-
PDF
- Split View
-
Views
-
Cite
Cite
Jens Olsson, Lena Bergström, Anna Gårdmark, Abiotic drivers of coastal fish community change during four decades in the Baltic Sea, ICES Journal of Marine Science, Volume 69, Issue 6, July 2012, Pages 961–970, https://doi.org/10.1093/icesjms/fss072
Close - Share Icon Share
Abstract
Evidence for long-term change of marine ecosystems is increasing worldwide. Coastal areas harbour the socio-economically and ecologically most vital aquatic ecosystems, but are under increasing anthropogenic pressure. Little is known, however, about how environmental perturbations affect the development of coastal systems. In this paper, datasets of coastal fish communities covering almost four decades (early/mid 1970s to 2008) in three different basins of the Baltic Sea were analysed. There were clear changes in species composition over time in all but one dataset and coherence among basins in the timing of change. Changes were mainly associated with variables related to climate (water temperature, salinity, and North Atlantic Oscillation index), but less so with those reflecting nutrient status (nutrient concentrations and loading). Despite the importance of local water temperature, regional climatic variables were more important for the temporal development of communities. The results indicate that Baltic coastal fish communities have undergone large structural changes governed by processes acting on both local and regional scales. The findings suggest that ecological targets should be set accounting for long-term changes in community structure and that a common management of coastal and offshore ecosystems would be beneficial.Olsson, J., Bergström, L., and Gårdmark, A. 2012. Abiotic drivers of coastal fish community change during four decades in the Baltic Sea – ICES Journal of Marine Science, 69: 961–970.
Introduction
Aquatic ecosystems worldwide have gone through substantial structural changes during recent decades (Hare and Mantua, 2000; Beugrand, 2004; Weijerman et al., 2005; Möllman et al., 2009; Conversi et al., 2010). A reorganization of a single trophic level, such as a decreased abundance of predators, might result in a lack of top–down control on consumers and alter the dynamics of the entire foodweb (Carpenter and Kitchell, 1993; Myers et al., 2007), as shown for example in the Baltic Sea pelagic ecosystem (Casini et al., 2008). Changes in ecosystem structure might thus influence the function of the system and result in profound loss of economical and ecological values. Structural changes in marine ecosystems have been associated with climatic forcing (e.g. Beamish et al., 2004; Möllman et al., 2009; Conversi et al., 2010), introduction of alien species (Daskalov et al., 2007), as well as eutrophication and overexploitation of key species (Österblom et al., 2007; Casini et al., 2009; Möllman et al., 2009). An integrated knowledge of different causes of ecosystem changes and their scales of action is required for implementing an ecosystem-based management, although research in this area is still in its infancy (Andersen et al., 2009).
Coastal ecosystems (here defined as inshore areas in the proximity of land), which are among the most productive and economically vital aquatic systems worldwide, are under increasing anthropogenic pressure. In contrast to ecosystems in the open sea, where mainly exploitation and large-scale climate forcing are influential, potential impacts on coastal ecosystems are more numerous, including also eutrophication, pollution, habitat degradation and invasive species (Collie et al., 2008). Another obvious difference is that coastal communities to a larger extent are locally structured, determined by the availability of suitable habitat and intra- or interspecific community processes (Wootton, 1998). To date, few studies have addressed the relative impacts of drivers on structural change of coastal communities (but see Lekve et al., 2003; Collie et al., 2008). In particular, knowledge of the relative importance of variables acting on different geographical scales and the generality of findings across areas and systems is lacking.
The Baltic Sea is the largest body of brackish water in the world, and is constituted of several basins with well-differentiated hydrological conditions. There is a strong gradient in salinity, which ranges from 1 to 2 psu in the innermost parts to ∼25 psu at the entrance to the North Sea (HELCOM, 1996). This variation in salinity has a strong impact on the species composition and diversity. Communities in the western part of the Baltic Sea (Kattegat) are mainly composed of marine species and have higher species diversity, whereas communities in the innermost parts have lower species diversity and contain a mixture of freshwater and marine species. Thus, the Baltic Sea offers an opportunity for studying variation in, and the generality of, drivers of ecosystem change in areas along this gradient in environmental condition, species composition, and diversity.
Offshore ecosystems in the Baltic Sea have gone through profound changes during recent decades (Casini et al., 2008; Möllman et al., 2008; Diekmann and Möllmann, 2010) as a result of large-scale climatic forcing and overexploitation of top-predators (Österblom et al., 2007; Möllman et al., 2009). Coastal and offshore areas are often managed separately, but an issue of strong relevance for marine management is whether changes in coastal areas are driven by the same processes as in offshore areas, or not (Estes et al., 1998).
In this study, datasets of coastal fish communities from three different basins of the Baltic Sea (Kattegat, Baltic Proper, and Bothnian Sea) covering almost four decades were analysed to assess causes of temporal changes in coastal fish communities from the aspects of generality and scale. The following questions were addressed. (i) Are there significant changes in species composition of coastal fish communities over time? (ii) Are there similarities in the timing of change across basins? (iii) What environmental variables are mainly associated with the temporal changes observed? (iv) At what geographical scale are the most important variables related to change operating; local, regional, or globally over all areas assessed?
Material and methods
Study areas
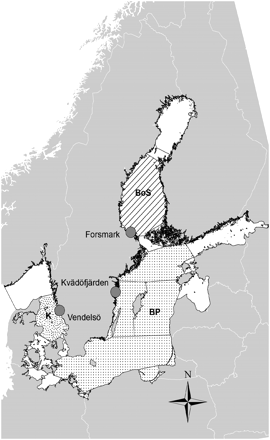
Species abundance data from monitoring areas in three different basins in the Baltic Sea: Kattegat (K), Baltic Proper (BP) and Bothnian Sea (BoS), were used (Figure 1). The monitoring area in Kattegat (Vendelsö) is located at a shallow, open coastline with a maximum depth of 10 m. Nearby areas are, however, as deep as 30 m. The average salinity is ∼17–20 psu, the bottom substrate is dominated by rock, with segments of sand, and the monitoring area covers ∼0.1 km2. In the Baltic Proper, the monitoring area Kvädöfjärden has an average salinity of 6–8 psu. The inner parts of the area are sheltered, with an average depth of ∼5 m, whereas the outer parts are more wave exposed and can be as deep as 25 m; the area covers ∼7.3 km2. The monitoring area in the Bothnian Sea (Forsmark) has an average salinity of 3–4 psu. About 40% of the total area is shallower than 3 m, although some parts are as deep as 30 m. The area covers ∼21.0 km2. Small islands and scerries are common, but the area is more wave exposed than Kvädöfjärden. In all areas, human population density is low, and the level of local anthropogenic impact stemming from land use is therefore low.
Location of areas for fish sampling in the Kattegat (K; Vendelsö), Baltic Proper (BP; Kvädöfjärden), and the Bothnian Sea (BoS; Forsmark).
Both the Forsmark and Vendelsö monitoring areas are used as reference areas for the surveillance programme of the nuclear power plants in Forsmark and Ringhals, respectively, monitoring the effects of cooling water discharges from these. Although located close to the power plants, both areas are not affected by the discharge of cooling water (Swedish Board of Fisheries, 2009, 2010). Kvädöfjärden serves as a reference area within the Swedish national environmental monitoring programme (HELCOM, 1996).
Fish data
Each area was represented by two fish community datasets, one representing the colder and one the warmer season, so that in total six time-series were used, starting between 1971 and 1976, and ending in 2002 or 2008 (Table 1). The rationale for using data from different seasons was that the species composition of coastal fish communities in the Baltic Sea generally differs throughout the year, as a result of species-specific seasonal differences in activity and migration behaviour (Thoresson, 1996; Neuman and Piriz, 2000). Species that have a temperature optimum >20°C are generally classified as ‘warm-water species’, whereas those having a temperature preference below 15°C are recognized as ‘cold-water species’ (Neuman, 1974). In the Baltic Proper and Bothnian Sea, species with a freshwater origin, e.g. perch (Perca fluvatilis) and roach (Rutilus rutilus), are generally more abundant in the warmer season and are with few exceptions recognized as ‘warm-water species’. Marine species such as Baltic herring (Clupea harengus membrans), eelpout (Zoarces viviparous), and cod (Gadus morhua), but also some freshwater species, such as whitefish (Coregonus maraena), fourhorned sculpin (Triglopsis quadrocornis), and smelt (Osmerus eperlanus), which have a lower temperature preference, are recognized as ‘cold-water species’, and are therefore more abundant in the cold season. In the Kattegat, almost all species are of a marine origin with a lower temperature preference, but during the warmer season, species preferring higher water temperatures, such as eel (Anguilla anguilla), gobids (Gobiidae), and wrasses (Labridae), dominate. In the colder season, species preferring lower water temperatures such as cod and eelpout (Zoarces vivparous) are more abundant. In all three basins, comparing the two types of time-series, species richness was generally higher in datasets representing the colder season (Mann–Whitney U-test, Z = –2.2, p = 0.028).
Environmental variables included in the analysis and output from the DISTLM models.
| Basin . | Season . | Dataset . | Years . | Local . | Regionald . | Global . | % Variation . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | Tla . | Trlb . | TotNc . | Te . | Sf . | DINg . | DIPg . | pHf . | NAO . | . |
| Kattegat | Cold | K Cold | 1976–2008 | 12.3 | * | 12.3 | |||||||
| Warm | K Warm | 1976–2008 | 13.9 | * | 13.0 | 26.9 | |||||||
| Baltic Proper | Cold | BP Cold | 1971–2008 | 6.9 | 23.9 | 9.7 | 40.5 | ||||||
| Warm | BP Warm | 1971–2008 | 13.7 | 21.3 | 35.0 | ||||||||
| Bothnian Sea | Cold | BoS Cold | 1975–2002 | 32.2 | 10.5 | 42.7 | |||||||
| Warm | BoS Warm | 1975–2008 | 11.0 | 29.6 | 40.6 | ||||||||
| Basin . | Season . | Dataset . | Years . | Local . | Regionald . | Global . | % Variation . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | Tla . | Trlb . | TotNc . | Te . | Sf . | DINg . | DIPg . | pHf . | NAO . | . |
| Kattegat | Cold | K Cold | 1976–2008 | 12.3 | * | 12.3 | |||||||
| Warm | K Warm | 1976–2008 | 13.9 | * | 13.0 | 26.9 | |||||||
| Baltic Proper | Cold | BP Cold | 1971–2008 | 6.9 | 23.9 | 9.7 | 40.5 | ||||||
| Warm | BP Warm | 1971–2008 | 13.7 | 21.3 | 35.0 | ||||||||
| Bothnian Sea | Cold | BoS Cold | 1975–2002 | 32.2 | 10.5 | 42.7 | |||||||
| Warm | BoS Warm | 1975–2008 | 11.0 | 29.6 | 40.6 | ||||||||
Tl, local temperature measured during fish sampling; Trl, local transparency measured during fish sampling; TotN, local yearly nutrient load.
Regional variables were T, offshore surface summer temperature; S, offshore surface salinity; DIN, winter concentrations of dissolved inorganic nitrogen; DIP, winter concentrations of dissolved inorganic phosphorus; pH, offshore surface pH. As a global variable we used the winter PC-based index of NAO. Values in each row show the percentage variation in fish community structure explained by each variable according to the DISTLM analyses. Underlined figures denote the main contributing variable of each dataset. Variables excluded from analysis due to collinearity (VIF-value >3) are indicated by *. Total variation explained is shown in the last column (% Variation).
aBottom temperature, average over all stations and days sampled, Swedish Board of Fisheries (SBF).
bMeasured as Secchi depth, average over all days sampled, SBF.
cBased on monthly averages of concentration and flow per county: for K Halland, for BP Östergötland, and for BoS Gävleborg, Swedish University of Agricultural Sciences.
dThe regional data for Kattegat were based on six stations (Fladen, Anholt E, L:a Middelgrund, S:t Middelgrund, Ålborg bugt, and Kattegat SW), for Baltic Proper 12 stations (BY10, BY15, BY20, BY29, BY31, BY32, BY38, BY1, BY2, BY4, BY5, and BCS III-10), and for Bothnian Sea six stations (SR5, MS4, C3, US3, US5B, and F26).
eAverage at 0–10 m depth in July–September for BP and June–September for BoS and K, Swedish Meteorological and Hydrological Institute (SMHI).
fAnnual average at 0–10 m depth, SMHI.
gAverage at 0–10 m depth in January–February for BoS and K, January–March for BP, SMHI.
Environmental variables included in the analysis and output from the DISTLM models.
| Basin . | Season . | Dataset . | Years . | Local . | Regionald . | Global . | % Variation . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | Tla . | Trlb . | TotNc . | Te . | Sf . | DINg . | DIPg . | pHf . | NAO . | . |
| Kattegat | Cold | K Cold | 1976–2008 | 12.3 | * | 12.3 | |||||||
| Warm | K Warm | 1976–2008 | 13.9 | * | 13.0 | 26.9 | |||||||
| Baltic Proper | Cold | BP Cold | 1971–2008 | 6.9 | 23.9 | 9.7 | 40.5 | ||||||
| Warm | BP Warm | 1971–2008 | 13.7 | 21.3 | 35.0 | ||||||||
| Bothnian Sea | Cold | BoS Cold | 1975–2002 | 32.2 | 10.5 | 42.7 | |||||||
| Warm | BoS Warm | 1975–2008 | 11.0 | 29.6 | 40.6 | ||||||||
| Basin . | Season . | Dataset . | Years . | Local . | Regionald . | Global . | % Variation . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | Tla . | Trlb . | TotNc . | Te . | Sf . | DINg . | DIPg . | pHf . | NAO . | . |
| Kattegat | Cold | K Cold | 1976–2008 | 12.3 | * | 12.3 | |||||||
| Warm | K Warm | 1976–2008 | 13.9 | * | 13.0 | 26.9 | |||||||
| Baltic Proper | Cold | BP Cold | 1971–2008 | 6.9 | 23.9 | 9.7 | 40.5 | ||||||
| Warm | BP Warm | 1971–2008 | 13.7 | 21.3 | 35.0 | ||||||||
| Bothnian Sea | Cold | BoS Cold | 1975–2002 | 32.2 | 10.5 | 42.7 | |||||||
| Warm | BoS Warm | 1975–2008 | 11.0 | 29.6 | 40.6 | ||||||||
Tl, local temperature measured during fish sampling; Trl, local transparency measured during fish sampling; TotN, local yearly nutrient load.
Regional variables were T, offshore surface summer temperature; S, offshore surface salinity; DIN, winter concentrations of dissolved inorganic nitrogen; DIP, winter concentrations of dissolved inorganic phosphorus; pH, offshore surface pH. As a global variable we used the winter PC-based index of NAO. Values in each row show the percentage variation in fish community structure explained by each variable according to the DISTLM analyses. Underlined figures denote the main contributing variable of each dataset. Variables excluded from analysis due to collinearity (VIF-value >3) are indicated by *. Total variation explained is shown in the last column (% Variation).
aBottom temperature, average over all stations and days sampled, Swedish Board of Fisheries (SBF).
bMeasured as Secchi depth, average over all days sampled, SBF.
cBased on monthly averages of concentration and flow per county: for K Halland, for BP Östergötland, and for BoS Gävleborg, Swedish University of Agricultural Sciences.
dThe regional data for Kattegat were based on six stations (Fladen, Anholt E, L:a Middelgrund, S:t Middelgrund, Ålborg bugt, and Kattegat SW), for Baltic Proper 12 stations (BY10, BY15, BY20, BY29, BY31, BY32, BY38, BY1, BY2, BY4, BY5, and BCS III-10), and for Bothnian Sea six stations (SR5, MS4, C3, US3, US5B, and F26).
eAverage at 0–10 m depth in July–September for BP and June–September for BoS and K, Swedish Meteorological and Hydrological Institute (SMHI).
fAnnual average at 0–10 m depth, SMHI.
gAverage at 0–10 m depth in January–February for BoS and K, January–March for BP, SMHI.
Datasets representing the warmer season were sampled in August in all areas (temperature range: 14–23°C). Datasets representing the colder season were sampled in April in the Kattegat and in October in the Baltic Proper and Bothnian Sea. No monitoring data were available for October in the Kattegat. Temperatures at fish sampling in the colder season were, however, similar in all basins (4–12°C). Sampling was performed using fykenets (K), net series (BP), or coastal survey nets (BoS). The fykenets (K) were set perpendicular to the shoreline at 2–5 m depth. During each season, six fixed stations were fished during nine and 12 consecutive nights per year, with two fykenets at a time (Thoresson, 1996; HELCOM, 2008). The fykenets representatively catch fish down to ∼9 cm length. For the net series (BP), 18 gillnets of seven different mesh sizes (21–60 mm) were set one at a time at one of four fixed stations, at the bottom at a 15–20 m depth (Thoresson, 1992). The coastal survey nets (BoS) were composed of two linked multimesh gillnets (with five different mesh sizes, 17–33 mm) at each station and were set at the bottom at 2–5 m depth in the warmer season, and at 15–20 m in the colder season. In each season, three fixed stations were fished repeatedly during three nights each year (Ådjers et al., 2006; HELCOM, 2008). To achieve a general estimate of the studied communities, an average per species, season, and year across all stations for each monitoring area was used as the input data for further analysis. As such, each dataset comprised a matrix of one observation per year and species. Both types of gillnet catch fish down to ∼14 cm length representatively. For all methods, data were sampled in the same way throughout the whole period assessed, by the same institute (Institute of Coastal Research).
In total, the datasets included 45 species (30 in K, 22 in BP, and 21 in BoS; Supplementary material, Table S1). These figures probably underestimate the true number of species in each area, since the gears do not sample all species present. The majority of the species were, however, sampled, and, in the text to follow, the term ‘community’ will therefore refer to the sampled part of the fish community. Since different sampling methods were used in the different basins, catch levels and community compositions could not be directly compared. The analyses were therefore restricted to assess relative changes in species compositions over time.
Environmental data
Data on environmental variables potentially influencing the temporal development of coastal fish community structure were defined as representing three geographical scales: local, regional, and global. Local variables representing temperature (Tl) and water transparency (Trl) were collected at the exact stations of fish sampling, whereas data on local nutrient loading (TotN) were based on calculated discharges of nitrogen from land within the county of each fish-monitoring area (see Table 1 for details ). Ideally, we would have data for nutrient loading at the very same scale as for Tl and TrL, but appropriate data were only available for K and BP, which showed good coherence with the data at the county level used here (r2 = 0.7–0.8). The regional scale was represented by data from the national open-sea monitoring programme in Sweden using monthly averages from all available monitoring stations in each of the basins (see Table 1 for further details). Variables related to climate on the regional scale were open-sea summer surface temperature (T), as well as surface salinity (S), and pH, which were included as averages over the year. Salinity levels in the Baltic are tightly linked to climate in that the inflow of saline water in the area is influenced by the direction and strength of prevailing winds. The link between climate and pH is that increased levels of atmospheric carbon dioxide (CO2) have resulted in increased uptake of CO2 by the oceans and thus a decrease in pH levels (Feely et al., 2004). Variables related to nutrient status were open-sea concentrations of dissolved inorganic nitrogen (DIN) and phosphorus (DIP), represented by winter values. The same regional data were used for both fish datasets within a basin. There was no spatial overlap between the fish-monitoring areas and the areas where the regional variables were sampled. The regional variables were, however, anticipated potentially to affect coastal fish communities either directly (via fish migration) or indirectly (affecting prey availability or local abiotic conditions). At the global scale, defined as influencing all basins, large-scale climate forcing was represented by the winter PC-based North Atlantic Oscillation (NAO) index (Hurell, 1995). This variable was anticipated potentially to influence coastal communities indirectly, through influence on the local abiotic environment (Lekve et al., 2003).
NAO is a climatic phenomenon reflecting differences in atmospheric pressure at the sea surface between the Arctic and the subtropical Atlantic (Hurell and Deser, 2009). It often has an impact on water surface and air temperature, precipitation, salinity, and wind directions over its area of influence (the North Atlantic Ocean and adjacent continents). The NAO index is commonly used as integrated measure of large-scale climatic events, potentially affecting both the growth and reproduction of aquatic organisms (Lekve et al., 2003), as well as the structure and functioning of marine ecosystems (Hurell and Deser, 2009). In the Baltic Sea, positive values of the NAO index generally coincide with elevated sea-surface temperatures and westerly winds, whereas negative values conform to the opposite (Kalnay et al., 1996). A correlation between NAO and other environmental variables included in the current study was seen for some datasets, but the relationship was relatively weak and showed inconsistent direction among datasets, suggesting that the variables to some extent reflected different aspects of environmental variation, with potentially different impacts on the communities assessed. The strongest correlation with NAO was seen for local temperature, regional nutrient levels (DIN and DIP), and pH (Supplementary material, Table S2). The strength and direction of these relationships did vary to some extent, but was generally positive with respect to local temperature and regional nutrient levels, and negative for regional pH.
Analyses
To identify significant changes in species composition over time, chronological clustering analysis (Legendre and Legendre, 1998), as implemented in Brodgar 2.5.7 (www.brodgar.com), was used. The analyses were based on the Bray–Curtis similarity index, which gives balanced weight to rare and abundant species (Zuur et al., 2007), and by which joint absences do not contribute to similarity between samples. The analyses were applied using a level of connectedness of 0.5; varying this level did not, however, affect the general results. A temporal change was interpreted as statistically significant from one year to the next at α = 0.01, to include only the strongest patterns in each dataset (Zuur et al., 2007). Given the differences in species composition and diversity among basins and seasons, the timing of change was considered similar across datasets if changes occurred within the same 2–3 years. In order to down-weight the influence of observations with high values, all analyses were performed on ln(x + 1)-transformed data as suggested by Clarke and Warwick (2001). Species with a frequency of occurrence >5% were excluded from further analyses.
The temporal development of the fish communities was further assessed by metric multidimensional scaling, using principal coordinate analysis (PCO; Zuur et al., 2007), as implemented in PERMANOVA+ of PRIMER v6 (Anderson et al., 2008). For consistency between analyses, the Bray–Curtis similarity index was used. Species with a multiple metric correlation >0.2 with any of the first two PCO axes were considered as contributing significantly to the temporal development of each dataset (Anderson et al., 2008). The temporal development of these species was also presented as anomaly graphs.
To test the relationship between community composition and environmental variables, distance-based linear modelling (DISTLM) was used. This is a multivariate multiple regression method where the ordination axes from a resemblance matrix of the response dataset is regressed against a matrix of explanatory variables (software PERMANOVA+ of PRIMER v6; Anderson et al., 2008). Prior to analysis, the datasets on environmental variables were checked for skewness using draftman plots (pairwise plots of all environmental variables; Clarke and Gorley, 2006) and for collinearity by analysis of variation inflation factors (VIFs), setting the limit for inclusion in analyses at VIF <3 (Table 1; Zuur et al., 2010). For each species dataset, the set of environmental variables was regressed on a similarity matrix based on the Bray–Curtis similarity index, using years as samples. Final models were selected using the BEST selection procedure in PRIMER v6 (Anderson et al., 2008), based on two selection criteria, the corrected Akaike information criterion (AICc; Burnham and Anderson, 2002) and Bayes information criterion (BIC; Schwarz, 1978). As models with AICc criteria within two units of the best model indicate some redundancy among models (Burnham and Anderson, 2002), we performed the model selection procedure in four separate steps. First, by minimizing the mean value of the two criteria (AICc and BIC) for all possible models following Anderson et al. (2008). The model with the smallest mean value for the two criteria was considered as superior. Second, we maximized the log-likelihood value for the AICc criterion for the models appearing within two units of the best model (Burnham and Anderson, 2002). This was done to assess whether redundancy among models was attributable to the inclusion of the penalty term for including additional variables (Burnham and Anderson, 2002). The model having a substantially higher log-likelihood value compared with the other models (commonly more than two units higher) was considered as superior. Third, we assessed the individual weights (i.e. the number of models in which a given variable occurs) of the variables included in all models appearing within two units of the best model according to the AICc selection criterion (Burnham and Anderson, 2002). The variables having the highest individual weights were considered as superior in explaining the observed changes in fish community response data. Fourth, by assessing the significance at α = 0.05 of variables in marginal F-tests as offered by the DISTLM routine. Variables exhibiting a significant relationship with the response dataset were considered superior. In all, the finally selected models were those fulfilling all of the four above-listed criteria. The partitioning of variation between the environmental variables included in the final model was then assessed using the sequential selection procedure (Anderson et al., 2008), and their temporal development was illustrated by anomaly graphs.
Results
Temporal changes of fish communities
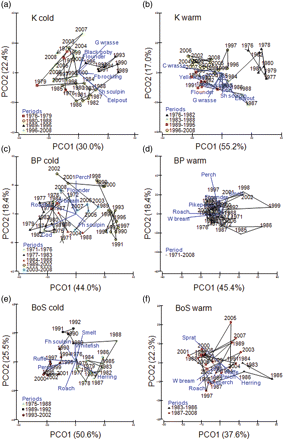
The first two ordination axes of the PCO analyses explained between 59.9% and 81.4% of the total variation in the different datasets (Figure 2). For the datasets representing the cold season, assessed communities have gone through substantial change. In the Kattegat colder season, a first period in the 1980s was characterized by species preferring lower water temperatures (shorthorn sculpin, Myoxocephalus scorpius, and eelpout, Figure 2a), followed by a period with high abundance of four-beard rockling (Enchelyopus cimbrius) and goldsinny wrasse (Ctenolabrus rupestris), both preferring warmer waters. From the mid 1990s, the abundance of black goby and flounder was high, whereas the abundances of species preferring low water temperatures were low (Figure 2a). In the Baltic Proper and the Bothnian Sea, fish communities in the colder season generally exhibited a decrease in marine species, and an increase in freshwater species over time (Figure 2c and e; Supplementary material, Figure S1). In both basins, the abundance of cod and herring was high until the late 1980s, after which it decreased strongly (correlation of herring with ordination axes in BP Cold; first = –0.18, second = –0.17, and cod in BoS Cold; first = 0.17, second = –0.17). The following years until the mid 1990s represented a transitional period in the Bothnian Sea and Baltic Proper, with relatively high abundances of freshwater species preferring lower water temperatures, such as fourhorned sculpin, smelt (BoS), and whitefish (BoS; Figure 2c and e). From the mid 1990s, freshwater species preferring warmer waters, such as perch and roach (both basins), white bream (Abramis bjoerkna; BP), and ruffe (Gymnocephalus cernus; BoS), increased. However, the latest years in the Baltic Proper dataset inclined towards similarity to the fish community in the late 1970s, as explained by an increased abundance of flounder (Platichthys flesus) and roach (Supplementary material, Figure S1).
PCO ordinations based on the Bray–Curtis similarity index for each fish community dataset. Projected vectors show changes in the abundance of species with a correlation >0.2 with any of the two first ordination axes. Periods with similar species composition according to the chronological clustering analyses (Table 2) are indicated by the same symbols. The line indicates the temporal trajectory. K, Kattegat; BP, Baltic Proper; BoS, Bothnian Sea. G wrasse, goldsinny wrasse; Fb rockling, four-beard rockling; Sh sculpin, shorthorn sculpin; C wrasse, corkwing wrasse; L forkbeard, lesser forkbeard; W bream, white bream; Fh sculpin, fourhorned sculpin.
Changes in community composition during the warmer season showed a general coherence with the cold seasons in the Kattegat. Species favoured by lower water temperatures (such as cod, eelpout, and shorthorn sculpin) were abundant during the early 1980s, after which they decreased (Figure 2b). Between the late 1980s and early 1990s, the fish community of the warmer season in Kattegatt was characterized by high abundances of goldsinny wrasse, flounder, and yellow eel, all preferring higher water temperatures. During the most recent years, the fish community was dominated by another species favoured by warmer waters, the corkwing wrasse (Symphodus melops). In both the Baltic Proper and Bothnian Sea warmer season datasets, increases in freshwater species preferring warmer water temperatures were seen, such as perch and pikeperch (both basins) and roach and white bream (BoS; Figure 2d and f; Supplementary material, Figure S1). There was also a recent increase in smelt in the BP (Supplementary material, Figure S1).
Timing of coastal fish community change
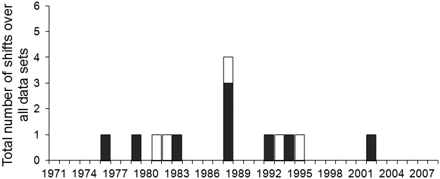
Significant changes in species composition over time were observed in all but one of the six datasets assessed and were distributed between 1976 and 2002 (Table 2, Figure 3). In general, changes occurred between two and four times in each dataset, with the highest intensity in the BP Cold and in both Kattegat datasets (Table 2). Parts of the changes occurred at the individual dataset level, probably as a result of differences in species composition, diversity, and to some extent also time-series length across datasets. Two of the changes observed were unique for the BP Cold dataset (1976/1977 and 2002/2003; Table 2). Of these, the earlier was mainly related to an increase in cod, and the later change by altered abundances of several species, such as white bream, four-horned sculpin, flounder, and roach (Supplementary material, Figure S1).
Number of changes in coastal fish community structure identified per year in all six datasets, according to chronological clustering analyses at α = 0.01. Bar colour denotes the season (white, warmer season; black, colder season).
Timing of significant changes in community structure for the studied datasets, according to chronological clustering analyses at α = 0.01.
| Basin . | Data Set . | Years . | Changes . | Intensity (no. of changes/year) . | |||
|---|---|---|---|---|---|---|---|
| . | . | . | 1970s . | 1980s . | 1990s . | 2000s . | . |
| Kattegat | K Cold | 1976–2008 | 1979/1980 | 1988/1989 | 1994/1995 | 0.09 | |
| K Warm | 1976–2008 | 1982/1983; 1988/1989 | 1995/1996 | 0.09 | |||
| Baltic Proper | BP Cold | 1971–2008 | 1976/1977 | 1983/1984; 1988/1989 | 2002/2003 | 0.11 | |
| BP Warm | 1971–2008 | 0 | |||||
| Bothnian Sea | BoS Cold | 1975–2002 | 1988/1989 | 1992/1993 | X | 0.08 | |
| BoS Warm | 1975–2008 | 1981/1982 | 1993/1994 | 0.06 | |||
| Basin . | Data Set . | Years . | Changes . | Intensity (no. of changes/year) . | |||
|---|---|---|---|---|---|---|---|
| . | . | . | 1970s . | 1980s . | 1990s . | 2000s . | . |
| Kattegat | K Cold | 1976–2008 | 1979/1980 | 1988/1989 | 1994/1995 | 0.09 | |
| K Warm | 1976–2008 | 1982/1983; 1988/1989 | 1995/1996 | 0.09 | |||
| Baltic Proper | BP Cold | 1971–2008 | 1976/1977 | 1983/1984; 1988/1989 | 2002/2003 | 0.11 | |
| BP Warm | 1971–2008 | 0 | |||||
| Bothnian Sea | BoS Cold | 1975–2002 | 1988/1989 | 1992/1993 | X | 0.08 | |
| BoS Warm | 1975–2008 | 1981/1982 | 1993/1994 | 0.06 | |||
Results are presented by decade between 1970 and 2000, X denotes that the dataset did not cover the decade. Intensity shows the number of changes observed per total number of years studied in each dataset. Abbreviations of dataset names are as in Table 1.
Timing of significant changes in community structure for the studied datasets, according to chronological clustering analyses at α = 0.01.
| Basin . | Data Set . | Years . | Changes . | Intensity (no. of changes/year) . | |||
|---|---|---|---|---|---|---|---|
| . | . | . | 1970s . | 1980s . | 1990s . | 2000s . | . |
| Kattegat | K Cold | 1976–2008 | 1979/1980 | 1988/1989 | 1994/1995 | 0.09 | |
| K Warm | 1976–2008 | 1982/1983; 1988/1989 | 1995/1996 | 0.09 | |||
| Baltic Proper | BP Cold | 1971–2008 | 1976/1977 | 1983/1984; 1988/1989 | 2002/2003 | 0.11 | |
| BP Warm | 1971–2008 | 0 | |||||
| Bothnian Sea | BoS Cold | 1975–2002 | 1988/1989 | 1992/1993 | X | 0.08 | |
| BoS Warm | 1975–2008 | 1981/1982 | 1993/1994 | 0.06 | |||
| Basin . | Data Set . | Years . | Changes . | Intensity (no. of changes/year) . | |||
|---|---|---|---|---|---|---|---|
| . | . | . | 1970s . | 1980s . | 1990s . | 2000s . | . |
| Kattegat | K Cold | 1976–2008 | 1979/1980 | 1988/1989 | 1994/1995 | 0.09 | |
| K Warm | 1976–2008 | 1982/1983; 1988/1989 | 1995/1996 | 0.09 | |||
| Baltic Proper | BP Cold | 1971–2008 | 1976/1977 | 1983/1984; 1988/1989 | 2002/2003 | 0.11 | |
| BP Warm | 1971–2008 | 0 | |||||
| Bothnian Sea | BoS Cold | 1975–2002 | 1988/1989 | 1992/1993 | X | 0.08 | |
| BoS Warm | 1975–2008 | 1981/1982 | 1993/1994 | 0.06 | |||
Results are presented by decade between 1970 and 2000, X denotes that the dataset did not cover the decade. Intensity shows the number of changes observed per total number of years studied in each dataset. Abbreviations of dataset names are as in Table 1.
Changes occurred in all basins during the early 1980s, the late 1980s, and the mid 1990s (Table 2; Figure 3). In the early 1980s, four changes occurred (K Cold, 1979/1980; K Warm, 1982/1983; BP Cold, 1983/1984; and BoS, 1981/1982), and during the late 1980s changes were seen in the colder season in all basins and also in the Kattegat warmer season. In all these cases, the changes occurred in 1988/1989 (Table 2). In the mid 1990s (1992–1995) changes occurred in both seasons in the Kattegat and the Bothnian Sea, but no change was observed in the Baltic Proper (Table 2).
Association with environmental variables
The final models according to the DISTLM analyses explained between 20.2% and 51.8% of the total variation in the datasets (Table 1). Water temperature (Tl) was the only local variable included in the final models. It was significant in all but the BoS Cold dataset, but never explained >13.9% of the total variation, and was only identified as the variable explaining most of the variation in the two Kattegat datasets (Table 1). The only regional variable included in the final models was salinity (S), found in four of the datasets. It explained in total almost twice as much of the variation as that captured by the local variable water temperature (Table 1). Salinity was the main contributing variable in the Baltic Proper and Bothnian Sea datasets, where on its own it always explained >20% of the total variation. In addition, the NAO index was included in three of the selected models (K Warm, BP Cold, and BoS Cold), explaining between 9.7% and 13% of the variation, but never identified as the strongest contributing variable (Table 1).
Less than half (five out of 12) of the variables included in the selected models exhibited a significant linear trend over time, whereas the others showed a more variable pattern (Supplementary material, Figure S1). Among the climate-related variables, local and regional temperature (Tl and T) showed a significant long-term increase in all three basins in the warmer season, but not in the cold season. Salinity showed a significant decrease in both the Baltic Proper and Bothnian Sea, but not in the Kattegat. The NAO index showed on average negative values in the 1970s, positive in the 1980s, increasing in the first half of 1990s, and decreasing again in the most recent years studied (Supplementary material, Figure S1). Among the nutrient-related variables, local water transparency increased in the Kattegat, whereas it decreased in the Baltic Proper.
Discussion
Despite their strong socio-economic and ecological importance (Harley et al., 2006), few studies have hitherto addressed causes of long-term structural change in coastal ecosystems (but see Jackson et al., 2001; Lekve et al., 2003; Collie et al., 2008). In this study, the temporal development of coastal fish communities in three different basins of the Baltic Sea during the last four decades was assessed in relation to environmental factors on local, regional, and global scales. Climate-related variables appeared to be more important than those related to nutrient levels, as observed at the local (temperature) as well as the global (NAO) scale in all three basins. In the Baltic Proper and Bothnian Sea communities, however, the environmental variable mainly associated with community changes was regional salinity. The results corroborate previous findings suggesting that coastal communities in the area to a large extent are local in their appearance (Saulamo and Neumann, 2002; Laikre et al., 2005; Olsson et al., 2011, 2012) and influenced by local environmental factors (Wottoon, 1998). However, they additionally suggest that variables acting on a regional (mainly salinity) and global (NAO) scale may be at least equally important for the temporal development of coastal fish communities.
Association with environmental variables
Gradients in abiotic conditions, species composition, and diversity are pronounced over the Baltic Sea area (HELCOM, 1996). Our study is, to the best of our knowledge, one of the first attempts to assess factors driving temporal changes at the community level along such a gradient. As expected, there were some unique basin- and season-specific responses to changes in environmental conditions, but several common patterns are also discernible. Generally, over the studied period, increasing sea surface water temperatures and decreasing salinity were observed, coinciding with a decrease in marine species and species preferring lower water temperatures of all fish communities in all three basins studied. At the same time, there was an increase in freshwater species, especially those favoured by higher water temperatures.
The variables most significant in relation to the observed changes in fish community composition were local temperature and regional salinity, both related to climate change. Regional salinity was included in the final models in both the Baltic Proper and the Bothnian Sea datasets, but not in the more marine Kattegat communities. This may be expected, as salinity is a limiting factor for the distribution of many marine and freshwater species in the Baltic Sea (Voipio, 1981), but less so in the Kattegat. The strong relationship with regional salinity observed in the colder season was rather expected, since coastal fish community composition in the Baltic Sea datasets at this time of the year is influenced by marine species immigrating from offshore areas. The association between salinity and fish community composition in the warmer season was more surprising, since the species dominating coastal fish communities in this season are typically of freshwater origin and have more local population structure (Saulamo and Neumann, 2002; Laikre et al., 2005; Olsson et al., 2011, 2012). As such, it is evident that the development of these types of coastal fish communities may also be influenced by large-scale environmental change. In all, these findings indicate the importance of large-scale climatic conditions for coastal fish communities across geographically separated basins, and also that open-sea and coastal ecosystems might be linked through pathways other than migrating fish species.
The weakest relationship with the studied environmental variables was seen for the Kattegat datasets, but these communities showed the strongest link to the increase in water temperatures experienced during the last four decades. In all communities, however, there were community responses to changes in local ambient temperatures. This is also manifested as an increase in species favoured by warmer waters in all communities assessed. In addition, the effect of local temperature might at least to some extent be a result of species-specific responses to temperature of catchability (Thoresson, 1996). For the datasets representing the warmer season, we considered a response at the community level more likely than catchability effects, since only a minority of the individual species exhibited a significant correlation between abundance and local temperature (JO, unpublished data). Moreover, local temperature at sampling in the warmer season either showed a significant linear increase over time or was strongly correlated with summer temperatures. Also in the colder season datasets, the relationship between species abundance and local temperature was only significant for a fraction of the species, but there was no or weak correlation between local temperature at fish sampling and summer temperature. As a result, changes in coastal community composition during the cold season might to some extent also partly reflect changes in migratory behaviour of the species due to ambient local temperatures (up to 6.9–2.3% of the total variation in two of the datasets).
Some effects of NAO on community composition were also discernible. Generally, the conditions over the Baltic were characterized by regular in-flows of saline water from the North Sea and lower sea surface temperatures between the 1970s and the end of the 1980s (i.e. on average negative values of NAO; Kalnay et al., 1996). During the following years, the index values were on average positive and the Baltic Sea relatively warmer and less saline. Thus, the effects of NAO on the coastal fish communities observed in this study are likely to be similar to those observed for salinity and local temperatures, but manifested on a larger geographical scale.
The level of unexplained variation in the final models was often rather high, probably reflecting that only abiotic variables were included in the analyses. Other potentially important drivers of change, such as pollution, habitat degradation, and exploitation of key species, or biological processes such as competition and predation were not considered in this study. These variables are known to impact community structure in fish assemblages (Wootton, 1998; Lekve et al., 2003; Österblom et al., 2007; Möllman et al., 2009). Despite the fact that the main results of this study are explicable only in light of the abiotic variables included, additional aspects, such as those listed above, should preferably be included in any further studies. Although the majority of species with a freshwater origin in the communities assessed are not targeted by large-scale fisheries, the decrease in coastal fisheries during the last decade might potentially be an important explanatory factor for the observed changes. Furthermore, incorporation of biotic explanatory variables may be especially important for the Kattegat datasets, where the weakest relationships with the abioic factors assessed were observed. For example, the increase in small-bodied species (wrasses and black goby) was mainly associated with increased temperatures in our study, but is probably also related to predatory release (Pihl, 1982; Pihl and Ulmestrand, 1993; Jackson et al., 2001; Myers et al., 2007; Savenkoff et al., 2007) as a result of the overexploitation of cod (ICES, 2010; Eriksson et al., 2011). Cod has also decreased in the Baltic Proper and Bothnian Sea during the past decades (ICES, 2010), concurrent with a cascading effect on lower trophic levels in coastal foodwebs caused by predatory release (Eriksson et al., 2011). In this study, a decrease in cod was observed in the Baltic Proper and Bothnian Sea datasets representing the colder season. However, most small-bodied species, including the most influential mesopredator in Baltic Sea coastal ecosystems, the three-spined sticklebacks (Gasterosteus aculeatus), were not representatively sampled in these two basins by the gears assessed in this study. Predatory release from cod on earlier life stages than those representatively sampled by these gears might however also have facilitated the increase of the freshwater species in the Baltic Proper and Bothnian Sea datasets since the early 1990s.
Timing of change
As might be expected from the variability in species composition across basins and seasons, specific changes at the dataset level did occur. Given that unique responses to external perturbation and internal dynamics across local fish communities over such a strong environmental gradient as in this study might be anticipated, we believe that the coherence in timing of change across basins and seasons is striking. During 1988/1989, changes in community composition were observed in all basins, but were mainly seen in datasets representing the colder season. Two other common periods of change were also identified, the late 1970s/early 1980s when changes occurred in all basins and seasons, and the mid 1990s when changes were restricted to the Kattegat and Bothnian Sea datasets. Dataset-specific timing of changes was most frequent in the communities assessed in the Baltic Proper. This may be explained by the higher degree of mixture of marine and freshwater species, making unique responses to environmental perturbation within this basin more likely.
In marine ecosystems, substantial structural changes (regime shifts; Andersen et al., 2009) have generally been seen to follow worldwide shifts in climatic–oceanic conditions during recent decades (1977, 1989, and 1998; reviewed in Beamish, 2004). In many North Pacific and North Atlantic ecosystems, including the offshore ecosystem of the Baltic Sea, particularly strong shifts have been observed in the late 1980s (e.g. Hare and Mantua, 2000; Link et al., 2002; Beugrand, 2004; Weijermann et al., 2005; Möllmann et al., 2009; Diekmann and Möllmann, 2010). The change in 1988/1989 together with the relationship between structural changes and climate-related variables, as derived in our study, indicate that changes in community structure in Baltic coastal areas are also linked to events on the global scale. In our analyses, however, we observed weaker support for a main change in 1977, as indicated in other marine systems (Beamish, 2004), and no support for a shift in 1998. Similar to the situation found for the coastal fish communities of the Kattegat and Bothnian Sea in this study, Diekmann and Möllmann (2010) also identified a structural change in offshore areas of the Baltic Sea in the mid 1990s. This was observed in all but one of six offshore systems assessed (Diekmann and Möllmann, 2010), but has, to our knowledge, not been reported for areas outside the Baltic Sea. These findings further support that the open-sea and coastal ecosystems might be linked through several pathways. Whether the changes in the late 1970s/early 1980s, as observed in this study, represent a unique coastal response has not yet been addressed due to shorter time-series in the offshore datasets from the Baltic Sea basins (cf. Diekmann and Möllmann, 2010; ICES, 2010).
At the species levels, however, coastal and offshore systems might exhibit different responses. For example, in the Bothnian Sea there has been a decrease in coastal herring (this study) concurrent with the increase in offshore stocks of herring (ICES, 2011). A likely explanation for this is that there are different spawning groups of coastal and open-sea herring in the Bothnian Sea (Ehnholm, 1951), and that the herring stock assessed in the Bothnian Sea therefore consists of several populations with potentially different population dynamics (ICES, 2011). The logic behind the similar development of the offshore and coastal herring in the Baltic Proper might be that the herring in this basin is less bound to the coast compared with the Bothnian Sea herring (Otterlind, 1976). Yet, at the community or foodweb level, our results suggest that changes in coastal community structures in the Baltic Sea are linked to events at larger spatial scale also affecting offshore foodwebs.
Since coastal communities are hypothesized as being of local appearance and the sampling areas used in this study are selected to represent one coastal habitat type in each basin, it is difficult to reconcile how representative our findings are on a basin-wide scale. We are, however, confident that the findings in this study could be generalized across Baltic Sea coastal areas for several reasons. First, the datasets extend over almost four decades and the overall transition from communities dominated by marine species and those favoured by cold water to a state characterized by species of a freshwater origin in favour of warmer waters is also manifested in the few other datasets existing that cover a similar time span (Swedish University of Agricultural Sciences, Institute of Coastal Research, unpublished data). Second, considering a shorter time-scale, there is a much better spatial coverage in the Baltic Proper and Bothnian Sea for datasets spanning over the last two decades. In these time-series there are local differences in the temporal dynamics of certain species, but they show a general pattern of increasing abundances of freshwater species and those favoured by higher water temperatures (HELCOM, in press). Third, the common results across the three geographically separated basins in this study suggest that the findings are general for coastal systems in the Baltic; there was a coherence in the timing of community change in all basins assessed, the temporal development of these communities has followed a similar pattern, and the same drivers across basins were related to community development despite the strong environmental gradient found across these basins (Voipio, 1981).
Conclusion
The results of this study show that changes in coastal fish communities may have common causes across geographically distant areas, and that offshore and coastal systems to some extent might respond to similar large-scale environmental conditions. These systems may be linked through several pathways, beyond species migrations, and the findings in this study advocate a wider geographical perspective in the management of coastal ecosystems and provide support for a common management of coastal and open-sea systems (see also Estes et al., 1998; Eriksson et al., 2011). Examples include the development of common long-term management plans and integrated monitoring programmes for open-sea and coastal ecosystems. Moreover, although variables related to large-scale climatic changes may not be manageable in the short term, the results in this study show that management of other human activities must be undertaken accounting for climate-related effects. The directional changes in coastal community structure observed in this study support the need for a flexible management, where management targets are continuously set in relation to community processes, such as altered productivity (ICES, 2008) or changes in species interactions (Collie and Gislason, 2001). In this context, assessments of changes in species composition, as outlined here, may complement the use of univariate indicators (e.g. indicator species; Möllmann et al., 2009) and provide a better representation of community changes that is still decomposable with respect to single species and interpretable in relation to external drivers.
Supplementary material
The supplementary material is available at ICESJMS online and cosists of a list of all species and their associated scientific names included in the analyses (Table S1), a cross-correlation matrix of the environmental variables used in each dataset (Table S2), and the development over time for the species contributing to the temporal development of each dataset (Figure S1).
Acknowledgements
We thank Kerstin Söderberg, four anonymous reviewers, the editor Verena Trenkel, and the ICES/HELCOM Working Group on Integrated Assessments of the Baltic Sea (WGIAB) for help and inspiration during the construction of this manuscript. This study is part of the project Integrated analyses of Baltic Sea ecosystems, financially supported by the Swedish Environmental Protection Agency, the Swedish Board of Fisheries, and the Swedish University of Agricultural Sciences.
References
Author notes
Handling editor: Verena Trenkel