-
PDF
- Split View
-
Views
-
Cite
Cite
P-M. Chouinard, J-D. Dutil, The structure of demersal fish assemblages in a cold, highly stratified environment, ICES Journal of Marine Science, Volume 68, Issue 9, September 2011, Pages 1896–1908, https://doi.org/10.1093/icesjms/fsr125
Close - Share Icon Share
Abstract
Fish are a major component of marine ecosystems, with many species co-occuring in the same habitats. Potential interactions among species and with the environment can be studied through the identification of species assemblages. Data from bottom trawl surveys (2004–2008) conducted in the estuary and northern Gulf of St Lawrence were analysed using multivariate methods (cluster, multidimensional scaling, and detrended canonical correspondence analysis) to describe the structure and composition of demersal fish assemblages, including rare and smaller non-commercial species. The spatial variability in environmental conditions that characterizes the study area has a significant impact on the composition of fish assemblages in the region. In all, 35 taxa were classified as key, and 6 main fish assemblages were described, based on catch in numbers. These assemblages had a coherent spatial distribution in the study area, associated with either depth, salinity and temperature, or dissolved oxygen. The analyses showed overall strong correlations between species abundance and prevalent environmental conditions and explained 18.4% of the variance in species abundance data and 79.2% of the variance in the species–environment relationship.Chouinard, P-M., and Dutil, J-D. 2011. The structure of demersal fish assemblages in a cold, highly stratified environment. – ICES Journal of Marine Science, 68: 1896–1908.
Introduction
The St Lawrence marine environment, by virtue of its size and the large amounts of freshwater discharge that it receives, is complex (Therriault, 1991). Tides and gyres give rise to a highly dynamic current system creating turbidity zones where visibility is limited. The system is vertically stratified (two layers in winter, three in summer) and characterized by water masses of varying density. Low salinity conditions prevail in the estuary and near the surface. Ice coverage varies from year to year, with low air temperature in winter giving rise to a permanent cold intermediate layer (CIL), with core temperatures <0°C in some locations (Galbraith et al., 2008). Nutrient-rich recharge areas at the head of deep channels are characterized by chronic hypoxic conditions (Gilbert et al., 2005) that have an adverse affect on the survival (Plante et al., 1998), growth (Chabot and Dutil, 1999), and swimming capacity of fish (Dutil et al., 2007). The seafloor has a variable geomorphology, with slopes, plateaus, and deep channels dotted with crests and humps. Depending on depth, it is overlaid by the surface layer, the CIL, or a mixture of cold Labrador Current and warm Gulf Stream waters. Maximum depth is 520 m (Dutil et al., 2011). These features, combined with strong seasonal variation in environmental conditions, result in a wide range of ecological niches likely to be suitable for many species originating from Arctic, Subarctic, and temperate environments (Dutil et al., 2009).
Species assemblages are acknowledged to be an important feature of marine ecosystems and contribute to shaping their structure, diversity, and stability (Francis et al., 2002). The term assemblage describes the set of species present at a defined location, whether or not there are interactions among them (Wootton, 1998). The description of species assemblages generates a great deal of interest because of its potential as a tool for characterizing and understanding interactions among species and relationships between species and their environment (Snelgrove and Haedrich, 1985; Mahon et al., 1998; Brown, 2000; Auster et al., 2001; Francis et al., 2002). Assemblage patterns are also a useful parameter to describe and monitor biodiversity. As direct and indirect interactions among species are often difficult to observe in the field, fish assemblage studies may reveal the existence of previously unsuspected connections among species and may provide information about both the structure and the quality of the habitat in which they live (Brown, 2000). Species belonging to the same assemblage tend to exhibit similar responses to environmental conditions (Cech et al., 1990; Legendre and Legendre, 1998; Brown, 2000). Studies on fish and environmental conditions in the St Lawrence have mostly dealt with individual species and focused mainly on the factors explaining distribution and abundance or different aspects of stock productivity (Castonguay et al., 1999; Dutil et al., 1999; Dutil and Brander, 2003). The connections between commercial species and functional groups have also been explored in terms of foodwebs and mass-balance models (Savenkoff et al., 2004). No study has yet examined species assemblages, or the connections between species assemblages and environmental conditions.
Diverse and severe environmental conditions affect to varying degrees the fish species in the St Lawrence. They should also have a marked effect on the composition of fish assemblages in the area. Demersal fish catches in bottom trawl surveys (2004–2008) conducted in the estuary and northern Gulf of the St Lawrence were examined using multivariate analysis methods [cluster, multidimensional scaling (MDS), and detrended canonical correspondence analysis (DCCA)]. We hypothesized that catches would reflect several different fish assemblages, with each assemblage being associated with a unique set of environmental conditions. Maintaining species diversity and anticipating the impacts of climate change on fisheries have become priorities. In this context, it is crucial to describe the current profile of fish assemblages and to understand how environmental factors structure them.
Material and methods
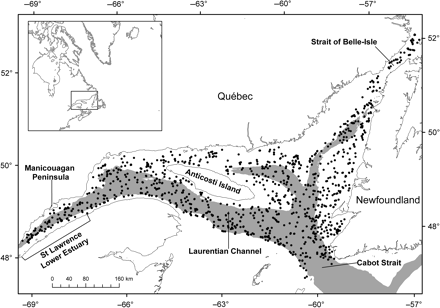
The study area extends over the entire lower estuary and the northern portion of the Gulf of St Lawrence, to the Strait of Belle Isle in the north, and south along the Laurentian Channel to Cabot Strait (Figure 1). The Department of Fisheries and Oceans (Canada) conducts annual research surveys in the study area to assess the abundance of northern shrimp and demersal fish of commercial importance. The survey follows a random stratified scheme with the level of effort in each depth stratum proportional to the relative size of the stratum. Between 2004 and 2008, the survey was conducted in August, using a large trawler (CCGS “Teleost”) and a Campelen shrimp trawl with a codend liner stretched mesh size of 12.7 mm; 870 stations were sampled at depths ranging from 41 to 513 m (Bourdages et al., 2007). Fish were identified to species, and the numbers caught were recorded. Bottom salinity and temperature and station depth were taken from a CTD Seabird (SBE911 Plus) profile at the different stations. Data for dissolved oxygen come from a consisting of a variety of sources. In 2004 and 2005, dissolved oxygen was measured using the Winkler method. The trawl was also equipped with an experimental probe comprising a datalogger, pressure and temperature probes, and a dissolved oxygen sensor (Aanderaa optode model 3830). At some stations, data were missing and were obtained from nearby stations or calculated by interpolation using a co-kriging method, with depth as a covariate, using ArcGIS software (version 9.2, ESRI, Inc.). More information on survey design and sampling protocols can be found in published reports (Bourdages et al., 2007; Nozères et al., 2010).
Map of the study area showing the lower estuary and northern Gulf of St Lawrence. The locations of trawl sets are shown as black dots. The grey area indicates the channels (depth >250 m).
This study examines catch composition and abundance at species level. In some cases, however, specimens were not identified to species for various reasons (species prone to damage by the trawl, no reliable or practical field method for identification). In such cases, data were treated within groups of species. Some were grouped at a family level, e.g. Myctophidae, Sternoptychidae, and Ceratiidae. The Myctophidae includes at least three species known from the study area: Benthosema glaciale, Lampadena speculigera, and Neoscopelus macrolepidotus (Dutil et al., 2009). Two species represent the Sternoptychidae, Argyropelecus gigas and Polyipnus clarus, and the Ceratiidae, Cryptopsaras couesii and Ceratias holboelli. Others were grouped at genus level, e.g. Ammodytes, of which two species are found in the study area, A. dubius and A. americanus. Similarly, two species of Sebastes (272 970 fish) were lumped, S. mentella and S. fasciatus; S. norvegicus (236 fish) was treated separately from these. Bathy- and bentho-pelagic species were included because they were considered potentially associated with the seafloor.
Abundance was defined as the number of fish of each species caught at each station; data were square-root transformed before analysis to minimize the gaps between abundant and rare species (Legendre and Legendre, 1998; Jørgensen et al., 2005). Numbers were favoured over biomass, given the marked differences in average size between the species considered. Multivariate analyses were conducted using Primer (Primer-E Ltd, version 6) and CANOCO software (CANOCO for Windows Version 4.55).
Groups of stations
The Bray–Curtis similarity index was used to calculate the similarity coefficient between stations and to build the similarity matrices required for subsequent analyses (Legendre and Legendre, 1998). Catch data were subjected to an average-linkage hierarchical cluster analysis (Primer CLUSTER analysis). The groups of stations were formed according to two criteria, the similarity index and the results of the similarity profile analysis (Primer SIMPROF procedure). Only groups consisting of stations with a between-station similarity coefficient of at least 40% were selected if the results of similarity profile permutation tests (SIMPROF) yielded a significant result (α = 0.05). MDS was carried out using the same similarity matrix as for the cluster analysis, to visualize the spatial structure of the groups of stations. Between-group differences in environmental conditions were tested by ANOVA and Tukey multiple comparison tests.
Species assemblages
Fish assemblages were described using catch composition. Species recorded at any of the stations within a group were considered part of a fish assemblage specific to that group of stations. The relative importance of a species in characterizing an assemblage was determined in two ways: the indicator value index determined using the Dufrêne and Legendre (1997) method and through a similarity analysis that determines the contribution of a species to between-station similarity for a group (SIMPER, Primer E, version 6 procedure). The value of Dufrêne and Legendre's (1997) index is 100% when a species is present at all stations in a group of stations, but is entirely absent from the stations of other groups. When the indicator value was maximal for a species across groups of stations, the species was considered as an indicator species for that assemblage and group of stations. Species with a maximum indicator value >15% were coined as “key species” of an assemblage, whereas those with a maximum indicator value <15% were termed “secondary species”. Other species were considered as having a lesser impact on the formation of assemblages. The richness and diversity index of Shannon–Wiener were determined based on unprocessed abundances for different groups using Primer. The distribution of assemblages was mapped using ArcGIS software (version 9.2, ESRI, Inc.).
Correlations with environmental parameters
Differences in environmental conditions between groups of stations and associated assemblages were tested using Statistical Analysis System JMP software (JMP version 7; SAS Institute, Cary, NC, USA). Relationships between groups of stations, species assemblages, and environmental conditions were explored using a DCCA. Data distribution followed an arch pattern, which was interpreted as resulting from the presence of rare species (Legendre and Legendre, 1998). Detrending of arch patterns was carried out by a second-degree polynomial (Hill and Gauch, 1980). The canonical correlations between species assemblages, groups of stations, and environmental parameters were tested against the distribution of eigenvalues obtained in the analysis using a Monte Carlo test with permutations (999 permutations, α = 0.05; ter Braak and Smilauer, 2002). The analysis focused on relationships among species (Hill's scaling focused on interspecies distances). The ordination plots were generated with Canodraw (ter Braak and Smilauer, 2002) and Sigmaplot (Sigmaplot version 10, Systat Software Appoints, Inc.) software.
Results
Groups of stations and species assemblages
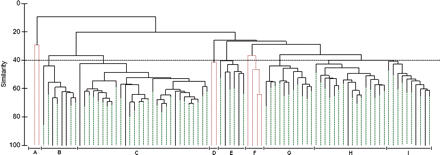
Hierarchical cluster analysis formed nine groups of stations at 40% similarity, each group consisting of similar stations in terms of species composition and abundance (Figure 2). Based on the number of stations, four groups of stations were most important (groups B, C, G, and H; 809 stations). Two other groups comprised fewer stations (groups E and I; 53 stations). The remaining groups can be considered as marginal because they represented very few stations (8). Those groups either did not meet the criterion of at least 40% similarity between stations (groups A and F) or were poorly structured in terms of the similarity analysis profile (group D, p > 0.05; Figure 2, dotted red lines).
Hierarchical cluster dendrogram of stations in the study area based on fish species composition and catch in numbers. Groups A–I are those for which the similarity coefficient between stations is greater than 40%. Groups rejected based on the similarity profile analysis (α = 0.05) are indicated as dotted lines. The Bray–Curtis coefficient was used to determine similarity, and data were square-root transformed.
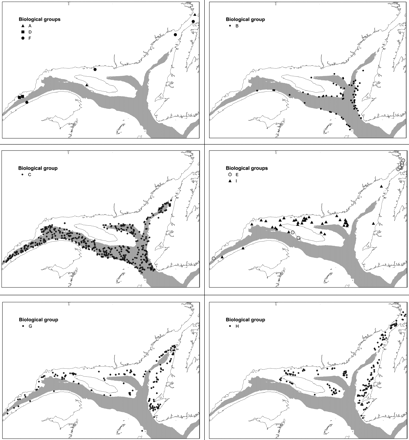
These groups of stations also clustered on an MDS graph (stress = 0.13) and followed different patterns of geographic distribution in the study area (Figure 3). Groups B and C were in the deep channels, and groups G and H plus the smaller groups E and I occupied plateaus and slopes. Therefore, the average depth of station and corresponding environmental conditions differed between groups of stations (Table 1). The three marginal groups of stations were closer to the coast. Stations in groups A and F were scattered in space, and group D near the Manicouagan Peninsula. The separation between deep and shallow waters is clear at a level of similarity of 20% (Figure 2).
Depth, salinity, temperature, and dissolved oxygen saturation for each of nine groups of stations, and species diversity (Shannon H′) and richness (number of species) of associated species assemblages in the lower estuary and northern Gulf of St Lawrence.
| Station group . | Number of stations . | Group similarity (%) . | Mean depth (m) . | Depth range (m) . | Salinity . | Temperature (°C) . | Oxygen saturation (%) . | Species diversity . | Total species richness . | Mean species richness . |
|---|---|---|---|---|---|---|---|---|---|---|
| A | 2 | 23 | 103.5 | 44–163 | 32.4 | −0.03 | 86.0 | 1.60 | 11 | 6.5 |
| B | 68 | 60 | 243.8 | 187–340 | 34.4 | 5.25 | 40.9 | 1.80 | 52 | 15.4 |
| C | 447 | 54 | 318.2 | 139–513 | 34.6 | 5.29 | 35.3 | 2.30 | 68 | 14.4 |
| D | 2 | 38 | 71.6 | 67–76 | 32.3 | 0.38 | 79.4 | 2.31 | 19 | 13.0 |
| E | 13 | 53 | 94.8 | 43–137 | 32.6 | −0.57 | 84.8 | 2.33 | 33 | 14.4 |
| F | 4 | 32 | 59.4 | 41–137 | 31.4 | 4.06 | 92.1 | 2.00 | 31 | 16.2 |
| G | 144 | 49 | 160.5 | 43–252 | 33.6 | 3.17 | 50.7 | 2.30 | 58 | 15.4 |
| H | 150 | 46 | 83.4 | 39–144 | 32.4 | 0.89 | 84.1 | 2.12 | 55 | 12.3 |
| I | 40 | 52 | 106.0 | 36–168 | 32.7 | 1.23 | 72.6 | 2.53 | 47 | 18.0 |
| Station group . | Number of stations . | Group similarity (%) . | Mean depth (m) . | Depth range (m) . | Salinity . | Temperature (°C) . | Oxygen saturation (%) . | Species diversity . | Total species richness . | Mean species richness . |
|---|---|---|---|---|---|---|---|---|---|---|
| A | 2 | 23 | 103.5 | 44–163 | 32.4 | −0.03 | 86.0 | 1.60 | 11 | 6.5 |
| B | 68 | 60 | 243.8 | 187–340 | 34.4 | 5.25 | 40.9 | 1.80 | 52 | 15.4 |
| C | 447 | 54 | 318.2 | 139–513 | 34.6 | 5.29 | 35.3 | 2.30 | 68 | 14.4 |
| D | 2 | 38 | 71.6 | 67–76 | 32.3 | 0.38 | 79.4 | 2.31 | 19 | 13.0 |
| E | 13 | 53 | 94.8 | 43–137 | 32.6 | −0.57 | 84.8 | 2.33 | 33 | 14.4 |
| F | 4 | 32 | 59.4 | 41–137 | 31.4 | 4.06 | 92.1 | 2.00 | 31 | 16.2 |
| G | 144 | 49 | 160.5 | 43–252 | 33.6 | 3.17 | 50.7 | 2.30 | 58 | 15.4 |
| H | 150 | 46 | 83.4 | 39–144 | 32.4 | 0.89 | 84.1 | 2.12 | 55 | 12.3 |
| I | 40 | 52 | 106.0 | 36–168 | 32.7 | 1.23 | 72.6 | 2.53 | 47 | 18.0 |
Depth, salinity, temperature, and dissolved oxygen saturation for each of nine groups of stations, and species diversity (Shannon H′) and richness (number of species) of associated species assemblages in the lower estuary and northern Gulf of St Lawrence.
| Station group . | Number of stations . | Group similarity (%) . | Mean depth (m) . | Depth range (m) . | Salinity . | Temperature (°C) . | Oxygen saturation (%) . | Species diversity . | Total species richness . | Mean species richness . |
|---|---|---|---|---|---|---|---|---|---|---|
| A | 2 | 23 | 103.5 | 44–163 | 32.4 | −0.03 | 86.0 | 1.60 | 11 | 6.5 |
| B | 68 | 60 | 243.8 | 187–340 | 34.4 | 5.25 | 40.9 | 1.80 | 52 | 15.4 |
| C | 447 | 54 | 318.2 | 139–513 | 34.6 | 5.29 | 35.3 | 2.30 | 68 | 14.4 |
| D | 2 | 38 | 71.6 | 67–76 | 32.3 | 0.38 | 79.4 | 2.31 | 19 | 13.0 |
| E | 13 | 53 | 94.8 | 43–137 | 32.6 | −0.57 | 84.8 | 2.33 | 33 | 14.4 |
| F | 4 | 32 | 59.4 | 41–137 | 31.4 | 4.06 | 92.1 | 2.00 | 31 | 16.2 |
| G | 144 | 49 | 160.5 | 43–252 | 33.6 | 3.17 | 50.7 | 2.30 | 58 | 15.4 |
| H | 150 | 46 | 83.4 | 39–144 | 32.4 | 0.89 | 84.1 | 2.12 | 55 | 12.3 |
| I | 40 | 52 | 106.0 | 36–168 | 32.7 | 1.23 | 72.6 | 2.53 | 47 | 18.0 |
| Station group . | Number of stations . | Group similarity (%) . | Mean depth (m) . | Depth range (m) . | Salinity . | Temperature (°C) . | Oxygen saturation (%) . | Species diversity . | Total species richness . | Mean species richness . |
|---|---|---|---|---|---|---|---|---|---|---|
| A | 2 | 23 | 103.5 | 44–163 | 32.4 | −0.03 | 86.0 | 1.60 | 11 | 6.5 |
| B | 68 | 60 | 243.8 | 187–340 | 34.4 | 5.25 | 40.9 | 1.80 | 52 | 15.4 |
| C | 447 | 54 | 318.2 | 139–513 | 34.6 | 5.29 | 35.3 | 2.30 | 68 | 14.4 |
| D | 2 | 38 | 71.6 | 67–76 | 32.3 | 0.38 | 79.4 | 2.31 | 19 | 13.0 |
| E | 13 | 53 | 94.8 | 43–137 | 32.6 | −0.57 | 84.8 | 2.33 | 33 | 14.4 |
| F | 4 | 32 | 59.4 | 41–137 | 31.4 | 4.06 | 92.1 | 2.00 | 31 | 16.2 |
| G | 144 | 49 | 160.5 | 43–252 | 33.6 | 3.17 | 50.7 | 2.30 | 58 | 15.4 |
| H | 150 | 46 | 83.4 | 39–144 | 32.4 | 0.89 | 84.1 | 2.12 | 55 | 12.3 |
| I | 40 | 52 | 106.0 | 36–168 | 32.7 | 1.23 | 72.6 | 2.53 | 47 | 18.0 |
Spatial distribution of nine groups of stations in the lower estuary and northern Gulf of St Lawrence, as defined by hierarchical cluster analysis and based on fish species composition and catch in numbers.
Of the 82 taxa caught during the surveys between 2004 and 2008, some played a greater role in structuring species assemblages associated with groups of stations. Hence, 35 taxa were classified as key species (IndVal >15%) and all others as secondary species (Tables 2 and 3). Except for Group C stations, which were characterized by 11 key and 20 secondary species, fish assemblages were in general characterized by roughly ten key or secondary species (Table 4).
Contribution to between-station similarity (SIMPER) and indicator value (IndVal) for species with an indicator value >15% for at least one of six groups of stations.
| . | Group B . | Group C . | Group E . | Group G . | Group H . | Group I . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species . | SIMPER . | IndVal . | SIMPER . | IndVal . | SIMPER . | IndVal . | SIMPER . | IndVal . | SIMPER . | IndVal . | SIMPER . | IndVal . |
| Amblyraja radiata | 2.60 | 17.03 | 6.46 | 31.82 | – | 0.01 | 5.41 | 29.54 | 1.08 | 1.89 | 0.91 | 3.76 |
| Anarhichas lupus | 0.52 | 11.79 | – | 0.01 | – | 0.37 | 1.55 | 15.87 | 1.91 | 11.28 | 0.68 | 3.75 |
| Arctozenus risso | 1.08 | 11.23 | 5.33 | 62.02 | – | 0.03 | 0.08 | 0.25 | 0.01 | 0.03 | – | 0.02 |
| Argentina silus | 0.09 | 21.24 | – | 0.03 | – | – | – | 0.02 | – | – | – | – |
| Artediellus atlanticus | 1.18 | 13.00 | 0.10 | 0.38 | – | 0.34 | 1.82 | 14.60 | 0.67 | 3.61 | 0.95 | 5.29 |
| Artediellus uncinatus | 0.03 | 0.17 | – | 0.01 | 3.95 | 42.02 | 0.05 | 0.12 | 0.32 | 1.50 | 0.89 | 5.21 |
| Centroscyllium fabricii | <0.01 | 0.02 | 0.38 | 22.91 | – | – | – | <0.01 | – | – | – | – |
| Enchelyopus cimbrius | 1.05 | 8.25 | 5.41 | 50.61 | – | – | 1.64 | 11.34 | – | <0.01 | 0.03 | 0.24 |
| Eumesogrammus praecisus | – | <0.01 | – | <0.01 | 13.19 | 46.04 | 0.14 | 0.21 | 5.17 | 6.29 | 3.92 | 11.57 |
| Eumicrotremus spinosus | – | – | – | <0.01 | 18.02 | 88.14 | 0.04 | 0.02 | 3.89 | 2.46 | 2.23 | 2.59 |
| Gadus morhua | 1.93 | 1.72 | 0.13 | 0.06 | 5.12 | 1.12 | 19.76 | 19.37 | 31.69 | 12.26 | 10.69 | 4.24 |
| Glyptocephalus cynoglossus | 3.62 | 20.79 | 9.98 | 42.08 | – | – | 4.41 | 13.39 | 0.35 | 0.58 | 0.11 | 0.12 |
| Gymnelus viridis | – | – | – | 0.00 | 3.48 | 67.86 | – | <0.01 | 0.23 | 0.69 | 0.10 | 0.57 |
| Gymnocanthus tricuspis | 0.01 | 0.02 | 0.02 | 0.03 | 8.09 | 26.66 | 0.10 | 0.47 | 1.16 | 3.91 | 4.38 | 31.02 |
| Hippoglossoides platessoides | 5.20 | 4.95 | 3.97 | 1.83 | 2.80 | 0.33 | 26.66 | 46.08 | 15.51 | 7.18 | 22.08 | 22.04 |
| Hippoglossus hippoglossus | 0.26 | 12.75 | 0.01 | 2.29 | – | – | 0.57 | 46.08 | 0.01 | 0.10 | – | 0.18 |
| Icelus spatula | – | – | – | – | 3.60 | 43.58 | 0.02 | 0.15 | 0.09 | 0.30 | 0.23 | 1.35 |
| Leptagonus decagonus | – | <0.01 | 0.01 | 0.02 | 0.81 | 3.72 | 0.74 | 2.73 | 0.20 | 0.29 | 6.34 | 28.98 |
| Leptoclinus maculatus | 0.01 | 0.01 | 0.05 | 0.02 | 4.25 | 7.48 | 3.24 | 8.36 | 1.57 | 1.73 | 13.85 | 69.65 |
| Liparis gibbus | – | – | – | <0.01 | 8.88 | 47.84 | 0.02 | 0.05 | 0.30 | 1.10 | 1.44 | 4.63 |
| Lumpenus lampretaeformis | 0.01 | 0.01 | – | <0.01 | – | – | 3.38 | 17.79 | 0.50 | 0.66 | 6.20 | 42.22 |
| Lycodes lavalaei | – | 0.01 | – | <0.01 | 6.99 | 37.10 | 0.39 | 1.30 | 1.31 | 5.02 | 3.28 | 18.48 |
| Lycodes vahlii | 0.48 | 3.53 | 0.15 | 1.03 | – | 0.02 | 4.20 | 45.29 | 0.03 | 0.08 | 1.61 | 13.30 |
| Malacoraja senta | 2.22 | 21.84 | 4.99 | 33.92 | 0.06 | 0.25 | 1.26 | 8.86 | 0.02 | 0.13 | 0.32 | 0.94 |
| Melanostigma atlanticum | 0.06 | 0.55 | 2.53 | 25.37 | – | 0.02 | 0.04 | 0.16 | – | <0.01 | – | 0.01 |
| Merluccius bilinearis | 0.18 | 24.62 | 0.02 | 0.66 | – | – | 0.01 | 0.29 | – | – | – | 0.05 |
| Myoxocephalus scorpius | – | – | – | 0.00 | 0.69 | 1.17 | 0.21 | 0.70 | 4.74 | 18.79 | 2.92 | 12.15 |
| Myxine glutinosa | 3.58 | 22.10 | 8.09 | 61.09 | – | – | 0.38 | 1.03 | – | <0.01 | – | – |
| Nezumia bairdii | 3.16 | 23.67 | 9.00 | 64.29 | – | 0.03 | 0.04 | 0.05 | – | <0.01 | 0.01 | 0.04 |
| Phycis chesteri | 0.26 | 7.44 | 1.53 | 31.67 | – | – | – | <0.01 | – | – | – | – |
| Reinhardtius hippoglossoides | 4.22 | 9.01 | 24.03 | 67.10 | – | <0.01 | 4.55 | 8.66 | 0.06 | 0.01 | 1.11 | 2.15 |
| Sebastes | 63.22 | 94.80 | 13.95 | 1.49 | – | <0.01 | 11.72 | 2.76 | 1.38 | 0.14 | 1.44 | 0.06 |
| Triglops murrayi | 0.04 | 0.03 | 0.03 | 0.01 | 15.79 | 24.35 | 3.36 | 4.78 | 23.65 | 32.51 | 8.50 | 12.86 |
| Ulcina olrikii | – | – | – | – | 1.47 | 49.48 | – | – | 0.05 | 0.22 | 0.02 | 0.24 |
| Urophycis tenuis | 2.73 | 63.19 | 1.07 | 9.34 | – | – | 0.04 | 0.13 | – | <0.01 | – | – |
| . | Group B . | Group C . | Group E . | Group G . | Group H . | Group I . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species . | SIMPER . | IndVal . | SIMPER . | IndVal . | SIMPER . | IndVal . | SIMPER . | IndVal . | SIMPER . | IndVal . | SIMPER . | IndVal . |
| Amblyraja radiata | 2.60 | 17.03 | 6.46 | 31.82 | – | 0.01 | 5.41 | 29.54 | 1.08 | 1.89 | 0.91 | 3.76 |
| Anarhichas lupus | 0.52 | 11.79 | – | 0.01 | – | 0.37 | 1.55 | 15.87 | 1.91 | 11.28 | 0.68 | 3.75 |
| Arctozenus risso | 1.08 | 11.23 | 5.33 | 62.02 | – | 0.03 | 0.08 | 0.25 | 0.01 | 0.03 | – | 0.02 |
| Argentina silus | 0.09 | 21.24 | – | 0.03 | – | – | – | 0.02 | – | – | – | – |
| Artediellus atlanticus | 1.18 | 13.00 | 0.10 | 0.38 | – | 0.34 | 1.82 | 14.60 | 0.67 | 3.61 | 0.95 | 5.29 |
| Artediellus uncinatus | 0.03 | 0.17 | – | 0.01 | 3.95 | 42.02 | 0.05 | 0.12 | 0.32 | 1.50 | 0.89 | 5.21 |
| Centroscyllium fabricii | <0.01 | 0.02 | 0.38 | 22.91 | – | – | – | <0.01 | – | – | – | – |
| Enchelyopus cimbrius | 1.05 | 8.25 | 5.41 | 50.61 | – | – | 1.64 | 11.34 | – | <0.01 | 0.03 | 0.24 |
| Eumesogrammus praecisus | – | <0.01 | – | <0.01 | 13.19 | 46.04 | 0.14 | 0.21 | 5.17 | 6.29 | 3.92 | 11.57 |
| Eumicrotremus spinosus | – | – | – | <0.01 | 18.02 | 88.14 | 0.04 | 0.02 | 3.89 | 2.46 | 2.23 | 2.59 |
| Gadus morhua | 1.93 | 1.72 | 0.13 | 0.06 | 5.12 | 1.12 | 19.76 | 19.37 | 31.69 | 12.26 | 10.69 | 4.24 |
| Glyptocephalus cynoglossus | 3.62 | 20.79 | 9.98 | 42.08 | – | – | 4.41 | 13.39 | 0.35 | 0.58 | 0.11 | 0.12 |
| Gymnelus viridis | – | – | – | 0.00 | 3.48 | 67.86 | – | <0.01 | 0.23 | 0.69 | 0.10 | 0.57 |
| Gymnocanthus tricuspis | 0.01 | 0.02 | 0.02 | 0.03 | 8.09 | 26.66 | 0.10 | 0.47 | 1.16 | 3.91 | 4.38 | 31.02 |
| Hippoglossoides platessoides | 5.20 | 4.95 | 3.97 | 1.83 | 2.80 | 0.33 | 26.66 | 46.08 | 15.51 | 7.18 | 22.08 | 22.04 |
| Hippoglossus hippoglossus | 0.26 | 12.75 | 0.01 | 2.29 | – | – | 0.57 | 46.08 | 0.01 | 0.10 | – | 0.18 |
| Icelus spatula | – | – | – | – | 3.60 | 43.58 | 0.02 | 0.15 | 0.09 | 0.30 | 0.23 | 1.35 |
| Leptagonus decagonus | – | <0.01 | 0.01 | 0.02 | 0.81 | 3.72 | 0.74 | 2.73 | 0.20 | 0.29 | 6.34 | 28.98 |
| Leptoclinus maculatus | 0.01 | 0.01 | 0.05 | 0.02 | 4.25 | 7.48 | 3.24 | 8.36 | 1.57 | 1.73 | 13.85 | 69.65 |
| Liparis gibbus | – | – | – | <0.01 | 8.88 | 47.84 | 0.02 | 0.05 | 0.30 | 1.10 | 1.44 | 4.63 |
| Lumpenus lampretaeformis | 0.01 | 0.01 | – | <0.01 | – | – | 3.38 | 17.79 | 0.50 | 0.66 | 6.20 | 42.22 |
| Lycodes lavalaei | – | 0.01 | – | <0.01 | 6.99 | 37.10 | 0.39 | 1.30 | 1.31 | 5.02 | 3.28 | 18.48 |
| Lycodes vahlii | 0.48 | 3.53 | 0.15 | 1.03 | – | 0.02 | 4.20 | 45.29 | 0.03 | 0.08 | 1.61 | 13.30 |
| Malacoraja senta | 2.22 | 21.84 | 4.99 | 33.92 | 0.06 | 0.25 | 1.26 | 8.86 | 0.02 | 0.13 | 0.32 | 0.94 |
| Melanostigma atlanticum | 0.06 | 0.55 | 2.53 | 25.37 | – | 0.02 | 0.04 | 0.16 | – | <0.01 | – | 0.01 |
| Merluccius bilinearis | 0.18 | 24.62 | 0.02 | 0.66 | – | – | 0.01 | 0.29 | – | – | – | 0.05 |
| Myoxocephalus scorpius | – | – | – | 0.00 | 0.69 | 1.17 | 0.21 | 0.70 | 4.74 | 18.79 | 2.92 | 12.15 |
| Myxine glutinosa | 3.58 | 22.10 | 8.09 | 61.09 | – | – | 0.38 | 1.03 | – | <0.01 | – | – |
| Nezumia bairdii | 3.16 | 23.67 | 9.00 | 64.29 | – | 0.03 | 0.04 | 0.05 | – | <0.01 | 0.01 | 0.04 |
| Phycis chesteri | 0.26 | 7.44 | 1.53 | 31.67 | – | – | – | <0.01 | – | – | – | – |
| Reinhardtius hippoglossoides | 4.22 | 9.01 | 24.03 | 67.10 | – | <0.01 | 4.55 | 8.66 | 0.06 | 0.01 | 1.11 | 2.15 |
| Sebastes | 63.22 | 94.80 | 13.95 | 1.49 | – | <0.01 | 11.72 | 2.76 | 1.38 | 0.14 | 1.44 | 0.06 |
| Triglops murrayi | 0.04 | 0.03 | 0.03 | 0.01 | 15.79 | 24.35 | 3.36 | 4.78 | 23.65 | 32.51 | 8.50 | 12.86 |
| Ulcina olrikii | – | – | – | – | 1.47 | 49.48 | – | – | 0.05 | 0.22 | 0.02 | 0.24 |
| Urophycis tenuis | 2.73 | 63.19 | 1.07 | 9.34 | – | – | 0.04 | 0.13 | – | <0.01 | – | – |
When a species has a maximum IndVal value for a given assemblage, the species is classified as a key indicator species and that value is italicized. The highest SIMPER values for a group (>50% cumulative SIMPER value) are shown emboldened.
Contribution to between-station similarity (SIMPER) and indicator value (IndVal) for species with an indicator value >15% for at least one of six groups of stations.
| . | Group B . | Group C . | Group E . | Group G . | Group H . | Group I . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species . | SIMPER . | IndVal . | SIMPER . | IndVal . | SIMPER . | IndVal . | SIMPER . | IndVal . | SIMPER . | IndVal . | SIMPER . | IndVal . |
| Amblyraja radiata | 2.60 | 17.03 | 6.46 | 31.82 | – | 0.01 | 5.41 | 29.54 | 1.08 | 1.89 | 0.91 | 3.76 |
| Anarhichas lupus | 0.52 | 11.79 | – | 0.01 | – | 0.37 | 1.55 | 15.87 | 1.91 | 11.28 | 0.68 | 3.75 |
| Arctozenus risso | 1.08 | 11.23 | 5.33 | 62.02 | – | 0.03 | 0.08 | 0.25 | 0.01 | 0.03 | – | 0.02 |
| Argentina silus | 0.09 | 21.24 | – | 0.03 | – | – | – | 0.02 | – | – | – | – |
| Artediellus atlanticus | 1.18 | 13.00 | 0.10 | 0.38 | – | 0.34 | 1.82 | 14.60 | 0.67 | 3.61 | 0.95 | 5.29 |
| Artediellus uncinatus | 0.03 | 0.17 | – | 0.01 | 3.95 | 42.02 | 0.05 | 0.12 | 0.32 | 1.50 | 0.89 | 5.21 |
| Centroscyllium fabricii | <0.01 | 0.02 | 0.38 | 22.91 | – | – | – | <0.01 | – | – | – | – |
| Enchelyopus cimbrius | 1.05 | 8.25 | 5.41 | 50.61 | – | – | 1.64 | 11.34 | – | <0.01 | 0.03 | 0.24 |
| Eumesogrammus praecisus | – | <0.01 | – | <0.01 | 13.19 | 46.04 | 0.14 | 0.21 | 5.17 | 6.29 | 3.92 | 11.57 |
| Eumicrotremus spinosus | – | – | – | <0.01 | 18.02 | 88.14 | 0.04 | 0.02 | 3.89 | 2.46 | 2.23 | 2.59 |
| Gadus morhua | 1.93 | 1.72 | 0.13 | 0.06 | 5.12 | 1.12 | 19.76 | 19.37 | 31.69 | 12.26 | 10.69 | 4.24 |
| Glyptocephalus cynoglossus | 3.62 | 20.79 | 9.98 | 42.08 | – | – | 4.41 | 13.39 | 0.35 | 0.58 | 0.11 | 0.12 |
| Gymnelus viridis | – | – | – | 0.00 | 3.48 | 67.86 | – | <0.01 | 0.23 | 0.69 | 0.10 | 0.57 |
| Gymnocanthus tricuspis | 0.01 | 0.02 | 0.02 | 0.03 | 8.09 | 26.66 | 0.10 | 0.47 | 1.16 | 3.91 | 4.38 | 31.02 |
| Hippoglossoides platessoides | 5.20 | 4.95 | 3.97 | 1.83 | 2.80 | 0.33 | 26.66 | 46.08 | 15.51 | 7.18 | 22.08 | 22.04 |
| Hippoglossus hippoglossus | 0.26 | 12.75 | 0.01 | 2.29 | – | – | 0.57 | 46.08 | 0.01 | 0.10 | – | 0.18 |
| Icelus spatula | – | – | – | – | 3.60 | 43.58 | 0.02 | 0.15 | 0.09 | 0.30 | 0.23 | 1.35 |
| Leptagonus decagonus | – | <0.01 | 0.01 | 0.02 | 0.81 | 3.72 | 0.74 | 2.73 | 0.20 | 0.29 | 6.34 | 28.98 |
| Leptoclinus maculatus | 0.01 | 0.01 | 0.05 | 0.02 | 4.25 | 7.48 | 3.24 | 8.36 | 1.57 | 1.73 | 13.85 | 69.65 |
| Liparis gibbus | – | – | – | <0.01 | 8.88 | 47.84 | 0.02 | 0.05 | 0.30 | 1.10 | 1.44 | 4.63 |
| Lumpenus lampretaeformis | 0.01 | 0.01 | – | <0.01 | – | – | 3.38 | 17.79 | 0.50 | 0.66 | 6.20 | 42.22 |
| Lycodes lavalaei | – | 0.01 | – | <0.01 | 6.99 | 37.10 | 0.39 | 1.30 | 1.31 | 5.02 | 3.28 | 18.48 |
| Lycodes vahlii | 0.48 | 3.53 | 0.15 | 1.03 | – | 0.02 | 4.20 | 45.29 | 0.03 | 0.08 | 1.61 | 13.30 |
| Malacoraja senta | 2.22 | 21.84 | 4.99 | 33.92 | 0.06 | 0.25 | 1.26 | 8.86 | 0.02 | 0.13 | 0.32 | 0.94 |
| Melanostigma atlanticum | 0.06 | 0.55 | 2.53 | 25.37 | – | 0.02 | 0.04 | 0.16 | – | <0.01 | – | 0.01 |
| Merluccius bilinearis | 0.18 | 24.62 | 0.02 | 0.66 | – | – | 0.01 | 0.29 | – | – | – | 0.05 |
| Myoxocephalus scorpius | – | – | – | 0.00 | 0.69 | 1.17 | 0.21 | 0.70 | 4.74 | 18.79 | 2.92 | 12.15 |
| Myxine glutinosa | 3.58 | 22.10 | 8.09 | 61.09 | – | – | 0.38 | 1.03 | – | <0.01 | – | – |
| Nezumia bairdii | 3.16 | 23.67 | 9.00 | 64.29 | – | 0.03 | 0.04 | 0.05 | – | <0.01 | 0.01 | 0.04 |
| Phycis chesteri | 0.26 | 7.44 | 1.53 | 31.67 | – | – | – | <0.01 | – | – | – | – |
| Reinhardtius hippoglossoides | 4.22 | 9.01 | 24.03 | 67.10 | – | <0.01 | 4.55 | 8.66 | 0.06 | 0.01 | 1.11 | 2.15 |
| Sebastes | 63.22 | 94.80 | 13.95 | 1.49 | – | <0.01 | 11.72 | 2.76 | 1.38 | 0.14 | 1.44 | 0.06 |
| Triglops murrayi | 0.04 | 0.03 | 0.03 | 0.01 | 15.79 | 24.35 | 3.36 | 4.78 | 23.65 | 32.51 | 8.50 | 12.86 |
| Ulcina olrikii | – | – | – | – | 1.47 | 49.48 | – | – | 0.05 | 0.22 | 0.02 | 0.24 |
| Urophycis tenuis | 2.73 | 63.19 | 1.07 | 9.34 | – | – | 0.04 | 0.13 | – | <0.01 | – | – |
| . | Group B . | Group C . | Group E . | Group G . | Group H . | Group I . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species . | SIMPER . | IndVal . | SIMPER . | IndVal . | SIMPER . | IndVal . | SIMPER . | IndVal . | SIMPER . | IndVal . | SIMPER . | IndVal . |
| Amblyraja radiata | 2.60 | 17.03 | 6.46 | 31.82 | – | 0.01 | 5.41 | 29.54 | 1.08 | 1.89 | 0.91 | 3.76 |
| Anarhichas lupus | 0.52 | 11.79 | – | 0.01 | – | 0.37 | 1.55 | 15.87 | 1.91 | 11.28 | 0.68 | 3.75 |
| Arctozenus risso | 1.08 | 11.23 | 5.33 | 62.02 | – | 0.03 | 0.08 | 0.25 | 0.01 | 0.03 | – | 0.02 |
| Argentina silus | 0.09 | 21.24 | – | 0.03 | – | – | – | 0.02 | – | – | – | – |
| Artediellus atlanticus | 1.18 | 13.00 | 0.10 | 0.38 | – | 0.34 | 1.82 | 14.60 | 0.67 | 3.61 | 0.95 | 5.29 |
| Artediellus uncinatus | 0.03 | 0.17 | – | 0.01 | 3.95 | 42.02 | 0.05 | 0.12 | 0.32 | 1.50 | 0.89 | 5.21 |
| Centroscyllium fabricii | <0.01 | 0.02 | 0.38 | 22.91 | – | – | – | <0.01 | – | – | – | – |
| Enchelyopus cimbrius | 1.05 | 8.25 | 5.41 | 50.61 | – | – | 1.64 | 11.34 | – | <0.01 | 0.03 | 0.24 |
| Eumesogrammus praecisus | – | <0.01 | – | <0.01 | 13.19 | 46.04 | 0.14 | 0.21 | 5.17 | 6.29 | 3.92 | 11.57 |
| Eumicrotremus spinosus | – | – | – | <0.01 | 18.02 | 88.14 | 0.04 | 0.02 | 3.89 | 2.46 | 2.23 | 2.59 |
| Gadus morhua | 1.93 | 1.72 | 0.13 | 0.06 | 5.12 | 1.12 | 19.76 | 19.37 | 31.69 | 12.26 | 10.69 | 4.24 |
| Glyptocephalus cynoglossus | 3.62 | 20.79 | 9.98 | 42.08 | – | – | 4.41 | 13.39 | 0.35 | 0.58 | 0.11 | 0.12 |
| Gymnelus viridis | – | – | – | 0.00 | 3.48 | 67.86 | – | <0.01 | 0.23 | 0.69 | 0.10 | 0.57 |
| Gymnocanthus tricuspis | 0.01 | 0.02 | 0.02 | 0.03 | 8.09 | 26.66 | 0.10 | 0.47 | 1.16 | 3.91 | 4.38 | 31.02 |
| Hippoglossoides platessoides | 5.20 | 4.95 | 3.97 | 1.83 | 2.80 | 0.33 | 26.66 | 46.08 | 15.51 | 7.18 | 22.08 | 22.04 |
| Hippoglossus hippoglossus | 0.26 | 12.75 | 0.01 | 2.29 | – | – | 0.57 | 46.08 | 0.01 | 0.10 | – | 0.18 |
| Icelus spatula | – | – | – | – | 3.60 | 43.58 | 0.02 | 0.15 | 0.09 | 0.30 | 0.23 | 1.35 |
| Leptagonus decagonus | – | <0.01 | 0.01 | 0.02 | 0.81 | 3.72 | 0.74 | 2.73 | 0.20 | 0.29 | 6.34 | 28.98 |
| Leptoclinus maculatus | 0.01 | 0.01 | 0.05 | 0.02 | 4.25 | 7.48 | 3.24 | 8.36 | 1.57 | 1.73 | 13.85 | 69.65 |
| Liparis gibbus | – | – | – | <0.01 | 8.88 | 47.84 | 0.02 | 0.05 | 0.30 | 1.10 | 1.44 | 4.63 |
| Lumpenus lampretaeformis | 0.01 | 0.01 | – | <0.01 | – | – | 3.38 | 17.79 | 0.50 | 0.66 | 6.20 | 42.22 |
| Lycodes lavalaei | – | 0.01 | – | <0.01 | 6.99 | 37.10 | 0.39 | 1.30 | 1.31 | 5.02 | 3.28 | 18.48 |
| Lycodes vahlii | 0.48 | 3.53 | 0.15 | 1.03 | – | 0.02 | 4.20 | 45.29 | 0.03 | 0.08 | 1.61 | 13.30 |
| Malacoraja senta | 2.22 | 21.84 | 4.99 | 33.92 | 0.06 | 0.25 | 1.26 | 8.86 | 0.02 | 0.13 | 0.32 | 0.94 |
| Melanostigma atlanticum | 0.06 | 0.55 | 2.53 | 25.37 | – | 0.02 | 0.04 | 0.16 | – | <0.01 | – | 0.01 |
| Merluccius bilinearis | 0.18 | 24.62 | 0.02 | 0.66 | – | – | 0.01 | 0.29 | – | – | – | 0.05 |
| Myoxocephalus scorpius | – | – | – | 0.00 | 0.69 | 1.17 | 0.21 | 0.70 | 4.74 | 18.79 | 2.92 | 12.15 |
| Myxine glutinosa | 3.58 | 22.10 | 8.09 | 61.09 | – | – | 0.38 | 1.03 | – | <0.01 | – | – |
| Nezumia bairdii | 3.16 | 23.67 | 9.00 | 64.29 | – | 0.03 | 0.04 | 0.05 | – | <0.01 | 0.01 | 0.04 |
| Phycis chesteri | 0.26 | 7.44 | 1.53 | 31.67 | – | – | – | <0.01 | – | – | – | – |
| Reinhardtius hippoglossoides | 4.22 | 9.01 | 24.03 | 67.10 | – | <0.01 | 4.55 | 8.66 | 0.06 | 0.01 | 1.11 | 2.15 |
| Sebastes | 63.22 | 94.80 | 13.95 | 1.49 | – | <0.01 | 11.72 | 2.76 | 1.38 | 0.14 | 1.44 | 0.06 |
| Triglops murrayi | 0.04 | 0.03 | 0.03 | 0.01 | 15.79 | 24.35 | 3.36 | 4.78 | 23.65 | 32.51 | 8.50 | 12.86 |
| Ulcina olrikii | – | – | – | – | 1.47 | 49.48 | – | – | 0.05 | 0.22 | 0.02 | 0.24 |
| Urophycis tenuis | 2.73 | 63.19 | 1.07 | 9.34 | – | – | 0.04 | 0.13 | – | <0.01 | – | – |
When a species has a maximum IndVal value for a given assemblage, the species is classified as a key indicator species and that value is italicized. The highest SIMPER values for a group (>50% cumulative SIMPER value) are shown emboldened.
Contribution to between-station similarity (SIMPER) and indicator value (IndVal) for species with an indicator value <15% for all groups of stations.
| . | Group B . | Group C . | Group E . | Group G . | Group H . | Group I . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species . | SIMPER . | IndVal . | SIMPER . | IndVal . | SIMPER . | IndVal . | SIMPER . | IndVal . | SIMPER . | IndVal . | SIMPER . | IndVal . |
| Ammodytes | – | 0.01 | – | 0.01 | – | – | – | 0.02 | <0.00 | 0.17 | 0.01 | 0.78 |
| Anarhichas minor | 0.06 | 5.30 | – | 0.05 | – | – | 0.02 | 1.42 | 0.10 | 3.18 | 0.02 | 2.66 |
| Anisarchus medius | – | – | – | – | – | – | – | <0.00 | <0.00 | <0.00 | – | 2.48 |
| Aspidophoroides monopterygius | 0.10 | 0.59 | 0.01 | 0.01 | 0.85 | 6.42 | 1.46 | 2.32 | 2.54 | 8.39 | 3.11 | 10.26 |
| Bathyraja spinicauda | – | – | – | 0.97 | – | – | – | 0.15 | <0.00 | 0.69 | – | – |
| Boreogadus saida | – | <0.01 | 0.01 | 0.03 | 1.53 | 10.56 | 0.13 | 1.37 | 0.11 | 0.22 | 0.69 | 5.92 |
| Careproctus reinhardti | – | 0.16 | 0.01 | 1.71 | – | – | 0.02 | 2.72 | <0.00 | 0.08 | – | 0.46 |
| Ceratiidae | – | – | – | 0.89 | – | – | – | – | <0.00 | – | – | – |
| Chauliodus sloani | – | – | – | 0.67 | – | – | – | – | – | – | – | – |
| Clupea harengus | 2.03 | 0.56 | 2.41 | 0.37 | 0.09 | 0.06 | 2.19 | 0.66 | 0.54 | 0.09 | 0.34 | 0.03 |
| Cottunculus microps | – | 2.45 | – | 0.11 | – | – | – | – | – | – | – | – |
| Cryptacanthodes maculatus | – | – | 0.03 | 7.17 | – | – | – | 0.49 | <0.00 | 0.03 | – | – |
| Cyclopterus lumpus | 0.02 | 0.64 | 0.03 | 0.70 | – | 0.28 | 0.28 | 10.56 | 0.16 | 1.85 | 0.55 | 14.18 |
| Cyclothone microdon | – | – | – | 2.91 | – | – | – | – | – | – | – | – |
| Gadus ogac | – | – | – | – | – | – | – | – | 0.40 | 4.65 | 0.04 | 0.65 |
| Gaidropsarus argentatus | – | – | – | 1.74 | – | – | – | 0.52 | <0.00 | – | – | 0.73 |
| Gasterosteus aculeatus | – | 0.02 | 0.01 | 3.26 | – | 1.10 | – | 0.09 | <0.00 | 0.24 | – | – |
| Hemitripterus americanus | – | 0.47 | – | 0.03 | – | – | 0.02 | 3.57 | <0.00 | 0.97 | – | 0.22 |
| Icelus bicornis | – | 0.01 | – | <0.00 | 0.35 | 12.19 | 0.04 | 0.42 | 0.17 | 2.19 | 0.96 | 12.49 |
| Leucoraja ocellata | – | 0.28 | – | 0.52 | – | – | – | – | <0.00 | 0.46 | – | – |
| Limanda ferruginea | – | – | – | – | – | – | – | 0.03 | 0.06 | 6.94 | 0.01 | 0.19 |
| Lophius americanus | 0.02 | 6.75 | 0.01 | 1.73 | – | – | – | 0.02 | – | – | – | – |
| Lycenchelys paxillus | – | – | 0.01 | 4.47 | – | – | – | – | <0.00 | – | – | – |
| Lycenchelys verrillii | 0.01 | 1.07 | 0.05 | 9.27 | – | – | – | 0.01 | <0.00 | 0.04 | – | 0.12 |
| Lycodes esmarkii | – | 1.24 | – | 1.94 | – | – | – | – | <0.00 | – | – | – |
| Lycodes terraenovae | – | 0.33 | – | 3.13 | – | – | – | – | <0.00 | – | – | – |
| Melanogrammus aeglefinus | – | 0.15 | – | – | – | – | 0.02 | 7.46 | – | – | – | – |
| Micromesistius poutassou | – | – | – | 0.22 | – | – | – | – | <0.00 | – | – | – |
| Myctophidae | – | 0.25 | 0.06 | 10.52 | – | – | – | 0.01 | <0.00 | – | – | – |
| Myoxocephalus aenaeus | – | – | – | – | – | – | – | – | – | 0.67 | – | – |
| Myoxocephalus octodecemspinosus | – | – | – | – | – | – | – | 0.18 | – | 2.32 | – | – |
| Paraliparis calidus | – | – | 0.02 | 7.38 | – | – | – | – | – | – | – | – |
| Paraliparis copei | – | – | – | 3.58 | – | – | – | – | – | – | – | – |
| Pholis gunnellus | – | – | – | – | – | – | – | – | <0.00 | 0.67 | – | – |
| Pollachius virens | – | 4.41 | – | – | – | – | – | – | – | – | – | – |
| Pseudopleuronectes americanus | – | – | – | 0.00 | – | – | – | – | <0.00 | 0.67 | – | – |
| Rajella fyllae | – | – | – | 0.33 | – | – | – | 0.35 | <0.00 | – | – | – |
| Sebastes norvegicus | – | 2.94 | – | – | – | – | – | – | <0.00 | – | – | – |
| Serrivomer beanii | – | – | – | 1.12 | – | – | – | – | <0.00 | – | – | – |
| Squalus acanthias | 0.01 | 6.99 | – | 0.04 | – | – | – | – | – | – | – | – |
| Sternoptychidae | – | – | – | 1.34 | – | – | – | – | – | – | – | – |
| Stichaeus punctatus | – | – | – | – | – | – | – | 0.01 | 0.68 | – | 0.10 | |
| Stomias boa | – | – | – | 0.22 | – | – | – | – | – | – | – | – |
| Synaphobranchus kaupii | – | – | – | 0.50 | – | – | – | 0.30 | – | – | – | – |
| Triglops nybelini | – | 0.23 | – | <0.00 | – | – | – | 0.10 | <0.00 | – | 0.01 | 5.72 |
| Triglops pingelii | – | – | – | – | – | 4.12 | – | <0.00 | <0.00 | 1.24 | – | – |
| . | Group B . | Group C . | Group E . | Group G . | Group H . | Group I . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species . | SIMPER . | IndVal . | SIMPER . | IndVal . | SIMPER . | IndVal . | SIMPER . | IndVal . | SIMPER . | IndVal . | SIMPER . | IndVal . |
| Ammodytes | – | 0.01 | – | 0.01 | – | – | – | 0.02 | <0.00 | 0.17 | 0.01 | 0.78 |
| Anarhichas minor | 0.06 | 5.30 | – | 0.05 | – | – | 0.02 | 1.42 | 0.10 | 3.18 | 0.02 | 2.66 |
| Anisarchus medius | – | – | – | – | – | – | – | <0.00 | <0.00 | <0.00 | – | 2.48 |
| Aspidophoroides monopterygius | 0.10 | 0.59 | 0.01 | 0.01 | 0.85 | 6.42 | 1.46 | 2.32 | 2.54 | 8.39 | 3.11 | 10.26 |
| Bathyraja spinicauda | – | – | – | 0.97 | – | – | – | 0.15 | <0.00 | 0.69 | – | – |
| Boreogadus saida | – | <0.01 | 0.01 | 0.03 | 1.53 | 10.56 | 0.13 | 1.37 | 0.11 | 0.22 | 0.69 | 5.92 |
| Careproctus reinhardti | – | 0.16 | 0.01 | 1.71 | – | – | 0.02 | 2.72 | <0.00 | 0.08 | – | 0.46 |
| Ceratiidae | – | – | – | 0.89 | – | – | – | – | <0.00 | – | – | – |
| Chauliodus sloani | – | – | – | 0.67 | – | – | – | – | – | – | – | – |
| Clupea harengus | 2.03 | 0.56 | 2.41 | 0.37 | 0.09 | 0.06 | 2.19 | 0.66 | 0.54 | 0.09 | 0.34 | 0.03 |
| Cottunculus microps | – | 2.45 | – | 0.11 | – | – | – | – | – | – | – | – |
| Cryptacanthodes maculatus | – | – | 0.03 | 7.17 | – | – | – | 0.49 | <0.00 | 0.03 | – | – |
| Cyclopterus lumpus | 0.02 | 0.64 | 0.03 | 0.70 | – | 0.28 | 0.28 | 10.56 | 0.16 | 1.85 | 0.55 | 14.18 |
| Cyclothone microdon | – | – | – | 2.91 | – | – | – | – | – | – | – | – |
| Gadus ogac | – | – | – | – | – | – | – | – | 0.40 | 4.65 | 0.04 | 0.65 |
| Gaidropsarus argentatus | – | – | – | 1.74 | – | – | – | 0.52 | <0.00 | – | – | 0.73 |
| Gasterosteus aculeatus | – | 0.02 | 0.01 | 3.26 | – | 1.10 | – | 0.09 | <0.00 | 0.24 | – | – |
| Hemitripterus americanus | – | 0.47 | – | 0.03 | – | – | 0.02 | 3.57 | <0.00 | 0.97 | – | 0.22 |
| Icelus bicornis | – | 0.01 | – | <0.00 | 0.35 | 12.19 | 0.04 | 0.42 | 0.17 | 2.19 | 0.96 | 12.49 |
| Leucoraja ocellata | – | 0.28 | – | 0.52 | – | – | – | – | <0.00 | 0.46 | – | – |
| Limanda ferruginea | – | – | – | – | – | – | – | 0.03 | 0.06 | 6.94 | 0.01 | 0.19 |
| Lophius americanus | 0.02 | 6.75 | 0.01 | 1.73 | – | – | – | 0.02 | – | – | – | – |
| Lycenchelys paxillus | – | – | 0.01 | 4.47 | – | – | – | – | <0.00 | – | – | – |
| Lycenchelys verrillii | 0.01 | 1.07 | 0.05 | 9.27 | – | – | – | 0.01 | <0.00 | 0.04 | – | 0.12 |
| Lycodes esmarkii | – | 1.24 | – | 1.94 | – | – | – | – | <0.00 | – | – | – |
| Lycodes terraenovae | – | 0.33 | – | 3.13 | – | – | – | – | <0.00 | – | – | – |
| Melanogrammus aeglefinus | – | 0.15 | – | – | – | – | 0.02 | 7.46 | – | – | – | – |
| Micromesistius poutassou | – | – | – | 0.22 | – | – | – | – | <0.00 | – | – | – |
| Myctophidae | – | 0.25 | 0.06 | 10.52 | – | – | – | 0.01 | <0.00 | – | – | – |
| Myoxocephalus aenaeus | – | – | – | – | – | – | – | – | – | 0.67 | – | – |
| Myoxocephalus octodecemspinosus | – | – | – | – | – | – | – | 0.18 | – | 2.32 | – | – |
| Paraliparis calidus | – | – | 0.02 | 7.38 | – | – | – | – | – | – | – | – |
| Paraliparis copei | – | – | – | 3.58 | – | – | – | – | – | – | – | – |
| Pholis gunnellus | – | – | – | – | – | – | – | – | <0.00 | 0.67 | – | – |
| Pollachius virens | – | 4.41 | – | – | – | – | – | – | – | – | – | – |
| Pseudopleuronectes americanus | – | – | – | 0.00 | – | – | – | – | <0.00 | 0.67 | – | – |
| Rajella fyllae | – | – | – | 0.33 | – | – | – | 0.35 | <0.00 | – | – | – |
| Sebastes norvegicus | – | 2.94 | – | – | – | – | – | – | <0.00 | – | – | – |
| Serrivomer beanii | – | – | – | 1.12 | – | – | – | – | <0.00 | – | – | – |
| Squalus acanthias | 0.01 | 6.99 | – | 0.04 | – | – | – | – | – | – | – | – |
| Sternoptychidae | – | – | – | 1.34 | – | – | – | – | – | – | – | – |
| Stichaeus punctatus | – | – | – | – | – | – | – | 0.01 | 0.68 | – | 0.10 | |
| Stomias boa | – | – | – | 0.22 | – | – | – | – | – | – | – | – |
| Synaphobranchus kaupii | – | – | – | 0.50 | – | – | – | 0.30 | – | – | – | – |
| Triglops nybelini | – | 0.23 | – | <0.00 | – | – | – | 0.10 | <0.00 | – | 0.01 | 5.72 |
| Triglops pingelii | – | – | – | – | – | 4.12 | – | <0.00 | <0.00 | 1.24 | – | – |
When a species has a maximum IndVal value for a given assemblage, the species is classified as a secondary indicator species and the value is italicized.
Contribution to between-station similarity (SIMPER) and indicator value (IndVal) for species with an indicator value <15% for all groups of stations.
| . | Group B . | Group C . | Group E . | Group G . | Group H . | Group I . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species . | SIMPER . | IndVal . | SIMPER . | IndVal . | SIMPER . | IndVal . | SIMPER . | IndVal . | SIMPER . | IndVal . | SIMPER . | IndVal . |
| Ammodytes | – | 0.01 | – | 0.01 | – | – | – | 0.02 | <0.00 | 0.17 | 0.01 | 0.78 |
| Anarhichas minor | 0.06 | 5.30 | – | 0.05 | – | – | 0.02 | 1.42 | 0.10 | 3.18 | 0.02 | 2.66 |
| Anisarchus medius | – | – | – | – | – | – | – | <0.00 | <0.00 | <0.00 | – | 2.48 |
| Aspidophoroides monopterygius | 0.10 | 0.59 | 0.01 | 0.01 | 0.85 | 6.42 | 1.46 | 2.32 | 2.54 | 8.39 | 3.11 | 10.26 |
| Bathyraja spinicauda | – | – | – | 0.97 | – | – | – | 0.15 | <0.00 | 0.69 | – | – |
| Boreogadus saida | – | <0.01 | 0.01 | 0.03 | 1.53 | 10.56 | 0.13 | 1.37 | 0.11 | 0.22 | 0.69 | 5.92 |
| Careproctus reinhardti | – | 0.16 | 0.01 | 1.71 | – | – | 0.02 | 2.72 | <0.00 | 0.08 | – | 0.46 |
| Ceratiidae | – | – | – | 0.89 | – | – | – | – | <0.00 | – | – | – |
| Chauliodus sloani | – | – | – | 0.67 | – | – | – | – | – | – | – | – |
| Clupea harengus | 2.03 | 0.56 | 2.41 | 0.37 | 0.09 | 0.06 | 2.19 | 0.66 | 0.54 | 0.09 | 0.34 | 0.03 |
| Cottunculus microps | – | 2.45 | – | 0.11 | – | – | – | – | – | – | – | – |
| Cryptacanthodes maculatus | – | – | 0.03 | 7.17 | – | – | – | 0.49 | <0.00 | 0.03 | – | – |
| Cyclopterus lumpus | 0.02 | 0.64 | 0.03 | 0.70 | – | 0.28 | 0.28 | 10.56 | 0.16 | 1.85 | 0.55 | 14.18 |
| Cyclothone microdon | – | – | – | 2.91 | – | – | – | – | – | – | – | – |
| Gadus ogac | – | – | – | – | – | – | – | – | 0.40 | 4.65 | 0.04 | 0.65 |
| Gaidropsarus argentatus | – | – | – | 1.74 | – | – | – | 0.52 | <0.00 | – | – | 0.73 |
| Gasterosteus aculeatus | – | 0.02 | 0.01 | 3.26 | – | 1.10 | – | 0.09 | <0.00 | 0.24 | – | – |
| Hemitripterus americanus | – | 0.47 | – | 0.03 | – | – | 0.02 | 3.57 | <0.00 | 0.97 | – | 0.22 |
| Icelus bicornis | – | 0.01 | – | <0.00 | 0.35 | 12.19 | 0.04 | 0.42 | 0.17 | 2.19 | 0.96 | 12.49 |
| Leucoraja ocellata | – | 0.28 | – | 0.52 | – | – | – | – | <0.00 | 0.46 | – | – |
| Limanda ferruginea | – | – | – | – | – | – | – | 0.03 | 0.06 | 6.94 | 0.01 | 0.19 |
| Lophius americanus | 0.02 | 6.75 | 0.01 | 1.73 | – | – | – | 0.02 | – | – | – | – |
| Lycenchelys paxillus | – | – | 0.01 | 4.47 | – | – | – | – | <0.00 | – | – | – |
| Lycenchelys verrillii | 0.01 | 1.07 | 0.05 | 9.27 | – | – | – | 0.01 | <0.00 | 0.04 | – | 0.12 |
| Lycodes esmarkii | – | 1.24 | – | 1.94 | – | – | – | – | <0.00 | – | – | – |
| Lycodes terraenovae | – | 0.33 | – | 3.13 | – | – | – | – | <0.00 | – | – | – |
| Melanogrammus aeglefinus | – | 0.15 | – | – | – | – | 0.02 | 7.46 | – | – | – | – |
| Micromesistius poutassou | – | – | – | 0.22 | – | – | – | – | <0.00 | – | – | – |
| Myctophidae | – | 0.25 | 0.06 | 10.52 | – | – | – | 0.01 | <0.00 | – | – | – |
| Myoxocephalus aenaeus | – | – | – | – | – | – | – | – | – | 0.67 | – | – |
| Myoxocephalus octodecemspinosus | – | – | – | – | – | – | – | 0.18 | – | 2.32 | – | – |
| Paraliparis calidus | – | – | 0.02 | 7.38 | – | – | – | – | – | – | – | – |
| Paraliparis copei | – | – | – | 3.58 | – | – | – | – | – | – | – | – |
| Pholis gunnellus | – | – | – | – | – | – | – | – | <0.00 | 0.67 | – | – |
| Pollachius virens | – | 4.41 | – | – | – | – | – | – | – | – | – | – |
| Pseudopleuronectes americanus | – | – | – | 0.00 | – | – | – | – | <0.00 | 0.67 | – | – |
| Rajella fyllae | – | – | – | 0.33 | – | – | – | 0.35 | <0.00 | – | – | – |
| Sebastes norvegicus | – | 2.94 | – | – | – | – | – | – | <0.00 | – | – | – |
| Serrivomer beanii | – | – | – | 1.12 | – | – | – | – | <0.00 | – | – | – |
| Squalus acanthias | 0.01 | 6.99 | – | 0.04 | – | – | – | – | – | – | – | – |
| Sternoptychidae | – | – | – | 1.34 | – | – | – | – | – | – | – | – |
| Stichaeus punctatus | – | – | – | – | – | – | – | 0.01 | 0.68 | – | 0.10 | |
| Stomias boa | – | – | – | 0.22 | – | – | – | – | – | – | – | – |
| Synaphobranchus kaupii | – | – | – | 0.50 | – | – | – | 0.30 | – | – | – | – |
| Triglops nybelini | – | 0.23 | – | <0.00 | – | – | – | 0.10 | <0.00 | – | 0.01 | 5.72 |
| Triglops pingelii | – | – | – | – | – | 4.12 | – | <0.00 | <0.00 | 1.24 | – | – |
| . | Group B . | Group C . | Group E . | Group G . | Group H . | Group I . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species . | SIMPER . | IndVal . | SIMPER . | IndVal . | SIMPER . | IndVal . | SIMPER . | IndVal . | SIMPER . | IndVal . | SIMPER . | IndVal . |
| Ammodytes | – | 0.01 | – | 0.01 | – | – | – | 0.02 | <0.00 | 0.17 | 0.01 | 0.78 |
| Anarhichas minor | 0.06 | 5.30 | – | 0.05 | – | – | 0.02 | 1.42 | 0.10 | 3.18 | 0.02 | 2.66 |
| Anisarchus medius | – | – | – | – | – | – | – | <0.00 | <0.00 | <0.00 | – | 2.48 |
| Aspidophoroides monopterygius | 0.10 | 0.59 | 0.01 | 0.01 | 0.85 | 6.42 | 1.46 | 2.32 | 2.54 | 8.39 | 3.11 | 10.26 |
| Bathyraja spinicauda | – | – | – | 0.97 | – | – | – | 0.15 | <0.00 | 0.69 | – | – |
| Boreogadus saida | – | <0.01 | 0.01 | 0.03 | 1.53 | 10.56 | 0.13 | 1.37 | 0.11 | 0.22 | 0.69 | 5.92 |
| Careproctus reinhardti | – | 0.16 | 0.01 | 1.71 | – | – | 0.02 | 2.72 | <0.00 | 0.08 | – | 0.46 |
| Ceratiidae | – | – | – | 0.89 | – | – | – | – | <0.00 | – | – | – |
| Chauliodus sloani | – | – | – | 0.67 | – | – | – | – | – | – | – | – |
| Clupea harengus | 2.03 | 0.56 | 2.41 | 0.37 | 0.09 | 0.06 | 2.19 | 0.66 | 0.54 | 0.09 | 0.34 | 0.03 |
| Cottunculus microps | – | 2.45 | – | 0.11 | – | – | – | – | – | – | – | – |
| Cryptacanthodes maculatus | – | – | 0.03 | 7.17 | – | – | – | 0.49 | <0.00 | 0.03 | – | – |
| Cyclopterus lumpus | 0.02 | 0.64 | 0.03 | 0.70 | – | 0.28 | 0.28 | 10.56 | 0.16 | 1.85 | 0.55 | 14.18 |
| Cyclothone microdon | – | – | – | 2.91 | – | – | – | – | – | – | – | – |
| Gadus ogac | – | – | – | – | – | – | – | – | 0.40 | 4.65 | 0.04 | 0.65 |
| Gaidropsarus argentatus | – | – | – | 1.74 | – | – | – | 0.52 | <0.00 | – | – | 0.73 |
| Gasterosteus aculeatus | – | 0.02 | 0.01 | 3.26 | – | 1.10 | – | 0.09 | <0.00 | 0.24 | – | – |
| Hemitripterus americanus | – | 0.47 | – | 0.03 | – | – | 0.02 | 3.57 | <0.00 | 0.97 | – | 0.22 |
| Icelus bicornis | – | 0.01 | – | <0.00 | 0.35 | 12.19 | 0.04 | 0.42 | 0.17 | 2.19 | 0.96 | 12.49 |
| Leucoraja ocellata | – | 0.28 | – | 0.52 | – | – | – | – | <0.00 | 0.46 | – | – |
| Limanda ferruginea | – | – | – | – | – | – | – | 0.03 | 0.06 | 6.94 | 0.01 | 0.19 |
| Lophius americanus | 0.02 | 6.75 | 0.01 | 1.73 | – | – | – | 0.02 | – | – | – | – |
| Lycenchelys paxillus | – | – | 0.01 | 4.47 | – | – | – | – | <0.00 | – | – | – |
| Lycenchelys verrillii | 0.01 | 1.07 | 0.05 | 9.27 | – | – | – | 0.01 | <0.00 | 0.04 | – | 0.12 |
| Lycodes esmarkii | – | 1.24 | – | 1.94 | – | – | – | – | <0.00 | – | – | – |
| Lycodes terraenovae | – | 0.33 | – | 3.13 | – | – | – | – | <0.00 | – | – | – |
| Melanogrammus aeglefinus | – | 0.15 | – | – | – | – | 0.02 | 7.46 | – | – | – | – |
| Micromesistius poutassou | – | – | – | 0.22 | – | – | – | – | <0.00 | – | – | – |
| Myctophidae | – | 0.25 | 0.06 | 10.52 | – | – | – | 0.01 | <0.00 | – | – | – |
| Myoxocephalus aenaeus | – | – | – | – | – | – | – | – | – | 0.67 | – | – |
| Myoxocephalus octodecemspinosus | – | – | – | – | – | – | – | 0.18 | – | 2.32 | – | – |
| Paraliparis calidus | – | – | 0.02 | 7.38 | – | – | – | – | – | – | – | – |
| Paraliparis copei | – | – | – | 3.58 | – | – | – | – | – | – | – | – |
| Pholis gunnellus | – | – | – | – | – | – | – | – | <0.00 | 0.67 | – | – |
| Pollachius virens | – | 4.41 | – | – | – | – | – | – | – | – | – | – |
| Pseudopleuronectes americanus | – | – | – | 0.00 | – | – | – | – | <0.00 | 0.67 | – | – |
| Rajella fyllae | – | – | – | 0.33 | – | – | – | 0.35 | <0.00 | – | – | – |
| Sebastes norvegicus | – | 2.94 | – | – | – | – | – | – | <0.00 | – | – | – |
| Serrivomer beanii | – | – | – | 1.12 | – | – | – | – | <0.00 | – | – | – |
| Squalus acanthias | 0.01 | 6.99 | – | 0.04 | – | – | – | – | – | – | – | – |
| Sternoptychidae | – | – | – | 1.34 | – | – | – | – | – | – | – | – |
| Stichaeus punctatus | – | – | – | – | – | – | – | 0.01 | 0.68 | – | 0.10 | |
| Stomias boa | – | – | – | 0.22 | – | – | – | – | – | – | – | – |
| Synaphobranchus kaupii | – | – | – | 0.50 | – | – | – | 0.30 | – | – | – | – |
| Triglops nybelini | – | 0.23 | – | <0.00 | – | – | – | 0.10 | <0.00 | – | 0.01 | 5.72 |
| Triglops pingelii | – | – | – | – | – | 4.12 | – | <0.00 | <0.00 | 1.24 | – | – |
When a species has a maximum IndVal value for a given assemblage, the species is classified as a secondary indicator species and the value is italicized.
Key (emboldened) and secondary indicator species of fish assemblages associated with six groups of stations in the lower estuary and northern Gulf of St Lawrence.
| Group B . | Group C . | ||
|---|---|---|---|
| Argentina silus | Amblyraja radiata | Bathyraja spinicauda | Lycodes terraenovae |
| Merluccius bilinearis | Arctozenus risso | Ceratiidae | Micromesistius poutassou |
| Sebastes | Centroscyllium fabricii | Chauliodus sloani | Myctophidae |
| Urophycis tenuis | Enchelyopus cimbrius | Cryptacanthodes maculatus | Paraliparis calidus |
| Anarhichas minor | Glyptocephalus cynoglossus | Cyclothone microdon | Paraliparis copei |
| Cottunculus microps | Malacoraja senta | Gaidropsarus argentatus | Serrivomer beanii |
| Lophius americanus | Melanostigma atlanticum | Gasterosteus aculeatus | Sternoptychidae |
| Pollachius virens | Myxine glutinosa | Leucoraja ocellata | Stomias boa |
| Sebastes norvegicus | Nezumia bairdii | Lycenchelys paxillus | Synaphobranchus kaupii |
| Squalus acanthias | Phycis chesteri | Lycenchelys verrillii | |
| Reinhardtius hippoglossoides | Lycodes esmarkii | ||
| Group E | Group G | Group H | Group I |
| Artediellus uncinatus | Anarhichas lupus | Myoxocephalus scorpius | Gymnocanthus tricuspis |
| Eumesogrammus praecisus | Artediellus atlanticus | Triglops murrayi | Leptagonus decagonus |
| Eumicrotremus spinosus | Gadus morhua | Limanda ferruginea | Leptoclinus maculatus |
| Gymnelus viridis | Hippoglossus hippoglossus | Myoxocephalus aenaeus | Lumpenus lampretaeformis |
| Icelus spatula | Hippoglossoides platessoides | Myoxocephalus octodecemspinosus | Ammodytes |
| Liparis gibbus | Lycodes vahlii | Pholis gunnellus | Anisarchus medius |
| Lycodes lavalaei | Careproctus reinhardti | Pseudopleuronectes americanus | Aspidophoroides monopterygius |
| Ulcina olrikii | Clupea harengus | Stichaeus punctatus | Cyclopterus lumpus |
| Boreogadus saida | Hemitripterus americanus | Gadus ogac | |
| Triglops pingelii | Melanogrammus aeglefinus | Icelus bicornis | |
| Rajella fyllae | Triglops nybelini | ||
| Group B . | Group C . | ||
|---|---|---|---|
| Argentina silus | Amblyraja radiata | Bathyraja spinicauda | Lycodes terraenovae |
| Merluccius bilinearis | Arctozenus risso | Ceratiidae | Micromesistius poutassou |
| Sebastes | Centroscyllium fabricii | Chauliodus sloani | Myctophidae |
| Urophycis tenuis | Enchelyopus cimbrius | Cryptacanthodes maculatus | Paraliparis calidus |
| Anarhichas minor | Glyptocephalus cynoglossus | Cyclothone microdon | Paraliparis copei |
| Cottunculus microps | Malacoraja senta | Gaidropsarus argentatus | Serrivomer beanii |
| Lophius americanus | Melanostigma atlanticum | Gasterosteus aculeatus | Sternoptychidae |
| Pollachius virens | Myxine glutinosa | Leucoraja ocellata | Stomias boa |
| Sebastes norvegicus | Nezumia bairdii | Lycenchelys paxillus | Synaphobranchus kaupii |
| Squalus acanthias | Phycis chesteri | Lycenchelys verrillii | |
| Reinhardtius hippoglossoides | Lycodes esmarkii | ||
| Group E | Group G | Group H | Group I |
| Artediellus uncinatus | Anarhichas lupus | Myoxocephalus scorpius | Gymnocanthus tricuspis |
| Eumesogrammus praecisus | Artediellus atlanticus | Triglops murrayi | Leptagonus decagonus |
| Eumicrotremus spinosus | Gadus morhua | Limanda ferruginea | Leptoclinus maculatus |
| Gymnelus viridis | Hippoglossus hippoglossus | Myoxocephalus aenaeus | Lumpenus lampretaeformis |
| Icelus spatula | Hippoglossoides platessoides | Myoxocephalus octodecemspinosus | Ammodytes |
| Liparis gibbus | Lycodes vahlii | Pholis gunnellus | Anisarchus medius |
| Lycodes lavalaei | Careproctus reinhardti | Pseudopleuronectes americanus | Aspidophoroides monopterygius |
| Ulcina olrikii | Clupea harengus | Stichaeus punctatus | Cyclopterus lumpus |
| Boreogadus saida | Hemitripterus americanus | Gadus ogac | |
| Triglops pingelii | Melanogrammus aeglefinus | Icelus bicornis | |
| Rajella fyllae | Triglops nybelini | ||
Key (emboldened) and secondary indicator species of fish assemblages associated with six groups of stations in the lower estuary and northern Gulf of St Lawrence.
| Group B . | Group C . | ||
|---|---|---|---|
| Argentina silus | Amblyraja radiata | Bathyraja spinicauda | Lycodes terraenovae |
| Merluccius bilinearis | Arctozenus risso | Ceratiidae | Micromesistius poutassou |
| Sebastes | Centroscyllium fabricii | Chauliodus sloani | Myctophidae |
| Urophycis tenuis | Enchelyopus cimbrius | Cryptacanthodes maculatus | Paraliparis calidus |
| Anarhichas minor | Glyptocephalus cynoglossus | Cyclothone microdon | Paraliparis copei |
| Cottunculus microps | Malacoraja senta | Gaidropsarus argentatus | Serrivomer beanii |
| Lophius americanus | Melanostigma atlanticum | Gasterosteus aculeatus | Sternoptychidae |
| Pollachius virens | Myxine glutinosa | Leucoraja ocellata | Stomias boa |
| Sebastes norvegicus | Nezumia bairdii | Lycenchelys paxillus | Synaphobranchus kaupii |
| Squalus acanthias | Phycis chesteri | Lycenchelys verrillii | |
| Reinhardtius hippoglossoides | Lycodes esmarkii | ||
| Group E | Group G | Group H | Group I |
| Artediellus uncinatus | Anarhichas lupus | Myoxocephalus scorpius | Gymnocanthus tricuspis |
| Eumesogrammus praecisus | Artediellus atlanticus | Triglops murrayi | Leptagonus decagonus |
| Eumicrotremus spinosus | Gadus morhua | Limanda ferruginea | Leptoclinus maculatus |
| Gymnelus viridis | Hippoglossus hippoglossus | Myoxocephalus aenaeus | Lumpenus lampretaeformis |
| Icelus spatula | Hippoglossoides platessoides | Myoxocephalus octodecemspinosus | Ammodytes |
| Liparis gibbus | Lycodes vahlii | Pholis gunnellus | Anisarchus medius |
| Lycodes lavalaei | Careproctus reinhardti | Pseudopleuronectes americanus | Aspidophoroides monopterygius |
| Ulcina olrikii | Clupea harengus | Stichaeus punctatus | Cyclopterus lumpus |
| Boreogadus saida | Hemitripterus americanus | Gadus ogac | |
| Triglops pingelii | Melanogrammus aeglefinus | Icelus bicornis | |
| Rajella fyllae | Triglops nybelini | ||
| Group B . | Group C . | ||
|---|---|---|---|
| Argentina silus | Amblyraja radiata | Bathyraja spinicauda | Lycodes terraenovae |
| Merluccius bilinearis | Arctozenus risso | Ceratiidae | Micromesistius poutassou |
| Sebastes | Centroscyllium fabricii | Chauliodus sloani | Myctophidae |
| Urophycis tenuis | Enchelyopus cimbrius | Cryptacanthodes maculatus | Paraliparis calidus |
| Anarhichas minor | Glyptocephalus cynoglossus | Cyclothone microdon | Paraliparis copei |
| Cottunculus microps | Malacoraja senta | Gaidropsarus argentatus | Serrivomer beanii |
| Lophius americanus | Melanostigma atlanticum | Gasterosteus aculeatus | Sternoptychidae |
| Pollachius virens | Myxine glutinosa | Leucoraja ocellata | Stomias boa |
| Sebastes norvegicus | Nezumia bairdii | Lycenchelys paxillus | Synaphobranchus kaupii |
| Squalus acanthias | Phycis chesteri | Lycenchelys verrillii | |
| Reinhardtius hippoglossoides | Lycodes esmarkii | ||
| Group E | Group G | Group H | Group I |
| Artediellus uncinatus | Anarhichas lupus | Myoxocephalus scorpius | Gymnocanthus tricuspis |
| Eumesogrammus praecisus | Artediellus atlanticus | Triglops murrayi | Leptagonus decagonus |
| Eumicrotremus spinosus | Gadus morhua | Limanda ferruginea | Leptoclinus maculatus |
| Gymnelus viridis | Hippoglossus hippoglossus | Myoxocephalus aenaeus | Lumpenus lampretaeformis |
| Icelus spatula | Hippoglossoides platessoides | Myoxocephalus octodecemspinosus | Ammodytes |
| Liparis gibbus | Lycodes vahlii | Pholis gunnellus | Anisarchus medius |
| Lycodes lavalaei | Careproctus reinhardti | Pseudopleuronectes americanus | Aspidophoroides monopterygius |
| Ulcina olrikii | Clupea harengus | Stichaeus punctatus | Cyclopterus lumpus |
| Boreogadus saida | Hemitripterus americanus | Gadus ogac | |
| Triglops pingelii | Melanogrammus aeglefinus | Icelus bicornis | |
| Rajella fyllae | Triglops nybelini | ||
Group B
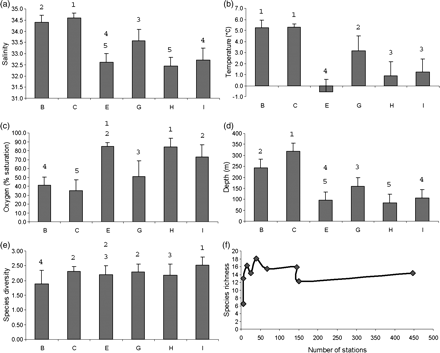
Group B was mainly located on the slopes bordering deep channels in the eastern part of the study area (Figure 3); the average depth was 244 m (Table 1). Except for temperature, which showed the same range as group C (Figure 4), environmental conditions differed from those of the other groups of stations. The group B species assemblage had a total richness of 52 species and a Shannon diversity index of 1.8, the lowest value among all groups. Sebastes spp. was the dominant taxon of the assemblage, contributing to 63% of the similarity between stations and an indicator value of 95% (Table 4). The assemblage was characterized by another three key species, Urophycis tenuis (63%), Merluccius bilinearis (25%), and Argentina silus (21%), and six secondary species.
Mean and standard error for (a) salinity, (b) temperature, (c) dissolved oxygen saturation, (d) depth, and (e) species diversity (Shannon H′), for six groups of stations in the lower estuary and northern Gulf of St Lawrence. Numbers above the bars show the results of pairwise comparisons (α = 0.05). (f) Relationship between average species richness and the number of stations.
Group C
Group C occupied deep channels and is characterized by greater depth than group A, an average of 318 m. This group had by far the largest number of stations (447 stations) and was associated with stations with the lowest oxygen levels (35% saturation) and higher average salinity (34.6). Except for temperature (see above), the environmental characteristics differed from all other groups (Figure 4). Species richness was high, 68 species, with a Shannon index of 2.3. The assemblage also contained the largest number of key (11) and secondary indicator species (20). Four taxa accounted for >50% of the similarity between stations: Reinhardtius hippoglossoides, Nezumia bairdii, Glyptocephalus cynoglossus, and Sebastes spp. These taxa represented an indicator value of >40%, except for Sebastes spp. (1.49), which nevertheless strongly contributed to similarity (14%).
Group E
Group E was geographically unique because it was located mainly north, in the Strait of Belle-Isle (Figure 3). The stations that made up this group were characterized by very low average temperatures (−0.6°C), i.e. lowest among all groups of stations. In terms of depth, group E was made up of shallow stations, with similar values of salinity and dissolved oxygen as groups H and I. The corresponding assemblage showed the lowest level of species richness (33 species), but a diversity index of 2.33, similar to groups C, G, and H (Figure 4). The group was made up of eight key indicator species and two secondary indicator species, all of a small size, in particular E. spinosus, with an indicator value of 88%. None of these species contributed much to between-station similarity of the group (maximum 18% for E. spinosus), yielding a total similarity, for all key indicator species, of 60%. The sculpin Triglops murrayi, although not a key indicator species, accounted for 16% of the similarity of the group.
Group G
Group G stations were widely distributed in the study area, with an average depth of 160 m. The stations occupied the edge of the slopes bordering the deep channels. Environmental characteristics for the group were representative of conditions prevailing below the CIL. Species richness was 58 species, and the diversity index (2.33) was similar to that of group C (deep channels). The species contributing most to between-station similarity were Hippoglossoides platessoides (27%), Gadus morhua (20%), and Sebastes spp. (12%). The other species each contributed at least 5% to within-group similarity, for a total value of 60%. The assemblage had ten indicator species, including six key ones, H. platessoides (46%), Hippoglossus hippoglossus (46%), Lycodes vahlii (45%), G. morhua (19%), Anarhichas lupus (16%), and Artediellus atlanticus (15%).
Group H
Stations of group H were close to the coast (average depth 83 m; Figure 3), sharing most characteristics with group E, except temperature, which it shared with group I (Figure 4). Stations were located in the eastern half of the study area, around Anticosti Island and on the west coast of Newfoundland. Species richness for the assemblage was 55 species, with an average diversity index of 2.12. Two species accounted for the cohesion of the group, G. morhua (32% of similarity) and T. murrayi (24% of similarity). The group contained only two key indicator species, T. murrayi and Myoxocephalus scorpius, plus six secondary ones, including Limanda ferruginea and Myoxocephalus octodecemspinosus.
Group I
Mainly located north of Anticosti Island, the stations of this group occupied cold, well-oxygenated waters at an average depth of 106 m, intermediate between groups G and H (Figure 3). They tended to be similar to stations in group H. Species richness for the assemblage was 47 species. The diversity index was the highest observed anywhere in the study (2.53; Figure 4). Four of the species classified as key indicator species: Leptoclinus maculatus (70%), Lumpenus lampretaeformis (42%), Gymnocanthus tricuspis (31%), and Leptagonus decagonus (29%), and seven as secondary indicator species. Group cohesion was mainly represented by four species: H. platessoides (22%), L. maculatus (14%), G. morhua (11%), and T. murrayi (9%).
Relationships between stations, indicator species, and environmental variables
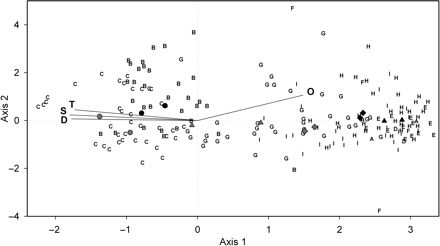
Scores for stations and key species (as symbols) and for environmental variables (as arrows) are shown as a triplot in Figure 5. The DCCA showed overall strong correlation between species abundance and environmental conditions prevailing at the stations sampled. The total variance of the values fitted in the DCCA (sum of all canonical eigenvalues) was 0.94, but only the first two axes were considered canonical (Monte Carlo permutation tests, p < 0.002). The first and the second axes explained 74% of the variation in abundance data, with eigenvalues of 0.60 and 0.14, respectively (Table 5). The first axis explained 14.9% of the variance in species data, and 64.0% of the variance in the species–environment relationship, whereas the second axis explained 3.7% of the variance in species data and 15.2% of the variance in the species–environment relationship. Therefore, our analysis explained 18.4% of the variance in species abundance data and 79.2% of the variance in the species–environment relationship. Species–environment correlations with ordination axes 1 and 2 were strong (0.97 and 0.74, respectively; Table 5).
Results of a DCCA on fish abundance data looking at the relationships between stations, species, and environmental conditions, with results shown for the first two axes of the ordination plot (p-values are those of the Monte Carlo permutation test).
| Parameter . | Axis 1 . | Axis 2 . | p-value . |
|---|---|---|---|
| Eigenvalue | 0.604 | 0.144 | |
| Species-environment correlations | 0.965 | 0.736 | |
| Cumulative percentage variance | |||
| of species data | 14.9 | 18.4 | |
| of species–environment relationship | 64.0 | 79.2 | |
| Correlation with axes of the ordination plot | |||
| Depth | −0.940 | 0.025 | 0.002 |
| Salinity | −0.955 | 0.085 | 0.002 |
| Temperature | −0.915 | 0.162 | 0.002 |
| Dissolved oxygen | 0.786 | 0.382 | 0.002 |
| Parameter . | Axis 1 . | Axis 2 . | p-value . |
|---|---|---|---|
| Eigenvalue | 0.604 | 0.144 | |
| Species-environment correlations | 0.965 | 0.736 | |
| Cumulative percentage variance | |||
| of species data | 14.9 | 18.4 | |
| of species–environment relationship | 64.0 | 79.2 | |
| Correlation with axes of the ordination plot | |||
| Depth | −0.940 | 0.025 | 0.002 |
| Salinity | −0.955 | 0.085 | 0.002 |
| Temperature | −0.915 | 0.162 | 0.002 |
| Dissolved oxygen | 0.786 | 0.382 | 0.002 |
Results of a DCCA on fish abundance data looking at the relationships between stations, species, and environmental conditions, with results shown for the first two axes of the ordination plot (p-values are those of the Monte Carlo permutation test).
| Parameter . | Axis 1 . | Axis 2 . | p-value . |
|---|---|---|---|
| Eigenvalue | 0.604 | 0.144 | |
| Species-environment correlations | 0.965 | 0.736 | |
| Cumulative percentage variance | |||
| of species data | 14.9 | 18.4 | |
| of species–environment relationship | 64.0 | 79.2 | |
| Correlation with axes of the ordination plot | |||
| Depth | −0.940 | 0.025 | 0.002 |
| Salinity | −0.955 | 0.085 | 0.002 |
| Temperature | −0.915 | 0.162 | 0.002 |
| Dissolved oxygen | 0.786 | 0.382 | 0.002 |
| Parameter . | Axis 1 . | Axis 2 . | p-value . |
|---|---|---|---|
| Eigenvalue | 0.604 | 0.144 | |
| Species-environment correlations | 0.965 | 0.736 | |
| Cumulative percentage variance | |||
| of species data | 14.9 | 18.4 | |
| of species–environment relationship | 64.0 | 79.2 | |
| Correlation with axes of the ordination plot | |||
| Depth | −0.940 | 0.025 | 0.002 |
| Salinity | −0.955 | 0.085 | 0.002 |
| Temperature | −0.915 | 0.162 | 0.002 |
| Dissolved oxygen | 0.786 | 0.382 | 0.002 |
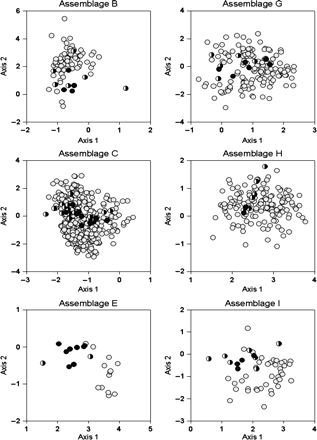
DCCA triplot (first two axes) showing the scores for groups of stations (letters A–I); not all stations are plotted for clarity. Scores for two key species with the highest indicator values are shown for groups B (black circles, Sebastes spp. and U. tenuis), C (grey circles, R. hippoglossoides and N. bairdii), E (black triangles, Eumicrotremus spinosus and Gymnelus viridis), G (grey triangles, H. platessoides and H. hippoglossus), H (black diamonds, T. murrayi and M. scorpius), and I (grey diamonds, L. maculatus and L. lampretaeformis). Environmental variable scores are shown as vectors: D, depth; S, salinity; T, temperature; O, dissolved oxygen saturation.
Some groups were strongly influenced by salinity and temperature, others more by dissolved oxygen, and the relationships varied among species of the same assemblage. Depth, salinity, and temperature were very strongly negatively correlated with ordination axis 1 (−0.94. −0.96, and −0.92), mainly influencing groups B and C (Table 5; Figure 5). These environmental variables were strongly correlated with each other and very weakly correlated with axis 2 (depth, 0.02; salinity, 0.09; temperature, 0.16). Dissolved oxygen was positively correlated with both axes 1 (0.79) and 2 (0.38) and influenced most of the other groups (Figure 5). The weighted average abundance values of key species for each assemblage with respect to standardized environmental variables are shown in Table 6.
Species–environment relations as weighted average species abundance for each environmental variable, with the highest absolute value for a given species shown emboldened, and weighted average values obtained from a DCCA.
| Assemblage . | Species . | Depth (m) . | Salinity (psu) . | Temperature (°C) . | Dissolved oxygen (psu) . |
|---|---|---|---|---|---|
| B | Argentina silus | 0.29 | 0.64 | 0.69 | 0.06 |
| Merluccius bilinearis | 0.04 | 0.39 | 0.59 | −0.55 | |
| Sebastes | 0.17 | 0.38 | 0.45 | −0.18 | |
| Urophycis tenuis | 0.32 | 0.56 | 0.63 | −0.50 | |
| C | Amblyraja radiata | 0.18 | 0.23 | 0.22 | −0.37 |
| Arctozenus risso | 0.96 | 0.76 | 0.62 | −0.29 | |
| Centroscyllium fabricii | 1.50 | 0.89 | 0.62 | −0.09 | |
| Enchelyopus cimbrius | 0.46 | 0.54 | 0.52 | −0.72 | |
| Glyptocephalus cynoglossus | 0.43 | 0.44 | 0.42 | −0.44 | |
| Malacoraja senta | 0.40 | 0.46 | 0.43 | −0.48 | |
| Melanostigma atlanticum | 0.76 | 0.63 | 0.57 | −0.76 | |
| Myxine glutinosa | 0.78 | 0.72 | 0.64 | −0.53 | |
| Nezumia bairdii | 1.02 | 0.78 | 0.64 | −0.34 | |
| Phycis chesteri | 1.31 | 0.87 | 0.63 | 0.09 | |
| Reinhardtius hippoglossoides | 0.49 | 0.54 | 0.53 | −0.72 | |
| E | Artediellus uncinatus | −1.07 | −1.24 | −1.30 | 1.21 |
| Eumesogrammus praecisus | −1.24 | −1.44 | −1.60 | 1.38 | |
| Eumicrotremus spinosus | −1.32 | −1.54 | −1.80 | 1.55 | |
| Gymnelus viridis | −1.38 | −1.62 | −2.07 | 1.75 | |
| Icelus spatula | −1.22 | −1.49 | −1.88 | 1.39 | |
| Liparis gibbus | −1.21 | −1.47 | −1.67 | 1.23 | |
| Lycodes lavalaei | −0.40 | −0.17 | −0.20 | −0.34 | |
| Ulcina olrikii | −1.27 | −1.47 | −2.24 | 1.74 | |
| G | Anarhichas lupus | −0.88 | −0.78 | −0.73 | 0.81 |
| Artediellus atlanticus | −0.51 | −0.40 | −0.34 | 0.37 | |
| Gadus morhua | −0.96 | −0.94 | −0.82 | 0.78 | |
| Hippoglossoides platessoides | −0.59 | −0.51 | −0.48 | 0.32 | |
| Hippoglossus hippoglossus | −0.19 | 0.12 | 0.22 | −0.45 | |
| Lycodes vahlii | −1.17 | −1.34 | −1.56 | 1.27 | |
| H | Myoxocephalus scorpius | −1.34 | −1.64 | −1.17 | 1.49 |
| Triglops murrayi | −1.23 | −1.42 | −1.40 | 1.35 | |
| I | Gymnocanthus tricuspis | −1.13 | −1.27 | −1.25 | 1.11 |
| Leptagonus decagonus | −0.91 | −1.01 | −.96 | 0.55 | |
| Leptoclinus maculatus | −0.94 | −0.96 | −1.08 | 0.75 | |
| Lumpenus lampretaeformis | −0.92 | −0.89 | −0.95 | 0.53 |
| Assemblage . | Species . | Depth (m) . | Salinity (psu) . | Temperature (°C) . | Dissolved oxygen (psu) . |
|---|---|---|---|---|---|
| B | Argentina silus | 0.29 | 0.64 | 0.69 | 0.06 |
| Merluccius bilinearis | 0.04 | 0.39 | 0.59 | −0.55 | |
| Sebastes | 0.17 | 0.38 | 0.45 | −0.18 | |
| Urophycis tenuis | 0.32 | 0.56 | 0.63 | −0.50 | |
| C | Amblyraja radiata | 0.18 | 0.23 | 0.22 | −0.37 |
| Arctozenus risso | 0.96 | 0.76 | 0.62 | −0.29 | |
| Centroscyllium fabricii | 1.50 | 0.89 | 0.62 | −0.09 | |
| Enchelyopus cimbrius | 0.46 | 0.54 | 0.52 | −0.72 | |
| Glyptocephalus cynoglossus | 0.43 | 0.44 | 0.42 | −0.44 | |
| Malacoraja senta | 0.40 | 0.46 | 0.43 | −0.48 | |
| Melanostigma atlanticum | 0.76 | 0.63 | 0.57 | −0.76 | |
| Myxine glutinosa | 0.78 | 0.72 | 0.64 | −0.53 | |
| Nezumia bairdii | 1.02 | 0.78 | 0.64 | −0.34 | |
| Phycis chesteri | 1.31 | 0.87 | 0.63 | 0.09 | |
| Reinhardtius hippoglossoides | 0.49 | 0.54 | 0.53 | −0.72 | |
| E | Artediellus uncinatus | −1.07 | −1.24 | −1.30 | 1.21 |
| Eumesogrammus praecisus | −1.24 | −1.44 | −1.60 | 1.38 | |
| Eumicrotremus spinosus | −1.32 | −1.54 | −1.80 | 1.55 | |
| Gymnelus viridis | −1.38 | −1.62 | −2.07 | 1.75 | |
| Icelus spatula | −1.22 | −1.49 | −1.88 | 1.39 | |
| Liparis gibbus | −1.21 | −1.47 | −1.67 | 1.23 | |
| Lycodes lavalaei | −0.40 | −0.17 | −0.20 | −0.34 | |
| Ulcina olrikii | −1.27 | −1.47 | −2.24 | 1.74 | |
| G | Anarhichas lupus | −0.88 | −0.78 | −0.73 | 0.81 |
| Artediellus atlanticus | −0.51 | −0.40 | −0.34 | 0.37 | |
| Gadus morhua | −0.96 | −0.94 | −0.82 | 0.78 | |
| Hippoglossoides platessoides | −0.59 | −0.51 | −0.48 | 0.32 | |
| Hippoglossus hippoglossus | −0.19 | 0.12 | 0.22 | −0.45 | |
| Lycodes vahlii | −1.17 | −1.34 | −1.56 | 1.27 | |
| H | Myoxocephalus scorpius | −1.34 | −1.64 | −1.17 | 1.49 |
| Triglops murrayi | −1.23 | −1.42 | −1.40 | 1.35 | |
| I | Gymnocanthus tricuspis | −1.13 | −1.27 | −1.25 | 1.11 |
| Leptagonus decagonus | −0.91 | −1.01 | −.96 | 0.55 | |
| Leptoclinus maculatus | −0.94 | −0.96 | −1.08 | 0.75 | |
| Lumpenus lampretaeformis | −0.92 | −0.89 | −0.95 | 0.53 |
Species–environment relations as weighted average species abundance for each environmental variable, with the highest absolute value for a given species shown emboldened, and weighted average values obtained from a DCCA.
| Assemblage . | Species . | Depth (m) . | Salinity (psu) . | Temperature (°C) . | Dissolved oxygen (psu) . |
|---|---|---|---|---|---|
| B | Argentina silus | 0.29 | 0.64 | 0.69 | 0.06 |
| Merluccius bilinearis | 0.04 | 0.39 | 0.59 | −0.55 | |
| Sebastes | 0.17 | 0.38 | 0.45 | −0.18 | |
| Urophycis tenuis | 0.32 | 0.56 | 0.63 | −0.50 | |
| C | Amblyraja radiata | 0.18 | 0.23 | 0.22 | −0.37 |
| Arctozenus risso | 0.96 | 0.76 | 0.62 | −0.29 | |
| Centroscyllium fabricii | 1.50 | 0.89 | 0.62 | −0.09 | |
| Enchelyopus cimbrius | 0.46 | 0.54 | 0.52 | −0.72 | |
| Glyptocephalus cynoglossus | 0.43 | 0.44 | 0.42 | −0.44 | |
| Malacoraja senta | 0.40 | 0.46 | 0.43 | −0.48 | |
| Melanostigma atlanticum | 0.76 | 0.63 | 0.57 | −0.76 | |
| Myxine glutinosa | 0.78 | 0.72 | 0.64 | −0.53 | |
| Nezumia bairdii | 1.02 | 0.78 | 0.64 | −0.34 | |
| Phycis chesteri | 1.31 | 0.87 | 0.63 | 0.09 | |
| Reinhardtius hippoglossoides | 0.49 | 0.54 | 0.53 | −0.72 | |
| E | Artediellus uncinatus | −1.07 | −1.24 | −1.30 | 1.21 |
| Eumesogrammus praecisus | −1.24 | −1.44 | −1.60 | 1.38 | |
| Eumicrotremus spinosus | −1.32 | −1.54 | −1.80 | 1.55 | |
| Gymnelus viridis | −1.38 | −1.62 | −2.07 | 1.75 | |
| Icelus spatula | −1.22 | −1.49 | −1.88 | 1.39 | |
| Liparis gibbus | −1.21 | −1.47 | −1.67 | 1.23 | |
| Lycodes lavalaei | −0.40 | −0.17 | −0.20 | −0.34 | |
| Ulcina olrikii | −1.27 | −1.47 | −2.24 | 1.74 | |
| G | Anarhichas lupus | −0.88 | −0.78 | −0.73 | 0.81 |
| Artediellus atlanticus | −0.51 | −0.40 | −0.34 | 0.37 | |
| Gadus morhua | −0.96 | −0.94 | −0.82 | 0.78 | |
| Hippoglossoides platessoides | −0.59 | −0.51 | −0.48 | 0.32 | |
| Hippoglossus hippoglossus | −0.19 | 0.12 | 0.22 | −0.45 | |
| Lycodes vahlii | −1.17 | −1.34 | −1.56 | 1.27 | |
| H | Myoxocephalus scorpius | −1.34 | −1.64 | −1.17 | 1.49 |
| Triglops murrayi | −1.23 | −1.42 | −1.40 | 1.35 | |
| I | Gymnocanthus tricuspis | −1.13 | −1.27 | −1.25 | 1.11 |
| Leptagonus decagonus | −0.91 | −1.01 | −.96 | 0.55 | |
| Leptoclinus maculatus | −0.94 | −0.96 | −1.08 | 0.75 | |
| Lumpenus lampretaeformis | −0.92 | −0.89 | −0.95 | 0.53 |
| Assemblage . | Species . | Depth (m) . | Salinity (psu) . | Temperature (°C) . | Dissolved oxygen (psu) . |
|---|---|---|---|---|---|
| B | Argentina silus | 0.29 | 0.64 | 0.69 | 0.06 |
| Merluccius bilinearis | 0.04 | 0.39 | 0.59 | −0.55 | |
| Sebastes | 0.17 | 0.38 | 0.45 | −0.18 | |
| Urophycis tenuis | 0.32 | 0.56 | 0.63 | −0.50 | |
| C | Amblyraja radiata | 0.18 | 0.23 | 0.22 | −0.37 |
| Arctozenus risso | 0.96 | 0.76 | 0.62 | −0.29 | |
| Centroscyllium fabricii | 1.50 | 0.89 | 0.62 | −0.09 | |
| Enchelyopus cimbrius | 0.46 | 0.54 | 0.52 | −0.72 | |
| Glyptocephalus cynoglossus | 0.43 | 0.44 | 0.42 | −0.44 | |
| Malacoraja senta | 0.40 | 0.46 | 0.43 | −0.48 | |
| Melanostigma atlanticum | 0.76 | 0.63 | 0.57 | −0.76 | |
| Myxine glutinosa | 0.78 | 0.72 | 0.64 | −0.53 | |
| Nezumia bairdii | 1.02 | 0.78 | 0.64 | −0.34 | |
| Phycis chesteri | 1.31 | 0.87 | 0.63 | 0.09 | |
| Reinhardtius hippoglossoides | 0.49 | 0.54 | 0.53 | −0.72 | |
| E | Artediellus uncinatus | −1.07 | −1.24 | −1.30 | 1.21 |
| Eumesogrammus praecisus | −1.24 | −1.44 | −1.60 | 1.38 | |
| Eumicrotremus spinosus | −1.32 | −1.54 | −1.80 | 1.55 | |
| Gymnelus viridis | −1.38 | −1.62 | −2.07 | 1.75 | |
| Icelus spatula | −1.22 | −1.49 | −1.88 | 1.39 | |
| Liparis gibbus | −1.21 | −1.47 | −1.67 | 1.23 | |
| Lycodes lavalaei | −0.40 | −0.17 | −0.20 | −0.34 | |
| Ulcina olrikii | −1.27 | −1.47 | −2.24 | 1.74 | |
| G | Anarhichas lupus | −0.88 | −0.78 | −0.73 | 0.81 |
| Artediellus atlanticus | −0.51 | −0.40 | −0.34 | 0.37 | |
| Gadus morhua | −0.96 | −0.94 | −0.82 | 0.78 | |
| Hippoglossoides platessoides | −0.59 | −0.51 | −0.48 | 0.32 | |
| Hippoglossus hippoglossus | −0.19 | 0.12 | 0.22 | −0.45 | |
| Lycodes vahlii | −1.17 | −1.34 | −1.56 | 1.27 | |
| H | Myoxocephalus scorpius | −1.34 | −1.64 | −1.17 | 1.49 |
| Triglops murrayi | −1.23 | −1.42 | −1.40 | 1.35 | |
| I | Gymnocanthus tricuspis | −1.13 | −1.27 | −1.25 | 1.11 |
| Leptagonus decagonus | −0.91 | −1.01 | −.96 | 0.55 | |
| Leptoclinus maculatus | −0.94 | −0.96 | −1.08 | 0.75 | |
| Lumpenus lampretaeformis | −0.92 | −0.89 | −0.95 | 0.53 |
The indicator species of group B exhibited greater weighted average abundance values with temperature, followed by salinity, except for M. bilinearis, which also showed a large weighted average abundance value for dissolved oxygen (Table 6). For group C, the key indicator species were divided between two significant factors; large positive weighted average abundance values with depth and large negative weighted average abundance values with dissolved oxygen. Most of group E showed a pronounced inverse correlation with temperature (weighted average abundance values less than −1.6); dissolved oxygen also played a general role in the distribution of these species. Species assigned to group G showed large negative weighted average abundance values with depth, except for L. vahlii and H. hippoglossus, which, respectively, were negatively correlated with temperature and dissolved oxygen. For species of groups H and I, negative weighted average abundance values were observed for depth, salinity, and temperature; only dissolved oxygen had positive values.
Key and secondary indicator species, shown, respectively, as filled and half-filled circles in Figure 6, fitted more or less closely the cloud of stations for their respective assemblages, as distributed in the ordination plot. Indicator species of groups C, G, and H were located centrally among stations of those groups, whereas those of groups B, E, and I were off-centre. The relative position of key and secondary indicator species was also not the same across groups (Figure 6). Some groups, e.g. group C, displayed greater cohesion of key and secondary indicator species than other groups, e.g. group H. Species within an assemblage that separated in the ordination plot likely responded slightly differently to the environmental parameters considered or may have responded to such other factors as other species within the same assemblage.
Ordination plot of stations and indicator species for six groups of stations and associated assemblages in the lower estuary and northern Gulf of St Lawrence. Stations, open circles; key species, filled circles; secondary species, half-filled circles.
Discussion
Fish assemblages in the Northwest Atlantic
Several studies have examined different aspects of fish diversity in the Northwest Atlantic. More effort has been devoted in recent years to developing an inventory of marine biodiversity worldwide. Sampling protocols in groundfish surveys conducted in recent years in the northern Gulf of St Lawrence have been modified accordingly (Nozères et al., 2010), making it possible to study the structure and environmental relationships of demersal fish in the area. Studies focusing on demersal fish have examined research survey data obtained with bottom trawls and considered short (González-Troncoso et al., 2006, and this study) or long time-series (Mahon and Smith, 1989; Gomes et al., 1992; Mahon et al., 1998). All provided conclusive evidence that fish species form spatially coherent assemblages, with their distribution explained by depth and depth-correlated patterns in environmental conditions.
Species composition appears to be stable over long periods of time (Mahon and Smith, 1989; Gomes et al., 1992), but changes in stock productivity and habitats associations do vary over time (e.g. Dutil and Brander, 2003; Swain and Benoit, 2006), resulting in changing dominance patterns within fish assemblages (Shackell and Frank, 2003). Differences in the composition of demersal assemblages among studies conducted in the Northwest Atlantic can be explained by the fact that size and location of study areas varied: Flemish Cap (González-Troncoso et al., 2006), Grand Bank (Gomes et al., 1992), Scotian Shelf (Mahon and Smith, 1989; Shackell and Frank, 2003), northern Gulf of St Lawrence (this study), and the whole east coast of North America (Mahon et al., 1998).
Given its large size and diversity of habitat (Dutil et al., 2011), the St Lawrence system creates conditions conducive to hosting a large number of species organized into well-structured assemblages. Heterogeneous environments are well-suited for the study of relationships between species, and between species and their environment. Complex oceanographic processes, such as observed in the St Lawrence (Therriault, 1991), result from predictable processes (Legendre and Legendre, 1998). This complexity and spatial and temporal heterogeneity add up to more niches to meet the needs of a greater number of species (Hutchinson, 1975).
In all, 82 fish species were reported in the bottom trawl surveys conducted in the northern Gulf of St Lawrence between 2004 and 2008 (Dutil et al., 2009), forming nine assemblages to which both commercial and non-commercial species contributed. Although there are differences in the make-up of assemblages between studies, both commercial and non-commercial species play a role in structuring the assemblages. Non-commercial and less abundant species are a source of information that should not be overlooked; they may be indicative of unique aspects of the environment to which an assemblage is associated (Souissi et al., 2001), and informative of factors shaping the structure of an ecosystem. Relationships among species and the structuring effect of the environment are the two main drivers forcing the association between species and explaining the spatial distribution of assemblages (Legendre and Legendre, 1998).
Multispecies approaches
Several methods can be used to group stations, define species assemblages, and determine the species best characterizing the assemblages. The method we used makes no a priori assumption on relationships among species or sectors, as is often the case in such studies (Gomes et al., 1992; Souissi et al., 2001; Jørgensen et al., 2005). In the present study, groups formed at a threshold value of 40% similarity were retained. That threshold was validated by similarity profile permutation tests (SIMPROF; p > 0.05) and was considered to maintain a balance between the number of groups formed and the scale at which environmental conditions varied in the study area.
Indicator species can also be defined by various metrics, including the Dufrêne and Legendre (1997) indicator value used here. That index is based on two criteria: fidelity and specificity. Fidelity is the frequency at which a species is present in the stations of the same group. Specificity is measured by the relative abundance of the species in the stations of the same group compared with stations of other groups. Dufrêne and Legendre (1997) used a threshold value of 25%, but did not make a specific proposal for a threshold. The 15% threshold level used here assigns more weight to rare species. It was set arbitrarily as a criterion to separate the main (>15%) and the secondary indicator species (<15%). Values <16% would indicate species present at ∼40% of the stations within a group with a relative abundance in that group of ∼40% (Dufrêne and Legendre, 1997).
Catchability and hence our perception of abundance varies between species, so the assemblages described here also reflect gear selectivity and sampling strategy. However, it is unlikely that the sampling effort (870 stations over a period of 5 years) was too weak to provide a complete account of species richness on the seafloor in the study area. The curve of cumulative number of species across groups clearly reached an asymptote, so increased sampling would be unlikely to have revealed many new species, even rare ones. Moreover, group species richness and average species richness of stations within groups did not show a general trend to increase with increasing number of stations when the latter numbered >50 (not shown). All fish sampled were identified, and each station was sampled by a set protocol, using the same vessel and gear within the same depth strata each year.
Fish assemblages and environmental conditions
Constrained ordination techniques (Legendre and Legendre, 1998) can be used to describe relationships between species assemblages and habitats (Mahon and Smith, 1989; Gomes et al., 1992; Dufrêne and Legendre, 1997; Mahon et al., 1998; Souissi et al., 2001; Jørgensen et al., 2005; González-Troncoso et al., 2006). Species belonging to the same assemblage generally show a similar response to environmental conditions (Snelgrove and Haedrich, 1985; Cech et al., 1990; Mahon et al., 1998; Brown, 2000). This can be interpreted as indicating that the species forming the assemblages, particularly the indicator species, seek out similar environmental conditions.
Interpretation of the results, however, needs to take into account the scale at which the study is conducted (Herbold, 1984; Rahel et al., 1984). If the size of the study area is such that it maximizes the variability of a parameter (and therefore a dependent variable in the model), this parameter can help in structuring assemblages. It follows that the formation of an assemblage, which is dictated by the choice of parameters, will also be dictated by the size of the study area or the ratio of the study area and the range of species or local populations (Legendre and Borcard, 2006). Physico-chemical variables often show large-scale vertical and horizontal gradients, and species occupy a range of conditions in these gradients (Mahon and Smith, 1989; Swain et al., 1998). Mobile species and species with a broad niche are likely to encounter a wide range of conditions. As the geographic distribution of each species is not necessarily covered entirely by the size of the study area, factors governing the distribution will not be taken into account in the formation of assemblages (Holyoak et al., 2005). Portions of the life cycle and some areas of great importance may not be taken into account (Boulinier et al., 2005).
Depth is a key variable to consider when explaining the structure of fish communities (Snelgrove and Haedrich, 1985; Swain and Benoit, 2006; Tamdradi, 2007). However, disentangling the relative weight of depth and other environmental parameters is not trivial because of the strong correlation generally observed between depth, salinity, and temperature in a stratified medium (Swain and Benoit, 2006). Fish assemblages in the northern Gulf of St Lawrence appear to be strongly associated with environmental variables considered in our analyses. The DCCA showed the overriding importance of depth on axis 1. The other two variables associated with axis 1, salinity and temperature, were strongly correlated with depth and to a lesser extent with dissolved oxygen, which is inversely correlated with depth.
In the study area, two large groups of stations were defined based on depth, a coastal and a deep station set (Tamdradi, 2007). Similarly, in our study, groups B and C were associated with deep stations and the other groups were derived based on their relative distance from the coast. Subdividing these two sets of assemblages (groups) based on their relationship with other environmental parameters is a challenge. A detailed description of other habitat features at different scales may be needed to understand better the habitat requirements of the assemblages, including groups A and F, which had very few stations.
Relevance to management
The implementation of ecosystem-based approaches to fishery and habitat management calls for greater integration of information on various components of the ecosystem, and including the natural and the anthropogenic processes having an impact on the resources. Multispecies approaches such as used here provide background information on the structure of large ecosystems over broad geographic areas. Key species were identified, potential interactions flagged, and associations with environmental conditions gauged. The multispecies approach allowed us to study the effect of natural and anthropogenic changes in environmental conditions across a community, or subset, of species forming the community and to monitor changes in species distributions at a finer scale by integrating the hierarchy existing between different species within assemblages. It also permitted us to monitor potential changes in species behaviour through observing the changing structure of the assemblage in time and space.
The inclusion of rare species, which are excluded from the vast majority of studies, allowed a dynamic representation of the ecological domain not possible when surveying only the most common and most abundant species, which are often less sensitive to fine-scale variations in their environment. Further, the integration of all species allowed an overview of the whole community, as opposed to focusing on a single species, which enabled us to take internal controls (inter- and intraspecific relationships, spatial dependence, population density) into account along with the external controls of environment and geographic location affecting populations.
Biodiversity and sustainable fisheries are subjects of ever-increasing concern. The study of fish assemblages can provide information on current ecosystem status and also be a tool for studying the association with large-scale habitats (Dutil et al., 2011) and for monitoring the response of rare or dominant species to management measures such as changes in environmental conditions resulting from global warming.
Acknowledgements
Part of this work was conducted in partial fulfilment of the requirements for P-MC of a Master's degree (Université du Québec à Rimouski) under the supervision of J-DD and Jean-Claude Brêthes. We are indebted to our many dedicated colleagues at Institut Maurice-Lamontagne (DFO) who participated in the field surveys aboard the CCGS “Teleost” and to Marilyn Thorne, Richard Larocque, Serge Proulx, and Denis Bernier who assisted with crucial aspects of data acquisition, validation, and mining. We also thank an anonymous reviewer for constructive comments and Cyrena Riley for proof-reading an early version.