-
PDF
- Split View
-
Views
-
Cite
Cite
Jon Egil Skjæraasen, Justin J. Meager, Ørjan Karlsen, Jeffrey A. Hutchings, Anders Fernö, Extreme spawning-site fidelity in Atlantic cod, ICES Journal of Marine Science, Volume 68, Issue 7, July 2011, Pages 1472–1477, https://doi.org/10.1093/icesjms/fsr055
Close - Share Icon Share
Abstract
Based on a 3-year mark-recapture study, evidence is provided of spawning-site fidelity in Atlantic cod (Gadus morhua) at a scale (<1 km) smaller than documented previously. Coastal regions where barriers to dispersal exist may allow for local population dynamics and adaptation to develop in broadcast-spawning marine fish at extremely fine spatial scales.Skjæraasen, J. E., Meager, J. J., Karlsen, Ø., Hutchings, J. A., and Fernö, A. 2011. Extreme spawning-site fidelity in Atlantic cod. – ICES Journal of Marine Science, 68: 1472–1477.
Introduction
Spatial scale is of basic importance to population dynamics and local adaptation. Knowledge of the geographic area within which population dynamic processes influence the mean and variance of per capita rates of population growth and within which genetic change in response to selection is likely is of crucial importance to resource managers, conservation biologists, and evolutionary ecologists.
The spatial scales at which these processes arise in widely distributed marine fish are poorly understood (Waples, 1998), although knowledge has been gained in the past decade (Hauser and Carvalho, 2008), particularly for species such as Atlantic cod, Gadus morhua. A broadcast-spawning species that provides its offspring with no parental care, cod are distributed throughout the North Atlantic. Putatively different populations, or stocks, have been managed for decades at broad spatial scales concordant with boundaries established by fisheries scientists in the mid-20th century (Halliday and Pinhorn, 1990). Following early studies of population structure based on analyses of variables such as vertebral counts (Templeman, 1981) and allozymes (Cross and Payne, 1978), it was perhaps not until the studies of Ruzzante et al. (2000) and Hutchinson et al. (2001) off Newfoundland and the British Isles, respectively, that it was appreciated that greater degrees of reproductive isolation could exist at considerably smaller spatial scales than thought previously. Smaller-scale (at distance of tens of kilometres) genetic variability has since been documented among cod inhabiting different fjords (based on in microsatellite DNA, μDNA; Knutsen et al., 2003) and even between cod inhabiting different parts of the same fjord (PAN I locus; Sarvas and Fevolden, 2005) in coastal Norway. Hutchings et al. (2007) documented genetic differences in key life-history traits at spatial scales not resolvable by μDNA in Canada, and Bradbury et al. (2010) found potentially adaptive associations between molecular markers and water temperature at various scales, using analyses of single-nucleotide polymorphisms throughout the species' range.
Notwithstanding the research undertaken thus far, many questions remain concerning cod population structure. One of these can be articulated as what are the smallest spatial scales at which population dynamic processes and adaptation arise in Atlantic cod? It was this question that served as primary motivation for this study of cod on the Norwegian west coast. Specifically, for a period encompassing several years, we studied the movements and homing behaviour of individual cod that comprised a spawning aggregation at Osen, Austevoll (60°04′N 05°13′E), a semi-enclosed embayment with a surface area of just ∼0.5 km2.
Material and methods
In spring 2007, 143 cod were released at Osen as part of a project examining the interactions between wild and farmed cod. Of these, 48 were implanted with ultrasonic tags and tracked with a stationary positioning system (VRAP, Vemco) for the duration of the 2007 spawning season (see Meager et al., 2009, 2010, for the results). Here, we combine new data obtained from a network of ultrasonic receivers (VR2W, Vemco) and fishing recaptures to analyse the broad-scale movement patterns and the subsequent fate of those cod up to March 2009.
Wild Atlantic cod were sourced from local fishers, using cod traps and nets in the immediate vicinity of the spawning ground (Figure 1), from December 2006 to early February 2007. After capture, fish were held in net-pens ∼3 km from the release site until tagging and subsequent release at the study site. F1-generation farmed fish were obtained from a commercial cod farm located at Fitjar (59°58′N 05°21′E). In January 2007, the farmed fish were transferred to two sea pens next to those containing the wild cod.
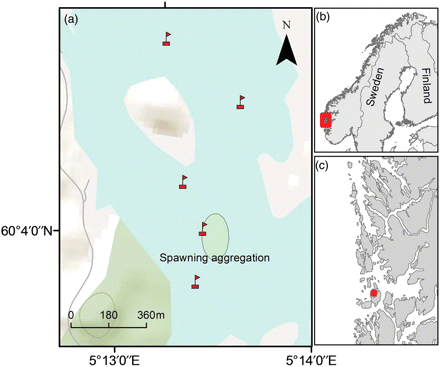
Position of the VR2W receivers when deployed in December 2007. (a) The area marked with an oval represents the main spawning aggregation in the 2007 spawning season (Meager et al., 2010). The two northernmost receivers are considered to have been placed outside the spawning ground proper. Between April 2008 and September 2008, three of the receivers disappeared from the study area, and only the receiver placed directly above the spawning ground and the northernmost receiver remained. During the balance of the study, the former northernmost receiver was redeployed at the approximate position of the missing former southernmost receiver. (b) Position of the study site on the Norwegian coast and (c) map showing the greater study area in western Norway.
Tagging, release, and monitoring
In mid-February 2007, all cod were sedated with benzocaine (0.5 g l−1), measured for weight and length, and their sex determined by ultrasound (following Karlsen and Holm, 1994). All fish (Table 1) were tagged externally beneath the dorsal fin with two individual tags; one possessed an identifier code only, and the other tag contained an identifier code and the text “The University of Bergen”. In addition, 12 fish of each type and sex were implanted with an ultrasonic transmitter. These were either continuous transmitters (Vemco V16P-4H, n = 8; Table 1) or individually coded transmitters (Vemco V16P-4H S256, n = 40; Table 1) that allow a large number of fish to be tracked on the same frequency (69 kHz). Tags weighed 12 g in water (diameter 16 mm, length 71 mm). Coded tags had a random delay of between 150 and 250 s between transmissions to minimize signal collisions. The coded tags had an expected battery life of ∼10 years, but the continuous transmitters lasted just ∼60 d. Given that the present study focuses on acoustic data recorded from November 2007 through March 2009, the results relate only to data obtained from the coded tags.
Length and weight of the cod released according to tagging category.
| Tag type . | Farmed cod . | Wild cod . | ||||
|---|---|---|---|---|---|---|
| Weight (g) . | Length (cm) . | Number released/recaptured . | Weight (g) . | Length (cm) . | Number released/recaptured . | |
| External only | 2 733 | 57.9 | 69/23 | 2 408 | 60.6 | 26/10 |
| Coded | 3 160 | 61.5 | 20/6 | 3 135 | 65.6 | 20/11 |
| Continuous | 3 396 | 63.0 | 4/2 | 2 931 | 65.3 | 4/2 |
| Tag type . | Farmed cod . | Wild cod . | ||||
|---|---|---|---|---|---|---|
| Weight (g) . | Length (cm) . | Number released/recaptured . | Weight (g) . | Length (cm) . | Number released/recaptured . | |
| External only | 2 733 | 57.9 | 69/23 | 2 408 | 60.6 | 26/10 |
| Coded | 3 160 | 61.5 | 20/6 | 3 135 | 65.6 | 20/11 |
| Continuous | 3 396 | 63.0 | 4/2 | 2 931 | 65.3 | 4/2 |
Length and weight of the cod released according to tagging category.
| Tag type . | Farmed cod . | Wild cod . | ||||
|---|---|---|---|---|---|---|
| Weight (g) . | Length (cm) . | Number released/recaptured . | Weight (g) . | Length (cm) . | Number released/recaptured . | |
| External only | 2 733 | 57.9 | 69/23 | 2 408 | 60.6 | 26/10 |
| Coded | 3 160 | 61.5 | 20/6 | 3 135 | 65.6 | 20/11 |
| Continuous | 3 396 | 63.0 | 4/2 | 2 931 | 65.3 | 4/2 |
| Tag type . | Farmed cod . | Wild cod . | ||||
|---|---|---|---|---|---|---|
| Weight (g) . | Length (cm) . | Number released/recaptured . | Weight (g) . | Length (cm) . | Number released/recaptured . | |
| External only | 2 733 | 57.9 | 69/23 | 2 408 | 60.6 | 26/10 |
| Coded | 3 160 | 61.5 | 20/6 | 3 135 | 65.6 | 20/11 |
| Continuous | 3 396 | 63.0 | 4/2 | 2 931 | 65.3 | 4/2 |
In late February 2007, 50 (25 females and 25 males) wild and 93 (48 females and 45 males) farmed cod (Table 1) were released at Osen. During the initial 2007 field season, fishers were encouraged not to fish on the spawning ground, although this encouragement did not extend to 2008 or 2009. Water current and temperature data were recorded at 10-min intervals during the study period by a flowmeter (SD6000, Saiv A/S, Bergen, Norway) located 30 m deep at the centre of the study site (15 m from the seabed).
From 14 December 2007 to 27 March 2009, stationary acoustic receivers/hydrophones (VR2W, Vemco) were deployed at the Osen spawning ground (Figure 1). These record ultrasonic signals transmitted within a certain range (∼600–700 m radius), which varies with factors such as ambient oceanic/weather conditions and bottom topography. The location of the spawning ground within a partially enclosed bay ensured that transmissions from fish on the spawning ground were only registered by receivers placed there (Figure 1), a distinction that could be verified by calibrating the system with a range-testing tag and using a sentinel tag positioned on the spawning ground. The sentinel tag (Vemco V16P-4H S256) was located ∼150 m northwest of the southernmost hydrophone (Figure 1). None of the 139 052 signals recorded from this tag during the study was obtained by the receivers positioned outside the spawning ground. The range-testing tag (Vemco V16P-4H) emitted an ultrasonic signal every 10 s at the same frequency and output power as the coded tags. It was fastened to a fishing line and towed in a grid over the spawning ground on 14 December for ∼1 h. We then examined the recordings obtained from various separate positions and confirmed that all ultrasonic signals transmitted on the spawning ground were received by at least one of the three hydrophones located on the spawning ground, but not by the two hydrophones located outside the spawning ground (Figure 1). Ultrasonic recordings were augmented with location data provided by local fishers who had been made aware of our offer to provide them with a monetary reward for returning tagged fish.
Data analyses
The distances moved by individual cod from release to recapture sites were estimated as the shortest swimming distance between the two sites. To explore the potential influences of fish dispersal, we employed linear modelling. In the initial model, the distance moved was treated as the response variable, and fish type and sex and their interaction as categorical explanatory variables together with the continuous explanatory variable time at liberty before recapture, i.e. days since release (ln-transformed). We also investigated whether there was a difference in the likelihood of recapture between (i) fish type, (ii) sexes, and (iii) tagging type (i.e. external only vs. internal + external). This was done by modelling the proportion of fish caught by logistic regression incorporating all these categorical variables and their interactions as explanatory variables. In both logistic regression and linear models, nested log-likelihood ratios (G2) were used to test for the significance of explanatory variables and to arrive at the final model (Faraway, 2006). All analyses were carried out using R (version 2.10.1; R Development Core Team, 2010).
Results
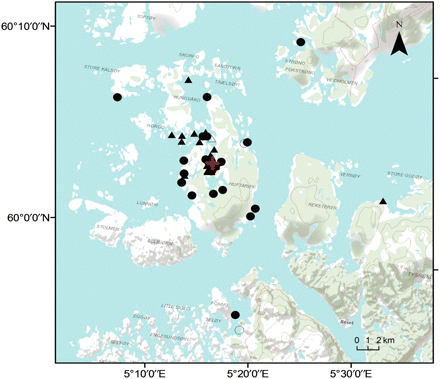
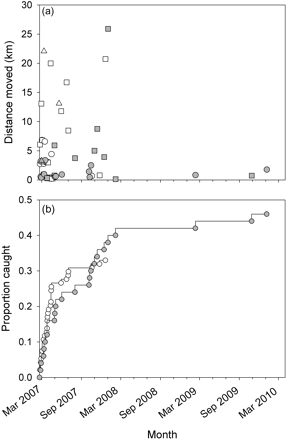
As of 31 March 2010, we had recovered 23 (12 female and 11 male) and 31 (14 female and 17 male) wild and farmed cod, respectively (Table 1, Figure 2). Broadly comparing recapture locations for the two types, 13% of the recaptured wild cod and 32% of the recaptured farmed cod were caught >5 km from the release site (Figure 3a). Male recapture distances were significantly greater than those of females (linear model, d.f. = 51, t = 2.93, p< 0.01); farmed males were recaptured on average 6.6 km from the release site and farmed females on average 2.1 km from the same point (Figure 3a); the corresponding numbers for wild fish were 5.3 vs. 1.4 km (Figure 3a). There was no association between distance moved from the release site and time at liberty or fish type (both p > 0.50). However, the latter estimate was heavily influenced by a wild male that had moved far from the release site (Figure 3a). If that fish was omitted from the analysis, there was a non-significant tendency for farmed cod to be recaptured farther from the release site than wild fish (linear model, d.f. = 49, t = 1.59, p = 0.12).
Recapture positions for wild (triangles) and farmed (circles) cod. Open symbols mark the second recapture location of individual cod released alive after their first recapture, and the red cross indicates the release location.
(a) Distance moved from the site of release, and (b) the total proportion of recaptured fish through time. Open and grey symbols denote farmed and wild fish, respectively, and squares and circles male and female cod, respectively. The three triangles in (a) represent the second recapture position of three fish that were released alive after we had been informed of their initial recapture position; these positions were not included in any analyses.
Initially, farmed cod tended to be recaptured with greater frequency than wild fish, but towards the end of the experiment proportionally more wild cod had been caught, although this difference was not statistically significant (χ2 test, p = 0.74; Figure 3b). Notably, whereas wild fish were recaptured between 19 February 2007 and 6 January 2010, no recaptures of farmed fish were reported after November 2007 (Figure 3b). Overall, fish type, sex, or tagging method did not influence the likelihood of recapture.
Ultrasonic telemetry
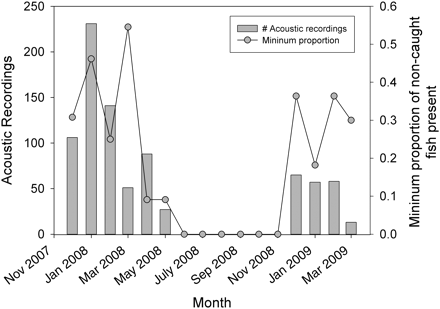
The data for fish tagged with ultrasonic transmitters showed a striking temporal pattern and were almost exclusively confined to wild cod (Figure 4). After receiver deployment in 2007, many of the fish were recorded on the spawning ground for several months, but this record decreased abruptly after the end of the presumed spawning period in March; from June 2008 to the end of November 2008, there was not a single ultrasonic recording. However, in December 2008, several fish reappeared on the spawning grounds and many recordings were made up to the time the receivers were retrieved in late March 2009 (Figure 4). Minima of 55 and 37% of the ultrasonically tagged wild fish still at liberty were present on the spawning ground in 2008 and 2009, respectively. Only 1 and 2 recordings (two different fish) were derived from farmed cod in 2008 and 2009, respectively, during the entire deployment period of the stationary receivers (data not shown). All ultrasonic recordings were obtained from receivers placed inside the spawning ground proper (Figure 1).
Acoustic recordings of wild fish at the Osen spawning ground. Shown are the total number of acoustic signals detected in different months, and the minimum proportion of fish equipped with acoustic transmitters still at large that were detected in a given month, i.e. this number is higher if there had been any natural mortality or unreported catches.
Discussion
The results of the study have identified the potential for genetic differentiation and population structure on a spatial scale considerably finer than that suggested previously for Atlantic cod. In this area of western Norway, for which the coastal waters are characterized by significant physical heterogeneity generated by many islands, bays, and fjords, the data suggest that this broadcast-spawning marine fish may, under some situations, be structured as spatially disparate breeding aggregations at scales of less than a few kilometres (see also genetic studies of Knutsen et al., 2003, 2007). Although the potential for dispersal is great during pelagic egg and larva stages (which can last for 2–3 months; Otterlei et al., 1999), dispersal distances during these early life stages are presumably inversely related to the probability of entrainment effected by local oceanographic features, such as water currents (Espeland et al., 2007; Ciannelli et al., 2010). The Osen spawning grounds are located several kilometres from the open ocean, the prevailing tidal current is weak (0–6.8 cm s−1), and the area is surrounded by islands on all sides (Figure 1). Under such conditions, we would expect dispersal of early life stages to be relatively modest and the behaviour of juvenile and adult cod to be important in producing locally distinct spawning aggregations. Bottom-dwelling juvenile cod, for example, exhibit relatively limited movements (Côté et al., 2003; Bradbury et al., 2008), and spawning adults exhibit strong fidelity and homing to spawning grounds (Robichaud and Rose, 2001; Windle and Rose, 2005). Although advection of eggs and larvae would be expected to be greater from areas less enclosed than this study site, egg retention is a characteristic of most spawning grounds of Norwegian coastal cod (Knutsen et al., 2007; Ciannelli et al., 2010). We therefore do not believe the Osen spawning ground to be unique in this respect.
Although natal homing and spawning-site fidelity has been documented throughout the species' range (Robichaud and Rose, 2001; Wright et al., 2006; Svedang et al., 2007), we are unaware of such fidelity on as small a geographic scale as documented here. Almost all recaptures of wild cod in the present study were within a few kilometres of the release site, and cod continued to be recaptured there 3 years after initial release at or about the time of spawning. Notwithstanding the likely spatially biased fishing effort (on the spawning aggregation), the large proportional representation of recaptured cod at or near the spawning grounds and the large proportion of acoustically tagged wild fish present at the spawning ground in both 2008 and 2009 are consistent with the hypothesis that cod in the area exhibit homing and fidelity to these spawning grounds. In fact, the actual proportion of fish on the spawning ground is likely higher because we do not have estimates of the reporting rate of recaptures or natural mortality, and we have accordingly not applied any correction to our data to account for these factors. In contrast to wild cod, farmed fish appear to attempt to join spawning shoals if they locate them post-escape, but do not establish themselves in local areas in the long run. Although farmed fish were present shortly after release during the 2007 spawning season (Meager et al., 2010), the recaptures of farmed fish tended to be made farther from the release site than those of wild fish and ceased after December 2007, results that were closely matched by data obtained from the acoustic transmitters. Interestingly, wild fish departed from the Osen area shortly after the end of the putative spawning period in March, but then reappeared there ∼2 months before the onset of the next spawning period. The area therefore appears to function mainly as a spawning area.
The data also suggest sex-differentiated post-spawning dispersal by adults. The recapture distances of males were greater than for females. Differences in dispersal rates between sexes are common among birds and mammals (Perrin and Mazalov, 1999). Much less is known of sex-biased dispersal in fish, but male brook trout of reproductive age disperse greater distances than females (Hutchings and Gerber, 2002). We are unaware of any other data suggesting that post-spawning dispersal distances may be greater among males than females in Atlantic cod. Although it has been hypothesized that the cod mating system is characteristic of that described by a lek (Morgan and Trippel, 1996; Hutchings et al., 1999; Nordeide and Folstad, 2000), it is not clear whether the more-intensive breeding activity expected of males in a lek-based system would also be associated with greater dispersal after spawning. However, if fishers were less likely to report captures farther from the study site, as may be the case, this could arguably affect the interpretation of the recapture results, so they should be treated with some caution.
Although not all the cod released were observed at the spawning ground 2 years post-release, the results of this work do provide evidence of homing and spawning-site fidelity at a spatial scale smaller than documented previously for Atlantic cod. Such philopatry provides a necessary condition for the evolution of local adaptations. Therefore, our work provides an empirical basis for exploring the potential existence of genetic differentiation in broadcast-spawning marine fish at finer scales than has been examined to date, particularly in coastal regions where geographic barriers to dispersal may exist.
Acknowledgements
The study was approved by the Norwegian Council for Animal Research. We thank the staff at Austevoll research station for help with the husbandry and sampling of fish, and T. Nilsen for statistical advice. Two anonymous reviewers provided valued comments on the submitted draft. The study was funded by Norwegian Research Council project 172649/S40, 177744/V40 and 190228.