-
PDF
- Split View
-
Views
-
Cite
Cite
David J. McLeod, Alistair J. Hobday, Jeremy M. Lyle, Dirk C. Welsford, A prey-related shift in the abundance of small pelagic fish in eastern Tasmania?, ICES Journal of Marine Science, Volume 69, Issue 6, July 2012, Pages 953–960, https://doi.org/10.1093/icesjms/fss069
Close - Share Icon Share
Abstract
Shifts in the relative abundance of small pelagic fish species have signalled a change in the ocean environment in a number of locations. Here we show that the replacement of jack mackerel, Trachurus declivis, with redbait, Emmelichthys nitidus, as the dominant small pelagic species from eastern Tasmania, following a period of high fishing pressure on jack mackerel, is consistent with altered zooplankton communities and long-term climate change. Stomach contents analysis and morphology measurements were conducted on both species to determine if they were functionally equivalent with regard to zooplankton prey. Diet varied between species and with fish size. Krill (Nyctiphanes australis) was consumed by both species, with redbait feeding more heavily on small copepods. The diet overlap and morphometric characteristics indicated that these species are not equivalent with regard to prey and therefore changes in prey availability may have contributed to the observed shifts in relative abundance. The continued poleward extension of the East Australian Current is expected to favour small warm-water copepods; thus, redbait may have an advantage over jack mackerel due to prey preferences. An increase in relative abundance of redbait has decreased effort in surface fisheries and may impact on surface-feeding higher predators in this region.McLeod, D. J., Hobday, A. J., Lyle, J. M., and Welsford, D. C. 2012. A prey-related shift in abundance of small pelagic fish in eastern Tasmania? – ICES Journal of Marine Science, 69: 953–960.
Introduction
Small pelagic fish are critical to ecosystem structure in many coastal regions (e.g. Cole and McGlade, 1998; Cury et al., 2000). Shifts in the dominance of small pelagic fish dramatically affect energy transfer between trophic levels, with major implications for ecosystem functioning and regional fisheries (Swartzlose et al., 1999; Cury et al., 2000; Jacobson et al., 2001). Oscillating regimes of species dominance have been observed in many of the world's small pelagic fish populations (Alheit and Hagen, 1997; Swartzlose et al., 1999; Bakun, 2001). The most widely documented example is the sardine (Sardinops spp.)–anchovy (Engraulis spp.) regime shifts; out-of-phase fluctuations of these genera have been documented over many centuries in the productive coastal waters of the Pacific and Atlantic oceans (Baumgartner et al., 1992). Both species are size-selective planktivores, with sardine preferring smaller particles than anchovy (Louw et al., 1998). Alheit and Niquen (2004) report that when large copepods are abundant, anchovies dominate over sardines, such as exemplified during the anchovy regime of the 1960s in the Humboldt Current off South America. Changes in the size structure of zooplankton communities, linked with differential feeding preferences of sardines and anchovies, can be used to help explain large fluctuations in the relative abundance of these species (Alheit and Niquen, 2004).
A comparable small pelagic example on the east coast of Tasmania is the replacement of jack mackerel, Trachurus declivis, with redbait, Emmelichthys nitidus (Welsford and Lyle, 2003). This change is concurrent with a warming trend on the east coast of Australia and Tasmania (Ridgway, 2007; Lough and Hobday, 2011). This region, one of the fastest warming in the southern hemisphere, has provided evidence of climate-related distributional change for a range of marine taxa, including phytoplankton (Hallegraeff, 2010), zooplankton (Johnson et al., 2011), invertebrates (Ling et al., 2009), and coastal fish (Last et al., 2011). A change in the relative abundance of the small pelagic fish in this region is indicated by changes in both catch composition and gear types used in the Tasmanian small pelagic fishery. During the 1980s, jack mackerel was the main target of a surface purse-seine fishery that was, at that time, the largest volume fishery in Australia (Marshall et al., 1993). Such intense fishing appears to have altered the population structure of jack mackerel, with Browne (2005) showing a reduction in mean size-at-age since 1987–1988, and a decline in the number of age classes present in the fishery; both well-documented signs of intense fishing pressure (Perry et al., 2010). In 2001, the fishery shifted to trawling subsurface redbait schools, with redbait previously only a bycatch species in the purse-seine jack mackerel fishery (Welsford and Lyle, 2003). Kirkwood et al. (2008) provide circumstantial evidence that the observed change in fishery catch composition is reflective of changes in relative abundance. Their study found that the proportion of redbait in the diet of the Australian fur seal (Arctocephalus pusillus doriferus) increased dramatically from <10% prior 2001 to >30% between 2002 and 2005.
There are a range of possible factors contributing to the observed change in catch composition: (i) a change in fishing method from surface purse-seine to midwater trawl; (ii) an influence of previous fishing pressure on jack mackerel populations; (iii) direct impacts on relative abundance as a result of climate-related warming; and (iv) indirect impacts on relative abundance via changing prey (zooplankton) composition, itself driven by environmental change. While (iii) and (iv) are closely related, this paper will address the hypothesis that the change in catch composition was indirectly caused by a change in the zooplankton community, as evidenced in the diet of redbait and jack mackerel.
The Tasmanian small pelagic fishery operates in an oceanographically dynamic area, which affects both phytoplankton and zooplankton community structure (Harris et al., 1987; Young et al., 1996). The east coast of Tasmania is influenced by two major water bodies, the southward flowing East Australian Current (EAC) and southern sub-Antarctic water (SAW). In the past 60 years the EAC has strengthened, resulting in greater poleward movement of warm water and a rate of ocean warming some four times faster than the global ocean average (Ridgway, 2007). The annual strength of the EAC has implications for seasonal productivity in the region (Harris et al., 1987; Ridgway, 2007), and over longer time periods there is evidence that the increased strength of the EAC has led to a change in the structure of zooplankton communities (Cazzasus, 2004; Johnson et al., 2011). Data from samples collected 30 years apart show a relative increase in small warm-water copepods that have extended southward ranges consistent with increased penetration of the EAC (Johnson et al., 2011). In addition, anecdotal evidence suggests that during the past 10 years there has been a reduction in the occurrence of large surface swarms of cold-water krill that were common in the east coast region of Tasmania (Johnson et al., 2011). During the 1970s and 1980s, stomach contents analysis of jack mackerel collected in this area showed that krill were, almost exclusively, the dominant prey item (Young et al., 1993). It is likely that krill are still relatively abundant, but appear to have changed their vertical distribution and, therefore, their availability to surface schools of small pelagic fish (K. Swadling, unpublished data).
If prey availability is changing, then determining the equivalence of small pelagic fish in regards to prey consumption may aid interpretation of the observed change in the zooplankton predator abundance. This requires the study of small pelagic fish feeding ecology and diet preferences. Information regarding the diet of fish is typically obtained through stomach contents analysis (e.g. Hyslop, 1980; Cortes, 1997), still one of the most accurate ways to achieve species identification and relative abundance. Here, we examine the diet of the two dominant small pelagic fish, redbait (E. nitidus) and jack mackerel (T. declivis), from the east coast of Tasmania. We focus on the components of the diet that determine ecological functional equivalence of the two species. We also use morphological characteristics to assist in explaining any differences in diets. Whether these species are functionally equivalent with regard to prey will have implications for their response to zooplankton dynamics and assist in explaining the observed shift in dominance from jack mackerel to redbait in this region.
Methods
Fish sampling
At the time of this study, the fishery had switched from purse-seine to exclusively midwater trawl capture methods. Commercial midwater trawl catches from the east coast of Tasmania were sampled to determine the diet and morphology of small pelagic fish. Regular haphazard catch samples (up to 30 kg) of redbait and jack mackerel were collected between January 2003 and December 2004 from a region bounded by 39°31′S 148°43′E and 43°55′S 147°36′E (Figure 1). Due to the greater abundance and, in particular, the wider size range of redbait available in the fishery samples, it was not possible to match numbers in each of the size classes for the two species.
Map of the study area on the east coast of Tasmania, Australia. Samples were obtained from a commercial fishing vessel operating within the rectangular inset.
Stomach contents analysis
Where possible, one individual of each species from every 10 mm size class from each fishery sample was measured for fork length (mm) and total weight (g), and the stomach removed. Each stomach was opened and the contents examined under a stereo dissecting microscope. Stomach contents were weighed en masse (to 0.01 g wet weight) to allow calculation of a wet weight index of stomach fullness (g stomach contents per kg fish weight). Prey items were identified to the lowest possible taxon. To increase speed of processing, prey items were counted into categories based on a log scale (base 3). Categories were assigned based on the midpoint (Table 1) and data were effectively log transformed, thus reducing any bias created by inaccurate counting of large numbers.
The log 3 scale used to assign numbers to prey items in the stomachs of (Emmelichthys nitidus) and jack mackerel (Trachurus declivis).
| Log 3 scale . | Category . | Range of numbers . | Midpoint . |
|---|---|---|---|
| 1 | 1 | 1–3 | 2 |
| 3 | 2 | 4–9 | 6 |
| 9 | 3 | 10–27 | 18 |
| 27 | 4 | 28–81 | 54 |
| 81 | 5 | 82–243 | 162 |
| Log 3 scale . | Category . | Range of numbers . | Midpoint . |
|---|---|---|---|
| 1 | 1 | 1–3 | 2 |
| 3 | 2 | 4–9 | 6 |
| 9 | 3 | 10–27 | 18 |
| 27 | 4 | 28–81 | 54 |
| 81 | 5 | 82–243 | 162 |
The midpoint was used in the calculation of N (%).
The log 3 scale used to assign numbers to prey items in the stomachs of (Emmelichthys nitidus) and jack mackerel (Trachurus declivis).
| Log 3 scale . | Category . | Range of numbers . | Midpoint . |
|---|---|---|---|
| 1 | 1 | 1–3 | 2 |
| 3 | 2 | 4–9 | 6 |
| 9 | 3 | 10–27 | 18 |
| 27 | 4 | 28–81 | 54 |
| 81 | 5 | 82–243 | 162 |
| Log 3 scale . | Category . | Range of numbers . | Midpoint . |
|---|---|---|---|
| 1 | 1 | 1–3 | 2 |
| 3 | 2 | 4–9 | 6 |
| 9 | 3 | 10–27 | 18 |
| 27 | 4 | 28–81 | 54 |
| 81 | 5 | 82–243 | 162 |
The midpoint was used in the calculation of N (%).
Mouth morphology
Functionally equivalent fish would be expected to share similar morphological characteristics (Douglas and Matthews, 1992). In order to relate relevant features of redbait and jack mackerel to their diet, fish were selected from a range of size classes (FL, mm) and measured for a number of mouth morphology characteristics. Following Castro and Hernandez-Garcia (1995), mouth gape (maximum dimension of protruded jaw), jaw length, average length of gill rakers, average gill raker gap, and gill arch size were measured. Jaw length and mouth gape were taken to 0.1 mm using dial callipers. All gill morphology measurements were obtained by removing the first gill arch from the mouth chamber and viewing under a stereo dissecting microscope equipped with graduated eye piece. To assess functional equivalence and variations in morphology between species, canonical discriminant analysis (CDA; Gittins, 1985) coupled with leave-one-out, cross-validation using all characters was carried out within the R software package (R Development Core Team, 2006). Given linear relationships and high correlations between fish size and each of the mouth morphology characters (0.81 ≥ r > 0.94), character measurements were divided by fork length prior to CDA, with resultant measures unrelated to fork length.
Data analysis
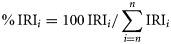
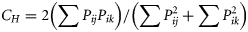
Multidimensional scaling (MDS) and cluster analysis were conducted on IRI data for five size classes (I, II, III, IV, and V) of both redbait (RBT) and jack mackerel (JMK; Table 2) to determine diet similarities using the Primer software package (version 5.2.9). Cluster and MDS analysis were based on Bray–Curtis similarity matrices between size classes using group average linkage and untransformed percentage IRI data. The Spearman rank correlation coefficient, rs (Zar, 1984), was also used on IRI data to determine diet overlap of size classes.
Labels used in the multivariate analyses to represent size classes of redbait (Emmelichthys nitidus) and jack mackerel (Trachurus declivis) collected from the east coast of Tasmania, 2003–2004.
| Size class (mm) . | Label . | E. nitidus (RBT) . | T. declivis (JMK) . |
|---|---|---|---|
| 100–149 | I | + | – |
| 150–199 | II | + | – |
| 200–249 | III | + | + |
| 250–299 | IV | + | + |
| 300–399 | V | – | + |
| Size class (mm) . | Label . | E. nitidus (RBT) . | T. declivis (JMK) . |
|---|---|---|---|
| 100–149 | I | + | – |
| 150–199 | II | + | – |
| 200–249 | III | + | + |
| 250–299 | IV | + | + |
| 300–399 | V | – | + |
Letters in parentheses are abbreviations of the species. + indicates that the size class present in that species; – indicates that the size class was absent from that species in the samples analysed.
Labels used in the multivariate analyses to represent size classes of redbait (Emmelichthys nitidus) and jack mackerel (Trachurus declivis) collected from the east coast of Tasmania, 2003–2004.
| Size class (mm) . | Label . | E. nitidus (RBT) . | T. declivis (JMK) . |
|---|---|---|---|
| 100–149 | I | + | – |
| 150–199 | II | + | – |
| 200–249 | III | + | + |
| 250–299 | IV | + | + |
| 300–399 | V | – | + |
| Size class (mm) . | Label . | E. nitidus (RBT) . | T. declivis (JMK) . |
|---|---|---|---|
| 100–149 | I | + | – |
| 150–199 | II | + | – |
| 200–249 | III | + | + |
| 250–299 | IV | + | + |
| 300–399 | V | – | + |
Letters in parentheses are abbreviations of the species. + indicates that the size class present in that species; – indicates that the size class was absent from that species in the samples analysed.
Results
Diet composition
The stomach contents of 341 redbait (107–298 mm FL; mean ± SD 188 ± 68 mm) and 164 jack mackerel (204–396 mm FL; mean ± SD 270 ± 68 mm were examined, with >90% of the stomachs containing food items (Table 3). In all, prey from 29 taxa, in seven broad taxonomic groups, were identified: Copepoda, Euphausiacea, Amphipoda, Decapoda, Gastropoda, Thaliacea, and ‘other’ (encompassing rarely seen taxa including foraminiferans, nematodes, and siphonophores; Table 3). The diets of both species were dominated by crustaceans, with IRI values of 98.1% and 69.9% for redbait and jack mackerel, respectively. Krill (Nyctiphanes australis) was the dominant crustacean consumed, with IRI values of 65.7% for redbait and 43.9% for jack mackerel. Of the other crustaceans, only copepods were of significance to the diets; IRI values of 33.2% for redbait and 14.7% for jack mackerel. Gastropods, the vast majority being pteropods, also featured significantly in the diet of jack mackerel (IRI of 26.1%) but were rare in the diet of redbait. In terms of overall composition differences, redbait stomachs contained more copepods than jack mackerel, while jack mackerel stomachs contained more gastropods.
Food items in the stomachs of redbait (Emmelichthys nitidus) and jack mackerel (Trachurus declivis) collected from the east coast of Tasmania 2003–2004.
| . | Emmelichthys nitidus (%nf = 91.5) . | Trachurus declivis (%nf = 97.6) . | ||||||
|---|---|---|---|---|---|---|---|---|
| Prey . | % FOO . | % N . | % W . | % IRI . | % FOO . | % N . | % W . | % IRI . |
| Crustacea | 43.6 | 85.7 | 87.5 | 98.1 | 36.7 | 37.7 | 59.4 | 69.9 |
| Euphausiacea | ||||||||
| Nyctiphanes australis | 24.6 | 32.4 | 77.4 | 65.7 | 34.5 | 14.4 | 56.4 | 43.9 |
| Copepoda | 24.6 | 45.7 | 9.8 | 33.2 | 12.7 | 22.2 | 1.5 | 14.7 |
| Candacia bipinnata | 9.0 | 3.9 | 0.6 | 3.9 | 1.9 | 0.0 | <0.1 | 0.1 |
| Euchirella spp. | 4.4 | 5.6 | 2.1 | 2.9 | 2.5 | 0.2 | <0.1 | 0.4 |
| Pleuromamma spp. | 8.4 | 15.0 | 0.7 | 13.8 | 10.1 | 6.2 | 0.3 | 9.3 |
| Caligus spp. | 1.6 | 0.1 | <0.1 | <0.1 | 1.9 | <0.1 | <0.1 | <0.1 |
| Calanoids | 1.9 | 11.4 | 5.4 | 2.9 | – | – | – | – |
| Unknown copepods | 6.5 | 13.1 | 1.1 | 9.4 | 1.3 | 2.3 | 1.5 | 4.8 |
| Decapoda | 1.2 | 0.1 | 0.3 | <0.1 | 2.5 | <0.1 | 0.5 | <0.1 |
| Jasus edwardsii (phyllasoma) | 0.3 | <0.1 | 0.2 | <0.1 | – | – | – | – |
| Decapod zoea | 0.9 | 0.1 | 0.1 | <0.1 | 2.5 | <0.1 | 0.5 | 1.4 |
| Amphipoda | 0.9 | 0.1 | 0.0 | <0.1 | 3.8 | 0.5 | 0.3 | 0.2 |
| Gammaridae | 0.3 | <0.1 | <0.1 | <0.1 | – | – | – | – |
| Hyperiidae | 0.6 | <0.1 | <0.1 | <0.1 | 3.8 | 0.5 | 0.3 | 3.2 |
| Cirripedia | 0.9 | <0.1 | <0.1 | <0.1 | 0.6 | <0.1 | <0.1 | <0.1 |
| Cladocera | 0.6 | <0.1 | <0.1 | <0.1 | – | – | – | – |
| Unknown Crustacea | 11.2 | <0.1 | 4.8 | 0.7 | 7.0 | <0.1 | 3.5 | 0.5 |
| Teleost | 3.7 | 0.3 | 2.9 | 0.2 | 9.5 | 1.1 | 14.6 | 2.9 |
| Myctophidae | ||||||||
| Lampanyctodes hectoris | 0.9 | <0.1 | 0.4 | <0.1 | 1.2 | 0.5 | 14.4 | 0.9 |
| Scorpaenidae | ||||||||
| Helicolenus percoides | 0.3 | <0.1 | <0.1 | <0.1 | – | – | – | – |
| Unidentified teleost | 2.5 | 0.1 | 1.4 | <0.1 | 6.7 | 0.4 | 0.3 | 0.2 |
| Gastropoda | 5.3 | 13.7 | 1.7 | 1.1 | 21.5 | 60.8 | 1.0 | 26.1 |
| Unidentified gastropoda | 2.8 | 0.2 | 0.1 | <0.1 | 8.2 | 0.8 | 0.1 | 0.3 |
| Pteropoda | 2.5 | 13.5 | 1.6 | 0.9 | 13.3 | 60.1 | 1.0 | 25.8 |
| Cephalopoda | – | – | – | – | 1.3 | 0.1 | 9.5 | 0.2 |
| Thaliacea | 3.1 | <0.1 | 0.9 | <0.1 | 2.5 | 0.1 | 6.4 | 0.3 |
| Other | 2.2 | 0.1 | 0.1 | <0.1 | 1.3 | <0.1 | <0.1 | – |
| Siphonophora | 1.2 | 0.1 | 0.1 | <0.1 | – | – | – | – |
| Foraminifera | 0.3 | <0.1 | <0.1 | – | – | – | – | – |
| Nematoda | 0.6 | <0.1 | <0.1 | – | 1.3 | <0.1 | <0.1 | – |
| . | Emmelichthys nitidus (%nf = 91.5) . | Trachurus declivis (%nf = 97.6) . | ||||||
|---|---|---|---|---|---|---|---|---|
| Prey . | % FOO . | % N . | % W . | % IRI . | % FOO . | % N . | % W . | % IRI . |
| Crustacea | 43.6 | 85.7 | 87.5 | 98.1 | 36.7 | 37.7 | 59.4 | 69.9 |
| Euphausiacea | ||||||||
| Nyctiphanes australis | 24.6 | 32.4 | 77.4 | 65.7 | 34.5 | 14.4 | 56.4 | 43.9 |
| Copepoda | 24.6 | 45.7 | 9.8 | 33.2 | 12.7 | 22.2 | 1.5 | 14.7 |
| Candacia bipinnata | 9.0 | 3.9 | 0.6 | 3.9 | 1.9 | 0.0 | <0.1 | 0.1 |
| Euchirella spp. | 4.4 | 5.6 | 2.1 | 2.9 | 2.5 | 0.2 | <0.1 | 0.4 |
| Pleuromamma spp. | 8.4 | 15.0 | 0.7 | 13.8 | 10.1 | 6.2 | 0.3 | 9.3 |
| Caligus spp. | 1.6 | 0.1 | <0.1 | <0.1 | 1.9 | <0.1 | <0.1 | <0.1 |
| Calanoids | 1.9 | 11.4 | 5.4 | 2.9 | – | – | – | – |
| Unknown copepods | 6.5 | 13.1 | 1.1 | 9.4 | 1.3 | 2.3 | 1.5 | 4.8 |
| Decapoda | 1.2 | 0.1 | 0.3 | <0.1 | 2.5 | <0.1 | 0.5 | <0.1 |
| Jasus edwardsii (phyllasoma) | 0.3 | <0.1 | 0.2 | <0.1 | – | – | – | – |
| Decapod zoea | 0.9 | 0.1 | 0.1 | <0.1 | 2.5 | <0.1 | 0.5 | 1.4 |
| Amphipoda | 0.9 | 0.1 | 0.0 | <0.1 | 3.8 | 0.5 | 0.3 | 0.2 |
| Gammaridae | 0.3 | <0.1 | <0.1 | <0.1 | – | – | – | – |
| Hyperiidae | 0.6 | <0.1 | <0.1 | <0.1 | 3.8 | 0.5 | 0.3 | 3.2 |
| Cirripedia | 0.9 | <0.1 | <0.1 | <0.1 | 0.6 | <0.1 | <0.1 | <0.1 |
| Cladocera | 0.6 | <0.1 | <0.1 | <0.1 | – | – | – | – |
| Unknown Crustacea | 11.2 | <0.1 | 4.8 | 0.7 | 7.0 | <0.1 | 3.5 | 0.5 |
| Teleost | 3.7 | 0.3 | 2.9 | 0.2 | 9.5 | 1.1 | 14.6 | 2.9 |
| Myctophidae | ||||||||
| Lampanyctodes hectoris | 0.9 | <0.1 | 0.4 | <0.1 | 1.2 | 0.5 | 14.4 | 0.9 |
| Scorpaenidae | ||||||||
| Helicolenus percoides | 0.3 | <0.1 | <0.1 | <0.1 | – | – | – | – |
| Unidentified teleost | 2.5 | 0.1 | 1.4 | <0.1 | 6.7 | 0.4 | 0.3 | 0.2 |
| Gastropoda | 5.3 | 13.7 | 1.7 | 1.1 | 21.5 | 60.8 | 1.0 | 26.1 |
| Unidentified gastropoda | 2.8 | 0.2 | 0.1 | <0.1 | 8.2 | 0.8 | 0.1 | 0.3 |
| Pteropoda | 2.5 | 13.5 | 1.6 | 0.9 | 13.3 | 60.1 | 1.0 | 25.8 |
| Cephalopoda | – | – | – | – | 1.3 | 0.1 | 9.5 | 0.2 |
| Thaliacea | 3.1 | <0.1 | 0.9 | <0.1 | 2.5 | 0.1 | 6.4 | 0.3 |
| Other | 2.2 | 0.1 | 0.1 | <0.1 | 1.3 | <0.1 | <0.1 | – |
| Siphonophora | 1.2 | 0.1 | 0.1 | <0.1 | – | – | – | – |
| Foraminifera | 0.3 | <0.1 | <0.1 | – | – | – | – | – |
| Nematoda | 0.6 | <0.1 | <0.1 | – | 1.3 | <0.1 | <0.1 | – |
%nf = percentage of fish stomachs containing prey; – = prey absent. See text for definitions of % frequency of occurrence (FOO), % number (N), % weight (W), and % index of relative importance (IRI).
Food items in the stomachs of redbait (Emmelichthys nitidus) and jack mackerel (Trachurus declivis) collected from the east coast of Tasmania 2003–2004.
| . | Emmelichthys nitidus (%nf = 91.5) . | Trachurus declivis (%nf = 97.6) . | ||||||
|---|---|---|---|---|---|---|---|---|
| Prey . | % FOO . | % N . | % W . | % IRI . | % FOO . | % N . | % W . | % IRI . |
| Crustacea | 43.6 | 85.7 | 87.5 | 98.1 | 36.7 | 37.7 | 59.4 | 69.9 |
| Euphausiacea | ||||||||
| Nyctiphanes australis | 24.6 | 32.4 | 77.4 | 65.7 | 34.5 | 14.4 | 56.4 | 43.9 |
| Copepoda | 24.6 | 45.7 | 9.8 | 33.2 | 12.7 | 22.2 | 1.5 | 14.7 |
| Candacia bipinnata | 9.0 | 3.9 | 0.6 | 3.9 | 1.9 | 0.0 | <0.1 | 0.1 |
| Euchirella spp. | 4.4 | 5.6 | 2.1 | 2.9 | 2.5 | 0.2 | <0.1 | 0.4 |
| Pleuromamma spp. | 8.4 | 15.0 | 0.7 | 13.8 | 10.1 | 6.2 | 0.3 | 9.3 |
| Caligus spp. | 1.6 | 0.1 | <0.1 | <0.1 | 1.9 | <0.1 | <0.1 | <0.1 |
| Calanoids | 1.9 | 11.4 | 5.4 | 2.9 | – | – | – | – |
| Unknown copepods | 6.5 | 13.1 | 1.1 | 9.4 | 1.3 | 2.3 | 1.5 | 4.8 |
| Decapoda | 1.2 | 0.1 | 0.3 | <0.1 | 2.5 | <0.1 | 0.5 | <0.1 |
| Jasus edwardsii (phyllasoma) | 0.3 | <0.1 | 0.2 | <0.1 | – | – | – | – |
| Decapod zoea | 0.9 | 0.1 | 0.1 | <0.1 | 2.5 | <0.1 | 0.5 | 1.4 |
| Amphipoda | 0.9 | 0.1 | 0.0 | <0.1 | 3.8 | 0.5 | 0.3 | 0.2 |
| Gammaridae | 0.3 | <0.1 | <0.1 | <0.1 | – | – | – | – |
| Hyperiidae | 0.6 | <0.1 | <0.1 | <0.1 | 3.8 | 0.5 | 0.3 | 3.2 |
| Cirripedia | 0.9 | <0.1 | <0.1 | <0.1 | 0.6 | <0.1 | <0.1 | <0.1 |
| Cladocera | 0.6 | <0.1 | <0.1 | <0.1 | – | – | – | – |
| Unknown Crustacea | 11.2 | <0.1 | 4.8 | 0.7 | 7.0 | <0.1 | 3.5 | 0.5 |
| Teleost | 3.7 | 0.3 | 2.9 | 0.2 | 9.5 | 1.1 | 14.6 | 2.9 |
| Myctophidae | ||||||||
| Lampanyctodes hectoris | 0.9 | <0.1 | 0.4 | <0.1 | 1.2 | 0.5 | 14.4 | 0.9 |
| Scorpaenidae | ||||||||
| Helicolenus percoides | 0.3 | <0.1 | <0.1 | <0.1 | – | – | – | – |
| Unidentified teleost | 2.5 | 0.1 | 1.4 | <0.1 | 6.7 | 0.4 | 0.3 | 0.2 |
| Gastropoda | 5.3 | 13.7 | 1.7 | 1.1 | 21.5 | 60.8 | 1.0 | 26.1 |
| Unidentified gastropoda | 2.8 | 0.2 | 0.1 | <0.1 | 8.2 | 0.8 | 0.1 | 0.3 |
| Pteropoda | 2.5 | 13.5 | 1.6 | 0.9 | 13.3 | 60.1 | 1.0 | 25.8 |
| Cephalopoda | – | – | – | – | 1.3 | 0.1 | 9.5 | 0.2 |
| Thaliacea | 3.1 | <0.1 | 0.9 | <0.1 | 2.5 | 0.1 | 6.4 | 0.3 |
| Other | 2.2 | 0.1 | 0.1 | <0.1 | 1.3 | <0.1 | <0.1 | – |
| Siphonophora | 1.2 | 0.1 | 0.1 | <0.1 | – | – | – | – |
| Foraminifera | 0.3 | <0.1 | <0.1 | – | – | – | – | – |
| Nematoda | 0.6 | <0.1 | <0.1 | – | 1.3 | <0.1 | <0.1 | – |
| . | Emmelichthys nitidus (%nf = 91.5) . | Trachurus declivis (%nf = 97.6) . | ||||||
|---|---|---|---|---|---|---|---|---|
| Prey . | % FOO . | % N . | % W . | % IRI . | % FOO . | % N . | % W . | % IRI . |
| Crustacea | 43.6 | 85.7 | 87.5 | 98.1 | 36.7 | 37.7 | 59.4 | 69.9 |
| Euphausiacea | ||||||||
| Nyctiphanes australis | 24.6 | 32.4 | 77.4 | 65.7 | 34.5 | 14.4 | 56.4 | 43.9 |
| Copepoda | 24.6 | 45.7 | 9.8 | 33.2 | 12.7 | 22.2 | 1.5 | 14.7 |
| Candacia bipinnata | 9.0 | 3.9 | 0.6 | 3.9 | 1.9 | 0.0 | <0.1 | 0.1 |
| Euchirella spp. | 4.4 | 5.6 | 2.1 | 2.9 | 2.5 | 0.2 | <0.1 | 0.4 |
| Pleuromamma spp. | 8.4 | 15.0 | 0.7 | 13.8 | 10.1 | 6.2 | 0.3 | 9.3 |
| Caligus spp. | 1.6 | 0.1 | <0.1 | <0.1 | 1.9 | <0.1 | <0.1 | <0.1 |
| Calanoids | 1.9 | 11.4 | 5.4 | 2.9 | – | – | – | – |
| Unknown copepods | 6.5 | 13.1 | 1.1 | 9.4 | 1.3 | 2.3 | 1.5 | 4.8 |
| Decapoda | 1.2 | 0.1 | 0.3 | <0.1 | 2.5 | <0.1 | 0.5 | <0.1 |
| Jasus edwardsii (phyllasoma) | 0.3 | <0.1 | 0.2 | <0.1 | – | – | – | – |
| Decapod zoea | 0.9 | 0.1 | 0.1 | <0.1 | 2.5 | <0.1 | 0.5 | 1.4 |
| Amphipoda | 0.9 | 0.1 | 0.0 | <0.1 | 3.8 | 0.5 | 0.3 | 0.2 |
| Gammaridae | 0.3 | <0.1 | <0.1 | <0.1 | – | – | – | – |
| Hyperiidae | 0.6 | <0.1 | <0.1 | <0.1 | 3.8 | 0.5 | 0.3 | 3.2 |
| Cirripedia | 0.9 | <0.1 | <0.1 | <0.1 | 0.6 | <0.1 | <0.1 | <0.1 |
| Cladocera | 0.6 | <0.1 | <0.1 | <0.1 | – | – | – | – |
| Unknown Crustacea | 11.2 | <0.1 | 4.8 | 0.7 | 7.0 | <0.1 | 3.5 | 0.5 |
| Teleost | 3.7 | 0.3 | 2.9 | 0.2 | 9.5 | 1.1 | 14.6 | 2.9 |
| Myctophidae | ||||||||
| Lampanyctodes hectoris | 0.9 | <0.1 | 0.4 | <0.1 | 1.2 | 0.5 | 14.4 | 0.9 |
| Scorpaenidae | ||||||||
| Helicolenus percoides | 0.3 | <0.1 | <0.1 | <0.1 | – | – | – | – |
| Unidentified teleost | 2.5 | 0.1 | 1.4 | <0.1 | 6.7 | 0.4 | 0.3 | 0.2 |
| Gastropoda | 5.3 | 13.7 | 1.7 | 1.1 | 21.5 | 60.8 | 1.0 | 26.1 |
| Unidentified gastropoda | 2.8 | 0.2 | 0.1 | <0.1 | 8.2 | 0.8 | 0.1 | 0.3 |
| Pteropoda | 2.5 | 13.5 | 1.6 | 0.9 | 13.3 | 60.1 | 1.0 | 25.8 |
| Cephalopoda | – | – | – | – | 1.3 | 0.1 | 9.5 | 0.2 |
| Thaliacea | 3.1 | <0.1 | 0.9 | <0.1 | 2.5 | 0.1 | 6.4 | 0.3 |
| Other | 2.2 | 0.1 | 0.1 | <0.1 | 1.3 | <0.1 | <0.1 | – |
| Siphonophora | 1.2 | 0.1 | 0.1 | <0.1 | – | – | – | – |
| Foraminifera | 0.3 | <0.1 | <0.1 | – | – | – | – | – |
| Nematoda | 0.6 | <0.1 | <0.1 | – | 1.3 | <0.1 | <0.1 | – |
%nf = percentage of fish stomachs containing prey; – = prey absent. See text for definitions of % frequency of occurrence (FOO), % number (N), % weight (W), and % index of relative importance (IRI).
Dietary overlap
The Simplified Morisita's index scores (CH) for pairwise overlap between redbait and jack mackerel stomach contents were moderate to high using all metrics. The highest overlap (92%) was found using the W metric, reflecting the similar proportions of krill in the stomachs of the two species. FOO (74%) and N (60%) overlaps were moderate, indicating some differentiation in the diet, whereas the IRI-based scores indicated even less overlap (50%).
Size-specific feeding
When stomach contents were analysed by fish size (size classes I–V), a trend towards larger prey items with increasing size of fish was apparent. The IRI for krill in small redbait (RBT I) was only 4.6%, while it dominated the stomach contents of larger size classes, ranging from 59% in RBT III to 73% in RBT IV. In jack mackerel, krill was absent in the smallest available size class (JMK III) but the IRI increased to 55% in the largest fish (JMK V). Copepods occurred in all size classes of both species; however, the general trend was for decreased occurrence with increasing fish size. Gastropods, primarily pteropods, were the dominant item in the smallest size class of jack mackerel, while this group was less important in size III and IV fish. In both redbait and jack mackerel stomachs, teleost items showed increased importance with size, as did cephalopods in jack mackerel.
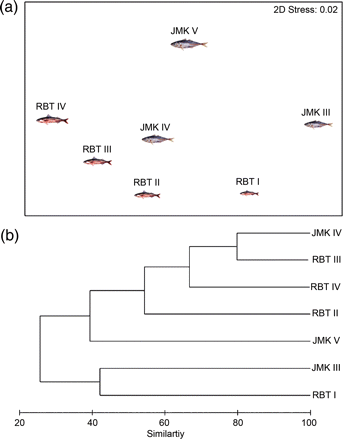
There was wide variation in the degree of dietary overlap between size classes for both species. The multivariate techniques (cluster analysis and MDS) indicated that the smallest redbait (RBT I) exhibited minimal overlap with the other size classes, whereas there were similarities between larger groups (RBT II, III, and IV; Figure 2). Jack mackerel displayed a similar trend, with intraspecific overlap appearing to be minimal except between IV and V size classes (rs = 0.67; Table 4). The cluster analysis and MDS revealed that the smallest size class of redbait (RBT I) appeared most similar to the smallest size class of jack mackerel (JMK III). Similarly, the stomach contents of the larger redbait (RBT III and IV) were highly correlated with the larger jack mackerel stomachs (JMK IV and V; Table 4). In general, the overlap was greater between the same size classes of redbait and jack mackerel, than between large and small individuals of the same species.
Diet similarity (based on % IRI) of size classes of redbait (Emmelichthys nitidus; RBT) and jack mackerel (Trachurus declivis; JMK) shown by (a) cluster analysis and (b) MDS based on untransformed data using a Bray–Curtis similarity matrix and group average linkage, collected from the east coast of Tasmania, 2003–2004.
Diet overlap between size classes of redbait (Emmelichthys nitidus; RBT) and jack mackerel (Trachurus declivis; JMK) collected from the east coast of Tasmania, 2003–2004 using the Spearman rank correlation rs.
| . | RBT I . | RBT II . | RBT III . | RBT IV . | JMK III . | JMK IV . |
|---|---|---|---|---|---|---|
| RBT II | 0.403 | |||||
| RBT III | –0.050 | 0.700 | ||||
| RBT IV | –0.233 | 0.444 | 0.951 | |||
| JMK III | 0.332 | –0.068 | –0.138 | –0.148 | ||
| JMK IV | 0.062 | 0.693 | 0.935 | 0.870 | 0.219 | |
| JMK V | –0.033 | 0.038 | 0.556 | 0.677 | 0.364 | 0.666 |
| . | RBT I . | RBT II . | RBT III . | RBT IV . | JMK III . | JMK IV . |
|---|---|---|---|---|---|---|
| RBT II | 0.403 | |||||
| RBT III | –0.050 | 0.700 | ||||
| RBT IV | –0.233 | 0.444 | 0.951 | |||
| JMK III | 0.332 | –0.068 | –0.138 | –0.148 | ||
| JMK IV | 0.062 | 0.693 | 0.935 | 0.870 | 0.219 | |
| JMK V | –0.033 | 0.038 | 0.556 | 0.677 | 0.364 | 0.666 |
High pairwise overlaps are indicated in bold.
Diet overlap between size classes of redbait (Emmelichthys nitidus; RBT) and jack mackerel (Trachurus declivis; JMK) collected from the east coast of Tasmania, 2003–2004 using the Spearman rank correlation rs.
| . | RBT I . | RBT II . | RBT III . | RBT IV . | JMK III . | JMK IV . |
|---|---|---|---|---|---|---|
| RBT II | 0.403 | |||||
| RBT III | –0.050 | 0.700 | ||||
| RBT IV | –0.233 | 0.444 | 0.951 | |||
| JMK III | 0.332 | –0.068 | –0.138 | –0.148 | ||
| JMK IV | 0.062 | 0.693 | 0.935 | 0.870 | 0.219 | |
| JMK V | –0.033 | 0.038 | 0.556 | 0.677 | 0.364 | 0.666 |
| . | RBT I . | RBT II . | RBT III . | RBT IV . | JMK III . | JMK IV . |
|---|---|---|---|---|---|---|
| RBT II | 0.403 | |||||
| RBT III | –0.050 | 0.700 | ||||
| RBT IV | –0.233 | 0.444 | 0.951 | |||
| JMK III | 0.332 | –0.068 | –0.138 | –0.148 | ||
| JMK IV | 0.062 | 0.693 | 0.935 | 0.870 | 0.219 | |
| JMK V | –0.033 | 0.038 | 0.556 | 0.677 | 0.364 | 0.666 |
High pairwise overlaps are indicated in bold.
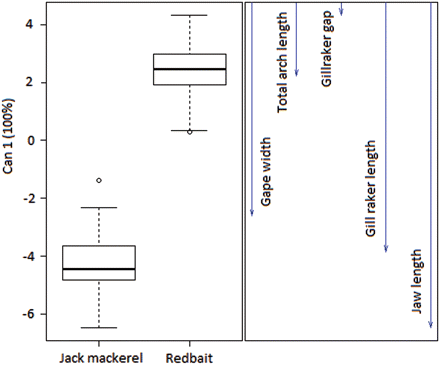
With regard to functional equivalence, evaluated using mouth morphology, the CDA completely separated redbait and jack mackerel along canonical variate 1 (CV1; Figure 3), and a multivariate analysis of variance (MANOVA) formalized these differences (MANOVA: F = 139.43, d.f. 5, 66; P < 000.1). Using leave-one-out, cross-validation, 100% of the individuals were classified to the correct species, indicative of a distinct separation of groups. Since there were only two a priori groups, there is only a single CV1 which explains 100% of the between-group variance, after rotation of the axes obtained from the eigen vectors of the within-group variance–covariance matrix. Therefore, the CV1 corresponds to the single linear discriminant function. All characters contributed to the division of species on CV1, but the gill raker length and jaw length vectors show the strongest influence (Figure 3).
Morphological variation in redbait (Emmelichthys nitidus) and jack mackerel (Trachurus declivis) collected from the east coast of Tasmania, 2003–2004, produced using canonical discriminant analysis (CDA) based on five mouth morphology measurements. Vectors on the right indicate the relative contribution of each character to the separation of the species. The CDA was conducted on data standardized for fish length.
Discussion
Shifts in dominance of small pelagic fish in a range of marine ecosystems have been linked to changes in physical ocean conditions. These physical changes are likely to cascade through to organisms at each trophic level; however, the way in which changes propagate through the food web is poorly recognized (Alheit, 2009). One possibility is that climate-driven changes to organisms low in the food chain then propagate to higher levels. If these higher species have different reliance on the range of prey species available in the system, changes in relative abundance may occur.
Functional equivalence with regard to prey of the small pelagic fish from the east coast of Tasmania was tested by analysing stomach contents and comparing overlap between species. The diet of redbait and jack mackerel consisted predominantly of pelagic crustaceans and other pelagic invertebrates. The moderate dietary overlap observed when using the IRI suggests that despite evidence for co-schooling (Welsford and Lyle, 2003), interspecific competition may be reduced by utilizing prey in differing proportions, particularly between size classes.
Morphological characteristics were used to determine if differences between species could provide further support for the diet-based finding that these species are functionally distinct. The combination of characteristics used in the CDA showed morphological separation, consistent with the diet differences. Overall, diet overlap and morphology suggest that these species are not playing identical ecological roles, and thus might respond to changes in prey size structure in different ways.
Ontogenetic diet shifts
Differences in prey consumption of species can also change over the size range of individuals, and can influence overall functional equivalence of a species (Xu et al., 2007). The diet composition of both redbait and jack mackerel showed changes as body size increased. As for many other fish species (e.g. Munoz and Ojeda, 1998; Persson and Bronmark, 2002), stomachs of smaller fish in this study generally contained smaller prey than larger fish. Maximum prey size is determined by mouth size (gape width) and retentive capabilities determined by gill raker morphology, with smaller prey retained by smaller gill raker gap (Alexander, 1967). Gape width and gill raker gap in both species increased linearly with size and are thus expected to influence the catchability of prey resources to fish in different size classes.
The ontogenetic diet changes observed in redbait and jack mackerel result in each size class consuming different prey items and in different proportions. Off the east coast of Tasmania, redbait and jack mackerel form mixed schools (Welsford and Lyle, 2003), with small redbait (size I and II) and small jack mackerel (size III) often co-occurring. Co-schooling fish typically feed on similar prey items (e.g. Louw et al., 1998). The general relationships observed here show that the intraspecific dietary overlap (between size classes of one species) is lower than the interspecific overlap (between species). Other studies also found dietary overlap between similar size classes of coastal fish species (Munoz and Ojeda, 1998; Kaeriyama et al., 2004). The ontogenetic shift observed in the diet of redbait and jack mackerel means that the dietary overlap is low between small and large representatives of both species, reducing competition for food resources between new recruits and older fish.
Given that small fish of each species also show an overlap in diet, an observed reduction in mean size-at-age for jack mackerel (Browne, 2005) is likely to exacerbate interspecific competition. Even though overall jack mackerel abundance may have declined, there are now relatively more small jack mackerel in the population competing for food with the more abundant redbait. This trophic competition between small pelagic fish (sensuBakun and Cury, 1999; Cury et al., 2000) may also be hampering the recovering of jack mackerel following intense fishing in the late 1980s.
Change in zooplankton abundance—who does it favour?
There is evidence for long-term warming on the east coast of Tasmania associated with the intrusion of EAC water onto the continental shelf off the east coast of Tasmania (Ridgway, 2007). With the continued extension of EAC water onto the shelf in the summer months, it would be expected to favour warmer water copepod communities and disadvantage krill populations (Cazassus, 2004; Johnson et al., 2011). A previous study by Young et al. (1993) on the diet of jack mackerel provides an opportunity to corroborate the observed change in major zooplankton communities. Young et al. (1993) found that the diet of jack mackerel sourced from the purse-seine fishery consisted almost exclusively of krill. In contrast, the IRI for krill in the present study was just 44%, with gastropods and copepods making up the other major food categories. These findings support the anecdotal evidence for a decrease in krill availability in the region (Johnson et al., 2011). Such a change in zooplankton community structure has been seen to initiate regime changes in small pelagic fish in other regions of the world due to differential feeding preferences (Swartzlose et al., 1999). It would be expected that the observed relative increase in small copepods would favour the small pelagic species that can utilize this prey resource more effectively. Results from this study indicate that redbait feeds more heavily upon copepods than does jack mackerel (33.2% and 14.7%, respectively). The relative increase in small copepods in eastern Tasmania (Cazzasus, 2004) would be expected to favour redbait due to feeding preferences. In addition, it appears that the intense fishing that occurred for jack mackerel coincided with a disappearance of surface swarms of krill and an increase in the relative abundance of redbait. Recall, krill made up almost 100% of the diet of jack mackerel in the late 1980s (Young et al., 1993) and only 44% in the study. Despite both redbait and jack mackerel consuming krill to some degree, the ability of redbait to feed on small copepods appears to have allowed it to exploit the niche space left by the removal of jack mackerel during the heavy fishing pressure of the 1980s. With the observed intensification of the EAC expected to continue and increase in intensity (Ridgway, 2007), it is likely that smaller warm-water copepod communities will also continue to expand their range poleward, as has been seen in other marine ecosystems such as the North Atlantic (Beaugrand et al., 2002; Beaugrand, 2004). The differential feeding preferences and the change in zooplankton communities off the east coast of Tasmania are thus predicted to favour redbait over jack mackerel in this region as the climate continues to warm.
The reduction in jack mackerel abundance inferred from the fishery data (Welsford and Lyle, 2003) may represent an example of the ‘school trap’ hypothesis proposed by Bakun and Cury (1999). Under this hypothesis, the innate requirement of small pelagic fish to join and remain within a school may force the numerically deficient species to live according to the needs of another more abundant species. Such a situation will reduce the productivity of the less abundant species (Bakun and Cury, 1999). Jack mackerel have been reported in mixed-species schools together with redbait on the east coast of Tasmania (Welsford and Lyle, 2003), although they have different diet composition. If this leads to reduced condition in jack mackerel, and perhaps to reduced reproductive performance, then recovery of the species in the future—if environmental conditions became favourable—would be impeded by the consequences of the ‘school trap’.
Effect on food webs and higher predators
In terms of ecosystem dynamics, if the two small pelagic fish species perform the same function within the ecosystem, then changes in their relative abundance may not have trophic impacts as long as the total abundance is maintained. If, however, these species are not functionally equivalent, then changes elsewhere in the foodweb are expected. This work has shown that redbait and jack mackerel are not functionally equivalent with regard to prey. The deeper feeding behaviour of the now dominant small pelagic species (redbait) probably reduces their availability to surface-feeding higher predators. Redbait and jack mackerel comprise a major portion of the diet for a range of large marine predators, including southern bluefin tuna (Young et al., 1997; 75% by weight, inshore waters) and the shy albatross (Hedd and Gales, 2001; 89% by weight). Impacts on these higher predators are often hard to measure and few data exist for the Tasmanian region at present. Elsewhere, a shift in the relative abundance of small pelagic fish has impacted the feeding success of higher predators due to prey availability (e.g. Chavez et al., 2003) and different energy values (e.g. Kirkham et al., 1985). The availability of small pelagic fish as prey for foraging seabirds and marine mammals is largely determined by their proximity to the ocean surface and, given the disappearance of surface schools of jack mackerel since 1989 (Young et al., 1993; Welsford and Lyle 2003), the changes off eastern Tasmania may also be impacting on higher predators.
Conclusion
Small pelagic fish from the shelf ecosystem off the east coast of Tasmania are not functionally equivalent with regard to diet and morphology. Redbait are able to feed on smaller prey, which are more available in warmer waters. However, ontogenetic diet shifts demonstrated for redbait and jack mackerel suggest that functionally equivalent groups may be size based rather than species based in this region. The shift to an increased relative abundance of redbait is likely to continue given the observed and projected ocean warming and likely increased presence of small warm-water copepods in this climate change hotspot.
Acknowledgements
We gratefully acknowledge Seafish Tasmania, in particular G. Geen and the crew of the FV ‘Ellidi’. We also thank David Ritz, Kerrie Swadling, and Camille White for their assistance with identification of copepods. Sally Russell, Roy Deng, Cathy Bulman, Kerrie Swadling, and the anonymous reviewers provided valuable assistance with the manuscript. We thank Steve Candy for his statistical advice.
References
Author notes
Handling editor: Bill Turrell