-
PDF
- Split View
-
Views
-
Cite
Cite
Matteo Sinerchia, Anthony J. Field, John D. Woods, Silvana Vallerga, Wes R. Hinsley, Using an individual-based model with four trophic levels to model the effect of predation and competition on squid recruitment, ICES Journal of Marine Science, Volume 69, Issue 3, May 2012, Pages 439–447, https://doi.org/10.1093/icesjms/fsr190
Close - Share Icon Share
Abstract
The Lagrangian Ensemble recruitment model (LERM) is the first prognostic model of fisheries recruitment based upon individuals. It incorporates five functional groups: phytoplankton (diatoms), herbivorous zooplankton (copepods), carnivorous zooplankton (squid paralarvae), and two top predators. Physiology and behaviour are described by equations derived from literature based on reproducible laboratory experiments. LERM is built using the Lagrangian Ensemble metamodel, in which the demography and biofeedback of each dynamic population are diagnostic properties, emerging from the life histories of individuals. The response of the plankton ecosystem and squid recruitment to different scenarios of exogenous forcing is investigated. Simulations were run at 41°N 27°W (Azores) under a stationary annual cycle of atmospheric forcing. The ecosystem adjusts to a stable attractor for each scenario. The emergent properties of each attractor are investigated, with focus on predation, competition for food, and spawning magnitude. Annual recruitment is a complex emergent property dependent on several factors, including food availability, predation, competition, and post-hatching growth rate, as proposed by Hjort's critical period theory, relating recruitment to predation mortality, depending on growth rate and hence food availability. The model provides a useful step towards linking small-scale processes governing the life histories of larvae and fisheries on the large scale.Sinerchia, M., Field, A. J., Woods, J. D., Vallerga, S., and Hinsley, W. R. 2012. Using an individual-based model with four trophic levels to model the effect of predation and competition on squid recruitment. – ICES Journal of Marine Science, 69: 439–447.
Introduction
There is evidence that although the abundance of fish stocks has been decreasing through overfishing, stocks of squid have been increasing as a consequence of reduced predation pressure from fish and lesser competition for food (Caddy and Rodhouse, 1998). Squid fisheries are therefore becoming increasingly important as a source of high-quality protein for human consumption and because of their possible role as an indicator of global ecological change driven by fishery exploitation in the oceans (Rodhouse, 2001). Despite the increased importance of squid stocks, their assessment and management remain difficult. Populations of short-lived, semelparous, opportunistic species, such as squid, are typically unstable, responding rapidly to changes in environmental conditions (Rodhouse, 2001; Boyle and Rodhouse, 2005). For squid, the exploited stock usually consists almost entirely of recently recruited animals of similar age (Agnew et al., 2002). Squid populations display great interannual variability in recruitment, but a strong relationship between breeding stock and new recruits has yet to be found (Agnew et al., 2000). Beddington et al. (1990) believe that squid are vulnerable to overexploitation because the successor stock consists entirely of recruits. The understanding of the mechanisms underlying recruitment variability in response to environmental conditions constitutes a big challenge for fisheries managers, who are concerned with maintaining stable recruitment (through the preservation of an adequate spawning-stock biomass known as “reproductive escapement”) while achieving optimal catch rates. However, the modelling and management of cephalopod fisheries is at an early stage and based on traditional methods adapted for finfish, using assumptions that are not totally appropriate for squid.
Individual-based models (IBMs) provide a powerful approach to addressing the issue stated above. They offer a tool to estimate the effects of variability in both environmental factors, especially during the critical period of post-hatching feeding, and specific biotic and abiotic external factors, such as temperature, predator abundance, and prey size and availability, on vital rates of fish larvae (Letcher et al., 1996; Lough et al., 2005). Each individual in an IBM follows an independent trajectory, so that at any point in time, the population is made up of individuals with differing internal states that depend on their respective life histories. There is therefore inherent intrapopulation variability. The demography of each population is computed by summing over the individual members of that population and is therefore an “emergent” property of the model (Grünbaum, 1994).
The adopted modelling method used here is the Lagrangian Ensemble (LE) metamodel (Woods, 2005), which defines a class of IBMs in which each individual represents a subpopulation of identical plankters. It provides a computationally efficient method for simulating the plankton ecosystem while retaining all the advantages of individual-based modelling (Grimm and Railsback, 2005). Each subpopulation has associated phenotypic equations for the behaviour and physiology of each species, derived from reproducible laboratory experiments, equations used to compute the life history of each individual and its descendants through a lineage. Intrapopulation variability arises as a result of the random displacement of individuals in turbulent water (i.e. in the surface mixing layer). Each individual is therefore exposed to a different ambient environment as it moves by sinking, swimming, or advection from turbulence.
The two-way interaction between the environment (temperature, light, nutrients, predator, prey, etc.) and the physiology (growth, digestion, respiration, egestion, excretion, etc.) and behaviour (swimming, ingestion, etc.) of the individuals in each population is therefore explicitly captured. The LE metamodel provides a logical framework for explaining emergent ecological properties, such as recruitment, in terms of external forcing and the biological model equations.
This paper presents the LE recruitment model (LERM) that uses the LE metamodel to model the process of squid recruitment. The main objective was to investigate the impact of environmental changes on the survival of squid paralarvae during the critical post-hatching period (Hjort, 1914; Cushing, 1996). We also investigated the sensitivity of predicted squid recruitment to changes in initial conditions, and explain those results in terms of the changing relationships between key ecosystem factors such as predation, inter- and intrapopulation competition, spawning magnitude, and the consequent post-hatching growth rate.
Methods
Lagrangian Ensemble recruitment model
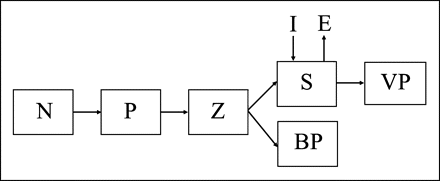
The LERM (Sinerchia, 2007) for predicting fisheries recruitment describes a classical food chain with four trophic levels: phytoplankton (diatoms, P), herbivorous zooplankton (calanoid copepods, Z), carnivorous zooplankton (planktonic squid, S), and two populations of top predator (basal predator, BP, and visual predator, VP; Figure 1).
The LERM community: N, nutrients; P, phytoplankton; Z, zooplankton; S, squid paralarvae; VP, visual predators; BP, basal predators; I, immigrant squid; E, emigrant squid.
The LERM was built using the Virtual Ecology Workbench (Hinsley et al., 2007) and is based on the LE metamodel (Woods, 2005). It uses phenotypic equations for the behaviour and physiology of individual organisms based on reproducible laboratory experiments and reported in the literature.
Simulations are performed in a one-dimensional virtual mesocosm, which extends from the sea surface to a depth of 500 m. The virtual mesocosm is divided vertically into 500 layers of 1 m, each with locally maintained variables for physics and chemistry, and anchored at a fixed location north of the Azores, 41°N 27°W. This is a familiar location (Woods et al., 2005), chosen because it lies close to the transatlantic line, where the annual surface heat budget is zero (solar heating equals cooling to the atmosphere). This yields a stationary annual cycle in the physical environment without recourse to advective heat flux divergence. A full description of LERM (Sinerchia, 2007; Sinerchia et al., 2008), including the list of phenotypic equations, parameters, and relevant literature, can be downloaded from http://www.virtualecology.org/docs/reports.cgi.
The diatoms are nourished by light, dissolved carbon, nitrate, ammonium, and silicate. They feature a dynamic concentration of chlorophyll controlled by nitrogen uptake, following the model of Geider et al. (1996, 1997, 1998). Silicate is taken up only when the biomass is sufficient for cell division, so both nitrogen and silicon are limiting nutrients: primary production in the mixed layer stops when these nutrients are exhausted by the spring bloom.
A generalized copepod population feeds on the diatoms. It is based on Calanus finmarchicus which, although typically found at higher latitudes, is the calanoid species most extensively studied in the laboratory, with phenotypic equations for staged growth, diel migration, and diapause (Carlotti and Wolf, 1998). Its behaviour is adaptive, in the sense that the equations describing the way individuals respond to the external environment and the internal state of the individual (e.g. if they are starving or well fed) change their attitude to risk during foraging, depending on recent feeding success (e.g. the starting date of overwintering, and midnight sinking). This adaptability in behaviour contributes to the heterogeneity in life histories of the individuals, resulting in intrapopulation variability. Copepods mature after a fixed number of successive development stages (staged growth), and moulting from one stage to the next is triggered by size (i.e. the protein pool). A copepod's physiological state is determined by ten biological state variables: carbon pool, protein pool, lipid pool, chitin carapace pool, nitrogen pool, gut content, gut fullness, gut volume, growth stage, and age.
The squid equations are based on the physiology and behaviour of Loligo opalescens, both of which have been studied extensively. In the simulations, adult squid enter the ecosystem to spawn on a prescribed date each year; this is an exogenous factor held constant for each simulation. Each adult is assumed to deposit an egg sac containing 200 eggs, which hatch after a period (days) that depends on the ambient temperature. Squid paralarvae begin to feed on the young copepods (Boyle and Rodhouse, 2005), which are themselves growing rapidly after hatching some days earlier at a rate that depends on prey size and abundance. Unlike copepods, whose growth is staged for moulting of the carapace, squid body growth is continuous and not staged. However, to allow for size-specific predation by the visual top predator, squid paralarvae have been allocated to the seven size classes S1–S7, based on their mantle length (ML). A small paralarva is less visible to visual top predators than a large one, but is slower in its escape. S1 represents the squid at hatching, and S7 the recruited squid. A squid can only be at one particular development stage at any time. As it grows and its ML increases by 1 mm, it moves into the next stage. We define annual recruitment as the proportion of eggs deposited that attain an ML of 8 mm, the size at which L. opalescens switches from a diet based on copepods to one consisting of mysid and shrimp larvae (Yang et al., 1986).
Top (visual) predators prey on the squid, which perform diel migration to reduce the risk of being seen and eaten. However, most are consumed, leaving a few survivors to continue to grow. The few that reach the prescribed ML of 8 mm are considered to have survived paralarval mortality and to become recruits to the adult squid population. At this final stage, the squid leave the virtual ecosystem (VE), which simulates only the planktonic stage of their life history. The life histories of new recruits once they reach adulthood are not modelled; in particular we do not compute the number that survives to spawn in the subsequent year. Each VE therefore has a new squid population initialized by an exogenous spawning event triggered at the same time each year of a multiyear simulation.
The model features two populations of top predator. These are special functional groups used to provide trophic closure to the model. The peculiarity of these top predators lies in the fact that their demography and size do not depend on their feeding history, but are instead prescribed by a model function. BPs feed on copepods and therefore compete with the planktonic squid for food; BP size and concentration are held constant throughout the year. VPs are larger squid-eating squid paralarvae. They feed on the planktonic squid at a rate that depends on predator size and concentration. They are set to grow at a rate of 2% of body weight per day, and their concentration decays exponentially (Sinerchia, 2007). Capture of squid paralarvae is via an interaction between individual predator agents and emergent prey concentration, which is computed by summing the prey subpopulations. Prey visibility is modelled using a parametrization devised by Woods (2005). The feature that distinguishes top predators from all other explicitly modelled functional groups is that temporal variations in their size, concentration, and distribution (with depth) are prescribed by exogenous equations and parameter values (Woods, 2005). A top predator population is represented by a single LE agent in each layer of the virtual mesocosm. Detailed explanation of model equations is provided in Sinerchia (2007) and Sinerchia et al. (2008).
Physical model and forcing
The physical model consists of solar radiation in 25 wavebands, Morel optics, and a model of the turbulent mixing layer as described in Woods and Barkmann (1986). Insolation (solar irradiance at the sea surface) is computed at each time-step from astronomical equations for solar elevation and cloud cover from Bunker (Isemer and Hasse, 1987). Surface fluxes of momentum and heat are derived from Bunker.
The initial profiles of nutrient concentrations were derived from the NOAA Ocean Atlas (NOAA, 2002).
Simulations
A reference model run (Base) was initialized with 2 × 108 individual diatoms m–2, represented by 4000 agents, 6000 individual copepods m–2, represented by 600 agents, 300 individual squid m–2, represented by 300 agents, and 3000 individual top predators m–2, represented by 100 agents each for BP and VP. The Base run (and all subsequent experiments) were initialized on 1 January and run for 25 years. On 10 April, each year a batch of squid eggs are released into the virtual mesocosm by an exogenous population of spawning adults. The Base run was used to allow the VE to adjust to a stable attractor, which was then used as the starting point for two groups of numerical experiments.
The first group of numerical experiments established the prerequisites for the project, demonstrating that, when driven by stationary annual forcing, the LERM achieves multiyear stability (i.e. the interannual variation in demographic variables from the multiyear average is low, i.e. ∼10 in Woods et al., 2005). The LE method can therefore be used successfully to compute the sensitivity of squid recruitment to changes in ecosystem conditions. The second group of numerical experiments explores the sensitivity of squid recruitment as a function of variations in exogenous factors.
Stability of the VE
The LERM is a stochastic model, because advection in the turbulent mixing layer is modelled by random displacement (Woods, 2005). The VEs generated by the LERM are, however, stable, in that the interannual variability of the ecosystem's emergent properties are within a few percentage points of their multiyear average, with a typical signal-to-noise ratio of ∼10. This condition is reached after an initial period of a few years during which the population balances with its ambient environment and becomes independent of the initial conditions; at this point, the VE is “on attractor” (Woods et al., 2005). Results are obtained by analysing a VE on attractor at a specified time each year, over several successive years. The independence of initial conditions was demonstrated by repeating the Base run with four variations of the initial P and Z concentrations specified above: (i) half P and half Z (P05Z05), (ii) double P and half Z (P2Z05), (iii) half P and double Z (P05Z2), and (iv) double P and double Z (P2Z2). The emergent noise in the attractor is reported below.
Sensitivity of squid recruitment to changes in exogenous factors
The experiments explored, one at a time, the sensitivity of recruitment to changes in three exogenous properties:
the concentration of VPs, varying between 0 and 12 000 ind. m–2, to investigate the effect of increasing levels of predator pressure on the population of squid paralarvae;
the concentration of BPs competing for squid food, varying between 0 and 12 000 ind. m–2, to investigate the effect of interpopulation competition for food (Z);
the number of squid eggs laid (Eggs), varying between 100 and 700 m–2, to investigate the effect of intrapopulation competition for food (Z).
Results
Table 1 shows the average biomass of P, Z, and S, and the interannual variation from the multiyear mean computed, first, over the entire 25-year simulation period, and, second, in the most recent 10 years, i.e. after a 15-year period of adjustment to initial conditions. The coefficient of variation (CV expressed as a percentage) of the biomass of a given species at a given point in the annual cycle (here, we choose 28 May) provides a measure of the stability when the VE reaches the attractor.
Diatom (P), copepod (Z), and squid (S) biomasses on 28 May at 06:00 (mmolC m–2), giving the interannual averages, standard deviations, and the coefficients of variation as a percentage (CV%) from the multiyear mean (full 25 years or most recent 10 years).
| . | . | Base . | P05Z05 . | P2Z05 . | P05Z2 . | P2Z2 . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Period . | Taxon . | Average . | CV% . | Average . | CV% . | Average . | CV% . | Average . | CV% . | Average . | CV% . |
| All (25 years) | P | 390.4 ± 27.8 | 7.1 | 393.0 ± 11.0 | 2.8 | 394.4 ± 0.3 | 0.1 | 396.1 ± 32.7 | 8.3 | 379.3 ± 38.5 | 10.2 |
| Z | 83.7 ± 8.0 | 9.6 | 81.4 ± 5.6 | 6.9 | 87.3 ± 6.2 | 7.1 | 99.8 ± 25.0 | 25.1 | 92.0 ± 17.5 | 19.0 | |
| S | 1.8 ± 0.4 | 21.4 | 1.6 ± 0.3 | 19.9 | 1.6 ± 0.1 | 9.0 | 1.8 ± 0.6 | 36.8 | 2.0 ± 1.2 | 59.5 | |
| Most recent (10 years) | P | 395.1 ± 9.4 | 2.4 | 395.7 ± 11.2 | 2.8 | 390.7 ± 3.8 | 1.0 | 404.3 ± 14.9 | 3.7 | 390.8 ± 8.9 | 2.3 |
| Z | 80.7 ± 4.9 | 6.1 | 83.9 ± 7.2 | 8.6 | 87.8 ± 4.5 | 5.2 | 90.4 ± 6.0 | 6.6 | 91.6 ± 4.0 | 4.4 | |
| S | 1.9 ± 0.2 | 9.2 | 1.5 ± 0.2 | 11.0 | 1.6 ± 0.1 | 5.3 | 1.7 ± 0.2 | 11.3 | 1.7 ± 0.2 | 8.9 | |
| . | . | Base . | P05Z05 . | P2Z05 . | P05Z2 . | P2Z2 . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Period . | Taxon . | Average . | CV% . | Average . | CV% . | Average . | CV% . | Average . | CV% . | Average . | CV% . |
| All (25 years) | P | 390.4 ± 27.8 | 7.1 | 393.0 ± 11.0 | 2.8 | 394.4 ± 0.3 | 0.1 | 396.1 ± 32.7 | 8.3 | 379.3 ± 38.5 | 10.2 |
| Z | 83.7 ± 8.0 | 9.6 | 81.4 ± 5.6 | 6.9 | 87.3 ± 6.2 | 7.1 | 99.8 ± 25.0 | 25.1 | 92.0 ± 17.5 | 19.0 | |
| S | 1.8 ± 0.4 | 21.4 | 1.6 ± 0.3 | 19.9 | 1.6 ± 0.1 | 9.0 | 1.8 ± 0.6 | 36.8 | 2.0 ± 1.2 | 59.5 | |
| Most recent (10 years) | P | 395.1 ± 9.4 | 2.4 | 395.7 ± 11.2 | 2.8 | 390.7 ± 3.8 | 1.0 | 404.3 ± 14.9 | 3.7 | 390.8 ± 8.9 | 2.3 |
| Z | 80.7 ± 4.9 | 6.1 | 83.9 ± 7.2 | 8.6 | 87.8 ± 4.5 | 5.2 | 90.4 ± 6.0 | 6.6 | 91.6 ± 4.0 | 4.4 | |
| S | 1.9 ± 0.2 | 9.2 | 1.5 ± 0.2 | 11.0 | 1.6 ± 0.1 | 5.3 | 1.7 ± 0.2 | 11.3 | 1.7 ± 0.2 | 8.9 | |
Diatom (P), copepod (Z), and squid (S) biomasses on 28 May at 06:00 (mmolC m–2), giving the interannual averages, standard deviations, and the coefficients of variation as a percentage (CV%) from the multiyear mean (full 25 years or most recent 10 years).
| . | . | Base . | P05Z05 . | P2Z05 . | P05Z2 . | P2Z2 . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Period . | Taxon . | Average . | CV% . | Average . | CV% . | Average . | CV% . | Average . | CV% . | Average . | CV% . |
| All (25 years) | P | 390.4 ± 27.8 | 7.1 | 393.0 ± 11.0 | 2.8 | 394.4 ± 0.3 | 0.1 | 396.1 ± 32.7 | 8.3 | 379.3 ± 38.5 | 10.2 |
| Z | 83.7 ± 8.0 | 9.6 | 81.4 ± 5.6 | 6.9 | 87.3 ± 6.2 | 7.1 | 99.8 ± 25.0 | 25.1 | 92.0 ± 17.5 | 19.0 | |
| S | 1.8 ± 0.4 | 21.4 | 1.6 ± 0.3 | 19.9 | 1.6 ± 0.1 | 9.0 | 1.8 ± 0.6 | 36.8 | 2.0 ± 1.2 | 59.5 | |
| Most recent (10 years) | P | 395.1 ± 9.4 | 2.4 | 395.7 ± 11.2 | 2.8 | 390.7 ± 3.8 | 1.0 | 404.3 ± 14.9 | 3.7 | 390.8 ± 8.9 | 2.3 |
| Z | 80.7 ± 4.9 | 6.1 | 83.9 ± 7.2 | 8.6 | 87.8 ± 4.5 | 5.2 | 90.4 ± 6.0 | 6.6 | 91.6 ± 4.0 | 4.4 | |
| S | 1.9 ± 0.2 | 9.2 | 1.5 ± 0.2 | 11.0 | 1.6 ± 0.1 | 5.3 | 1.7 ± 0.2 | 11.3 | 1.7 ± 0.2 | 8.9 | |
| . | . | Base . | P05Z05 . | P2Z05 . | P05Z2 . | P2Z2 . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Period . | Taxon . | Average . | CV% . | Average . | CV% . | Average . | CV% . | Average . | CV% . | Average . | CV% . |
| All (25 years) | P | 390.4 ± 27.8 | 7.1 | 393.0 ± 11.0 | 2.8 | 394.4 ± 0.3 | 0.1 | 396.1 ± 32.7 | 8.3 | 379.3 ± 38.5 | 10.2 |
| Z | 83.7 ± 8.0 | 9.6 | 81.4 ± 5.6 | 6.9 | 87.3 ± 6.2 | 7.1 | 99.8 ± 25.0 | 25.1 | 92.0 ± 17.5 | 19.0 | |
| S | 1.8 ± 0.4 | 21.4 | 1.6 ± 0.3 | 19.9 | 1.6 ± 0.1 | 9.0 | 1.8 ± 0.6 | 36.8 | 2.0 ± 1.2 | 59.5 | |
| Most recent (10 years) | P | 395.1 ± 9.4 | 2.4 | 395.7 ± 11.2 | 2.8 | 390.7 ± 3.8 | 1.0 | 404.3 ± 14.9 | 3.7 | 390.8 ± 8.9 | 2.3 |
| Z | 80.7 ± 4.9 | 6.1 | 83.9 ± 7.2 | 8.6 | 87.8 ± 4.5 | 5.2 | 90.4 ± 6.0 | 6.6 | 91.6 ± 4.0 | 4.4 | |
| S | 1.9 ± 0.2 | 9.2 | 1.5 ± 0.2 | 11.0 | 1.6 ± 0.1 | 5.3 | 1.7 ± 0.2 | 11.3 | 1.7 ± 0.2 | 8.9 | |
For all experiments, the biomass on 28 May for the full 25 years is compared with that observed in the past 10 years (Table 1). For all experiments, the VEs converge to an attractor, as shown by the reduced variability in biomass of the VE in the past 10 years compared with the 25-year period (CV%). In particular, the variability from the interannual mean in the most recent 10 years was small, not >3.7% for P, >8.6% for Z, and >11.3% for S, and all VEs converge to the same attractor independently of initial conditions. Once on attractor, the biomasses on 28 May for P, Z, and S varied, respectively, between 390.8 ± 8.9 and 404.3 ± 14.9, between 80.7 ± 4.9 and 91.6 ± 4.0, and between 1.5 ± 0.2 and 1.9 ± 0.2.
Sensitivity of squid recruitment to changes in exogenous factors
Table 2 shows the average rate of mortality, either by starvation (St) or predation (Pr), and the recruitment (R) of squid paralarvae during the most recent 10 years, when the VE has reached a stable attractor, in each simulation of the three sensitivity studies.
Average rate of squid paralarva mortality (by starvation, St, and predation, Pr) and recruitment, R, for the most recent 10 years of simulation, i.e. when the VE is on attractor.
| . | St . | Pr . | R . | . | St . | Pr . | R . | . | St . | Pr . | R . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| VP × 103 . | (ind. m–2 year–1) . | BP × 103 . | (ind. m–2 year–1) . | Eggs × 102 . | (ind. m–2 year–1) . | ||||||
| 0 | 297.0 | 0.0 | 3.0 | 0 | 61.4 | 238.6 | 0.0 | 1 | 0.0 | 95.4 | 4.6 |
| 1 | 90.9 | 204.9 | 4.2 | 1 | 0.0 | 291.3 | 8.7 | 2 | 0.0 | 195.4 | 4.6 |
| 2 | 0.4 | 286.6 | 13.0 | 2 | 0.4 | 289.1 | 10.5 | 3 | 0.0 | 295.8 | 4.2 |
| 3 | 0.0 | 296.0 | 4.0 | 3 | 0.0 | 296.0 | 4.0 | 4 | 0.0 | 395.8 | 4.1 |
| 4 | 0.0 | 297.3 | 2.7 | 4 | 0.0 | 296.5 | 3.5 | 5 | 41.4 | 457.4 | 2.2 |
| 5 | 0.2 | 298.1 | 1.8 | 5 | 0.0 | 296.2 | 3.8 | 6 | 14.8 | 584.5 | 0.8 |
| 6 | 0.0 | 298.4 | 1.6 | 6 | 1.3 | 296.0 | 2.7 | 7 | 39.5 | 660.4 | 0.0 |
| 7 | 0.0 | 298.7 | 1.3 | 7 | 2.0 | 295.0 | 3.0 | ||||
| 8 | 0.0 | 297.9 | 2.1 | 8 | 10.6 | 288.4 | 1.0 | ||||
| 9 | 0.0 | 298.5 | 1.5 | 9 | 8.9 | 290.0 | 1.1 | ||||
| 10 | 0.0 | 297.6 | 2.4 | 10 | 11.3 | 288.1 | 0.6 | ||||
| 11 | 0.0 | 298.0 | 2.0 | 11 | 13.3 | 286.0 | 0.7 | ||||
| 12 | 0.0 | 298.6 | 1.4 | 12 | 16.0 | 284.0 | 0.0 | ||||
| . | St . | Pr . | R . | . | St . | Pr . | R . | . | St . | Pr . | R . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| VP × 103 . | (ind. m–2 year–1) . | BP × 103 . | (ind. m–2 year–1) . | Eggs × 102 . | (ind. m–2 year–1) . | ||||||
| 0 | 297.0 | 0.0 | 3.0 | 0 | 61.4 | 238.6 | 0.0 | 1 | 0.0 | 95.4 | 4.6 |
| 1 | 90.9 | 204.9 | 4.2 | 1 | 0.0 | 291.3 | 8.7 | 2 | 0.0 | 195.4 | 4.6 |
| 2 | 0.4 | 286.6 | 13.0 | 2 | 0.4 | 289.1 | 10.5 | 3 | 0.0 | 295.8 | 4.2 |
| 3 | 0.0 | 296.0 | 4.0 | 3 | 0.0 | 296.0 | 4.0 | 4 | 0.0 | 395.8 | 4.1 |
| 4 | 0.0 | 297.3 | 2.7 | 4 | 0.0 | 296.5 | 3.5 | 5 | 41.4 | 457.4 | 2.2 |
| 5 | 0.2 | 298.1 | 1.8 | 5 | 0.0 | 296.2 | 3.8 | 6 | 14.8 | 584.5 | 0.8 |
| 6 | 0.0 | 298.4 | 1.6 | 6 | 1.3 | 296.0 | 2.7 | 7 | 39.5 | 660.4 | 0.0 |
| 7 | 0.0 | 298.7 | 1.3 | 7 | 2.0 | 295.0 | 3.0 | ||||
| 8 | 0.0 | 297.9 | 2.1 | 8 | 10.6 | 288.4 | 1.0 | ||||
| 9 | 0.0 | 298.5 | 1.5 | 9 | 8.9 | 290.0 | 1.1 | ||||
| 10 | 0.0 | 297.6 | 2.4 | 10 | 11.3 | 288.1 | 0.6 | ||||
| 11 | 0.0 | 298.0 | 2.0 | 11 | 13.3 | 286.0 | 0.7 | ||||
| 12 | 0.0 | 298.6 | 1.4 | 12 | 16.0 | 284.0 | 0.0 | ||||
Average rate of squid paralarva mortality (by starvation, St, and predation, Pr) and recruitment, R, for the most recent 10 years of simulation, i.e. when the VE is on attractor.
| . | St . | Pr . | R . | . | St . | Pr . | R . | . | St . | Pr . | R . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| VP × 103 . | (ind. m–2 year–1) . | BP × 103 . | (ind. m–2 year–1) . | Eggs × 102 . | (ind. m–2 year–1) . | ||||||
| 0 | 297.0 | 0.0 | 3.0 | 0 | 61.4 | 238.6 | 0.0 | 1 | 0.0 | 95.4 | 4.6 |
| 1 | 90.9 | 204.9 | 4.2 | 1 | 0.0 | 291.3 | 8.7 | 2 | 0.0 | 195.4 | 4.6 |
| 2 | 0.4 | 286.6 | 13.0 | 2 | 0.4 | 289.1 | 10.5 | 3 | 0.0 | 295.8 | 4.2 |
| 3 | 0.0 | 296.0 | 4.0 | 3 | 0.0 | 296.0 | 4.0 | 4 | 0.0 | 395.8 | 4.1 |
| 4 | 0.0 | 297.3 | 2.7 | 4 | 0.0 | 296.5 | 3.5 | 5 | 41.4 | 457.4 | 2.2 |
| 5 | 0.2 | 298.1 | 1.8 | 5 | 0.0 | 296.2 | 3.8 | 6 | 14.8 | 584.5 | 0.8 |
| 6 | 0.0 | 298.4 | 1.6 | 6 | 1.3 | 296.0 | 2.7 | 7 | 39.5 | 660.4 | 0.0 |
| 7 | 0.0 | 298.7 | 1.3 | 7 | 2.0 | 295.0 | 3.0 | ||||
| 8 | 0.0 | 297.9 | 2.1 | 8 | 10.6 | 288.4 | 1.0 | ||||
| 9 | 0.0 | 298.5 | 1.5 | 9 | 8.9 | 290.0 | 1.1 | ||||
| 10 | 0.0 | 297.6 | 2.4 | 10 | 11.3 | 288.1 | 0.6 | ||||
| 11 | 0.0 | 298.0 | 2.0 | 11 | 13.3 | 286.0 | 0.7 | ||||
| 12 | 0.0 | 298.6 | 1.4 | 12 | 16.0 | 284.0 | 0.0 | ||||
| . | St . | Pr . | R . | . | St . | Pr . | R . | . | St . | Pr . | R . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| VP × 103 . | (ind. m–2 year–1) . | BP × 103 . | (ind. m–2 year–1) . | Eggs × 102 . | (ind. m–2 year–1) . | ||||||
| 0 | 297.0 | 0.0 | 3.0 | 0 | 61.4 | 238.6 | 0.0 | 1 | 0.0 | 95.4 | 4.6 |
| 1 | 90.9 | 204.9 | 4.2 | 1 | 0.0 | 291.3 | 8.7 | 2 | 0.0 | 195.4 | 4.6 |
| 2 | 0.4 | 286.6 | 13.0 | 2 | 0.4 | 289.1 | 10.5 | 3 | 0.0 | 295.8 | 4.2 |
| 3 | 0.0 | 296.0 | 4.0 | 3 | 0.0 | 296.0 | 4.0 | 4 | 0.0 | 395.8 | 4.1 |
| 4 | 0.0 | 297.3 | 2.7 | 4 | 0.0 | 296.5 | 3.5 | 5 | 41.4 | 457.4 | 2.2 |
| 5 | 0.2 | 298.1 | 1.8 | 5 | 0.0 | 296.2 | 3.8 | 6 | 14.8 | 584.5 | 0.8 |
| 6 | 0.0 | 298.4 | 1.6 | 6 | 1.3 | 296.0 | 2.7 | 7 | 39.5 | 660.4 | 0.0 |
| 7 | 0.0 | 298.7 | 1.3 | 7 | 2.0 | 295.0 | 3.0 | ||||
| 8 | 0.0 | 297.9 | 2.1 | 8 | 10.6 | 288.4 | 1.0 | ||||
| 9 | 0.0 | 298.5 | 1.5 | 9 | 8.9 | 290.0 | 1.1 | ||||
| 10 | 0.0 | 297.6 | 2.4 | 10 | 11.3 | 288.1 | 0.6 | ||||
| 11 | 0.0 | 298.0 | 2.0 | 11 | 13.3 | 286.0 | 0.7 | ||||
| 12 | 0.0 | 298.6 | 1.4 | 12 | 16.0 | 284.0 | 0.0 | ||||
Sensitivity to changes in VP concentration
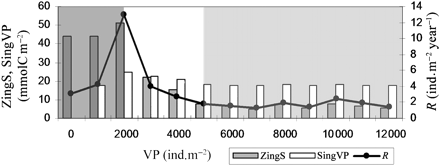
The effect of predation pressure on squid mean annual recruitment is shown in Figure 2. Mean annual recruitment varied between 1.3 ± 0.1 individual squid m–2 year–1, when VP was 7000 individual top predators m–2, and 13.0 ± 2.8 individual squid m–2 year–1, when VP was 2000 individual top predators m–2. In particular, the sensitivity of mean annual recruitment to predator concentration can be divided into three levels, depicted below.
At low predator concentrations (VP ≤ 2000 ind. m–2), mean annual recruitment increased rapidly with increasing VP, peaking at 13 ind. m–2 year–1. This increase in recruitment with increasing predator concentration is a counter-intuitive emergent response of the numerical experiments. However, it is because low predator concentration reduces the number of squid eaten, but also causes an increase in intrapopulation competition for the limited food present. This is reflected by the causes of mortality. At this level, recruitment is limited by food availability, and starvation mortality only takes place when predator concentration is ≤2000 ind. m–2 (Table 2). The mean annual copepod biomass ingested by the squid population (ZingS) exceeded 43 mmolC m–2, with a maximum of 51 mmolC m–2 corresponding to the highest level of recruitment (VP 2000 ind. m–2). At this level, the mean annual squid biomass removed by predation (SingVP) increased from 0 to 25 mmolC m–2.
With increasing VP concentrations above 2000 ind. m–2, mean annual recruitment decreased quickly from 13.0 ± 2.8 to 1.8 ± 0.3 ind. m–2 year–1 (VP 5000 ind. m–2). Increasing VP concentration from 2000 to 3000 ind. m–2 more than halves the mean annual zooplankton biomass ingested by the squid population, from 51 to 22 mmolC m–2, whereas the mean annual squid biomass removed by predation decreased only slightly, from 25 to 23 mmolC m–2. Note too that at a VP concentration of ∼3000 ind. m–2, the mean annual biomass ingested by the predator became higher than that ingested by squid.
Above 5000 ind. m–2, mean annual recruitment stabilized at 1.3–2.4 ind. m–2 year–1, whereas the mean zooplankton biomass ingested by the squid population never exceeded 10 mmolC m–2 and the mean annual squid biomass removed by predation was always <20 mmolC m–2.
Mean squid recruitment (R, line), mean zooplankton biomass ingested by the squid population (ZingS), and mean squid biomass ingested by the VP population (SingVP) during the most recent 10 years of simulation. The shaded areas illustrate the three levels of recruitment sensitivity to VP.
Sensitivity to changes in BP concentration
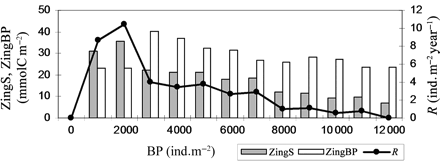
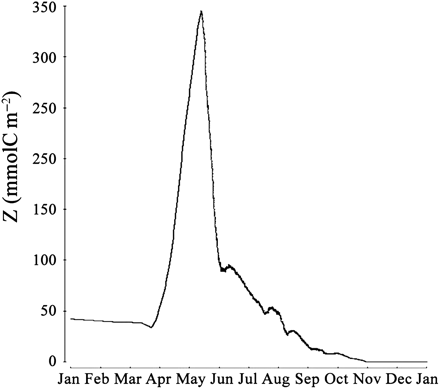
The effect of interpopulation competition for food (Z) on the variation in squid mean annual recruitment is shown in Figure 3. Mean annual recruitment varied between 0 ind. m–2 year–1, when BP concentration was 0 or ≥12 000 ind. m–2, to a maximum of 10.5 ± 4.6 ind. m–2 year–1, when BP was 2000 ind. m–2. A surprising observation is that when BPs were absent from the VE, copepods became extinct and squid failed to recruit. The absence of BPs caused an overall decrease in predation on the copepod population, which was effectively only eaten by squid during the time between hatching and recruitment. On 28 May, copepod biomass peaked at almost 250 mmolC m–2 (Figure 4), about three times higher than the average annual biomass for that date (Table 1). This triggered a density-dependent decrease in the amount of food available, as for squid in the VP experiments. In these food-limited conditions, copepods did not store sufficient energy to enter the overwintering stage nor to survive through winter (Figure 4). As a consequence of copepod extinction, squid were deprived of their food source, and recruitment was zero.
Mean annual squid recruitment (R, line), and mean annual zooplankton biomass ingested by the squid population (ZingS) and by the BP population (ZingBP) during the most recent 10 years of simulation.
Squid recruitment increased as BP concentration increased from 0 to 2000 ind. m–2. During squid presence in the mesocosm, when BP concentration was 1000–2000 ind. m–2, squid ingested more zooplankton biomass than BPs, and recruitment was more than double that at higher concentrations of BPs. With increasing BP concentration above 2000 ind. m–2, BPs ingested more zooplankton biomass than squid, and recruitment decreased progressively to 0 ind. m–2 year–1, when BP concentration was 12 000 ind. m–2. Mortality attributable to predation was the principal cause of squid death, ranging from a minimum of 238.6 in the total absence of food (i.e. when the copepods became extinct), to a maximum of 296.5 squid ingested annually (Table 2). Mortality as a result of starvation occurred in the absence of food (BP = 0 ind. m–2), and increasingly with BP concentrations ≥6000 ind. m–2.
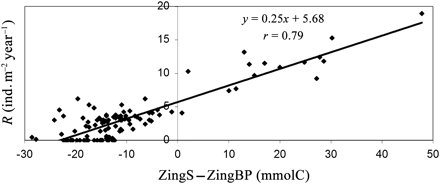
Figure 5 is generated using the data from each year of the 13 runs (BP1–B12 + BASE, 130 datapoints), and shows how squid recruitment was linearly correlated with the difference between carbon ingested by squid and BPs.
Correlation between recruitment and the difference between carbon ingested by squid and BPs.
Sensitivity to changes in the number of squid eggs deposited
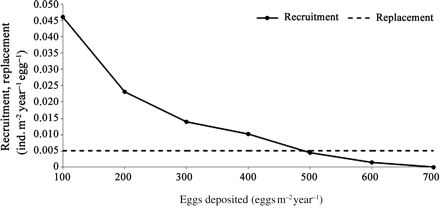
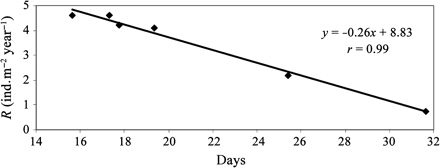
With reference to Table 2, mean annual squid recruitment decreased from 4.6 to 0 ind. m–2 year–1, when the number of eggs deposited rose from 100 to 700 eggs m–2 year–1. Figure 6 shows the mean annual recruitment optimization curve and replacement line (where at least 1 of the 200 eggs in a sac attains 8 mm ML and therefore recruits) as a function of the number of eggs deposited per square metre. The proportion of eggs deposited that recruited decreases progressively as more eggs are deposited annually, exceeding the carrying capacity of the system when 500 or more eggs were deposited per square metre per year (Figure 6). Annual recruitment did not vary when 100 or 200 eggs were deposited, and it decreased slightly when 300 and 400 eggs were deposited. When 500 or more eggs were deposited, annual recruitment decreased progressively, until there was no recruitment when 700 eggs were deposited. When 100–400 eggs were deposited, mortality was exclusively caused by predation; when more eggs were deposited, it was caused by a combination of starvation and predation (Table 2). The residence time of squid in stages S1 and S2 increased with the number of eggs deposited. Almost all (99%) the recruitment variability is explained by the time spent in stages S1 and S2 (Figure 7).
Squid recruitment per egg deposited as a function of the total number of eggs deposited.
Discussion
Squid mortality was caused by a mixture of starvation and predation. Mortality attributable to starvation arose only when food availability became limiting, causing annual recruitment to decrease. This was a consequence of increased competition for food either at an intrapopulation (more squid competing for limited food) or at an interpopulation (more BPs) level. This outcome concurs with speculation that stocks of squid have increased through relaxed competition for food (Caddy and Rodhouse, 1998), but the suggested hypotheses cannot be validated empirically owing to the lack of field data quantifying the extent of ecosystem trophic interactions (Shepherd and Cushing, 1980; Caddy and Rodhouse, 1998; Arkhipkin and Middleton, 2002).
Stability experiments
The Base experiment demonstrated that the VE created by using LERM under the LE metamodel is stable, in the sense that the interannual variability of the ecosystem emergent properties is within a few percentage points of their multiyear average (Woods et al., 2005). The stability experiments, in which the Base run was repeated with four different initial concentrations of P and Z, prove that the VEs created converge to a stable attractor within 15 years, independent of initial conditions, thereafter responding only to external forcing. The convergence of the VEs to a stable attractor, independent of initial conditions, is illustrated in Table 1. This is shown in all runs (the Base run and the four variations with different initial conditions), by the reduced variability in biomass of the VE populations once the ecosystem is on attractor (in the most recent 10 years) compared with the whole 25-year period. This allows one to appreciate the effect of variations in the pressure induced, for example, by predator or competitor abundance on ecosystem variables such as recruitment.
Sensitivity experiments
Predation (VPs)
Results from the numerical experiments presented suggest that, in the VE created, predation was the most important cause of squid mortality during the critical post-hatching period (Table 2). The bars in Figure 2 are used to compare the average carbon transferred from Z to S (ZingS) and from S to VP (SingVP) during the period of squid permanence. This provides an indication of the effect of increasing predation and its effect on squid recruitment. Increasing the number of VPs has the obvious effect of increasing squid mortality. On the other hand, increased predation has the beneficial effect of reducing the number of competing squid. Low levels of predation caused a density-dependent decrease in the food available for individual squid, with a consequent slower growth rate and therefore more exposure to predation, as suggested for fish populations (Ricker and Foerster, 1948; Shepherd and Cushing, 1980). Larger squid are better swimmers, so are more capable of escaping VP attack than small squid. Also, they are better able to counter turbulence in their attempt to swim downwards to avoid being visible to predators. A squid that grows slowly spends more time at a set stage, upon which the maximum swimming speed depends, and is more liable to be eaten than a squid growing faster.
Annual recruitment was determined by a combination of the density-dependent effect, in particular when VP was <3000 ind. m–2, and predation pressure at higher concentrations of VPs. At VP concentration >5000 ind. m–2, the heavier predation rate on squid was compensated by a decrease in competition for food, leading to the surviving squid enjoying rapid growth rates, reaching the size for recruitment before being eaten.
Several studies have suggested that stocks of squid are sensitive to predation pressure (Caddy and Rodhouse, 1998; Arkhipkin and Middleton, 2002). However, there are insufficient data to allow rigorous quantitative analysis of the significance of cephalopods (or other prey) in the diets of predatory fish. Even the most comprehensive studies do not facilitate prediction, because their findings relate only to the period of each study (Boyle and Rodhouse, 2005).
Interpopulation competition for food (BPs)
Some studies have pointed to the importance of interpopulation competition on the abundance and recruitment of squid in different areas of the world (Caddy and Rodhouse, 1998; Arkhipkin and Middleton, 2002). The results from our numerical experiments have shown that, in the VE created, competition for food among members of different populations was indeed a significant factor influencing annual recruitment success (Figure 3). As for intrapopulation competition, abundance of competitor populations greatly influenced squid mortality indirectly through predation mortality caused by a Ricker and Foerster (1948) effect (Table 2): lesser abundance of food per squid led to suboptimal growth, exposing newly hatched squid to greater risks of predation for longer periods (Figures 3–5). The effect of interpopulation competition for food becomes great when BP concentration exceeds 5000 ind. m–2, as shown by the extent of squid mortality caused by starvation. However, when no BPs were present in the VE to compete with squid for food, copepod biomass rose rapidly and caused a correspondingly large reduction in phytoplankton biomass. This, in turn, caused a density-dependent effect in the copepod population, in that the depleted phytoplankton biomass was insufficient to sustain copepod growth. As a result, copepods had to share the limited phytoplankton biomass, and none built up sufficient reserves to overwinter in the first year (Figure 4). Copepods therefore became extinct and squid recruitment was always zero. This condition, in which no other animal feeds on copepods, is an extreme one. However, it does show the overwhelming influence of bottom-up processes on the control of recruitment in squid populations.
A modest increase in BP concentration (up to 3000 ind. m–2) allowed copepods to store sufficient reserves for overwintering, while reducing the competition for food for the squid. As a consequence, when BP was <3000 ind. m–2, squid recruitment was double that at higher concentrations of BPs. Analysis of fisheries statistics suggested that the increased cephalopods landings observed over the past 30 years, in particular in areas where cephalopods and finfish have both been fished intensively in the past (e.g. Mediterranean Sea, NW Pacific, and the central East Atlantic), may be attributable to a reduction in competition for food (Caddy and Rodhouse, 1998). This could be the result of the typically shorter lifespans and faster growth rates, and therefore increased spawning potential, of cephalopods under intensive fishing compared with finfish populations (Caddy and Rodhouse, 1998). Possible competitive interactions between Illex argentinus and Loligo gahi have been reported in Falkland Islands waters, with a strong negative correlation between abundance, possibly through competition for limited food resources or direct predation. (Arkhipkin and Middleton, 2002).
Intrapopulation competition for food (Eggs)
Competition for food between members of the same population was a significant factor influencing the magnitude of recruitment. Annual recruitment as a function of egg production showed a typical Ricker domed shape (Ricker, 1954): it increased with egg production up to a point beyond which density-dependent processes were so strong that they overcompensated for changes in biomass, so that increased egg production led to decreased recruitment (Figure 6; Shepherd, 1982). This phenomenon has been observed in nature for Loligo pealei (Rosenberg et al., 1996) and L. gahi (Agnew et al., 2000). The latter authors suggested that this was caused by a density-dependent effect, but they could only hypothesize that this could be attributable either to cannibalism or competition for food. Rodhouse (2001) suggested that the proposed density-dependent mechanism must be different from cannibalism, because in squid such as L. gahi, the parent stock dies soon after spawning and is therefore unable to cannibalize the next generation when it starts to grow.
Results from our numerical experiments on sensitivity of recruitment as a function of egg production suggest that this density-dependent effect could be caused by intrapopulation competition for food. When the magnitude of spawning exceeds 400 eggs m–2, mortality increases mainly through predation and, to a much lesser extent, starvation, leading to lower survival rates and recruitment (Table 2). In particular, the carrying capacity of the system was exceeded when >500 eggs m–2 were deposited (Figure 6). At a population level, the annual quantity of protein ingested increased with the number of eggs deposited, especially by newly hatched paralarvae. However, this was shared between more squid, making it increasingly insufficient for rapid growth and survival. The average quantity of protein ingested per squid per unit time decreased with increasing squid competing for the limited food, causing them to grow more slowly and rendering them more vulnerable to predation (Figure 7). The interaction between density-dependent larval growth and predation during the critical period results in density-dependent survival of the larvae. This emergent mechanism has been suggested to be an important factor in shaping the recruitment of fish populations (Ricker and Foerster, 1948; Shepherd and Cushing, 1980; Cushing and Horwood, 1994).
Conclusions
The results of the numerical experiments suggest that annual recruitment is a complex emergent property dependent on changes in relationships between predation, intra- and interpopulation competition for food, spawning magnitude, and the consequent speed of growth of newly hatched squid paralarvae.
The results support the Hjort critical-period thesis that recruitment is primarily determined by predation mortality during early larval stages, the duration of which depends on growth rate and hence food availability. Intra- and interpopulation competition for food were significant factors influencing the magnitude of recruitment. Squid recruitment appears to have an overcompensatory relationship with the number of eggs deposited, as observed for L. gahi (Agnew et al., 2000). This is caused by the interaction of density-dependent growth and predation producing density-dependent survival: when competition for a limited food resource is great, larvae grow slowly and are vulnerable to predation for longer (Ricker and Foerster, 1948; Shepherd and Cushing, 1980).
Finally, the LE metamodel and the LERM have proven successful in providing a plausible description of the mechanisms involved in determining squid annual recruitment. However, the method needs to be refined before it can be used operationally as a contribution to fishery management.
Acknowledgements
The research was supported by the Department of Earth Sciences and Engineering of Imperial College, London. We thank the Consiglio Nazionale delle Ricerche (Italy) and ICES for financial support to attend the Symposium in Chile, and Roger Wiley for many helpful discussions relating to the work. Our results broadly support the match–mismatch theory of fisheries recruitment promoted by the late David Cushing, who was a staunch supporter of our work on the LE metamodel.
References
Author notes
Handling editor: Andrew Payne