-
PDF
- Split View
-
Views
-
Cite
Cite
Kyle J. Krumsick, George A. Rose, Atlantic cod (Gadus morhua) feed during spawning off Newfoundland and Labrador, ICES Journal of Marine Science, Volume 69, Issue 10, December 2012, Pages 1701–1709, https://doi.org/10.1093/icesjms/fss158
Close - Share Icon Share
Abstract
We test a current assumption that Atlantic cod (Gadus morhua) off Newfoundland and Labrador, Canada, do not feed during the protracted spawning season (March–September). Stomach contents were analysed from 10 473 cod from four Northwest Atlantic Fisheries Organization regions (2J, 3K, 3L, and 3Ps) over 9 years from which gonads were also analysed to determine sex and maturity status. Adult cod in spawning condition did feed in all regions, usually at rates equivalent to or even greater than non-spawning fish and juveniles. Both sexes fed during spawning, though females consumed lesser amounts. Regional differences were evident. The total fullness index was greater in the northern (2J) than the southern (3Ps) region, with no consistent differences between spawners and non-spawners. The most southerly region (3Ps) exhibited the greatest prey diversity, the northern region (2J) the least. Shrimp was the major diet item in the northern regions. Capelin, zooplankton, crab, and other fish increased in importance to the south. Differences in prey items between non-spawning and spawning individuals of both sexes were possibly related to spawning behaviour. Models using consumption rates should not assume that cod do not feed during the protracted spawning season in these waters.Krumsick, K. J., and Rose, G. A. 2012. Atlantic cod (Gadus morhua) feed during spawning off Newfoundland and Labrador. – ICES Journal of Marine Science, 69: 1701–1709.
Introduction
Knowledge of diet and consumption of dominant predatory species such as Atlantic cod (Gadus morhua) are pivotal to ecosystem models and approach to fisheries management (Bundy and Fanning, 2005). Much of the information on diet has been derived from stomach content studies, many limited to a single season within one or more years with assumptions of how this relates to annual consumption (Popova, 1963; Lilly and Fleming, 1981; Methven and Piatt, 1989; Casas and Paz, 1996; Gerasimova and Kiseleva, 1998; Adlerstein and Welleman, 2000). More comprehensive information on annual cod feeding has been obtained from studies utilizing data over entire years (Turuk, 1971; Daan, 1973; Klemetsen, 1982; Albikovskaya and Gerasimova, 1993; Schwalme and Chouinard, 1999; Mello and Rose, 2005; Link et al., 2009).
In the Newfoundland–Labrador area, cod spawning is protracted over 40–50 days, typically occurring between March and August (Templeman and Fleming, 1962; Templeman and May, 1965; Fitzpatrick and Miller, 1979; Hutchings and Myers, 1993; Myers et al., 1993). Female cod release eggs in batches over spawning intervals of 2 or more days (Kjesbu, 1989; Fordham and Trippel, 1999). Known spawning locations around Newfoundland and Labrador are widespread, including outer portions of the Labrador shelf (notably the Hamilton Bank and Hawke Channel), northeast Newfoundland Shelf, northern and southern Grand Banks, Halibut Channel and St Pierre Bank, as well as several inshore areas, such as Trinity and Placentia Bays, though spawning may also occur at varying locations during migration (Templeman and May, 1965; Serebryakov, 1967; Fitzpatrick and Miller, 1979; Hutchings et al., 1993; Rose, 1993; Smedbol and Wroblewski, 1997).
A prevailing assumption for Northwest Atlantic cod, in particular the stocks of Newfoundland and Labrador, is that these fish do not feed during the protracted spawning period. This assumption has been based largely on studies conducted on captive fish in which feeding appeared to cease during spawning (Brawn, 1961; Templeman, 1965; Fordham and Trippel, 1999). Many models and findings relating to cod consumption and growth have incorporated this assumption (Hutchings, 1999; Skjæraasen et al., 2004; Jørgensen and Fiksen, 2006). The same assumption has been made for Northeast Atlantic cod, but was recently shown to be invalid (Michalsen et al., 2008). North Sea cod have long been known to feed during spawning (Daan, 1973). These findings led to a questioning of the assumption that cod off the coasts of Newfoundland and Labrador cease feeding during spawning.
In this study, we test a base hypothesis that spawning cod cease feeding in Newfoundland and Labrador waters. We also tested four null hypotheses relating to feeding behaviour during the spawning period. Specifically, we test for no difference in: (i) feeding amount in spawning, non-spawning, and juvenile fish; (ii) prey species; (iii) diversity of prey; and (iv) prey consumption between sexes. These hypotheses are assessed using data for cod populations in four Northwest Atlantic Fisheries Organization (NAFO) subdivisions off Newfoundland and Labrador.
Methods
Stomach analysis
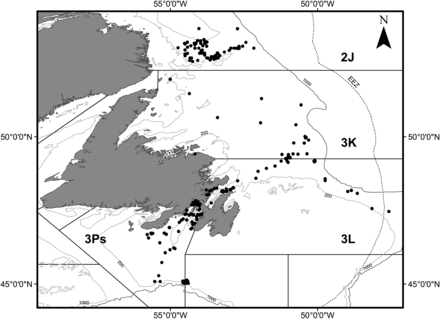
In all, 10 473 cod stomachs were collected between March and September from trawl (Campelen 1800 and Yankee 36) and handline catches during surveys from 1997 to 2003, 2008, and 2011 in NAFO subdivisions 2J, 3K, 3L, and 3Ps off Newfoundland and Labrador (Tables 1 and 2, Figure 1). Spawning occurs in each of these regions (Myers et al., 1993). We compared the diets of cod on this geographical basis, recognizing that within these regions a number of cod spawning groups exist. The broadscale geographic basis of our comparisons is thought to have maximized the likelihood that any small-scale differences in diet, or small sample size, would not overly influence the large-scale questions posed here.
Sample size, percentage of empty stomachs, mean TFI, and Simpson's diversity index (D) of sampled cod divided by region, spawning status, and sex.
| Region . | Spawning status . | Sex . | Sample size (n) . | % Empty stomachs . | TFI . | D . |
|---|---|---|---|---|---|---|
| 2J | Immature | M | 335 | 26.3 | 0.686 | 0.393 |
| F | 760 | 19.9 | 0.696 | 0.347 | ||
| Non-spawning | M | 875 | 17.5 | 0.713 | 0.389 | |
| F | 802 | 25.0 | 0.624 | 0.404 | ||
| Spawning | M | 150 | 27.5 | 0.581 | 0.356 | |
| F | 73 | 20.4 | 0.471 | 0.304 | ||
| 3K | Immature | M | 215 | 25.1 | 0.807 | 0.655 |
| F | 355 | 22.1 | 0.731 | 0.485 | ||
| Non-spawning | M | 96 | 10.5 | 0.845 | 0.600 | |
| F | 96 | 12.4 | 1.056 | 0.582 | ||
| Spawning | M | 38 | 13.2 | 0.988 | 0.543 | |
| F | 12 | 25.0 | 0.421 | 0.333 | ||
| 3L | Immature | M | 395 | 34.6 | 0.749 | 0.747 |
| F | 636 | 35.7 | 0.775 | 0.690 | ||
| Non-spawning | M | 309 | 28.2 | 0.461 | 0.634 | |
| F | 337 | 20.8 | 0.552 | 0.627 | ||
| Spawning | M | 49 | 12.2 | 0.588 | 0.601 | |
| F | 26 | 23.0 | 0.360 | 0.620 | ||
| 3Ps | Immature | M | 588 | 35.0 | 0.778 | 0.650 |
| F | 887 | 24.0 | 0.794 | 0.646 | ||
| Non-spawning | M | 1518 | 29.6 | 0.607 | 0.632 | |
| F | 1266 | 21.5 | 0.790 | 0.633 | ||
| Spawning | M | 406 | 23.4 | 0.768 | 0.631 | |
| F | 238 | 29.0 | 0.422 | 0.612 |
| Region . | Spawning status . | Sex . | Sample size (n) . | % Empty stomachs . | TFI . | D . |
|---|---|---|---|---|---|---|
| 2J | Immature | M | 335 | 26.3 | 0.686 | 0.393 |
| F | 760 | 19.9 | 0.696 | 0.347 | ||
| Non-spawning | M | 875 | 17.5 | 0.713 | 0.389 | |
| F | 802 | 25.0 | 0.624 | 0.404 | ||
| Spawning | M | 150 | 27.5 | 0.581 | 0.356 | |
| F | 73 | 20.4 | 0.471 | 0.304 | ||
| 3K | Immature | M | 215 | 25.1 | 0.807 | 0.655 |
| F | 355 | 22.1 | 0.731 | 0.485 | ||
| Non-spawning | M | 96 | 10.5 | 0.845 | 0.600 | |
| F | 96 | 12.4 | 1.056 | 0.582 | ||
| Spawning | M | 38 | 13.2 | 0.988 | 0.543 | |
| F | 12 | 25.0 | 0.421 | 0.333 | ||
| 3L | Immature | M | 395 | 34.6 | 0.749 | 0.747 |
| F | 636 | 35.7 | 0.775 | 0.690 | ||
| Non-spawning | M | 309 | 28.2 | 0.461 | 0.634 | |
| F | 337 | 20.8 | 0.552 | 0.627 | ||
| Spawning | M | 49 | 12.2 | 0.588 | 0.601 | |
| F | 26 | 23.0 | 0.360 | 0.620 | ||
| 3Ps | Immature | M | 588 | 35.0 | 0.778 | 0.650 |
| F | 887 | 24.0 | 0.794 | 0.646 | ||
| Non-spawning | M | 1518 | 29.6 | 0.607 | 0.632 | |
| F | 1266 | 21.5 | 0.790 | 0.633 | ||
| Spawning | M | 406 | 23.4 | 0.768 | 0.631 | |
| F | 238 | 29.0 | 0.422 | 0.612 |
Sample size, percentage of empty stomachs, mean TFI, and Simpson's diversity index (D) of sampled cod divided by region, spawning status, and sex.
| Region . | Spawning status . | Sex . | Sample size (n) . | % Empty stomachs . | TFI . | D . |
|---|---|---|---|---|---|---|
| 2J | Immature | M | 335 | 26.3 | 0.686 | 0.393 |
| F | 760 | 19.9 | 0.696 | 0.347 | ||
| Non-spawning | M | 875 | 17.5 | 0.713 | 0.389 | |
| F | 802 | 25.0 | 0.624 | 0.404 | ||
| Spawning | M | 150 | 27.5 | 0.581 | 0.356 | |
| F | 73 | 20.4 | 0.471 | 0.304 | ||
| 3K | Immature | M | 215 | 25.1 | 0.807 | 0.655 |
| F | 355 | 22.1 | 0.731 | 0.485 | ||
| Non-spawning | M | 96 | 10.5 | 0.845 | 0.600 | |
| F | 96 | 12.4 | 1.056 | 0.582 | ||
| Spawning | M | 38 | 13.2 | 0.988 | 0.543 | |
| F | 12 | 25.0 | 0.421 | 0.333 | ||
| 3L | Immature | M | 395 | 34.6 | 0.749 | 0.747 |
| F | 636 | 35.7 | 0.775 | 0.690 | ||
| Non-spawning | M | 309 | 28.2 | 0.461 | 0.634 | |
| F | 337 | 20.8 | 0.552 | 0.627 | ||
| Spawning | M | 49 | 12.2 | 0.588 | 0.601 | |
| F | 26 | 23.0 | 0.360 | 0.620 | ||
| 3Ps | Immature | M | 588 | 35.0 | 0.778 | 0.650 |
| F | 887 | 24.0 | 0.794 | 0.646 | ||
| Non-spawning | M | 1518 | 29.6 | 0.607 | 0.632 | |
| F | 1266 | 21.5 | 0.790 | 0.633 | ||
| Spawning | M | 406 | 23.4 | 0.768 | 0.631 | |
| F | 238 | 29.0 | 0.422 | 0.612 |
| Region . | Spawning status . | Sex . | Sample size (n) . | % Empty stomachs . | TFI . | D . |
|---|---|---|---|---|---|---|
| 2J | Immature | M | 335 | 26.3 | 0.686 | 0.393 |
| F | 760 | 19.9 | 0.696 | 0.347 | ||
| Non-spawning | M | 875 | 17.5 | 0.713 | 0.389 | |
| F | 802 | 25.0 | 0.624 | 0.404 | ||
| Spawning | M | 150 | 27.5 | 0.581 | 0.356 | |
| F | 73 | 20.4 | 0.471 | 0.304 | ||
| 3K | Immature | M | 215 | 25.1 | 0.807 | 0.655 |
| F | 355 | 22.1 | 0.731 | 0.485 | ||
| Non-spawning | M | 96 | 10.5 | 0.845 | 0.600 | |
| F | 96 | 12.4 | 1.056 | 0.582 | ||
| Spawning | M | 38 | 13.2 | 0.988 | 0.543 | |
| F | 12 | 25.0 | 0.421 | 0.333 | ||
| 3L | Immature | M | 395 | 34.6 | 0.749 | 0.747 |
| F | 636 | 35.7 | 0.775 | 0.690 | ||
| Non-spawning | M | 309 | 28.2 | 0.461 | 0.634 | |
| F | 337 | 20.8 | 0.552 | 0.627 | ||
| Spawning | M | 49 | 12.2 | 0.588 | 0.601 | |
| F | 26 | 23.0 | 0.360 | 0.620 | ||
| 3Ps | Immature | M | 588 | 35.0 | 0.778 | 0.650 |
| F | 887 | 24.0 | 0.794 | 0.646 | ||
| Non-spawning | M | 1518 | 29.6 | 0.607 | 0.632 | |
| F | 1266 | 21.5 | 0.790 | 0.633 | ||
| Spawning | M | 406 | 23.4 | 0.768 | 0.631 | |
| F | 238 | 29.0 | 0.422 | 0.612 |
Mutliway ANOVA of percentage of empty stomachs and Simpson's diversity index.
| Parameter . | Percentage of empty stomachs [F (p-value)] . | Simpon's diversity index [F (p-value)] . |
|---|---|---|
| Region | 15.11 (<0.001) | 36.87 (<0.01) |
| Spawning/non-spawning | 8.82 (0.003) | 0.11 (0.74) |
| Mature/immature | 0.32 (0.57) | 0.22 (0.64) |
| Sex | 0.27 (0.61) | 0.14 (0.71) |
| Region × spawning | 7.37 (0.01) | 0.33 (0.80) |
| Region × mature | 0.20 (0.65) | 0.41 (0.75) |
| Region × sex | 2.30 (0.13) | 1.55 (0.20) |
| Spawning × sex | 3.93 (0.15) | 3.22 (0.07) |
| Mature × sex | 1.05 (0.31) | 1.24 (0.27) |
| Parameter . | Percentage of empty stomachs [F (p-value)] . | Simpon's diversity index [F (p-value)] . |
|---|---|---|
| Region | 15.11 (<0.001) | 36.87 (<0.01) |
| Spawning/non-spawning | 8.82 (0.003) | 0.11 (0.74) |
| Mature/immature | 0.32 (0.57) | 0.22 (0.64) |
| Sex | 0.27 (0.61) | 0.14 (0.71) |
| Region × spawning | 7.37 (0.01) | 0.33 (0.80) |
| Region × mature | 0.20 (0.65) | 0.41 (0.75) |
| Region × sex | 2.30 (0.13) | 1.55 (0.20) |
| Spawning × sex | 3.93 (0.15) | 3.22 (0.07) |
| Mature × sex | 1.05 (0.31) | 1.24 (0.27) |
Mutliway ANOVA of percentage of empty stomachs and Simpson's diversity index.
| Parameter . | Percentage of empty stomachs [F (p-value)] . | Simpon's diversity index [F (p-value)] . |
|---|---|---|
| Region | 15.11 (<0.001) | 36.87 (<0.01) |
| Spawning/non-spawning | 8.82 (0.003) | 0.11 (0.74) |
| Mature/immature | 0.32 (0.57) | 0.22 (0.64) |
| Sex | 0.27 (0.61) | 0.14 (0.71) |
| Region × spawning | 7.37 (0.01) | 0.33 (0.80) |
| Region × mature | 0.20 (0.65) | 0.41 (0.75) |
| Region × sex | 2.30 (0.13) | 1.55 (0.20) |
| Spawning × sex | 3.93 (0.15) | 3.22 (0.07) |
| Mature × sex | 1.05 (0.31) | 1.24 (0.27) |
| Parameter . | Percentage of empty stomachs [F (p-value)] . | Simpon's diversity index [F (p-value)] . |
|---|---|---|
| Region | 15.11 (<0.001) | 36.87 (<0.01) |
| Spawning/non-spawning | 8.82 (0.003) | 0.11 (0.74) |
| Mature/immature | 0.32 (0.57) | 0.22 (0.64) |
| Sex | 0.27 (0.61) | 0.14 (0.71) |
| Region × spawning | 7.37 (0.01) | 0.33 (0.80) |
| Region × mature | 0.20 (0.65) | 0.41 (0.75) |
| Region × sex | 2.30 (0.13) | 1.55 (0.20) |
| Spawning × sex | 3.93 (0.15) | 3.22 (0.07) |
| Mature × sex | 1.05 (0.31) | 1.24 (0.27) |
Sampling Locations in the NAFO regions surrounding Newfoundland and Labrador.
All stomachs were collected whole and frozen. Everted stomachs resulting from an erupted swimbladder were discarded. In the laboratory, stomach contents were weighed and identified to the lowest taxon feasible. Slow-dissolving morphological prey components, such as otoliths, exoskeletons, and squid beaks, were frequently utilized for identification. Prey items of particular interest were capelin (Mallotus villosus), cod (G. morhua), sand lance (Ammodytes sp.), herring (C. harengus), other gadids, shrimp (Pandalus sp., Crangon sp., etc.), crab, eggs, and zooplankton. Additional categories for remaining benthic prey and any additional fish species consumed were also established. These classifications represent the dominant prey items with categories such as benthic prey covering a range of species. Each prey item was quantified by weight.
Analysis of maturity
Gonads were analysed at the time of sampling to determine maturity and spawning status. Males were classified as immature, maturing (no milt but gonads turgid), partially spent (spawning with milt present, gonads turgid), and spent (gonads non-turgid but developed). Females were classified as immature, maturing with no hydrated eggs, spawning with less than 50% hydrated eggs, spawning with over 50% hydrated eggs, or spent (deflated gonads) according to Morrison (1990).
Data analysis
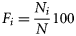
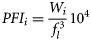
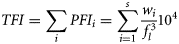
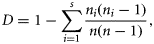
The stomach content data always exhibited non-normal error structure; thus, non-parametric analysis was used to examine differences between groups. The Mann–Whitney or the Wilcoxon test was used to test differences between two samples such as between spawning states or males and females. Testing of regional variation among groups employed the Kruskal–Wallis one-way analysis of variance. Percentages of empty stomachs were compared using a logistic link function with binomial error. Diversity was analysed using a mixed-effects model with the Poisson error structure. All analyses were done in R statistical software.
Results
Prevalence of spawning individuals
Spawning individuals were present in all regions during the months sampled (Table 1). Handline catches had a higher frequency of spawning individuals than did trawl catches, likely because they more closely targeted active spawning areas. The most complete sampling occurred in 3Ps where spawning fish were present from March to September (7 months). The proportions of spawning fish across regions could only be compared in March and June and in both these months were higher in 3Ps than the other regions (5% in March with 0–2% in other regions and 15% in June with 4–8% in other regions). These differences are not thought to represent differences in the proportions spawning in the different regions but reflect more focused sampling on spawning fish in 3Ps.
Differences in feeding amount
The percentage of empty stomachs ranged from 0 to 36% among the various region-maturity stage groups (Table 1) with region being the most significant explanatory factor (p < 0.001; Table 2). The northern regions had lower percentages of empty stomachs than did southern regions (2J, 21.6; 3K, 19.9; 3L, 30.4; 3Ps, 26.3%). Spawning state was found to be important for explaining the proportion of empty stomachs (p = 0.003), but interaction between spawning status and region was significant (p = 0.007). In the northern regions, 3K and 2J, spawning fish had slightly higher percentages of empty stomachs than did non-spawning fish (16.0 vs. 11.4% for 3K and 25.2 vs. 21.1% for 2J), whereas the reverse was true for the southern regions of 3L and 3Ps (16.0 vs. 24.3% for 3L and 23.3 vs. 25.9% for 3Ps). Hence, there was no consistent difference in the proportion of empty stomachs between spawning and non-spawning cod.
Mean TFIs also varied among regions (0.364 for 2J, 0.295 for 3K, 0.082 for 3L, and 0.147 for 3Ps, p ≤ 0.001) with the northern regions showing higher total gut contents (Table 3). TFIs of spawning and non-spawning fish within each region did not differ except in 3Ps where non-spawning individuals had a higher TFI (0.156) than did spawning individuals (0.076; p ≤ 0.001). In all other regions, spawning individuals had higher TFIs than did non-spawners, although these differences were not significant (p = 0.055 for 2J, 0.651 for 3K, 0.143 for 3L).
Mean PFI and TFI values for each prey species across the regions, sexes, and spawning status.
| NAFO . | Spawning status . | Sex . | Mean PFI . | TFI . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Capelin . | Sand lance . | Cod . | Herring . | Gadids . | Other fish . | Shrimp . | Zooplankton . | Crab . | Benthic prey . | Other . | ||||
| 2J | Immature | M | 0.030 | 0 | 0 | 0 | 0 | 0.033 | 0.594*§ | 0.005 | 0.010 | 0.014§ | <0.001 | 0.686 |
| F | 0.010 | 0 | 0 | 0 | 0.006 | 0.024§ | 0.635* | 0.005 | 0.005 | 0.011 | <0.001 | 0.696§ | ||
| Non-spawning | M | 0.017 | 0† | <0.001 | 0 | 0 | 0.049 | 0.625†* | 0.005 | 0.008 | 0.008 | <0.001 | 0.713* | |
| F | 0.006 | 0 | 0 | 0 | 0 | 0.050 | 0.540* | 0.006 | 0.014 | 0.005 | 0.001 | 0.624* | ||
| Spawning | M | 0.004 | 0.007† | 0 | 0 | 0 | 0.039 | 0.514† | 0.003 | 0.004 | 0.009 | 0 | 0.581 | |
| F | 0 | 0 | 0 | 0 | 0 | 0.036 | 0.431 | 0.003 | 0.001 | 0.002 | <0.001 | 0.471 | ||
| 3K | Immature | M | 0.147 | 0 | 0 | 0 | 0 | 0.113* | 0.487 | 0.017§ | 0.016 | 0.026 | 0.001 | 0.807 |
| F | 0.074 | 0 | 0 | 0 | 0 | 0.030* | 0.586§ | 0.014§ | 0.005 | 0.023 | 0.003§ | 0.731 | ||
| Non-spawning | M | 0.137 | 0 | 0 | 0 | 0 | 0.076* | 0.618 | 0.008 | 0.001 | 0.003 | <0.001 | 0.845 | |
| F | 0.069 | 0 | 0 | 0 | <0.001 | 0.011* | 0.951 | 0.008 | 0.007 | 0.008 | 0.001 | 1.056 | ||
| Spawning | M | 0.151 | 0 | 0 | 0 | 0 | 0.020 | 0.788 | 0.004 | 0 | 0.025 | 0 | 0.988 | |
| F | 0.166 | 0 | 0 | 0 | 0 | 0 | 0.249 | 0.002 | 0 | 0.004 | 0 | 0.421 | ||
| 3L | Immature | M | 0.378 | 0§ | 0 | 0 | 0 | 0.054 | 0.197§ | 0.035§ | 0.045*§ | 0.038 | 0.002 | 0.749§ |
| F | 0.412 | <0.001§ | 0 | 0 | 0.004 | 0.063 | 0.220§ | 0.020§ | 0.030* | 0.023 | 0.003 | 0.775§ | ||
| Non-spawning | M | 0.278 | 0.003 | 0 | 0.001 | 0.002 | 0.064 | 0.056† | 0.027 | 0.002 | 0.027 | 0.001† | 0.461 | |
| F | 0.378 | 0.003 | 0.021 | 0.001 | 0.004 | 0.039 | 0.054 | 0.022 | 0.014 | 0.013 | 0.002 | 0.552 | ||
| Spawning | M | 0.345 | 0 | 0 | 0 | 0 | 0.051 | 0.106† | 0.072 | 0.001 | 0.003 | 0.009† | 0.588 | |
| F | 0.176 | 0 | 0 | 0 | 0 | 0.032 | 0.055 | 0.046 | 0.046 | <0.001 | 0.001 | 0.360 | ||
| 3Ps | Immature | M | 0.288 | 0.13§ | <0.001 | 0.003 | 0 | 0.075 | 0.046 | 0.151 | 0.032§ | 0.039 | 0.006 | 0.778§ |
| F | 0.315 | 0.095§ | 0.002 | 0.006 | 0 | 0.744 | 0.042§ | 0.195§ | 0.020§ | 0.038 | 0.005§ | 0.794 | ||
| Non-spawning | M | 0.200†* | 0.033 | 0.006 | 0.004 | <0.001 | 0.072 | 0.038†* | 0.106 | 0.093* | 0.043 | 0.007† | 0.607* | |
| F | 0.268* | 0.039† | 0.017 | 0.006 | 0† | 0.084 | 0.055†* | 0.111† | 0.154†* | 0.047† | 0.009 † | 0.790†* | ||
| Spawning | M | 0.319† | 0.013 | 0* | 0 | 0 | 0.080 | 0.102†* | 0.184* | 0.034* | 0.021* | 0.015†* | 0.768* | |
| F | 0.139 | 0.002† | 0.051* | 0 | 0.012† | 0.034 | 0.006†* | 0.140†* | 0.023†* | 0.012†* | 0.004†* | 0.422†* | ||
| NAFO . | Spawning status . | Sex . | Mean PFI . | TFI . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Capelin . | Sand lance . | Cod . | Herring . | Gadids . | Other fish . | Shrimp . | Zooplankton . | Crab . | Benthic prey . | Other . | ||||
| 2J | Immature | M | 0.030 | 0 | 0 | 0 | 0 | 0.033 | 0.594*§ | 0.005 | 0.010 | 0.014§ | <0.001 | 0.686 |
| F | 0.010 | 0 | 0 | 0 | 0.006 | 0.024§ | 0.635* | 0.005 | 0.005 | 0.011 | <0.001 | 0.696§ | ||
| Non-spawning | M | 0.017 | 0† | <0.001 | 0 | 0 | 0.049 | 0.625†* | 0.005 | 0.008 | 0.008 | <0.001 | 0.713* | |
| F | 0.006 | 0 | 0 | 0 | 0 | 0.050 | 0.540* | 0.006 | 0.014 | 0.005 | 0.001 | 0.624* | ||
| Spawning | M | 0.004 | 0.007† | 0 | 0 | 0 | 0.039 | 0.514† | 0.003 | 0.004 | 0.009 | 0 | 0.581 | |
| F | 0 | 0 | 0 | 0 | 0 | 0.036 | 0.431 | 0.003 | 0.001 | 0.002 | <0.001 | 0.471 | ||
| 3K | Immature | M | 0.147 | 0 | 0 | 0 | 0 | 0.113* | 0.487 | 0.017§ | 0.016 | 0.026 | 0.001 | 0.807 |
| F | 0.074 | 0 | 0 | 0 | 0 | 0.030* | 0.586§ | 0.014§ | 0.005 | 0.023 | 0.003§ | 0.731 | ||
| Non-spawning | M | 0.137 | 0 | 0 | 0 | 0 | 0.076* | 0.618 | 0.008 | 0.001 | 0.003 | <0.001 | 0.845 | |
| F | 0.069 | 0 | 0 | 0 | <0.001 | 0.011* | 0.951 | 0.008 | 0.007 | 0.008 | 0.001 | 1.056 | ||
| Spawning | M | 0.151 | 0 | 0 | 0 | 0 | 0.020 | 0.788 | 0.004 | 0 | 0.025 | 0 | 0.988 | |
| F | 0.166 | 0 | 0 | 0 | 0 | 0 | 0.249 | 0.002 | 0 | 0.004 | 0 | 0.421 | ||
| 3L | Immature | M | 0.378 | 0§ | 0 | 0 | 0 | 0.054 | 0.197§ | 0.035§ | 0.045*§ | 0.038 | 0.002 | 0.749§ |
| F | 0.412 | <0.001§ | 0 | 0 | 0.004 | 0.063 | 0.220§ | 0.020§ | 0.030* | 0.023 | 0.003 | 0.775§ | ||
| Non-spawning | M | 0.278 | 0.003 | 0 | 0.001 | 0.002 | 0.064 | 0.056† | 0.027 | 0.002 | 0.027 | 0.001† | 0.461 | |
| F | 0.378 | 0.003 | 0.021 | 0.001 | 0.004 | 0.039 | 0.054 | 0.022 | 0.014 | 0.013 | 0.002 | 0.552 | ||
| Spawning | M | 0.345 | 0 | 0 | 0 | 0 | 0.051 | 0.106† | 0.072 | 0.001 | 0.003 | 0.009† | 0.588 | |
| F | 0.176 | 0 | 0 | 0 | 0 | 0.032 | 0.055 | 0.046 | 0.046 | <0.001 | 0.001 | 0.360 | ||
| 3Ps | Immature | M | 0.288 | 0.13§ | <0.001 | 0.003 | 0 | 0.075 | 0.046 | 0.151 | 0.032§ | 0.039 | 0.006 | 0.778§ |
| F | 0.315 | 0.095§ | 0.002 | 0.006 | 0 | 0.744 | 0.042§ | 0.195§ | 0.020§ | 0.038 | 0.005§ | 0.794 | ||
| Non-spawning | M | 0.200†* | 0.033 | 0.006 | 0.004 | <0.001 | 0.072 | 0.038†* | 0.106 | 0.093* | 0.043 | 0.007† | 0.607* | |
| F | 0.268* | 0.039† | 0.017 | 0.006 | 0† | 0.084 | 0.055†* | 0.111† | 0.154†* | 0.047† | 0.009 † | 0.790†* | ||
| Spawning | M | 0.319† | 0.013 | 0* | 0 | 0 | 0.080 | 0.102†* | 0.184* | 0.034* | 0.021* | 0.015†* | 0.768* | |
| F | 0.139 | 0.002† | 0.051* | 0 | 0.012† | 0.034 | 0.006†* | 0.140†* | 0.023†* | 0.012†* | 0.004†* | 0.422†* | ||
†95% significant differences in PFI between spawning and non-spawning within sex for each region.
*95% significant differences in PFI between male and female for each region and spawning group.
§95% significant differences in PFI difference between immature and mature individuals within sex for each region.
Mean PFI and TFI values for each prey species across the regions, sexes, and spawning status.
| NAFO . | Spawning status . | Sex . | Mean PFI . | TFI . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Capelin . | Sand lance . | Cod . | Herring . | Gadids . | Other fish . | Shrimp . | Zooplankton . | Crab . | Benthic prey . | Other . | ||||
| 2J | Immature | M | 0.030 | 0 | 0 | 0 | 0 | 0.033 | 0.594*§ | 0.005 | 0.010 | 0.014§ | <0.001 | 0.686 |
| F | 0.010 | 0 | 0 | 0 | 0.006 | 0.024§ | 0.635* | 0.005 | 0.005 | 0.011 | <0.001 | 0.696§ | ||
| Non-spawning | M | 0.017 | 0† | <0.001 | 0 | 0 | 0.049 | 0.625†* | 0.005 | 0.008 | 0.008 | <0.001 | 0.713* | |
| F | 0.006 | 0 | 0 | 0 | 0 | 0.050 | 0.540* | 0.006 | 0.014 | 0.005 | 0.001 | 0.624* | ||
| Spawning | M | 0.004 | 0.007† | 0 | 0 | 0 | 0.039 | 0.514† | 0.003 | 0.004 | 0.009 | 0 | 0.581 | |
| F | 0 | 0 | 0 | 0 | 0 | 0.036 | 0.431 | 0.003 | 0.001 | 0.002 | <0.001 | 0.471 | ||
| 3K | Immature | M | 0.147 | 0 | 0 | 0 | 0 | 0.113* | 0.487 | 0.017§ | 0.016 | 0.026 | 0.001 | 0.807 |
| F | 0.074 | 0 | 0 | 0 | 0 | 0.030* | 0.586§ | 0.014§ | 0.005 | 0.023 | 0.003§ | 0.731 | ||
| Non-spawning | M | 0.137 | 0 | 0 | 0 | 0 | 0.076* | 0.618 | 0.008 | 0.001 | 0.003 | <0.001 | 0.845 | |
| F | 0.069 | 0 | 0 | 0 | <0.001 | 0.011* | 0.951 | 0.008 | 0.007 | 0.008 | 0.001 | 1.056 | ||
| Spawning | M | 0.151 | 0 | 0 | 0 | 0 | 0.020 | 0.788 | 0.004 | 0 | 0.025 | 0 | 0.988 | |
| F | 0.166 | 0 | 0 | 0 | 0 | 0 | 0.249 | 0.002 | 0 | 0.004 | 0 | 0.421 | ||
| 3L | Immature | M | 0.378 | 0§ | 0 | 0 | 0 | 0.054 | 0.197§ | 0.035§ | 0.045*§ | 0.038 | 0.002 | 0.749§ |
| F | 0.412 | <0.001§ | 0 | 0 | 0.004 | 0.063 | 0.220§ | 0.020§ | 0.030* | 0.023 | 0.003 | 0.775§ | ||
| Non-spawning | M | 0.278 | 0.003 | 0 | 0.001 | 0.002 | 0.064 | 0.056† | 0.027 | 0.002 | 0.027 | 0.001† | 0.461 | |
| F | 0.378 | 0.003 | 0.021 | 0.001 | 0.004 | 0.039 | 0.054 | 0.022 | 0.014 | 0.013 | 0.002 | 0.552 | ||
| Spawning | M | 0.345 | 0 | 0 | 0 | 0 | 0.051 | 0.106† | 0.072 | 0.001 | 0.003 | 0.009† | 0.588 | |
| F | 0.176 | 0 | 0 | 0 | 0 | 0.032 | 0.055 | 0.046 | 0.046 | <0.001 | 0.001 | 0.360 | ||
| 3Ps | Immature | M | 0.288 | 0.13§ | <0.001 | 0.003 | 0 | 0.075 | 0.046 | 0.151 | 0.032§ | 0.039 | 0.006 | 0.778§ |
| F | 0.315 | 0.095§ | 0.002 | 0.006 | 0 | 0.744 | 0.042§ | 0.195§ | 0.020§ | 0.038 | 0.005§ | 0.794 | ||
| Non-spawning | M | 0.200†* | 0.033 | 0.006 | 0.004 | <0.001 | 0.072 | 0.038†* | 0.106 | 0.093* | 0.043 | 0.007† | 0.607* | |
| F | 0.268* | 0.039† | 0.017 | 0.006 | 0† | 0.084 | 0.055†* | 0.111† | 0.154†* | 0.047† | 0.009 † | 0.790†* | ||
| Spawning | M | 0.319† | 0.013 | 0* | 0 | 0 | 0.080 | 0.102†* | 0.184* | 0.034* | 0.021* | 0.015†* | 0.768* | |
| F | 0.139 | 0.002† | 0.051* | 0 | 0.012† | 0.034 | 0.006†* | 0.140†* | 0.023†* | 0.012†* | 0.004†* | 0.422†* | ||
| NAFO . | Spawning status . | Sex . | Mean PFI . | TFI . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Capelin . | Sand lance . | Cod . | Herring . | Gadids . | Other fish . | Shrimp . | Zooplankton . | Crab . | Benthic prey . | Other . | ||||
| 2J | Immature | M | 0.030 | 0 | 0 | 0 | 0 | 0.033 | 0.594*§ | 0.005 | 0.010 | 0.014§ | <0.001 | 0.686 |
| F | 0.010 | 0 | 0 | 0 | 0.006 | 0.024§ | 0.635* | 0.005 | 0.005 | 0.011 | <0.001 | 0.696§ | ||
| Non-spawning | M | 0.017 | 0† | <0.001 | 0 | 0 | 0.049 | 0.625†* | 0.005 | 0.008 | 0.008 | <0.001 | 0.713* | |
| F | 0.006 | 0 | 0 | 0 | 0 | 0.050 | 0.540* | 0.006 | 0.014 | 0.005 | 0.001 | 0.624* | ||
| Spawning | M | 0.004 | 0.007† | 0 | 0 | 0 | 0.039 | 0.514† | 0.003 | 0.004 | 0.009 | 0 | 0.581 | |
| F | 0 | 0 | 0 | 0 | 0 | 0.036 | 0.431 | 0.003 | 0.001 | 0.002 | <0.001 | 0.471 | ||
| 3K | Immature | M | 0.147 | 0 | 0 | 0 | 0 | 0.113* | 0.487 | 0.017§ | 0.016 | 0.026 | 0.001 | 0.807 |
| F | 0.074 | 0 | 0 | 0 | 0 | 0.030* | 0.586§ | 0.014§ | 0.005 | 0.023 | 0.003§ | 0.731 | ||
| Non-spawning | M | 0.137 | 0 | 0 | 0 | 0 | 0.076* | 0.618 | 0.008 | 0.001 | 0.003 | <0.001 | 0.845 | |
| F | 0.069 | 0 | 0 | 0 | <0.001 | 0.011* | 0.951 | 0.008 | 0.007 | 0.008 | 0.001 | 1.056 | ||
| Spawning | M | 0.151 | 0 | 0 | 0 | 0 | 0.020 | 0.788 | 0.004 | 0 | 0.025 | 0 | 0.988 | |
| F | 0.166 | 0 | 0 | 0 | 0 | 0 | 0.249 | 0.002 | 0 | 0.004 | 0 | 0.421 | ||
| 3L | Immature | M | 0.378 | 0§ | 0 | 0 | 0 | 0.054 | 0.197§ | 0.035§ | 0.045*§ | 0.038 | 0.002 | 0.749§ |
| F | 0.412 | <0.001§ | 0 | 0 | 0.004 | 0.063 | 0.220§ | 0.020§ | 0.030* | 0.023 | 0.003 | 0.775§ | ||
| Non-spawning | M | 0.278 | 0.003 | 0 | 0.001 | 0.002 | 0.064 | 0.056† | 0.027 | 0.002 | 0.027 | 0.001† | 0.461 | |
| F | 0.378 | 0.003 | 0.021 | 0.001 | 0.004 | 0.039 | 0.054 | 0.022 | 0.014 | 0.013 | 0.002 | 0.552 | ||
| Spawning | M | 0.345 | 0 | 0 | 0 | 0 | 0.051 | 0.106† | 0.072 | 0.001 | 0.003 | 0.009† | 0.588 | |
| F | 0.176 | 0 | 0 | 0 | 0 | 0.032 | 0.055 | 0.046 | 0.046 | <0.001 | 0.001 | 0.360 | ||
| 3Ps | Immature | M | 0.288 | 0.13§ | <0.001 | 0.003 | 0 | 0.075 | 0.046 | 0.151 | 0.032§ | 0.039 | 0.006 | 0.778§ |
| F | 0.315 | 0.095§ | 0.002 | 0.006 | 0 | 0.744 | 0.042§ | 0.195§ | 0.020§ | 0.038 | 0.005§ | 0.794 | ||
| Non-spawning | M | 0.200†* | 0.033 | 0.006 | 0.004 | <0.001 | 0.072 | 0.038†* | 0.106 | 0.093* | 0.043 | 0.007† | 0.607* | |
| F | 0.268* | 0.039† | 0.017 | 0.006 | 0† | 0.084 | 0.055†* | 0.111† | 0.154†* | 0.047† | 0.009 † | 0.790†* | ||
| Spawning | M | 0.319† | 0.013 | 0* | 0 | 0 | 0.080 | 0.102†* | 0.184* | 0.034* | 0.021* | 0.015†* | 0.768* | |
| F | 0.139 | 0.002† | 0.051* | 0 | 0.012† | 0.034 | 0.006†* | 0.140†* | 0.023†* | 0.012†* | 0.004†* | 0.422†* | ||
†95% significant differences in PFI between spawning and non-spawning within sex for each region.
*95% significant differences in PFI between male and female for each region and spawning group.
§95% significant differences in PFI difference between immature and mature individuals within sex for each region.
Prey differences
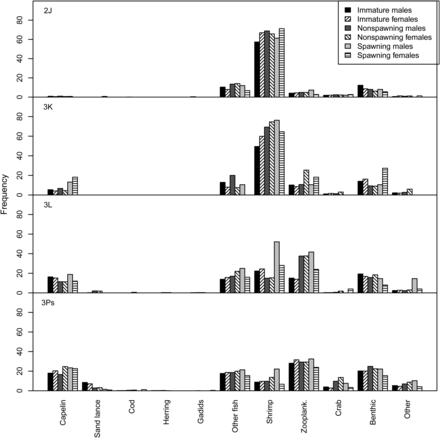
In all, 103 different prey items were identified in cod stomachs. Prey composition and intensity of feeding did not vary consistently between gear types. Overall, the dominant prey species were capelin (31.6% by weight), shrimp (21.3%), other fish (11.0%), crab (10.5%), and zooplankton (10.0%). Regional differences were paramount (Table 3). In the most northern region, 2J, the diet was dominated by shrimp (87.1%) and other fish (7.4%), whereas in 3K, shrimp (78.1%), capelin (13.5%), and other fish (5.3%) were dominant. In 3L, capelin (61.3%), shrimp (12.0%), other fish (9.0%), and cod (5.3%) were consumed. In the most southerly region studied, 3Ps, the diet was more diverse and comprised of capelin (33.5%), zooplankton (14.9%), crab (14.4%), other fish (12.6%), sand lance (6.7%), benthic organisms (5.7%), and shrimp (5.2%). The frequency of occurrence of each prey type parallels the regional variations in the amounts of prey in the stomachs (Figure 2).
Frequency of occurrence of prey items in cod stomachs for the four NAFO regions by maturity stage and sex.
Spawning fish tended to consume more lipid-rich prey, especially capelin (p < 0.001), than did non-spawners that consumed more crab and sand lance (Table 3). Although spawning individuals were on average 2.92 cm larger than non-spawning individuals (53.36 and 56.28 cm for non-spawning and spawning, respectively), the difference in prey eaten is not likely related to size differences. Regional differences were again apparent. Spawning individuals in 2J consumed more sand lance, whereas capelin was more common in spawners from 3K (p = 0.024), shrimp in 3L, and shrimp and sand lance in 3Ps.
Differences between sexes
No consistent differences were evident between the percentage of empty stomachs in males and females or in interactions with the region, maturity state, and spawning state (Table 2). For fish that had fed, spawning males had higher TFIs than did spawning females in all regions (Table 3). In contrast, non-spawning females had higher TFIs than did males in all regions except 2J. Immature fish TFIs did not differ between males and females. Higher TFIs in spawning males reflected PFIs of the dominant prey items: in 2J and 3K largely shrimp; in 3L capelin and shrimp; and in 3Ps capelin, sand lance, and most other diet items, with the notable exception that spawning females consumed more small cod. As in spawning fish, the higher TFIs of non-spawning females reflected higher PFIs of shrimp in 2J and 3K, capelin in 3L, and capelin, shrimp, and crab in 3Ps. Diets of immature fish were mostly near identical, with only minor differences evident.
Immature vs. adult diet
The overall percentage of empty stomachs did not differ between immature and mature individuals, although some regional variation was evident (Table 2). Regions 3L and 3Ps varied in amount fed between immature and mature individuals. Mature cod from regions 3L and 3Ps had reduced TFIs when compared with immature individuals (p < 0.001 for both regions). Immature cod in southern regions were therefore found to reduce the frequency of feeding on greater quantities of prey, whereas northern regions fed at the same frequency on approximately the same amounts of prey.
As expected, immature cod typically consumed greater quantities of zooplankton, shrimp, and sand lance whereas mature individuals consumed more crab, cod, and other fish (Table 3), though the frequency of occurrence shows immature individuals had a lesser occurrence of such prey in their stomachs (Figure 2). Regional variation was observed. In 2J, mature individuals consumed more benthic prey and shrimp, whereas immature cod consumed more gadids (most often Arctic cod, Boreogadus saida). Mature individuals consumed more shrimp and zooplankton in 3K, more zooplankton, shrimp, other fish, and sand lance in 3L, and more shrimp in 3Ps than did immature fish in their respective regions.
Diversity of prey
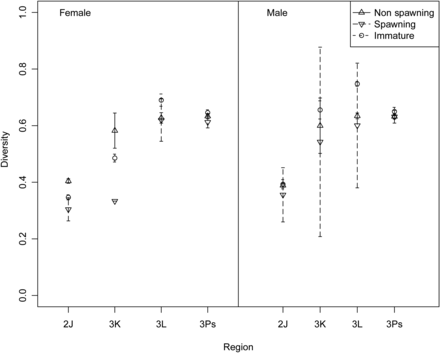
Region was the only significant factor explaining the variation observed in the diversity index (Table 2). The diversity of prey items was highest in southern regions 3L and 3Ps (Table 1, Figure 3) and lowest in 2J where shrimp dominated the diet (Figure 2). No interactive effects were evident.
Simpson's diversity index of stomach contents by region, sex, and spawning status (error bars signify ±1 SE).
Discussion
Base hypothesis
Our results indicate that spawning cod around eastern Newfoundland and Labrador continue to feed during spawning. The presence of food in the stomachs of spawning cod in addition to the lack of any consistent increase in the percentage of empty stomachs among spawners indicates that feeding persists throughout the spawning period. Hence, the base hypothesis that cod cease feeding during spawning can be rejected.
Differences in feeding amount
Feeding amounts differed between spawning and non-spawning fish but in diverse ways. TFIs were elevated in males during spawning in all regions. Females in one region (3Ps) showed decreased food consumption during spawning. Juvenile TFIs did not differ from those of adults. In the more northern regions (2J and 3K), the higher percentages of empty stomachs suggest that spawning cod will feed less frequently than non-spawning cod, but often consume more. This difference was not evident in southern regions and may reflect the wider availability of potential food items in the southern regions.
The cod at higher latitudes were found to have higher average TFIs than those of southern populations. Despite these elevated amounts present in the stomachs, the growth rates are lower in the northern NAFO subdivisions than in southern ones (Olsen et al., 2005), which is counter to the counter-gradient effects suggested by Billerbeck et al. (2001). Low temperatures will negatively influence the growth of cod (Björnsson et al., 2007); hence, the increased consumption and fewer empty stomachs may counter the lower-quality shrimp prey available in the north. It appears from these results that prey quality and energy content, capelin rich and shrimp poor (see Rose and O'Driscoll, 2002 for summary), trumps latitude in terms of gradients in growth.
Prey species
Our PFI data show that the types of food consumed differed among regions. Northern fish (2J and 3K), both mature and immature, spawning and not spawning, fed primarily on shrimp. In the southern regions (3L and 3Ps), the diet included large quantities of capelin, sand lance, and many other prey. These regional differences are consistent with the southerly shift in capelin that occurred in the early 1990s and has endured over the period of the present study (Frank et al., 1996). Differences in diet between spawning and non-spawning fish in all regions except 3Ps were infrequent and mostly related to total feeding (TFI).
Spawning individuals were found to consume more lipid-rich prey, particularly capelin. As the sizes of spawning and non-spawning individuals were approximately the same as non-spawning ones (56.2 and 53.2 cm, respectively), the increase in lipid-rich prey consumption is likely not the result of gape size. The foraging behaviour may therefore not be limiting. It seems likely that energetic needs during spawning may explain the need to pursue better-quality prey.
The primary prey of cod around Newfoundland and Labrador has been reported to be capelin, zooplankton, crab, and shrimp (Pandalus sp.; Popova, 1963; Turuk, 1971; Methven and Piatt, 1989; Lilly, 1991; Mello and Rose, 2005). Although these species comprise the majority of the overall cod diet, regional variation was quite large. Capelin predation depends on their migration (Lilly and Fleming, 1981; Methven and Piatt, 1989; Rose and Leggett, 1990), leading to a large observed variation in the capelin PFI in northern regions. Consistent with our results, spatial variation in prey items has been observed off the coast of Newfoundland such that northern populations consume greater quantities of shrimp while southern populations consume greater quantities of fish and crab (Gerasimova and Kiseleva, 1998). Prey consumed by northern populations was overall of lower energy content than the food consumed by the southern populations. This fact likely reflects prey availability rather than cod selectivity. Cod in northern regions have less access to lipid-rich prey than do southern populations, which likely contributes to observed low growth and survival rates (Olsen et al., 2005).
Diversity
Our data indicate that prey diversity differed among regions and increased with decreasing latitude, as expected with increased diversity of potential prey in southern regions. Casas and Paz (1996) and Powles (1958) observed an increase in diet diversity with increased age in cod from the Flemish Cap stock (NAFO 3M) and the Gulf of St Lawrence, respectively, but this was not observed in the present study. Our results indicate that mature cod had higher consumption of benthic or fish prey, primarily oil-rich fish such as capelin (M. villosus), herring (C. harengus), and sand lance (Ammodytes sp.) than immature fish, but comparable diet diversity. Neither spawning status nor sex was a significant factor in describing the observed variation in diet.
Differences between sexes
Our data suggest that variation may exist between male and female cod diets, though differences between regions were of greater magnitude. Explanations for sex differences include possible prey selectivity or spatial variation in cod residency on the spawning grounds (Robichaud and Rose, 2003), which could result in different food availability.
Conclusion
Our goal was to test the hypothesis that Atlantic cod do not feed during spawning in Newfoundland and Labrador waters. We have rejected that hypothesis. In brief, although some region-sex variations were evident in the amount and frequency of feeding, cod do not restrict their feeding during the protracted spawning season in Newfoundland and Labrador waters. This finding has important implications for bioenergetics and ecosystem-based models based on the consumption of key predators such as Atlantic cod.
Acknowledgements
Cod stomachs and data were collected by sea research programs of the Natural Sciences and Engineering Research Council of Canada (NSERC) Chair in Fisheries Conservation (CFC) and the Centre for Fisheries Ecosystems Research (CFER) funded by the Department of Fisheries and Aquaculture, Newfoundland and Labrador, at the Marine Institute of Memorial University. Laboratory work was funded by grants to George Rose. Kyle Krumsick was funded by a scholarship from the Research Development Corporation of Newfoundland and Labrador. We thank many members of the CFC and CFER who assisted in the collection and analysis of these data, especially Susan Fudge.
References
Author notes
Handling editor: Howard Browman