-
PDF
- Split View
-
Views
-
Cite
Cite
Anabela A. Berasategui, Mónica S. Hoffmeyer, M. Sofía Dutto, Florencia Biancalana, Seasonal variation in the egg morphology of the copepod Eurytemora americana and its relationship with reproductive strategy in a temperate estuary in Argentina, ICES Journal of Marine Science, Volume 69, Issue 3, May 2012, Pages 380–388, https://doi.org/10.1093/icesjms/fsr192
Close - Share Icon Share
Abstract
Seasonal variation in the morphology of Eurytemora americana eggs and its relationship with reproductive strategy were studied in the Bahía Blanca Estuary. Eggs from field females were analysed morphologically by optical (OM) and electron microscopy [scanning electron microscopy (SEM)–transmission electron microscopy (TEM)] during the population pulse. Additionally, egg-carrying females were incubated simulating in situ environmental conditions to evaluate the resting egg character. Subitaneous and resting eggs were indistinguishable by OM, but showed different chorion appearance under SEM and TEM. Sinuous patterned chorion eggs, mainly produced during the period of population growth, were classed as subitaneous eggs based on their high level of hatching success. Eggs with a sponge-like appearance, produced after the population peaked, were classed as diapause eggs based on their inability to hatch even under favourable conditions. There were significant differences in egg size through the population pulse and diapause eggs were significantly bigger than subitaneous eggs. The observation of both morphotypes in egg-carrying females after peak population abundance confirms the existence of a transitional reproductive stage before the exclusive production of diapause eggs at the end of the pulse. Diapause egg production enables E. americana to resist adverse conditions, ensuring its survival in temperate estuaries.Berasategui, A. A., Hoffmeyer, M. S., Dutto, M. S., and Biancalana, F. 2012. Seasonal variation in the egg morphology of the copepod Eurytemora americana and its relationship with reproductive strategy in a temperate estuary in Argentina. – ICES Journal of Marine Science, 69: 380–388.
Introduction
Egg morphology has been used as an appropriate tool for systematic studies and population dynamics research in copepods (Belmonte and Puce, 1994; Chen and Marcus, 1997; Belmonte, 1998; Castro-Longoria and Williams, 1999). Copepod eggs can differ between species and even within those species that produce resting and subitaneous eggs during their reproductive cycles. Moreover, specific features found in resting eggs may be helpful in determining the distribution of seasonal species (Belmonte and Puce, 1994; Castro-Longoria, 2001; Castellani and Lucas, 2003). Most species of calanoid copepod show morphological differences between subitaneous and diapause eggs (Zillioux and Gonzalez, 1972; Belmonte, 1992, 1998), but often, eggs are not readily distinguishable from each other using conventional microscope techniques (Kasahara et al., 1974; Ianora and Santella, 1991; Onoue et al., 2004; Hansen et al., 2010; Samchyshyna and Santer, 2010). Diapause eggs are produced by some copepods in response to cues preceding the onset of environmental adversity (Grice and Marcus, 1981; Marcus, 1982; Hairston and Olds, 1984; Ban, 1992; Belmonte and Pati, 2007). This type of resting egg, unlike subitaneous quiescent eggs, stays in an arrested development state and does not hatch, even under favourable conditions, until the refractory phase is complete (Watson and Smallman, 1971; Marcus, 1996; Chen and Marcus, 1997; Castro-Longoria, 2001; Katajisto, 2006). It has been reported that the calanoid copepod Eurytemora americana probably produces diapause eggs as resting eggs (Marcus, 1984; Hoffmeyer et al., 2008) like its congener E. affinis (Ban, 1992; Ban and Minoda, 1992; Hirche, 1992; Katajisto, 2006), but there are no studies to date on the dormancy strategy of E. americana. Although the morphological description of copepod eggs is of ecological importance, data on these features of E. americana eggs are still lacking in the literature.
Eurytemora americana was introduced into the Bahía Blanca Estuary (BBE) through ballast water in the mid-1980s (Hoffmeyer, 1994; Hoffmeyer et al., 2000). Each year, it develops a brief planktonic pulse in the estuary during the cold austral season from June to October, then disappears from the water column (Hoffmeyer, 2004; Hoffmeyer et al., 2008). The first E. americana nauplii are recruited from benthic resting eggs at the start of the cold season, with minimal surface temperatures (6–7.2°C), low daily irradiation (5 MJ m−2), and high phytoplankton biomass (Hoffmeyer et al., 2008, 2009). Over approximately the past 20 years, this calanoid copepod has become one of the most abundant species within the mesozooplankton in the BBE, so has been considered a key species in the trophic web of the estuary (Hoffmeyer et al., 2009).
Only two studies have focused on the reproductive biology of E. americana and the influence of environmental factors on its fecundity (Berasategui et al., 2009a, b). Those studies revealed that the species has two markedly distinct reproductive patterns in the BBE, depending on the temporal environment. During the period of population growth, from July to early September, it produces large clutches of subitaneous eggs with high hatching success. When environmental conditions become unfavourable from September to October (early spring; temperature > 12°C, Chl a < 8 µg l−1), however, clutch size (CS) is small and hatching success is poor. The latter is associated with the production of resting eggs to ensure population recruitment in the BBE. Although an increase in egg size at the end of the planktonic pulse has been reported, suggesting them to be resting eggs (Berasategui et al., 2009b), no studies have confirmed this. The aims of the present study were therefore to (i) describe the morphology of the different types of egg produced by E. americana and to ascertain whether there are morphological variations between subitaneous and resting eggs, (ii) confirm that the eggs produced by E. americana at the end of the population pulse are resting eggs, possibly diapause eggs, and (iii) analyse the relationship between the morphological features observed and environmental variables, population abundance (PA), females size, and CS to supplement knowledge of the reproductive strategy of E. americana in the BBE.
Methods
The BBE is a temperate, highly turbid, mesotidal system (Perillo et al., 2007) located in the SW Atlantic in Argentina (38°45–39°40′S 61°45–62°30′W). The sampling site, Puerto Cuatreros (Figure 1), is located in the innermost zone of the estuary. It is a eutrophic area characterized by marked seasonal variation in terms of temperature (mean 5.1°C in winter, 26.4°C in summer) and salinity (17.9 and 41.3, respectively). Hypersalinity is usually registered during hot dry summers when elevated evaporation rates produce high salt concentrations in the adjacent tidal marshes (Freije et al., 2008).
Map of the BBE, showing the locations of Bahía Blanca City, the port of Ingeniero White (Ing. White), and the sampling site, Puerto Cuatreros.
Sampling took place during the 2007 E. americana planktonic pulse on nine occasions: 3 and 17 July, 1, 6, and 15 August, 5 and 20 September, and 2 and 8 October. On each sampling date, field females were collected by two replicate oblique tows, using a plankton net with 200 μm mesh. One sample was preserved in 4% formalin to obtain fixed females, and one was kept live and transported in a temperature-controlled 5 l container to maintain field temperature until arrival at the laboratory. In situ temperature and salinity were recorded using a HORIBA® multiparameter probe. Surface water was collected to determine Chl a according to Lorenzen (1967) and for use in the experimental incubations (10–20 l). The photoperiod was estimated as the number of light hours (h) per sampling day.
Seasonal patterns of prosome length (PL), CS, and PA, measured during the period of the present study, were described by Berasategui et al. (2009b) and provide context for the results presented here. The period of E. americana population growth, from June to early September, was defined as the period in which the abundance increased until 5 September, when the population peaked. A period of population decline was registered thereafter, until the final disappearance of the copepod from the water column in November.
Hatching pattern
To evaluate the resting character of the eggs produced by E. americana at the end of the population pulse, egg-carrying females were sorted from live mesozooplankton samples and incubated simulating the in situ environmental conditions recorded. On each sampling date, 10–30 egg-carrying females (with full sacs and empty oviducts) were placed in plastic bottles filled with 5 l of filtered seawater (<60 µm). The experimental incubation period varied from 2 to 7 d, until the eggs/nauplii were released. After this period, incubation water was filtered through sieves of 200 and 30 μm to retain and count the nauplii and the unhatched eggs (N°UE). The percentage of unhatched eggs (%UE) was estimated taking into account the total number of eggs produced (N°TE = N°UE + nauplii). Incubations were performed in a culture room under regulated temperature conditions. The salinity and the temperature of the incubated water were measured using a digital multisensor probe. For the high-salinity condition, salinity was adjusted by adding distilled water. Oxygen concentration was recorded using a Hanna® oximeter, regulated by aerator and sealing of the incubation devices. The natural photoperiod was simulated during the incubation period by controlling the light hours in the culture room.
Unhatched eggs were subsequently incubated for 3 months under in situ nauplii recruitment conditions according to Hoffmeyer et al. (2009). They were placed in glass dishes filled with 200 ml of filtered seawater (≤30 µm) at 6–7.2°C and low daily irradiation conditions. Incubation conditions, i.e. temperature, salinity, oxygen concentration, photoperiod, and hatching success, were monitored every 7 d.
Egg morphology
To examine the morphology of the eggs produced by E. americana on each sampling date, 15–30 egg-carrying females with full sacs and empty oviducts were selected from fixed mesozooplankton samples. The eggs of each female were isolated for measurement under an optical microscope (OM), and the average diameter (egg size ES) was obtained on each occasion (n = 20–30 eggs). To cover the entire population pulse, 227 eggs from the different sampling dates were measured optically. To describe the surface of the eggs under scanning electron microscope (SEM), 30–40 eggs were selected for each population period (growth, maximum, decline, end). These eggs were selected from a set of eggs produced by different females previously isolated for each population period. Observations of the appearance of the eggs on full-sac egg-carrying females (5–10 for each population period) were also made under SEM. The eggs processed by SEM were pre-fixed in vials with 4% formalin for 1 month, as advised by Belmonte and Puce (1994). After pre-fixation, they were prepared following the Sorrivas de Lozano and Morales (1986) and Castro-Longoria (2001) protocols. The eggs were subsequently rinsed in seawater (≤0.2 μm), then cleaned by ultrasound in 1% hydrochloric acid solution for 3–6 min. Post-fixation was then applied overnight at 18–20°C with formalin acetic acid ethanol, after which the eggs were dehydrated in ethanol series, then dried by a critical point. Finally, they were mounted on stubs and sputter-coated with gold.
To describe and analyse the structure of the eggs under transmission electron microscopy (TEM), 30–40 eggs were selected from egg-carrying females isolated from live mesozooplankton samples of each population period. These eggs were processed following the protocols of Couch et al. (2001), with modifications taken from Sorrivas de Lozano and Morales (1986). They were pre-fixed in vials with 3% glutaraldehyde at 5°C for 3–5 d. A secondary post-fixation technique was applied using 1% buffered osmium tetroxide cacodylate (0.2 M) for 2.5 h at room temperature (pH 7.4). Thereafter, the eggs were rinsed in distilled water and dehydrated in a series of ethanol dilutions (50, 70 and 95%) for 45 min each. They were then submerged overnight in acetone with a first solution of 50:50 alcohol–acetone, then transferred to 90% acetone for 60 min at room temperature. Infiltration in Spurr's resin (1969) was performed in increasing concentrations (1:1 acetone–resin for 60 min, 1:2 acetone–resin for 60 min, and resin overnight). Polymerization was conducted at 60°C for 48 h. As a contrast, the block-stain method was performed with 3% uranyl acetate and 0.5% lead citrate (pH 11.2). Visualization was performed under a transmission microscope (JEOL-Erlangshen CCD camera model 785ES1000W) with a potential of 80 kV and digital program GATAN.
Statistical analysis
Non-parametric tests were used because the variables considered did not meet normality and homoscedasticity assumptions. To analyse the differences in ES, PL, and CS among sampling dates (nine campaigns), a Kruskal–Wallis test was applied. ES was also analysed throughout the population pulse using the same test followed by a Tukey–Kramer test to determine the sampling dates on which differences were significant. A Mann–Whitney test was applied to seek statistical differences in ES between the periods of population growth and decline. In addition, to supplement the information on E. americana reproductive strategy, relationships among environmental variables, PL, CS, ES, and PA, were analysed using Spearman's rank correlations. PL, CS, and PA data (n = 227) were taken from Berasategui et al. (2009b) for reanalysis along with the results of the present study.
Results
Field conditions
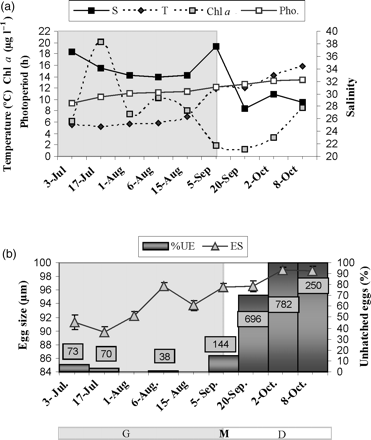
Low temperatures (5.25–7°C), high salinities (32.73–37.51), and high values of Chl a (8.07–20.09 μg l−1) were observed from July to early September 2007, coinciding with the period of E. americana population growth (Figures 2a and 3a). The Chl a pattern revealed two maxima during this period (20.09 and 10.32 μg l−1), the largest magnitude on 17 July, corresponding to the winter phytoplankton bloom. A decrease in Chl a (values generally <3.4 mg l−1) and an increase in temperature (≥12°C) and daylight (>12 h) were recorded from 15 August on. Salinity remained high (37.51–27.62) during the whole study period, with values <30 from September on (Figure 3a).
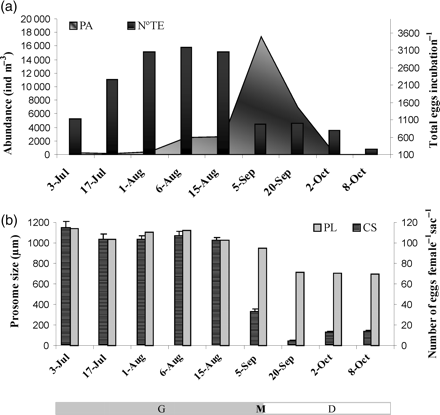
Seasonal pattern of the biological variables. Seasonal patterns of (a) PA and total egg production in each incubation (N°TE), and (b) PL and CS. PL, CS, and PA data were taken from Berasategui et al. (2009b) and correspond to the same population pulse under study. G, period of population growth; M, maximum (peak) abundance; D, period of population decline.
Seasonal patterns of (a) in situ environmental/experimental variables, and (b) egg size (ES) and the percentage of unhatched eggs (%UE). Unhatched eggs are expressed as the percentage of the total number of eggs (N°TE). The boxes in each column represent the number of unhatched eggs (N°UE). G, period of population growth; M, maximum (peak) abundance; D, period of population decline.
Hatching pattern
There was an increase in the total number of eggs produced (N°TE) in the incubations performed during the period of population growth, but it then decreased, after the population peaked (Figure 2a). A low %UE was found in the incubations with females from the period of population growth, and large numbers of nauplii were counted. In contrast, %UE increased in the experimental incubation with females from the period of population decline (Figure 3b), and unhatched eggs were released in mass form (Figure 4f). This pattern coincided with an increase in temperature to ≥12°C and photoperiod, and a decrease in salinity and Chl a (Figure 3a and b). The unhatched eggs did not hatch, although they had been incubated for 3 months under favourable in situ recruitment conditions.
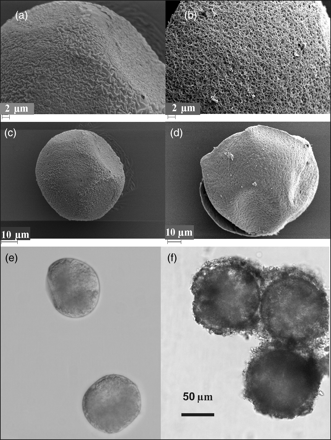
Egg morphology by optical and SEM techniques. Appearance of eggs produced (a, c, and e) mainly during the period of peak population growth and (b, d, and f) during the period of population decline. (e and f) Optical images. (a–d) Details of the chorion surface under SEM.
Egg morphology
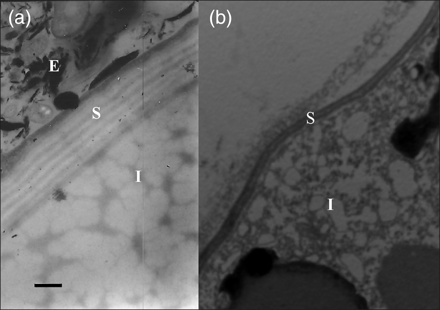
During the period of population growth, ES ranged from 89.86 ± 0.72 μm (n = 22) on 17 July to 96.56 ± 0.48 μm (n = 30) on 6 August, and during the period of population decline, from 96.39 ± 0.61 μm (n = 30) on 5 September to 98.88 ± 0.45 μm (n = 30) on 8 October (Figure 3b). No differences were observed (OM) in the morphology of the eggs produced at the start and those produced at the end of the pulse (Figure 4e and f), although SEM results indicated morphological differences in the surface of the chorion between the eggs produced during the two periods (Figure 4a and c vs. Figure 4b and d). Eggs produced during the period of population growth showed a sinuous patterned chorion with concave marks, possibly caused by the effect of the pressure from adjacent eggs inside the sac (Figure 4a and c), whereas the chorion of those produced at the end of the pulse showed a sponge-like appearance (Figure 4b and d). Eggs with a sponge-like appearance and a small proportion (≤15%) of sinuous patterned chorion eggs were found within the same sac in egg-carrying females collected after the population had peaked. TEM revealed that the eggs with a sponge-like appearance were surrounded by an egg envelope and that the shell consisted of 4–5 layers 1.25 µm thick (Figure 5a). The eggs with a sinuous appearance consisted of a single shell <0.15 µm thick (Figure 5b).
Egg morphology by the TEM technique. Appearance of eggs produced during the period of (a) population decline, and (b) population growth. E, outer envelope of egg; S, inner layer of egg shell; I, egg interior. Scale bar = 0.5 µm.
Egg morphology and relationships between environmental and biological variables
Smaller eggs coincided with larger females and larger CS (101–114 eggs per sac per female) during the period of population growth (Figures 2b and 3b). After peak PA, however, the average ES increased from 96.39 ± 0.61 (n = 30) to 98.88 ± 0.45 µm (n = 30), CS decreased from 32 to 4 eggs per sac per female, and PL decreased from 0.95 ± 0.02 to 0.67 ± 0.01 mm (n = 30). Medium-sized females, compared with larger females at the start and smaller females at the end of the pulse, were found near the peak period of PA. These females were carrying both eggs of a sponge-like appearance and sinuous patterned chorion eggs. Eggs with a sponge-like appearance were smaller during the population peak than at the end of the pulse (Figure 3b). Very few females typical of the start of the population pulse, i.e. large females with large CS and small ES, were observed during this transitional period.
Highly significant differences were found in ES during the E. americana population pulse (Kruskal–Wallis: H= 100.26, p< 0.001, n = 227). The Tukey–Kramer test revealed that the first three sampling dates differed significantly from the last four, whereas the other dates (4 and 5th) were significantly different from the first and last dates, respectively (Table 1). Significant differences in terms of ES were also found when comparing the periods of growth and decline (Mann–Whitney test: Z = 8.03, p < 0.01, n = 122 and n = 105, for each period, respectively).
The Tukey–Kramer multiple comparison test among date groups using egg diameter as the variable analysed, showing significantly different groups (p < 0.05).
| Date . | Date group . | n . | Mean ± s.d. . | Different from groups . |
|---|---|---|---|---|
| 3 July | 1 | 10 | 91.29 ± 3.50 | 6, 4, 7, 9, 8 |
| 17 July | 2 | 22 | 89.87 ± 3.37 | 5, 6, 4, 7, 9, 8 |
| 1 August | 3 | 30 | 92.20 ± 3.75 | 6, 4, 7, 9, 8 |
| 6 August | 4 | 30 | 96.56 ± 2.66 | 2, 1, 3 |
| 15 August | 5 | 30 | 93.73 ± 4.21 | 2, 9, 8 |
| 5 September | 6 | 30 | 96.39 ± 3.32 | 2, 1, 3 |
| 20 September | 7 | 30 | 96.56 ± 4.67 | 2, 1, 3 |
| 2 October | 8 | 30 | 98.88 ± 2.49 | 2, 1, 3, 5 |
| 8 October | 9 | 15 | 98.83 ± 2.64 | 2, 1, 3, 5 |
| Date . | Date group . | n . | Mean ± s.d. . | Different from groups . |
|---|---|---|---|---|
| 3 July | 1 | 10 | 91.29 ± 3.50 | 6, 4, 7, 9, 8 |
| 17 July | 2 | 22 | 89.87 ± 3.37 | 5, 6, 4, 7, 9, 8 |
| 1 August | 3 | 30 | 92.20 ± 3.75 | 6, 4, 7, 9, 8 |
| 6 August | 4 | 30 | 96.56 ± 2.66 | 2, 1, 3 |
| 15 August | 5 | 30 | 93.73 ± 4.21 | 2, 9, 8 |
| 5 September | 6 | 30 | 96.39 ± 3.32 | 2, 1, 3 |
| 20 September | 7 | 30 | 96.56 ± 4.67 | 2, 1, 3 |
| 2 October | 8 | 30 | 98.88 ± 2.49 | 2, 1, 3, 5 |
| 8 October | 9 | 15 | 98.83 ± 2.64 | 2, 1, 3, 5 |
The Tukey–Kramer multiple comparison test among date groups using egg diameter as the variable analysed, showing significantly different groups (p < 0.05).
| Date . | Date group . | n . | Mean ± s.d. . | Different from groups . |
|---|---|---|---|---|
| 3 July | 1 | 10 | 91.29 ± 3.50 | 6, 4, 7, 9, 8 |
| 17 July | 2 | 22 | 89.87 ± 3.37 | 5, 6, 4, 7, 9, 8 |
| 1 August | 3 | 30 | 92.20 ± 3.75 | 6, 4, 7, 9, 8 |
| 6 August | 4 | 30 | 96.56 ± 2.66 | 2, 1, 3 |
| 15 August | 5 | 30 | 93.73 ± 4.21 | 2, 9, 8 |
| 5 September | 6 | 30 | 96.39 ± 3.32 | 2, 1, 3 |
| 20 September | 7 | 30 | 96.56 ± 4.67 | 2, 1, 3 |
| 2 October | 8 | 30 | 98.88 ± 2.49 | 2, 1, 3, 5 |
| 8 October | 9 | 15 | 98.83 ± 2.64 | 2, 1, 3, 5 |
| Date . | Date group . | n . | Mean ± s.d. . | Different from groups . |
|---|---|---|---|---|
| 3 July | 1 | 10 | 91.29 ± 3.50 | 6, 4, 7, 9, 8 |
| 17 July | 2 | 22 | 89.87 ± 3.37 | 5, 6, 4, 7, 9, 8 |
| 1 August | 3 | 30 | 92.20 ± 3.75 | 6, 4, 7, 9, 8 |
| 6 August | 4 | 30 | 96.56 ± 2.66 | 2, 1, 3 |
| 15 August | 5 | 30 | 93.73 ± 4.21 | 2, 9, 8 |
| 5 September | 6 | 30 | 96.39 ± 3.32 | 2, 1, 3 |
| 20 September | 7 | 30 | 96.56 ± 4.67 | 2, 1, 3 |
| 2 October | 8 | 30 | 98.88 ± 2.49 | 2, 1, 3, 5 |
| 8 October | 9 | 15 | 98.83 ± 2.64 | 2, 1, 3, 5 |
ES showed a significant negative correlation with CS (r = −0.47, p < 0.01, d.f. = 225), PL (r = −0.45, p < 0.01, d.f. = 225), salinity (r = −0.41, p < 0.01, d.f. = 225), and Chl a (r = −0.29, p < 0.05, d.f. = 225), and a significant positive correlation with temperature (r = 0.62, p < 0.01, d.f. = 225) and photoperiod (r = 0.62, p < 0.01, d.f. = 225). The correlation between ES and PA was not significant (r = −0.01, p ≥ 0.05, d.f. = 225).
Discussion
The results of the experimental incubations of field-collected, egg-carrying females suggest that E. americana begins to produce resting eggs after the peak PA at temperatures ≥12°C and with increased photoperiod (≥12 h), as predicted by Berasategui et al. (2009b). Egg hatching decreased in the incubations conducted after PA peaked, when environmental conditions turned unfavourable, suggesting that these unhatched eggs were resting or non-viable eggs. No hatched eggs were found in the incubations conducted with females taken from the end of the pulse, suggesting that E. americana produces resting eggs only at the termination of the pulse, agreeing with the findings of Hoffmeyer et al. (2008) and with those reported by Ban and Minoda (1991) for the congener E. affinis. The possible resting character of these eggs was confirmed in the present study by their typical behaviour of failing to hatch even under favourable conditions (Watson and Smallman, 1971; Marcus, 1996; Chen and Marcus, 1997; Castro-Longoria, 2001; Katajisto, 2006). The findings here therefore suggest that the resting eggs produced by E. americana are diapause eggs that do not hatch, probably because they require a refractory phase before embryo development can continue. Nevertheless, to arrive at a better understanding of diapause eggs as a dormancy strategy, it is necessary to perform more studies on eggs from benthic banks.
The eggs with a sinuous patterned chorion were mainly produced during the period of population growth and classed as subitaneous eggs owing to their high rate of hatching success (Castro-Longoria, 2001; Katajisto, 2006). The low percentage of unhatched eggs during this period could correspond to non-viable subitaneous eggs; some of the unhatched eggs recorded during and after peak PA are also likely to be non-viable subitaneous eggs (Marcus, 1996; Chen and Marcus, 1997). On the other hand, eggs whose chorion had a sponge-like appearance and which appeared after the population peaked were categorized as diapause eggs because they failed to hatch even under favourable conditions (Watson and Smallman, 1971; Katajisto, 2006). The SEM observation of both morphotypes after peak PA confirmed the existence of a reproductive transitional stage, previously mentioned by Berasategui et al. (2009a), before the exclusive production of diapause eggs (as a dormancy strategy) at the end of the pulse. The findings also agree with the observations of Ban and Minoda (1991) for E. affinis. Those authors reported that, usually, all E. affinis eggs within a single sac could be identified as either subitaneous or diapause, although a few subitaneous eggs were occasionally found in a diapause egg sac during the switch in production from subitaneous to diapause eggs.
The variations observed in the morphology of E. americana eggs during the population pulse coincide with those reported in previous studies on other copepods (Chen and Marcus, 1997; Belmonte, 1998; Castro-Longoria, 2001) and depict the reproductive strategy of this species in the estuary. The difference in size between egg samples from the start and the end of the pulse became more pronounced, diapause eggs being larger than subitaneous eggs. Eurytemora americana, like many calanoid copepods (Ianora and Santella, 1991; Cuoc et al., 1994), produces subitaneous and diapause eggs that are indistinguishable by OM but whose chorions are shown by electron microscopy to be different. The differences in surface ornamentation between eggs classed as subitaneous and the diapause eggs reported here are in accord with the findings of Onoue et al. (2004) and Hansen et al. (2010) for other calanoid copepods. The variation in the thickness of the chorion of the two egg types, subitaneous eggs having a thicker shell than diapause eggs, has been reported too by Ban and Minoda (1991) for E. affinis and by Castellani and Lucas (2003) for Temora longicornis.
The chorion structure of resting eggs was consistent with the diapause type (Santella and Ianora, 1990; Ianora and Santella, 1991; Belmonte and Puce, 1994; Belmonte, 1998; Cuoc et al., 1994; Couch et al., 2001). The thick shell covering diapause eggs allows them to resist stress such as desiccation (Hairston and Walton, 1986; Brendonck, 1996), toxins (Naess, 1991), and anoxia (Marcus and Lutz, 1998) during their resting period in the bottom sediments of an estuary. Previous studies in the inner zone of the BBE have suggested the presence of E. americana eggs in the bottom sediments of tidal flats (Diodato et al., 2006), which are exposed to environmental changes driven by tidal cycles and the impact of sewage (Marcovecchio et al., 2008). The particular morphology and ultrastructure of diapause eggs enable them to adapt better than quiescent eggs and hence to survive for long periods in the sediment (Hairston and Olds, 1984; Marcus, 1996; Støttrup et al., 1999), providing E. americana with a survival advantage over other copepods in a competitive environment. In fact, over the past 20 years, this invasive species has caused temporal exclusion of the historically most common copepod, Acartia tonsa from the BBE (Hoffmeyer et al., 2009). Although A. tonsa has been reported to produce diapause eggs in other estuaries (Zillioux and Gonzalez, 1972; Grice and Marcus, 1981; Marcus, 1996; Castro-Longoria, 2001), in the BBE, it may produce resting eggs that behave as transitional eggs between quiescent and diapause (AAB, pers. obs.). In this sense, E. americana may have a survival advantage over A. tonsa, because it produces eggs structurally more resistant to adverse environmental conditions. However, further comparative study is required to test this hypothesis rigorously.
As the history of the female plays a basic role in its fecundity (Stearns et al., 1989; Tiselius et al., 1995; Runge and Roff, 2000), it is difficult to determine the factors that trigger diapause egg production in studies conducted with field-collected females. However, the present findings suggest that, as for E. affinis (Ban, 1992; Ban and Minoda, 1992; Katajisto, 2006), photoperiod and temperature could induce diapause egg production in E. americana, but in a different manner. Field female E. americana began to produce diapause eggs under late spring conditions, with rising temperature and increasing photoperiod, whereas female E. affinis produced diapause eggs exclusively under late autumn conditions, with a shortening photoperiod and lower temperature under laboratory conditions (Ban, 1992; Ban and Minoda, 1992; Katajisto, 2006). On the other hand, as observed in E. affinis, overpopulation may also induce diapause egg production (Ban, 1992). High population density may therefore have influenced the switch to diapause egg production in E. americana, because diapause eggs appeared after the peak in PA. However, further laboratory experiments under controlled temperature, salinity, photoperiod, food, and density conditions are required to test this hypothesis adequately.
Seasonal variation in terms of body size has been reported in E. americana by Hoffmeyer and Torres (2001), linked to temperature and food conditions. Those authors also reported that females became smaller at the end of the phytoplankton bloom, when temperatures began to rise. During the E. americana population pulse under study, a gradual increase in ES was accompanied by a decrease in the size of females. Therefore, according to Berasategui et al. (2009b), the smaller females from the end of the pulse could be the result of a metabolic balance in favour of diapause egg production under unfavourable conditions, leaving little reserves to be accumulated in body mass.
The variations observed in the morphology of E. americana eggs along the reproductive strategy in the BBE are probably related to a modified dormancy strategy in its reproductive behaviour, triggered by an increase in temperature, photoperiod, and peak PA. Diapause egg production would appear, therefore, to enable E. americana to withstand adverse environmental conditions in the bottom sediments, ensuring the survival of the population in future years.
Acknowledgements
The study is based partly on the PhD thesis of the first author and was supported by grant PICT1713-2006 (ANPCYT) and a CONICET fellowship. We thank the Instituto Argentino de Oceanografía and the staff of the Marine Chemistry Laboratory for their collaboration, and Viviana Sorrivas for providing supplies for the TEM–SEM study. The assistance of the staff of the Electron Microscope Lab (CCT-CONICET-BB) is also much appreciated. Finally, we thank two anonymous reviewers for their valued comments on the manuscript submitted.