-
PDF
- Split View
-
Views
-
Cite
Cite
Todd C. Stevenson, Brian N. Tissot, Jan Dierking, Fisher behaviour influences catch productivity and selectivity in West Hawaii's aquarium fishery, ICES Journal of Marine Science, Volume 68, Issue 5, May 2011, Pages 813–822, https://doi.org/10.1093/icesjms/fsr020
Close - Share Icon Share
Abstract
In 1999, marine protected areas (MPAs) were implemented along the west coast of the Big Island of Hawaii, closing ∼35% of the coastline to aquarium fishing. Catch per unit effort and total catch of the most commonly targeted fish, yellow tang (Zebrasoma flavescens), have increased since the implementation of the MPAs, yet its abundance has declined by 45% in areas open to aquarium fishing between 1999 and 2007. How effort allocation, harvesting efficiencies, and job satisfaction influence catch productivity and selectivity in West Hawaii's aquarium fishery are investigated, and how these dynamics explain the discrepancy between catch rates and relative abundance for yellow tang is discussed. Cross-sectional fisher questionnaires, semi-structured fisher interviews, and in situ and ex situ catch analyses were performed. The results indicate that fishers dive deeper when reef fish recruitment is perceived as weak, increase harvest efficiency with larger fishing teams, and intensively harvest “coral-friendly” reef fish to supply the global aquarium fish trade. Experienced fishers were less likely to exit the fishery, and job satisfaction was high despite declining fish stocks. These findings may help explain harvesting efficiencies and fleet investment, underscore the importance for evaluating fisher behaviours, and have potential management implications for other aquarium fisheries.Stevenson, T. C., Tissot, B. N., and Dierking, J. 2011. Fisher behaviour influences catch productivity and selectivity in West Hawaii's aquarium fishery. – ICES Journal of Marine Science, 68: 813–822.
Introduction
It has been suggested that the collapse of a fishery can be attributed to misunderstanding fishing fleet dynamics rather than the resource (Hilborn, 1985) and that understanding fisher behaviour is a key component for effective fisheries management (Wilen et al., 2002). Fleet dynamics describe attributes that influence catch variability, including fleet investment and disinvestment, effort allocation, harvesting efficiencies, and discarding practices (Hilborn, 1985). The literature on fleet dynamics primarily focuses on large commercial fisheries (Branch et al., 2006), with a limited number of publications focusing on smaller, artisanal fisheries, and evidence suggests that factors other than economic profit may have greater influence on smaller fishing fleets than on larger commercial ones (Béné and Tewfik, 2001; Salas and Gaertner, 2004). One common thread between large- and small-scale fleet dynamics is that changes in the above-mentioned elements can contribute to variation in catch efficiency and hence influence catch productivity (Salas and Gaertner, 2004; Branch et al., 2006).
Understanding why people enter and exit a fishery is a key factor for elucidating fleet investment and disinvestment strategies, and social scientists are increasingly recognizing the importance of fisher job satisfaction as an underlying mechanism that influences these dynamics. Commercial fishing often fulfils the psychocultural needs of people who seek adventure and thrill, which sometimes supersede the needs of income generation (Pollnac and Poggie, 1988), as demonstrated by fishers subsidizing their fishing efforts with other income to maintain their lifestyle (Gatewood and McCay, 1990). Some economists argue that a target species will become economically extinct before becoming biologically extinct because of the low profitability of harvesting low-density populations (Dulvy et al., 2003); however, other studies have concluded that commercial fishing has considerable non-monetary benefits and suggest that not all fishers will quit fishing when economic models predict they should (Gatewood and McCay, 1990; Pollnac and Poggie, 2008). Understanding how job satisfaction and other job-related attributes influence fisher choice for entering or exiting a fishery has important implications for effective management, especially when fisher job satisfaction is strong in fisheries with declining stocks.
The global trade in ornamental fish is a major industry involving ∼30 million fish annually with a retail value estimated between US$90 million and US$300 million (Wood, 2001a). Nearly all specimens harvested for the marine ornamental trade are from wild stocks in coral environments (Wood, 2001b). The State of Hawaii's aquarium fishery developed rapidly and expanded in the early 1970s, grossing $3.2 million in sales, with industry profits at $1.2 million in 2002, making it Hawaii's most lucrative nearshore fishery (Walsh et al., 2004). The majority of the State's aquarium fish catch value is now generated from the west coast of the Big Island of Hawaii (hereinafter West Hawaii; Walsh et al., 2004), where ∼37 aquarium fishers were considered active in 2007 (i.e. reporting an annual catch of more than 1000 yellow tang, Zebrasoma flavescens, the most commonly targeted species in the region; Williams et al., 2009). In 1999, to improve management of the fishery, some 35% of West Hawaii's coastline was closed to aquarium fishing via a network of marine protected areas (MPAs; Tissot et al., 2009).
Walsh et al. (2004) and Williams et al. (2009) showed that catch per unit effort (cpue) and total catch for yellow tang, respectively, increased since the establishment of the MPAs in West Hawaii, suggesting that they may have enhanced the aquarium fishery. In addition, Williams et al. (2009) detected evidence of spillover of adult yellow tang near the boundaries of several MPAs. Although these effects of MPAs may influence catch productivity positively outside the protected areas, the aquarium fishery targets juvenile yellow tang (Williams et al., 2009), which display high site fidelity and limited mobility (Claisse, 2009). Therefore, although MPAs may enhance recruitment by increasing the adult spawning stock, fishers targeting juvenile yellow tang are unlikely to benefit significantly from spillover dynamics, i.e. juvenile fish moving across MPA boundaries into fishable waters. Additionally, despite the presence of the MPAs, the abundance of yellow tang has declined by 45% in areas open to aquarium fishing between 1999 and 2007 (Williams et al., 2009), which is likely the result of increased fishing pressure from clustering fishers in permissible fishing areas after the MPAs were established. Fishery managers frequently assume fish abundance is proportional to cpue, which is often misleading (Maunder et al., 2006). Detecting an increase in cpue while stock abundance declines is a common pattern observed in commercial fisheries known as hyperstability, and it can manifest when the data used to calculate cpue are standardized by removing attributes associated with fleet efficiency (Hilborn and Walters, 1992; Maunder et al., 2006).
Several studies have provided a brief description of the methods used for capturing and processing reef fish and the catch composition of Hawaii's aquarium fishery (Randall, 1987; Miyasaka, 1994, 1997; Ogawa and Brown, 2001; Cesar et al., 2002), but these efforts have not examined the fleet factors that may contribute to catch variability within the fishery. Here, we attempt to explain catch trends for yellow tang in West Hawaii by describing the aquarium fishery and investigating how effort allocation, harvesting efficiencies, and job satisfaction influence catch productivity and selectivity. To our knowledge, this is the first study to investigate fishing fleet dynamics for a marine aquarium fishery anywhere in the world.
Methods
Interviews
In 2002, semi-structured in-person interviews were conducted with ten active aquarium fishers from West Hawaii to determine fisher age and fishing effort. The interview questions were determined a priori, and each participant was asked the same questions. In 2007, we obtained answers to the same questions using mailed surveys and in-person interviews to assess changes in these attributes over time. Additionally, in 2007 and 2008, we performed pre- and post-survey fisher interviews with 12 fishers who had a wide range of experience. The pre-survey interviews served to ascertain information about the social environment and perceptions surrounding the aquarium fishery, as well as to establish a rapport with the fishers for the planned surveys and in situ observations (see below). The post-survey interviews served to validate overall responses received from the surveys. Any detected incongruities in the responses were noted.
Surveys
The results from the pre-survey interviews were used to develop a cross-sectional survey to evaluate changes within the aquarium fishery after the establishment of the MPAs. We used income satisfaction, willingness to encourage new fishers to enter the fishery, and willingness to exit the fishery if training for another job with a similar income as surrogates for job satisfaction. Three aquarium fishers and the State fishery biologist responsible for West Hawaii pre-tested the survey before it was disseminated, to evaluate clarity and appropriateness.
Each fisher received a cover letter explaining the research objectives and a statement of confidentially, a survey, a self-addressed stamped envelope for returning the survey, and instructions on how to obtain a modest incentive for participating in the study. The State Division of Aquatic Resources (hereinafter DAR) sent the survey packets to all permit-holding aquarium fishers in West Hawaii (n = 67) on our behalf, and 23 surveys were returned. In addition to using the DAR's assistance, we also used fisher informants to help disseminate the survey to their peers who were less likely to respond to a survey sent from the state government. The returned surveys were coded and the responses were saved in a database. The Kruskal–Wallis test and the Mann–Whitney U-test were used to analyse the survey and catch data, and results were considered significant at p < 0.05. Fisher perceptions were assessed both for fishers as an aggregate group and for fishers grouped by the level of experience (0–5, 6–10, 11–15, 16–20, and 21+ years in the fishery).
Observations and catch analysis
Fisher observations and catch analyses were conducted between June and July 2007 and in November 2008, to document the methods used by aquarium fishers for capturing reef fish and to document and measure the target species and size of the fish caught, respectively. The number of fish caught per species (used here to calculate relative catch abundance), size (standard length in cm), depth and habitat of capture, capture mortality, and number of discarded fish per species were documented. Discarded fish were defined as those that were captured, brought to the surface, and released alive from the boat. Depth was measured using our scuba depth gauge at sites where nets were placed and fish collected. When possible, photographs at collection sites were used to classify preferred fisher habitats using methods developed by Ortiz and Tissot (2008). Overall, fishing effort per fisher in 2002 and 2007 was calculated by multiplying the mean fishing intensity (i.e. the number of dives per fishing trip) by the mean fishing frequency (i.e. the number of trips per week) in those years. Finally, cpue was determined by counting the number of individuals of each species caught per dive duration.
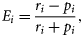
Ei ranges between −1 and +1. Positive values indicate preference (a taxon overrepresented in the catch composition in relation to its availability in the environment), and negative values avoidance (a taxon underrepresented in the catch composition in relation to its availability; Lechowicz, 1982). The abundance data for reef fish were obtained from the West Hawaii Aquarium Project (WHAP) database, using methods described in Tissot et al. (2004). Although these data represent the best available estimates of reef-fish density, we acknowledge that abundance data from the WHAP may not correspond to actual reef-fish densities because cryptic or nocturnal species are generally undercounted during scuba surveys, monitoring data may not represent the same range and distribution of habitats where fish are harvested, and fish behaviour changes in the presence of divers (Williams et al., 2006).
When fishers sell their catch to an exporter, they are given an invoice stating the number of fish caught, the number of fish purchased by the exporter, the price per fish, and the names of the fishers who caught the fish. To assess the influence of team size on the number of yellow tang caught per dive, we analysed 247 sale invoices from one fisher who dived solo (102 invoices) and with one (127 invoices) or two (18 invoices) additional divers on different occasions. We assessed differences in the number of yellow tang caught by dive teams of different size using a Mann–Whitney test, with probability values corrected for multiple comparisons (i.e. one vs. two divers, one vs. three divers, and two vs. three divers). Variables that may have confounded the results from these analyses (e.g. depth, location, season, team dynamics) were not available, and therefore could not be included; however, the fish sold were always caught using the same method.
Results
In 2007, 23 fishers completed the survey and 12 were interviewed. The mean age, the number of years participating in the fishery, and the number of fishing days per week for those who completed the 2007 survey was 47 years, 16 years, and 3 d per week, respectively, and 74% of fishers originally acquired their permits on the Island of Hawaii (Supplementary Figure S1).
Harvesting methods and efficiencies
In 2007, equipment and methods used for locating and capturing reef fish were observed and documented. In general, reef fish were corralled and pushed into monofilament barriernets set by the fishers; however, the specific process varied among fishers. Methods for locating fish varied from using dive masks or viewing scopes while on the boat to using underwater scooters (hand-held motorized submersible devices that enable scuba divers to increase their mobility and speed with minimal exertion). Dive masks or viewing scopes were most commonly used for identifying yellow tang, both allowing a fisher to search the reef for fish while remaining on the boat. When yellow tang were located, fishers anchored their boat and commenced fishing using scuba. A less-common technique involved using underwater scooters to scout large areas of reef for yellow tang. Fishers would initially scout the reef using the scooter, identifying the locations of reef fish. They then corralled smaller clusters of fish into larger groups before capture. Both methods are highly effective for locating aggregating yellow tang.
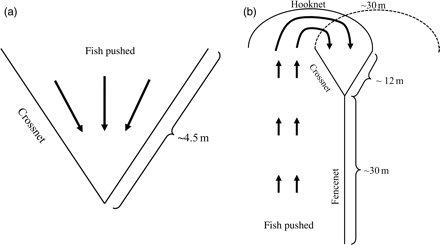
During our investigation, we documented three primary approaches for capturing reef fish in West Hawaii. The two most common methods used two distinct monofilament barriernet designs: (i) crossnet and (ii) multiple-net designs (Figure 1). Barriernets have a float line at the top edge of the net and a lead line at the bottom to keep the net floating upright. The crossnet method involves using a single net that commonly measures ∼9 m long by 2 m high, with a 1-cm mesh size. The method requires positioning the net in a V-shape, with the back lower end of the net forming a pocket, and the lead line placed flush with the substratum (Figure 1a). If available, fishers will set their nets near natural barriers (e.g. next to sand patches, large boulders, or corals) to control fish movement. Using the crossnet design enables fishers to set and adjust their net in different locations on the reef multiple times per dive, based on where the fish are located. The fishers often use two 1.3-cm diameter fibreglass sticks that are sometimes referred to as “tickle sticks”, or probes that vary in length. Fishers use these devices to cluster small groups of reef fish into larger ones before corralling them into their nets. The number of times a fisher will set their crossnet and push fish into it can range from ∼5 to 10 times per dive. Most fishers will set their net as few times as possible because it is strenuous and increases a diver's demand for air, diminishing bottom time. Once the fish are in the net, fishers will extract them using their hands or a handnet and place them into an underwater live-well basket for the remainder of the dive. Whether a fisher uses his hand or a handnet to remove the fish from the barriernet is a matter of preference, but some claim that using their hands damages a fish and reduces its value. People who prefer the crossnet method usually dive solo or with one or occasionally two other fisher(s) and largely target surgeonfish such as yellow tang. Lastly, fishers decompressed their catch by slowly raising their live-well basket to the surface of the water after the return to the vessel, and residual trauma was relieved by purging air from the fish's swimbladder using a hypodermic needle. The multiple-net method involves the use of much longer fence-, hook-, and crossnets, which are all barriernets, but are differentiated based on their function (Figure 1b). Fishers who used this method sometimes operated individually, but more often in small teams of 2–4. The fishers divide an area of reef by placing a fencenet commonly measuring ∼30 m long and 2 m high along the reef substratum. Next, they place the apex of a crossnet on one end of the fencenet, then adjoin a hooknet and “hook” it around the opposite end of the crossnet (Figure 1b). These crossnets are ∼24 m long and 4 m high, and the hooknet ∼30 m long and 2 m high. The fisher(s) will corral the fish and push them into the crossnet, then collect them as described above. Next, they will switch the hooknet and adjoin it to the opposite end of the crossnet and repeat the steps into the newly placed crossnet. Once both sides of the fencenet are fished, the fisher(s) will repeat the process by reversing the direction of the cross-, fence-, and hooknets. Depending on the number of people fishing this net design, it takes ∼4–5 tanks of compressed air (or nitrox–oxygen-enriched air usually ranging between 32 and 36% oxygen, the higher percentage of oxygen increasing dive recovery time and bottom time, but reducing the operable depth through possible oxygen toxicity) to complete a set. Like crossnet fishers, people who use the multiple-net design primarily target surgeonfish.
Top view of crossnet (a) and multiple-net (b) designs. The dashed hooknet represents the reset on the opposite crossnet end.
Another method observed for capturing reef fish involved the use of handnets to target individual high-value fish that often take refuge in finger-coral habitat, e.g. juvenile Ctenochaetus hawaiiensis, Centropyge potteri, and Centropyge loriculus. Handnet size ranged from ∼21 to 30 cm in diameter, with 21 cm being the most common. Fishers insert their tickle sticks into finger coral and coax hiding fish into the open, where they use a handnet to capture them. Handnets are also used to capture smaller fish like cleaner wrasses (Labroides phthirophagus) and docile, deep-water reef fish that inhabit depths >60 m, e.g. Chaetodon tinkeri and Apolemichthys arcuatus. From our post-survey interviews, we estimated that ≤5 fishers within the fleet harvest deep-water reef fish with regularity. Lastly, handnets were also used to retrieve fish from cross- and multiple-net designs before placing them in the underwater baskets.
Post-survey interviews also revealed that gear and technology changed before and after the MPAs were implemented in 1999. The use of underwater scooters and nitrox was introduced to the fishery during the late 1990s. Approximately 5–8 people use scooters and nearly the entire fleet uses nitrox, except for a few people who use compressed air when targeting species found in deeper water. At least five fishers adopted global positioning system (GPS) devices between 2002 and 2005 and regularly used this technology in 2007. Finally, at least three fishers used artificial material that mimics sand patches and is placed on the top of the coral reefs to control fish movement and prevent them from taking refuge. We were unable to determine when this method of capture was first introduced.
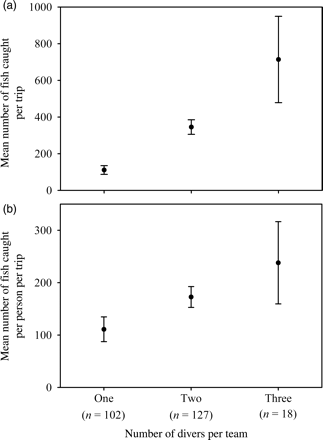
Respondents in 2007 indicated that 18.2% fished solo, 45.5% fished with one additional diver, 22.7% fished with two additional divers, and 13.6% fished with more than three additional divers (n = 22). Sale invoices revealed a significant difference in the total number of yellow tang caught per team size (H = 105.59; p < 0.001; n = 247), with the mean numbers of fish caught equalling 111, 345, and 714 per one-, two-, and three-person team, respectively (one vs. two divers: U = 6981.5; p < 0.0001; one vs. three divers: U = 5394.5; p < 0.0001; two vs. three divers: U = 8683.0; p < 0.001; Figure 2a). More importantly, the mean number of yellow tang caught per person per trip per team size was 111, 173, and 237 fish, increasing significantly with dive team size (one vs. two divers: U = 8857.5, p < 0.005; one vs. three divers: U = 5656.0, p < 0.005; two vs. three divers: U = 8996.5, p > 0.05; Figure 2b).
The mean number of yellow tang caught (a) per trip, and (b) per person per trip. Both were influenced significantly by the number of divers per fishing team.
Effort allocation
Most fishers collected fish at depths between 12.5 and 18 m, but preferred fishing depth differed significantly between periods with different levels of juvenile fish recruitment. Specifically, preferred fishing depth was deeper when juvenile reef fish recruitment was perceived as weak as opposed to strong (U = 422.0; p < 0.05; n = 45; adjusted for ties; Table 1). Fishers also adjusted their fishing choices in response to weak juvenile fish recruitment (Table 2). Of the 21 fishers who indicated they were affected by weak juvenile fish recruitment, those with 11–15 years experience were most affected and most commonly stated they collected larger, older fish as a response. Fishers with 0–5 and 21+ years of fishing experience were the two most common groups reporting they collected fish deeper in response to weak juvenile fish recruitment. Mean fishing effort per fisher remained constant between 2002 and 2007 at 11 dives per week. In 2002 and 2007, the estimated mean time per dive was 45 and 52 min, respectively.
Fisher dive and operating depth preference during perceived strong and weak recruitment years for reef fish in 2007.
| Depth (m) . | Strong recruitment (%) . | Weak recruitment (%) . |
|---|---|---|
| 0 and 12 | 18.2 | 13.0 |
| 12.5 and 18 | 72.7 | 43.5 |
| 18.5 and 24 | 9.1 | 39.1 |
| >24.5 | 0 | 4.3 |
| Total respondents | 22 | 23 |
| Depth (m) . | Strong recruitment (%) . | Weak recruitment (%) . |
|---|---|---|
| 0 and 12 | 18.2 | 13.0 |
| 12.5 and 18 | 72.7 | 43.5 |
| 18.5 and 24 | 9.1 | 39.1 |
| >24.5 | 0 | 4.3 |
| Total respondents | 22 | 23 |
Fisher dive and operating depth preference during perceived strong and weak recruitment years for reef fish in 2007.
| Depth (m) . | Strong recruitment (%) . | Weak recruitment (%) . |
|---|---|---|
| 0 and 12 | 18.2 | 13.0 |
| 12.5 and 18 | 72.7 | 43.5 |
| 18.5 and 24 | 9.1 | 39.1 |
| >24.5 | 0 | 4.3 |
| Total respondents | 22 | 23 |
| Depth (m) . | Strong recruitment (%) . | Weak recruitment (%) . |
|---|---|---|
| 0 and 12 | 18.2 | 13.0 |
| 12.5 and 18 | 72.7 | 43.5 |
| 18.5 and 24 | 9.1 | 39.1 |
| >24.5 | 0 | 4.3 |
| Total respondents | 22 | 23 |
Fisher responses shown as a percentage (%) for adjusting to perceived weak juvenile fish recruitment in 2007.
| Collected fish deeper | 26.5 |
| Collected larger, older fish | 20.6 |
| Depended more on a pre-existing, non-fishing job | 17.6 |
| Increased fish collecting intensity | 8.8 |
| Took a new job | 5.9 |
| Sold my boat, property, equipment, or gear | 2.9 |
| Relied on government assistance | 2.9 |
| Relied on other fisheries | 2.9 |
| Other | 11.8 |
| Total number of responses | 34a |
| Collected fish deeper | 26.5 |
| Collected larger, older fish | 20.6 |
| Depended more on a pre-existing, non-fishing job | 17.6 |
| Increased fish collecting intensity | 8.8 |
| Took a new job | 5.9 |
| Sold my boat, property, equipment, or gear | 2.9 |
| Relied on government assistance | 2.9 |
| Relied on other fisheries | 2.9 |
| Other | 11.8 |
| Total number of responses | 34a |
aMultiple responses were accepted.
Fisher responses shown as a percentage (%) for adjusting to perceived weak juvenile fish recruitment in 2007.
| Collected fish deeper | 26.5 |
| Collected larger, older fish | 20.6 |
| Depended more on a pre-existing, non-fishing job | 17.6 |
| Increased fish collecting intensity | 8.8 |
| Took a new job | 5.9 |
| Sold my boat, property, equipment, or gear | 2.9 |
| Relied on government assistance | 2.9 |
| Relied on other fisheries | 2.9 |
| Other | 11.8 |
| Total number of responses | 34a |
| Collected fish deeper | 26.5 |
| Collected larger, older fish | 20.6 |
| Depended more on a pre-existing, non-fishing job | 17.6 |
| Increased fish collecting intensity | 8.8 |
| Took a new job | 5.9 |
| Sold my boat, property, equipment, or gear | 2.9 |
| Relied on government assistance | 2.9 |
| Relied on other fisheries | 2.9 |
| Other | 11.8 |
| Total number of responses | 34a |
aMultiple responses were accepted.
The depth at the collection sites included in our in situ catch analyses ranged from 13.7 to 27.4 m. Preferred habitat for collecting fish was located in deep coral-rich habitat with finger (Porites compressa), lobe (P. lobata), and mixed coral, as well as in uncolonized rubble, which is found at depths between 8 and 30 m (Ortiz and Tissot, 2008). Over a total of 45 fishing hours, the five divers observed in 2007 and two divers observed in 2008 collected 4611 fish, a cpue of ∼100 fish per dive-hour. The most commonly targeted family of fish was the Acanthuridae, constituting 89% of the total catch. Yellow tang alone made up 69% (n = 3200) of the total catch. Acanthurids remained the most commonly targeted family of fish even with yellow tang excluded from the analysis, constituting 65% of the remaining catch. Some 75% of these acanthurids were Ctenochaetus strigosus, with Naso lituratus and Acanthurus nigrofuscus each yielding an additional 16%. The remaining catch consisted of Pomacentridae (12%), Chaetodontidae (8%), Labridae (7%), and other families (7%). Only three principally corallivorous fish were collected: speckled butterflyfish (Chaetodon multicinctus; n = 32), ornate butterflyfish (C. ornatissimus; n = 1), and fourspot butterflyfish (C. quadrimaculatus; n = 1). Fishers frequently released unwanted species underwater at the collection sites; such fish included ornate butterflyfish, saddle wrasses (Thalassoma duperrey), agile chromis (Chromis agilis), brown surgeonfish (A. nigrofuscus), larger chevron tangs (C. hawaiiensis), longnose butterflyfish (Forcipiger sp.), yellow tang, and goldring surgeonfish. When the relative importance of the fish caught was compared with their abundance on the reef, psychedelic wrasse (Anampses chrysocephalus), goldrim surgeonfish (Acanthurus nigricans), Hawaiian domino damselfish (Dascyllus albisella), and yellow tang had the highest values of electivity (Table 3).
Target species size and Ivlev's electivity index values (Ei) from 2007 and 2008 in situ catch analyses.
| Species . | Mean standard length (cm) . | s.d. . | Range (cm) . | n . | Ei . |
|---|---|---|---|---|---|
| Zebrasoma flavescens | 6.1 | 1.61 | 3.0–12.8 | 2 633 | 0.52 |
| Ctenochaetus strigosus | 7.8 | 1.10 | 4.0–11.5 | 583 | −0.38 |
| Naso lituratus | 13.0 | 3.00 | 7.5–20.0 | 73 | 0.18 |
| Forcipiger flavissimus | 9.7 | 1.40 | 7.5–12.5 | 46 | 0.36 |
| Dascyllus albisella | 9.6 | 1.20 | 7.2–11.5 | 35 | 0.52 |
| Acanthurus achilles | 8.2 | 2.00 | 3.0–12.0 | 34 | 0.40 |
| Chaetodon multicinctus | 7.0 | 0.90 | 5.6–8.9 | 20 | −0.83 |
| Halichoeres ornatissimus | 9.9 | 1.40 | 6.8–1.5 | 20 | −0.59 |
| Myripristis berndti | 13.2 | 0.80 | 12.0–14.0 | 17 | −0.25 |
| Acanthurus olivaceus | 12.2 | 3.60 | 8.5–21.5 | 16 | 0.07 |
| Ctenochaetus hawaiiensis | 6.4 | 6.40 | 3.5–13.0 | 14 | 0.07 |
| Anampses chrysocephalus | 9.1 | 1.30 | 7.3–12.0 | 13 | 0.90 |
| Acanthurus nigrofuscus | 7.1 | 1.10 | 5.1–9.7 | 12 | −0.94 |
| Acanthurus nigricans | 8.4 | 1.20 | 7.0–10.5 | 11 | 0.53 |
| Sufflamen bursa | 11.4 | 2.00 | 7.0–13.7 | 10 | −0.48 |
| Species . | Mean standard length (cm) . | s.d. . | Range (cm) . | n . | Ei . |
|---|---|---|---|---|---|
| Zebrasoma flavescens | 6.1 | 1.61 | 3.0–12.8 | 2 633 | 0.52 |
| Ctenochaetus strigosus | 7.8 | 1.10 | 4.0–11.5 | 583 | −0.38 |
| Naso lituratus | 13.0 | 3.00 | 7.5–20.0 | 73 | 0.18 |
| Forcipiger flavissimus | 9.7 | 1.40 | 7.5–12.5 | 46 | 0.36 |
| Dascyllus albisella | 9.6 | 1.20 | 7.2–11.5 | 35 | 0.52 |
| Acanthurus achilles | 8.2 | 2.00 | 3.0–12.0 | 34 | 0.40 |
| Chaetodon multicinctus | 7.0 | 0.90 | 5.6–8.9 | 20 | −0.83 |
| Halichoeres ornatissimus | 9.9 | 1.40 | 6.8–1.5 | 20 | −0.59 |
| Myripristis berndti | 13.2 | 0.80 | 12.0–14.0 | 17 | −0.25 |
| Acanthurus olivaceus | 12.2 | 3.60 | 8.5–21.5 | 16 | 0.07 |
| Ctenochaetus hawaiiensis | 6.4 | 6.40 | 3.5–13.0 | 14 | 0.07 |
| Anampses chrysocephalus | 9.1 | 1.30 | 7.3–12.0 | 13 | 0.90 |
| Acanthurus nigrofuscus | 7.1 | 1.10 | 5.1–9.7 | 12 | −0.94 |
| Acanthurus nigricans | 8.4 | 1.20 | 7.0–10.5 | 11 | 0.53 |
| Sufflamen bursa | 11.4 | 2.00 | 7.0–13.7 | 10 | −0.48 |
Target species size and Ivlev's electivity index values (Ei) from 2007 and 2008 in situ catch analyses.
| Species . | Mean standard length (cm) . | s.d. . | Range (cm) . | n . | Ei . |
|---|---|---|---|---|---|
| Zebrasoma flavescens | 6.1 | 1.61 | 3.0–12.8 | 2 633 | 0.52 |
| Ctenochaetus strigosus | 7.8 | 1.10 | 4.0–11.5 | 583 | −0.38 |
| Naso lituratus | 13.0 | 3.00 | 7.5–20.0 | 73 | 0.18 |
| Forcipiger flavissimus | 9.7 | 1.40 | 7.5–12.5 | 46 | 0.36 |
| Dascyllus albisella | 9.6 | 1.20 | 7.2–11.5 | 35 | 0.52 |
| Acanthurus achilles | 8.2 | 2.00 | 3.0–12.0 | 34 | 0.40 |
| Chaetodon multicinctus | 7.0 | 0.90 | 5.6–8.9 | 20 | −0.83 |
| Halichoeres ornatissimus | 9.9 | 1.40 | 6.8–1.5 | 20 | −0.59 |
| Myripristis berndti | 13.2 | 0.80 | 12.0–14.0 | 17 | −0.25 |
| Acanthurus olivaceus | 12.2 | 3.60 | 8.5–21.5 | 16 | 0.07 |
| Ctenochaetus hawaiiensis | 6.4 | 6.40 | 3.5–13.0 | 14 | 0.07 |
| Anampses chrysocephalus | 9.1 | 1.30 | 7.3–12.0 | 13 | 0.90 |
| Acanthurus nigrofuscus | 7.1 | 1.10 | 5.1–9.7 | 12 | −0.94 |
| Acanthurus nigricans | 8.4 | 1.20 | 7.0–10.5 | 11 | 0.53 |
| Sufflamen bursa | 11.4 | 2.00 | 7.0–13.7 | 10 | −0.48 |
| Species . | Mean standard length (cm) . | s.d. . | Range (cm) . | n . | Ei . |
|---|---|---|---|---|---|
| Zebrasoma flavescens | 6.1 | 1.61 | 3.0–12.8 | 2 633 | 0.52 |
| Ctenochaetus strigosus | 7.8 | 1.10 | 4.0–11.5 | 583 | −0.38 |
| Naso lituratus | 13.0 | 3.00 | 7.5–20.0 | 73 | 0.18 |
| Forcipiger flavissimus | 9.7 | 1.40 | 7.5–12.5 | 46 | 0.36 |
| Dascyllus albisella | 9.6 | 1.20 | 7.2–11.5 | 35 | 0.52 |
| Acanthurus achilles | 8.2 | 2.00 | 3.0–12.0 | 34 | 0.40 |
| Chaetodon multicinctus | 7.0 | 0.90 | 5.6–8.9 | 20 | −0.83 |
| Halichoeres ornatissimus | 9.9 | 1.40 | 6.8–1.5 | 20 | −0.59 |
| Myripristis berndti | 13.2 | 0.80 | 12.0–14.0 | 17 | −0.25 |
| Acanthurus olivaceus | 12.2 | 3.60 | 8.5–21.5 | 16 | 0.07 |
| Ctenochaetus hawaiiensis | 6.4 | 6.40 | 3.5–13.0 | 14 | 0.07 |
| Anampses chrysocephalus | 9.1 | 1.30 | 7.3–12.0 | 13 | 0.90 |
| Acanthurus nigrofuscus | 7.1 | 1.10 | 5.1–9.7 | 12 | −0.94 |
| Acanthurus nigricans | 8.4 | 1.20 | 7.0–10.5 | 11 | 0.53 |
| Sufflamen bursa | 11.4 | 2.00 | 7.0–13.7 | 10 | −0.48 |
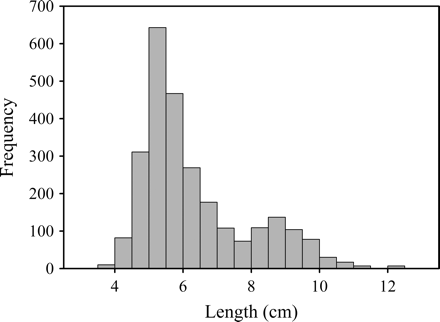
The mean standard length for the yellow tang caught was 6.1 cm (Table 3, Figure 3). Additionally, yellow tang caught during November 2008 were significantly smaller (mean = 5.92 cm; n = 2382) than those caught during June/July 2007 (mean = 7.78 cm; n = 251; H = 296.1; p < 0.001; n = 2633). Yellow tang harvested during November 2008 were bimodally distributed with the two modes at 5.0 and 8.5 cm. Mortality and discarded fish for observations made during November 2008 over 33 h of fishing effort was <1% (n = 230) of the total catch. The total number of discarded fish was 216, primarily C. agilis (53%), Z. flavescens (16%), and A. nigrofuscus (15%). All discarded fish were either commercially unimportant (e.g. C. agilis), blemished (e.g. natural discolouration and deformation, or laden with parasites), or injured (e.g. fin damage). Mortality was observed in D. albisella (n = 7), Z. flavescens (n = 4), C. loriculus (n = 1), Ostracion meleagris (n = 1), and Halichoeres ornatissimus (n = 1) on the boat after the fish were decompressed.
Standard length (cm) and frequency histogram for yellow tang measured during June/July 2007 and November 2008 (n = 2633).
Job satisfaction
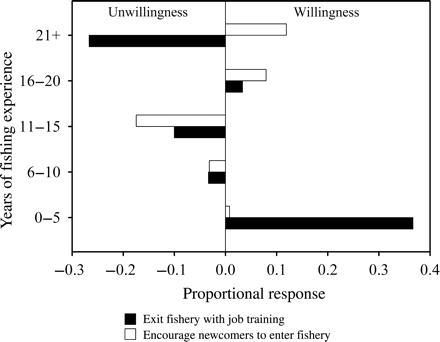
Fishers were extremely satisfied (n = 3), satisfied (n = 12), or held neutral opinions (n = 6) regarding their level of income earned from the fishery; none was dissatisfied or strongly dissatisfied. Of the fishers surveyed, 71% indicated they would not exit the aquarium fishery if training for another job that was equally profitable were provided. The difference between those fishers who would vs. would not exit the fishery with job training was significant (H = 12.27; p < 0.001; n = 21). Fishers with 0–5 years of fishing experience were most willing to exit the fishery with training for another job that was equally profitable, whereas fishers with 21+ years of experience were least willing (Figure 4). The difference between respondents who would encourage vs. not encourage younger fishers to enter the fishery was significant (H = 15.75; p < 0.001; n = 23), and regulations, conflict, competition, and initial start-up costs were reasons given for not encouraging newcomers. Fishers with 16–20 and 21+ years experience had a stronger proportional response for encouraging younger fishers to enter the fishery (Figure 4). Finally, the most consistent responses for what fishers liked most about their occupation included autonomy and exposure to nature, whereas bureaucracy, conflict, and poor industry reputation were the most consistent responses for what they liked least (Table 4).
Fishers' stated preference for what they liked most and least about West Hawaii's aquarium fishery in 2007.
| Liked most . | Per cent . | Liked least . | Per cent . |
|---|---|---|---|
| Exposure to nature | 25.8 | Bureaucracy and conflict | 20.7 |
| Autonomy | 25.8 | Poor industry reputation | 17.2 |
| Scuba diving | 12.9 | Bad weather | 13.8 |
| The challenge | 9.7 | Poor water conditions | 13.8 |
| Total responses | 31a | Total responses | 29a |
| Liked most . | Per cent . | Liked least . | Per cent . |
|---|---|---|---|
| Exposure to nature | 25.8 | Bureaucracy and conflict | 20.7 |
| Autonomy | 25.8 | Poor industry reputation | 17.2 |
| Scuba diving | 12.9 | Bad weather | 13.8 |
| The challenge | 9.7 | Poor water conditions | 13.8 |
| Total responses | 31a | Total responses | 29a |
aMultiple responses were accepted.
Fishers' stated preference for what they liked most and least about West Hawaii's aquarium fishery in 2007.
| Liked most . | Per cent . | Liked least . | Per cent . |
|---|---|---|---|
| Exposure to nature | 25.8 | Bureaucracy and conflict | 20.7 |
| Autonomy | 25.8 | Poor industry reputation | 17.2 |
| Scuba diving | 12.9 | Bad weather | 13.8 |
| The challenge | 9.7 | Poor water conditions | 13.8 |
| Total responses | 31a | Total responses | 29a |
| Liked most . | Per cent . | Liked least . | Per cent . |
|---|---|---|---|
| Exposure to nature | 25.8 | Bureaucracy and conflict | 20.7 |
| Autonomy | 25.8 | Poor industry reputation | 17.2 |
| Scuba diving | 12.9 | Bad weather | 13.8 |
| The challenge | 9.7 | Poor water conditions | 13.8 |
| Total responses | 31a | Total responses | 29a |
aMultiple responses were accepted.
Proportional response of fishers who reported willingness to (i) exit the aquarium fishery in West Hawaii if training for another job with equal income earning potential was provided, and (ii) encourage new fishers into the fishery by years of fishing experience.
Discussion
To our knowledge, this is the first in-depth study investigating the fleet dynamics for any marine aquarium fishery. Our findings demonstrate that the decisions fishers make regarding the number of divers, operating depth, technology used, and fishing methods deployed may influence catch productivity in West Hawaii's aquarium fishery. Additionally, fishers selectively target coral-friendly species such as yellow tang for the marine ornamental fish trade. Finally, fisher job satisfaction remained high despite declining yellow tang abundance, and fishers with more experience are less likely to exit the fishery. To that end, we now discuss the implications of our findings regarding catch productivity and selectivity for yellow tang in West Hawaii, and then explore the broader implications of our study for global aquarium fisheries.
Harvesting methods and efficiencies
The adoption of new technologies and equipment for catching fish can improve a fleet's catch efficiency (Salas and Gaertner, 2004; Branch et al., 2006; Marchal et al., 2007). The use of GPS by aquarium fishers in West Hawaii likely started during the past decade when the technology became more readily available and affordable, and GPS is used by some members in the fleet to chart areas and to mark desirable locations to fish the reef systematically. Robins et al. (1998) found that fishers increased their catch efficiency by 2 or 3% each year that they used GPS technology for the first 3 years, and increased fishing efficiency by 12% over 5 years, despite effort reductions of 39% in a prawn fishery in northern Australia. The increasing use of GPS among fishers in the West Hawaii fishery, revealed by our post-survey interviews, may therefore also result in increasing fishing efficiency. Similarly, studies have shown that using nitrox increases bottom time and extends scuba operating days by shortening diver recovery time (Mastro and Dinsmore, 1989). The increasing use of nitrox in West Hawaii improves the overall bottom time of fishers and likely contributes to increased catch.
Fishers are often exceptionally knowledgeable about the behavioural characteristics of their target species (Johannes et al., 2001; Moreno et al., 2007). In West Hawaii, juvenile yellow tang show strong habitat preferences for finger coral (Ortiz and Tissot, 2008), making them susceptible to capture in high densities even when overall abundance is low (Hilborn and Walters, 1992). Using a dive mask, underwater viewing scope, or scooter to locate yellow tang decreases search time and hence increases a fisher's catch efficiency. Some aquarium fishers have learned to capitalize on juvenile yellow tang that avoid swimming over sand patches by laying materials that mimic sand over the reef to prevent the fish from taking refuge in the coral. This improves a fisher's ability to move fish underwater and into their nets. The extent to which fishers have learned to use these behavioural characteristics of yellow tang is therefore likely to have a strong potential to affect catch efficiency.
The sale-invoice analysis revealed that fishing team size has a significant effect on catch rates in West Hawaii's aquarium fishery, because larger teams augment processes associated with searching, setting net (net management), and moving and corralling fish. Additionally, this is the first time that differences in the net designs applied in aquarium fish collections (cross- vs. multiple-net designs) have been documented for the West Hawaii fishery. It is uncertain if the multiple-net design is more efficient at catching fish because more fishers are generally needed to deploy it, or if having multiple nets decreases efficiency. Future studies examining the differences in catching power between these methods may well be fruitful, because catching power may vary significantly.
The cumulative effects of adopting new technology and equipment, acquiring knowledge of target species behaviour as experience is gained, and variation in fishing team size have likely influenced catch efficiency and hence catch rates. These dynamics will likely change over time and influence overall catch productivity as fisher turnover rates fluctuate.
Effort allocation
Understanding fishing-effort allocation is important for effective fisheries management (Hilborn, 1985). In dive fisheries, effort is usually associated with physical limitations, such as ability and willingness to take risks (Béné and Tewfik, 2001). Nitrogen accumulation in fishers is a risk associated with fisheries requiring scuba, and these risks limit fishing effort. Therefore, it was not surprising that fishing effort, measured as the number of dives per week, remained stable between 2002 and 2007. Because of these limitations, it also appears unlikely that changes in fishing effort will influence future catch rates significantly in West Hawaii's aquarium fishery, unless new methods or technologies allow fishers to overcome the physical limitation associated with conventional scuba diving.
An interesting adaptation of fishers to the strength of recruitment of juvenile reef fish was the change in preferred dive depth, with a shift to deeper when juvenile fish recruitment was perceived as weak, particularly among fishers with 0–5 and 21+ years of experience. Recruiting and juvenile yellow tang commonly inhabit deep, coral-rich reefs, sandy rubble, mid-depth reefs, and boulder habitats. They then migrate to shallow turf-rich boulder habitat as they mature (Ortiz and Tissot, 2008; Claisse et al., 2009). Deeper water can act as a refuge for juvenile fish and other species commonly targeted in coral reef fisheries (Tyler et al., 2009). Shifting reef fish harvesting deeper when recruitment is weak may allow fishers to maintain high catch productivity by increasing their operating area and allowing them to target recruits (as opposed to shallower juveniles) and other species not typically caught. At the same time, this behaviour is likely to affect depth-refuge benefits negatively (Morato et al., 2006). Additionally, fishers mentioned in interviews that newly recruited fish are more sensitive to chemicals used to control parasites and diseases in exporter holding facilities, making them more susceptible to mortality. In turn, post-collection mortality from handling, holding, and shipping in the aquarium fish trade presumably increases fishing pressure, because fishers will harvest more fish to offset losses (Wood, 2001b). As mentioned above, fishers who use scuba are physically limited by the number of dives they can complete safely, so greater post-collection mortality may not encourage fishers to dive more frequently, although it may encourage them to work harder to replace the lost catch the next time they dive, which may influence catch productivity.
A comparison of our results on catch composition with previous state-wide reports in Hawaii shows that catches from the aquarium fishery have become much more specialized on surgeonfish, particularly yellow tang, in the past 10 years. Previously, aquarium fishers targeted a more diverse catch, including corallivorous reef fish (Miyasaka, 1994). For example, in the 1970s, surgeonfish and butterflyfish comprised ∼40 and ∼30%, respectively, of the total catch in Hawaii (W. Walsh, pers. comm.). This stands in stark contrast to the overwhelming importance of yellow tang and other surgeonfish in the catch recorded in this study, with butterflyfish contributing a mere 8% to the catch. This change in relative importance cannot be explained by changes in abundance in the wild, but rather reflects active choice by fishers, as indicated by high positive electivity (i.e. higher relative importance in the catches than the relative abundance on reefs would suggest) for species such as goldrim surgeonfish and yellow tang, and avoidance of species such as C. multicinctus. The electivity data also show that fishing pressure diverges between species. The underlying reason for the shift from butterflyfish to surgeonfish may lie in market dynamics. In particular, one interviewed aquarium fish exporter in Hawaii and one US-based wholesaler stated that home aquarium-keepers have shifted away from buying corallivorous reef fish (e.g. ornate butterflyfish) because they are problematic when placed in live coral tanks, which have grown increasingly popular since the mid-1990s (Rhyne et al., 2009). Additionally, fishers told us that soft-bodied fish, e.g. butterflyfish and angelfish, are more prone to mortality, making the hardier surgeonfish more desirable for aquarium fishers and home aquarium-keepers. The hypothesis that catch composition is strongly influenced by active choice of fishers is further supported by the observation that fishers in our study frequently released certain species underwater immediately after capture, primarily multiband butterflyfish (C. multicinctus) and ornate butterflyfish. The choice for increasingly selecting coral-friendly surgeonfish may explain the amplified number of yellow tang caught in West Hawaii's aquarium fishery over the past decade.
In contrast, not all patterns in catch characteristics observed in this study were determined by fisher choice alone. In particular, a straightforward explanation for the differences in yellow tang size in catches in early summer 2007 and autumn 2008 lies in the recruitment patterns for this species. Fishers preferably target juvenile yellow tang that have grown for at least 3 months after recruiting to the reef, because this allows the fish to reach market size and increases survivorship in exporting holding facilities. Recruitment of yellow tang is strongly seasonal, with the main peak in late spring and summer (Bushnell et al., 2010). This means that fish would reach harvestable size for the first time in autumn, which explains the small mean harvested size in November. By the following spring, these fish would have grown, consistent with the higher mean harvested size of yellow tang then.
Generally, the bulk of catch for most regional aquarium fisheries focuses on a few key species (Wood, 2001a). These species-specific fisheries have greater potential to impact ecosystem functioning than fisheries with a more diverse exploitation strategy (Zhou et al., 2010), a concern for marine ornamental fisheries (Rhyne et al., 2009). The increasing trend towards selecting herbivorous reef fish to accommodate coral-friendly home aquarium tanks is potentially problematic on a global scale given the international nature of the industry, but particularly in Hawaii where low abundance of herbivorous reef fish coupled with increased anthropogenic nutrient inputs can facilitate ecological phase shifts (Smith et al., 2001; Tissot and Hallacher, 2003). Considering that yellow tang in Hawaii are among the most abundant herbivores on the reefs and that catches of juveniles for the aquarium fish trade result in declines in adult abundance (Claisse et al., 2009), the fishery for yellow tang could ultimately be reflected in declining levels of herbivory. In addition, several larger herbivorous species in Hawaii are overexploited by food fisheries (Williams et al., 2008), meaning that the synergistic effects of different fisheries may jointly affect the herbivory level.
In this context, sustainability of the aquarium fishery is an important consideration. The significant decline in abundance of yellow tang over the past 12 years in areas open to fishing despite closing ∼35% of the coastline to fish harvesting, and the +75% greater abundance of yellow tang inside the MPAs vs. outside underscores the fact that the fishery is having a strong effect on its resource (Williams et al., 2009). Unfortunately, despite the increase in fish abundance inside the MPAs, the MPAs may in part be responsible for the decline in the resource, because they concentrate fishers into fewer areas, while increasing (or at least maintaining) pre-MPA fishing pressure by allowing the numbers of fishers to increase over time. At the same time, the resilience of fish populations to continuous fishing appears to be relatively high, considering that reef fish have been harvested for the aquarium trade in West Hawaii for decades. This suggests that the aquarium fishery has the potential to be sustainable if catch levels could be controlled better; however, further biological and socio-economic research in this direction is necessary.
Despite their high value, our finding that few fishers target deep-water species such as C. tinkeri and A. arcuatus with any regularity is likely explained by the necessity to dive to >60 m to capture these species. Diving at such depths necessitates more logistical planning, can only be performed for short intervals, and incurs greater physical risks that often outweigh the potential benefits. However, if new equipment, e.g. a closed-circuit breathing apparatus or blended gas mixtures, replaces conventional scuba, the accessibility to harvest deep-water species will increase. Population assessments for deep-water fish have not been performed, so it is uncertain if existing fishing pressure is impacting these species.
Job satisfaction
Although fisher income satisfaction was high, nearly all fishers indicated that non-monetary benefits generated from the fishery are what they liked most about their occupation (Table 4). This may explain the retention of older fishers despite the arduous labour associated with the fishery. However, the fact that fishers with 0–5 years of experience were more likely to exit the fishery if training for another job with equal income potential was available suggests that economic stimuli may be more important for newer operators. It is intriguing that despite a 45% decline in yellow tang abundance in areas open to fishing (Williams et al., 2009), most fishers are still satisfied with their occupation. This may support the hypothesis that non-monetary benefits and psychocultural needs influence job satisfaction (Anderson, 1980; Smith, 1981; Pollnac and Poggie, 1988, 2006). At the same time, considering that the total catch of yellow tang has not decreased despite their population decline implies that the fishery remains profitable. In the end, both monetary and non-monetary benefits from the fishery could be operating synergistically to produce high levels of job satisfaction.
Conclusion
To our knowledge, this is the first study to have investigated fishing fleet dynamics for a marine aquarium fishery. Our results indicate that changes in fleet dynamics can significantly influence catch productivity (i.e. the number of fish caught) and selectivity (i.e. the targeted species). We have shown that aquarium fishers in West Hawaii adopted and used new technology and methods for capturing reef fish and that dive team size significantly influences catch productivity for yellow tang. Additionally, job satisfaction is high and non-monetary incentives are important to aquarium fishers, which may encourage some fishers to maintain participation in the fishery despite declining fish stocks. Finally, the increased selection of coral-friendly fish by fishers is likely associated with consumer demand that has consequently put greater pressure on surgeonfish and contributed to the increased catch of yellow tang. Although reef fish abundance is significantly greater in the MPAs, it is unlikely that juvenile spillover dynamics have contributed significantly to the increased number of yellow tang caught since their implementation. We acknowledge too that West Hawaii experiences high periodic recruitment of juvenile yellow tang, which is potentially associated with the MPAs and may influence catch rates via replenishment (Christie et al., 2010). We argue that changes in fleet dynamics influenced by fisher behaviour and consumer demands for surgeonfish better explains the discrepancy between increasing catch rates and decreasing relative abundance of yellow tang in areas open to fishing in West Hawaii.
Although our study is regionally focused, the aquarium fish trade is a global industry. Our findings regarding effort allocation and harvesting efficiencies enhance our understanding of factors influencing the harvest of reef fish and therefore have the potential to contribute to the development of appropriate management strategies. In particular, in addition to developing management plans based on reef-fish abundance or fish import/export data for the aquarium trade, our results show that it will be essential to understand how and where fishers operate.
Supplementary material
Acknowledgements
We thank the aquarium fishers in West Hawaii who provided their time, cooperation, and support. Richard Pollnac, Cheryl Schultz, and Veronica Dujon also provided important feedback and support. Thanks are also due to William Walsh, Steve Cotton, Laura Livnat, and Sara Peck, and the Benthic Ecology Laboratory at Washington State University for their logistical support and guidance, to Delisse Ortiz, Andrew Harwood, and Andrew Erickson for their assistance with data collection and organization, and to William Brown for his hospitality. Finally, we are grateful to two anonymous reviewers for their suggestions on improvements to the manuscript. Funding was provided by NOAA's Coral Reef Conservation Program grant NA08NMF4630457 and Washington State University Vancouver.