-
PDF
- Split View
-
Views
-
Cite
Cite
Mary Mar P. Noblezada, Wilfredo L. Campos, Chaetognath assemblages along the Pacific Coast and adjacent inland waters of the Philippines: relative importance of oceanographic and biological factors, ICES Journal of Marine Science, Volume 69, Issue 3, May 2012, Pages 410–420, https://doi.org/10.1093/icesjms/fsr209
Close - Share Icon Share
Abstract
When studying plankton distribution, it is necessary to investigate the biology of the target organisms and the surrounding physical environment. Station and species groupings are only useful if they provide insight into the environmental associations of the species in the group. The study covers two geographic regions: the Pacific Coast (Bicol Shelf) and inland waters (San Bernardino Strait, Ticao Pass, Sibuyan, and Visayan Seas) of the Philippines. Comprehensive information is provided on chaetognath assemblages and distribution within the regions. The findings are integrated with oceanographic conditions and phenomena that define the characteristics of the subareas and consideration given to how these conditions affect chaetognath ecology. A comparison is also provided of the community structure of the two regions, and the possible use of chaetognaths as indicator species of water mass movement and oceanographic phenomena explored. In all, 28 284 specimens were examined, and 33 species from 17 genera were identified. Chaetognath distributions, abundance, and community structure were analysed using dissimilarity indices and multiple regression. The results show that the distribution of chaetognaths agrees well with the movement of oceanic water from the Pacific into the central part of the archipelago.Noblezada, M. M. P., and Campos, W. L. 2012. Chaetognath assemblages along the Pacific Coast and adjacent inland waters of the Philippines: relative importance of oceanographic and biological factors. – ICES Journal of Marine Science, 69: 410–420.
Introduction
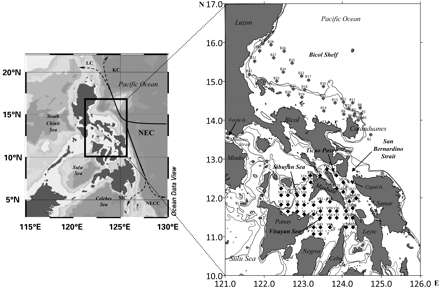
The Philippine archipelago has long been considered one of the most diverse areas of the world's oceans (Carpenter and Springer, 2005). It consists of several basins of varying size, configuration, and topography (Figure 1). Water exchange between the basins is regulated by hydrographic processes, bathymetry, and topographic features, particularly at their interfaces. As a whole, the basins are influenced by the oceanic waters that border them: the South China Sea and Sulu Sea to the west and the Pacific Ocean to the east.
Map showing the bifurcation of the North Equatorial Current (NEC) in the Philippine Sea and station locations in the area surveyed in April and May 2001. LC, Luzon Current; KC, Kuroshio Current; MC, Mindanao Current; and NECC, North Equatorial Counter Current. The 200-m isobath is shown as a grey line surrounding the main islands, and the dashed line represents the 500-m isobath.
A series of large-scale, interbasin oceanographic surveys was recently conducted in the eastern central Philippines wherein the overall compositions of the ichthyoplankton and zooplankton assemblages were characterized (Campos et al., 2002; Estremadura et al., 2002). However, correlating the plankton to hydrographic phenomena and inferring the movement of water from plankton distribution, abundance, and composition have been limited by the rather coarse taxonomic levels employed in those studies. Examining the community structure using a finer resolution (species level) could provide greater insight into the relationships between the organisms and their environment.
Chaetognaths occupy a prominent place among the plankton (Reeve, 1970, 1971). Like other planktonic organisms, they are subjected to dispersion and changing physico-chemical conditions of the water. Several species are reportedly related to specific water masses so are excellent indicators of water mass movement (Bieri, 1959; Alvariño, 1965; Cheney, 1985; Nagai et al., 2006, 2008; Tse et al., 2007). Chaetognaths are active predators, strictly carnivorous but not entirely selective, and they play a major role in structuring the zooplankton community on which they feed at several trophic levels (Baier and Purcell, 1997). Their biomass in the pelagic realm of the world's oceans is estimated to be 30% of that of copepods (Baier and Terazaki, 2005) and their main prey (Baier and Purcell, 1997). They are considered both as competitors of fish larvae and important predators on them, potentially causing significant mortality (Feigenbaum, 1991; Baier and Purcell, 1997; Casanova, 1999). In turn, they themselves are prey for adult fish such as Pacific saury (Cololabis saira) and walleye pollock (Theragra chalcogramma; Johnson and Terazaki, 2003). Hence, they play an important role in the transfer of energy from copepods to higher trophic levels and can greatly influence the dynamics of plankton assemblages.
This study examines chaetognath assemblages along the Pacific Coast and adjacent inland waters of the Philippines, and specifically how their distributions are related to hydrographic features, physico-chemical and biological variables of the area, and water mass movement.
Material and methods
The area covered by the study includes the Pacific coast of the Bicol Peninsula in the eastern central Philippines and inland waters including San Bernardino Strait, Ticao Pass, and the Visayan and Sibuyan Seas (124.80–122.42°E 11.26–15.80°N; Figure 1). The spatial distribution of chaetognaths off the northern Bicol Shelf is described by Noblezada and Campos (2008) and the hydrographic features during the survey by Amedo et al. (2002). The latter area is included to comparison and characterization of the influence of Pacific water on the inland waters.
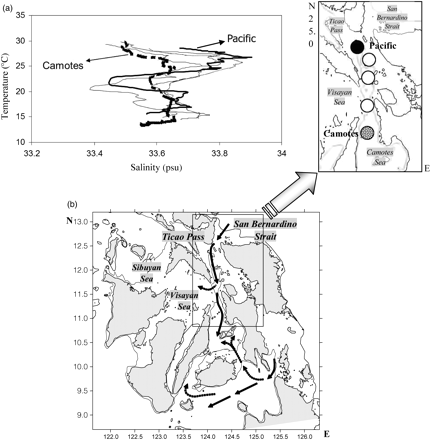
The inland waters included in this study consist of some very productive basins. The Visayan Sea is the shallowest of the basins, with an average station depth of ∼38 m and with most areas <50 m deep and a maximum depth of just 150 m. It is located in the middle of the Visayan Islands that constitute the central Philippines, covering a total area of 5184 km2, and is centred at ∼124°E 12°N. It is connected to the Sulu Sea to the west and receives input of water from the South China Sea via the Mindoro Strait and Verde Island Passage to the northwest. Along the eastern side of the survey area is the San Bernardino Strait, which, although being fairly shallow itself, continues south into a relatively deep channel (100–300 m). Pacific Ocean water flows directly into the internal basins through the San Bernardino Strait. The channel leading to the strait is orientated north–south and is split by Capul Island, directing the flow of water towards Ticao Pass and the Sibuyan Sea to the north and towards the Visayan Sea to the south (Campos et al., 2002; Figure 2).
(a) Temperature–salinity diagram (station location to the right) of water between San Bernardino Strait and the Camotes Sea, and (b) the path of water movement into the central Philippines proposed by Campos et al. (2002).
Sampling
Two oceanographic cruises were conducted, the first covering 34 stations on the Bicol Shelf on board the MV “DA-BFAR” of the Bureau of Fisheries and Aquatic Resources, 1–11 April 2001, and the second covering 46 stations from San Bernardino Strait, into the Visayan Sea, the Sibuyan Sea, and Ticao Pass, on board the TRV “Sardinella” of the University of the Philippines–Visayas, from 26 April to 2 May 2001 (Figure 1).
Zooplankton and ichthyoplankton were sampled by double oblique tows to a maximum depth of 100 m at deep stations or to 5 m above the seabed at shallow stations, using a 60-cm bongo net with 335-μm mesh. A mechanical flowmeter was mounted across the mouth of the net to measure the volume of water filtered. For each haul, the length of towline paid out was adjusted using its angle of declination to maintain a standard depth of sampling.
Only the methods of collection of oceanographic data in inland waters are presented here. Oceanographic data on temperature, salinity, and dissolved oxygen were measured with a Seacat Profiler, and water samples for the determination of nutrients and chlorophyll a concentration were collected with Van Dorn bottles set at 0–1 m (surface), 10 m, and near the seabed (i.e. just above the maximum depth of deployment). Nutrient (NO2, NO3, NH3, PO4, and Si) and chlorophyll a concentrations were determined on board employing spectrometric methods. However, procedural errors with some of the on-board analyses led to the loss of some samples.
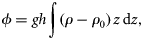
The plots and contours of the hydrographic data were prepared using Ocean Data Viewer (ODV) mp-version 3.3.1 (Schlitzer, 2007) and Surfer 8.
Processing and data analysis
Samples were preserved in 10% buffered formalin. The zooplankton biomass at each station was determined by measuring the displaced volume of each sample and is expressed as ml m–3. Chaetognaths were sorted and identified to the species level and densities calculated as the number of individuals per m3.
The Shannon diversity index (H′; Shannon, 1948) and species evenness (J′) and richness (number of species; Pielou, 1966) were computed to compare species diversity between the two regions and among stations. To explore differences and distributional similarities of the different species, cluster analyses (Q and R) were performed based on relative abundance. The Q-mode cluster analysis was performed to form station clusters and the R-mode analysis to form assemblages of species showing similar relative abundances at the same stations. Species composition and densities were summarized and presented by station cluster in a two-way coincidence table with column (stations) and row (species) sequences following the results of the cluster analyses.
Stepwise multiple regression was performed on the following dependent variables: species abundance, overall chaetognath density, Shannon diversity index, species evenness, and species richness. The independent variables were physical (temperature, salinity, and stratification index ϕ), chemical (dissolved oxygen, NO2, NO3, and PO4), and biological (chlorophyll a, zooplankton biomass, and densities of fish larvae and eggs). Abundance variables were natural log-(x+ 1)-transformed when normality tests failed. Standardized β-coefficients were used as basis for determining the importance of the various factors. Statistical analysis was performed at α = 0.05, using STATISTICA 6.1 (Statsoft Inc.).
Results
Hydrography
The hydrographic features of the northern Bicol Shelf are presented in Noblezada and Campos (2008) and detailed hydrographic features during the survey are described by Amedo et al. (2002) and Primavera et al. (2002). The results presented here focus mainly in the inland waters, which include San Bernardino Strait, Visayan Sea, Sibuyan Sea, and Ticao Pass.
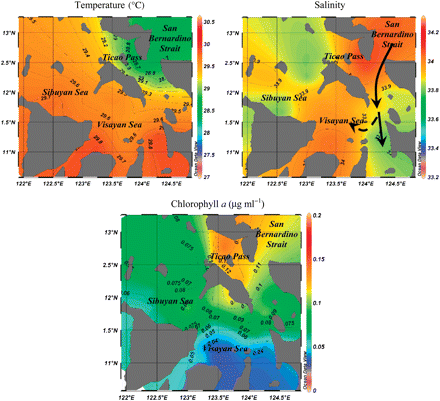
The horizontal distributions of temperature, salinity, and chlorophyll a in the inland waters are illustrated in Figure 3. Mean surface temperature ranged from 27 to 30°C. Surface salinity ranged from 33.2 to 34.3. The integrated (from surface and bottom samples) chlorophyll a concentrations showed mean values ranging from 0.001 to 0.67 μg ml–1, with highest concentrations in Ticao Pass.
Horizontal distribution of temperature (°C) and salinity in inland waters of the Philippines in April and May 2001. The black arrows denote inferred water movement from the Pacific into inland waters (Campos et al., 2002).
Spatial distribution, composition, and abundance
In all, 28 284 chaetognaths were examined from the 77 samples analysed (33 species belonging to 17 genera). Species composition, mean density, and relative abundance among the areas surveyed are shown in Table 1.
Mean density (m−3) and relative abundance (%) of chaetognath species recorded in the various basins surveyed in April and May 2001.
| Taxon . | Bicol Shelf . | Sibuyan Sea . | Ticao Pass . | San Bernardino Strait . | Visayan Sea . | All areas . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean . | Percentage . | Mean . | Percentage . | Mean . | Percentage . | Mean . | Percentage . | Mean . | Percentage . | Mean . | Percentage . | |
| Aidanosagitta neglecta | 0.5 | 12.3 | 3.3 | 37.2 | 0.2 | 18.8 | 1.3 | 14.9 | 19.4 | 79.7 | 5.8 | 54.7 |
| Flaccisagitta enflata | 1.9 | 43.0 | 2.9 | 32.4 | 0.4 | 34.6 | 3.5 | 39.4 | 3.1 | 12.9 | 2.5 | 23.8 |
| Serratosagitta serratodentata | 0.4 | 10.0 | 1.1 | 12.2 | >0.0 | 3.0 | 1.4 | 15.5 | 0.2 | 0.7 | 0.6 | 5.5 |
| Sagitta bipunctata | 0.3 | 7.2 | 0.2 | 2.3 | 0.1 | 9.1 | 0.4 | 4.1 | 0.1 | 0.5 | 0.2 | 2.3 |
| Ferosagitta ferox | 0.2 | 5.6 | 0.2 | 2.7 | >0.0 | 1.2 | 0.6 | 7.2 | 0.1 | 0.3 | 0.2 | 2.2 |
| Mesosagitta minima | 0.1 | 2.3 | 0.2 | 2.5 | 0.1 | 9.2 | 0.2 | 2.8 | 0.2 | 0.8 | 0.2 | 1.5 |
| Pterosagitta draco | 0.0 | 0.1 | 0.2 | 1.8 | >0.0 | 0.5 | 0.5 | 5.3 | 0.3 | 1.3 | 0.2 | 1.5 |
| Zonosagitta bedoti | 0.2 | 4.6 | 0.1 | 0.7 | >0.0 | 2.5 | 0.1 | 1.0 | 0.1 | 0.6 | 0.1 | 1.3 |
| Ferosagitta robusta | 0.1 | 3.1 | >0.0 | 0.5 | >0.0 | 0.4 | 0.1 | 1.0 | 0.2 | 0.6 | 0.1 | 1.0 |
| Decipisagitta decipiens | 0.1 | 1.6 | 0.1 | 1.5 | >0.0 | 3.4 | 0.2 | 2.5 | 0.1 | 0.2 | 0.1 | 0.9 |
| Aidanosagitta oceanica | 0.1 | 3.2 | >0.0 | 0.1 | >0.0 | 0.4 | >0.0 | 0.1 | 0.1 | 0.5 | 0.1 | 0.8 |
| Aidanosagitta regularis | >0.0 | 0.3 | >0.0 | 0.4 | >0.0 | 1.9 | 0.1 | 0.6 | 0.2 | 0.9 | 0.1 | 0.7 |
| Caecosagitta macrocephala | >0.0 | 0.5 | 0.2 | 1.8 | >0.0 | 0.3 | 0.2 | 2.1 | >0.0 | 0.1 | 0.1 | 0.6 |
| Serratosagitta pacifica | 0.1 | 1.6 | >0.0 | 0.4 | 0.1 | 10.0 | 0.1 | 0.8 | >0.0 | 0.1 | 0.1 | 0.5 |
| Sagitta juvenile | 0.1 | 2.3 | – | – | – | – | – | – | – | – | >0.0 | 0.3 |
| Flaccisagitta hexaptera | >0.0 | 1.0 | >0.0 | 0.5 | >0.0 | 0.2 | >0.0 | 0.3 | >0.0 | >0.0 | >0.0 | 0.3 |
| Aidanosagitta johorensis | >0.0 | 0.9 | >0.0 | 0.1 | >0.0 | 0.9 | >0.0 | 0.1 | >0.0 | 0.1 | >0.0 | 0.2 |
| Zonosagitta pulchra | >0.0 | 0.1 | 0.1 | 0.7 | >0.0 | 0.1 | >0.0 | 0.4 | >0.0 | 0.1 | >0.0 | 0.2 |
| Sagitta juvenile 1 | – | – | >0.0 | 0.5 | >0.0 | 0.5 | >0.0 | >0.0 | >0.0 | 0.1 | >0.0 | 0.1 |
| Unidentified | – | – | >0.0 | 0.5 | – | – | >0.0 | >0.0 | >0.0 | 0.1 | >0.0 | 0.1 |
| Eukrohnia fowleri | >0.0 | >0.0 | >0.0 | 0.2 | >0.0 | 0.4 | >0.0 | 0.5 | >0.0 | 0.1 | >0.0 | 0.1 |
| Spadella cephaloptera | – | – | >0.0 | 0.3 | – | – | >0.0 | >0.0 | >0.0 | 0.1 | >0.0 | 0.1 |
| Zonosagitta nagae | >0.0 | 0.1 | >0.0 | 0.2 | >0.0 | 1.6 | >0.0 | 0.4 | >0.0 | >0.0 | >0.0 | 0.1 |
| Solidosagitta planctonis | – | – | >0.0 | 0.1 | >0.0 | 0.1 | >0.0 | 0.4 | >0.0 | >0.0 | >0.0 | >0.0 |
| Serratosagitta tasmanica | >0.0 | 0.2 | >0.0 | >0.0 | – | – | >0.0 | 0.4 | >0.0 | >0.0 | >0.0 | >0.0 |
| Krohnitta subtilis | >0.0 | >0.0 | >0.0 | 0.1 | >0.0 | 0.2 | >0.0 | 0.1 | >0.0 | >0.0 | >0.0 | >0.0 |
| Pseudosagitta lyra | – | – | >0.0 | 0.1 | – | – | – | – | >0.0 | >0.0 | >0.0 | >0.0 |
| Krohnitta pacifica | >0.0 | >0.0 | >0.0 | >0.0 | – | – | >0.0 | >0.0 | >0.0 | >0.0 | >0.0 | >0.0 |
| Paraspadella sp. | – | – | – | – | – | – | – | – | >0.0 | >0.0 | >0.0 | >0.0 |
| Sagitta juvenile 2 | – | – | – | – | – | – | >0.0 | 0.1 | – | – | >0.0 | >0.0 |
| Aidanosagitta bedfordii | >0.0 | >0.0 | – | – | – | – | >0.0 | >0.0 | – | – | >0.0 | >0.0 |
| Sagitta sp. 1. | >0.0 | >0.0 | – | – | – | – | – | – | >0.0 | >0.0 | >0.0 | >0.0 |
| Solidosagitta zetesios | – | – | >0.0 | >0.0 | >0.0 | 0.6 | >0.0 | >0.0 | – | – | >0.0 | >0.0 |
| Sagitta sp. 2. | – | – | – | – | – | – | >0.0 | 0.1 | – | – | >0.0 | >0.0 |
| Krohnitta sp. | – | – | >0.0 | >0.0 | – | – | >0.0 | >0.0 | – | – | >0.0 | >0.0 |
| Aidanosagitta septata | >0.0 | >0.0 | >0.0 | >0.0 | – | – | >0.0 | >0.0 | – | – | >0.0 | >0.0 |
| Parasagitta friderici | – | – | – | – | – | – | >0.0 | >0.0 | – | – | >0.0 | >0.0 |
| Parasagitta setosa | >0.0 | >0.0 | – | – | – | – | – | – | – | – | >0.0 | >0.0 |
| Spadella sp. | >0.0 | >0.0 | – | – | – | – | – | – | – | – | >0.0 | >0.0 |
| Total number of genera | 14 | 15 | 13 | 15 | 16 | 17 | ||||||
| Total number of species | 26 | 28 | 22 | 29 | 26 | 33 | ||||||
| Density of all species | 4.4 | 8.9 | 1.1 | 8.9 | 24.3 | 25.6 | ||||||
| Taxon . | Bicol Shelf . | Sibuyan Sea . | Ticao Pass . | San Bernardino Strait . | Visayan Sea . | All areas . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean . | Percentage . | Mean . | Percentage . | Mean . | Percentage . | Mean . | Percentage . | Mean . | Percentage . | Mean . | Percentage . | |
| Aidanosagitta neglecta | 0.5 | 12.3 | 3.3 | 37.2 | 0.2 | 18.8 | 1.3 | 14.9 | 19.4 | 79.7 | 5.8 | 54.7 |
| Flaccisagitta enflata | 1.9 | 43.0 | 2.9 | 32.4 | 0.4 | 34.6 | 3.5 | 39.4 | 3.1 | 12.9 | 2.5 | 23.8 |
| Serratosagitta serratodentata | 0.4 | 10.0 | 1.1 | 12.2 | >0.0 | 3.0 | 1.4 | 15.5 | 0.2 | 0.7 | 0.6 | 5.5 |
| Sagitta bipunctata | 0.3 | 7.2 | 0.2 | 2.3 | 0.1 | 9.1 | 0.4 | 4.1 | 0.1 | 0.5 | 0.2 | 2.3 |
| Ferosagitta ferox | 0.2 | 5.6 | 0.2 | 2.7 | >0.0 | 1.2 | 0.6 | 7.2 | 0.1 | 0.3 | 0.2 | 2.2 |
| Mesosagitta minima | 0.1 | 2.3 | 0.2 | 2.5 | 0.1 | 9.2 | 0.2 | 2.8 | 0.2 | 0.8 | 0.2 | 1.5 |
| Pterosagitta draco | 0.0 | 0.1 | 0.2 | 1.8 | >0.0 | 0.5 | 0.5 | 5.3 | 0.3 | 1.3 | 0.2 | 1.5 |
| Zonosagitta bedoti | 0.2 | 4.6 | 0.1 | 0.7 | >0.0 | 2.5 | 0.1 | 1.0 | 0.1 | 0.6 | 0.1 | 1.3 |
| Ferosagitta robusta | 0.1 | 3.1 | >0.0 | 0.5 | >0.0 | 0.4 | 0.1 | 1.0 | 0.2 | 0.6 | 0.1 | 1.0 |
| Decipisagitta decipiens | 0.1 | 1.6 | 0.1 | 1.5 | >0.0 | 3.4 | 0.2 | 2.5 | 0.1 | 0.2 | 0.1 | 0.9 |
| Aidanosagitta oceanica | 0.1 | 3.2 | >0.0 | 0.1 | >0.0 | 0.4 | >0.0 | 0.1 | 0.1 | 0.5 | 0.1 | 0.8 |
| Aidanosagitta regularis | >0.0 | 0.3 | >0.0 | 0.4 | >0.0 | 1.9 | 0.1 | 0.6 | 0.2 | 0.9 | 0.1 | 0.7 |
| Caecosagitta macrocephala | >0.0 | 0.5 | 0.2 | 1.8 | >0.0 | 0.3 | 0.2 | 2.1 | >0.0 | 0.1 | 0.1 | 0.6 |
| Serratosagitta pacifica | 0.1 | 1.6 | >0.0 | 0.4 | 0.1 | 10.0 | 0.1 | 0.8 | >0.0 | 0.1 | 0.1 | 0.5 |
| Sagitta juvenile | 0.1 | 2.3 | – | – | – | – | – | – | – | – | >0.0 | 0.3 |
| Flaccisagitta hexaptera | >0.0 | 1.0 | >0.0 | 0.5 | >0.0 | 0.2 | >0.0 | 0.3 | >0.0 | >0.0 | >0.0 | 0.3 |
| Aidanosagitta johorensis | >0.0 | 0.9 | >0.0 | 0.1 | >0.0 | 0.9 | >0.0 | 0.1 | >0.0 | 0.1 | >0.0 | 0.2 |
| Zonosagitta pulchra | >0.0 | 0.1 | 0.1 | 0.7 | >0.0 | 0.1 | >0.0 | 0.4 | >0.0 | 0.1 | >0.0 | 0.2 |
| Sagitta juvenile 1 | – | – | >0.0 | 0.5 | >0.0 | 0.5 | >0.0 | >0.0 | >0.0 | 0.1 | >0.0 | 0.1 |
| Unidentified | – | – | >0.0 | 0.5 | – | – | >0.0 | >0.0 | >0.0 | 0.1 | >0.0 | 0.1 |
| Eukrohnia fowleri | >0.0 | >0.0 | >0.0 | 0.2 | >0.0 | 0.4 | >0.0 | 0.5 | >0.0 | 0.1 | >0.0 | 0.1 |
| Spadella cephaloptera | – | – | >0.0 | 0.3 | – | – | >0.0 | >0.0 | >0.0 | 0.1 | >0.0 | 0.1 |
| Zonosagitta nagae | >0.0 | 0.1 | >0.0 | 0.2 | >0.0 | 1.6 | >0.0 | 0.4 | >0.0 | >0.0 | >0.0 | 0.1 |
| Solidosagitta planctonis | – | – | >0.0 | 0.1 | >0.0 | 0.1 | >0.0 | 0.4 | >0.0 | >0.0 | >0.0 | >0.0 |
| Serratosagitta tasmanica | >0.0 | 0.2 | >0.0 | >0.0 | – | – | >0.0 | 0.4 | >0.0 | >0.0 | >0.0 | >0.0 |
| Krohnitta subtilis | >0.0 | >0.0 | >0.0 | 0.1 | >0.0 | 0.2 | >0.0 | 0.1 | >0.0 | >0.0 | >0.0 | >0.0 |
| Pseudosagitta lyra | – | – | >0.0 | 0.1 | – | – | – | – | >0.0 | >0.0 | >0.0 | >0.0 |
| Krohnitta pacifica | >0.0 | >0.0 | >0.0 | >0.0 | – | – | >0.0 | >0.0 | >0.0 | >0.0 | >0.0 | >0.0 |
| Paraspadella sp. | – | – | – | – | – | – | – | – | >0.0 | >0.0 | >0.0 | >0.0 |
| Sagitta juvenile 2 | – | – | – | – | – | – | >0.0 | 0.1 | – | – | >0.0 | >0.0 |
| Aidanosagitta bedfordii | >0.0 | >0.0 | – | – | – | – | >0.0 | >0.0 | – | – | >0.0 | >0.0 |
| Sagitta sp. 1. | >0.0 | >0.0 | – | – | – | – | – | – | >0.0 | >0.0 | >0.0 | >0.0 |
| Solidosagitta zetesios | – | – | >0.0 | >0.0 | >0.0 | 0.6 | >0.0 | >0.0 | – | – | >0.0 | >0.0 |
| Sagitta sp. 2. | – | – | – | – | – | – | >0.0 | 0.1 | – | – | >0.0 | >0.0 |
| Krohnitta sp. | – | – | >0.0 | >0.0 | – | – | >0.0 | >0.0 | – | – | >0.0 | >0.0 |
| Aidanosagitta septata | >0.0 | >0.0 | >0.0 | >0.0 | – | – | >0.0 | >0.0 | – | – | >0.0 | >0.0 |
| Parasagitta friderici | – | – | – | – | – | – | >0.0 | >0.0 | – | – | >0.0 | >0.0 |
| Parasagitta setosa | >0.0 | >0.0 | – | – | – | – | – | – | – | – | >0.0 | >0.0 |
| Spadella sp. | >0.0 | >0.0 | – | – | – | – | – | – | – | – | >0.0 | >0.0 |
| Total number of genera | 14 | 15 | 13 | 15 | 16 | 17 | ||||||
| Total number of species | 26 | 28 | 22 | 29 | 26 | 33 | ||||||
| Density of all species | 4.4 | 8.9 | 1.1 | 8.9 | 24.3 | 25.6 | ||||||
Mean density (m−3) and relative abundance (%) of chaetognath species recorded in the various basins surveyed in April and May 2001.
| Taxon . | Bicol Shelf . | Sibuyan Sea . | Ticao Pass . | San Bernardino Strait . | Visayan Sea . | All areas . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean . | Percentage . | Mean . | Percentage . | Mean . | Percentage . | Mean . | Percentage . | Mean . | Percentage . | Mean . | Percentage . | |
| Aidanosagitta neglecta | 0.5 | 12.3 | 3.3 | 37.2 | 0.2 | 18.8 | 1.3 | 14.9 | 19.4 | 79.7 | 5.8 | 54.7 |
| Flaccisagitta enflata | 1.9 | 43.0 | 2.9 | 32.4 | 0.4 | 34.6 | 3.5 | 39.4 | 3.1 | 12.9 | 2.5 | 23.8 |
| Serratosagitta serratodentata | 0.4 | 10.0 | 1.1 | 12.2 | >0.0 | 3.0 | 1.4 | 15.5 | 0.2 | 0.7 | 0.6 | 5.5 |
| Sagitta bipunctata | 0.3 | 7.2 | 0.2 | 2.3 | 0.1 | 9.1 | 0.4 | 4.1 | 0.1 | 0.5 | 0.2 | 2.3 |
| Ferosagitta ferox | 0.2 | 5.6 | 0.2 | 2.7 | >0.0 | 1.2 | 0.6 | 7.2 | 0.1 | 0.3 | 0.2 | 2.2 |
| Mesosagitta minima | 0.1 | 2.3 | 0.2 | 2.5 | 0.1 | 9.2 | 0.2 | 2.8 | 0.2 | 0.8 | 0.2 | 1.5 |
| Pterosagitta draco | 0.0 | 0.1 | 0.2 | 1.8 | >0.0 | 0.5 | 0.5 | 5.3 | 0.3 | 1.3 | 0.2 | 1.5 |
| Zonosagitta bedoti | 0.2 | 4.6 | 0.1 | 0.7 | >0.0 | 2.5 | 0.1 | 1.0 | 0.1 | 0.6 | 0.1 | 1.3 |
| Ferosagitta robusta | 0.1 | 3.1 | >0.0 | 0.5 | >0.0 | 0.4 | 0.1 | 1.0 | 0.2 | 0.6 | 0.1 | 1.0 |
| Decipisagitta decipiens | 0.1 | 1.6 | 0.1 | 1.5 | >0.0 | 3.4 | 0.2 | 2.5 | 0.1 | 0.2 | 0.1 | 0.9 |
| Aidanosagitta oceanica | 0.1 | 3.2 | >0.0 | 0.1 | >0.0 | 0.4 | >0.0 | 0.1 | 0.1 | 0.5 | 0.1 | 0.8 |
| Aidanosagitta regularis | >0.0 | 0.3 | >0.0 | 0.4 | >0.0 | 1.9 | 0.1 | 0.6 | 0.2 | 0.9 | 0.1 | 0.7 |
| Caecosagitta macrocephala | >0.0 | 0.5 | 0.2 | 1.8 | >0.0 | 0.3 | 0.2 | 2.1 | >0.0 | 0.1 | 0.1 | 0.6 |
| Serratosagitta pacifica | 0.1 | 1.6 | >0.0 | 0.4 | 0.1 | 10.0 | 0.1 | 0.8 | >0.0 | 0.1 | 0.1 | 0.5 |
| Sagitta juvenile | 0.1 | 2.3 | – | – | – | – | – | – | – | – | >0.0 | 0.3 |
| Flaccisagitta hexaptera | >0.0 | 1.0 | >0.0 | 0.5 | >0.0 | 0.2 | >0.0 | 0.3 | >0.0 | >0.0 | >0.0 | 0.3 |
| Aidanosagitta johorensis | >0.0 | 0.9 | >0.0 | 0.1 | >0.0 | 0.9 | >0.0 | 0.1 | >0.0 | 0.1 | >0.0 | 0.2 |
| Zonosagitta pulchra | >0.0 | 0.1 | 0.1 | 0.7 | >0.0 | 0.1 | >0.0 | 0.4 | >0.0 | 0.1 | >0.0 | 0.2 |
| Sagitta juvenile 1 | – | – | >0.0 | 0.5 | >0.0 | 0.5 | >0.0 | >0.0 | >0.0 | 0.1 | >0.0 | 0.1 |
| Unidentified | – | – | >0.0 | 0.5 | – | – | >0.0 | >0.0 | >0.0 | 0.1 | >0.0 | 0.1 |
| Eukrohnia fowleri | >0.0 | >0.0 | >0.0 | 0.2 | >0.0 | 0.4 | >0.0 | 0.5 | >0.0 | 0.1 | >0.0 | 0.1 |
| Spadella cephaloptera | – | – | >0.0 | 0.3 | – | – | >0.0 | >0.0 | >0.0 | 0.1 | >0.0 | 0.1 |
| Zonosagitta nagae | >0.0 | 0.1 | >0.0 | 0.2 | >0.0 | 1.6 | >0.0 | 0.4 | >0.0 | >0.0 | >0.0 | 0.1 |
| Solidosagitta planctonis | – | – | >0.0 | 0.1 | >0.0 | 0.1 | >0.0 | 0.4 | >0.0 | >0.0 | >0.0 | >0.0 |
| Serratosagitta tasmanica | >0.0 | 0.2 | >0.0 | >0.0 | – | – | >0.0 | 0.4 | >0.0 | >0.0 | >0.0 | >0.0 |
| Krohnitta subtilis | >0.0 | >0.0 | >0.0 | 0.1 | >0.0 | 0.2 | >0.0 | 0.1 | >0.0 | >0.0 | >0.0 | >0.0 |
| Pseudosagitta lyra | – | – | >0.0 | 0.1 | – | – | – | – | >0.0 | >0.0 | >0.0 | >0.0 |
| Krohnitta pacifica | >0.0 | >0.0 | >0.0 | >0.0 | – | – | >0.0 | >0.0 | >0.0 | >0.0 | >0.0 | >0.0 |
| Paraspadella sp. | – | – | – | – | – | – | – | – | >0.0 | >0.0 | >0.0 | >0.0 |
| Sagitta juvenile 2 | – | – | – | – | – | – | >0.0 | 0.1 | – | – | >0.0 | >0.0 |
| Aidanosagitta bedfordii | >0.0 | >0.0 | – | – | – | – | >0.0 | >0.0 | – | – | >0.0 | >0.0 |
| Sagitta sp. 1. | >0.0 | >0.0 | – | – | – | – | – | – | >0.0 | >0.0 | >0.0 | >0.0 |
| Solidosagitta zetesios | – | – | >0.0 | >0.0 | >0.0 | 0.6 | >0.0 | >0.0 | – | – | >0.0 | >0.0 |
| Sagitta sp. 2. | – | – | – | – | – | – | >0.0 | 0.1 | – | – | >0.0 | >0.0 |
| Krohnitta sp. | – | – | >0.0 | >0.0 | – | – | >0.0 | >0.0 | – | – | >0.0 | >0.0 |
| Aidanosagitta septata | >0.0 | >0.0 | >0.0 | >0.0 | – | – | >0.0 | >0.0 | – | – | >0.0 | >0.0 |
| Parasagitta friderici | – | – | – | – | – | – | >0.0 | >0.0 | – | – | >0.0 | >0.0 |
| Parasagitta setosa | >0.0 | >0.0 | – | – | – | – | – | – | – | – | >0.0 | >0.0 |
| Spadella sp. | >0.0 | >0.0 | – | – | – | – | – | – | – | – | >0.0 | >0.0 |
| Total number of genera | 14 | 15 | 13 | 15 | 16 | 17 | ||||||
| Total number of species | 26 | 28 | 22 | 29 | 26 | 33 | ||||||
| Density of all species | 4.4 | 8.9 | 1.1 | 8.9 | 24.3 | 25.6 | ||||||
| Taxon . | Bicol Shelf . | Sibuyan Sea . | Ticao Pass . | San Bernardino Strait . | Visayan Sea . | All areas . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean . | Percentage . | Mean . | Percentage . | Mean . | Percentage . | Mean . | Percentage . | Mean . | Percentage . | Mean . | Percentage . | |
| Aidanosagitta neglecta | 0.5 | 12.3 | 3.3 | 37.2 | 0.2 | 18.8 | 1.3 | 14.9 | 19.4 | 79.7 | 5.8 | 54.7 |
| Flaccisagitta enflata | 1.9 | 43.0 | 2.9 | 32.4 | 0.4 | 34.6 | 3.5 | 39.4 | 3.1 | 12.9 | 2.5 | 23.8 |
| Serratosagitta serratodentata | 0.4 | 10.0 | 1.1 | 12.2 | >0.0 | 3.0 | 1.4 | 15.5 | 0.2 | 0.7 | 0.6 | 5.5 |
| Sagitta bipunctata | 0.3 | 7.2 | 0.2 | 2.3 | 0.1 | 9.1 | 0.4 | 4.1 | 0.1 | 0.5 | 0.2 | 2.3 |
| Ferosagitta ferox | 0.2 | 5.6 | 0.2 | 2.7 | >0.0 | 1.2 | 0.6 | 7.2 | 0.1 | 0.3 | 0.2 | 2.2 |
| Mesosagitta minima | 0.1 | 2.3 | 0.2 | 2.5 | 0.1 | 9.2 | 0.2 | 2.8 | 0.2 | 0.8 | 0.2 | 1.5 |
| Pterosagitta draco | 0.0 | 0.1 | 0.2 | 1.8 | >0.0 | 0.5 | 0.5 | 5.3 | 0.3 | 1.3 | 0.2 | 1.5 |
| Zonosagitta bedoti | 0.2 | 4.6 | 0.1 | 0.7 | >0.0 | 2.5 | 0.1 | 1.0 | 0.1 | 0.6 | 0.1 | 1.3 |
| Ferosagitta robusta | 0.1 | 3.1 | >0.0 | 0.5 | >0.0 | 0.4 | 0.1 | 1.0 | 0.2 | 0.6 | 0.1 | 1.0 |
| Decipisagitta decipiens | 0.1 | 1.6 | 0.1 | 1.5 | >0.0 | 3.4 | 0.2 | 2.5 | 0.1 | 0.2 | 0.1 | 0.9 |
| Aidanosagitta oceanica | 0.1 | 3.2 | >0.0 | 0.1 | >0.0 | 0.4 | >0.0 | 0.1 | 0.1 | 0.5 | 0.1 | 0.8 |
| Aidanosagitta regularis | >0.0 | 0.3 | >0.0 | 0.4 | >0.0 | 1.9 | 0.1 | 0.6 | 0.2 | 0.9 | 0.1 | 0.7 |
| Caecosagitta macrocephala | >0.0 | 0.5 | 0.2 | 1.8 | >0.0 | 0.3 | 0.2 | 2.1 | >0.0 | 0.1 | 0.1 | 0.6 |
| Serratosagitta pacifica | 0.1 | 1.6 | >0.0 | 0.4 | 0.1 | 10.0 | 0.1 | 0.8 | >0.0 | 0.1 | 0.1 | 0.5 |
| Sagitta juvenile | 0.1 | 2.3 | – | – | – | – | – | – | – | – | >0.0 | 0.3 |
| Flaccisagitta hexaptera | >0.0 | 1.0 | >0.0 | 0.5 | >0.0 | 0.2 | >0.0 | 0.3 | >0.0 | >0.0 | >0.0 | 0.3 |
| Aidanosagitta johorensis | >0.0 | 0.9 | >0.0 | 0.1 | >0.0 | 0.9 | >0.0 | 0.1 | >0.0 | 0.1 | >0.0 | 0.2 |
| Zonosagitta pulchra | >0.0 | 0.1 | 0.1 | 0.7 | >0.0 | 0.1 | >0.0 | 0.4 | >0.0 | 0.1 | >0.0 | 0.2 |
| Sagitta juvenile 1 | – | – | >0.0 | 0.5 | >0.0 | 0.5 | >0.0 | >0.0 | >0.0 | 0.1 | >0.0 | 0.1 |
| Unidentified | – | – | >0.0 | 0.5 | – | – | >0.0 | >0.0 | >0.0 | 0.1 | >0.0 | 0.1 |
| Eukrohnia fowleri | >0.0 | >0.0 | >0.0 | 0.2 | >0.0 | 0.4 | >0.0 | 0.5 | >0.0 | 0.1 | >0.0 | 0.1 |
| Spadella cephaloptera | – | – | >0.0 | 0.3 | – | – | >0.0 | >0.0 | >0.0 | 0.1 | >0.0 | 0.1 |
| Zonosagitta nagae | >0.0 | 0.1 | >0.0 | 0.2 | >0.0 | 1.6 | >0.0 | 0.4 | >0.0 | >0.0 | >0.0 | 0.1 |
| Solidosagitta planctonis | – | – | >0.0 | 0.1 | >0.0 | 0.1 | >0.0 | 0.4 | >0.0 | >0.0 | >0.0 | >0.0 |
| Serratosagitta tasmanica | >0.0 | 0.2 | >0.0 | >0.0 | – | – | >0.0 | 0.4 | >0.0 | >0.0 | >0.0 | >0.0 |
| Krohnitta subtilis | >0.0 | >0.0 | >0.0 | 0.1 | >0.0 | 0.2 | >0.0 | 0.1 | >0.0 | >0.0 | >0.0 | >0.0 |
| Pseudosagitta lyra | – | – | >0.0 | 0.1 | – | – | – | – | >0.0 | >0.0 | >0.0 | >0.0 |
| Krohnitta pacifica | >0.0 | >0.0 | >0.0 | >0.0 | – | – | >0.0 | >0.0 | >0.0 | >0.0 | >0.0 | >0.0 |
| Paraspadella sp. | – | – | – | – | – | – | – | – | >0.0 | >0.0 | >0.0 | >0.0 |
| Sagitta juvenile 2 | – | – | – | – | – | – | >0.0 | 0.1 | – | – | >0.0 | >0.0 |
| Aidanosagitta bedfordii | >0.0 | >0.0 | – | – | – | – | >0.0 | >0.0 | – | – | >0.0 | >0.0 |
| Sagitta sp. 1. | >0.0 | >0.0 | – | – | – | – | – | – | >0.0 | >0.0 | >0.0 | >0.0 |
| Solidosagitta zetesios | – | – | >0.0 | >0.0 | >0.0 | 0.6 | >0.0 | >0.0 | – | – | >0.0 | >0.0 |
| Sagitta sp. 2. | – | – | – | – | – | – | >0.0 | 0.1 | – | – | >0.0 | >0.0 |
| Krohnitta sp. | – | – | >0.0 | >0.0 | – | – | >0.0 | >0.0 | – | – | >0.0 | >0.0 |
| Aidanosagitta septata | >0.0 | >0.0 | >0.0 | >0.0 | – | – | >0.0 | >0.0 | – | – | >0.0 | >0.0 |
| Parasagitta friderici | – | – | – | – | – | – | >0.0 | >0.0 | – | – | >0.0 | >0.0 |
| Parasagitta setosa | >0.0 | >0.0 | – | – | – | – | – | – | – | – | >0.0 | >0.0 |
| Spadella sp. | >0.0 | >0.0 | – | – | – | – | – | – | – | – | >0.0 | >0.0 |
| Total number of genera | 14 | 15 | 13 | 15 | 16 | 17 | ||||||
| Total number of species | 26 | 28 | 22 | 29 | 26 | 33 | ||||||
| Density of all species | 4.4 | 8.9 | 1.1 | 8.9 | 24.3 | 25.6 | ||||||
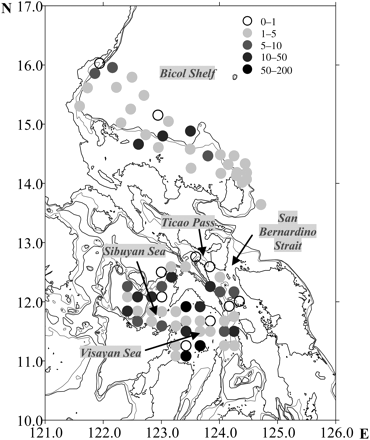
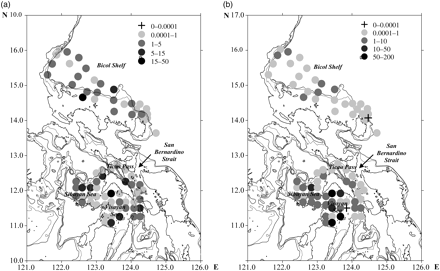
Chaetognath densities ranged from 0.3 to 197.3 m–3 with an overall mean of 10.6 m–3. Overall mean density was greatest in the Visayan Sea (mean 24.3 m–3), with the other internal waters, the Sibuyan Sea and San Bernardino Strait, showing moderately high densities except Ticao Pass, where chaetognath density was low. High density in the Visayan Sea is attributed to high concentrations at three stations, whereas the rest of the stations in the basin had low to moderate densities (Figure 4). Along the Pacific coast, chaetognath concentrations were greatest at stations located at the shelf break, particularly over the central portion of the shelf (Figure 4), with gradually decreasing abundance towards the east and west.
Spatial distribution of chaetognath density (m−3) along the Pacific Coast and inland waters of the Philippines in April and May 2001. The 200-m isobath is shown as a grey line surrounding the major islands, and the dashed line represents the 500-m isobath.
Aidanosagitta neglecta was the most abundant species, comprising 54.7% of all chaetognaths recorded (Table 1). Among the top five species also were Flaccisagitta enflata (23.9%), Serratosagitta serratodentata (5.6%), Sagitta bipunctata (2.3%), and Ferosagitta ferox (2.2%). Together, these five species contributed 88.7% of all the chaetognaths recorded.
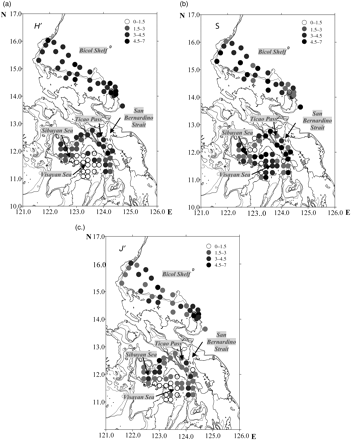
The distributions of the Shannon diversity (H′), species richness (S), and evenness (J′) indices in the area surveyed are displayed in Figure 5a–c. In general, diversity decreased gradually from the Pacific coast towards internal waters. Diversity was greatest at stations located in the southeastern portion of the Bicol Shelf. Values were moderate in the basins surrounding the Visayan Sea (San Bernardino Strait, Ticao Pass, and Sibuyan Sea), then decreased dramatically further into the Visayan Sea (Figure 5a). There was good correspondence between evenness (Figure 5c) and diversity (Figure 5a). Both indices were lowest in the Visayan Sea. In contrast, species richness was moderate to high in that area, but low in stations north of Catanduanes (Figure 5b).
The distribution of diversity indices: (a) Shannon index (H′), (b) species richness (S), and (c) evenness (J′) in the area surveyed in April and May 2001.
Community structure and pattern of distribution
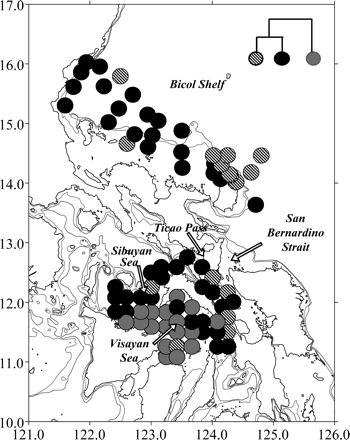
The cluster analysis revealed three main station clusters, which corresponded closely to the distribution of diversity and can be grouped “roughly” geographically as follows (Figure 6):
Pacific inflow, consisting of stations in San Bernardino Strait and north of Catanduanes (stripes), which seem to correspond to Pacific water entering the internal seas via the San Bernardino Strait;
Bicol Shelf and the Visayan Sea Shelf margin, consisting of stations on the Bicol Shelf, in the Ticao Pass, and deep stations in the Visayan and Sibuyan Seas (black);
the Visayan Sea Shelf (grey), which includes stations located in the inner and shallow portions of the Visayan Sea and the southeastern Sibuyan Sea.
Map of the geographic location of station clusters: Pacific inflow (striped circles), Bicol Shelf and Visayan Sea Shelf margin (black circles), and Visayan Sea Shelf (dark grey circles).
The analysis also formed four major chaetognath species assemblages, whose distribution in the various station clusters is summarized in a two-way coincidence table (Figure 7). Assemblage A consists of species at high density and frequency in all three station clusters (Figure 7). Of these, A. neglecta and F. enflata were most dense and common. Flaccisagitta enflata was recorded at all stations surveyed and in comparable concentrations at all station clusters, except ober the shelf of the Visayan Sea, where abundance was lower. On the other hand, A. neglecta was not recorded at 3 of the 77 stations and was clearly at greater concentration over the shelf of the Visayan Sea. Serratosagitta serratodentata, A. oceanica, Z. bedoti, S. bipunctata, F. robusta, and Ferosagitta ferox were present at similar frequencies and concentrations on the Bicol Shelf, in the Pacific inflow, and at the margins of the Visayan Sea, but were infrequent over the shelf of the Visayan Sea. Assemblage (B) consisted of Z. pulchra, F. hexaptera, M. macrocephala, P. draco, A. regularis, D. decipiens, S. Pacifica, and M. Minima and consisted of generally ubiquitous species at moderate density. They were most abundant along the margins of the Visayan Sea Shelf but much less frequent on the Bicol Shelf and other station clusters. Assemblage (C) had just four species at low density and in patches, but occasionally at high frequencies of occurrence in the margin of the Visayan Sea. Eukrohnia fowleri and S. planctonis were recorded primarily at the margins of the Visayan Sea Shelf, and Aidanosagitta johorensis and Z. nagae were distributed similarly along the borders of the shelf of the Visayan Sea and over the Bicol Shelf. Assemblage (D) consisted of 13 species (P. setosa, Paraspadella sp., Spadela sp., S. tasmanica, A. bedfordii, K. pacifica, S. zetosis, P. lyra, S. cephaloptera, K. subtilis, A. septata, P. friederici, and Krohnitta sp.) that showed no recognizable distribution pattern, aside from being rare species found in patches at low density. As an assemblage, they were essentially absent from the Visayan Sea Shelf and the Pacific inflow station clusters.
Two-way coincidence table indicating the relative abundance of the different chaetognath species at the different stations surveyed in April and May 2001. Stations (columns) and species (rows) are arranged as station clusters and species assemblages, respectively, following the results of the cluster analysis. The colours denote relative abundance (%): black (≥20), dark grey (<20 ≥ 5), light grey (<5 ≥ 0.001), and white (absent).
Of the 33 species included in this study, just five (15%) showed significant regressions with the various factors (Table 2). The r2 values were generally low, with values ranging from 0.003 to 0.400. There was no significant relationship between temperature and abundance of any species. Among the variables, the biological factors appeared to be the most important drivers of the distribution. Of the abiotic factors, salinity appeared to be the most notable, followed by the stratification index and dissolved oxygen. Diversity indices (Shannon, evenness, and species richness) showed significant correlations with zooplankton, fish larvae, fish eggs, salinity, and NO2.
Summary of stepwise multiple regression analysis (n = 77) showing only those regressions with r2 ≥ 0.20.
| . | . | Independent variables . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | Physical . | Chemical . | Biological . | |||||
| Dependent variable . | r2 . | Salinity . | Stratification index . | Dissolved oxygen . | NO2 . | Chl a . | Zooplankton . | Fish larvae . | Fish eggs . |
| P. septata | 0.31 | 0.00001*** | 0.00001*** | ||||||
| Z. pulchra | 0.25 | 0.01** | 0.002** | ||||||
| A. bedfordi | 0.24 | 0.000011*** | 0.00001*** | ||||||
| Z. bedoti | 0.21 | 0.0001*** | 0.01** | ||||||
| F. hexaptera | 0.2 | 0.03* | 0.02* | ||||||
| Shannon index (H′) | 0.4 | 0.0002** | 0.002** | ||||||
| Evenness (J′) | 0.38 | 0.00002*** | 0.003** | ||||||
| Species richness (S) | 0.21 | 0.01** | 0.02* | 0.01** | |||||
| . | . | Independent variables . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | Physical . | Chemical . | Biological . | |||||
| Dependent variable . | r2 . | Salinity . | Stratification index . | Dissolved oxygen . | NO2 . | Chl a . | Zooplankton . | Fish larvae . | Fish eggs . |
| P. septata | 0.31 | 0.00001*** | 0.00001*** | ||||||
| Z. pulchra | 0.25 | 0.01** | 0.002** | ||||||
| A. bedfordi | 0.24 | 0.000011*** | 0.00001*** | ||||||
| Z. bedoti | 0.21 | 0.0001*** | 0.01** | ||||||
| F. hexaptera | 0.2 | 0.03* | 0.02* | ||||||
| Shannon index (H′) | 0.4 | 0.0002** | 0.002** | ||||||
| Evenness (J′) | 0.38 | 0.00002*** | 0.003** | ||||||
| Species richness (S) | 0.21 | 0.01** | 0.02* | 0.01** | |||||
Tabulated values are the standardized β-coefficients with significance levels ≤0.05.
*p ≤ 0.05.
**p ≤ 0.01.
***p ≤ 0.0001.
Summary of stepwise multiple regression analysis (n = 77) showing only those regressions with r2 ≥ 0.20.
| . | . | Independent variables . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | Physical . | Chemical . | Biological . | |||||
| Dependent variable . | r2 . | Salinity . | Stratification index . | Dissolved oxygen . | NO2 . | Chl a . | Zooplankton . | Fish larvae . | Fish eggs . |
| P. septata | 0.31 | 0.00001*** | 0.00001*** | ||||||
| Z. pulchra | 0.25 | 0.01** | 0.002** | ||||||
| A. bedfordi | 0.24 | 0.000011*** | 0.00001*** | ||||||
| Z. bedoti | 0.21 | 0.0001*** | 0.01** | ||||||
| F. hexaptera | 0.2 | 0.03* | 0.02* | ||||||
| Shannon index (H′) | 0.4 | 0.0002** | 0.002** | ||||||
| Evenness (J′) | 0.38 | 0.00002*** | 0.003** | ||||||
| Species richness (S) | 0.21 | 0.01** | 0.02* | 0.01** | |||||
| . | . | Independent variables . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | Physical . | Chemical . | Biological . | |||||
| Dependent variable . | r2 . | Salinity . | Stratification index . | Dissolved oxygen . | NO2 . | Chl a . | Zooplankton . | Fish larvae . | Fish eggs . |
| P. septata | 0.31 | 0.00001*** | 0.00001*** | ||||||
| Z. pulchra | 0.25 | 0.01** | 0.002** | ||||||
| A. bedfordi | 0.24 | 0.000011*** | 0.00001*** | ||||||
| Z. bedoti | 0.21 | 0.0001*** | 0.01** | ||||||
| F. hexaptera | 0.2 | 0.03* | 0.02* | ||||||
| Shannon index (H′) | 0.4 | 0.0002** | 0.002** | ||||||
| Evenness (J′) | 0.38 | 0.00002*** | 0.003** | ||||||
| Species richness (S) | 0.21 | 0.01** | 0.02* | 0.01** | |||||
Tabulated values are the standardized β-coefficients with significance levels ≤0.05.
*p ≤ 0.05.
**p ≤ 0.01.
***p ≤ 0.0001.
Discussion
All chaetognath species in the study have already been reported from the same waters in previous investigations (Michael, 1919; Alvariño, 1967; Jumao-as and von Westernhagen, 1978; Rottman, 1978; Cordero, 2006; Johnson et al., 2006). In general, chaetognaths are grouped under general categories based primarily on their observed horizontal (oceanic, neritic, warm, cold, and mixed) and vertical (epipelagic, mesopelagic, bathypelagic, and benthic) distribution, as well as hydrographic conditions (e.g. eurythermal and euryhaline; Alvariño, 1965; Vinogradov, 1970; Rottman, 1978; Pierrot-Bults, 1982, 1991; Johnson et al., 2006; Nagai et al., 2008). Their vertical and geographical distributions therefore vary with latitude and in different regions owing to differences in temperature, salinity, dissolved oxygen, and other local hydrological conditions, as well as the time/season of sampling (Noblezada and Campos, 2008).
Most chaetognaths are considered to be oceanic (Thompson, 1947; Bieri, 1959; Casanova, 1999; Pierrot-Bults and van der Spoel, 2003; Nagai et al., 2006; Tse et al., 2007). Of those identified during this study, such species include Flaccisagitta enflata, A. regularis, F. robusta, F. ferox, K. pacifica, K. subtilis, P. draco, D. decipiens, F. hexaptera, and S. serratodenatata (Bieri, 1959; Pierrot-Bults, 1982, 1991; Johnson et al., 2006). Zonosagitta bedoti, A. neglecta, Z. pulchra, P. friderici, and P. setosa, on the other hand, are considered to be neritic (Bieri, 1959; Casanova, 1999; Pierrot-Bults and van der Spoel, 2003). However, the overall abundance and species composition of the northern Bicol Shelf and inland Philippine waters were similar. Both hydrographic and behavioural factors are important in determining whether oceanic species can live successfully in shallow, neritic environments (Bieri, 1959; Alvariño, 1965; Rottman, 1978; Nagai et al., 2008). This requires a wide range of tolerance to environmental factors such as temperature and salinity, and currents that allow known oceanic species to penetrate shallow environments. In the study area, oceanic water from the Pacific enters through San Bernardino Strait then flows west to Ticao Pass and south towards the Visayan Sea (Figure 3; Campos et al., 2002; Meñez et al., 2006). Such intrusions can explain the abundance of oceanic species in inland waters of the central Philippines.
One of the most notable findings of this study is the contrasting distribution of the two most abundant and common species in the study, Aidanosagitta neglecta and Flaccisagitta enflata (Figure 8). Although A. neglecta was the most abundant species overall, its dominance was restricted to two basins: the Sibuyan Sea and the Visayan Sea. The Visayan Sea is relatively shallow (depth range 15–75 m; Estremadura et al., 2002), whereas the Sibuyan Sea has both shelf and deep-water parts. Aidanosagitta neglecta has been described as neritic, which is consistent with its observed distribution in the present study. Although present at small numbers at almost all stations of the Bicol Shelf, it was clearly more abundant in the shallow Visayan Sea (Figure 8b). Flaccisagitta enflata, on the other hand, is a cosmopolitan species in temperate and warm waters (Alvariño, 1965). It is generally categorized as oceanic, but has been found to tolerate a wide range of salinity (Tokioka, 1962; Alvariño, 1965; Kehayias et al., 1994; Terazaki, 1996; Kehayias, 2003; Giesecke and Gonzalez, 2004, 2008) typical of shallower, nearshore waters. In this study, F. enflata was second only to A. neglecta in overall abundance, but was the only species recorded at all stations (Figure 8a). It occurred in low densities at stations located within the shallow portion (∼50 m) of the Visayan Sea, where densities of A. neglecta were greatest (Figure 8b) and where salinities were also slightly lower (Figure 2), but was at higher density in the deeper basins bordering the Visayan Sea (i.e. the Sibuyan Sea, San Bernardino Strait, and Ticao Pass), which are influenced more by oceanic waters originating from the Pacific Ocean to the east and the South China Sea and the Sulu Sea to the west (Meñez et al., 2006). This indicates a preference by F. enflata for more saline or oceanic conditions, which is consistent with findings in other areas (Rottman, 1978; Kehayias et al., 1994; Marazzo and Nogueria, 1996; Nagai et al., 2006, 2008). As high salinity water enters from the Pacific Ocean through the San Bernardino Strait, it mixes with water of lower salinity in the inner basins, resulting in a salinity gradient decreasing from the Strait inwards, particularly in the Visayan Sea. This is accompanied by an increase in the abundance of the neritic species A. neglecta and a parallel decrease in the abundance of the oceanic species F. enflata. Freshwater run-off from tributaries and rivers on the islands bordering the Visayan Sea render its salinity lower than in adjacent basins (e.g. the Sibuyan Sea, Ticao Pass, and the San Bernardino Strait), in turn making conditions favourable for neritic species such as A. neglecta while limiting the abundance and occurrence of other oceanic species.
Horizontal distribution of (a) F. enflata and (b) A. neglecta. The 200-m isobath is shown as a grey line surrounding the major islands, and the dashed line represents the 500-m isobath.
In practice, categorization of chaetognath habitats has been based on where they are most concentrated or commonly found over large scales (Noblezada and Campos, 2008). The ocean, however, is a dynamic environment, and species may be transported to areas where they are otherwise uncommon. In this study, of the 33 species recorded in the epipelagic zone (i.e. the upper 100 m as covered by the double oblique tows employed), only 23 have been previously described as epipelagic. Serratosagitta serratodentata, S. tasmanica, F. hexaptera, K. subtilis, and K. pacifica, though well represented in the epipelagic layer studied, have been reported as mesopelagic in the South Atlantic (Casanova, 1999), in the Caribbean Sea (Michel, 1984), and also in the Philippines (Michael, 1919). Similarly, species rarely recorded here, e.g. E. fowleri, Paraspadella sp., and Spadella spp. (Table 1), have been reported previously as benthic (Bieri, 1959; Alvariño, 1965; Cheney, 1985), but may have been transported to the upper water layer particularly over the Bicol Shelf by upwelling (Noblezada and Campos, 2008). However, the presence of primarily oceanic species in internal waters may be brought about by water exchange across channels and into adjacent waters such as the San Bernardino Strait, Ticao Pass, the Sibuyan Sea, and the Visayan Sea (Campos et al., 2002; Meñez et al., 2006). In such cases, the resulting geographic distribution would likely be similar outside and inside the internal waters, as observed, or with greater abundance and/or frequency outside.
The pelagic environment plays an important role in structuring chaetognath distribution (Pierrot-Bults and van der Spoel, 2003). Species richness and evenness showed contrasting distributions in the Pacific and internal water basins, with the greatest difference in the shallow Visayan Sea (Figure 5). Patterns were also contrasting for stations north of Catanduanes, where species richness was relatively low but evenness moderate to high. In a similar manner, species richness was relatively high near San Bernardino Strait, but evenness was low. The Visayan Sea Shelf, however, showed the least evenness despite the moderate-to-high species richness. Low evenness and hence diversity in this cluster is attributed to the numerical dominance of A. neglecta (Figure 8b). It is unknown which factors favour the dominance of this species, but previous studies have argued that changes in the number of species are the consequences of changes in major abiotic factors, so that the ecosystem effects of species richness (number of species) per se are expected to be both comparatively small and difficult to isolate (Waide et al., 1999). Nevertheless, it is likely that high productivity over the Visayan Sea Shelf, as reflected by high chlorophyll a concentrations (Figure 2c), allows A. neglecta to attain and maintain high levels of abundance.
The Visayan Sea Shelf cluster was characterized by an assemblage of mostly neritic species, markedly dominated by the neritic A. neglecta. This cluster had lower salinity (Figure 2) and the lowest evenness indices (Figure 5), but the greatest densities (Figure 4). The dominance of a neritic species in the assemblage can be explained by its supposed tolerance of wide-ranging conditions (e.g. salinity 29.1–35; Bieri, 1959; Alvariño, 1965; Jumao-as and von Westernhagen, 1978), whereas the presence of several oceanic species can be attributed to the intrusion and mixing of oceanic (Pacific) with internal waters.
The overall results here link the spatial distribution of chaetognaths with the hydrography of the area, although even if a particular species is closely associated with a particular water mass, the combination of physico-chemical factors characterizing that water mass may not be the only issue governing the distribution of the species. Whereas the regression analysis was exploratory, the limited results provide insights on potential interactions between abundance, overall density, diversity, and physico-chemical and biological variables. The distribution of chaetognaths is also influenced by interactions between individuals and their reactions to their biological environment, including a response to patches of potential food organisms and predators. It has long been known that chaetognaths are an active predator and competitor for food of various zooplankton groups. This may be one of the reasons for the relative importance of biological variables in the regression results.
These results are currently the most geographically extensive on chaetognaths in Philippine waters. Similar efforts in adjacent basins, such as the South China Sea and the Sulu Sea, can further improve the understanding of how water from such sources moves and mixes within the archipelago. Together with the adequate coverage of seasonal variability, such information provides more insight into the larger context of the dispersal of planktonic propagules, their subsequent recruitment, and their maintenance of marine biodiversity within the country's waters.
Acknowledgements
We thank the members of the OceanBio and Marine Biological Laboratories at the University of the Philippines Visayas for their support and assistance, the officers and crews of the TRV “Sardinella” and MV “DA-BFAR” for assistance in field sampling, and GLOBEC, PICES, ICES, and the local organizers of the 5th International Zooplankton Production Symposium in Chile and the Atmosphere and Ocean Research Institute of The University of Tokyo for support. The study forms part of the Pacific Seaboard Research and Development Programme Phase 1, with funding from the Philippine Council for Aquatic and Marine Research and Development (PCAMRD) of the Department of Science and Technology.
References
Author notes
Handling editor: Andy Payne