties in the distribution and abundance of VME indicator species, and similar uncertainties in the link between fishing effort and significant adverse impacts. In the face of these uncertainties, precautionary approaches should be the overarching requirement. Much remains to be understood about the taxonomy, population biology, reproductive biology, functional role, and resilience of species that comprise VMEs. In fact, comprehensive studies of such communities in areas of the high seas have only just begun in many areas, and syntheses with broad geographic applicability are few (but see Pitcher et al., 2007).
Although research focused on the biological attributes of deep coral communities in the Northeast Atlantic has been conducted at a significant pace in the past decade (Roberts et al., 2009), studies of the biological attributes of seamount coral communities in the Northwest Atlantic have only recently been conducted (2001–2005; i.e. New England and Corner Rise Seamounts). In the CCAMLR area, records of habitat-forming Scleractinia are scarce and restricted to the northern limits of the region, but other VMEs formed by, for example, hydrocorals and sponges are well known and have been identified (CCAMLR, 2008a). Seamount communities in the Southern Ocean, in general, are poorly known (Arntz et al., 2006). Recent results indicate that coral communities across seamount chains and across depth ranges within seamounts can vary over small spatial scales in terms of composition and distribution (Rogers et al., 2007; Cho, 2008). That is, all seamounts within a region are not equal and management of impacts should consider spatial variation at relatively small spatial scales, such as within seamount chains. These basic patterns are consistent with the analyses of the distribution of coral communities across the Northeast Atlantic (Hall-Spencer et al., 2007).
The geographic relationships within and between coral species across seamounts appear complex and variable between taxa, and it is difficult to predict general patterns of connectivity from studies limited to a few areas or species. Analyses of mitochondrial genes from corals of the subfamily Keratoisidinae from across the Pacific Ocean revealed widespread occurrence of haplotypes across very large geographic distances, suggesting long-distance dispersal of coral larvae (Smith et al., 2004). In contrast, Corallium lauuense on the seamounts and islands in Hawaii showed low, but significant, genetic differentiation within continuous coral beds, between beds on the slopes of the same island or seamount, and between islands or islands and seamounts (Baco and Shank, 2005). This suggests that C. lauuense forms largely self-recruiting populations, with only occasional long-distance dispersal, in contrast to the high levels of long-distance dispersal and connectivity shown by Keratoisidinae populations.
A similarly complex picture has arisen from studies of Paramuricea collected from 16 locations across the western North Atlantic (New England and Corner Rise seamounts, submarine canyons along the continental margin of North America, and deep basins in the Gulf of Maine) at depths between 200 and 2200 m. Among 89 colonies sampled, genetic data show that there are at least four genotypes corresponding to three or four species (Thoma et al., 2009). Two of these (clades B and C) are evolutionarily older lineages, and the other two (A and D) are more recently derived and closely related. All types were found on at least some seamounts, but only clade A was found on the continental margin (canyons and Gulf of Maine). Clades B and C were widely distributed on seamounts across the sampled region, although clade C was absent from the four easternmost locations in the Corner Rise Seamounts, and clade B was absent from the two westernmost locations (Bear and Retriever seamounts). All clades were observed only on Kelvin Seamount. Observations of associations between a range of echinoderm and crustacean species with specific corals across the New England and Corner Rise seamounts suggest obligate relationships (Cho, 2008; Mosher and Watling, 2009), indicating that distribution ranges and connectivity of these other ecosystem components are tied to those of their coral associates.
Recently, Watling (2007) described four new species and one new genus of Chrysogorgiid octocoral from specimens collected across the New England Seamount Chain. Work is ongoing regarding new descriptions of bamboo corals with the validity of two genera in question (Lepidisis and Keratoisis). Some coral species are known from only a single location (Cairns, 2006). Fifteen species of black coral were also collected, including seven that have not previously been observed on the seamounts (S. France, pers. comm.). In the northeastern Atlantic, some coral species have been observed exclusively on seamounts or mostly occur only on seamounts (Hall-Spencer et al., 2007). Others are restricted in distribution mainly to the continental slope, but overall coral communities are different on the continental slope of Europe, the flanks of oceanic islands, and seamounts in this region (Hall-Spencer et al., 2007).
In contrast, in the northwestern Atlantic, observations of fish on seamounts at 900–2500 m depth suggest that while multiple species interact with seamount habitats, only Neocyttus helgae has at least a facultative relationship with fan and whip octocoral-dominated habitats (Auster et al., 2005; Moore et al., 2008). Associations of fish species of economic importance with octocoral, scleractinian, and sponge habitats appear more common on continental slopes and deep-shelf regions (e.g. Auster, 2005; Costello et al., 2005; Koenig et al., 2005; Parrish, 2006; Stone, 2006). Various mechanisms and reasons for association between fish and deep-water coral ecosystems have been proposed. For example, limited observations from seamounts suggest that the base of corals and sponges can collect vertically migrating macrozooplankton and serve as sites for enhanced feeding opportunities for fish (unpublished observations). However, we currently know little about the functional role of corals across seasons and life history stages for most deep-sea fish species, and interpretation of distribution patterns and relationships between fish and corals is tentative (Auster, 2007).
Effort–impact information with regard to particular gear types used in different deep-sea habitats is also limited (Freese et al., 1999; Roberts et al., 2000; Freese, 2001; Koslow et al., 2001; Krieger, 2001; Fosså et al., 2002; Hall-Spencer et al., 2002; Grehan et al., 2005; Wheeler et al., 2005; Waller et al., 2007). The types and directions of impacts of fishing gears are well known, however, from the global literature on the subject (Collie et al., 2000; Kaiser et al., 2006) and significant damage to both scleractinian, soft coral, and sponge communities are known from single impacts of mobile gear such as trawls (Freese et al., 1999), as well as static gear such as benthic longlines and traps (Krieger, 2001; Grehan et al., 2004; Stone, 2006; Edinger et al., 2007). Such information could be used to predict and differentiate the potential for particular types of fishing operation to produce significant adverse impacts to VMEs.
Recent reviews of RFMOs have shown that the current management of deep-sea fisheries resources is inadequate to ensure sustainability of such resources, or of the ecosystems within which they occur, and that new or expanding deep-water fisheries should therefore be developed in accordance with the precautionary principle (Arbuckle et al., 2008). In the absence of a strictly precautionary approach to managing these fisheries (by minimizing exploitation rates and impacts), population assessments and biological reference points will be required for exploited and bycatch species not currently managed, such as alfonsino, orange and Mediterranean roughy, blue ling, and other taxa targeted or that occur as bycatch in fisheries (e.g. based on catch detailed in Vinnichenko, 1997). Such assessments are extremely difficult for deep-water species and relatively few have been conducted.
Clearly, there are problems and uncertainties in defining the species within major taxonomic groups that are indicative of VMEs, the geographic extent of their populations, the distribution of interacting communities, the functional role of VME species, and links to patterns of biological diversity, patterns of recruitment, and resilience to disturbance. Defining and designating VMEs in a geospatial context will necessarily remain case-specific, and will be highly dependent on the mix of data available for individual locations. Although the research continues to generate adequate data to develop robust habitat prediction models and to design effective management approaches to protect these systems, given the current sense of urgency for advice needed by managers under the timeline for implementing the UNGA resolution, a high degree of precaution in interim measures appears to be necessary.
The move-on rule
Although there are no explicit target values for what would constitute evidence or proof of fisheries interaction with VMEs (e.g. based on diversity, biomass per unit area, etc., as indicated by benthic bycatch during fishing operations) used for explicit designations, there are generalized provisions in the guidelines to address detection of potential VMEs based on encounters with VME indicator species captured in the fishing process. To date, such provisions have been implemented via encounter protocols designed to trigger a “move-on” rule. A move-on rule is based on the premise that a fishing vessel will move a minimum distance from a location where species indicating the presence of a VME are captured by the gear. RFMOs have set threshold weights or volumes that are considered (by the respective RFMO science processes and participants) to constitute “evidence of a VME” for such cases, as well as distances vessels must move upon an encounter (Table 1). For example, in the NAFO area, if a vessel brings on board more than 60 kg of live corals or 800 kg of sponges, it must move a minimum of 2 nautical miles from the fished area. In the CCAMLR region, more precautionary rules have been established for longlines. A bycatch of 5 kg or 5 VME units (i.e. 1 VME unit equals 1 l in a 10-l container or 1 kg in weight) in a segment of longline or pot line (1200 m line length or 1000 hooks) requires notification, while a catch of 10 kg or 10 VME units on a gear segment requires notification and moving on with a subsequent closure of 1 nautical mile around the encounter point (CCAMLR, 2008b, c, d).
Comparison of provisions of encounter and move-on rules across regions as of December 2009
| RFMO . | Provisions of move-on rule . |
|---|---|
| CCAMLR | Move-on rule adopted for bottom longlines and pots when more than 10 l or a 10-kg basket of indicator species is caught by a 1200-m gear segment or 1 000 hooks. Area within 1 nautical mile radius closed to all vessels until reviewed by SC and management actions determined by Commission. 100% scientific observer coverage required |
| NAFO; NEAFC; SEAFO | Move-on rule is implemented if more than 60 kg of live corals or 800 kg of sponges are in catch. For the existing fishing areas, vessels move 2 nautical miles from area, but overall area remains open to others. All new fishing areas are closed to all vessels until scientific review process is completed. Observer coverage is only required in “new” fishing areas |
| SPRFMO | Interim move-on rule proposed with weight thresholds from 1 to 50 kg for different taxonomic groups, plus an index of biodiversity |
| RFMO . | Provisions of move-on rule . |
|---|---|
| CCAMLR | Move-on rule adopted for bottom longlines and pots when more than 10 l or a 10-kg basket of indicator species is caught by a 1200-m gear segment or 1 000 hooks. Area within 1 nautical mile radius closed to all vessels until reviewed by SC and management actions determined by Commission. 100% scientific observer coverage required |
| NAFO; NEAFC; SEAFO | Move-on rule is implemented if more than 60 kg of live corals or 800 kg of sponges are in catch. For the existing fishing areas, vessels move 2 nautical miles from area, but overall area remains open to others. All new fishing areas are closed to all vessels until scientific review process is completed. Observer coverage is only required in “new” fishing areas |
| SPRFMO | Interim move-on rule proposed with weight thresholds from 1 to 50 kg for different taxonomic groups, plus an index of biodiversity |
RFMO, Regional Fishery Management Organization; CCAMLR, Commission for the Conservation of Antarctic Living Marine Resources; NAFO, Northwest Atlantic Fisheries Organization; NEAFC, Northeast Atlantic Fisheries Commission; SEAFO, South East Atlantic Fisheries Organization; SPRFMO, South Pacific Regional Fisheries Management Organization.
Comparison of provisions of encounter and move-on rules across regions as of December 2009
| RFMO . | Provisions of move-on rule . |
|---|---|
| CCAMLR | Move-on rule adopted for bottom longlines and pots when more than 10 l or a 10-kg basket of indicator species is caught by a 1200-m gear segment or 1 000 hooks. Area within 1 nautical mile radius closed to all vessels until reviewed by SC and management actions determined by Commission. 100% scientific observer coverage required |
| NAFO; NEAFC; SEAFO | Move-on rule is implemented if more than 60 kg of live corals or 800 kg of sponges are in catch. For the existing fishing areas, vessels move 2 nautical miles from area, but overall area remains open to others. All new fishing areas are closed to all vessels until scientific review process is completed. Observer coverage is only required in “new” fishing areas |
| SPRFMO | Interim move-on rule proposed with weight thresholds from 1 to 50 kg for different taxonomic groups, plus an index of biodiversity |
| RFMO . | Provisions of move-on rule . |
|---|---|
| CCAMLR | Move-on rule adopted for bottom longlines and pots when more than 10 l or a 10-kg basket of indicator species is caught by a 1200-m gear segment or 1 000 hooks. Area within 1 nautical mile radius closed to all vessels until reviewed by SC and management actions determined by Commission. 100% scientific observer coverage required |
| NAFO; NEAFC; SEAFO | Move-on rule is implemented if more than 60 kg of live corals or 800 kg of sponges are in catch. For the existing fishing areas, vessels move 2 nautical miles from area, but overall area remains open to others. All new fishing areas are closed to all vessels until scientific review process is completed. Observer coverage is only required in “new” fishing areas |
| SPRFMO | Interim move-on rule proposed with weight thresholds from 1 to 50 kg for different taxonomic groups, plus an index of biodiversity |
RFMO, Regional Fishery Management Organization; CCAMLR, Commission for the Conservation of Antarctic Living Marine Resources; NAFO, Northwest Atlantic Fisheries Organization; NEAFC, Northeast Atlantic Fisheries Commission; SEAFO, South East Atlantic Fisheries Organization; SPRFMO, South Pacific Regional Fisheries Management Organization.
The South Pacific Regional Fisheries Management Organization (SPRFMO) was only recently organized and has not yet formally adopted VME encounter protocols. Participants have agreed, however, to a benthic assessment framework which does contain a VME encounter protocol (Parker et al., 2009). Weight thresholds for taxonomic groups under this protocol vary based on the analysis of historical bycatch weights, ranging from 1 kg for identified Antipatharia, Alcyonacea, or Gorgonacea, to 30 kg for Scleractinia, to 50 kg for Porifera, with an additional biodiversity score for all other benthic species. Move-on is either triggered by a threshold catch of one group or any catch of a number of groups.
There are a number of implicit assumptions in all of these protocols that are required to justify the premise that such actions have a conservation value. Here we discuss a number of these assumptions and suggest that the threshold values for encounters and associated move-on distances currently in use are inadequate to ensure conservation of VMEs in the face of current ecological or technological realities.
Threshold values for coral and sponge bycatch that trigger the move-on rule are not supported by any explicit demonstration of biomass–density relationships that produce some critical threshold for a VME, nor any related evaluation about catch efficiency in fishing gear. Justification that move-on constitutes protection therefore requires substantial (unsubstantiated) assumptions regarding the level of bycatch that indicates presence of a VME, and what area such a VME might be expected to occupy around the encounter site. It is true that evaluation of these factors would be a difficult task given that there are few studies linking the density or biomass of species or communities with bycatch. Limited studies that do address this issue, however, indicate that bycatch may be a very poor indicator of seabed species composition and density, largely because bottom-fishing gear (trawlnets and longlines) designed to catch fish is a very poor sampling tool for most sessile benthic organisms.
Freese et al. (1999) quantified catch efficiency of trawl-caught invertebrates by comparing density estimates based on area swept of the trawl with density estimates from seabed imagery at deep-water sites (206–274 m depth) off southeast Alaska. Density based on catch compared with photographic estimates was <1% for asteroids, echinoids, and molluscs, and 4.6% for holothurians. There were no quantifiable estimates of octocorals or sponges, probably because the size and fragility of species encountered meant that they were not retained by the nets. Light, flexible, and fragile specimens tend to be fragmented in the catch process and are extruded through the mesh. Heavy specimens that fragment can drop through the meshes in the belly of the net. Specimens that are retained in the codend are those that are larger than the mesh of the gear throughout or at least have a density that allows transport to the codend in the flow regime within the net. Furthermore, specimens must be resistant to abrasion by the gear. A comparison of taxa observed via video, trawl, and longline catch at stations along the northern Mid-Atlantic Ridge produced similar results, with underwater video capturing the greatest species richness (Mortensen et al., 2008). Although the aforementioned studies contain a limited set of observations focused on taxa that explicitly comprise VMEs, we acknowledge that a gradient of catchability and catch efficiency accounts for variable patterns in catch composition of corals and sponges observed elsewhere (Wassenberg et al., 2002).
There are few published accounts of coral bycatch in cold-water deep-sea fisheries. Coral bycatch from research survey trawl tows off eastern Newfoundland and Labrador in the Northwest Atlantic reveal a large disparity in catch based on taxonomic and morphological differences of corals (Edinger et al., 2007). Empirical data of coral bycatch were based on 15-min survey tows. When extrapolated to 4-h tows that are common in commercial fisheries on the high seas, only the highest catch rates of species classified as soft corals and large gorgonian corals might meet or exceed the 60-kg threshold value for the move-on rule (shorter tows would almost never trigger the rule). Virtually no catches composed entirely of small gorgonians or other soft corals, and no catches of sea pens and cup corals would ever exceed the threshold value, although there are areas where such taxa dominate (Cho, 2008; LW, S. France, and PJA, unpublished data). Threshold values based on the 50th percentile weight by taxonomic group from historic observer trawl catch data were used to establish evidence of a VME encounter in the SPRFMO region (Parker et al., 2009). The authors acknowledge that the level of bycatch that is biologically significant is not known, and simply chose the median historical (50th percentile) weight as the proposed basis to trigger the move-on rule.
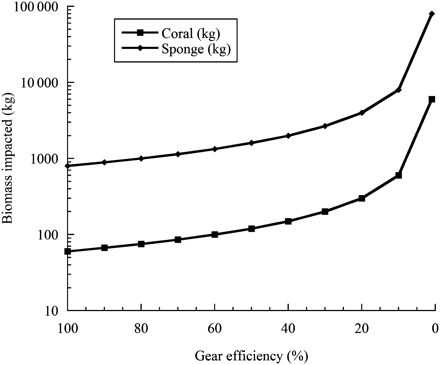
It is therefore important to consider the possible relationship between catch, catch efficiency, and the biomass of the species of concern impacted in such scenarios. Using the threshold values of 60 kg of live coral or 800 kg of sponge that requires vessels to move on in the NAFO and NEAFC regions of the North Atlantic as reference points, we predict the biomass of impacted taxa across a gradient of catch efficiencies (Figure 2). For example, at a 10% catch efficiency level for both corals and sponges, 600 kg of coral and 8000 kg of sponges would actually be impacted. At 1% efficiency, a level more in accordance with the study by Freese et al. (1999), 6000 kg of coral or 80 000 kg of sponge would be impacted based on a bycatch large enough to trigger the move-on rules concerned. Smaller catches than these levels would not require a vessel to move on.
A comparison of the weight of coral and sponge taxa impacted by mobile gear based on a gradient of catch efficiencies. The origin of each line is the encounter threshold catch weight of 60 kg of corals and 800 kg of sponges that triggers the move-on rule in the NAFO region.
In one of the few studies to examine the relationship between coral biomass and the diversity of associated fauna, Jensen and Frederiksen (1992) used dredges to recover coral blocks from two banks off the Faroe Islands. They counted 4625 individuals representing 256 species on 25 coral blocks with a total weight of 18.5 kg. Given that dredges are not 100% efficient, specimens not clearly associated with coral blocks and individual members of colonial forms (including the coral polyps themselves) were not counted, the impact of removing 6000 kg could result in mortality of millions of individuals of hundreds of species.
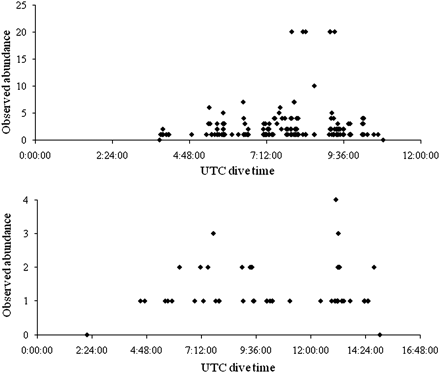
Of course, such catch rates and efficiency measures assume that these species are evenly distributed over the landscape that is targeted by fishing gear. This is not the case in many (or perhaps most) ecological settings. Corals and sponges are suspension-feeders and generally are distributed along the edges and peaks of topographic rises and in areas of significant current or enhanced food supply (Figure 3; see Genin et al., 1986; Frederiksen et al., 1992; Klitgaard et al., 1997; Klitgaard and Tendal, 2001; Freiwald et al., 2004; White et al., 2005; Mienis et al., 2007; Dolan et al., 2008; Davies et al., 2009; Guinan et al., 2009; Roberts et al., 2009). Distributions of organisms are therefore often extremely patchy when variation in the surface morphology of hard substrata is considered (Figure 4). Furthermore, cold-water coral reefs may comprise only a thin veneer of living coral over a complex framework of dead and eroding coral (Rogers, 1999). Many of the species associated with such reefs are confined to dead coral framework, so a move-on rule that specifies a catch of 60 kg of “live” coral may in fact cause far more extensive damage to a coral-based VME than is apparent from live coral catch. On the New England Seamounts, LW (unpublished) describes a new species of bamboo coral that has a “bramble”-like colony form and lives almost exclusively on beds of sub-fossil Desmophyllum dianthus. Removing this dead coral from the seamount will reduce the habitat of this rare species. Dead coral may serve as habitat for juvenile stages of deep-sea fish as well, although direct observations are limited (Moore and Auster, 2009).
Examples of patchiness in the distribution of octocorals at Manning (top) and Bear seamounts (bottom) in the New England Seamounts chain. Observations are based on numbers of coral colonies observed in the field of view of the camera at each time, rather than explicit measures of density, during dives in 2004 (Manning from 1933 to 1662 m depth; Bear from 1869 to 1395 m depth). Zero values on either end of the series of counts indicate time on and off bottom during each dive.
Examples of octocoral-dominated communities that could be classified as vulnerable marine ecosystems from New England and Corner Rise seamounts. (a) Patch of Paragorgia sp. spaced at approximately 2-m intervals on the basalt pavement (Manning Seamount, depth ca. 1320 m), (b) Paracalytrophora sp. on patch of exposed basalt substratum (Manning Seamount, depth ca. 1680 m), (c) dense patch of small soft corals, primarily Acanthogorgia armata (Retriever Seamount, 2004, depth ca. 2045 m), (d) patch of whip corals Lepidisis sp., antipitharian coral and false boarfish Neocyttus helgae (Manning2 Seamount, 2004, depth ca. 1820 m), (e) edge of sediment patch and exposed basalt pavement with Paragorgia sp. colony and roundnose grenadier Coryphaenoides rupestris (Manning Seamount, depth ca. 1330 m). All images by Deep Atlantic Stepping Stones Science Team/IFE/NOAA.
A related problem is that fishing effort is not evenly distributed. Effectively, most deep-sea fishing is confined to the upper reaches and summits of seamounts and similar features with abrupt topographies. Where there is an association (whether dependent or coincidental) between specific seabed features and both benthic coral communities and fish aggregations (which is the case for seamount summits), triggering the move-on rule as currently implemented is unlikely to result in a simple distance move away from the area probably containing VMEs to an area that does not, but rather to another area probably containing both fish and VMEs. The accumulative impact of resulting fishing operations when carried out in accordance with the move-on rule as promulgated has the potential to be substantial, with vessels continually moving on to similarly vulnerable areas. The specific concern with such move-on behaviour and resulting cumulative impacts being steadily extended to other areas is that coral-structured communities are typically fragile and have very long recovery times such that even low fishing effort can inflict lasting damage (Clark and Koslow, 2007). For example Grehan et al. (2004) documented the complete removal of coral reef from the summit of a deep-sea mound off the west coast of Ireland following the prosecution of a short-lived trawl fishery for orange roughy.
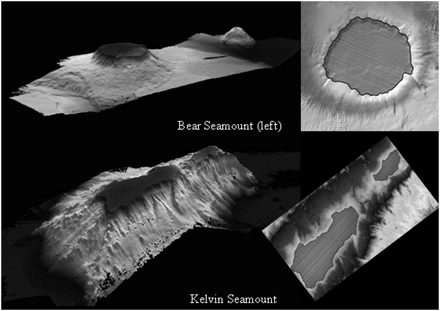
An obvious question, therefore, is: can this approach prevent significant adverse impacts to VMEs or to areas likely to contain VMEs? In a study on seamounts we used continuous coverage multibeam maps of three seamounts in the New England Seamounts chain to estimate the amount of effort required to impact the “fishable” area of each feature. Assuming that the relatively flat summits of Bear, Kelvin, and Manning Seamounts are the fishable regions (Figure 5), and the gear is towed in a fashion to remain on the seabed throughout each tow, then only 32–61 tows would impact the entirety of each summit (see Table 2 for details of calculations). Such a small number of possible tows with repeated move-on events could impact the entire summit fauna with few vessels targeting each feature.
Examples of fishable area delineated for two seamounts used in the effort calculations in Table 2. Oblique view from multibeam sonar records of each seamount to the left. Note the steep sides assessed as unfishable. Fishable areas of summits outlined on orthogonal views of bathymetric coverages to the right.
Predicted fishable area and trawl fishing effort for three seamounts in the New England Seamount chain, assuming that there is no overlap in area of coverage of tows
| Seamount . | Fishable area (km2) . | Number of tows (AOC) . | Number of tows (straight line) . |
|---|---|---|---|
| Bear | 76.5 | 32 | 129 |
| Kelvin | 147.2 | 61 | 158 |
| Manning | 96.8 | 41 | 209 |
| Seamount . | Fishable area (km2) . | Number of tows (AOC) . | Number of tows (straight line) . |
|---|---|---|---|
| Bear | 76.5 | 32 | 129 |
| Kelvin | 147.2 | 61 | 158 |
| Manning | 96.8 | 41 | 209 |
Effort calculations assumed 80-m trawl width including doors and bridles, tow speed of 4 knots (7.4 km h−1) and 4-h tow times. The number of tows was calculated based solely on matching fishable area of coverage (AOC) to area impacted for each tow (i.e. 4-h tows impact 2.4 km2 for 80 m width gear). This assumes that the fishing vessel continuously navigates the gear over the summit and does not overlap previous tows. Straight line tows assumed an 80-m separation and that the gear touches down along one edge and crosses the seamount, ending the tow in midwater. Individual tow times would be significantly <4 h. For example, a maximum tow time of 1.5 h on the seafloor was calculated for the longest distance across Bear Seamount.
Predicted fishable area and trawl fishing effort for three seamounts in the New England Seamount chain, assuming that there is no overlap in area of coverage of tows
| Seamount . | Fishable area (km2) . | Number of tows (AOC) . | Number of tows (straight line) . |
|---|---|---|---|
| Bear | 76.5 | 32 | 129 |
| Kelvin | 147.2 | 61 | 158 |
| Manning | 96.8 | 41 | 209 |
| Seamount . | Fishable area (km2) . | Number of tows (AOC) . | Number of tows (straight line) . |
|---|---|---|---|
| Bear | 76.5 | 32 | 129 |
| Kelvin | 147.2 | 61 | 158 |
| Manning | 96.8 | 41 | 209 |
Effort calculations assumed 80-m trawl width including doors and bridles, tow speed of 4 knots (7.4 km h−1) and 4-h tow times. The number of tows was calculated based solely on matching fishable area of coverage (AOC) to area impacted for each tow (i.e. 4-h tows impact 2.4 km2 for 80 m width gear). This assumes that the fishing vessel continuously navigates the gear over the summit and does not overlap previous tows. Straight line tows assumed an 80-m separation and that the gear touches down along one edge and crosses the seamount, ending the tow in midwater. Individual tow times would be significantly <4 h. For example, a maximum tow time of 1.5 h on the seafloor was calculated for the longest distance across Bear Seamount.
The move-on rule based on a single weight threshold also ignores any variation in fishing gear configuration or effort. For example, an 80-m-wide net (here considered the width of all gear in contact with the seabed) towed at 7.4 km h−1 (ca. 4 knots) for 4 h travels approximately 30 km and has a footprint of approximately 2.4 km2. A 120-m-wide net towed for the same time and distance impacts a footprint of 3.6 km2. Assuming a catch efficiency of 10%, a generous assumption for most coral and sponge species based on the previous discussion, the mean weight per unit area for corals on the seabed to reach the threshold value of the move-on rule discussed above is 253.4 kg km−2 for the 80-m net and 168.9 kg km−2 for the 120-m net (to reach the threshold weight for sponges the mean biomass value would be 3378.4 kg km−2 for the 80-m net and 2252.3 kg km−2 for the 120-m net). Note that the smaller gear requires nearly 50% more biomass per unit area to trigger the move-on provision. Furthermore, tow lengths can vary based on seabed morphology and patterns of catch. Some tows will be much shorter in time. This could have the consequence of bringing up less bycatch with each tow, but could have more widespread impacts. Without some explicit consideration of gear configuration, catch efficiency, tow time, and distribution of indicator taxa, the ecological realities to trigger the move-on rule are widely disparate.
Where to go from here?
There are clearly problems with the current management approaches adopted by some RFMOs regarding the detection of VMEs during fishing operations that will have to be rectified to provide the level of precaution needed for conservation if fisheries are to continue on the high seas. Greatly reducing the threshold to trigger a move-on rule to any detectable catch of VME indicator species (i.e. a simple presence–absence rule, rather than actual weight thresholds) would better match ecological realities with obligations under UNGA 61/105 and the FAO deep-water guidelines, although this may not greatly reduce the likelihood of future encounters with VMEs, given that fishing grounds typically overlap areas where VMEs are likely to be found. At present, the approach adopted by CCAMLR appears to be a more precautionary one and is currently being evaluated and monitored to assess effectiveness.
The alternative approach envisaged in the guidelines, but rarely implemented in a sufficient manner, is prior environmental impact assessments, followed by prior implementation of measures to “prevent significant impacts to VMEs”, before fishing is conducted. Paragraph 47 of the Guidelines calls for Flag States and RFMO/As (FAO, 2009) to “conduct assessments to establish if deep-sea fishing activities are likely to produce significant adverse impacts in a given area”. The elements of such an impact assessment are clearly spelled out in the same paragraph:
An alternative solution, which would require strict monitoring, is simply not to allow bottom-contact fishing. The use of net acoustics can keep gear just above but not on the seabed. Observers can be used to verify such acoustic records and composition of the catch.Such an impact assessment should address:
(i) type(s) of fishing conducted or contemplated, including vessels and gear types, fishing areas, target and potential bycatch species, fishing effort levels, and duration of fishing (harvesting plan);
(ii) best available scientific and technical information on the current state of fishery resources and baseline information on the ecosystems, habitats, and communities in the fishing area, against which future changes are to be compared;
(iii) identification, description, and mapping of VMEs known or likely to occur in the fishing area;
(iv) data and methods used to identify, describe, and assess the impacts of the activity, the identification of gaps in knowledge, and an evaluation of uncertainties in the information presented in the assessment;
(v) identification, description, and evaluation of the occurrence, scale, and duration of likely impacts, including cumulative impacts of activities covered by the assessment on VMEs and low-productivity fishery resources in the fishing area;
(vi) risk assessment of likely impacts by the fishing operations to determine which impacts are likely to be significant adverse impacts, particularly impacts on VMEs and low productivity fishery resources; and
(vii) the proposed mitigation and management measures to be used to prevent significant adverse impacts on VMEs and ensure long-term conservation and sustainable utilization of low-productivity fishery resources, and the measures to be used to monitor effects of the fishing operations.
If RFMOs and States seek to prevent significant adverse impacts to VMEs without the use of individual impact assessments, a concerted effort across jurisdictions that evaluates VME species, communities, and habitats at the scale of ocean basins is needed. Such an effort can mine the existing knowledge and datasets to develop robust predictive models (Bryan and Metaxas, 2007; Tittensor et al., 2009), define the diversity of VMEs in explicit ecological terms, respond to new data arising from scientific cruises, use inference to predict locations of VMEs, link decision-rules for encounters to VME definitions, and set directions for strategic investment in survey and research needs. To some extent, such large-scale evaluations are now being considered by states, RFMOs, and regional scientific advisory bodies, and have resulted in the designation of large-scale closed or protected areas that serve to prevent significant adverse impacts on any VMEs contained therein (CCAMLR, 2008a; ICES, 2008). Such strategies will allow management to move from precaution to preventive and corrective approaches as information is produced and synthesized (sensuAuster, 2001).
It is likely, given the overlap between fishing grounds and location of VMEs, that an ecosystem approach to management of deep-sea fisheries will have to include judicious use of prior impact assessments, closed areas, gear restrictions, and fishing effort controls to prevent significant adverse impacts to VMEs. For example, most coral and sponge species found on the New England and Corner Rise Seamounts to date are delicate and fragile, so they almost by definition need to be classified as “vulnerable” to fishing. An alternative approach to addressing this issue might be to demonstrate that a seamount or other benthic community is actually resilient enough to withstand a gradient of fishing pressures. Rather than rely on the inadequate and imprecise move-on rule to prevent inadvertent impacts to VMEs when all the appropriate measures have been taken, we consider that as an additional precautionary measure, large-scale closed or protected areas should also be an integral and precautionary component of each regional fishery management strategy for deep-sea fisheries. This should have high priority in future assessments regarding progress in the implementation of the UNGA 61/105 resolution.
Acknowledgements
PJA, AR, and KG were part of the FAO Expert Consultation in Bangkok where they developed the decision support diagram, and thank all the other group members for productive discussions about management of fisheries in areas beyond national jurisdictions. NOAA's National Undersea Research Program and Office of Ocean Exploration provided support for PJA and LW to conduct the fieldwork that produced the insights reported here. ADR acknowledges the Lighthouse Foundation for funding of the Azores Deep-Water Coral Ecosystems Project. ADR and AJG both benefit from support received from the CoralFISH project (grant agreement number 213144) funded by the European Commission under the Seventh Framework Programme (FP7) Theme 6: Environment; Activity 6.2: Sustainable Management of Resources. The opinions expressed here are those of the authors and do not necessarily represent the views of the funding agencies.