-
PDF
- Split View
-
Views
-
Cite
Cite
M. Heino, F. M. Porteiro, T. T. Sutton, T. Falkenhaug, O. R. Godø, U. Piatkowski, Catchability of pelagic trawls for sampling deep-living nekton in the mid-North Atlantic, ICES Journal of Marine Science, Volume 68, Issue 2, January 2011, Pages 377–389, https://doi.org/10.1093/icesjms/fsq089
Close - Share Icon Share
Abstract
Material collected in summer 2004 from the Mid-Atlantic Ridge between Iceland and the Azores with three pelagic trawls was used to estimate relative catchabilities of common fish, cephalopod, decapod, and jellyfish species. Catchability is defined as the ratio of numbers caught between two trawls, standardized for towed distance. Taxon-specific catchability coefficients were estimated for two large pelagic trawls with graded meshes, using a smaller pelagic trawl with a uniform mesh size as the reference trawl. Two of the trawls were equipped with multiple opening–closing codends that allowed sampling of different depth layers. Generalized linear and mixed models suggest that most of the taxa have catchabilities much lower than expected from the area of opening alone, indicating that only a few species are herded by the large mesh at the mouth of larger trawls. Catchability coefficients across taxa show a very large spread, indicating that the sampled volume for the larger trawls with graded meshes was highly taxon-specific. Part of this variability can be explained by body size and taxonomic group, the latter probably reflecting differences in body form and behaviour. The catchability estimates presented here form the basis for combining data for quantitative analyses of community structure.Heino, M., Porteiro, F. M., Sutton, T. T., Falkenhaug, T., Godø, O. R., and Piatkowski, U. 2011. Catchability of pelagic trawls for sampling deep-living nekton in the mid-North Atlantic. – ICES Journal of Marine Science, 68: 377–389.
Introduction
Trawls are an effective and widely used method for collecting nekton because they sample large volumes of often sparsely distributed organisms and allow direct species identification and individual-level observations from specimens taken on board (e.g. length measurements, ageing, and stomach-content analysis). One type of trawl, however, cannot perform well for all types of nekton, which range in size from a few millimetres to several metres. Overall trawl size, which largely determines its ability to capture fast-swimming organisms, has to be traded off against mesh size, which determines the retention of small organisms. Furthermore, fine-meshed trawls cannot be towed at speeds high enough to capture species that show avoidance behaviour. A natural solution is to use more than one type of trawl with complementary characteristics, but combining the data from different gears is not easy (e.g. Kashkin and Parin, 1983; Wassenberg et al., 1997; Pelletier, 1998; von Szalay and Brown, 2001; Fock et al., 2002; West, 2002; Helser et al., 2004; Lewy et al., 2004; Porteiro, 2005).
Patterns and Processes of the Ecosystems of the Northern Mid-Atlantic (MAR-ECO) is a Census of Marine Life project that was set up to describe and understand the patterns of distribution, abundance, and trophic relationships of the organisms inhabiting the mid-oceanic North Atlantic and to identify and model ecological processes that cause variability in these patterns (Bergstad and Godø, 2002; Bergstad et al., 2008; see also www.mar-eco.no). A major contribution to this project was a 2-month cruise of the RV “G.O. Sars” in summer 2004 surveying the ecosystems along the Mid-Atlantic Ridge from Iceland to the Azores (Wenneck et al., 2008). To get quantitative and representative samples from various types and size classes of pelagic nekton, three different trawls were used (Table 1): a macrozooplankton trawl and two fish trawls, the medium-sized Åkra trawl and the larger Egersund trawl. These trawls differ substantially in their overall size as well as in mesh sizes. Both the Åkra and macrozooplankton trawls were used systematically, following a predetermined sampling scheme (15 and 17 successful hauls, respectively), whereas the Egersund trawl was used opportunistically to sample acoustically “interesting” registrations (four successful hauls). To analyse these data, e.g. to characterize the species assemblages (Sutton et al., 2008), it would be desirable to combine data from all three gear types. Simply merging the data across gears, however, would be questionable because the trawls differ considerably in their essential characteristics, which determine how efficient they are at catching pelagic organisms.
Trawls used on the RV “G.O. Sars” during the MAR-ECO cruise in summer 2004
| Trawl . | Description . | Mesh size (stretched) in the codend (mm) . | Approximate opening area (m2) . | Ratio of opening areas (macrozooplankton trawl = 1) . | Typical towing speed (knots) . |
|---|---|---|---|---|---|
| Macrozooplankton | Five codends, uniform meshes | 6 | 36 | 1 | 2 |
| “Åkra” (medium-sized fish trawl) | Three codends, graded meshes | 22 | 660 | 18 | 3 |
| “Egersund” (large fish trawl) | One codend, graded meshes | 50 | 5 000 | 137 | 3 |
| Trawl . | Description . | Mesh size (stretched) in the codend (mm) . | Approximate opening area (m2) . | Ratio of opening areas (macrozooplankton trawl = 1) . | Typical towing speed (knots) . |
|---|---|---|---|---|---|
| Macrozooplankton | Five codends, uniform meshes | 6 | 36 | 1 | 2 |
| “Åkra” (medium-sized fish trawl) | Three codends, graded meshes | 22 | 660 | 18 | 3 |
| “Egersund” (large fish trawl) | One codend, graded meshes | 50 | 5 000 | 137 | 3 |
See Wenneck et al. (2008) for further details. Macrozooplankton and Åkra trawls were equipped with a “MultiSampler” that enabled opening and closing several codends at pre-programmed depths (Engås et al., 1997).
Trawls used on the RV “G.O. Sars” during the MAR-ECO cruise in summer 2004
| Trawl . | Description . | Mesh size (stretched) in the codend (mm) . | Approximate opening area (m2) . | Ratio of opening areas (macrozooplankton trawl = 1) . | Typical towing speed (knots) . |
|---|---|---|---|---|---|
| Macrozooplankton | Five codends, uniform meshes | 6 | 36 | 1 | 2 |
| “Åkra” (medium-sized fish trawl) | Three codends, graded meshes | 22 | 660 | 18 | 3 |
| “Egersund” (large fish trawl) | One codend, graded meshes | 50 | 5 000 | 137 | 3 |
| Trawl . | Description . | Mesh size (stretched) in the codend (mm) . | Approximate opening area (m2) . | Ratio of opening areas (macrozooplankton trawl = 1) . | Typical towing speed (knots) . |
|---|---|---|---|---|---|
| Macrozooplankton | Five codends, uniform meshes | 6 | 36 | 1 | 2 |
| “Åkra” (medium-sized fish trawl) | Three codends, graded meshes | 22 | 660 | 18 | 3 |
| “Egersund” (large fish trawl) | One codend, graded meshes | 50 | 5 000 | 137 | 3 |
See Wenneck et al. (2008) for further details. Macrozooplankton and Åkra trawls were equipped with a “MultiSampler” that enabled opening and closing several codends at pre-programmed depths (Engås et al., 1997).
In this paper, we aim to estimate relative catchabilities for the three different midwater trawls used on the RV “G.O. Sars” in summer 2004 (Wenneck et al., 2008). Catchability is defined as the expected ratio of catch in numbers for two trawls fishing in the same area with the same effort (here, the distance trawled). Catchability can be defined at different levels of biological organization; here we focus on species and higher taxonomic levels. A first indication of catchability is provided by the ratio of opening areas (Table 1). Nominal opening area, however, is just one major factor affecting catchability. In general, catchability is determined by both the properties of the trawl and the characteristics of the organisms encountered, and the interactions between them. The following are four major factors that are expected to cause systematic differences in the catchability of the trawls used in this study: The estimated catchability coefficient will reflect all the above-mentioned factors, plus measurement noise arising from, for example, spatial heterogeneity and variability in gear performance (Byrne et al., 1981; Pelletier, 1998).
Area of opening. Filtered volume is proportional to the mouth area of trawl, but strict proportionality between the filtered volume and the catches is expected only when there is no avoidance and all individuals in the filtered volume are retained (Barkley, 1972). Expected effect on catchabilities: Egersund > Åkra > macrozooplankton.
Ease of avoidance. This is closely related to the size of trawl (Barkley, 1964, 1972; Bethke et al., 1999) and the towing speed (Barkley, 1964, 1972; Winger et al., 2000; Gabriel et al., 2005). For organisms showing avoidance behaviour, increasing the diameter of a trawl should increase the catchability, and increasing the towing speed should have a similar effect, to the extent that the so-called bucket effect does not come into the play. Rigging may also affect the noise and the bioluminescence caused by the approaching trawl (Jamieson et al., 2006) and thus the likelihood of early detection and avoidance, but we have no data on these parameters. Expected effect on catchabilities: Egersund > Åkra > macrozooplankton.
Retention through mesh selection. Mesh selection depends on the mesh size relative to the size of individuals as well as their body shape and form (Barkley, 1972; Gartner et al., 1989; Millar, 1992; Wileman et al., 1996; Bethke et al., 1999). Expected effect on catchabilities: Egersund < Åkra < macrozooplankton.
Herding effect. In pelagic trawls with decreasing meshes towards the codend, capture is based not only on filtering but also on behavioural response known as herding (Lee et al., 1996; Valdemarsen, 2001). Fish inside the trawl try to avoid the meshes and do not swim through the meshes even if they could do so, but are instead herded in the middle of the trawl, eventually encountering meshes that are small enough for retention. In bottom trawls, trawl doors and bridles cause herding (Wardle, 1993; Ramm and Xiao, 1995; Sangster and Breen, 1998; Winger et al., 2004), but the extent to which this happens in pelagic trawls is unknown. Visual detection of trawls in deep water is made possible by bioluminescence caused by the trawl itself (Jamieson et al., 2006). Expected effect on catchabilities: potentially important in Egersund and Åkra trawls, probably unimportant in the macrozooplankton trawl.
The value of catchability estimates comes from three sources. First, catchability coefficients form the quantitative basis on which data collected with different gears can be compared. Furthermore, catchability coefficients allow for a description of the performance of trawls (e.g. effective mouth area). Taken together, catchability estimation contributes to improved monitoring strategies for the deep ocean. Finally, catchability estimates also provide indirect information on the behaviour of deep-living biota.
Material and methods
Wenneck et al. (2008) give a detailed account of the methods employed in collecting the material. We include fish, cephalopods, decapods, and large medusae (disc diameter >1 cm) in our analyses. The analyses were run at five taxonomic levels, at the level of species, genus, family, order, and class, following taxonomy by Nelson (2006) for fish, Sweeney and Roper (1998) for cephalopods, and Crosnier and Forest (1973) and Vereshchaka (2000) for decapods. Atolla, Mastigoteuthis, and Hymenodora were not identified to species level, but for simplicity we refer to them also as “species”.
Sampling was based on predetermined “superstations” where both the macrozooplankton and the Åkra trawls were used, whereas the Egersund trawl was used opportunistically (Wenneck et al., 2008). The macrozooplankton and the Åkra trawls were equipped with a “MultiSampler” (Engås et al., 1997), a multiple opening–closing device that enabled five and three samples, respectively, to be obtained from pre-programmed depths during a single haul. Because an estimation of the sampling volume was straightforward only for the macrozooplankton trawl, this trawl was used as the reference trawl against which the Åkra and the Egersund trawls were compared. In a statistical sense, the sampling unit was a specific depth layer and superstation where both gears being compared were successfully used. In analyses specific to a taxon, data from sampling units where the taxon was not observed in either trawl were omitted. The data therefore contain informative zeros from sampling units where only one gear captured the taxon and are balanced with respect to the trawl.
Although the macrozooplankton and the Åkra trawls were equipped with a multiple opening–closing device, surface contamination can occur. When single specimens of abundant epi- or mesopelagic species were captured well below their continuous depth distribution in the current data and below their reported depth range, they were considered contaminants and removed from the data. This led to the deletion of a few observations of Entelurus aequoreus, Maurolicus muelleri, and seven species of myctophid.
In comparisons with the Åkra trawl, the macrozooplankton trawl catches were aggregated into three layers that showed the closest match with the depth layers sampled by the other trawl at the same stations; sometimes a macrozooplankton trawl sample had to be discarded because there was no corresponding Åkra trawl sample (e.g. the horizontal macrozooplankton trawl hauls). This led to a balanced set-up, where samples could be compared as pairs representing the same station and similar depth interval but different trawl (Supplementary material Table S1). Because the Egersund trawl was used opportunistically outside the predetermined standard stations, the samples were paired thereafter by matching stations based on the geographic distance and species composition (Supplementary material Table S2).
In the final analyses involving the Åkra trawl, we only included taxa that had three or more positive records with both trawls being compared; species that were not frequent enough for species-level analyses still contributed to analyses at higher taxonomic levels. For species-level analyses involving the Åkra trawl, our material includes 52 fish species, 19 species of crustaceans, five species of cephalopod, and two species of medusa (total 78 species). Because the Egersund trawl was successfully used only four times, we relaxed the data-selection criterion and included taxa that had two or more positive records with both the Egersund and the macrozooplankton trawls. The material includes eight fish species, five decapods, one cephalopod, and two medusae.
Samples were classified as daytime, dusk, night, or dawn samples, using sunrise and sunset times calculated for each sampling location and date. Sunrise and sunset times were calculated using the CBM model of Forsythe et al. (1995) to estimate daylength and the equation of time and longitude to estimate the solar noon. A dusk sample was defined as a sample that was at least partially taken during the period from 1 h before sunset to 1 h after. Similarly, dawn samples were those that overlapped with the period from 1 h before sunrise to 1 h after sunrise. Our sampling was unbalanced with respect to gear and diel phase: the macrozooplankton trawl was used more often at night (11 samples or 26% of the total) than the Åkra trawl (one sample, 2.4%); the proportions of dusk and dawn samples were similar (respectively, six and seven samples).
Statistical methods
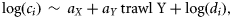
Including additional explanatory variables could improve catchability estimates in terms of precision and accuracy. We considered depth and diel variation in comparisons between the macrozooplankton and the Åkra trawls; for the Egersund trawl, there were too few observations. Alas, diel effects could not be routinely considered because for many species data were too unbalanced, with not all combinations of day and night vs. gear type being present at those superstations where a species occurred. Therefore, diel effects were considered only for species with sufficiently balanced data as an additional check of robustness of the results.
Depth, calculated as the average of a haul's starting and finishing depth (Supplementary material Table S1), could be used routinely. Because our measure of depth is not precise, however, we did not use depth for species that had a relatively narrow vertical range of <500 m (E. aequoreus and five myctophids: Lampanyctus pusillus, Vinciguerria poweriae, Diaphus rafinesquii, Symbolophorus veranyi, and Electrona risso). For all other species, we centred the depth data so that the species-specific mean depth was zero, and estimated models with linear and/or quadratic depth terms (the quadratic term allows for the catch rates to peak at intermediate depths). The model that had the lowest Akaike Information Criterion (with correction for small sample sizes, AICc) was chosen as the final model. A depth term was included for 51 out of 78 species in our data (65%). Nevertheless, in most cases, the estimates of catchability were little influenced by consideration of depth effects. In a few cases where changes were larger, these were supported by non-negligible improvements in the AICc and were considered biologically sensible. For example, catchability for Lampanyctus crocodilus was ρ = 0.43 without a depth effect, and ρ = 1.2 with a linear depth effect (ΔAICc = −4.7); neither estimate is significantly different from 1 but the latter is more reasonable for a relatively larger species. Furthermore, when the best model involved a depth term, the standard error for the catchability was usually somewhat smaller than without the depth term.
All analyses were carried out in R 2.9.0 (R Development Core Team, 2009). We used function “glm.nb” by Venables and Ripley (2002) for fitting the negative binomial models. When taxon was included as an explanatory variable and treated as a random effect, package “lme4” by Bates and Maechler (2009) was used for fitting generalized mixed models. When exact p-values for hypothesis testing are not given, p = 0.05 is used as the limit of statistical significance.
Results
Macrozooplankton vs. Åkra trawl
Catchability of the Åkra trawl relative to the macrozooplankton trawl for all fish was 2.3 (95% CI for catchability: 1.6–3.4, aY = 0.838, s.e. 0.197) for catch in numbers. For all cephalopods, the catchability of the Åkra trawl was estimated to be 0.38 (95% CI: 0.14–1.03, aY = –0.966, s.e. 0.510). For large medusae, the catchability of the Åkra trawl was estimated to be 3.05 (95% CI: 0.50–19, aY = 1.12, s.e. 0.926). For decapods, the catchability of the Åkra trawl was estimated to be 0.57 (95% CI: 0.35–0.93, aY = –0.566, s.e. 0.251). Therefore, the Åkra trawl was more efficient at catching fish than the macrozooplankton trawl, whereas the opposite was true for decapods. For medusae and cephalopods, the results were inconclusive, although the results were suggestive of a tendency of the macrozooplankton trawl to catch more cephalopods than the Åkra trawl.
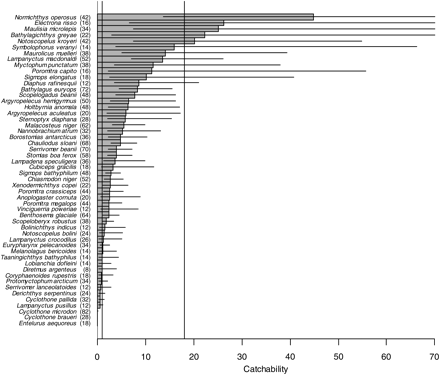
We were able to estimate catchability for 52 fish species (Figure 1). The estimates range from 0.0066 (snake pipefish, E. aequoreus) to 45 (platytroctid, Normichthys operosus). For 31 species (60%), the Åkra trawl was significantly more efficient than the macrozooplankton trawl (ρ > 1), but only for 12 species (23%) was the theoretical catchability derived from the ratio of mouth areas (ρ = 18) was within the confidence limits of the estimate. There were three species, however, for which the macrozooplankton trawl was significantly more efficient, all of them small (two species of bristlemouth, Cyclothone) or very thin-bodied (E. aequoreus).
Estimates of catchability of 52 fish species with the Åkra trawl, a medium-sized pelagic trawl with graded meshes, relative to the macrozooplankton trawl. Horizontal bars give 95% confidence limits (for N. operosus, E. risso, M. microlepis, and B. greyae these extend outside the plot area to, respectively, 148, 104, 85, and 170). Vertical lines give reference values that correspond to equal catchability (1) and to the ratio of opening areas (18). Sample size is indicated in parenthesis after the species name.
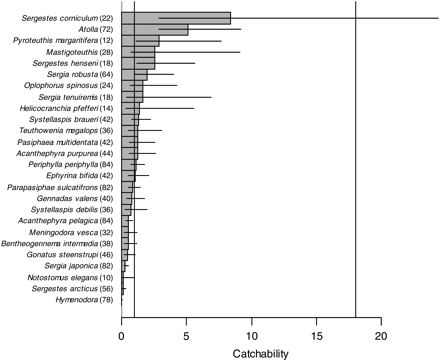
We estimated catchability for 26 invertebrate species (Figure 2). For the majority of these (65%), the Åkra and the macrozooplankton trawls were not significantly different, and only for the decapod shrimp Sergestes corniculum did the confidence limits overlap with the theoretical catchability derived from the ratio of mouth areas (ρ = 18). Decapods in general showed a very large spread of catchabilities, ranging from 0.033 in Hymenodora to 8.4 in S. corniculum, with five species having catchability significantly less than 1, whereas two species (both from genus Sergestes) had catchability that was significantly larger than 1. Also one medusa (Atolla) and one cephalopod (Pyroteuthis margaritifera) had catchabilities significantly larger than 1.
Estimates of catchability of 2 medusa, 5 cephalopod, and 19 decapod species (or genera) with the Åkra trawl relative to the macrozooplankton trawl. Horizontal bars give 95% confidence limits. Vertical lines give reference values that correspond to equal catchability (1) and to the ratio of opening areas (18). Sample size is indicated in parenthesis after the species name.
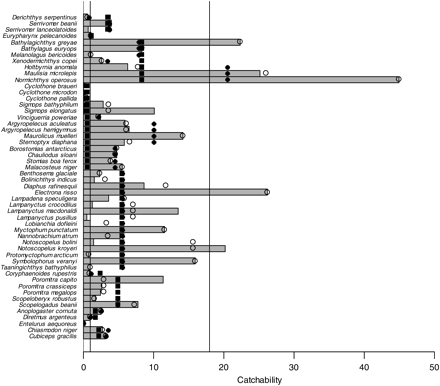
We estimated catchability also at higher taxonomic levels (Figure 3). In some cases, there were considerable differences within a genus or family. Of families represented by more than one species, the Platytroctidae had the highest catchability whereas the Gonostomatidae had the lowest. The estimate for the Gonostomatidae was strongly influenced by small but abundant Cyclothone species, whereas other genera in the family had higher catchabilities.
Estimates of catchability (in numbers) of fish taxa for the Åkra trawl relative to the macrozooplankton trawl. For each fish for which the catchability was estimated at the species level (grey bars), we also give the estimates at the generic (open circles), familial (black circles), and ordinal levels (black squares). For some orders, there was only one species and all estimates are identical. The taxa are sorted following Nelson (2006).
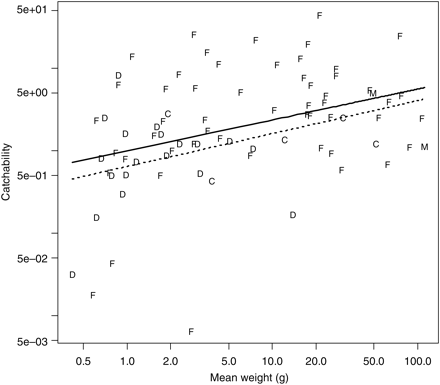
Some variability in the catchability estimates can be explained by body size: catchability was positively related to mean body weight (linear model with log-transformation of both variables: F1,76 = 12.0, p = 0.001), and on average, doubling the body weight increased the catchability by a factor of 1.46 (95% CI: 1.18–1.80). However, the relationship was noisy (Figure 4) and only a small proportion of variability in the data could be explained (r2 = 14%). Taxon-specific differences remained: including “order” as an explanatory variable significantly improved the fit (F12,64 = 3.28, p = 0.001, r2 = 47%); the effect was weaker but still significant (F9,64 = 2.16, p = 0.037, r2 = 36%) if three orders represented by only one species (Gadiformes, Saccopharyngiformes, Syngnathiformes) were excluded. Without monospecific orders and using the abundantly sampled lanternfish (Myctophiformes) as the reference order, we saw that eels (order Anguilliformes), decapods, and cephalopods (Oegopsida) had a lower catchability than their weight would suggest; medusae and other fish orders were not significantly different from lanternfish. Similarly, the fit could be improved using family (instead of order) as an explanatory variable, either with (F27,49 = 2.86, p = 0.001, r2 = 66%) or without monospecific families (F12,49 = 3.41, p = 0.001, r2 = 58%). Without mono-specific families and using the abundantly sampled lanternfish (Myctophidae) as the reference family, we saw that when accounting for weight differences, two fish (Gonostomatidae and Serrivomeridae), one decapod (Oplophoridae), and one cephalopod (Cranchiidae) families had a lower catchability than their weight would suggest. Treating order or family as a random effect, instead of a fixed effect as above, gave a similar estimate for the average effect of doubling the body size (order as a random effect: 1.49, 95% CI: 1.17–1.90; family as a random effect: 1.58, 95% CI: 1.25–1.99) as obtained above for the model without taxonomic information (1.46). We also considered taxon-specific weight effects on catchability, but our data were too few to allow detecting significant effects.
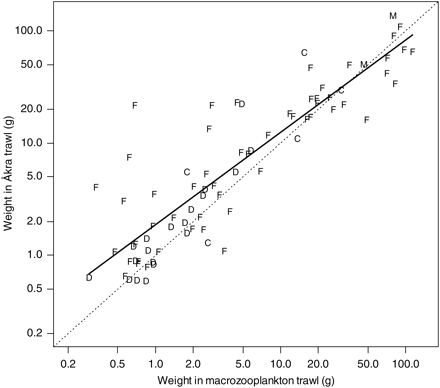
Relationship between the mean species-specific weight and the estimated catchability for the Åkra trawl relative to the macrozooplankton. Letters are used to indicate a taxon: F = fish, D = decapod, C = cephalopod, M = medusae. Mean weight is calculated as the mean individual weight (catch weight/catch numbers) over all the hauls in the comparison. The thick regression line is for an ordinary regression, and the dotted regression line is for a mixed model treating order as a random effect. Note the logarithmic scale on both axes.
Diel effects could also influence catchability. Our data were imbalanced, however, such that diel and gear effects could become confounded. To reduce this problem, we analysed diel effects only at higher taxonomic levels. For fish, including diel phase (day, dusk, night, and dawn; see the “Material and methods” section) did not significantly improve the model where gear was used as the explanatory variable (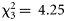 , p = 0.236), but it did so when “order” was also included (
, p = 0.236), but it did so when “order” was also included (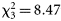 , p = 0.037). The latter model suggested that daytime catches tended to be higher than night-time catches; dawn and dusk catches were not significantly different from night catches. This effect could arise from the fact that the Åkra trawl had more daytime samples than the macrozooplankton trawl.
, p = 0.037). The latter model suggested that daytime catches tended to be higher than night-time catches; dawn and dusk catches were not significantly different from night catches. This effect could arise from the fact that the Åkra trawl had more daytime samples than the macrozooplankton trawl.
To make the data more balanced, we regrouped dawn and dusk catches with night-time catches. Analysing the data by order suggested that night-time catches were significantly higher for the orders Osmeriformes and Syngnathiformes. A significant gear × day/night interaction was detected for Anguilliformes, Osmeriformes, and Stomiiformes, suggesting that the Åkra trawl was relatively more efficient during darkness for the two first orders, but the opposite held true for the last one. For cephalopods, a significant diel effect was apparent (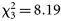 , p = 0.042), but this disappeared if a single large dusk catch of Gonatus steenstrupi was omitted. Also for medusae, the data suggested a diel effect (
, p = 0.042), but this disappeared if a single large dusk catch of Gonatus steenstrupi was omitted. Also for medusae, the data suggested a diel effect (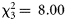 , p = 0.046): dawn catches appeared on average higher than night-time catches. In contrast to the aforementioned groups, diel effects appeared relatively strong in decapods: inclusion of the diel phase greatly improved the model fit (
, p = 0.046): dawn catches appeared on average higher than night-time catches. In contrast to the aforementioned groups, diel effects appeared relatively strong in decapods: inclusion of the diel phase greatly improved the model fit (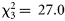 , p < 0.001), with dusk catches being much higher than night-time catches. Furthermore, there was a significant interaction between the trawl and the diel phase (
, p < 0.001), with dusk catches being much higher than night-time catches. Furthermore, there was a significant interaction between the trawl and the diel phase (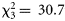 , p < 0.001): the Åkra trawl appeared less efficient in catching decapods during the day and dusk compared with the macrozooplankton trawl.
, p < 0.001): the Åkra trawl appeared less efficient in catching decapods during the day and dusk compared with the macrozooplankton trawl.
In addition to the Åkra and the macrozooplankton trawls often catching different numbers of individuals of a species for the same effort, they also had a tendency to catch differently sized individuals: for 56 of 78 species, mean individual weight was higher in the Åkra than in the macrozooplankton trawl (Figure 5). This tendency was evident across the main taxonomic groups, but was more pronounced in small species; linear regression fitted on a log–log scale yielded a significantly positive intercept but a slope that was significantly less than 1.
Relationship between the mean species-specific weight between the macrozooplankton and Åkra trawl catches. The corresponding regression model is illustrated by a thick line (r2 = 78%). Letters are used to indicate a taxon: F = fish, D = decapod, C = cephalopod, M = medusa. Mean weight is calculated as the mean individual weight (catch weight/catch numbers) for each combination of species and trawl type. The diagonal is shown as a dotted line. Note the logarithmic scale on both axes.
Macrozooplankton vs. Egersund trawl
Because the Egersund trawl was only used four times, catchability of the Egersund trawl relative to the macrozooplankton trawl could only be estimated for a few species. Note also that the material only included relatively large species because smaller ones were not caught often enough by the large-meshed Egersund trawl.
The catchability of the Egersund trawl relative to the macrozooplankton trawl for fish in general was 57 (95% CI: 19–168, aY = 4.04, s.e. 0.55). For all decapods, the catchability of the Egersund trawl was estimated to be 0.35 (95% CI: 0.01–18, aY = –827, s.e. 1.91). For medusae, the catchability of the Egersund trawl was estimated to be 7.8 (95% CI: 0.06–1070, aY = 2.06, s.e. 2.51). Only one cephalopod, G. steenstrupi, was common enough for estimation, and even the estimate for this species was highly uncertain (2.8, 95% CI: 0.37–21). The Egersund trawl was therefore more efficient than the macrozooplankton trawl for fish, but for the other groups there was no detectable difference.
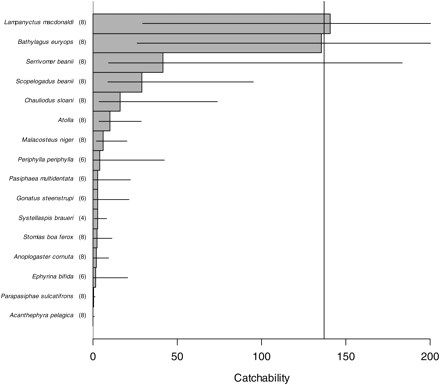
Figure 6 shows catchability estimates obtained for all species fulfilling our data-selection criteria. For one species (decapod, Acanthephyra pelagica), the macrozooplankton trawl was significantly more efficient than the Egersund trawl, whereas the Egersund trawl was significantly better at catching six fish and one medusa species. The ratio of opening areas (137) was within the confidence limits of catchability estimates for three fish species; for two of these species, the point estimate was similar to the ratio of opening areas, but the estimate was very imprecise.
Estimates of catchability of eight fish and eight invertebrate species with the Egersund trawl, a large pelagic trawl with graded meshes, relative to the macrozooplankton trawl. Horizontal bars give 95% confidence limits (for Lampanyctus macdonaldi this extends outside the plot to 674 and for Bathylagus euryops to 697). The vertical line gives a reference value that corresponds to the ratio of opening areas (137). Sample size is indicated in parenthesis after the species name.
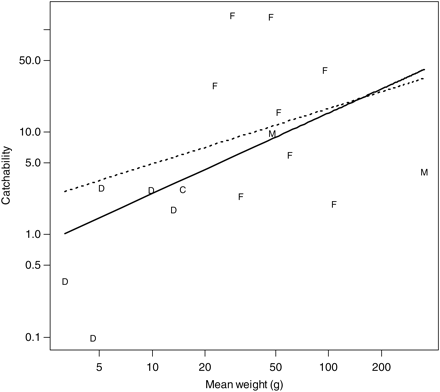
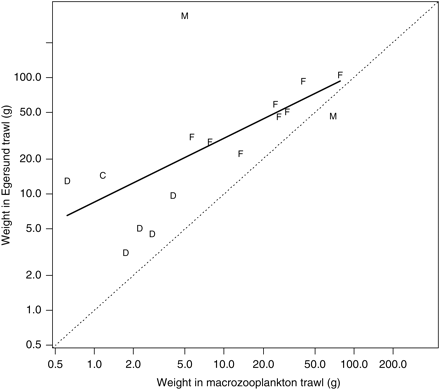
Regressing log-catchability against log body weight showed a significant positive effect of body weight on catchability; the regression could explain 26% of the variance (Figure 7). However, the relationship was heavily influenced by decapods that are relatively small and had low catchabilities; treating order as an explanatory variable resulted in a weaker positive weight effect that no longer was significant (p = 0.51). The Egersund trawl had a marked tendency to catch larger individuals of a certain species than the macrozooplankton trawl (Figure 8).
Relationship between the mean species-specific weight and the estimated catchability for the Egersund trawl relative to the macrozooplankton trawl. See Figure 4 for further explanation.
Relationship between the mean species-specific weight between the macrozooplankton and Egersund trawl catches. The corresponding regression model is illustrated by a thick line (r2 = 42%). See Figure 5 for further explanation.
Discussion
The catchability estimates presented in this paper showed large variability among different species of fish, cephalopods, and large medusae. Towing the relatively small macrozooplankton trawl at the same depth and area for the same distance as the medium-sized Åkra trawl would be expected to yield, on average, 150 times as many pipefish, E. aequoreus, but only ∼1/45 of the catch of the platytroctid N. operosus. Many of the smallest species caught with the macrozooplankton trawl were entirely missed by the large Egersund trawl. These findings call for care when data from different gears are synthesized.
Our analysis was based on pairs of hauls taken with two gears being compared, which is the standard approach in gear comparisons (Wileman et al., 1996). However, because comparing the catchability of different trawls was not the primary goal of the sampling, the pairs are inherently more different from what could be achieved in a targeted study (Pelletier, 1998; von Szalay and Brown, 2001; Lewy et al., 2004). In particular, depth ranges were not always closely matching. This is likely to add noise to our data but not introduce a systematic bias. Furthermore, because total tow durations were long and only a single vessel was used, samples were often taken under different light regimes. This is potentially more problematic because the macrozooplankton trawl was used more often during darkness than the Åkra trawl. Diel migrations, however, do not change overall abundance of organisms at the station level, so the potential for bias arises only if the night-time samples with the macrozooplankton trawl were distributed unevenly between the depth layers. At the level of the whole data, the distribution was only mildly uneven (5, 3, and 3 samples from the depth layers 1–3), but for individual species, imbalance might be more serious. In conclusion, we do not expect diel migrations to bias our catchability estimates in general, but for individual species this can happen.
Some species often get entangled in large meshes in the forenet and never enter the codend (e.g. Kashkin and Parin, 1983). This applies in particular to cephalopods, large specimens of jellyfish, and species like eels and the dragonfish Stomias boa ferox. The cause of entanglement could be fully passive (jellyfish), or an active behavioural response, i.e. an animal attacking the trawl (possibly triggered by bioluminescence) as suggested by Stomias, which were often found hanging with their teeth in the net.
Catchabilities showing the macrozooplankton trawl to be more efficient per towed distance than the larger trawls (ρ < 1) probably reflect mesh selection in the codend (e.g. Gartner et al., 1989; Wileman et al., 1996). These are mostly small species (Figures 4 and 7). Our results also show that the small-meshed macrozooplankton trawl catches, on average, smaller specimens than the large-meshed trawls (Figures 5 and 8). Mesh selection is probably contributing to this difference, but the ability of larger trawls to catch large specimens able to avoid the smaller trawls might be important. Disentangling these mechanisms requires individual size data that we did not collect systematically; the size data we have suggest that both mechanisms are operating but not always simultaneously (unpublished results).
For a perfectly herded species where mesh selection in the forenet is unimportant, we would expect a catchability similar to the ratio of the opening areas. For a number of fish species, the estimated catchability was near this theoretical catchability (with the theoretical catchability within the confidence limits; Figure 1). The species with the highest catchability estimates included two platytroctids, a deep-sea smelt and a number of lanternfish. Because the body size of these species was small to moderate (the largest individuals had a total length of ∼20 cm), much of the opening area of the larger trawls had such large meshes that retention could not possibly account for the high catchability. Two complementary explanations then remain. First, herding and avoidance of large forenet meshes were important. Second, these species were relatively successful in avoiding the smaller trawl. With our data, it is not possible to disentangle these mechanisms, and both probably played some role.
Both mechanisms mentioned above imply that the fish species with a high catchability must be able to maintain relatively high swimming speeds for some time. ROV observations provide some support for this statement (Trenkel et al., 2004; J. Moore, pers. comm.). This contradicts the stereotypical view of deep-sea fish, at least the non-migrant ones, being typically phlegmatic energy savers. This view might have been overly influenced by sit-and-wait predators, such as dragonfish. The high catchability estimates for some species in our material, together with their relatively sleek body shapes, suggest that perhaps they are more active predators than previously thought.
Only very few invertebrates had a catchability greater than 1. For one decapod, S. corniculum, the best estimate was rather high, and the confidence limit overlaps with the theoretical catchability (Figure 2). This is a relatively small species (average body weight <1 g) that must be capable of quite a high swimming speed relative to its body size to be able to display behaviour implied by its catchability estimate; indeed, S. corniculum is known for its extensive vertical migration (Roe, 1984). Alternatively, it could be that the “true” catchability is much less than the best current estimate. Catchability could be estimated for two other, though slightly smaller, Sergestes species, one of which had a catchability just barely larger than 1, whereas the other, and the most common of the three, S. arcticus, had a catchability much less than 1. One medusa, Atolla, also had a relatively high catchability. As Atolla are poor swimmers but often quite large, mesh selection outside the codend is the probable explanation for the catchability of this animal.
A trawl does not necessarily scare off all animals. A trawl moving in water stimulates bioluminescence (Jamieson et al., 2006); this light can attract fish and is often used in fish capture (Pascoe, 1990; Gabriel et al., 2005). To what extent this process influences the catchability of deep-pelagic nekton is unknown, although attaching electric lights to trawls is known to increase their catchability, at least, for certain species (Clarke and Pascoe, 1985, 1998; Clarke et al., 1986; Swinney et al., 1986), and to decrease the catchability of certain other species (Clarke et al., 1986). Whether attraction caused by bioluminescence is differently influencing the trawls considered here is unknown. Another source of attraction is the animals in the trawl itself: codend feeding by active predators such as cephalopods is known (Herring, 2002). Such predators are unlikely to be caught by the trawl, but their feeding in the codend would reduce the catches of prey species. Also species not attracted by the catch but opportunistically feeding in the codend would have a similar effect. Although codend feeding is difficult to show, there was nothing suggesting that this was important in our samples.
Our analyses suggest some diel effects on catchability. Because we sampled more or less the whole water column, diel migrations alone are not sufficient to cause systematic diel catchability effects. Imbalanced day- and night-time sampling with respect to the trawl, however, could give rise to artefactual diel effects. This could explain the higher daytime catches when gear × day/night interaction was not allowed. With the interaction term present, the analyses tended to suggest higher catches during darkness. This is compatible with visual avoidance of trawls in the upper parts of the water column with some daylight.
Traditionally, trawl comparisons have focused primarily on differences in size selectivity (e.g. Millar, 1992; Erickson et al., 1996; Wileman et al., 1996; Millar and Holst, 1997; Millar and Fryer, 1999; Bethke et al., 1999; Kvamme and Isaksen, 2004). There has been less focus on differences in catch rates at the species level (Wassenberg et al., 1997; Sangster and Breen, 1998; Fock et al., 2002; West, 2002; Lewy et al., 2004; Porteiro, 2005). Studies of the fishing power of survey vessels may involve different trawls but these are confounded by vessel effects (von Szalay and Brown, 2001; Helser et al., 2004). Common to most of these studies is the methodological similarity to this study in that they analysed effort-standardized catch rates using linear statistical models. Porteiro (2005) adopted a different approach, using multivariate statistics to account for gear differences. The studies by Wassenberg et al. (1997), West (2002), Lewy et al. (2004), and Porteiro (2005) point to big differences between different trawls in catchability as well as species that are caught. On the other hand, von Szalay and Brown (2001) and Helser et al. (2004), comparing research and commercial fishing vessels using bottom trawls, showed moderate differences in catchability of key species and that combining data from different platforms is possible and possibly worthwhile.
Helser et al. (2004) treated gear (or more precisely, vessel) as a random effect. This is sensible when many gears are being compared and one is interested in overall gear effects, not specific gear types. In this paper, gear was treated as a fixed effect because there were only three trawl types (of which only two could be compared at a time) and we were interested in those very trawls, so that the data from different trawls could ultimately be merged. Our approach necessitates choosing one trawl as the reference trawl, here the macrozooplankton trawl. Dividing catches obtained with one of the large trawls by the corresponding catchability estimate gives an estimate of catch that would have been caught with the macrozooplankton trawl, given the same effort in terms of towed distance. As the effective mouth area of the macrozooplankton trawl is known, catches per towed distance with the other trawls can be converted to density estimates in volume that would have been caught with the macrozooplankton trawl. Note, however, that this does not imply that the estimate is “correct”, even if the catchability estimate is correct. If a species is rather successful in avoiding the macrozooplankton and less so with a larger trawl (this would be seen as a catchability estimate exceeding the ratio of the opening areas), converting the observations from the large trawl to the macrozooplankton trawl scale underestimates the abundance. Using the macrozooplankton trawl as the reference trawl must therefore be seen as a pragmatic choice.
The primary application of our catchability estimates is community characterization of pelagic fauna along the Mid-Atlantic Ridge. If data from different gears are analysed together, ordination methods tend to cluster them separately, as observed in other studies (e.g. West, 2002). Correction with catchability estimates, however, nests the Åkra trawl samples within the macrozooplankton trawl samples in multivariate analysis (Sutton et al., 2008). Therefore, the systematic differences between the gears appear to be successfully removed. Of course, the catchability estimates obtained here only apply to the material studied in this paper. The estimates provide some guidance for other areas and times, but care should be taken, especially during different seasons and where populations with different size composition are encountered.
The focus of this paper on catchability tends to highlight challenges rather than the benefits arising from complementary characteristics of different gears. The first impression is that relatively little is gained or lost with using larger trawls. For the Åkra trawl, catchabilities estimated for major taxonomic groups showed that the macrozooplankton trawl was significantly more efficient than the Åkra trawl for decapods, whereas the opposite was true for fish; for other groups the difference was insignificant and none of the differences were large in magnitude. The results are similar for the Egersund trawl, except that the efficiency gain for fish was substantial. This ignores, however, the facts that the Egersund trawl missed many smaller species, the specimens in the catch were more damaged, and the trawl is more time-consuming to operate. On the other hand, even within a species, the small and large trawls did not necessarily catch similar specimens: larger trawls with large meshes tended to miss smaller specimens, but also to catch larger specimens than the small trawls. Indeed, some of the specimens appeared unusually large for the species. A study targeting the whole life cycle of a species might therefore need to use both small and large trawls.
Furthermore, different trawls may catch entirely different species. Because of the data selection applied here, our results only apply to species caught with both trawl types under comparison. Several species, however, were caught only with one trawl type (corresponding to a catchability approaching either zero or infinity). The macrozooplankton trawl caught 31 fish species not caught with the Åkra trawl, whereas the corresponding number for the Åkra trawl is 96; 108 species were caught with both trawls. For rare species this is likely to be by chance alone, and the total sampling effort in terms of distance trawled was greater for the Åkra trawl, so care is needed before drawing conclusions from these numbers. Preliminary analyses using a randomization approach (e.g. Manly, 1997), pooling the macrozooplankton trawl samples so that the distance trawled was similar to the Åkra trawl samples, suggested that both Åkra and macrozooplankton trawls caught slightly more species than expected by chance, but that the differences are not significant. Results for cephalopods were similar.
Using different gears to sample an ecosystem is both an opportunity and a challenge. The results presented here and in Sutton et al. (2008) suggest that the challenges are potentially manageable. It must be acknowledged that two trawls will sample a broader range of species as well as a broader size spectrum within a species than a single trawl, and that something is lost if only one trawl type can be employed. Whether the extra effort and costs needed to operate more than one trawl type are warranted will depend on the specific goals. For routine monitoring the answer might well be negative, whereas more comprehensive ecosystem studies or faunal inventories should seriously consider using more than one trawl. Indeed, the need to use more than one sampling method is often acknowledged in faunal surveys of terrestrial and freshwater systems (e.g. Southwood and Henderson, 2000; Gunzburger, 2007; Ribeiro-Júnior et al., 2008), but less so in deep oceanic surveys. If one then chooses a multi-trawl approach, care is needed so that the sampling design is sufficiently balanced to allow quantitative merging of the data from different sources.
Supplementary material
The supplementary material is available at ICESJMS online.
Acknowledgements
We thank personnel and our colleagues on board RV “G.O. Sars” for their collaboration during the survey, Census of Marine Life for support for our work, and J. Moore for helpful comments on the manuscript. We also acknowledge support from the Network of Excellence “Marine Biodiversity and Ecosystem Functioning” (MarBEF), funded by the European Community's Sixth Framework Programme (contract no. GOCE-CT-2003-505446). MH acknowledges support from the Bergen Research Foundation.