-
PDF
- Split View
-
Views
-
Cite
Cite
Andreia Braga-Henriques, Marina Carreiro-Silva, Filipe M. Porteiro, Valentina de Matos, Íris Sampaio, Oscar Ocaña, Sérgio P. Ávila, The association between a deep-sea gastropod Pedicularia sicula (Caenogastropoda: Pediculariidae) and its coral host Errina dabneyi (Hydrozoa: Stylasteridae) in the Azores, ICES Journal of Marine Science, Volume 68, Issue 2, January 2011, Pages 399–407, https://doi.org/10.1093/icesjms/fsq066
Close - Share Icon Share
Abstract
The Azores region harbours the richest communities of stylasterid corals in the Northeast Atlantic area. Of the nine deep-water species found there, Errina dabneyi seems to be the most abundant species; it is commonly collected as bycatch from longline fishing. E. dabneyi host Pedicularia gastropods on their branches, and a detailed study of shell shape and morphometry at different growth stages, complemented by shell characterization through scanning electron microscopy, allows the individuals to be identified as Pedicularia sicula. The incidence of this species on E. dabneyi was high (69.8%), with abundances ranging between 1 and 223 individuals per colony. The pediculariids exhibited a high degree of plasticity and produced evident traces on the stylasterid skeletons at their fixation points, suggesting that they are ectoparasites and not predators of E. dabneyi. The stylasterid colonies also hosted a rich associated fauna dominated by suspension-feeders using the coral as substratum and for protection.Braga-Henriques, A., Carreiro-Silva, M., Porteiro, F. M., de Matos, V., Sampaio, Í., Ocaña, O., and Ávila, S., P. 2011. The association between a deep-sea gastropod Pedicularia sicula (Caenogastropoda: Pediculariidae) and its coral host Errina dabneyi (Hydrozoa: Stylasteridae) in the Azores. – ICES Journal of Marine Science, 68: 399–407.
Introduction
The genus Pedicularia Swainson, 1840 (Caenogastropoda: Pediculariidae) encompasses 10 gastropod species (Simone, 2005; Lorenz and Fehse, 2009), commonly reported as symbionts of stylasterid corals (Zibrowius and Cairns, 1992; Bouchet and Warén, 1993; Goud and Hoeksema, 2001). Many gastropods living in association with corals are described in the literature as parasites (Goh et al., 1999) or predators (Théodor, 1967; Robertson, 1970; Cate, 1973; Morton, 1988; Wu et al., 1990; Goh and Chou, 1994; Oliverio et al., 1995; Schiaparelli et al., 2005). It is sometimes difficult to distinguish between parasitism and predation, so their functional classification remains uncertain (Buhl-Mortensen and Mortensen, 2004).
The association between pediculariids and stylasterids has been reported in 12 host species and one subspecies from several locations in the Atlantic and the Mediterranean (Table 1), with western Atlantic records rarer than those from the Northeast Atlantic and Mediterranean (Zibrowius and Cairns, 1992). Records for the Azores were mostly based on traces and identification to species level was never achieved (Cairns, 1991; Zibrowius and Cairns, 1992), except a few observations made by Wisshak et al. (2009); however, no method of identification was provided.
Pedicularia and their stylasterid hosts known for the Atlantic and the Mediterranean.
| Symbiont . | Host . | Locality . | Depth range (m) . | Reference . |
|---|---|---|---|---|
| One specimen + traces | Errina atlantica Hickson, 1912 | Azores | 810–983 | Zibrowius and Cairns (1992) |
| One specimen + traces | Errina dabneyi Pourtalès, 1871 | Azores | 215–560 | Zibrowius and Cairns (1992) |
| Traces only | Lepidopora eburnea Calvet, 1903 | Azores | 480–895 | Zibrowius and Cairns (1992) |
| P. sicula Swainson, 1840 | Errina dabneyi Pourtalès, 1871 | Southern Faial Channel, Azores | 475; 490 | Wisshak et al. (2009) |
| P. splendida Lorenz, 2008 | Errina atlantica Hickson, 1912 | Hyères and Atlantis Seamounts | 845–1520 | Lorenz (2008) |
| Traces only | Stenohelia maderensis Johnson, 1862 | Madeira | 300–340 | Zibrowius and Cairns (1992) |
| Traces only | Stylaster erubescens Pourtalès, 1868 | Blake Plateau, W Atlantic | 505 | Zibrowius and Cairns (1992) |
| Traces only | Stylaster complanatus Pourtalès, 1867 | Cuba, W Atlantic | – | Zibrowius and Cairns (1992) |
| Traces only | Crypthelia peircei Pourtalès, 1867 | Guadaloupe and St Vincent, W Atlantic | – | Zibrowius and Cairns (1992) |
| Traces only | Conopora sp. | NW Brazil, W Atlantic | – | Zibrowius and Cairns (1992) |
| P. tibia Simone, 2005 | Stylaster sp. | Canopus Bank, Brazil, W Atlantic | 60 | Simone (2005) |
| P. bonfigliolii Cossignani, 2006a | Stylaster sp. | Canopus Bank, Brazil, W Atlantic | 60 | Cossignani (2006) |
| P. sicula Swainson, 1840b | Stylaster ibericus Zibrowius and Cairns, 1992 | Bay of Biscay | 500; 515 | Zibrowius and Cairns (1992) |
| Traces only | Pliobothrus symmetricus Pourtalès, 1868 | Celtic Sea | 380; 1050 | Zibrowius and Cairns (1992) |
| Traces only | Stylaster erubescens britannicus Zibrowius and Cairns, 1992 | Celtic Sea | 1080 | Zibrowius and Cairns (1992) |
| P. sicula Swainson, 1840 | Errina aspera Linnaeus, 1767 | Strait of Gibraltar | 61–443 | Álvarez-Pérez et al. (2005) |
| P. sicula Swainson, 1840 | Errina aspera Linnaeus, 1767 | Mediterranean Sea | about 100 | Arnaud and Zibrowius (1979) |
| P. sicula Swainson, 1840 | Errina aspera Linnaeus, 1767 | Mediterranean Sea | 181–236 | di Natale and Mangano (1985) |
| P. sicula Swainson, 1840b | Errina aspera Linnaeus, 1767 | Mediterranean Sea | 220–633 | Zibrowius and Cairns (1992) |
| P. sicula Swainson, 1840 | Errina aspera Linnaeus, 1767 | Strait of Messina, Sicily | 100–300 + | Giudice (1990) |
| Symbiont . | Host . | Locality . | Depth range (m) . | Reference . |
|---|---|---|---|---|
| One specimen + traces | Errina atlantica Hickson, 1912 | Azores | 810–983 | Zibrowius and Cairns (1992) |
| One specimen + traces | Errina dabneyi Pourtalès, 1871 | Azores | 215–560 | Zibrowius and Cairns (1992) |
| Traces only | Lepidopora eburnea Calvet, 1903 | Azores | 480–895 | Zibrowius and Cairns (1992) |
| P. sicula Swainson, 1840 | Errina dabneyi Pourtalès, 1871 | Southern Faial Channel, Azores | 475; 490 | Wisshak et al. (2009) |
| P. splendida Lorenz, 2008 | Errina atlantica Hickson, 1912 | Hyères and Atlantis Seamounts | 845–1520 | Lorenz (2008) |
| Traces only | Stenohelia maderensis Johnson, 1862 | Madeira | 300–340 | Zibrowius and Cairns (1992) |
| Traces only | Stylaster erubescens Pourtalès, 1868 | Blake Plateau, W Atlantic | 505 | Zibrowius and Cairns (1992) |
| Traces only | Stylaster complanatus Pourtalès, 1867 | Cuba, W Atlantic | – | Zibrowius and Cairns (1992) |
| Traces only | Crypthelia peircei Pourtalès, 1867 | Guadaloupe and St Vincent, W Atlantic | – | Zibrowius and Cairns (1992) |
| Traces only | Conopora sp. | NW Brazil, W Atlantic | – | Zibrowius and Cairns (1992) |
| P. tibia Simone, 2005 | Stylaster sp. | Canopus Bank, Brazil, W Atlantic | 60 | Simone (2005) |
| P. bonfigliolii Cossignani, 2006a | Stylaster sp. | Canopus Bank, Brazil, W Atlantic | 60 | Cossignani (2006) |
| P. sicula Swainson, 1840b | Stylaster ibericus Zibrowius and Cairns, 1992 | Bay of Biscay | 500; 515 | Zibrowius and Cairns (1992) |
| Traces only | Pliobothrus symmetricus Pourtalès, 1868 | Celtic Sea | 380; 1050 | Zibrowius and Cairns (1992) |
| Traces only | Stylaster erubescens britannicus Zibrowius and Cairns, 1992 | Celtic Sea | 1080 | Zibrowius and Cairns (1992) |
| P. sicula Swainson, 1840 | Errina aspera Linnaeus, 1767 | Strait of Gibraltar | 61–443 | Álvarez-Pérez et al. (2005) |
| P. sicula Swainson, 1840 | Errina aspera Linnaeus, 1767 | Mediterranean Sea | about 100 | Arnaud and Zibrowius (1979) |
| P. sicula Swainson, 1840 | Errina aspera Linnaeus, 1767 | Mediterranean Sea | 181–236 | di Natale and Mangano (1985) |
| P. sicula Swainson, 1840b | Errina aspera Linnaeus, 1767 | Mediterranean Sea | 220–633 | Zibrowius and Cairns (1992) |
| P. sicula Swainson, 1840 | Errina aspera Linnaeus, 1767 | Strait of Messina, Sicily | 100–300 + | Giudice (1990) |
aPedicularia bonfigliolii was recently synonymized with the western Atlantic species Pedicularia decussata (Gould, 1855; see Lorenz and Fehse, 2009).
bIdentity of Pedicularia sicula (and its synonymy) is given or confirmed by Bouchet and Warén (1993).
Pedicularia and their stylasterid hosts known for the Atlantic and the Mediterranean.
| Symbiont . | Host . | Locality . | Depth range (m) . | Reference . |
|---|---|---|---|---|
| One specimen + traces | Errina atlantica Hickson, 1912 | Azores | 810–983 | Zibrowius and Cairns (1992) |
| One specimen + traces | Errina dabneyi Pourtalès, 1871 | Azores | 215–560 | Zibrowius and Cairns (1992) |
| Traces only | Lepidopora eburnea Calvet, 1903 | Azores | 480–895 | Zibrowius and Cairns (1992) |
| P. sicula Swainson, 1840 | Errina dabneyi Pourtalès, 1871 | Southern Faial Channel, Azores | 475; 490 | Wisshak et al. (2009) |
| P. splendida Lorenz, 2008 | Errina atlantica Hickson, 1912 | Hyères and Atlantis Seamounts | 845–1520 | Lorenz (2008) |
| Traces only | Stenohelia maderensis Johnson, 1862 | Madeira | 300–340 | Zibrowius and Cairns (1992) |
| Traces only | Stylaster erubescens Pourtalès, 1868 | Blake Plateau, W Atlantic | 505 | Zibrowius and Cairns (1992) |
| Traces only | Stylaster complanatus Pourtalès, 1867 | Cuba, W Atlantic | – | Zibrowius and Cairns (1992) |
| Traces only | Crypthelia peircei Pourtalès, 1867 | Guadaloupe and St Vincent, W Atlantic | – | Zibrowius and Cairns (1992) |
| Traces only | Conopora sp. | NW Brazil, W Atlantic | – | Zibrowius and Cairns (1992) |
| P. tibia Simone, 2005 | Stylaster sp. | Canopus Bank, Brazil, W Atlantic | 60 | Simone (2005) |
| P. bonfigliolii Cossignani, 2006a | Stylaster sp. | Canopus Bank, Brazil, W Atlantic | 60 | Cossignani (2006) |
| P. sicula Swainson, 1840b | Stylaster ibericus Zibrowius and Cairns, 1992 | Bay of Biscay | 500; 515 | Zibrowius and Cairns (1992) |
| Traces only | Pliobothrus symmetricus Pourtalès, 1868 | Celtic Sea | 380; 1050 | Zibrowius and Cairns (1992) |
| Traces only | Stylaster erubescens britannicus Zibrowius and Cairns, 1992 | Celtic Sea | 1080 | Zibrowius and Cairns (1992) |
| P. sicula Swainson, 1840 | Errina aspera Linnaeus, 1767 | Strait of Gibraltar | 61–443 | Álvarez-Pérez et al. (2005) |
| P. sicula Swainson, 1840 | Errina aspera Linnaeus, 1767 | Mediterranean Sea | about 100 | Arnaud and Zibrowius (1979) |
| P. sicula Swainson, 1840 | Errina aspera Linnaeus, 1767 | Mediterranean Sea | 181–236 | di Natale and Mangano (1985) |
| P. sicula Swainson, 1840b | Errina aspera Linnaeus, 1767 | Mediterranean Sea | 220–633 | Zibrowius and Cairns (1992) |
| P. sicula Swainson, 1840 | Errina aspera Linnaeus, 1767 | Strait of Messina, Sicily | 100–300 + | Giudice (1990) |
| Symbiont . | Host . | Locality . | Depth range (m) . | Reference . |
|---|---|---|---|---|
| One specimen + traces | Errina atlantica Hickson, 1912 | Azores | 810–983 | Zibrowius and Cairns (1992) |
| One specimen + traces | Errina dabneyi Pourtalès, 1871 | Azores | 215–560 | Zibrowius and Cairns (1992) |
| Traces only | Lepidopora eburnea Calvet, 1903 | Azores | 480–895 | Zibrowius and Cairns (1992) |
| P. sicula Swainson, 1840 | Errina dabneyi Pourtalès, 1871 | Southern Faial Channel, Azores | 475; 490 | Wisshak et al. (2009) |
| P. splendida Lorenz, 2008 | Errina atlantica Hickson, 1912 | Hyères and Atlantis Seamounts | 845–1520 | Lorenz (2008) |
| Traces only | Stenohelia maderensis Johnson, 1862 | Madeira | 300–340 | Zibrowius and Cairns (1992) |
| Traces only | Stylaster erubescens Pourtalès, 1868 | Blake Plateau, W Atlantic | 505 | Zibrowius and Cairns (1992) |
| Traces only | Stylaster complanatus Pourtalès, 1867 | Cuba, W Atlantic | – | Zibrowius and Cairns (1992) |
| Traces only | Crypthelia peircei Pourtalès, 1867 | Guadaloupe and St Vincent, W Atlantic | – | Zibrowius and Cairns (1992) |
| Traces only | Conopora sp. | NW Brazil, W Atlantic | – | Zibrowius and Cairns (1992) |
| P. tibia Simone, 2005 | Stylaster sp. | Canopus Bank, Brazil, W Atlantic | 60 | Simone (2005) |
| P. bonfigliolii Cossignani, 2006a | Stylaster sp. | Canopus Bank, Brazil, W Atlantic | 60 | Cossignani (2006) |
| P. sicula Swainson, 1840b | Stylaster ibericus Zibrowius and Cairns, 1992 | Bay of Biscay | 500; 515 | Zibrowius and Cairns (1992) |
| Traces only | Pliobothrus symmetricus Pourtalès, 1868 | Celtic Sea | 380; 1050 | Zibrowius and Cairns (1992) |
| Traces only | Stylaster erubescens britannicus Zibrowius and Cairns, 1992 | Celtic Sea | 1080 | Zibrowius and Cairns (1992) |
| P. sicula Swainson, 1840 | Errina aspera Linnaeus, 1767 | Strait of Gibraltar | 61–443 | Álvarez-Pérez et al. (2005) |
| P. sicula Swainson, 1840 | Errina aspera Linnaeus, 1767 | Mediterranean Sea | about 100 | Arnaud and Zibrowius (1979) |
| P. sicula Swainson, 1840 | Errina aspera Linnaeus, 1767 | Mediterranean Sea | 181–236 | di Natale and Mangano (1985) |
| P. sicula Swainson, 1840b | Errina aspera Linnaeus, 1767 | Mediterranean Sea | 220–633 | Zibrowius and Cairns (1992) |
| P. sicula Swainson, 1840 | Errina aspera Linnaeus, 1767 | Strait of Messina, Sicily | 100–300 + | Giudice (1990) |
aPedicularia bonfigliolii was recently synonymized with the western Atlantic species Pedicularia decussata (Gould, 1855; see Lorenz and Fehse, 2009).
bIdentity of Pedicularia sicula (and its synonymy) is given or confirmed by Bouchet and Warén (1993).
Of the nine stylasterid species recorded for the Azores (reviewed by Zibrowius and Cairns, 1992), Errina dabneyi (Pourtalès, 1871) is by far the most common. Unlike most stylasterids, which appear widely distributed throughout insular areas (Cairns, 1992), this species is known to occur only in the Azores and the Mid-Atlantic Ridge southwest of the Azores, mainly at upper bathyal depths (Zibrowius and Cairns, 1992). It possesses a uniplanar fan-shaped calcified skeleton up to 40 cm high and wide with a reticulate-granular texture, which is covered by a thin layer of tissue (Zibrowius and Cairns, 1992).
Many specimens of E. dabneyi hosting Pedicularia on their branches have been collected in the Azores. The main goal of this study was to contribute to our knowledge of the association between pediculariids and stylasterids, because most of the available records of Pedicularia consist of empty shells or dead animals in mixed debris (Goud and Hoeksema, 2001), lacking any reference to the host species. Specific aims were: (i) to identify the Pedicularia individuals present in E. dabneyi colonies, and describe shell morphology at various growth stages; (ii) to characterize the Pedicularia traces found on the host; and (iii) to provide new insights on the nature of this symbiotic relationship. The epifauna present on E. dabneyi is also briefly described.
Material and methods
Collection and examination of specimens
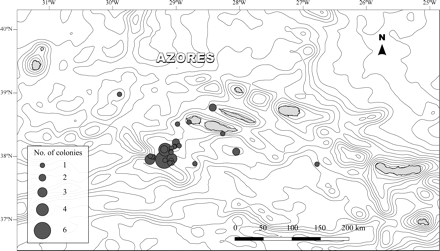
In situ observations of E. dabneyi hosting Pedicularia were made with the manned submersible “Lula” from the Rebicoff-Niggeler Foundation. The dive was carried out on the southern slope of the Faial-Pico Channel (start position 38°29′260′′N 28°37′310′′W; end position 38°29′400′′N 28°37′260′′W) between depths of 330 and 480 m, on 4 September 2008. Specimens used for detailed examination and measurement were obtained as bycatch from longline fishing cruises with RV “Arquipélago” (ARQDAÇO-03-P95; ARQDAÇO-29-P08) and from the local longline fleet. A total of 43 living colonies of E. dabneyi have been collected over the past 14 years within the Azores EEZ, from depths between 201 and 1097 m (Figure 1). The material is deposited in the collection of the Department of Oceanography and Fisheries of the University of the Azores (DOP-UAz).
Distribution of the localities within the Azores Archipelago where Errina dabneyi was collected. The size of grey circles is proportional to the number of colonies obtained at each locality. Spacing of contour lines is 300 m.
Each colony was carefully examined for the presence of Pedicularia or their traces with the aid of a stereomicroscope. The number of Pedicularia individuals per colony was counted and a few individuals with intact shell aperture were preserved in 70% ethanol for later identification to species level (n = 107). These specimens were identified by comparison with related Atlantic species [Pedicularia sicula Swainson, 1840, Pedicularia decussata (Gould, 1855), Pedicularia splendida Lorenz, 2009, and Pedicularia tibia Simone, 2005]. The species P. tibia and Pedicularia bonfigliolii Cossignani, 2006, both described from the same type of locality (Canopus Bank, Brazil), were treated by Lorenz and Fehse (2009) as synonyms of P. decussata. This classification is accepted for P. bonfigliolii, but is still controversial for P. tibia (L. Simone, pers. comm.). Therefore, the latter species was considered as a valid species.
Some of the preserved specimens were soaked in distilled water for 48 h without their shells for observation of anatomical characters. Finally, 10 squashes of Pedicularia stomachs were examined under a light microscope to look for the presence of nematocysts (i.e. proteinaceous capsules found in all cnidarians).
Morphometric characterization
The width and height of 63 Pedicularia individuals were measured to the nearest millimetre using a Leica MZ 16FA stereomicroscope and the software Leica Application Suite version 2.8.1. Given that juveniles have distinct shell characters, only size data of sedentary adults (n = 54) were used for the morphometric analysis within localities. The shell morphology of distinct morphotypes of adult Pedicularia was characterized through high-magnification images by scanning electron microscopy (SEM), obtained with a JEOL JFM-5410 at the Department of Biology of the University of the Azores (DBUA). The samples were covered with gold/palladium 40/60 in a vacuum evaporator JEOL JEE 400 before scanning. Particular attention was paid to the protoconch and to the microsculpture of the shell. This material is deposited in the DBUA reference collection.
Results
In the present study we have identified the pediculariids associated with the stylasterid E. dabneyi in the Azores Archipelago as belonging to the species P. sicula. The presence of P. sicula was recorded in colonies collected between depths of 201 and 548 m. Details of the material examined in this study are available in the Supplementary data online.
Taxonomic description
Gastropoda Cuvier, 1797
Caenogastropoda Cox, 1959
Cypraeoidea Rafinesque, 1815
Pediculariidae H. and A. Adams, 1855
Pedicularia sicula Swainson, 1840: 357, Fig. 44.
Synonyms: Calyptrea polymorpha Calcara, 1842: 17; Thyreus paradoxus Philippi, 1844: 92, p1. 18 Fig. 1; Pedicularia decurvata Locard, 1897: 96–99, p1. 3 Fig. 12–15; Pedicularia sicula var. sublaevigata Locard, 1897: 99; Mioseguenzia cimbrica recens Nordsieck, 1973; Mioseguenzia conica Nordsieck, 1973; Pedicularia sicula Swainson, 1840: Bouchet and Warén 1993: 746–752, Figs 1772–1787.
Description
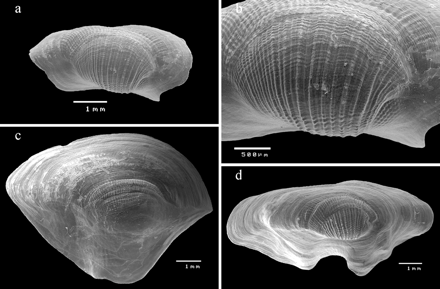
The embryonic shell (protoconch I) consists of about one whorl of 156–197 µm width, 90 µm height, and shows the typical finely pitted sculpture of P. sicula (Figure 2a and b). Protoconch II consists of about four whorls with a diagonally reticulated surface (Figure 2a–d). The transition between protoconch I and II is smooth. The first whorl of protoconch II shows, in apical view, two distinct horizontal rows that disappear at the beginning of the second whorl (Figure 2b). Protoconch II has a width of about 662 µm (Figure 2c) and a height of 652 µm (Figure 2d). These planktonic veliger larvae are ovate in shape and possess a distinct beak on the aperture of the larval shell (Figure 2d) that is still visible after the first whorl of the teleoconch has grown. The axis of the protoconch is not tilted against the axis of the teleoconch.
Pedicularia sicula (a) lateral view showing protoconch I and the first two whorls of protoconch II, DBUA Suppl 191–3; (b) frontal view of the protoconch I, DBUA Suppl 191–3; (c) apical view of the shell (male phase), DBUA Suppl 191–3; (d) male phase of the shell, DBUA Suppl 191–3. The white arrow shows the beak on the aperture of the larval shell; (e) male phase of the shell (“Trivia” stage sensu Bouchet and Warén, 1993), DBUA Suppl 191–4; (f) male phase of the shell, with crenulated outer lip (3.82 × 2.36 mm), DOP-1107; (g) ventral view of female with brood pouch, DOP-1457.
The growth of the teleoconch progressively covers the protoconch during the male phase of the shell (up to around 4 mm height; Figure 2d and e). The male shell (∼3.2 mm height, 2.2 mm width) was called the “Trivia” stage by Bouchet and Warén (1993: 752) and it has a very consistent shape (Figure 2e). The labrum forms a rounded anterior and posterior extremity and the columella has a parietal shield. In more calloused shells, the columellar ridge and the labral edge are more developed and fine transverse ridges are visible all around the aperture; in this phase, the outer lip is usually crenulated (Figure 2f).
Average shell height of adult P. sicula was 5.99 mm (3.45–9.88), average shell width was 2.75 mm (1.41–4.09), and the height/width ratio ranged from 1.51 to 3.08 mm (Table 2). Most adult specimens were females presenting a reddish colouration, with a few individuals cream-coloured. Usually, shells >4 mm in height are females. The largest females observed were collected at Açor Bank3 (38°04′48″N 28°02′24″W; 402 m depth) and measured 9.88 × 3.25 mm. In ventral view, females show the brood-pouch, a cavity which extends along the foot and where the embryos are brooded (Figure 2g). The shells were irregular and sub-patelliform, with a very long aperture channelled at both ends, and exhibited a high level of plasticity (Figure 3). The surfaces of the female shells have irregular girdles with fine transverse vertical ribs (Figure 3a); sometimes, these are almost smooth and porcellaneous. The top surface of the shell is sculptured by about 16–20 vertical, narrow, uniformly distributed spiral lines that form tubercules with the additional perpendicular growth lines; the space between the lines is about twice the width of the lines (Figure 3b), with alternating strong and fine ridges. Younger regions of the shell expand widely, the transverse ridges are absent, and growth lines are present (Figure 3c and d).
Body size measurements of sedentary adults of Pedicularia sicula from different localities.
| Locality . | n . | Height (mm) . | Width (mm) . | H/W ratio . |
|---|---|---|---|---|
| Açor Bank2 | 2 | 7.20 (6.85–7.55) | 3.35 (2.60–4.07) | 2.23 (1.86–2.60) |
| Açor Bank3 | 14 | 6.16 (4.31–9.88) | 2.66 (1.90–3.74) | 2.35 (1.70–3.08) |
| Açor Bank4 | 6 | 5.79 (4.95–7.20) | 2.68 (2.19–3.57) | 2.19 (1.87–2.69) |
| Açor Bank5 | 4 | 6.61 (5.45–7.39) | 3.21 (2.76–3.64) | 2.07 (1.83–2.48) |
| Açor Bank6 | 11 | 6.09 (5.23–7.17) | 2.78 (2.15–4.09) | 2.23 (1.60–2.70) |
| Açor Bank7 (35 miles) | 1 | 7.64 | 2.74 | 2.79 |
| Gigante Seamount | 1 | 5.33 | 2.82 | 1.89 |
| Princesse Alice Bank3 | 6 | 5.84 (4.90–6.37) | 2.57 (2.37–3.11) | 2.29 (2.01–2.57) |
| Princesse Alice Bank4 | 3 | 6.12 (6.09–6.13) | 3.31 (3.04–3.53) | 1.86 (1.72–2.02) |
| Princesse Alice Bank5 | 3 | 5.07 (4.29–6.47) | 2.16 (1.41–2.55) | 2.45 (1.76–3.04) |
| Rosais, São Jorge Island | 3 | 4.32 (3.45–5.02) | 2.57 (2.29–2.73) | 1.67 (1.51–1.84) |
| Locality . | n . | Height (mm) . | Width (mm) . | H/W ratio . |
|---|---|---|---|---|
| Açor Bank2 | 2 | 7.20 (6.85–7.55) | 3.35 (2.60–4.07) | 2.23 (1.86–2.60) |
| Açor Bank3 | 14 | 6.16 (4.31–9.88) | 2.66 (1.90–3.74) | 2.35 (1.70–3.08) |
| Açor Bank4 | 6 | 5.79 (4.95–7.20) | 2.68 (2.19–3.57) | 2.19 (1.87–2.69) |
| Açor Bank5 | 4 | 6.61 (5.45–7.39) | 3.21 (2.76–3.64) | 2.07 (1.83–2.48) |
| Açor Bank6 | 11 | 6.09 (5.23–7.17) | 2.78 (2.15–4.09) | 2.23 (1.60–2.70) |
| Açor Bank7 (35 miles) | 1 | 7.64 | 2.74 | 2.79 |
| Gigante Seamount | 1 | 5.33 | 2.82 | 1.89 |
| Princesse Alice Bank3 | 6 | 5.84 (4.90–6.37) | 2.57 (2.37–3.11) | 2.29 (2.01–2.57) |
| Princesse Alice Bank4 | 3 | 6.12 (6.09–6.13) | 3.31 (3.04–3.53) | 1.86 (1.72–2.02) |
| Princesse Alice Bank5 | 3 | 5.07 (4.29–6.47) | 2.16 (1.41–2.55) | 2.45 (1.76–3.04) |
| Rosais, São Jorge Island | 3 | 4.32 (3.45–5.02) | 2.57 (2.29–2.73) | 1.67 (1.51–1.84) |
Height value = mean (min–max); width value = mean (min–max); H/W value (height/width ratio) = mean (min–max).
Body size measurements of sedentary adults of Pedicularia sicula from different localities.
| Locality . | n . | Height (mm) . | Width (mm) . | H/W ratio . |
|---|---|---|---|---|
| Açor Bank2 | 2 | 7.20 (6.85–7.55) | 3.35 (2.60–4.07) | 2.23 (1.86–2.60) |
| Açor Bank3 | 14 | 6.16 (4.31–9.88) | 2.66 (1.90–3.74) | 2.35 (1.70–3.08) |
| Açor Bank4 | 6 | 5.79 (4.95–7.20) | 2.68 (2.19–3.57) | 2.19 (1.87–2.69) |
| Açor Bank5 | 4 | 6.61 (5.45–7.39) | 3.21 (2.76–3.64) | 2.07 (1.83–2.48) |
| Açor Bank6 | 11 | 6.09 (5.23–7.17) | 2.78 (2.15–4.09) | 2.23 (1.60–2.70) |
| Açor Bank7 (35 miles) | 1 | 7.64 | 2.74 | 2.79 |
| Gigante Seamount | 1 | 5.33 | 2.82 | 1.89 |
| Princesse Alice Bank3 | 6 | 5.84 (4.90–6.37) | 2.57 (2.37–3.11) | 2.29 (2.01–2.57) |
| Princesse Alice Bank4 | 3 | 6.12 (6.09–6.13) | 3.31 (3.04–3.53) | 1.86 (1.72–2.02) |
| Princesse Alice Bank5 | 3 | 5.07 (4.29–6.47) | 2.16 (1.41–2.55) | 2.45 (1.76–3.04) |
| Rosais, São Jorge Island | 3 | 4.32 (3.45–5.02) | 2.57 (2.29–2.73) | 1.67 (1.51–1.84) |
| Locality . | n . | Height (mm) . | Width (mm) . | H/W ratio . |
|---|---|---|---|---|
| Açor Bank2 | 2 | 7.20 (6.85–7.55) | 3.35 (2.60–4.07) | 2.23 (1.86–2.60) |
| Açor Bank3 | 14 | 6.16 (4.31–9.88) | 2.66 (1.90–3.74) | 2.35 (1.70–3.08) |
| Açor Bank4 | 6 | 5.79 (4.95–7.20) | 2.68 (2.19–3.57) | 2.19 (1.87–2.69) |
| Açor Bank5 | 4 | 6.61 (5.45–7.39) | 3.21 (2.76–3.64) | 2.07 (1.83–2.48) |
| Açor Bank6 | 11 | 6.09 (5.23–7.17) | 2.78 (2.15–4.09) | 2.23 (1.60–2.70) |
| Açor Bank7 (35 miles) | 1 | 7.64 | 2.74 | 2.79 |
| Gigante Seamount | 1 | 5.33 | 2.82 | 1.89 |
| Princesse Alice Bank3 | 6 | 5.84 (4.90–6.37) | 2.57 (2.37–3.11) | 2.29 (2.01–2.57) |
| Princesse Alice Bank4 | 3 | 6.12 (6.09–6.13) | 3.31 (3.04–3.53) | 1.86 (1.72–2.02) |
| Princesse Alice Bank5 | 3 | 5.07 (4.29–6.47) | 2.16 (1.41–2.55) | 2.45 (1.76–3.04) |
| Rosais, São Jorge Island | 3 | 4.32 (3.45–5.02) | 2.57 (2.29–2.73) | 1.67 (1.51–1.84) |
Height value = mean (min–max); width value = mean (min–max); H/W value (height/width ratio) = mean (min–max).
SEM images of adult sedentary specimens of Pedicularia sicula showing variation of sculpture and shape. (a) Frontal view, DOP-1378, DBUA Suppl 224–2; (b) detail of the teleoconch, DOP-1378, DBUA Suppl 224–2; (c) frontal view, DOP-1114 DBUA Suppl 223–1; (d) frontal view, DOP 1114, DBUA Suppl 222–1.
The head of P. sicula has two tentacles, both with a black eye at the base. The foot is slightly shorter than the shell and has two lobes in front. Juveniles were morphologically distinct from the adult specimens.
Diagnosis
All the specimens examined were identified as P. sicula, according to the distinguishing characters summarized in Table 3. P. sicula differs from P. tibia by possessing five whorls in the protoconch against four in P. tibia, by the lower number of teleoconch vertical spiral lines (ribs)—16–20 for P. sicula, 35–40 for P. tibia—and by the differences detected in the width of the spaces between the teleoconch spiral lines, which are about the same width as the lines for P. tibia, and about twice the width of the lines for P. sicula.
Main distinguishing characters of Atlantic species of Pedicularia.
| Diagnostic characters . | P. sicula Swainson, 1840 . | P. splendida Lorenz, 2009 . | P. tibia Simone, 2005 . |
|---|---|---|---|
| Number of protoconch whorls | 5 | 5 | 4 |
| Axis protoconch/teleoconch tilted | No | Yes (8–10°) | No |
| Teleoconch spiral lines | 16–20 | Fine; teleoconch smooth-like | 35–40 |
| Space between teleoconch spiral lines | Twice the width of the lines | Indistinct | Same width as the lines |
| Diagnostic characters . | P. sicula Swainson, 1840 . | P. splendida Lorenz, 2009 . | P. tibia Simone, 2005 . |
|---|---|---|---|
| Number of protoconch whorls | 5 | 5 | 4 |
| Axis protoconch/teleoconch tilted | No | Yes (8–10°) | No |
| Teleoconch spiral lines | 16–20 | Fine; teleoconch smooth-like | 35–40 |
| Space between teleoconch spiral lines | Twice the width of the lines | Indistinct | Same width as the lines |
Main distinguishing characters of Atlantic species of Pedicularia.
| Diagnostic characters . | P. sicula Swainson, 1840 . | P. splendida Lorenz, 2009 . | P. tibia Simone, 2005 . |
|---|---|---|---|
| Number of protoconch whorls | 5 | 5 | 4 |
| Axis protoconch/teleoconch tilted | No | Yes (8–10°) | No |
| Teleoconch spiral lines | 16–20 | Fine; teleoconch smooth-like | 35–40 |
| Space between teleoconch spiral lines | Twice the width of the lines | Indistinct | Same width as the lines |
| Diagnostic characters . | P. sicula Swainson, 1840 . | P. splendida Lorenz, 2009 . | P. tibia Simone, 2005 . |
|---|---|---|---|
| Number of protoconch whorls | 5 | 5 | 4 |
| Axis protoconch/teleoconch tilted | No | Yes (8–10°) | No |
| Teleoconch spiral lines | 16–20 | Fine; teleoconch smooth-like | 35–40 |
| Space between teleoconch spiral lines | Twice the width of the lines | Indistinct | Same width as the lines |
Pedicularia sicula differs from P. splendida in the axis between protoconch and teleoconch, which is tilted (8–10°) in P. splendida and not tilted in P. sicula; also P. splendida lacks a dorsal sculpture in the adult shell, in contrast to both P. sicula and P. tibia.
Neither P. decurvata nor the western Atlantic species P. decussata, both considered as valid species by Lorenz (2008) and Lorenz and Fehse (2009), were observed. The specimens examined differ from these two species by having a stronger dorsal sculpture, while P. decurvata has obscured dorsal ribs and P. decussata has rather fine and decussate dorsal striae.
Distribution
Pedicularia sicula is distributed from the northern part of the Bay of Biscay southwards along the continental slope to the Iberian Peninsula and Gulf of Morocco. It also occurs in the Mediterranean, as far east as the Strait of Messina and the sill of the Sicilian Channel. In the Atlantic Ocean, P. sicula is a bathyal species, while in the Strait of Messina it occurs as shallow as 100 m.
Description of the association
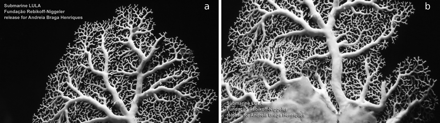
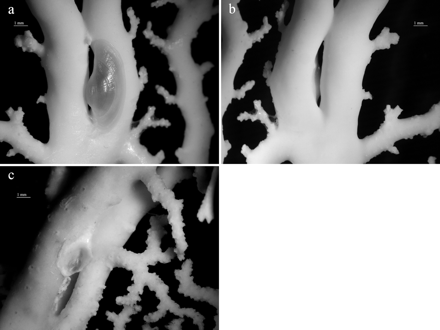
In situ footage from the southern slope of the Faial-Pico Channel revealed that most living E. dabneyi colonies had P. sicula on their surface (Figure 4). A total of 30 of the 43 colonies obtained as bycatch (69.8%) also carried P. sicula on their main and secondary branches (Figure 5). Abundances ranged between 1 and 223 individuals per colony (30.6 ± 46.2; mean ± s.d.), with higher densities on the concave side of the stylasterid. Colour matching between the pediculariid and the host (pure white) was never observed.
In situ observations of Pedicularia sicula on Errina dabneyi captured with LULA submersible on the southern slope of the Faial-Pico Channel, Azores Archipelago, (38°29′N 28°37′W) at 450 m.
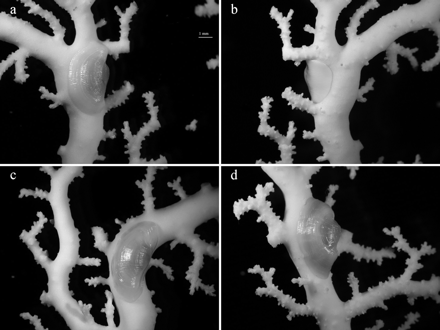
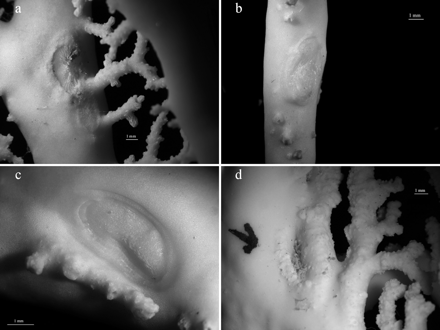
Pedicularia sicula on Errina dabneyi at several fixation points. (a) and (b) Front and back side view of one of the colony branches; (c) and (d) pediculariid fully adjusted to the morphology of the host; scale bar = 1 mm.
The traces produced by P. sicula were often not clearly visible to the naked eye, only becoming evident under the stereomicroscope. They consisted of a depression on the stylasterid exoskeleton with the size and shape of the shell margin, delimited by a thick and prominent border. Whereas P. sicula traces on distal and tip branches were elongated, when the pediculariid adhered to the branch axis an ovulated trace was created. Several traces were also found in some colonies lacking P. sicula individuals. Skeleton injuries at the fixation point of the pediculariid were observed in all E. dabneyi (Figure 6). Many of these consisted of prominent calcareous crests. Moreover, occasional branch anastomosis (i.e. branch fusion) with a pediculariid or a scar between the two branches was found in some colonies (Figure 7).
Skeleton injuries caused by Pedicularia sicula. Each letter corresponds to an Errina dabneyi colony.
Branch anastomosis in Errina dabneyi. (a) and (b) Pedicularia sicula, front and back side view, DOP-2107; (c) Pedicularia trace, DOP-422.
The analysis of stomach contents did not reveal the presence of nematocysts.
Other epibionts
Several invertebrate species belonging to different taxonomic groups, such as sponges, hydroids, anthozoans, bryozoans, polychaetes, and decapods, were found attached to both dead and living sections of E. dabneyi skeletons. Among the anthozoans, many solitary scleractinians were recorded, of which Caryophyllia cyathus was the most common species (dead and alive). A few live specimens of Javania cailleti were observed on the thicker branches of an E. dabneyi colony collected at the Açor seamount at 201 m. The latter also had one C. cyathus specimen adhered to the base of the corallum. Moreover, soft corals (order Corallimorpharia) were found on the branch surface of some colonies. A few E. dabneyi hosted many barnacles of an unidentified species on the surface of the main branches and growing tips. Other colonies were infested by tube worms, with the stylasterid growing over them and leaving only the opening at the top exposed. Two squat lobsters were also recorded inhabiting one E. dabneyi from Princesse Alice Bank at 201 m.
Some E. dabneyi were growing over dead exoskeletons of Errina atlantica, sharing the available surface with other epibionts (e.g. sponges, bryozoans, polychaetes, and young colonies of the primnoid octocoral Candidella imbricata).
Discussion
We have documented here the association between two species in the Azores, a pediculariid gastropod and its cnidarian host. The pediculariid found on the E. dabneyi has been identified as P. sicula, which agrees with the known geographical distribution for this species, the Northeast Atlantic and the Mediterranean (Bouchet and Warén, 1993). In addition to Errina aspera and Stylaster ibericus (see Table 1), E. dabneyi is now also confirmed as a host of P. sicula, reinforcing the evidence that P. sicula can inhabit multiple hosts.
Species description
Specimens of P. sicula were well adjusted to the morphology of their host, displaying a high degree of plasticity even within the same host. The infested E. dabneyi hosted pediculariids of different size and shell shape, depending on the corallum fixation point. Shape irregularity was also reported by Schmieder (1982) for Pedicularia californica Newcomb, 1864, living on Stylantheca. In the review by Bouchet and Warén (1993), it is stated that the shell shape of Pedicularia depends on the branch morphology of the stylasterid host. In fact, the identification of Pedicularia has been troublesome because of the variable shell shape, making taxonomic studies difficult (Schmieder, 1980; Bouchet and Warén, 1993). Several authors have argued that P. decussata, which occurs in the West Atlantic, should be considered as being P. sicula (Scheltema, 1971; Settepassi, 1971; Bouchet and Warén, 1993). However, because of the differences found in the teleoconch sculpture, P. decussata was never synonymized with the European species (Settepassi, 1971; Bouchet and Warén, 1993). Furthermore, Schmieder (1980) argues that Pedicularia species can exhibit an intermediate growth form which could be misidentified as another species, as for Pedicularia ovuliformis Berry, 1946, shown to be a growth phase of P. californica.
Hedley (1903) suggested that the colouration displayed by Pedicularia individuals is given by their hosts. Although that may be true for some species, such as P. californica associated with Allopora californica (Ostarello, 1973), this has not been observed in the present study. A wide variety of colours has been reported for Pedicularia vanderlandi (Goud and Hoeksema, 2001), which lives on the shallow-water stylasterid Distichopora vervoorti, as well as for Pedicularia japonica Dall, 1871, living on Stylaster, providing further evidence of no clear connection between the colour of the pediculariid and its host (Goud and Hoeksema, 2001).
Description of the association
Some authors suggested that the exoskeleton of the host is prevented from growing at the fixation point of Pedicularia by the deposition of a lime layer by the snail to optimize the foot's fixation (Zibrowius and Cairns, 1992; Bouchet and Warén, 1993). All traces observed in this study consisted of depressions on the corallum limited by a thick border, indicating that only the surrounding areas of the skeleton were able to grow after attachment of the pediculariid. Prominent crests within traces were observed, and although Zibrowius and Cairns (1992) argued that they are a result of the lime layer deposition, some of these injuries could also be radular marks. Some authors mention that Pedicularia feeds on stylasterids, among them Errina (Habe, 1955; Robertson, 1970). For instance, Giudice (1990) suggests that P. sicula feeds on the humours of the polyps of E. aspera. No direct observations were made to support these assumptions, and what the snail feeds on is not clearly known. The radular marks could indicate that P. sicula feeds by scraping off the mucus from the coral surface with its radular teeth, as suggested by Liltved (1989) for Pedicularia elegantissima Deshayes, 1863. Furthermore, the analysis of stomach contents did not reveal the presence of nematocysts, which would be expected if P. sicula feeds on coenenchyme tissues.
The high density of P. sicula per colony as well as the presence of many skeleton injuries suggests that P. sicula is best characterized as an ectoparasite rather than a predator. The anastomosis on branches with pediculariids or traces may indicate a defence mechanism developed by the coral, which is trying to expel the snails from its skeleton. Therefore, like other gastropod species living on corals (Goh et al., 1999), P. sicula seems to benefit from this close association whereas the host is being harmed, which can be defined as parasitism (Goh et al., 1999; Buhl-Mortensen and Mortensen, 2004).
E. dabneyi epibionts
The abundant and diverse epibiontic fauna found attached to E. dabneyi confirms that this species is an important habitat provider for sessile invertebrates in the deep sea, where hard substratum can be a limiting resource (Buhl-Mortensen and Mortensen, 2004). Those epibionts probably also receive protection from harsh environmental conditions and predators (Metaxas and Davies, 2005). Moreover, access to food may be increased, given the fan-shaped morphology of E. dabneyi (Wainwright and Dillon, 1969; Wainwright and Koehl, 1976). Filter-feeding organisms can also feed on detritus or micro-organisms trapped in the mucus secreted by gorgonians (Patton, 1972). Mobile epifauna, like the two squat lobsters found, probably not only have access to the food trapped on the skeleton of E. dabneyi, but also receive protection from predators.
Stylasterids are an important structural component of coral gardens (Roberts et al., 2006), and are highly susceptible to disturbance by fishing activities. All the material examined was coral bycatch, which means that although bottom trawling is forbidden in the Azores, E. dabneyi and its associated fauna are still vulnerable to the local fishery. Buhl-Mortensen and Mortensen (2004) argue that because the number of obligate symbionts is lower than that of their hosts, when the abundance of corals in a given habitat decreases, the successful reproduction of their symbionts could be compromised. For that reason detailed information on the species living in association with E. dabneyi as well as on the nature of their relationships is needed.
Supplementary material
Supplementary material is available at ICESJMS online.
Acknowledgements
We thank the captain (Fernando Serpa), chief scientists (O. Melo, D. Catarino, G. Menezes) and crew of RV “Arquipélago” for collecting the specimens, Kirsten Jakobsen and Joachim Jakobsen (Fundação Rebikoff-Niggeler) for their cooperation and imaging data, and Jorge Medeiros (CIRN/UAç) for the SEM photos. We are also grateful to R. Rosa and local fishers, in particular J. Gonçalves, for collection of specimens. This study was supported by the following projects: (1) BANCOMAC (funded by INTERREG IIIB programme); (2) “Taxonomy of cold-water corals of the Azores' seamounts” (funded by CenSeam mini-grant/2008); (3) CORAZON (funded by Fundação para a Ciência e a Tecnologia, FCT/PTDC/MAR/72169/2006); and (4) CoralFISH (funded by the EC-FP7 Programme under the priority “Sustainable Management of Resources”, grant agreement no. 213144). AB-H is funded by an FRCT Doctoral grant (ref. M3.1.2/F/016/2008) and MC-S by a post-doctoral grant (SFRH/BPD/34634/2007).