-
PDF
- Split View
-
Views
-
Cite
Cite
Gemma Jennings, Derek J. McGlashan, Robert W. Furness, Responses to changes in sprat abundance of common tern breeding numbers at 12 colonies in the Firth of Forth, east Scotland, ICES Journal of Marine Science, Volume 69, Issue 4, May 2012, Pages 572–577, https://doi.org/10.1093/icesjms/fss022
Close - Share Icon Share
Abstract
Breeding numbers collected in 12 common tern Sterna hirundo colonies in the Firth of Forth, Scotland, along with sprat landings data for the area, were used to investigate how the dynamics of a shared prey resource may affect different colonies in a region. Between 1969 and 2010, breeding numbers fluctuated much more at individual colonies than across the region as a whole, with the largest colonies showing opposite trends, suggesting relocation by birds. This indicates that data from individual colonies may be less useful than regional numbers when using seabirds as indicators. Tern breeding numbers in the region were reduced when the sprat stock (Sprattus sprattus) collapsed in the early 1980s after targeted fishing, but recovered during recent decades when the stock was unfished. This should be considered for reopening the Firth of Forth sprat fishery, as well as in the management of other shared prey stocks.Jennings, G., McGlashan, D. J. and Furness, R. W. 2012. Responses to changes in sprat abundance of common tern breeding numbers at 12 colonies in the Firth of Forth, east Scotland. – ICES Journal of Marine Science, 69: 572–577.
Introduction
Many of the world's fish stocks are considered to be fully fished or overfished (FAO, 2010), and for small pelagic fish, there is growing concern over the effects of stock depletion on top predators such as seabirds (Furness and Tasker, 2000; Gjøsæter et al., 2009). There is a need to understand how seabirds respond to prey stock depletion and to develop indicators for ecosystem management of fisheries taking account of the needs of top predators (Kabuta and Laane, 2003; Cury and Christensen, 2005; Livingston et al., 2005). One such indicator might be breeding numbers of seabirds in colonies. There are clear examples of seabird population crashes resulting from the depletion of small pelagic prey fish stocks, particularly from upwelling areas of high productivity (Crawford et al., 2007; Pichegru et al., 2010) and simple foodwebs (Barrett and Krasnov, 1996; Frederiksen et al., 2004; Gjøsæter et al., 2009). However, in many regions, the factors affecting seabird breeding population sizes are a complex mixture of top–down and bottom–up effects, and changes in breeding numbers can rarely be attributed with confidence to the depletion of specific prey-fish stocks (Mitchell et al., 2004). In part, this is due to buffering in the life history of seabirds. As long-lived animals, seabirds may show strong responses of breeding success to reduced food supply, but in poor years may abandon breeding to protect adult survival rates, which are the main driver of population change, thus maximizing lifetime reproductive success (Piatt et al., 2007). There is also clear variation among seabird species in their responses to changes in food abundance (Furness and Tasker, 2000; Diamond and Devlin, 2003). Terns are particularly likely to experience breeding failure, to abandon breeding colonies, and to move between colonies within a region when food stocks are reduced (Becker and Ludwigs, 2004; Crawford, 2009), whereas many larger seabirds with greater foraging ranges are highly site-faithful even under adverse environmental conditions (Pichegru et al., 2010). Terns are particularly sensitive to fluctuations in the abundance of small pelagic fish (Schaffner, 1986; Furness and Tasker, 2000; Mitchell et al., 2004; Crawford, 2009; Dänhardt and Becker, 2011). Theory predicts that tern breeding success should show stronger responses to reductions in food supply than tern breeding numbers, since seabirds as long-lived animals buffer their survival against stresses of food shortage and will abandon breeding attempts (Monaghan, 1996; Becker and Ludwigs, 2004; Piatt et al., 2007). However, breeding success of terns can also be strongly influenced by weather conditions and by predators (Becker and Specht, 1991; Furness and Tasker, 2000; Mitchell et al., 2004). It has also been shown that common terns Sterna hirundo select which breeding colony they recruit into based on their experience as pre-breeders in the area (Dittmann et al., 2005). As a result of both these processes, tern breeding success may be less clearly related to food abundance than breeding numbers.
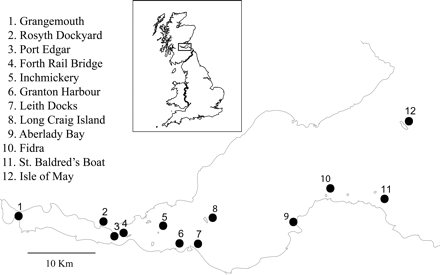
There are many long-term datasets on the breeding success of seabirds, and, in some cases, these can be related to measured changes in abundance of their prey fish (Furness, 2007; Cury et al., 2011; Dänhardt and Becker, 2011). Although there are some long-term datasets on the breeding numbers of seabirds at individual colonies (e.g. in Mitchell et al., 2004), there are rather few datasets for all colonies within a specific region. It has been suggested that trends in breeding numbers may vary among colonies within particular regions (Mitchell et al., 2004). If so, measures of the regional breeding population, rather than of individual colonies, are likely to be more appropriate to relate to changes in food abundance. Here, we examine changes in breeding numbers of common terns in the Firth of Forth region, east Scotland (Figure 1). There is a detailed time-series for the years 1969–2010 on breeding numbers in all the 12 colonies within this region. During this period, the abundance of their main prey in the Firth of Forth, the sprat Sprattus sprattus (Jennings et al., 2010), though not assessed analytically, has changed considerably.
Map of the Firth of Forth, showing the locations of the 12 common tern colonies, numbered 1–12 from west to east.
The sprat stock of the Firth of Forth was the target of a locally based reduction fishery (a fishery to manufacture fishmeal and oil) that harvested a total of 88 000 t between 1966 and 1980 (Marine Scotland Database). As mean sprat mass in this fishery was only a few grammes (Appendix 2 in Fernandez et al., 2005), this is equivalent to tens of billions of fish, a large harvest from a relatively small sea area (93 km long with a 4655-km2 drainage basin; Elliott and Neill, 2007). This fishery was mainly carried out by small boats from ports in Fife, on the north shore of the Firth of Forth, using light trawl gear (Fernandez et al., 2005). After 1980, catches fell to extremely low levels as the stock collapsed (Fernandez et al., 2005), and the fishery was progressively abandoned by local fishers in the early 1980s due to the lack of a profitable catch rate. It ceased completely in 1985. It has never reopened, although sprat abundance in the Firth of Forth has subsequently recovered (Fernandez et al., 2005). There have been many attempts since the late 1990s by local fishers to argue that sprat fishing in the Firth of Forth should be allowed to resume. To date, these have been rejected by the then Scottish Executive (now Scottish Government; http://www.theyworkforyou.com/sp/?id=2005-10-27.20112.2), and so the Firth of Forth sprat stock has been unfished for the last 25 years. The sprat fishery took only a trivial bycatch of juvenile herring Clupea harengus (Fernandez et al., 2005), which are very rarely found in the diet of common terns in the Firth of Forth (Jennings et al., 2010). Therefore, variations in herring abundance are unlikely to complicate the analysis of relationships between common terns and sprats in this region.
Here, we first test the hypothesis that tern numbers at individual colonies will vary much more than across the region as a whole, so that individual colony sizes are less suitable as indicators of seabird–fish stock relationships than the sum of all colonies within a region. Second, we test the hypothesis that breeding numbers of common terns in the Firth of Forth would decrease when the sprat stock collapsed after 1980, but would recover after sprats increased during the unfished period in recent years.
Methods
Long-term data: breeding common terns and sprat fishery
Seabird surveys of breeding numbers have been performed annually in the Firth of Forth since 1969. Data were obtained from the Forth Seabird Group database and the Joint Nature Conservation Committee's (JNCC) Seabird Monitoring Programme database for 12 sites in the Firth of Forth, listed from west to east: Grangemouth, Rosyth Dockyard, Port Edgar, Forth Rail Bridge, Inchmickery, Granton Harbour, Leith Docks, Long Craig Island, Aberlady Bay, Fidra, St Baldred's Boat, and the Isle of May (Figure 1). Data were available from 1969 to 2010. There were several years with missing data for some sites, but usually it is likely that the site was not counted because breeding terns were absent or numbers were negligible. There are no unmonitored colonies within this region. The standard count unit is apparently occupied nests, which equates closely to breeding pairs (Walsh et al., 1995).
Sprat has made up from 69 to more than 90% of the diet fed to chicks by breeding common terns in the Firth of Forth in recent years (Jennings et al., 2010), so changes in sprat abundance will be a critical factor for this tern population. Annual landing data (tonnes of sprat) dating back to 1960 were obtained from the Marine Scotland Database for the Firth of Forth (ICES rectangles 41E6 and 41E7). Data were for all vessels landing in Scotland and for all types of gear.
Data analyses
Variation in common tern breeding numbers within and between colonies
To test the hypothesis that tern numbers at individual colonies (Figure 2) will vary more than across the Firth of Forth region as a whole, the mean, variance, and coefficient of variation (CV) were calculated for numbers of breeding terns at each of the 12 colonies and for the whole region, for the period 1969–2010. We predicted that the CV would be lower for the whole Firth of Forth than for individual colonies. We then correlated breeding numbers at the four largest colonies (Leith Docks, Inchmickery, Aberlady Bay, and the Isle of May) across years to test whether numbers at different colonies followed similar patterns responding to changes in food abundance, showed independent dynamics, or showed inverse relationships. Inverse relationships would indicate a local redistribution of a total regional population that was likely to be food limited, in response to local changes in colony habitat quality.
Numbers of breeding pairs of common terns each year from 1969 to 2010 at the nine largest colonies in the Firth of Forth [three further colonies (Granton, Port Edgar, and Forth Rail Bridge) that held on average fewer than ten pairs are not shown].
Relationship between common tern breeding numbers and the status of the sprat fishery
Sprat stock biomass is difficult to measure, and ICES recently concluded that even at the scale of the North Sea, there were no reliable data on annual variations in sprat stock biomass (ICES, 2009). There are no analytical data on sprat stock biomass in the Firth of Forth (ICES, 2009). We cannot use annual sprat catch data as a proxy for sprat stock biomass, as catch varies in part as a function of effort. However, we can categorize years into periods of differing sprat stock status. When the fishery started, the sprat abundance was high, leading to large catches in 1969–1980 (Figure 3), so the period 1969–1980 was categorized as “harvest period”. Between 1981 and 1990, landings were greatly reduced and the fishery was eventually abandoned by local fishers due to the collapse of the sprat stock (Figure 3). This period was thus labelled “collapse”. The 10-year period 1991–2000 was labelled “initial no-take period”; it is likely that the reduced sprat stock was in a state of recovery during this time, but there are no data to confirm this. However, after 2001, the stock had clearly recovered and fishers were lobbying to reopen the fishery, so we define this fourth period as “recent” and infer that sprat stock biomass was relatively high during this period. In summary, these periods, based on the inferred status of the sprat stock of the Firth of Forth, were defined as follows. (i) 1969–1980,“harvest period”; (ii) 1981–1990, “collapse”; (iii) 1991–2000, “initial no-take period”; and (iv) 2001–2010, “recent”. We compared common tern breeding numbers between these four periods. The response variable (numbers of breeding common terns) was overdispersed, so a generalized linear model with a quasipoisson distribution was applied to the data using the software package “R”.
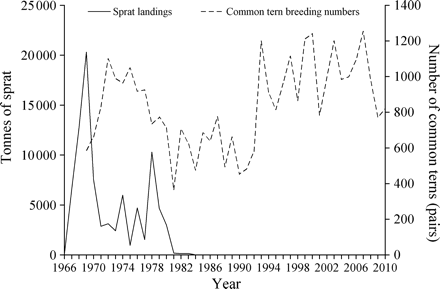
Sprat landings and numbers of breeding common terns in the Firth of Forth. Annual landings of sprats from the Firth of Forth fishery from 1966 to 2010. Data are for statistical rectangles 41E6 and 41E7 from Scottish Government Fisheries Management Database. Total catch of 88 000 t of clupeids, assessed as >98% sprat, <2% herring. Common tern numbers for each year are the sum of numbers of breeding pairs at each of the 12 colonies shown in Figure 1, for the years 1969 to 2010.
Results
Variation in common tern breeding numbers within and between colonies
There was considerable interannual variation in numbers of breeding pairs at individual colonies, with some colonies being abandoned during the study period and other new colonies being formed. The CV in common tern breeding numbers at the 12 colonies in the Firth of Forth varied from 0.48 at Grangemouth to 3.37 at Granton Harbour (Table 1) with half of the colonies having a CV of >1. The CV for the entire Firth of Forth was much lower, at 0.31. Clearly the breeding population of the Firth of Forth was relatively more stable over the period 1969–2010 than were numbers at any single colony within the region.
Mean and CV in breeding numbers of common terns in the Firth of Forth at individual colonies (listed individually below from west to east; Figure 1) and for the whole area, from 1969 to 2010.
| Colony . | Mean . | CV . |
|---|---|---|
| Grangemouth | 82.3 | 0.48 |
| Rosyth | 32.4 | 0.71 |
| Port Edgar | 6.2 | 1.14 |
| Forth Rail Bridge | 5.1 | 0.71 |
| Inchmickery | 200.3 | 1.20 |
| Granton Harbour | 1.3 | 3.37 |
| Leith Docks | 407.1 | 0.73 |
| Long Craig Island | 73.7 | 0.56 |
| Aberlady Bay | 113.0 | 1.35 |
| Fidra | 37.4 | 2.10 |
| St Baldred's Boat | 40.7 | 0.78 |
| Isle of May | 82.7 | 1.23 |
| Firth of Forth region | 822.8 | 0.31 |
| Colony . | Mean . | CV . |
|---|---|---|
| Grangemouth | 82.3 | 0.48 |
| Rosyth | 32.4 | 0.71 |
| Port Edgar | 6.2 | 1.14 |
| Forth Rail Bridge | 5.1 | 0.71 |
| Inchmickery | 200.3 | 1.20 |
| Granton Harbour | 1.3 | 3.37 |
| Leith Docks | 407.1 | 0.73 |
| Long Craig Island | 73.7 | 0.56 |
| Aberlady Bay | 113.0 | 1.35 |
| Fidra | 37.4 | 2.10 |
| St Baldred's Boat | 40.7 | 0.78 |
| Isle of May | 82.7 | 1.23 |
| Firth of Forth region | 822.8 | 0.31 |
Mean and CV in breeding numbers of common terns in the Firth of Forth at individual colonies (listed individually below from west to east; Figure 1) and for the whole area, from 1969 to 2010.
| Colony . | Mean . | CV . |
|---|---|---|
| Grangemouth | 82.3 | 0.48 |
| Rosyth | 32.4 | 0.71 |
| Port Edgar | 6.2 | 1.14 |
| Forth Rail Bridge | 5.1 | 0.71 |
| Inchmickery | 200.3 | 1.20 |
| Granton Harbour | 1.3 | 3.37 |
| Leith Docks | 407.1 | 0.73 |
| Long Craig Island | 73.7 | 0.56 |
| Aberlady Bay | 113.0 | 1.35 |
| Fidra | 37.4 | 2.10 |
| St Baldred's Boat | 40.7 | 0.78 |
| Isle of May | 82.7 | 1.23 |
| Firth of Forth region | 822.8 | 0.31 |
| Colony . | Mean . | CV . |
|---|---|---|
| Grangemouth | 82.3 | 0.48 |
| Rosyth | 32.4 | 0.71 |
| Port Edgar | 6.2 | 1.14 |
| Forth Rail Bridge | 5.1 | 0.71 |
| Inchmickery | 200.3 | 1.20 |
| Granton Harbour | 1.3 | 3.37 |
| Leith Docks | 407.1 | 0.73 |
| Long Craig Island | 73.7 | 0.56 |
| Aberlady Bay | 113.0 | 1.35 |
| Fidra | 37.4 | 2.10 |
| St Baldred's Boat | 40.7 | 0.78 |
| Isle of May | 82.7 | 1.23 |
| Firth of Forth region | 822.8 | 0.31 |
Breeding numbers at individual colonies showed strong changes across years, with different or even contrasting trends between single colonies (Figure 2). Spearman's correlations between numbers at the five largest colonies (Table 2) showed strong negative correlations between numbers breeding at Leith Docks and Inchmickery, the two largest colonies, and between numbers at Leith Docks and Aberlady Bay, the third largest colony. In contrast, numbers at Inchmickery and Aberlady Bay showed a strong positive correlation over the 42-year period. Numbers at Leith Docks and the Isle of May showed a positive correlation over this period (Table 2).
Spearman's correlations between numbers of common terns nesting at the four largest colonies in the Firth of Forth from 1969 to 2010.
| Colony . | Inchmickery . | Aberlady Bay . | Isle of May . | Fidra . |
|---|---|---|---|---|
| Leith Docks | −0.81* | −0.83* | 0.72* | −0.24 |
| Inchmickery | 0.59* | −0.62* | 0.03 | |
| Aberlady Bay | −0.87* | 0.42 | ||
| Isle of May | −0.38* |
| Colony . | Inchmickery . | Aberlady Bay . | Isle of May . | Fidra . |
|---|---|---|---|---|
| Leith Docks | −0.81* | −0.83* | 0.72* | −0.24 |
| Inchmickery | 0.59* | −0.62* | 0.03 | |
| Aberlady Bay | −0.87* | 0.42 | ||
| Isle of May | −0.38* |
Significant correlations are denoted by an asterisk (p < 0.05).
Spearman's correlations between numbers of common terns nesting at the four largest colonies in the Firth of Forth from 1969 to 2010.
| Colony . | Inchmickery . | Aberlady Bay . | Isle of May . | Fidra . |
|---|---|---|---|---|
| Leith Docks | −0.81* | −0.83* | 0.72* | −0.24 |
| Inchmickery | 0.59* | −0.62* | 0.03 | |
| Aberlady Bay | −0.87* | 0.42 | ||
| Isle of May | −0.38* |
| Colony . | Inchmickery . | Aberlady Bay . | Isle of May . | Fidra . |
|---|---|---|---|---|
| Leith Docks | −0.81* | −0.83* | 0.72* | −0.24 |
| Inchmickery | 0.59* | −0.62* | 0.03 | |
| Aberlady Bay | −0.87* | 0.42 | ||
| Isle of May | −0.38* |
Significant correlations are denoted by an asterisk (p < 0.05).
Relationship between common tern breeding numbers and the status of the sprat fishery
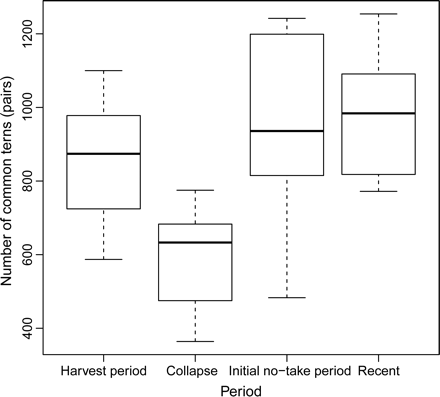
Across the region as a whole, there was a significant difference between numbers of breeding pairs of common terns present during the “harvest period”, “initial no-take period”, and “recent” periods compared with the “collapse” period, with smaller numbers of breeding pairs of common terns during the “collapse” (Figure 4; ANOVA, F = 9.5947, d.f. = 3, p < 0.0001). During the early presence of the fishery, common tern breeding numbers initially showed an increase from 587 pairs in 1969 to 1110 in 1972. After this, landings declined (apparently due to a sprat stock collapse within the region) and the sprat fishery was closed by 1985. This collapse was followed by a period of reduced common tern numbers throughout the 1980s. Eight years after the closure of the sprat fishery, in 1993, the common tern population increased and has remained at large numbers ever since.
Numbers of common terns in the Firth of Forth over four periods: (i) 1969–1980, “harvest period”; (ii) 1981–1990, “collapse”; (iii) 1991–2000, “initial no-take period”; and (iv) 2001–2010, “recent”. The median values of the data are indicated within each box, with the lower and upper edges indicating the 25th and 75th percentiles, respectively. Whiskers show the minimum and maximum data values.
Discussion
Variation in common tern breeding numbers within and between colonies
There was a considerably lower CV in numbers of common tern pairs in the whole of the Firth of Forth compared to numbers at individual colonies (whether large or small), indicating that numbers at individual colonies varied much more than the total population of the region. Based on the strong negative correlations between breeding numbers at the largest colonies, we refute the hypotheses that numbers vary independently among colonies and that numbers at each colony respond similarly across years to changes in abundance of their main food, the Firth of Forth sprat stock. We propose that the strong negative correlations between the largest colonies indicate a redistribution of the Firth of Forth breeding population of common terns from one colony to another, either as a result of large differences in recruitment or as a result of the movement of adults between sites. Numbers fluctuate at individual colonies for a wide variety of reasons, which include local effects of predators, food shortage, human disturbance, exposure to weather extremes, and local environmental change (Becker and Specht, 1991; Craik, 1992, 1997; Becker and Ludwigs, 2004; Forrester et al., 2007). Terns are particularly sensitive to impacts of gulls, which eat eggs and chicks as well as displacing terns from nesting habitat (Eggeling, 1974; Forrester et al., 2007). Within the Firth of Forth, common terns abandoned the Isle of May during the 1950s as gull numbers increased (Eggeling, 1974). After extensive culling of gulls at that site, common terns returned to the Isle of May in 1979, though the colony has never regained its status of the 1940s, as the largest common tern colony in the region (Wanless, 1988).
Relationship between common tern breeding numbers and the status of the sprat fishery
Long-term data for common terns breeding at all sites in the Firth of Forth show that overall numbers fluctuated considerably between 1969 and 2010. A comparison of tern breeding numbers across four fishery periods supports the hypothesis that breeding numbers would decrease when the sprat stock collapsed after 1980 but would recover after sprats increased during the recent unfished period. During the initial presence of the sprat fishery, common tern numbers showed an increase. But when the sprat stock collapsed in the 1980s, tern numbers declined. Following the collapse and subsequent fishery closure, the number of common terns in the Firth of Forth remained considerably reduced for a 10-year period. The state of the sprat stock during this post-fishery period is unknown, but it is likely that the sprat population would have required a number of years to recover to unfished levels of abundance (Hutchings, 2000; Worm et al., 2009; Hammer et al., 2010; Murawski, 2010). Subsequent stock growth, even in the absence of a fishery may be slow, requiring a considerable recovery period (Worm et al., 2009; Hammer et al., 2010; Murawski, 2010). In 1993, the tern population increased and since then has been higher than it was during the fishery or during the 10-year period that followed the collapse of the sprat stock. The data clearly show that tern numbers were reduced in the region when sprat abundance was too low to sustain a fishery and that numbers subsequently recovered to be similar to numbers before the sprat stock collapse. These data indicate that while breeding numbers at individual colonies fluctuated considerably, the total population of the region changed in relation to the inferred variations in sprat abundance. Although sprat catch data do not act as a proxy for annual stock biomass, the change from an abundant stock supporting a fishery in 1969–1980 (“harvest period”) to a collapsed stock in 1981–1990 with very low catch represents a large qualitative change in status with consequences for terns. Such major changes in key food fish stocks, with consequent impacts on seabirds, are not uncommon and have been described in the North Sea (sandeels Ammodytes marinus; Furness and Tasker, 2000; Frederiksen et al., 2004), the Wadden Sea (sprat and herring C. harengus; Dänhardt and Becker, 2011), the Barents Sea (capelin Mallotus villosus; Barrett and Krasnov, 1996; Gjøsæter et al., 2009), and the Benguela ecosystem in southern Africa (anchovy Engraulis encrasicolus; Crawford et al., 2007).
Conclusions
The high concentration of common terns at Leith Docks in recent years is an interesting move by the birds to nest in an industrial site where both predation risk and human disturbance exist, but currently at low levels (Jennings et al., 2010). We suggest that numbers at individual colonies are strongly affected particularly by local influences of predation, whereas numbers in the region as a whole are more strongly influenced by food supply. Dänhardt and Becker (2011) showed that breeding success of common terns at colonies in the Wadden Sea correlated with annual estimates of North Sea herring recruitment and Wadden Sea sprat abundance. However, at some common tern colonies, impacts of predation can be so severe that any relationship with food supply is completely obscured by catastrophic breeding failures caused by predators (e.g. Eggeling, 1974; Craik, 1997; Forrester et al., 2007; Dänhardt and Becker, 2011). We suggest that, in regions where food supply is good but some colonies are affected by predators, common terns will readily relocate or will recruit predominantly into colonies where predation impacts are absent or small (Dittmann et al., 2005). Such behaviour will result in regional breeding numbers showing closer relationships with forage fish abundance and individual colony sizes being driven more by local predation impacts. This has important implications for seabird monitoring studies and conservation and should be considered when using seabirds as indicators. With the implementation of policies such as the EU Marine Strategy Framework Directive, there is now an increased need to establish appropriate indicators, and consideration of regional seabird breeding numbers is of particular relevance to ecosystem-based management of shared fish stocks. Future research should carefully consider the dynamics of individual colonies when evaluating how generally applicable conclusions drawn from single or a small number of seabird colonies may be for management on a broader scale.
Currently, there is no sprat fishery in the Firth of Forth but the sprat stock is now considered to be at a high level (http://www.theyworkforyou.com/sp/?id=2005-10-27.20112.2). Any assessments considering the reopening of the Firth of Forth sprat fishery clearly should consider the potential impact that changes in sprat abundance may have on dependent predators in the region, in particular the population of common terns since the largest colony, at Leith Docks (now holding about 90% of the Firth of Forth population), is protected under European Law as a Special Protection Area for common terns.
Acknowledgements
We thank Dr Paul Fernandes at Marine Scotland for providing sprat catch data for the Firth of Forth from the Scottish Government Fisheries Management Database and the Joint Nature Conservation Committee and Forth Seabird Group for data on breeding numbers of common terns. The corresponding author conducted this research while being funded by a studentship from Forth Ports Limited. We thank two anonymous referees for helpful advice on revising the MS.
References
Author notes
Handling editor: Rochelle Seitz



![Numbers of breeding pairs of common terns each year from 1969 to 2010 at the nine largest colonies in the Firth of Forth [three further colonies (Granton, Port Edgar, and Forth Rail Bridge) that held on average fewer than ten pairs are not shown].](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/icesjms/69/4/10.1093_icesjms_fss022/1/m_fss02202.gif?Expires=1716403842&Signature=fyxYIB7nyPQGrp4U93b4qRihl2t-~ihaH9Nk9YEmI9wZUbZ9jm5xQ1ivb75fymUtwHl6XX9c4aG1Mq9b4bPs-Y8NJRq~Rplk41VUNb0CapCBl6j2TZfME-QluXtldKmAnDRxaHYEFjy2bILvsn1P4vGU0Zy0QrOCEH~o9Lovhmwtq6Nmrf3U5Gwif5EBklAevDZhwuXvy20a6XpQTRfQD-457b~Q0Ortb2sl~B3e2ulNQIncuxXalIxTvnl6Y6z7N51NxBUYZji3Iln6yiozNvsa3~LxrAn6ISxejaJvvvp7DINX5XCrwkclHwAbHqq78aTogWFszTbkLPGcVULlBw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)