-
PDF
- Split View
-
Views
-
Cite
Cite
Ana Sabatés, Paloma Martín, Vanesa Raya, Changes in life-history traits in relation to climate change: bluefish (Pomatomus saltatrix) in the northwestern Mediterranean, ICES Journal of Marine Science, Volume 69, Issue 6, July 2012, Pages 1000–1009, https://doi.org/10.1093/icesjms/fss053
Close - Share Icon Share
Abstract
This study shows the strong relationship between the increasing surface temperature in the NW Mediterranean and the expansion northwards of the bluefish distribution range with the species reproducing in the new distribution areas. Two shifts in temperature were detected: the first one in the early 1980s and the second around 1997. This last shift, explained by warmer springs (April–June), when the species migrates for spawning, led to the observed changes in bluefish. In the western Mediterranean basin, a significant increase in bluefish landings was observed by the mid nineties, whereas in the Catalan coast, the northern edge of the species distribution, a northward expansion was observed from 2000. At present, spawning takes place all along the Catalan coast (June to September), including the new distribution areas, being 21°C the threshold for the presence of larvae in the plankton. This temperature was not attained in June two decades ago. The highest concentrations of larvae were located near the mouth of the Ebro River and their distribution to the north did not extend beyond the thermal front. Bluefish has taken the advantage of the changing environmental conditions and is established in new northernmost distribution areas.Sabatés, A., Martín, P., and Raya, V. 2012. Changes in life-history traits in relation to climate change: bluefish (Pomatomus saltatrix) in the northwestern Mediterranean. – ICES Journal of Marine Science, 69: 1000–1009.
Introduction
There is now ample evidence that climate change has important impacts on the geographic distribution, demography, and phenology of a broad range of organisms (e.g. Stenseth et al., 2002; Beaugrand, 2009). Climate-driven changes in temperature can impact fish both directly, by affecting their physiology and behaviour, and indirectly, by affecting the structure and productivity of ecosystems (Beaugrand, 2009; Brander, 2010).
It has long been recognized that temperature greatly influences fish ecology and physiology, as it affects their reproductive capacity, growth, survival, and migration (Magnuson et al., 1979). Furthermore, temperature is an important factor in defining the range of suitable habitats for marine fish and in determining their distribution within habitats (Murawski, 1993). Consequently, long-term temperature shifts due to climate change are expected to result in contractions or expansions in fish distribution ranges (Perry et al., 2005; Rijnsdorp et al., 2009). These changes are most evident near the boundaries of a species’ range where warming or cooling drives fish to higher or lower latitudes (Rose, 2005; Sabatés et al., 2006). Therefore, climate-driven changes in temperature can modify the phenology of annual migrations to feeding and/or spawning grounds of temperate marine species (Edwards and Richardson, 2004; Jansen and Gislason, 2011).
In the western Mediterranean, a consistent warming trend has been reported in the deep (Bethoux et al., 1999; Rixen et al., 2005; Vargas-Yáñez et al., 2005) and upper (Vargas-Yáñez et al., 2010) layers throughout the 20th century. This warming trend has been particularly evident since the 1980s and at the end of the 1990s (Rixen et al., 2005; Vargas-Yáñez et al., 2005). The warming in the western Mediterranean is similar to that of adjacent areas, such as the North Atlantic or even in the northern hemisphere (Vargas-Yáñez et al., 2010). On the northern Catalan coast, the temperature has increased around 1.1°C in the uppermost waters (0–50 m) and around 0.7°C at 80 m over the last 35 years (1974–2009; Calvo et al., 2011), at a similar rate to that inferred from satellite observations between 1985 and 2006 for the western Mediterranean (0.03°C year−1, Nykjaer, 2009).
Several distinctive features make the Mediterranean Sea a very sensitive area to climate change (Lejeusne et al., 2010; Calvo et al., 2011). It is characterized by a well-defined seasonality, with relatively cold winters in the north and long hot summers in the south. This latitudinal gradient determines the species distribution. Therefore, subtropical species are mainly found in the eastern basin and southern Mediterranean, where the water temperature is higher than average, whereas cold-temperate species inhabit the colder northern areas (Gulf of Lions, Ligurian Sea, and northern Adriatic), with seasonal variation at the surface ranging from 13 to 25°C (Salat, 1996; Bianchi and Morri, 2000). In the Mediterranean, climate change is undoubtedly affecting the basic biology and the ecology of organisms as well as the ecosystem functioning (e.g. Molinero et al., 2008; Conversi et al., 2010; Lejeusne et al., 2010; Calvo et al., 2011; Martín et al., 2012). The long-term temperature increase has been demonstrated to affect the boundaries of biogeographic regions, with some warm-water species extending their ranges and colonizing new areas where they were previously absent (CIESM, 2008). The northward migration of species with an affinity for warm waters has been demonstrated in different Mediterranean regions (Francour et al., 1994; Astraldi et al., 1995; Bianchi and Morri, 2000; Sabatés et al., 2006). Other authors predict the species niche reductions by the middle and the end of the 21st century that might result from sea temperature increase (Ben Rais Lasram et al., 2010).
Bluefish, Pomatomus saltatrix, is a migratory coastal pelagic species found in temperate and tropical marine waters throughout the world (Juanes et al., 1996). In the Mediterranean, it is more abundant in the southern and eastern warm waters (Tortonese, 1986). In the western Mediterranean Basin, the southern Catalan coast had been proposed as the northern boundary edge of the species distribution (Sabatés and Martin, 1993). In that area, the only study on the biology of bluefish is that of Sabatés and Martin (1993), which deals with its distribution and spawning in the early eighties. Other studies refer to the association of the species to fish-farms sea cages (Sanchez-Jerez et al., 2008) and to its population genetic structure (Pardiñas et al., 2010). Hence, the biology, behaviour, and migration of the bluefish in the western Mediterranean still remain largely unknown.
The life cycle, distribution, seasonal migration, and spawning of P. saltatrix are closely linked to temperature. The species is usually found at temperatures from 14–16 to 30°C (Fahay et al., 1999). Specific temperature ranges have been reported for seasonal migration linked to reproduction depending on the geographic area, and 20–26°C has been found to be the preferential surface temperature range for spawning (e.g. Norcross et al., 1974; Kendall and Walford, 1979; Ditty and Shaw, 1995; Juanes et al., 1996). The migration patterns share common characteristics. Therefore, bluefish spends the colder months in warm-water areas and, when the surface temperature reaches a certain value, migrates towards colder waters where the species spawns once a threshold temperature has been attained. Within the Mediterranean, reproduction-related migrations have been described to take place in the eastern basin, from the Aegean Sea to the Black Sea in spring where spawning takes place, returning in autumn (Gordina and Klimova, 1996).
To understand the complex effects of climate on a species, it is necessary an integrated life cycle approach that identifies the responses of the species at its different life stages. Considering the warming of the western Mediterranean waters and the close link between bluefish biology and temperature, the aim of this study was to analyse the likely changes in the distribution and spawning of the species driven by the increase in temperature over the last decades.
Material and methods
Study area
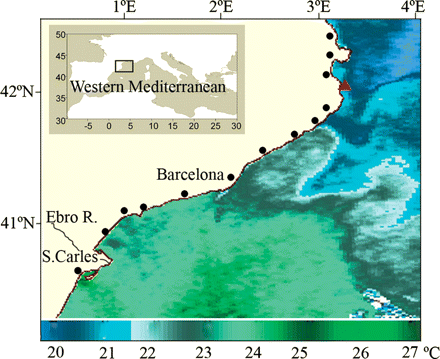
The Catalan coast, located in the NW Mediterranean Sea, is characterized by a quite narrow continental shelf, which only widens clearly in the southernmost area around the Ebro River Delta and in the north between the main submarine canyons. The general surface circulation in the NW Mediterranean region is well established with a main shelf-slope current, the Northern Current, along the entire northern continental slope (Millot, 1999). This current flows southwestwards in front of the Catalan coast at ∼30 cm s−1 at the surface (Salat, 1996). The northern sector of the Catalan coast, which is more directly influenced by strong northerly winds, is generally colder than the central and southern areas (Figure 1). This can be clearly observed in satellite thermographies in which a surface thermal front perpendicular to the coast roughly coincides with the limit of frequent northerly winds (Sabatés et al., 2007a; Figure 1). Continental water inputs play an important role in the region. The southern shelf of the Catalan coast receives a large input of continental water from the Ebro River, and thus the surface chlorophyll levels are higher in this area than in the north.
Study area. AVHRR/NOAA satellite image from 4 July 2004. The dots indicate the fishing ports located along the Catalan coast and the triangle L'Estartit meteorological station.
Sea surface temperature
Time-trend of sea surface temperature (SST, °C) was analysed at the monthly and annual scales. Data on monthly SST for the period 1974–2010 were obtained from L'Estartit Meteorological Station, located at the north of the study area (http://www.meteoestartit.cat; Figure 1). These data were used to assess the temperature changes in the study area, i.e. the identification of local trends and their duration. To this aim, the monthly SST time-series was detrended by replacing each monthly value by its deviance from the mean value of the corresponding month over the period 1974–2010, and the series of cumulative sum of residuals of the detrended SST monthly series was calculated. Successive negative residuals produce a decreasing slope, whereas successive positive residuals generate an increasing slope and values not very different from the mean show no slope. To detect regime shifts, the STARS method, based on sequential t-test analysis (Rodionov and Overland, 2005), was applied to the annual SST dataseries. The method consists in calculating a regime shift index, which represents a cumulative sum of normalized anomalies relative to a critical value, and provides a probability level for the identified year of regime shift. Taking into account the characteristics displayed by the monthly series of the cumulative sum of residuals, a cut-off length of 8 years was chosen. To explore the link between the years of shift identified through STARS and the time of the year assumed to determine the presence of bluefish close to the coast, the monthly time-series values of April, May, and June were plotted against the corresponding monthly means. In addition, to highlight the very close link between bluefish monthly landings and SST, monthly SST data (1988–2010) from the southern study area, where bluefish is particularly abundant, were taken from the Comprehensive Ocean-Atmosphere Data Set (COADS; see Slutz et al., 1985; Woodruff et al., 1998). These data comprise monthly means for 1° latitude and 1° longitude units; the time-series centred at 40.51°N 1.51°E was used.
Landings of P. saltatrix
Bluefish annual landings in the western Mediterranean (1974–2008) were obtained from the FAO Fisheries Statistical Database (http://www.fao.org/fishery/statistics/en). The STARS method was also applied to these data. Data on monthly and annual landings (1988–2010) along the Catalan coast and data on the number of vessels were taken from fishing statistics of the Spanish Ministry of Agriculture and Fisheries and the Autonomous Government of Catalonia. Bluefish is mainly fished by the small-scale and trawl fleets. These fleets operate close to the base port, which allows the identification of the areas where the species is present. The occasional records of bluefish landings by the purse-seine fleet were not considered because given the mobility of this fleet, the landing port may not correspond to the area where the bluefish were caught. To the landings analysis, the “northern area” was defined based on Sabatés and Martin (1993) and includes the ports located to the north of Barcelona (Figure 1). In the early eighties, the species did not reproduce in the “northern area” and landings were practically nil.
Larvae of P. saltatrix
Bluefish larvae were sampled on the continental shelf and slope along the Catalan coast (northwestern Mediterranean) during four oceanographic surveys covering the reproductive period of P. saltatrix in the western Mediterranean (Sabatés and Martin, 1993): 18–25 July and 11–20 September, 2003, and 23 June–1 July and 21–29 July, 2004. In each survey, 66 sampling stations were located on transects perpendicular to the shoreline, from near the coast to the slope. In each transect, stations were placed 7.5 nautical miles apart and the distance between transects was 10 nautical miles.
Vertical profiles of the basic hydrographic variables (temperature, salinity, and fluorescence) were obtained with a Neil Brown Mark III-CTD (WOCE standard) equipped with a Sea-Tech fluorometer. The vertical profiles were interpolated to 1-m depth intervals. At each station, water samples for chlorophyll a (Chl a) determinations were collected using a rosette system at three depths down to 70 m during both the day and the night to calibrate the fluorometer. The Chl a concentration (µg l−1) was determined fluorometrically (Yentsch and Menzel, 1963) on board. Samples from 100 to 200 ml were filtered through Whatman GF/F filters. Chl a was extracted from filters immersed in 6 ml of 90% acetone (24 h at 4°C in the darkness). The extract was analysed with a Turner Designs fluorometer calibrated with pure Chl a (Sigma Co.). The relationship between the Chl a concentration vs. fluorescence (flu) obtained in each survey was used to convert the continuous CTD fluorescence register into the Chl a concentration. The calibration was similar in surveys performed in the same year: Chl a = 1.69 × flu + 0.0001 (July 2003); Chl a = 1.62 × flu − 0.0222 (September 2003); Chl a = 2.14 × flu − 0.0341 (June 2004); and Chl a = 2.04 × flu − 0.0223 (July 2004).
Fish larvae were sampled by oblique tows, from a maximum depth of 200 m to the surface, using a Bongo net with a 60 cm diameter opening and a mesh size of 300 µm. The volume of filtered water was estimated by a flowmeter placed at the centre of the net mouth. Zooplankton samples were fixed in 5% formaldehyde buffered with sodium tetraborate.
In the laboratory, fish eggs and larvae were sorted and identified from the preserved samples. The number of P. saltatrix larvae collected at each station was standardized to the number of larvae per 10 m2. The standard length (SL) of bluefish larvae was measured to the nearest 0.1 mm. Larvae were grouped in 0.5 mm size classes and abundance per size class was standardized to the number of larvae per 10 m2.
Relationships between the abundance of P. saltatrix larvae and environmental conditions were explored using generalized additive modelling (GAM) to define the set of parameters that best describes the conditions associated with bluefish larval abundance. GAM is a form of non-parametric multiple regression that models a response (dependent) variable as a function of one or more predictor (independent) variables (Hastie and Tibshirani, 1990; Wood, 2000). GAMs in this study are given by: Yi = g(Xi)+ ɛi, where Yi is the value of the response variable (larval abundance) at station i, g(Xi) the predictor function, and ɛi the residual. The predictor function g(Xi) is given by: g(Xi)= α + s(Xi), where Xi is the explanatory variable (environmental variable), α the intercept, and s(Xi) the smoothing function. The GAMs were implemented in R (using Brodgar software package, Highland Statistics Ltd, http://www.brodgar.com). Based on the residual plots of preliminary runs, we specified a Poisson distribution function for the error structure of the dependent variable (larval abundance) with a log-link relating the dependent variable to the predictors (surface temperature, salinity, and Chl a). The predictor variables were modelled as cubic splines with a degree of smoothing estimated by the mgcv routine (Wood, 2000).
Results
Time-trend of temperature
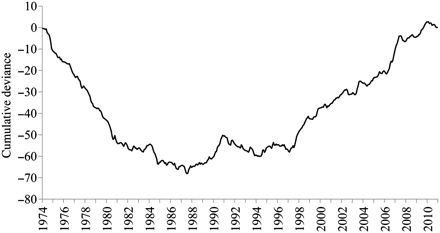
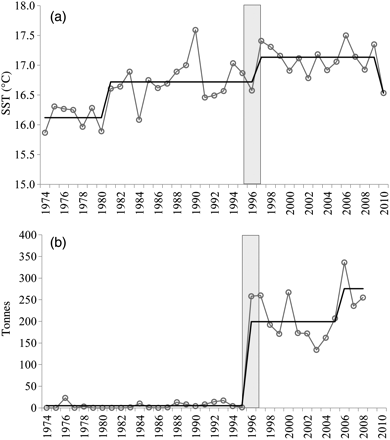
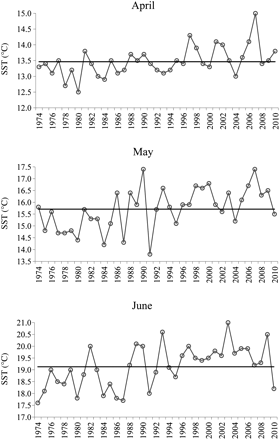
The series of cumulative sum of residuals of the detrended monthly SST time-series from L'Estartit (1974–2010) pointed out three main periods. From 1974 to 1981, the series was characterized by a decreasing trend, with monthly values lower than the corresponding monthly means, followed by a transition period from 1982 to 1996, and a period of increasing trend over 1997–2009. The curve minimum corresponded to 1987. It is worth noting that at the end of the series, 2010 displayed monthly values lower than the mean (Figure 2). STARS applied to the annual SST time-series identified 1981, 1997, and 2010 as years of shifts (p = 0.05; Figure 3). As for spring months (April, May, and June), and taking as reference 1997, when the increasing trend of SST started, it can be observed that most of the SST values were higher than the mean, in particular May and June. In 2010, SST in these 2 months fell below the mean (Figure 4).
Time-trend of the monthly surface temperature during 1974–2010: cumulative deviation of the detrended monthly surface temperature dataseries from L'Estartit meteorological station (Figure 1).
Shifts in the annual surface temperature (a) and in the P. saltatrix annual landings (b) in the western Mediterranean as detected by the STARS method (Rodionov and Overland, 2005). The identified years of shift are 1981, 1997, and 2010 for surface temperature and 1996 and 2006 for P. saltatrix landings (p = 0.05). Data sources: L'Estartit meteorological station and FAO statistics.
Time-trend of the surface temperature in spring, months of April, May, and June, over 1974–2010. The mean value of each series is also shown (black line). Data from the L'Estartit meteorological station.
Spatio-temporal patterns of P. saltatrix landings
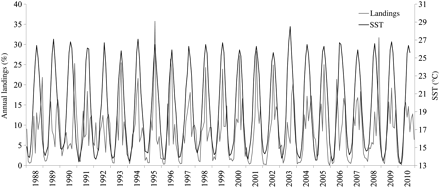
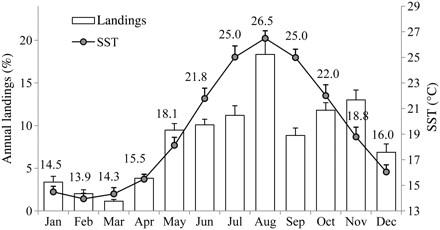
Bluefish annual landings in the western Mediterranean (1970–2008) underwent a significant shift in 1996, as detected by STARS, from <50 t to around 200 t (Figure 3). In the Catalan coast (1988–2010), landings were much higher in the southern part in the fishing grounds located over the shelf in front of the Ebro River Delta. Landings from the fishing port of Sant Carles de la Ràpita, which is located in this area, represented more than 55% of the annual landings on the entire Catalan coast. The seasonal pattern of the monthly landings in this port over the year was closely linked to that of the SST (Figure 5). Bluefish landings were almost nil during the colder months and started increasing when the SST also began to increase. The peak of both the monthly landings and the SST occurred in August, then the landings decreased as the SST decreased. The annual cycles for landings and SST are shown in more detail in Figure 6. Monthly values for both the landings and SST correspond to the means over the period 1988–2010. Landings started to increase in the period from April to May, when SST increased from 15.5 to 18.1°C, peaked in August with an SST of 26.5°C, and later decreased, with minima coinciding with the coldest months, at an SST around 14°C.
Relationship between monthly surface temperature (black) and P. saltatrix landings (grey) over 1988–2010. Data sources: monthly landings from the fishing port of Sant Carles de la Ràpita expressed as the percentage of the annual landings; monthly surface temperature from the COADS database, 1° latitude × 1° longitude square centre 40.51°N 1.51°E.
Relationship between surface temperature and P. saltatrix landings during the year. Values are the means over 1988–2010 (bars correspond to the standard deviation in temperature and standard error in landings). Data sources: monthly landings from the fishing port of Sant Carles de la Ràpita; monthly surface temperature from the COADS database, 1° latitude × 1° longitude square centre 40.51°N 1.51°E.
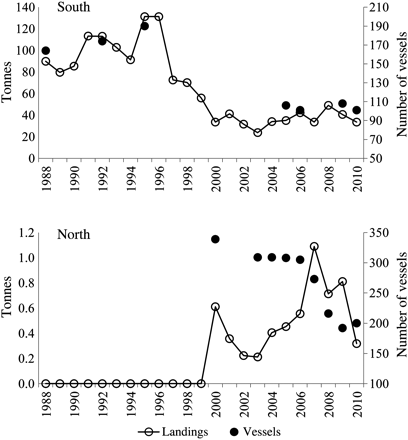
As stated above, bluefish were much more abundant in the southern part of the study area. Annual landings from Sant Carles de la Ràpita fluctuated between 90 and 130 t during 1988–1996 and were around 50 t during the last decade (Figure 7). The decrease in bluefish landings is not related to a decrease in the abundance of the species, but rather to the dramatic reduction in the fishing effort (the number of vessels shifted from 160–190 during 1988–1996 to around 100 in recent years; Figure 7). Nevertheless, it is remarkable that it was not until the year 2000 that bluefish landings, although very low, started to be recorded on the northern Catalan coast. The number of vessels has always been much higher in the northern part of the study area (Figure 7); thus, the landings registered from 2000 are indicative of the presence of bluefish on the northern Catalan coast. In 2010, landings decreased both in the southern and northern parts of the study area.
Pomatomus saltatrix annual landings during 1988–2010 and the number of vessels in the northern (fishing ports to the north of Barcelona) and the southern study area (fishing port of Sant Carles de la Ràpita; Figure 1).
Pomatomus saltatrix larvae
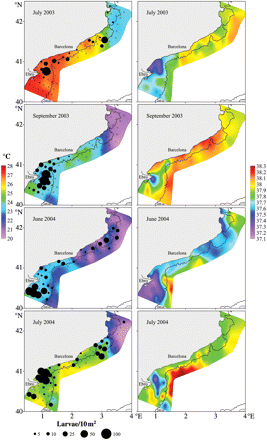
The horizontal temperature and salinity distributions at 5-m depth, during the four surveys, are shown in Figure 8. In all situations, the temperature was higher in the southern part of the study area. North of Barcelona, a marked thermal front, around 41°30′N, was evident across the shelf, although its orientation, gradient, and position varied slightly among surveys. The surface temperature was highest in July 2003, ranging between 23°C in the north and 28°C in the south, and lowest in June 2004, between 20 and 24°C. During this survey, the thermal front was evident somewhat further north, around 42°N (Figure 8). Surface salinity distributions showed relatively uniform values (around 37.9) in the entire area, except in the southern part, where low-salinity patches (<37.4) were detected in association with run-off from the Ebro River. The position and extension of these low-salinity patches varied among surveys (Figure 8). The surface Chl a distribution showed very low values in the entire study area in the four surveys. There were, however, some patches of relatively high surface Chl a near the Ebro River Delta (>0.5 µg l−1) whose position coincided with that of the low surface salinity patches.
Surface temperature and P. saltatrix larval distribution and abundance (left) and surface salinity (right) during the oceanographic surveys conducted in 2003 (July and September) and 2004 (June and July).
The abundances of P. saltatrix larvae were highest in July 2004. During all samplings, larvae were mostly collected over the continental shelf at <200-m depth (Figure 8). The highest concentrations were located in the southern part of the study area, on the Ebro River continental shelf, associated with the low-salinity and high Chl a surface waters, where the temperature was higher than in the north. Larval distribution to the north did not extend beyond the thermal front. In September 2003, the presence of larvae was limited to the southern part of the study area, coinciding with the end of the reproductive period (Figure 8).
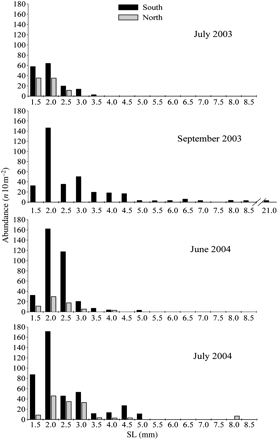
The size frequency distributions of bluefish larvae in each survey were fairly similar in the northern and southern parts of the study area (Figure 9). The size frequency distributions of larvae were indicative of the decreasing abundance from smaller to larger size classes that characterizes localized, stationary spawning. Most larvae were very small, ranging between 1.5 and 5.0 mm SL, being the 2-mm size class larvae the most abundant. In September, end of the spawning period, larval size classes showed a wider range, from 1.5 to 21 mm SL.
SL size frequency distributions of P. saltatrix larvae in the southern and the northern (north of Barcelona) study area during the oceanographic surveys conducted in 2003 (July and September) and 2004 (June and July).
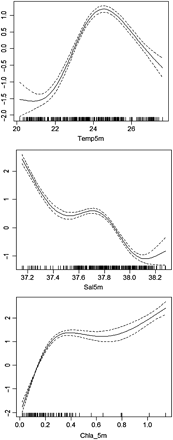
In the univariate GAMs proposed, the three explanatory variables chosen were highly significant (n = 263; p < 0.001). The deviance of the larval abundance explained by the surface temperature, salinity, and Chl a was similar (18.1, 21, and 20.8%, respectively). Larval abundance showed a non-linear relationship with surface temperature. The results allowed the identification of the most favourable SST range for bluefish larvae between 23 and 27°C. As for the other environmental variables, larval abundance was highest at lower salinities (<37.4) and higher Chl a concentrations (>0.5 µg l−1; Figure 10).
GAM smoothing curves fitted to effects of surface temperature, salinity, and Chl a on P. saltatrix larval abundance. The solid lines are the estimated smoother and the dashed lines represent 95% confidence intervals around the main effects. The black lines at the bottom of each plot indicate where the data values lie.
Discussion
The present study has demonstrated a strong relationship between the increasing surface temperature in the NW Mediterranean and basic traits of bluefish biology, such as northward expansion, and timing and location of spawning. Two shifts in temperature were detected: the first one in the early 1980s and the second around 1997. This last shift, with a pronounced overall increasing trend until 2010, led to the observed changes in bluefish. In particular, the spring temperatures, which determine the coastal migration and trigger the reproduction of the species, have undergone a marked increase since 1997. The temperature increase in the Mediterranean over the last two decades is well documented (e.g. Rixen et al., 2005; Vargas-Yáñez et al., 2010). Nevertheless, this increase is not constant throughout the year. Large seasonal variability has been observed in the western basin, in which spring display the highest warming rate (Sabatés et al., 2006; Nykjaer, 2009; Skliris et al., 2012).
In the western Mediterranean Basin, a significant increase in bluefish landings was observed by the mid nineties (1996), whereas the temperature shift was detected in 1997. This 1-year mismatch could be explained by the different spatial scales of both dataseries. Therefore, bluefish landings correspond to the whole western basin, including southern waters, where the species is more abundant and temperatures are higher than in the north, whereas the temperature data refer to one station at the northern Mediterranean. The increase in sea temperature in the western Mediterranean has been gradual, from south to north (Sabatés et al., 2006), and therefore, it could be expected that the overall increase in fish abundance took place earlier than temperature shift in the north. In the Catalan coast, northern distribution limit of the species in the western Mediterranean, bluefish has been always present in the warmer southern part in the Ebro River Delta. Nevertheless, it was not until 2000 that landings of this species were recorded in the northern colder part, which is related to the detected SST shift. This shift has been shown to be explained by warmer springs, from April to June, a crucial period when bluefish migrate closer to the coast for reproduction. The timing of the arrival was related to temperatures between 15 and 18°C, just before spawning. Therefore, as soon as conditions become favourable to the north of the species’ spatial distribution edge, the bluefish can extend its range northwards. Azzurro et al. (2011) also identified the late 1990s as the breakpoint for northward expansion of warm-water fish species in the Mediterranean. It could be argued whether the observed changes in the landings in the northern part of the study area are the response to fishing pressure. In fact, an important issue in assessing the impact of climate change on fish populations is the disentanglement of its effects from those of other drivers, such as fishing (Brander, 2010). We have to stress that, in the study area, P. saltatrix is not a fishing target but a bycatch species, and it is not under high fishing pressure. Hence, the observed changes would be a response to environmental changes that favour the presence of the species in northern areas.
Bluefish migrations linked to reproduction, referred to in the Introduction, have been described for different areas around the world. All coincide in that the species moves to colder waters for reproduction and then returns to warmer waters where it stays in the colder months of the year. No information is available for the western Mediterranean which allows conjecture about the migration pattern of bluefish in the study area. It is not known whether the increase in bluefish abundance in spring is a consequence of the arrival of individuals from southern areas or from offshore waters. The only available information on bluefish in coastal waters in the western Mediterranean regards the presence of the species in association with fish-farms sea cages in spring and summer (Valle et al., 2007; Sanchez-Jerez et al., 2008).
Bluefish has voracious behaviour and uses different habitats throughout its life cycle. Habitat selection, in addition to being temperature-dependent, has been shown to be at least partially explained by the spatio-temporal dynamics in prey composition. The adult diet is dominated by schooling species, such as squid, butterfish, and small pelagic fish (Juanes and Conover, 1994). In the Mediterranean, southwards of the study area, the diet of bluefish is dominated by small pelagic species, mainly round sardinella (Sardinella aurita; Sanchez-Jerez et al., 2008). Likewise for bluefish, an increasing abundance and expansion northwards has been reported for S. aurita in the NW Mediterranean in recent years (Sabatés et al., 2006). The highest bluefish abundance is located in the southern part of the study area, in front of the Ebro River Delta, where anchovy (Engraulis encrasicolus) and sardine (Sardina pilchardus) fishing grounds are located. The seasonal pattern of bluefish landings is the same as that of anchovy, with landings peaking in the summer and minimum in winter (Martín et al., 2008). The impact of bluefish on its prey populations in the Mediterranean is unknown, although it could be large.
The small size of larvae showed that bluefish reproduces all along the study area. Spawning was more protracted in the southern part, extending from June to September. The absence of larvae in the north by the end of the spawning period (September) is related to the colder temperature in this area since the temperature begins to decrease earlier than in the south (Sabatés et al., 2007a). Larvae were particularly abundant in front of the Ebro River Delta, where adults were also more abundant and the temperatures were higher than in the north. Spawning took place at temperatures ranging between 21 and 27°C, although the highest concentrations of larvae appeared between 23 and 27°C. In a previous study conducted in the same area in the early eighties, Sabatés and Martin (1993) reported the presence of larvae in the plankton from July to September, in a narrower temperature range (between 25 and 26°C), being larvae always absent in the northern area. Therefore, based on the results of the present study, we can conclude that currently the spawning area has extended to the north and that spawning starts earlier (end of spring, June). Furthermore, 21°C appears to be the threshold temperature for the presence of larvae in the plankton, and this threshold was not attained in June in the early eighties (Sabatés and Martin, 1993). Therefore, it can be concluded that the advancement in the onset of the spawning is related to the temperature increase detected in spring, before spawning. In the Mid-Atlantic Bight, Callihan et al. (2008) already indicated that the temperature influenced the timing of the spawning peak in bluefish, with earlier peak activity in warmer conditions. Phenology, or the timing of repeated seasonal activities such as migrations and reproduction, is highly sensitive to sea warming (Edwards and Richardson, 2004; Jansen and Gislason, 2011). Progressively earlier breeding and spawning have been observed in fish species due to the warmer spring temperatures (Edwards and Richardson, 2004; Genner et al., 2010).
The conditions that determined the occurrence of early life-history stages of bluefish observed in the present study are consistent with those indicated in other geographical areas. In the western North Atlantic, the timing and the duration of spawning along the latitudinal gradient where migration takes place have been associated with surface temperatures ranging from 18 to 26°C (Norcross et al., 1974; Kendall and Walford, 1979; Hare and Cowen, 1996). In the eastern Atlantic, on the coasts of Mauritania and Senegal, the spawning peak was observed at >24°C (Champagnat et al., 1983), whereas on the east coast of Australia, the highest larval densities were found between 19.5 and 22.4°C (Ward et al., 2003). In the Black Sea and the Sea of Marmara, bluefish spawns at water temperatures of 20–26°C (Gordina and Klimova, 1996; Ceyhan et al., 2007).
In the present study, we observed a clear association between P. saltatrix larvae and low-salinity and high Chl a concentrations in surface waters (Figures 8 and 10). These conditions were found near the coast in the southern part of the study area over the wide shelf in front of the Ebro River Delta. Bluefish larvae have been reported to tolerate a wide salinity range of between 17 and 38 (see the review in Juanes et al., 1996 and references therein). The high surface productivity on the Ebro shelf is characteristic of this area (Salat, 1996). It should be taken into account that in the Mediterranean summer period, when P. saltatrix reproduces, is characterized by a stratified water column with a marked thermocline, and as a result primary production remains limited to a deep chlorophyll maximum, below the thermocline. However, the discharges from the Ebro River during the stratified season lead to small areas of surface productivity and high concentrations of zooplankton, prey of fish larvae, have been reported associated with these waters (Sabatés et al., 2008). Taking into account that P. saltatrix larvae are located close to the surface, mainly in the upper 10 m and above the thermocline (Sabatés and Martin, 1993), the Ebro shelf would be a favourable habitat for the development and survival of these larvae. In the northern Gulf of Mexico, Ditty and Shaw (1995) reported that the main spawning areas of P. saltatrix were around frontal zones of the Mississippi River Delta.
The fact that bluefish eggs and larvae inhabit surface waters makes them vulnerable to being transported by surface advective mechanisms. In other geographic areas, alongshore transport of larvae from spawning grounds to nursery areas has been described in association with different hydrodynamic mechanisms (e.g. Beckley and Connell, 1996; Hare and Cowen, 1996; Juanes et al., 1996). In our study area, larval transport from the main spawning grounds (the Ebro Delta) to the north is unlikely since the dominant current, the Northern Current, flows in opposite direction, from the colder northern waters southwestwards along the continental slope (Millot, 1999). It is also unlikely that the larvae collected in the northern study area had been transported from further north areas by the Northern Current. The spatial distribution of larvae showed that they were virtually absent north of the thermal front (Figure 8) and this area is under the direct influence of the Northern Current (Sabatés et al., 2009). Therefore, if spawning of P. saltatrix occurred further north on the Catalan coast, part of the larvae would probably be transported, as already described for anchovy larvae in the same area (Sabatés et al., 2007b). Furthermore, most of the larvae collected in the northern area were very small, demonstrating that they were of local origin (Figure 9). The northwards expansion of the species and the presence of small larvae in the colder northern part of the Catalan coast suggest that the species reproduces at the northern edge of the distribution range in the western Mediterranean.
In summary, an expansion northwards of the bluefish distribution range has taken place in the western Mediterranean, with the species reproducing in the new distribution areas. These changes are related to the increase in temperature during spring months, as these temperatures are crucial for migration and reproduction events. The warmer spring months would also account for the earlier onset of spawning. The evidence presented in this study highlights how P. saltatrix is able to take the advantage of the changing environmental conditions and become established in new areas.
Acknowledgements
This work was supported by the EU Project VECTORS (FP7 OCEAN-2010, 266445) and by the Spanish project MAR-CTM2010-18874. We acknowledge Josep Pascual and the General Direction of Fishing and Maritime Affairs of the Catalan Government for providing sea temperature and bluefish landings data.
References
Author notes
Handling editor: Howard Browman