-
PDF
- Split View
-
Views
-
Cite
Cite
Jarrod A. Santora, Stephen Ralston, William J. Sydeman, Spatial organization of krill and seabirds in the central California Current, ICES Journal of Marine Science, Volume 68, Issue 7, July 2011, Pages 1391–1402, https://doi.org/10.1093/icesjms/fsr046
Close - Share Icon Share
Abstract
The hypothesis that krill and krill–predator spatial organization and abundance co-vary interannually was tested by investigating the spatial organization of krill and planktivorous seabirds in the central California Current ecosystem over 5 years of varying oceanographic conditions, 2002–2006. To measure the abundance and distribution of krill, data were integrated from large-scale hydroacoustic surveys and station-based net samples, and these data linked to concurrent shipboard visual surveys of seabirds. Acoustically based estimates of the relative abundance of krill were correlated with net samples of Euphausia pacifica, suggesting that acoustic signals mainly reflected the distribution of this numerically dominant species. The distribution and abundance of krill displayed marked changes over years, but the characteristic spatial scale of krill and seabirds remained similar (1–4 nautical miles), confirming the hypothesis of covariance in spatial structure. Krill and the seabird species investigated showed similar habitat associations, i.e. the outer shelf and shelf–slope region, showing that the at-sea distributions of seabirds can provide information on the presence/absence of krill patches. The results also underscore the importance of measuring spatial organization as well as relative abundance in promoting better understanding of predator–prey and marine ecosystem dynamics.Santora, J. A., Ralston, S., and Sydeman, W. J. 2011. Spatial organization of krill and seabirds in the central California Current. – ICES Journal of Marine Science, 68: 1391–1402.
Introduction
Understanding how spatial patterns interact with ecological processes is a central component of ecology. In particular, the study of time-varying predator–prey spatial associations is basic in marine ecology to the development of spatially explicit management strategies for marine resources. In many marine ecosystems, krill, crustaceans of the family Euphausiidae, are important and provide many links in epipelagic foodwebs (Brinton, 1962; Murphy et al., 1988; Batchelder et al., 2002; Field et al., 2006). Studies of krill and their predators have been conducted from the North Pacific to the Southern Ocean, with an emphasis on understanding apparent spatial organization, habitat selection, and associations of krill predators relative to the distribution and abundance of krill (Veit et al., 1993; Mackas et al., 1997; Fiedler et al., 1998; Reid et al., 2004; Croll et al., 2005; Murase et al., 2009). Most often, however, these studies have been limited to a single season or a few years, but in dynamic coastal environments, longer-term studies are required to characterize the spatial structure of prey adequately and to model predator–prey spatial associations effectively across time (Santora et al., 2009, 2010).
Here, we examine the spatial organization of krill and seabirds over 5 years in the variable, but productive, California Current ecosystem (hereafter CCE; Checkley and Barth, 2009), with the goal of examining interannual variation in spatial structure of krill and its planktivorous seabird predators. Two species of krill, Thysanoessa spinifera and Euphausia pacifica, form critical linkages between lower and upper trophic levels in the system (Brinton, 1962; Batchelder et al., 2002; Field et al., 2006; Ainley et al., 2009). Many fish, mammals, and birds depend on krill directly or indirectly as a primary food resource, e.g. Pacific hake Merluccius productus, juvenile rockfish Sebastes spp., salmonids, whales, and auklets. For example, euphausiids are the most important prey item by weight for Pacific sardine (Sardinops sagax) in outer shelf waters (Emmett et al., 2005); sardine in turn can be important prey items for seabirds (Sydeman et al., 2001). Recent substantial fluctuations in the productivity of some fish (Brodeur et al., 2003, 2007; Lindley et al., 2009) and birds (Sydeman et al., 2006) point to changes in krill availability as a likely cause. Previous work on krill in the southern (Brinton and Townsend, 2003), northern (Tanasichuk, 1998; Feinberg and Peterson, 2003), and central (Ainley et al., 1996; Marinovic et al., 2002; Croll et al., 2005; Abraham and Sydeman, 2006; Jahncke et al., 2008) CCE has shown substantial intra- and interannual variation in relative abundance and distribution in relation to oceanographic conditions. Yet, despite the critical importance of euphausiids to the CCE and top predator foraging and population dynamics, understanding of the spatio-temporal dynamics of krill populations and their linkage to krill predators remains fragmentary.
This problem was approached by developing and integrating a series of indicators of krill abundance, distribution, and spatial organization with similar indicators for krill-feeding seabirds. We tested the overarching hypothesis that krill and seabird abundance and spatial organization in the central CCE co-vary interannually, and specifically investigated the relationships between krill and two species of seabird (Cassin's auklet, Ptychoramphus aleuticus, and sooty shearwater, Puffinus griseus). To test this hypothesis, hydroacoustic data, data from net samples, and visual surveys of seabirds collected as part of the National Marine Fisheries Service (NMFS) Juvenile Rockfish/Ecosystem Survey (JRS) were analysed, the latter focusing primarily on estimating the prerecruit abundance of rockfish (Sebastes spp.). For krill predators, we chose auklets and shearwaters as focal species because both are strongly affected by interannual ocean climate variability (Veit et al., 1997; Sydeman et al., 2006), consume large quantities of krill (Briggs and Chu, 1987), and display contrasting life history and feeding strategies. Cassin's auklets are small (180 g) pursuit-diving seabirds capable of exploiting krill over a larger vertical dimension than shearwaters, but are constrained in foraging ambit during the survey period by breeding/offspring care responsibilities on the nearby Farallon Islands (Figure 1). Sooty shearwaters (∼750 g) are migrants to the CCE and are not constrained to a particular location, but feed near the surface, typically to depths of ∼11 m (Briggs and Chu, 1987).
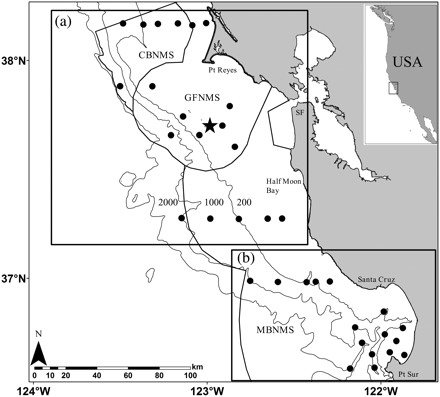
Survey area in the central California Current sampled during May/June of 2002–2006: (a) Gulf of Farallones (north) and (b) Monterey Bay (south). Dots indicate the fixed net-sampling locations (n = 35), the star the location of the Farallon Islands, SF is San Francisco, and the lines demarcate boundaries of the National Marine Sanctuaries (CBNMS, Cordell Bank; GFNMS, Gulf of Farallones; MBNMS, Monterey Bay). The depth contours shown are 200, 1000, and 2000 m.
Methods
Shipboard sampling included underway hydroacoustics, net sampling, and estimation of seabird distribution and abundance (Table 1). Data were collected during May/June each year from 2002 to 2006 aboard the RV “David Starr Jordan”. Here, we restricted all data and analyses to the core survey domain of 38.2°N (∼Pt Reyes, CA, USA) to 36.5oN (∼Pt Sur, CA, USA) because survey coverage was consistent and comprehensive within this area each year (Figure 1). Surveys north of Pt Reyes and south of Pt Sur were intermittent (see Yen et al., 2004, for an example of this variation). Coverage in the east–west (longitudinal) dimension was similar between years. Next, the core domain was subdivided into two regions based on distinct geographic, bathymetric, and oceanographic differences that relate to an upwelling centre (Rosenfeld et al., 1994). The bathymetry along the coast changes drastically at 37.18°N, following the contour around the coastal cape at Pigeon Point and Ano Nuevo into Monterey Bay (Rosenfeld et al., 1994; Figure 1). Hence, these regions broadly reflect the difference between the wide shelf habitat to the north and the large submarine canyon in the south, the Gulf of Farallones (38.2–37.15°N) representing the north and Monterey Bay the south (37.15–36.5°N; Figure 1).
Survey effort conducted during the years 2002–2006.
| Year . | Krill acoustics (miles of trackline) . | Seabird visual surveys (miles of trackline) . | Net-hauls (stations sampled) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| North . | South . | Total . | North . | South . | Total . | North . | South . | Total . | |
| 2002 | 897 | 748 | 1 645 | 510 | 423 | 933 | 35 | 46 | 81 |
| 2003 | 487 | 715 | 1 202 | 392 | 373 | 765 | 51 | 50 | 101 |
| 2004 | 1 186 | 834 | 2 020 | 313 | 219 | 532 | 38 | 50 | 88 |
| 2005 | 566 | 561 | 1 127 | 300 | 185 | 485 | 35 | 50 | 85 |
| 2006 | 772 | 709 | 1 481 | 198 | 282 | 480 | 41 | 36 | 77 |
| Total | 3 908 | 3 567 | 7 475 | 1 712 | 1 481 | 3 193 | 201 | 232 | 432 |
| Mean | 781.6 | 713.4 | 1 495.0 | 342.6 | 296.4 | 639.0 | 40.0 | 46.4 | 86.4 |
| Year . | Krill acoustics (miles of trackline) . | Seabird visual surveys (miles of trackline) . | Net-hauls (stations sampled) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| North . | South . | Total . | North . | South . | Total . | North . | South . | Total . | |
| 2002 | 897 | 748 | 1 645 | 510 | 423 | 933 | 35 | 46 | 81 |
| 2003 | 487 | 715 | 1 202 | 392 | 373 | 765 | 51 | 50 | 101 |
| 2004 | 1 186 | 834 | 2 020 | 313 | 219 | 532 | 38 | 50 | 88 |
| 2005 | 566 | 561 | 1 127 | 300 | 185 | 485 | 35 | 50 | 85 |
| 2006 | 772 | 709 | 1 481 | 198 | 282 | 480 | 41 | 36 | 77 |
| Total | 3 908 | 3 567 | 7 475 | 1 712 | 1 481 | 3 193 | 201 | 232 | 432 |
| Mean | 781.6 | 713.4 | 1 495.0 | 342.6 | 296.4 | 639.0 | 40.0 | 46.4 | 86.4 |
Survey effort conducted during the years 2002–2006.
| Year . | Krill acoustics (miles of trackline) . | Seabird visual surveys (miles of trackline) . | Net-hauls (stations sampled) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| North . | South . | Total . | North . | South . | Total . | North . | South . | Total . | |
| 2002 | 897 | 748 | 1 645 | 510 | 423 | 933 | 35 | 46 | 81 |
| 2003 | 487 | 715 | 1 202 | 392 | 373 | 765 | 51 | 50 | 101 |
| 2004 | 1 186 | 834 | 2 020 | 313 | 219 | 532 | 38 | 50 | 88 |
| 2005 | 566 | 561 | 1 127 | 300 | 185 | 485 | 35 | 50 | 85 |
| 2006 | 772 | 709 | 1 481 | 198 | 282 | 480 | 41 | 36 | 77 |
| Total | 3 908 | 3 567 | 7 475 | 1 712 | 1 481 | 3 193 | 201 | 232 | 432 |
| Mean | 781.6 | 713.4 | 1 495.0 | 342.6 | 296.4 | 639.0 | 40.0 | 46.4 | 86.4 |
| Year . | Krill acoustics (miles of trackline) . | Seabird visual surveys (miles of trackline) . | Net-hauls (stations sampled) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| North . | South . | Total . | North . | South . | Total . | North . | South . | Total . | |
| 2002 | 897 | 748 | 1 645 | 510 | 423 | 933 | 35 | 46 | 81 |
| 2003 | 487 | 715 | 1 202 | 392 | 373 | 765 | 51 | 50 | 101 |
| 2004 | 1 186 | 834 | 2 020 | 313 | 219 | 532 | 38 | 50 | 88 |
| 2005 | 566 | 561 | 1 127 | 300 | 185 | 485 | 35 | 50 | 85 |
| 2006 | 772 | 709 | 1 481 | 198 | 282 | 480 | 41 | 36 | 77 |
| Total | 3 908 | 3 567 | 7 475 | 1 712 | 1 481 | 3 193 | 201 | 232 | 432 |
| Mean | 781.6 | 713.4 | 1 495.0 | 342.6 | 296.4 | 639.0 | 40.0 | 46.4 | 86.4 |
Acoustic krill indices
Routinely, echosounder data are used to map and track changes in krill distribution, abundance, and spatial distribution worldwide (Mackas et al., 1997; Hewitt and Demer, 2000; Reiss et al., 2008; Murase et al., 2009). During the NMFS JRS, acoustic volume-backscattering data (Sv, dB) were collected using a multifrequency echosounder (Simrad EK500) configured with downlooking 38, 120, and 200 kHz split-beam transducers mounted on the hull of the ship at a depth of ∼4 m. During surveys, pulses were transmitted every 2 s at 1 kW for 1 ms duration. Geographic positions were obtained from the ship's GPS and logged every 2 s. Acoustic data were analysed only when the ship was travelling at speeds >5 knots during daylight.
SonarData Echoview was used to visualize and process echograms for horizontally based euphausiid abundance (Hewitt and Demer, 2000; Santora et al., 2009). Differences in volume-backscattering strength measured at different frequencies were used to identify the backscattering from krill (Watkins and Brierley, 2002). We added a 10-m buffer from the depth of the transducers to ensure the removal of noise caused by turbulence along the hull and across the transducers, i.e. aeration, ringing. In addition, a 5-m buffer zone was added above the seabed on all echograms to ensure the removal of erroneous scatterings. Krill were delineated from other scatterers by the use of a three-frequency ▵Sv method (Watkins and Brierley, 2002), with a constant range of size of E. pacifica and T. spinifera (5–25 mm). Volume backscattering of the 120–200-kHz difference was averaged, integrating signals over horizontal segments of 1 nautical mile (hereafter mile) and from a depth of 400 m or the bottom (e.g. 50 m) to the near-surface (to the upper buffer boundary). The mean adult length of E. pacifica and T. spinifera off central California and Oregon at an age of 13 months is 22 mm (Marinovic et al., 2002; Feinberg and Peterson, 2003), but because of the similar body dimensions of the two target species, it is not possible to separate them using acoustic data (Fiedler et al., 1998). We calculated the Nautical Acoustic Scattering Coefficient (NASC mile−1) as our basic measurement of horizontal krill distribution and abundance (MacLennan and Simmonds, 2005; Reiss et al., 2008; Santora et al., 2009). This measurement may contain other scatterers of similar size, but as others have suggested, the NASC provides a valuable index for investigating the spatial organization, e.g. relative abundance and clustering, of plankton patches (Hewitt and Demer, 2000; Santora et al., 2009), so is appropriate to the study of krill and krill–predator associations.
Net-based krill indices
In all, 35 fixed stations were sampled using a midwater trawl several times during each annual survey (Brodeur et al., 2003; Sakuma et al., 2006; Figure 1). Net-sampling targeted a headrope depth of 30 m. The dimension of the trawl opening was 12 × 12 m. The net was equipped with a 9-mm codend liner. Typically 5–7 midwater trawls, each of 15 min duration at a target depth of 30 m, were conducted every night between 21:30 and 05:30 Pacific Standard Time.
At sea, following the sorting of fish, krill were volumetrically subsampled (∼5% of each sample) and sorted to species under a dissecting microscope. The total number of krill per haul was estimated by extrapolating the subsample species composition to the total krill volume, which in some cases exceeded 120 l. Krill species regularly caught included E. pacifica and T. spinifera, and less often Nematoscelis difficilis and Nyctiphanes simplex. For this study, we focus on interannual variability of E. pacifica and T. spinifera because they are the most common euphausiids in the northern and central California Current (Brinton, 1962, 1976) and are, as noted above, key to the diet of the seabirds being investigated (Briggs and Chu, 1987; Abraham and Sydeman, 2006). For E. pacifica and T. spinifera, we calculated the catch per unit effort (cpue; number haul−1) using each haul as the sampling unit. Hauls with zero captures were included in the analysis. Overall, 432 hauls conducted from 2002 to 2006 within the region from Pt Reyes to Pt Sur were used for cpue calculations.
Seabird abundance index
Standard strip-transect methods were used to collect data on the abundance and distribution of seabirds (Tasker et al., 1984; for methodological details of this survey, see Yen et al., 2004). Two experienced observers counted birds continuously from the ship's flying bridge (12 m above sea level) during daylight and while the vessel was underway at speeds >5 knots. A rangefinder was used to estimate the width of the survey transect, and only those birds sighted within a 300-m arc from the bow (directly ahead) to 90° off the side with best visibility (e.g. least glare) were logged into a field computer. The relative abundance of seabirds is expressed as number km−2. During the period 2002–2006, the at-sea abundance of sooty shearwaters comprised 36–72% of the total seabird community, whereas Cassin's auklets accounted for just 3–4%.
Statistical analyses
The objective of the study was to develop quantitative indices of interannual variability of krill spatial organization and abundance based on acoustics and net samples and to relate these to the spatial organization and relative abundance of seabirds. Integration of net-hauls, acoustic sampling, and visual surveys of seabirds was carried out in a GIS framework. In addition to position (latitude/longitude), bathymetry data (www.dfg.ca.gov/biogeodata/gis/mr.asp) were used to estimate depth as a covariate to link with continuous underway acoustics and bird distributions.
In the first set of analyses, a general linear model (ANCOVA) was used to test whether acoustically and net-determined krill abundance and seabird abundance (log-transformed) varied among years and between regions (northern vs. southern portions of the study area; Figure 1). The design included year and region as categorical factors, and continuous covariates latitude (°N) and longitude (°W; Legendre and Legendre, 1998). We used a Bonferroni post hoc test to examine differences among years and to test the interaction between year and region. For net-samples, ANOVA was used to test whether the abundance of E. pacifica and T. spinifera varied among years and regions. Mean ± s.e. abundance indices of acoustic and net-sampled krill were compared using Spearman's rank correlation to test for covariation between the sampling techniques. Two indices of krill were used: the mean acoustic index (NASC mile−1) for comparison with seabird abundance and the mean cpue (number haul−1) for net-samples. We tested whether the annual abundance estimates of krill and seabirds covaried, using Spearman's rank correlation.
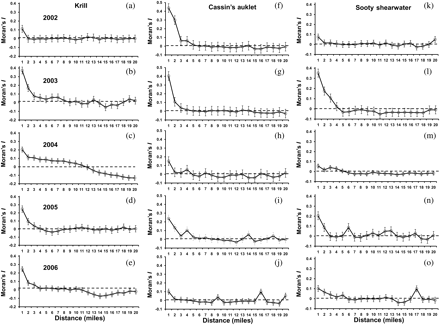
In the second set of analyses, the focus was on describing the spatial variability (organization) of acoustically determined krill and seabirds for the entire study area, so two-dimensional Moran's I correlograms (isotropic) were calculated to examine separately the interannual variability of the spatial autocorrelation of the acoustic krill index and of birds (Legendre and Legendre, 1998). Lag size was defined as an interval of 1 (nautical) mile. The characteristic patch scale was assessed qualitatively by counting the number of lags (≥1) with positive values before becoming negative or close to zero, depending on the standard error estimated by randomizations at each lag (Reid et al., 2004; Yen et al., 2004).
In the third set of analyses, generalized additive models (GAMs) were used to investigate how krill and seabirds covaried in space and whether they responded similarly to geospatial features, e.g. longitude and depth (Wood and Augustin, 2002). A GAM is a non-parametric regression technique useful for investigating non-linear relationships between response variables and covariates, using smoothing terms to fit the model (Wood and Augustin, 2002). GAMs have been implemented successfully in studies relating environmental factors to the spatial distribution of fish (Swartzman et al., 1992), krill (Murase et al., 2009), and seabirds (Wright and Begg, 1997) and are an appropriate tool for investigating the krill–seabird spatial associations in this study. In preparation for GAM fitting, the correlations between latitude, longitude, and depth (from survey trackline data) were examined to determine their relationships, i.e. highly correlated covariates could lead to overfitting of the model. Latitude was significantly correlated with depth (r = −0.8, p < 0.001), which is attributed to the differences between survey effort in the deep waters of Monterey Canyon and the wide shelf region of the Gulf of Farallones in the north (Figure 1). Longitude was not significantly correlated with depth (r = 0.1, p = 0.06). Therefore, we used longitude (east/west variation) and depth as spatial proxies for variation in the position of the shelf break (i.e. nearshore and offshore habitat), which changes markedly along the central California coastline (Figure 1) and is thought to be important habitat for E. pacifica (Brinton, 1962). The fitted GAM for the acoustic krill index was specified with a Gaussian distribution with an identity-link function: krill = year + s(depth) + s(longitude) + te(depth, longitude), where s() and te() are the smooth functions (regression spline) for depth and longitude. To examine the spatial covariation between birds and krill, survey effort for bird observations was linked to underway acoustic krill sampling. Effort for seabird observations did not match acoustic sampling for krill, so required the merging of survey periods when krill and birds were sampled simultaneously (Table 1). The fitted GAM for birds was specified with a Poisson distribution with a log-link function: bird = year + s(depth) + s(longitude) + s(krill) + te(depth, longitude) + te(krill, depth, longitude), where bird refers to the abundance of Cassin's auklet or sooty shearwater. The GAM analysis was carried out using the “mgcv” package (version 1.6–2) in R (R Development Core Team, 2009); the percentage deviance explained and adjusted r2 were determined as indicators of model performance. Model selection procedures were not followed owing to the simplicity of the models, but the effect of each covariate included in the GAM was plotted to inspect the functional form and to determine whether krill and birds exhibited similar peaks or changes in relation to depth and longitude. In addition, the effect of krill on bird density was plotted as a means of quantifying how birds were responding to krill abundance.
Results
Distribution of krill and seabirds
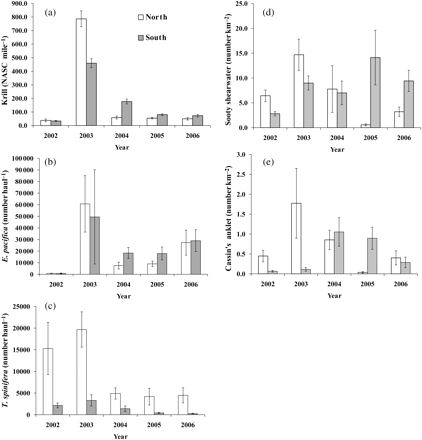
Krill and seabird abundance and spatial distribution varied significantly during the 5-year study, with strong coherence in regional variability (Figure 2). The acoustic index of krill displayed significant interannual and spatial variability (Table 2), with a clear peak in abundance in 2003 (Figure 3); excluding 2003 did not change the results. The significant interaction between year and region (Table 2) for krill and the seabirds indicated that regional variation depended on year. Specifically, in 2003, more krill were found in the north (p < 0.001), and in 2004, more were found in the south (p < 0.001, Figure 3a); there was no difference between regions in other years. Similarly, the at-sea relative abundance of auklets and shearwaters displayed a significant interaction with year and region (Table 2). Although Cassin's auklet was generally more abundant in the north (as expected given the proximity to the large Farallon Islands breeding colony; Figure 3d), the ANCOVA indicated that in 2005 and 2006, more birds were present in the southern than the northern region (p < 0.001). Sooty shearwaters (Figure 3d) were distributed north in 2003 (p < 0.001), and south in 2005 (p < 0.001).
Results of ANCOVA for comparison of interannual variability of abundance for krill, Cassin's auklet, and sooty shearwater in the central California Current, 2002–2006.
| Parameter . | Acoustic krill index . | Cassin's auklet . | Sooty shearwater . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| d.f. . | Mean square . | F-value . | p-value . | d.f. . | Mean square . | F-value . | p-value . | d.f. . | Mean square . | F-value . | p-value . | |
| Intercept | 1 | 4.42 | 1.01 | 0.31 | 1 | 1.49 | 6.51 | 0.01 | 1 | 18.84 | 15.4 | <0.0001 |
| Latitude | 1 | 54.21 | 12.44 | 0.0004 | 1 | 5.70 | 24.78 | <0.0001 | 1 | 3.94 | 3.22 | 0.07 |
| Longitude | 1 | 25.17 | 5.77 | 0.016 | 1 | 4.24 | 18.42 | <0.0001 | 1 | 19.16 | 15.66 | <0.0001 |
| Year | 4 | 2 907.19 | 667.11 | <0.0001 | 4 | 1.65 | 7.18 | <0.0001 | 4 | 31.54 | 25.79 | <0.0001 |
| Region | 1 | 1.07 | 0.24 | 0.62 | 1 | 1.31 | 5.68 | 0.02 | 1 | 1.35 | 1.10 | 0.29 |
| Year × region | 4 | 247.96 | 55.90 | <0.0001 | 4 | 3.13 | 13.56 | <0.0001 | 4 | 15.0 | 12.27 | <0.0001 |
| Error | 7 463 | 4.36 | 3 181 | 0.23 | 3 181 | 1.22 | ||||||
| Parameter . | Acoustic krill index . | Cassin's auklet . | Sooty shearwater . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| d.f. . | Mean square . | F-value . | p-value . | d.f. . | Mean square . | F-value . | p-value . | d.f. . | Mean square . | F-value . | p-value . | |
| Intercept | 1 | 4.42 | 1.01 | 0.31 | 1 | 1.49 | 6.51 | 0.01 | 1 | 18.84 | 15.4 | <0.0001 |
| Latitude | 1 | 54.21 | 12.44 | 0.0004 | 1 | 5.70 | 24.78 | <0.0001 | 1 | 3.94 | 3.22 | 0.07 |
| Longitude | 1 | 25.17 | 5.77 | 0.016 | 1 | 4.24 | 18.42 | <0.0001 | 1 | 19.16 | 15.66 | <0.0001 |
| Year | 4 | 2 907.19 | 667.11 | <0.0001 | 4 | 1.65 | 7.18 | <0.0001 | 4 | 31.54 | 25.79 | <0.0001 |
| Region | 1 | 1.07 | 0.24 | 0.62 | 1 | 1.31 | 5.68 | 0.02 | 1 | 1.35 | 1.10 | 0.29 |
| Year × region | 4 | 247.96 | 55.90 | <0.0001 | 4 | 3.13 | 13.56 | <0.0001 | 4 | 15.0 | 12.27 | <0.0001 |
| Error | 7 463 | 4.36 | 3 181 | 0.23 | 3 181 | 1.22 | ||||||
Region refers to north and south (see Figure 1).
Results of ANCOVA for comparison of interannual variability of abundance for krill, Cassin's auklet, and sooty shearwater in the central California Current, 2002–2006.
| Parameter . | Acoustic krill index . | Cassin's auklet . | Sooty shearwater . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| d.f. . | Mean square . | F-value . | p-value . | d.f. . | Mean square . | F-value . | p-value . | d.f. . | Mean square . | F-value . | p-value . | |
| Intercept | 1 | 4.42 | 1.01 | 0.31 | 1 | 1.49 | 6.51 | 0.01 | 1 | 18.84 | 15.4 | <0.0001 |
| Latitude | 1 | 54.21 | 12.44 | 0.0004 | 1 | 5.70 | 24.78 | <0.0001 | 1 | 3.94 | 3.22 | 0.07 |
| Longitude | 1 | 25.17 | 5.77 | 0.016 | 1 | 4.24 | 18.42 | <0.0001 | 1 | 19.16 | 15.66 | <0.0001 |
| Year | 4 | 2 907.19 | 667.11 | <0.0001 | 4 | 1.65 | 7.18 | <0.0001 | 4 | 31.54 | 25.79 | <0.0001 |
| Region | 1 | 1.07 | 0.24 | 0.62 | 1 | 1.31 | 5.68 | 0.02 | 1 | 1.35 | 1.10 | 0.29 |
| Year × region | 4 | 247.96 | 55.90 | <0.0001 | 4 | 3.13 | 13.56 | <0.0001 | 4 | 15.0 | 12.27 | <0.0001 |
| Error | 7 463 | 4.36 | 3 181 | 0.23 | 3 181 | 1.22 | ||||||
| Parameter . | Acoustic krill index . | Cassin's auklet . | Sooty shearwater . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| d.f. . | Mean square . | F-value . | p-value . | d.f. . | Mean square . | F-value . | p-value . | d.f. . | Mean square . | F-value . | p-value . | |
| Intercept | 1 | 4.42 | 1.01 | 0.31 | 1 | 1.49 | 6.51 | 0.01 | 1 | 18.84 | 15.4 | <0.0001 |
| Latitude | 1 | 54.21 | 12.44 | 0.0004 | 1 | 5.70 | 24.78 | <0.0001 | 1 | 3.94 | 3.22 | 0.07 |
| Longitude | 1 | 25.17 | 5.77 | 0.016 | 1 | 4.24 | 18.42 | <0.0001 | 1 | 19.16 | 15.66 | <0.0001 |
| Year | 4 | 2 907.19 | 667.11 | <0.0001 | 4 | 1.65 | 7.18 | <0.0001 | 4 | 31.54 | 25.79 | <0.0001 |
| Region | 1 | 1.07 | 0.24 | 0.62 | 1 | 1.31 | 5.68 | 0.02 | 1 | 1.35 | 1.10 | 0.29 |
| Year × region | 4 | 247.96 | 55.90 | <0.0001 | 4 | 3.13 | 13.56 | <0.0001 | 4 | 15.0 | 12.27 | <0.0001 |
| Error | 7 463 | 4.36 | 3 181 | 0.23 | 3 181 | 1.22 | ||||||
Region refers to north and south (see Figure 1).
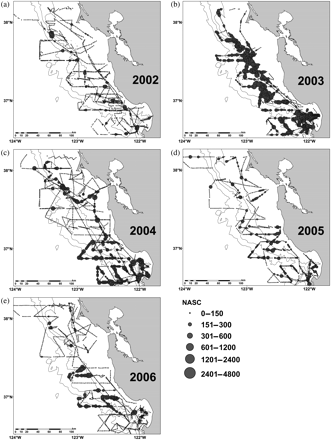
Distribution and abundance of krill estimated by acoustics: (a) 2002, (b) 2003, (c) 2004, (d) 2005, and (e) 2006. The depth contours are 200, 1000, and 2000 m.
Interannual variability of mean (±s.e.) abundance of (a) acoustic krill index, (b) E. pacifica, (c) T. spinifera (number haul−1), (d) sooty shearwaters, and (e) Cassin's auklet (number km−2). North refers to the Gulf of Farallones and south to Monterey Bay.
The cpue of E. pacifica and T. spinifera caught by nets varied significantly among years (F4,423 = 14.55, p < 0.0001, and F4,423 = 54.34, p < 0.0001, respectively). The highest value of cpue was in 2003 (Figure 3b and c). The abundance of E. pacifica increased by some 5 orders of magnitude between 2002 and 2003, decreased by about the same magnitude in 2004, then increased slightly up to 2006 (Figure 3b). Thysanoessa spinifera abundance was greatest in 2002 and 2003, and some 3 orders of magnitude lower during 2004–2006 (Figure 3c). In relation to regions, there was no difference in the cpue of E. pacifica (F1,423 = 0.03, p = 0.86), but the cpue of T. spinifera was higher in the north in all years (F1,423 = 29.11, p < 0.0001).
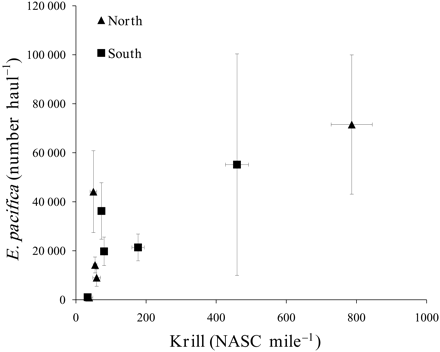
The acoustic krill index was positively correlated with cpue for E. pacifica (ρ = 0.76, p = 0.01; Figure 4), but not for T. spinifera (ρ = −0.27, p = 0.44). Shearwaters were significantly positively correlated with the acoustic krill index (ρ = 0.78, p = 0.007), but not correlated with the cpue of E. pacifica (ρ = 0.55, p = 0.09) or T. spinifera (ρ = −0.17, p = 0.63). Auklets were not correlated with the acoustic krill index (ρ = 0.54, p = 0.11), E. pacifica (ρ = 0.26, p = 0.47), or T. spinifera (ρ = 0.33, p = 0.35).
Association between the relative abundance of E. pacifica and the acoustic krill index, with north being the Gulf of Farallones and south being Monterey Bay.
Spatial organization of and relationships between krill and seabirds
Based on acoustic surveys, the characteristic spatial scale (patch size) of krill was identified. In 2002, krill were weakly spatially autocorrelated, with a characteristic patch size of ∼1–2 miles (Figure 5a). In 2003, the scale of krill patchiness was positively autocorrelated out to 4 miles (Figure 5b). During 2004, the spatial correlation of krill patches displayed the largest characteristic patch scale of 10 miles (Figure 5c). In 2005 and 2006, the characteristic patch scale was 1–3 miles (Figure 5d and e). In general, the characteristic spatial scale of Cassin's auklet and sooty shearwater was similar to krill, ranging from 1 to 3 miles (Figure 5f–o).
Interannual spatial variability (Moran's I correlogram) for the period 2002–2006 for (a–e) acoustic krill index, (f–j) Cassin's auklet, and (k–o) sooty shearwater. The characteristic spatial scale is defined as the number of lags (≥1) with successive positive values before becoming negative or close to zero depending on the standard errors estimated at each lag.
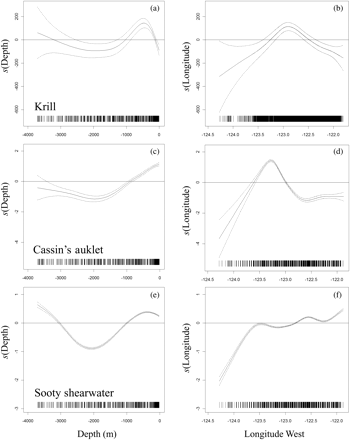
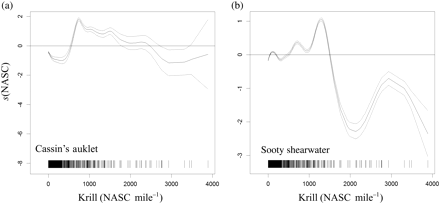
The GAM indicated that the acoustic krill index and at-sea abundance of Cassin's auklet and sooty shearwater were related to depth and longitude (Table 3). In terms of depth, krill and the locally breeding Cassin's auklet displayed similar changes in relation to bathymetry, with a preference for water depth <1000 m (Figure 6a and c). In contrast, the effect of depth on sooty shearwaters showed that birds were utilizing both shelf–slope and oceanic regions (Figure 6e). In terms of longitudinal variation, krill and Cassin's auklet showed similar responses and peaks, suggesting that they generally associated with the position of the shelf break (Figure 6b and d). Sooty shearwaters displayed a more variable response to longitudinal variation, possibly through their use of nearshore and offshore water (Figure 6f). By including krill as a term in the model for seabirds, changes in bird abundance and distribution in relation to krill (Figure 7) were quantified. The GAM indicated that the effect of krill on the spatial distribution and abundance of Cassin's auklet and sooty shearwater was significant (Table 3) and positive (Figure 7), suggesting that birds and krill are spatially associated within similar marine habitats.
GAM results for factors with smoothing terms affecting the acoustic krill index (gamma distribution, log-link function) and those of seabirds (Poisson distribution, log-link function).
| Variable . | Adjusted r2 . | Percentage deviance . | Depth . | Longitude . | Krill . | Depth, longitude . | Krill, depth, longitude . |
|---|---|---|---|---|---|---|---|
| Krill | 0.26 | 26.5 | (6.73)** | (8.83)** | – | (7.50)** | – |
| Cassin's auklet | 0.84 | 76.5 | (8.60)** | (8.10)** | (9.00)** | (21.91)** | (90.2)** |
| Sooty shearwater | 0.41 | 53.6 | (8.98)** | (8.99)** | (8.99)** | (21.19)** | (99.6)** |
| Variable . | Adjusted r2 . | Percentage deviance . | Depth . | Longitude . | Krill . | Depth, longitude . | Krill, depth, longitude . |
|---|---|---|---|---|---|---|---|
| Krill | 0.26 | 26.5 | (6.73)** | (8.83)** | – | (7.50)** | – |
| Cassin's auklet | 0.84 | 76.5 | (8.60)** | (8.10)** | (9.00)** | (21.91)** | (90.2)** |
| Sooty shearwater | 0.41 | 53.6 | (8.98)** | (8.99)** | (8.99)** | (21.19)** | (99.6)** |
Values in parenthesis are the estimated degrees of freedom.
**p < 0.001.
GAM results for factors with smoothing terms affecting the acoustic krill index (gamma distribution, log-link function) and those of seabirds (Poisson distribution, log-link function).
| Variable . | Adjusted r2 . | Percentage deviance . | Depth . | Longitude . | Krill . | Depth, longitude . | Krill, depth, longitude . |
|---|---|---|---|---|---|---|---|
| Krill | 0.26 | 26.5 | (6.73)** | (8.83)** | – | (7.50)** | – |
| Cassin's auklet | 0.84 | 76.5 | (8.60)** | (8.10)** | (9.00)** | (21.91)** | (90.2)** |
| Sooty shearwater | 0.41 | 53.6 | (8.98)** | (8.99)** | (8.99)** | (21.19)** | (99.6)** |
| Variable . | Adjusted r2 . | Percentage deviance . | Depth . | Longitude . | Krill . | Depth, longitude . | Krill, depth, longitude . |
|---|---|---|---|---|---|---|---|
| Krill | 0.26 | 26.5 | (6.73)** | (8.83)** | – | (7.50)** | – |
| Cassin's auklet | 0.84 | 76.5 | (8.60)** | (8.10)** | (9.00)** | (21.91)** | (90.2)** |
| Sooty shearwater | 0.41 | 53.6 | (8.98)** | (8.99)** | (8.99)** | (21.19)** | (99.6)** |
Values in parenthesis are the estimated degrees of freedom.
**p < 0.001.
Fitted GAM results showing the relationship between the covariates water depth (m) and longitude (decimal °W) on changes in (a and b) acoustically determined krill, (c and d) Cassin's auklet, and (e and f) sooty shearwater. Data availability is indicated on the x-axis.
Fitted GAM results showing the relationship between acoustically determined krill abundance and seabirds: (a) Cassin's auklet, (b) sooty shearwater. Data availability is indicated on the x-axis.
Discussion
Krill are the forage base for a wide variety of mid- and upper-trophic-level predators, and their dynamics vital for understanding ecosystem variability and the viability of commercially and recreationally valuable and threatened species in the central CCE. However, until now, despite work on interannual variability in krill relative abundance (Brinton and Townsend, 2003; Sydeman et al., 2006; Jahncke et al., 2008), there have been few studies on the spatial organization of krill and top predators in the central CCE (but see Briggs et al., 1988; Croll et al., 2005; Ainley et al., 2009). Indices of krill abundance and spatial organization were developed here using an integrated approach, coupling acoustic signatures with net surveys and relating them to the distribution of planktivorous seabirds. The acoustically derived krill abundance index was clearly related to the abundance of E. pacifica, and the annual acoustic index was positively related to shearwaters and auklets. There was no relationship between net-based estimates of krill abundance and seabird abundance. In general, over the 5 years studied, the characteristic spatial scale of krill and seabirds was similar (1–4 miles), despite the distribution and abundance of krill displaying marked changes over the study period. Moreover, the use of GAMs to explore the spatial relationships between krill, seabirds, and their environment showed that krill and seabirds are spatially associated within similar habitats, i.e. the shelf–slope region.
Here, we contend that the acoustic signatures reflect mainly the distribution, abundance, and spatial organization of the dominant euphausiid species in the ecosystem, E. pacifica, though the acoustic index does reflect a variety of scatters that may be attributed to plankton organisms of similar size. The contention is based on the significant covariation for indices of relative abundance between acoustic and net-sampling techniques for E. pacifica, and the lack of correspondence between acoustic statistics and T. spinifera. Few studies have found accord in net and acoustic estimates of krill abundance (Mackas et al., 1997; Reiss et al., 2008), nor pinpointed the species that are important in multispecies euphausiid systems. The relative abundance in net samples of N. simplex or N. difficilis was not assessed against acoustically based NASC estimates, because those two species are scarce in the region during May and June (Brinton and Townsend, 2003), so would not have accounted for much of the acoustic signal. Euphausia pacifica is numerically dominant in the region, and more generally in the CCE (Brinton, 1962). Less is known about the population dynamics of T. spinifera, but it is generally thought to favour inner shelf waters <180 m deep (Feinberg and Peterson, 2003). Our net-sampling supports this hypothesis, with 86% of all T. spinifera taken in water depths <150 m (NMFS, unpublished data). In contrast, E. pacifica is distributed over a wider range of depths; ∼40% of our catches were on the shelf, whereas the rest were farther offshore. The relative difference in the relationship between acoustic estimates and the cpue of these species could also be related to a bias in the surveys to outer shelf habitat, where E. pacifica is most prevalent. Also, Smith and Adams (1988) found that T. spinifera swarmed at the surface during daylight in the Gulf of Farallones; surface swarms would not be detected by hull-mounted echosounders and probably would not be well-sampled during net operations even if they were in the correct habitat for T. spinifera (Feinberg and Peterson, 2003). Despite the lack of association between acoustic indices and the cpue of T. spinifera, the relative abundance of T. spinifera was highest in 2002 and 2003 and decreased in later years. This interannual variability relates to the breeding success of some seabirds, notably auklets, in the region, and may have been responsible for the unprecedented breeding failure of auklets in 2005 (Sydeman et al., 2006).
Krill and seabird spatial organization
Seabirds have been advanced as indicators of change in the abundance of prey populations (Cairns, 1987), and the relationships between krill and krill predators have been well-studied in the Southern Ocean. Indeed, the relationships between krill and predator breeding performance and foraging behaviour have and are playing a large role in the Convention for the Conservation of Antarctic Marine Living Resources (CCAMLR) ecosystem monitoring programme, in which krill predators serve as indicators of krill distribution (Reid et al., 2005; Hill et al., 2009). As in past studies on krill spatial distribution and its relationship with krill predators in the Southern Ocean and California system (Veit et al., 1993; Fiedler et al., 1998; Reid et al., 2004, 2005; Croll et al., 2005; Santora et al., 2009, 2010), we sought to examine the hypothesis that the spatial organization, i.e. patchiness, of krill and seabirds is related.
A clear advantage in using acoustic surveys coupled with net-sampling and seabird surveys lies in the ability to map and measure patch characteristics over a range of spatial and temporal scales (Hewitt and Demer, 2000). Using an index of spatial clustering (Moran's I), we determined that the characteristic spatial scale of krill and seabirds was similar (generally 1–4 miles) over the 5 years studied. Although this has been documented for other ecosystems (Fauchald and Erikstad, 2002; Santora et al., 2009, 2010), this is the first study to describe krill and krill–predator patchiness in the CCE over multiple years. Interestingly, the spatial organization of krill was similar in years of high and low abundance of krill, indicating that it is possible to observe low krill abundance and simultaneously to detect spatial clustering, or conversely, a few locations containing a relatively high abundance of krill (Figure 2). This observation has been made previously in the Southern Ocean (Santora et al., 2009), as well as in studies of seabirds and schooling fish in the Barents Sea (Fauchald and Erikstad, 2002), and it has important implications both for the resilience of krill populations and for the foraging ecology of krill predators. If krill abundance is low, and there are fewer patches, it seems reasonable to assume that krill predators would face an energetically costly foraging situation (Santora et al., 2009). However, if, as has been described here and elsewhere, krill abundance is low but clustered (high patchiness), predator–prey relationships may be maintained depending on the abilities of the predators to find and use isolated patches. If under a situation of low abundance, patches are located in the right place, the predator's foraging success may be good, with consequences for predator demography. Indeed, that appears to have been the case in 2002 when krill were relatively less abundant, yet auklets breeding on the Farallon Islands demonstrated the best productivity values on record (Sydeman et al., 2006). From the perspective of krill, there may be locations where krill concentrate and that are perhaps predictable, even when overall conditions are unfavourable for growth, reproduction, and recruitment. Those locations may depend on the coupling of favourable hydrographic (e.g. fronts) and benthic (e.g. bathymetric discontinuities, submarine canyons) habitat characteristics (Mackas et al., 1997; Marinovic et al., 2002) that promote the spatial persistence of krill geographically on seasonal and annual scales.
In addition to demonstrating that the characteristic spatial scale of krill and seabirds was similar within and between years, we found considerable geospatial covariation in the aggregative response of krill and seabirds to broad-scale habitat characteristics, depth, and longitude. The GAM indicated that krill abundance declined in water depths >500 m and were centred around 123°W, the location of the widest shelf–slope in the study area. Similarly, Cassin's auklet, an obligate plankton-feeder and resident breeder in the Gulf of Farallones, displayed the same response to changes in depth and longitude that were found for krill. Sooty shearwaters demonstrated a more variable response to depth and longitude and tended to occupy shelf and oceanic waters. This difference between auklets and shearwaters is likely attributable to shearwaters being seasonal visitors that migrate to higher latitudes (i.e. Gulf of Alaska) or track and target offshore populations of pelagic fish. Moreover, the GAM fitted to seabirds showed that krill had a positive effect on the abundance and distribution of auklets and shearwaters, associating seabirds with krill distribution, but how the birds track fine-scale spatio-temporal changes (over days) in krill distribution or the importance of krill hotspots is uncertain. However, the results do provide insight into geospatial processes that may be useful for spatial management of the northern and central CCE, although more work is required to determine whether there are predictable locations where krill patches persist on seasonal and annual time-scales.
The spatial organization of krill described here deserves further investigation and evaluation relative to the response of other krill predators, e.g. fish and whales, in conjunction with studies of seabirds. There have been advances in understanding of krill patch dynamics in the CCE through studies involving foraging baleen whales. In the Southern California Bight, Fiedler et al. (1998) investigated the relationships between blue whales (Balaenoptera musculus) and krill distribution and found that whales selectively fed on E. pacifica along the 200-m isobath. The results here are in keeping with these findings on the importance of the shelf-break/slope region to E. pacifica and foraging planktivorous predators. Studies by Fiedler et al. (1998) and Croll et al. (2005) also found that blue whales preferentially targeted patches of adult krill, showing that the age and sexual maturity of krill may be an important component for understanding predator–prey interactions. Therefore, information on krill size, condition, and sexual maturity may provide additional insight on the importance and significance of spatial organization of krill and krill predators. Long-term data are available to link information on krill demography and growth to the at-sea spatial organization of krill predators in this system (Brinton, 1962; Marinovic et al., 2002; Brinton and Townsend, 2003). In the Southern Ocean too, studies of krill demography have important implications for the interannual variability of krill biomass (Reiss et al., 2008) and the breeding performance and survival of land-based krill predators (Reid et al., 2005). This identifies a topic for future research in the California Current study system too.
Conclusions
The abundance and spatial organization of krill in the northern and central California Current are dynamic and have undergone substantial fluctuations recently. This may be attributed to ocean climate, specifically variability in upwelling (Schwing et al., 2006) or possibly advective processes that may transport krill into or out of the region or influence local reproductive success and recruitment in these populations (Brinton, 1976; Tanasichuk, 1998; Marinovic et al., 2002; Brinton and Townsend, 2003; Feinberg and Peterson, 2003). Spatial patterns of krill may be useful in testing spatial matches and mismatches between krill and their predators, and these in turn could play a role in better understanding of fish and seabird population fluctuations.
Moreover, monitoring the spatial organization of krill may be useful in ecosystem-based approaches to management (Reid et al., 2005; Field et al., 2006; Hill et al., 2009), including place-based management (e.g. Marine Protected Areas), and fisheries management (e.g. integrating krill information in stock assessment). Indeed, Field and Francis (2006) reported that a large proportion of the energy flux in the CCE flows through krill, underscoring the critical role krill play in regulating ecosystem productivity. Currently in the CCE, there is no framework to account for seasonal or interannual spatial variability of krill and the probable consequences on fish, seabirds, and marine mammals. Models that include both a temporal and a spatial component of krill (Hill et al., 2009) in the CCE would therefore provide information on the potential energy transfer to protected and commercially important species in the CCE.
Acknowledgements
We thank members of the NOAA–NMFS JRS team, including Keith Sakuma, Ken Baltz, and John Field, as well as Baldo Marinovic, for providing information from net data on relative abundance. Acoustic processing was greatly enhanced through collaboration with the NOAA–NMFS US Antarctic Marine Living Resources programme, and we are grateful for the assistance and support of Anthony Cossio and Christian Reiss in facilitating the acoustic analyses. We also thank several anonymous reviewers and Keith Reid for suggestions that greatly improved the paper. Funding for the project was made possible through a grant to WJS and Steven J. Bograd from the California Ocean Protection Council and California Sea Grant (OPC-ENV-07) and to Francisco Chavez and Brian Wells from NASA (project NNX09AU39G).