-
PDF
- Split View
-
Views
-
Cite
Cite
Padmini Dalpadado, Randi B. Ingvaldsen, Leif Christian Stige, Bjarte Bogstad, Tor Knutsen, Geir Ottersen, Bjørnar Ellertsen, Climate effects on Barents Sea ecosystem dynamics, ICES Journal of Marine Science, Volume 69, Issue 7, September 2012, Pages 1303–1316, https://doi.org/10.1093/icesjms/fss063
Close - Share Icon Share
Abstract
Effects of climate variability and change on sea temperature, currents, and water mass distribution are likely to affect the productivity and structure of high-latitude ecosystems. This paper focuses on the Barents Sea (BS), a productive Arcto–boreal shelf ecosystem sustaining several ecologically and economically important fish species. The water masses in the region are classified as Atlantic, Arctic, and mixed, each having a distinct ecological signature. The pronounced increase in temperature and a reduction in the area covered by Arctic water that has taken place during the past decade have affected the ecology of the region. An increase in biomass of lipid-rich euphausiids in recent years, possibly linked to the temperature increase, has apparently provided good feeding and growth conditions for several species, including capelin and young cod. The observed reduction in Arctic zooplankton may on the other hand have negative implications for polar cod and other zooplankton predators linked to the Arctic foodweb. Despite these changes, the BS at present seems to maintain relatively stable levels of boreal zooplankton biomass and production, with no significant changes in the abundances of Calanus finmarchicus or the episodic immigrant C. helgolandicus.Dalpadado, P., Ingvaldsen, R. B., Stige, L. C., Bogstad, B., Knutsen, T., Ottersen, G., and Ellertsen, B. 2012. Climate effects on Barents Sea ecosystem dynamics. – ICES Journal of Marine Science, 69: .
Introduction
According to Wassmann et al. (2011), the number of well-documented changes in planktonic systems in the Arctic attributable to climate change is unexpectedly low, implying the need for further investigation. The current investigation explores the structural changes in the Barents Sea (BS) plankton community as they relate to climate variability, and their possible consequences on BS ecosystem productivity. Within this context, we investigate the roles of advection and climate as driving forces on zooplankton dynamics, explore long-term trends in key zooplankton groups in response to climate variability and consider possible consequences of changes in zooplankton attributable to climate change on higher trophic levels.
Recent studies suggest that climate fluctuations may affect whole pelagic ecosystems from phytoplankton to zooplankton to higher trophic levels (Richardson, 2008; Reygondeau and Beaugrand, 2011, and references therein). To the south and upstream of the study area in the BS, both the North Sea and the southern Norwegian Sea have experienced changes in their zooplankton communities during the past decade. These have been linked to both top–down (fishing, high predation) and bottom–up (climate impact) effects (Kirby et al., 2009; Holst and Huse, 2011). In the central and northern North Sea, the warm-water-associated species Calanus helgolandicus has increased in abundance relative to its more cold-water-orientated congener C. finmarchicus, which in the past was the most abundant species in both numbers and biomass (Helaouët and Beaugrand, 2007; Falkenhaug and Omli, 2011). Southern parts of the Norwegian Sea are now experiencing similar changes (Ellertsen and Melle, 2011), and a progressive reduction in zooplankton biomass has been observed since 2002 (Melle, 2008). The structural and productivity changes occurring at lower trophic levels in the North Sea have affected higher trophic levels, the most prominent example being the once large, but now dwindling, cod (Gadus morhua) stock. Its collapse is undoubtedly related to heavy fishing pressure, but it is also related to the aforementioned changes in the plankton community (Beaugrand et al., 2003; Kirby et al., 2009; Olsen et al., 2011).
Plankton are transported north and east from the Norwegian Sea in the Coastal and Atlantic currents, into the BS (Figure 1). This transport allows plankton species to expand their distribution (Speirs et al., 2006), modifying to regional biodiversity, abundance, and production levels. The dominant zooplankton species transported into the BS is C. finmarchicus, which overwinters in the deeper regions of the Norwegian Sea and in spring migrate upwards towards the surface. At that time, large numbers are transported onto the Norwegian shelf. Indeed, model studies by Torgersen and Huse (2005) suggest that most of the C. finmarchicus advected into the BS originate from along the Norwegian coast, indicating that shelf dynamics are important in transporting them northwards. Hence, recent structural and production changes in the upstream regions of the North Sea and the southern Norwegian Sea, especially related to changes in Calanus composition, could, in the longer term, affect the productivity of the BS ecosystem through changes in the advection of zooplankton via inflowing currents. Therefore, in addition to the objectives stated above, another central question we address here is whether the changes to the zooplankton in the North and Norwegian seas are also taking place in the BS.
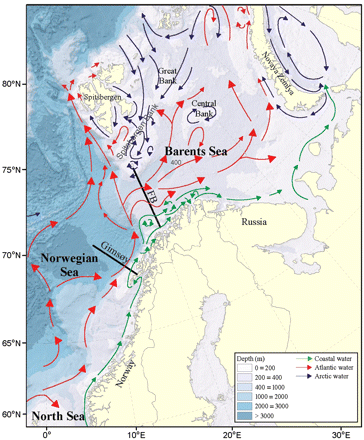
A schematic representation of the main currents and topography in the BS. The location of the two standard sections; the FB section at the western entrance to the BS, and the Gimsøy section in the Norwegian Sea, is indicated.
Before presenting a discussion of the data used in this study, we provide a brief introduction to the BS, its physical and biological variability, and its zooplankton community. The BS is an Arcto-boreal shelf sea that extends from the shelf break constituting the border with the Norwegian Sea, eastwards to Novaya Zemlya and northwards from the coasts of Norway and Russia to the shelf break towards the Arctic Ocean. Its latitude ranges from around 68.5–82.5°N, its area is ∼1.6 million km2, and its average depth is 230 m (Figure 1; Carmack et al., 2006). The BS is characterized by large interannual spatial and temporal variations in ice cover, spring phytoplankton bloom dynamics, zooplankton abundance, and fish recruitment. In addition to physical conditions, which act as a primary driving force for ecosystem variability, biological interactions affect the state of the ecosystem. Zooplankton (copepods, euphausiids, amphipods) are major prey items for many pelagic plankton-feeding fish, the dominant species in the BS being young herring (Clupea harengus), capelin (Mallotus villosus), and polar cod (Boreogadus saida). Zooplankton also constitute a large part of the diet, for especially juvenile, demersal fish such as cod and haddock (Melanogrammus aeglefinus). Moreover, higher trophic level predators such as mammals and seabirds benefit by feeding directly on zooplankton or indirectly by consuming planktivorous fish.
The BS ecosystem has a rich and diverse zooplankton community, and major zooplankton species are usually associated with different water masses (Atlantic, Arctic, and mixed). The mesozooplankton fraction is generally dominated by Calanus copepods, with Calanus finmarchicus dominating Atlantic water (AW) masses and C. glacialis and C. hyperboreus being abundant in Arctic waters (ArWs). Calanus finmarchicus is a key species and the principal food of fish larvae in the northern North Atlantic (Skjoldal et al., 2004).
Euphausiids are another important plankton group in the BS. Thysanoessa inermis is regarded as a shelf species and is dominant in the west, especially around the Svalbard archipelago. The areas of high abundance of T. inermis overlap with those of capelin, and the species can constitute up to 60% of the diet of adult capelin (Dolgov et al., 2011; Dalpadado and Mowbray, in press). Thysanoessa raschii prefers colder, less-saline water and is mostly confined to shallow waters in the east. The largest euphausiid species, Meganyctiphanes norvegica, is usually restricted to slope waters of the Norwegian Sea. However, in the past decade, it has extended its distribution northwards, most likely because of increased temperatures, and is now common in the western BS (Zhukova et al., 2009; Orlova et al., 2011).
The large amphipod species Themisto libellula, which is found in vast concentrations in ArWs of the BS (Auel et al., 2002; Dalpadado et al., 2009), is key prey for cod, polar cod, mammals, and other species living close to the ice edge. The smaller amphipod species, T. abyssorum, on the other hand, is an important component of the foodweb in Atlantic/boreal waters.
Material and methods
Oceanographic data
Temperature and volume of AW flowing into the BS was measured at the standard section Fugløya–Bear Island (FB), located at the western entrance to the BS (FB section, along ∼20°E, Figure 1, Table 1). We used time-series of temperature at 50–200 m, representing the main core of the Atlantic flow. The volume flux of AW along the section has been monitored by current-meter moorings since 1997 (Ingvaldsen et al., 2004). The monthly mean values of volume flux from August 1997 to June 2010 were used in the analysis.
Datasets used in this study.
| Type . | Period . | Area . | Equipment . | Sampling frequency . | Sampling depth . | Source . |
|---|---|---|---|---|---|---|
| Temperature | 1970–2010 | BS | CTD and water bottles | Once a year | Entire water column | IMR/PINRO |
| Volume flux | 1997–2010 | FB section | Current-meter moorings | Monthly | Entire water column | IMR |
| Mesozooplankton | 1981–20101 | BS | WP2 plankton net | Once a year | Entire water column | IMR |
| Mesozooplankton | 1995–2010 | FB section | WP2 plankton net | Up to six times per year | Entire water column | IMR |
| Mesozooplankton | 1997–2010 | Gimsøy section | WP2 plankton net | Up to six times per year | 0–200 m | IMR |
| Amphipod | 1984–2010 | BS | Pelagic trawl | Once a year | 0–100 m | IMR |
| Krill index | 1984–2005 | BS | Net attached to the headline of BT | Once a year | 6–10 m from bottom | PINRO |
| Pelagic fish stocks | 1983–2010 | BS | Pelagic trawl | Once a year | Entire water column | ICES (2011) |
| Diet of cod aged 1 and 2 | 1984–2010 | BS | Mainly bottom trawl | Three times per year | Close to bottom | IMR/PINRO |
| Type . | Period . | Area . | Equipment . | Sampling frequency . | Sampling depth . | Source . |
|---|---|---|---|---|---|---|
| Temperature | 1970–2010 | BS | CTD and water bottles | Once a year | Entire water column | IMR/PINRO |
| Volume flux | 1997–2010 | FB section | Current-meter moorings | Monthly | Entire water column | IMR |
| Mesozooplankton | 1981–20101 | BS | WP2 plankton net | Once a year | Entire water column | IMR |
| Mesozooplankton | 1995–2010 | FB section | WP2 plankton net | Up to six times per year | Entire water column | IMR |
| Mesozooplankton | 1997–2010 | Gimsøy section | WP2 plankton net | Up to six times per year | 0–200 m | IMR |
| Amphipod | 1984–2010 | BS | Pelagic trawl | Once a year | 0–100 m | IMR |
| Krill index | 1984–2005 | BS | Net attached to the headline of BT | Once a year | 6–10 m from bottom | PINRO |
| Pelagic fish stocks | 1983–2010 | BS | Pelagic trawl | Once a year | Entire water column | ICES (2011) |
| Diet of cod aged 1 and 2 | 1984–2010 | BS | Mainly bottom trawl | Three times per year | Close to bottom | IMR/PINRO |
FB, Fugløya–Bear island; BT, bottom trawl; IMR, Institute of Marine Research, Norway; PINRO, Polar Research Institute of Marine Fisheries and Oceanography, Russia.
1Years 1982 and 1983 not included.
Datasets used in this study.
| Type . | Period . | Area . | Equipment . | Sampling frequency . | Sampling depth . | Source . |
|---|---|---|---|---|---|---|
| Temperature | 1970–2010 | BS | CTD and water bottles | Once a year | Entire water column | IMR/PINRO |
| Volume flux | 1997–2010 | FB section | Current-meter moorings | Monthly | Entire water column | IMR |
| Mesozooplankton | 1981–20101 | BS | WP2 plankton net | Once a year | Entire water column | IMR |
| Mesozooplankton | 1995–2010 | FB section | WP2 plankton net | Up to six times per year | Entire water column | IMR |
| Mesozooplankton | 1997–2010 | Gimsøy section | WP2 plankton net | Up to six times per year | 0–200 m | IMR |
| Amphipod | 1984–2010 | BS | Pelagic trawl | Once a year | 0–100 m | IMR |
| Krill index | 1984–2005 | BS | Net attached to the headline of BT | Once a year | 6–10 m from bottom | PINRO |
| Pelagic fish stocks | 1983–2010 | BS | Pelagic trawl | Once a year | Entire water column | ICES (2011) |
| Diet of cod aged 1 and 2 | 1984–2010 | BS | Mainly bottom trawl | Three times per year | Close to bottom | IMR/PINRO |
| Type . | Period . | Area . | Equipment . | Sampling frequency . | Sampling depth . | Source . |
|---|---|---|---|---|---|---|
| Temperature | 1970–2010 | BS | CTD and water bottles | Once a year | Entire water column | IMR/PINRO |
| Volume flux | 1997–2010 | FB section | Current-meter moorings | Monthly | Entire water column | IMR |
| Mesozooplankton | 1981–20101 | BS | WP2 plankton net | Once a year | Entire water column | IMR |
| Mesozooplankton | 1995–2010 | FB section | WP2 plankton net | Up to six times per year | Entire water column | IMR |
| Mesozooplankton | 1997–2010 | Gimsøy section | WP2 plankton net | Up to six times per year | 0–200 m | IMR |
| Amphipod | 1984–2010 | BS | Pelagic trawl | Once a year | 0–100 m | IMR |
| Krill index | 1984–2005 | BS | Net attached to the headline of BT | Once a year | 6–10 m from bottom | PINRO |
| Pelagic fish stocks | 1983–2010 | BS | Pelagic trawl | Once a year | Entire water column | ICES (2011) |
| Diet of cod aged 1 and 2 | 1984–2010 | BS | Mainly bottom trawl | Three times per year | Close to bottom | IMR/PINRO |
FB, Fugløya–Bear island; BT, bottom trawl; IMR, Institute of Marine Research, Norway; PINRO, Polar Research Institute of Marine Fisheries and Oceanography, Russia.
1Years 1982 and 1983 not included.
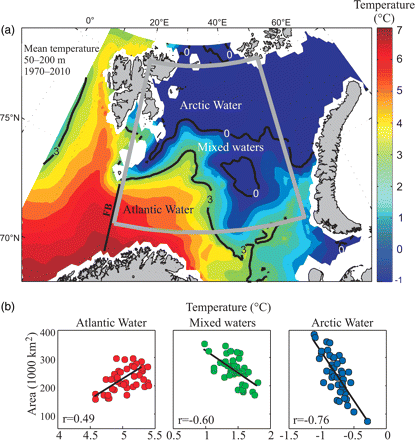
Temperature data, collected using a CTD (conductivity, temperature, depth) system fitted with a water-bottle rosette sampler during annual scientific surveys to the BS, have been interpolated into a horizontal grid with 1/6° meridional resolution (18 km) and 1/2° zonal resolution (10–14 km). No extrapolation or smoothing was performed, except for the implicit effect of the interpolation. From the gridded fields, mean temperature fields in the 50–200-m depth range were calculated, and the areas and mean temperature of AW (T > 3°C), ArW (T < 0°C), and mixed waters (0°C < T < 3°C) were estimated. To ensure complete data coverage each year, the area calculations were restricted to the area 72–80°N 20–50°E (Figure 2a).
(a) Mean temperature, 50–200 m, August to early October, based on observations from 1970 to 2010. The water masses are defined as AW (T > 3°C), ArW (T < 0°C), and mixed waters (0°C < T < 3°C). The grey outline shows the domain for which the area and mean temperatures calculations are performed. The standard FB section is indicated by a black line. (b) Regression analyses between the areas of AW, ArW, and mixed waters and mean temperature in the three water masses.
Biological data
Mesozooplankton
Zooplankton were collected with WP2 plankton nets (Table 1) along the standard sections and during the ecosystem cruises (large-scale surveys). Standard sections were sampled up to six times per year, covering all seasons, whereas ecosystem cruises were restricted to autumn (August to early October). The WP2 is a plankton net with 180 µm mesh and a diameter of 56 cm, towed vertically between the bottom and the surface. The samples obtained were usually divided into two using a Motoda splitter; one-half was preserved in 4% formalin for the analysis of species composition and abundance at the IMR laboratory, and the other was used for biomass estimation. The latter half was fractionated successively through three sieves: 2 mm, 1 mm, and 180 µm. The content on each sieve was rinsed briefly with freshwater (to remove the salt) and transferred to preweighed aluminium trays. These were then dried at 60°C for a minimum of 24 h (to achieve constant dry weight) and later weighed to obtain dry weight biomass. For larger organisms, the drying period was prolonged until a constant weight was obtained. By taking into account the volume of water filtered through the net and the sampling depth interval, the results were expressed as wet or dry weight biomass per m3 of seawater, or per m2 of water column, for the <1, 1–2, and >2 mm size fractions. A factor of 5 was used to convert dry weight to wet weight (Skjoldal et al., 2004).
The Calanus composition (C. finmarchicus, C. glacialis, and C. hyperboreus) was investigated based on WP2 samples along the FB section. In addition, we examined the presence of the warm-water species, C. helgolandicus. Since the taxonomic separation of C. finmarchicus and C. helgolandicus is rather time-consuming, limited numbers of individuals of the later stages (up to 40 copepodite stage V and females) were examined at each station to establish the proportion between the species. Therefore, the results on C. helgolandicus in the study reflect only the proportion relative to C. finmarchicus and not absolute numbers.
Calculations of advected biomass
To calculate the zooplankton biomass advected into the BS, we used zooplankton biomass data (WP2 net, 0–200 m) from Coastal and AW masses on the Gimsøy transect (Table 1, Figure 1). A zonal restriction (east of 10°E) was performed to ensure the capture of the fraction of water and zooplankton transported into the BS. Advected biomass per month was calculated by multiplying the biomass at Gimsøy with monthly mean volume flux across the FB section. In doing this, we assume that the biomass is representative for the given sampling month. The calculations were only performed for months (a total of 72) when both zooplankton and volume flux data were available. The seasonal cycle in the advected biomass was computed as monthly mean values across the years with available data. The accumulated advected biomass over the year was calculated based on the seasonal cycle.
Macrozooplankton
For euphausiids, dataseries from 1984 to 2005 from the Polar Research Institute of Marine Fisheries and Oceanography (PINRO) were used in the analysis (Anon., 1983, 1996, 2000; Zhukova et al., 2009, the data used with permission). The macrozooplankton survey by PINRO includes the annual monitoring of abundance and distribution of euphausiids during the period of the autumn–winter trawl/acoustic survey of demersal fish. The gear used is a modified egg net with a mouth opening of 0.2 m2 and a mesh size of 564 μm, fastened to the headline of the bottom trawl. The net samples a layer 6–10 m from the bottom. It is assumed that by late autumn, the euphausiids have descended to deeper layers to overwinter, so that the catches represent the total abundance of euphausiids present in the water column.
Amphipod catches were obtained by a pelagic trawl from cruises conducted during the period 1984–2010. The catches were limited to the upper 80 m (Dingsør, 2005), and the data in this investigation were restricted to the Norwegian Economic Zone. The pelagic trawl has a 20 × 20-m mouth opening, seven panels, and a codend. Each panel consists of meshes of different sizes, the mesh size varying from 100 mm in the first to 30 mm in the last panel. In this study, we only used catches north of 74°N, because most samples from farther south contained no amphipods. Based on published results and observations made on cruises, we deduced that the pelagic trawl catches were dominated by the pelagic Arctic amphipod T. libellula (maximum ∼50 mm), which forms a key component of the Arctic ecosystem (Auel et al., 2002; Dalpadado, 2002). To study the effect of climate on the zooplankton community and its predators, we calculated correlations between an annual amphipod biomass index (kg wet weight per nautical mile) corrected for interannual variation in the sampling location (see below), the fraction of ArW in the BS (72–80°N 20–50°E), and the estimates of consumption by cod aged 1 and 2 (see below).
Pelagic fish stocks
Biomass of pelagic fish in the BS was extracted from the following reports and publications: age 1+ capelin, acoustic estimates in September (ICES, 2011); age 1 and 2 herring, virtual population analysis (VPA) estimates (ICES, 2010), using the standard weights at age (9 g at age 1, 20 g at age 2); age 1+ polar cod and blue whiting (Micromesistius poutassou), acoustic estimates in September (Anon., 2010); estimates of the 0-group biomass of cod, haddock, and herring, corrected for catching efficiency (Anon., 2010; Eriksen et al., 2011). Biomasses of 0-group fish were incorporated because such fish may exhibit considerable predation on zooplankton.
Diet data
Cod diet was estimated using data from the Joint Norwegian–Russian stomach database (Dolgov et al., 2007). On average, ∼9000 stomachs were sampled annually. The stomach content data were combined with a temperature-dependent model for the stomach evacuation rate (dos Santos and Jobling, 1995) and estimates of cod stock size to give the total consumption by cod of various prey species. The following prey categories were used: capelin, herring, polar cod, blue whiting, cod, haddock, redfish (Sebastes spp.), long rough dab (Hippoglossoides platessoides), Greenland halibut (Reinhardtius hippoglossoides), shrimp (Pandalus borealis), krill, amphipods, and “other”. The calculation method is described by Bogstad and Mehl (1997), and updated time-series are presented in ICES (2011). The consumption estimates (1000 t wet weight) were calculated separately by cod age group, area (west, east, and north), and for the first and second half of the year.
Statistical analyses
To correct for bias from interannual variability in sampling location and sampling gear, we constructed annual indices of amphipod biomass and biomasses of zooplankton of different size fractions using generalized additive models (Wood, 2006), using the mgcv package version 1.7–2 in the program R.
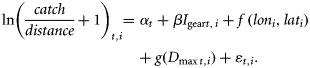
The biomass of zooplankton sampled by WP2 nets was analysed similarly, using ln(mg wet weight + 1) as response, and a spatial term and a year-specific intercept as predictor variables, to calculate the annual indices of total zooplankton biomass and the biomasses of zooplankton >2, 1–2, and <1 mm.
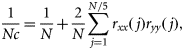
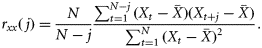
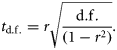
Results
Long-term changes in temperature and water mass index
AW, usually defined by temperature >3°C and salinity >35 (Loeng, 1991), dominates the southern region of the BS (Figure 2a). Within this water mass, there was a statistically significant linear relation between area and mean temperature (Figure 2b), so there were high AW temperatures when this water mass occupied large areas. ArW, characterized by temperatures below zero and low salinity dominated the northern BS. In ArW, there was a negative relation between area and mean temperature, indicating simultaneous large areas and low temperatures. In mixed waters (0°C < T < 3°C), the same pattern was found as in ArW, with large areas of occupation associated with low temperatures.
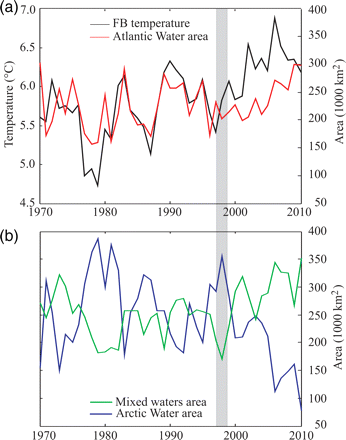
Time-series of temperature and the area of AW revealed a pronounced shift in co-variability and amplitude in the years 1996–1998 (Figure 3a). Before the shift, there were strong and pronounced signals and high temperature in the inflow region (FB temperature), causing correspondingly high temperatures and large areas of AW within the BS. Although this pattern was also present after the shift, it was substantially weaker.
(a) Plot of mean temperature at the FB section and the area of AW from August to early October, 1970–2010. (b) Annual variation in the area of mixed waters and ArW during the same season and period.
The recent (post-2000) high temperatures along the FB section have been associated with neither particularly large areas nor high mean temperatures of the AW within the BS. Instead, this heat input has been distributed over a large area, causing substantial changes in the areas occupied by both ArW and mixed waters (Figure 3a and b). Since the years 1996–1998, the areas occupied by AW and ArW water masses have increased and decreased, respectively, beyond the range observed earlier in the time-series. The area of ArW decreased by 228 000 km2 (74%), with a corresponding temperature increase of ∼0.5°C (Figure 2b) between the late 1990s and 2010. Over the same period, the area of mixed waters almost doubled, with a corresponding temperature decrease of ∼0.8°C (Figure 3b). Hence, an area, which from 1996 to 1998 was dominated by ArW, was gradually replaced by mixed waters with temperatures above zero. This change in water mass characteristics was most conspicuous over the Central Bank, where the Polar Front weakened substantially or vanished (not shown), and it was also evident in the northwestern BS (Lind and Ingvaldsen, 2012).
Advective processes and quantification
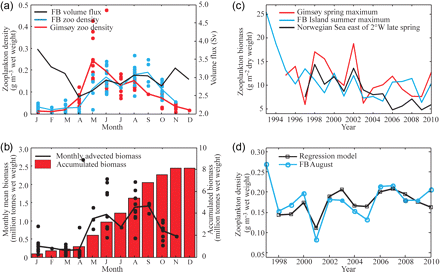
The mean volume flux into the BS was highest in winter during the period 1997–2010, with the mean monthly volume flux varying from ca. 2.5 Sv in April to ca. 4.0 Sv in January (Figure 4a). The advected water brings heat, which sustains ice-free areas and provides improved growth conditions for boreal zooplankton, as well as bringing plankton and nutrients into the BS. The peak in zooplankton density on the Gimsøy section is in May (Figure 4a), 1 month earlier than the spring peak on the FB section. However, zooplankton density (g m–3 wet weight) on the FB section reached its maximum in late summer (August/September; Figure 4a), when the density is about twice as high as on the Gimsøy section. Accumulated over a year, an average of 8 million tonnes wet weight of plankton is advected towards the BS (Figure 4b). On interannual time-scales, this biomass varied between 7 and 9.2 million tonnes over the period 1997–2010.
(a) Monthly variation in volume flux (Atlantic and Coastal waters), zooplankton density along the FB and Gimsøy sections shown as dots (monthly values) and lines (monthly means) for the period 1997–2010. (b) Monthly biomasses (dots, individual observations; black line, monthly means) and accumulated biomass (histograms) advected into the BS. (c) Variation in mean May (late spring) biomass in the Norwegian Sea (after Ellertsen and Melle, 2011), spring and summer maximum along the FB and Gimsøy sections. (d) Zooplankton density along the FB section in August compared with predictions using a linear regression model including volume flux and zooplankton density along the Gimsøy section in March/April.
We explored the relationships between the zooplankton biomass of the Norwegian Sea (average biomass east of 2°W; from Ellertsen and Melle, 2011), and maximum biomass values in spring and summer, respectively, on the Gimsøy and FB sections (Table 2, Figure 4c). The results show that the decreasing trend in biomass observed in the eastern Norwegian Sea is not equally reflected in the spring and summer biomass along the two transects, indicating that the biomass of zooplankton advected into the BS was not much affected by the changes in the Norwegian Sea.
Average zooplankton biomass (g m–2 dry weight) in the Norwegian Sea (May) and on the standard sections Gimsøy (spring) and FB (summer) during two periods, 1997–2002 and 2003–2010.
| Area . | 1997–2002 . | 2003–2010 . | Difference . | Source . |
|---|---|---|---|---|
| Norwegian Sea east of 2°W (mean biomass in the upper 200 m) | 11.0 | 6.7 | 4.3 | Ellertsen and Melle (2011) |
| Gimsøy east of 10°E (maximum) | 12.2 | 9.4 | 2.8 | This study |
| FB (maximum) | 10.9 | 8.9 | 2.0 | This study |
| Area . | 1997–2002 . | 2003–2010 . | Difference . | Source . |
|---|---|---|---|---|
| Norwegian Sea east of 2°W (mean biomass in the upper 200 m) | 11.0 | 6.7 | 4.3 | Ellertsen and Melle (2011) |
| Gimsøy east of 10°E (maximum) | 12.2 | 9.4 | 2.8 | This study |
| FB (maximum) | 10.9 | 8.9 | 2.0 | This study |
Average zooplankton biomass (g m–2 dry weight) in the Norwegian Sea (May) and on the standard sections Gimsøy (spring) and FB (summer) during two periods, 1997–2002 and 2003–2010.
| Area . | 1997–2002 . | 2003–2010 . | Difference . | Source . |
|---|---|---|---|---|
| Norwegian Sea east of 2°W (mean biomass in the upper 200 m) | 11.0 | 6.7 | 4.3 | Ellertsen and Melle (2011) |
| Gimsøy east of 10°E (maximum) | 12.2 | 9.4 | 2.8 | This study |
| FB (maximum) | 10.9 | 8.9 | 2.0 | This study |
| Area . | 1997–2002 . | 2003–2010 . | Difference . | Source . |
|---|---|---|---|---|
| Norwegian Sea east of 2°W (mean biomass in the upper 200 m) | 11.0 | 6.7 | 4.3 | Ellertsen and Melle (2011) |
| Gimsøy east of 10°E (maximum) | 12.2 | 9.4 | 2.8 | This study |
| FB (maximum) | 10.9 | 8.9 | 2.0 | This study |
Focusing on interannual variability for the FB zooplankton density maximum in late summer, the regression analysis shows that 61% of the total variability (r = 0.78) is explained by volume flux and zooplankton density on the Gimsøy section, measured in March/April (Figure 4d). The volume flux alone explains 56% (r = 0.75) and including the zooplankton density on the Gimsøy section only adds 5% to the explanation. All months other than March and April gave less-significant correlations, indicating that the spring inflow is most important for zooplankton advection.
Mesozooplankton variability
The indices of annual mean biomass of the different size classes of zooplankton in the BS in August and September showed large interannual fluctuations (Figure 5). The fluctuations in zooplankton were inversely related to the fluctuations in capelin, and capelin stock size could explain 40% of the interannual variation in total zooplankton biomass during the period 1984–2010 (r = −0.64, n = 29, Nc = 11.1, p = 0.03, accounting for autocorrelation; both time-series on a ln scale).
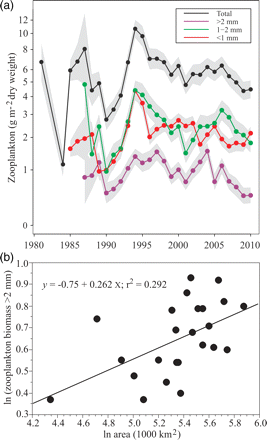
(a) Mean annual zooplankton biomass for the total and three different size categories, <1, 1–2, and >2 mm. Samples were obtained from WP2 plankton nets, so the biomass represents mostly mesozooplankton, mainly copepods. The autumn biomass estimates are calculated using generalized additive models, accounting for interannual differences in sampling locations. The grey areas indicate approximate 95% confidence intervals (not accounting for spatial correlation). (b) Regression analysis between the area of ArW and the largest mesozooplankton (>2 mm, the same values as illustrated in Figure 5a in a natural logarithmic scale) fraction.
The three zooplankton size categories (<1, 1–2, and >2 mm) co-varied more or less up to the late 1990s. After that, the intermediate and largest size fractions seemed to decrease compared with the smallest fraction. The biomass of the largest size fraction was significantly positively correlated with the area of the BS covered by ArW for the period 1987–2010 (r = 0.54, n = 24, Nc = 15.6, p = 0.03, accounting for autocorrelation; Figure 5b). In 2010, when the ArW area coverage was least (76 900 km2), one of the lowest mean annual biomass values (1.4 g m–2 dry weight) was recorded for the largest size category.
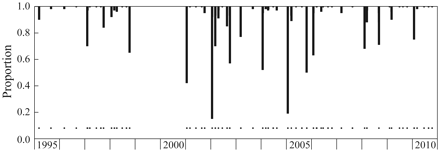
Investigation of the Calanus species composition on the FB section during the periods 1995–1998 and 2001–2010 demonstrated that the abundance of C. finmarchicus (mean 30 000 ind. m–2) was roughly 30 and 300 times higher than that of C. glacialis and C. hyperboreus, respectively (Table 3). Further, those two Arctic species declined in abundance, particularly after 2004. A non-parametric Mann–Whitney test was performed to reveal whether there were significantly lesser abundances of C. finmarchicus, C. glacialis, and C. hyperboreus after vs. before 2004 (Table 3). The test results showed no change in C. finmarchicus abundance (p > 0.2), a nearly significant decrease in C. glacialis (p = 0.07), and a significant decrease in C. hyperboreus (p = 0.02). Another important question relates to the appearance of southern forms in the BS. In this study, there were occasionally high ratios of C. helgolandicus to C. finmarchicus between 1995 and the present, particularly during midwinter, when C. finmarchicus is generally scarce. There was, however, no increase in the proportion of C. helgolandicus over time, suggesting that this species has not increased in abundance at the western entrance to the BS (Figure 6).
Mean annual abundance (numbers m–2) of Calanus spp. along the FB section located at the western entrance to the BS.
| Year . | Calanus finmarchicus . | Calanus glacialis . | Calanus hyperboreus . |
|---|---|---|---|
| 1995 | 50 746 | 984 | 123 |
| 1996 | 28 580 | 1 047 | 161 |
| 1997 | 30 844 | 5 473 | 63 |
| 1998 | 24 158 | 2 541 | 120 |
| 2001 | 16 436 | 730 | 128 |
| 2002 | 23 915 | 489 | 294 |
| 2003 | 15 777 | 476 | 204 |
| 2004 | 9 992 | 483 | 254 |
| 2005 | 26 477 | 103 | 67 |
| 2006 | 30 508 | 821 | 129 |
| 2007 | 19 639 | 675 | 89 |
| 2008 | 15 840 | 10 | 4 |
| 2009 | 16 337 | 310 | 15 |
| 2010 | 90 156 | 681 | 22 |
| Year . | Calanus finmarchicus . | Calanus glacialis . | Calanus hyperboreus . |
|---|---|---|---|
| 1995 | 50 746 | 984 | 123 |
| 1996 | 28 580 | 1 047 | 161 |
| 1997 | 30 844 | 5 473 | 63 |
| 1998 | 24 158 | 2 541 | 120 |
| 2001 | 16 436 | 730 | 128 |
| 2002 | 23 915 | 489 | 294 |
| 2003 | 15 777 | 476 | 204 |
| 2004 | 9 992 | 483 | 254 |
| 2005 | 26 477 | 103 | 67 |
| 2006 | 30 508 | 821 | 129 |
| 2007 | 19 639 | 675 | 89 |
| 2008 | 15 840 | 10 | 4 |
| 2009 | 16 337 | 310 | 15 |
| 2010 | 90 156 | 681 | 22 |
Mean annual abundance (numbers m–2) of Calanus spp. along the FB section located at the western entrance to the BS.
| Year . | Calanus finmarchicus . | Calanus glacialis . | Calanus hyperboreus . |
|---|---|---|---|
| 1995 | 50 746 | 984 | 123 |
| 1996 | 28 580 | 1 047 | 161 |
| 1997 | 30 844 | 5 473 | 63 |
| 1998 | 24 158 | 2 541 | 120 |
| 2001 | 16 436 | 730 | 128 |
| 2002 | 23 915 | 489 | 294 |
| 2003 | 15 777 | 476 | 204 |
| 2004 | 9 992 | 483 | 254 |
| 2005 | 26 477 | 103 | 67 |
| 2006 | 30 508 | 821 | 129 |
| 2007 | 19 639 | 675 | 89 |
| 2008 | 15 840 | 10 | 4 |
| 2009 | 16 337 | 310 | 15 |
| 2010 | 90 156 | 681 | 22 |
| Year . | Calanus finmarchicus . | Calanus glacialis . | Calanus hyperboreus . |
|---|---|---|---|
| 1995 | 50 746 | 984 | 123 |
| 1996 | 28 580 | 1 047 | 161 |
| 1997 | 30 844 | 5 473 | 63 |
| 1998 | 24 158 | 2 541 | 120 |
| 2001 | 16 436 | 730 | 128 |
| 2002 | 23 915 | 489 | 294 |
| 2003 | 15 777 | 476 | 204 |
| 2004 | 9 992 | 483 | 254 |
| 2005 | 26 477 | 103 | 67 |
| 2006 | 30 508 | 821 | 129 |
| 2007 | 19 639 | 675 | 89 |
| 2008 | 15 840 | 10 | 4 |
| 2009 | 16 337 | 310 | 15 |
| 2010 | 90 156 | 681 | 22 |
The proportion of CV and female C. helgolandicus (bars) to C. finmarchicus from individual samples obtained along the FB section during the periods 1995–1998 and 2001–2010. The samples from 1999 and 2000 are currently being analysed. Samples were collected using a WP2 plankton net. Black dots represents sampling events. The largest proportion of C. helgolandicus is generally at coastal stations. In some years, seasonal coverage was scarce owing to limited cruise time or poor weather.
Trends in macrozooplankton biomass
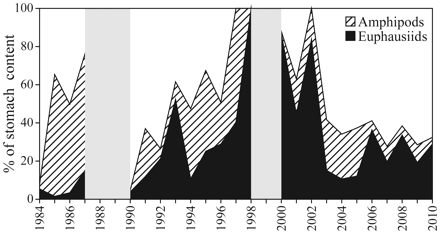
The proportions of amphipods and euphausiids in the diet of cod aged 1 and 2 were related to the in situ abundance of plankton and climate (ArW index = area of Arctic water, standardized to zero mean and unit variance; Figure 7). There was a shift in the proportion of amphipods to euphausiids in the diet over the study period, with more euphausiids and less amphipods in the diet since the late 1990s. The euphausiid abundance index explained 29% of the interannual variation in the proportion of euphausiids in the diet for cod aged 1 for the years 1984–2005 (r = 0.54, n = 22, Nc = 17.0, p = 0.03, corrected for autocorrelation).
Proportion of krill and amphipods in the diet of cod aged 1 in the northern area in the second half of the year (north of 74°N). The years 1988, 1989, and 1999 are omitted (grey area) because of the small sample sizes.
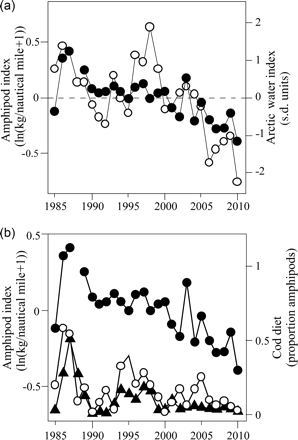
The in situ abundance of amphipods and the ArW index co-varied over the study period, with both decreasing markedly over the past decade (Figure 8a). The ArW index explained 54% of the interannual variation in the abundance of amphipods in situ (r = 0.71, n = 25, Nc = 11.8, p = 0.01, corrected for autocorrelation). The category Amphipods in Figures 7 and 8 consisted mostly of hyperiids of the genus Themisto, but occasionally gammariids were also present. These groups are usually not determined to the species level when cod stomachs are analysed, but Themisto spp. seem to be dominated by T. libellula, although T. abyssorum is occasionally observed (Dalpadado, 2002; Dalpadado and Bogstad, 2004; IMR database). The in situ amphipod abundance was positively correlated with the level of amphipods found in cod aged 1 and 2 (age 1: r = 0.57, n = 25, Nc = 21.0, p < 0.01; age 2: r = 0.65, n = 25, Nc = 22.7, p < 0.001, corrected for autocorrelation; Figure 8b).
(a) Time-series of in situ amphipod biomass index (dots) and ArW area index (open circles) expressed as anomalies. (b) Amphipod in situ biomass index (dots) and the proportions of amphipods in the diet of cod aged 1 and 2 (open circles and filled triangles, respectively).
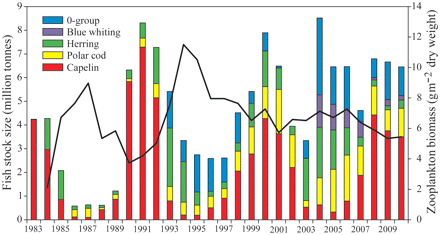
Development of pelagic fish biomass
The biomass of pelagic fish in the BS, particularly that of capelin, but also of blue whiting and herring, fluctuated considerably during the period 1983–2010 (Figure 9). The fluctuations in total pelagic fish biomass (including 0-group) and in plankton abundance were, however, much weaker in the 2000s than in the 1980s and 1990s. Note that for blue whiting, the main distribution area is farther south and west and that normally only juvenile herring are found in the BS, so Figure 9 does not reflect the development in the total abundance of these two stocks.
Development of the pelagic fish stocks in the BS. The biomasses of pelagic fish species in the BS are taken from the following reports and publications: capelin, ICES (2011); herring, ICES (2010); polar cod and blue whiting, Anon. (2010); 0-group cod, haddock, and herring, Eriksen et al. (2011) and Anon. (2010). Note that polar cod, 0-group, and blue whiting estimates are available only after 1986, 1993, and 2004, respectively. The black line represents the estimated average autumn zooplankton biomass in the BS, calculated using generalized additive models accounting for interannual differences in sampling location.
Discussion
Hydrographic shifts and Calanus dynamics
Marked shifts in the three water mass indices (AW, ArW, and mixed) were observed starting in ∼2000. The shifts were especially evident for ArW, for which a drastic reduction in the area was observed (Figure 3b). Corresponding to these climate-related shifts, some ecological changes were observed.
The ArW is an important habitat for large lipid-rich C. glacialis as well as for ice-associated amphipods (Hyperiidae and Gammaridae), which support large colonies of seabirds such as little auks (Alle alle; Anker-Nilssen et al., 2000; Karnovsky et al., 2003). There are indications that the foraging trip durations for the little auk from Kongsfjorden (Spitsbergen, Svalbard) to find Arctic zooplankton are now the longest ever recorded (Welcker et al., 2009). Further, it has been suggested that some fish-eating seabirds, e.g. the black-legged kittiwake (Rissa tridactyla), have changed their diet from polar cod towards capelin (Gabrielsen and Hop, 2009). This may be related to the current general high abundance of capelin relative to polar cod (Figure 9), but it could also result from changes in the distribution patterns in either or both of these two fish species.
An intriguing question is how the changes in the extension of various water masses have affected the areal distribution of C. finmarchicus, C. glacialis, and C. hyperboreus. To quantify how the distributions of these species are related to particular water masses is indeed challenging. Dvoretsky (2011) states that C. finmarchicus prefer water temperatures in the range 2–13°C to reproduce successfully. The substantially increased area of mixed waters (0°C < T < 3°C) in the BS over the past 10 years suggests that new regions may be opening up for improved survival and possibly reproductive success for C. finmarchicus. This is supported by the modelling studies of Reygondeau and Beaugrand (2011), who suggest a progressive reduction in spatial habitat for C. finmarchicus in some regions (e.g. in the North Sea) and an increase in abundance at the northern edge of the species distribution range (e.g. into the BS). However, Hirche and Kosobokova (2007) state that temperature per se may not be limiting reproduction. Other factors, such as the match of food availability and intrinsic timing of development, may also be important. In contrast, the significant reduction in the areal coverage of ArW (T < 0°C) suggests that habitats for the true Arctic species, C. glacialis and C. hyperboreus, are diminishing, causing these species to retreat north. Kosobokova (1999) found that when daily average surface temperatures reached 5°C, C. glacialis left the surface layers and stopped spawning, which suggests that mixed water regions in the BS should be able to support reproduction and survival of C. glacialis.
Northward colonization of C. glacialis habitats by C. finmarchicus will also depend on its ability to establish an overwintering population, and its need for an earlier start in phytoplankton development. Minor but persistent changes in favour of C. finmarchicus, however, including rising temperatures, could in time cause the ecosystem to pass a tipping point, leading to impaired reproduction and diminishing populations of both C. glacialis and C. hyperboreus. In addition, C. glacialis is a shelf species, and its distribution is limited to the marginal seas of the Arctic Ocean, such as the BS. If these become too warm under climate warming, C. glacialis may be forced out of the shallow shelf waters and only be able to maintain viable populations in cold Arctic fjords (Slagstad et al., 2011).
Published data indicate that in the past few decades, the zooplankton community has changed considerably throughout the North Atlantic basin (Beaugrand et al., 2002; Beaugrand, 2004; Licandro et al., 2011) in response to changes in sea temperature, and this may have led to recruitment failure for some of the key fish species, particularly in the North Sea.
In the southwestern parts of the BS, a different challenge facing the existing mesozooplankton community is the introduction of warm-water species from more southern areas. Although the peak spawning of C. finmarchicus in spring is tightly coupled to the spawning of abundant fish stocks such as Arcto–Norwegian cod and Norwegian spring-spawning herring, the warm-water congener C. helgolandicus spawns in autumn, and its naupliar offspring are therefore unavailable for most fish larvae during their critical survival period in spring. The presence of C. helgolandicus at stations on the FB section was mostly restricted to coastal waters and to the period between January and March. Calanus helgolandicus is probably advected north in the Norwegian Coastal Current, with water taking up to 4 months (using surface current speeds from Koszalka et al., 2011) to get from the southern part of the Norwegian Sea to the western entrance of the BS. However, there are no specific trends in the 14 years of data available, showing that the ratio of C. helgolandicus to C. finmarchicus has not changed at the entrance to the BS. Those observations suggest that a biogeographic shift similar to that observed in the North Sea should not be a major concern with respect to the pelagic ecosystem of the BS, in the short term at least.
Hydrographic shifts and amphipods, krill, and foodweb dynamics
A reduction in the abundance of amphipods (mainly T. libellula) was significantly correlated with the area of ArW present (Figure 8a). In the 25-year dataseries, the ArW index was at its minimum in 2010, when the biomass of amphipods was also lowest. Themisto libellula is regarded as an indicator of the presence of ArW (Søreide et al., 2003). Therefore, a persistent reduction in the area of ArW and thereby their habitat will most certainly result in a negative impact on the population level, with respect to their overall abundance and distribution.
As T. libellula is a key prey species in the Arctic foodweb (Auel et al., 2002; Dalpadado, 2002), species at higher trophic levels may also be negatively influenced by the development described above. This may, for instance, be the case for polar cod, which feed heavily on this Arctic amphipod off Svalbard (Bogstad et al., 2011; Dolgov et al., 2011). Polar cod abundance has likely reduced in West Spitsbergen fjords (e.g. Kongsfjorden), as indicated by their lower contribution to the diets of fish-eating seabirds since 2007 (H. Hop, Norwegian Polar Institute, pers. comm.). The shelf edge north of Svalbard and Franz Josef Land may limit how far north polar cod and other fish species can move in instances of higher temperatures.
Euphausiids, which are Atlantic boreal species, are likely favoured by both increases in the temperature and expanded areas of the Atlantic and mixed water masses. Recent observational studies also seem to indicate that euphausiid abundance is usually associated with warmer years (Zhukova et al., 2009; Orlova et al., 2010). Although a clear pattern in krill abundance related to hydrographic shifts is neither visible nor statistically evident, the krill dataseries of PINRO (Johannesen et al., 2012) shows that their abundances in the southern BS were higher during the past decade than during the three preceding decades.
Euphausiids are a major prey of capelin in the BS (Orlova et al., 2002, 2010; Dalpadado and Mowbray, in press), as well as for young cod and herring (Dalpadado et al., 2009; Dolgov et al., 2011), so variability in their abundance is crucial for the feeding success, growth, and overall condition of capelin (see below).
Cod feed throughout the foodweb from zooplankton to higher trophic levels. Young, small cod are to much greater degree plankton-feeders than older, larger individuals. It is evident from the current investigation that the ratio of euphausiids (krill) to amphipods in their diet has increased over time (Figure 8b). This change is most probably related to the in situ abundances of the prey species, associated with changes in the environment. The recent change in the euphausiid/amphipod ratio in the diet of cod aged 1 and 2 (Figure 8b) can also be detected in older cod, although the diet for those age groups is usually dominated by fish prey (Link et al., 2009).
Lipid-rich euphausiids may provide a suitable food source for both capelin and cod. Several studies have shown that the fatness indices of capelin were higher when they fed extensively on euphausiids than on copepods (Orlova et al., 2002, and references therein), leading to better growth. Amphipods are not important as prey for capelin (Dalpadado and Mowbray, in press). For cod feeding principally on capelin (ICES, 2011), the present conditions should also be beneficial. Similarly, other species higher up the food chain, e.g. seabirds such as black-legged kittiwakes, which depend on capelin as prey for successful breeding in the southwestern BS (Sandvik et al., 2005; Barrett, 2007), may gain from the present conditions in the ecosystem.
The status of the zooplankton community
Although results from this study indicate a decrease in Arctic zooplankton in general, the average total mesozooplankton biomass in the BS has remained relatively stable during the past decade, even during periods when predation pressure from pelagic fish was high. Using an average annual mesozooplankton biomass of 5 g m–2 dry weight (FB mean during the period 1993–2010) and a production/biomass (P/B) ratio of 5 (Skjoldal et al., 2004), we calculate an annual average production level of ∼120 million tonnes wet weight in the BS for the area covered by waters warmer than 0°C (940 000 km2). The biomass data from the FB section are used to represent the biomass in the southern part of the BS for two reasons: first, the FB section has good seasonal coverage, providing sufficient data to estimate the annual average value, and second, the large-scale survey in August/September, which covers both the FB section and large parts of the BS, shows that the section is fairly representative for the whole BS with regard to the average zooplankton biomass. From a simulation run with the Norwegian Ecological coupled physical-biological model system, NORWECOM.E2E (Hjøllo et al., 2012), a secondary production level of 16.5 million tonnes of carbon can be found for the same part of the BS in 1997 (S. Hjøllo, IMR, Bergen, Norway, pers. comm.). NORWECOM.E2E is a fully coupled physical/primary production/individual-based C. finmarchicus end-to-end model system, which is currently being developed to link other modules such as krill and key fish species. Using a conversion factor of 6.67 (Hirche et al., 2001) from carbon to wet weight, and assuming that Calanus biomass is 70% of the total (Jashnov, 1939; Dvoretsky and Dvoretsky, 2009), the calculated production value is ∼157 million tonnes wet weight for the mesozooplankton fraction in 1997. The production level based on annual average data on the FB section only from 1997 is ∼137 million tonnes wet weight. This indicates that the annual biomass values computed in this study are comparable with those generated by the model described by Hjøllo et al. (2012).
As the imported zooplankton (8 million tonnes wet weight) may continue to grow and reproduce in the BS, they may contribute to production of up to ∼40 million tonnes wet weight (assuming the same P/B ratio as above), or up to 33% of the total production in the region. The above estimates are based on many assumptions, so should be interpreted with caution. Nevertheless, the estimates are consistent with what has been shown before: namely, that the contribution from the advective biomass is important for maintaining high zooplankton production levels in the BS (Skjoldal and Rey, 1989; Falk-Petersen et al., 2000; Edvardsen et al., 2003; Torgersen and Huse, 2005). However, these results also indicate that the most important factor is local production, which according to our calculations can contribute ∼67% of total production.
In fact, the observed temperature rise over the past decade might have increased production within the area. A temperature increase from 3 to 5°C can result in a doubling of production (from 4 to 8 g C m–2) over summer, and an additional increase in temperature to 7°C can increase the projected production by 50% (from 8 to 12 g C m–2; Tande, 1991). Moreover, the biomass of plankton in the region of the inflowing Atlantic and Coastal currents was generally higher than the average May maximum in the Norwegian Sea (Figure 4c). Along with the general temperature increase in the BS, the inflow of substantial quantities of zooplankton to the region may explain why the BS has not experienced a decrease in zooplankton biomass of a magnitude similar to that in the Norwegian Sea.
It is not only higher temperature per se that is important to allow for higher productivity, although it is often stated as a requirement. Other factors are also important, such as the availability of “new” nutrients, the seasonal timing of phytoplankton growth (e.g. bloom dynamics) vs. that of zooplankton reproduction, and the quality and type of phytoplankton available for key zooplankton species and their larvae (Sakshaug, 1997; Søreide et al., 2010; Leu et al., 2011). Important mechanisms governing the biomass and productivity dynamics of phytoplankton in the BS are discussed in Sakshaug (1997), and they may affect secondary production too. We do not, however, address this issue further here because no time-series data are available that could allow for more quantitative analyses.
The advected plankton biomass reported in this study falls well within the values reported by Edvardsen et al. (2003). Those authors estimated (when converted) the biomass of advected plankton to be 2.6 million tonnes in June 1998 and 0.02 million tons in July 1998. The large variations in their estimates reveal the varying water-volume transport, which was also evident during this study. Our study supports the hypothesis that it is the early spring (March and April) conditions that are the most important for determining peak zooplankton density on the FB section in August. The dominant copepod, C. finmarchicus, overwinters at depths >400 m until early March, well below the sill depth of the BS in winter (Østvedt, 1955; Diel and Tande, 1992; Melle et al., 2004). Once above this depth, individuals caught in the Atlantic and Coastal currents are brought north towards the BS. Recruitment to copepodite stages CI–CIII starts in April in the eastern area of the AW masses and in March in coastal waters.
The transport of individuals from the Gimsøy area to the western entrance to the BS can take 2–6 months, depending on their horizontal and vertical location in the current (Vikebø et al., 2007; Gascard and Mork, 2008; Koszalka et al., 2011). Moreover, the new generation (G1) can develop to stage V and adults within 1.5 months (Hygum et al., 2000), and under favourable conditions (temperature, food), this generation could spawn and give rise to a new generation (G2) in about July or August. Analysis of Calanus composition (background data for Table 3) indicates that old stages of G1 and young G2 are present on the FB section in August. Hence, the peak density on the FB section in August depends on the currents and zooplankton density to the southwest in March and April, which determines initial conditions for plankton transport from the Norwegian Sea into the BS, and on the development of successive generations that might be produced while being transported north. Calanus finmarchicus produces 1–3 generations per year along the Norwegian coast (Pedersen et al., 2001).
It is intuitive and has also been shown empirically that capelin exert the greatest predation pressure on zooplankton when the stock is large. However, in recent years, the mesozooplankton biomass has remained relatively stable (5–6 g m–2 dry weight), even at high capelin stock size and expected high levels of predation (Figure 9). This indicates favourable conditions for mesozooplankton production for the whole BS in recent years partly counteracting high predation levels.
Ecological responses to climate fluctuations
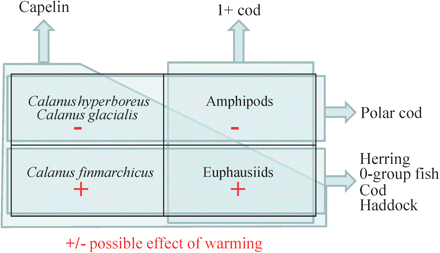
There has been a strong overall increase in temperature in the BS, and the past decade has been the warmest on record. A schematic presentation of anticipated possible effects of warming illustrates that many ecologically and economically important fish, such as herring and 0-group fish (mostly in AW), cod (AW and mixed waters), and capelin (mixed waters), benefit from good feeding conditions (e.g. high abundances of krill and mesozooplankton) brought on by current ecosystem conditions (Figure 10). For capelin and adult cod, their geographic distributions have in recent years expanded north to 81 and 80°N, respectively (ICES, 2011), probably associated with higher temperatures, higher stock abundance, and food availability. The observed distribution patterns for both species are the northernmost recorded to the present. One may anticipate similar changes in the abundances and geographical distributions for several zooplankton and fish species if the current warming trend persists. This could also affect the overlap between stocks and hence their roles as predator and prey. Moreover, species associated with the Arctic foodweb may face population declines, because of a reduction in their habitat and prey abundance and composition. A physical-biologically coupled model study by Slagstad et al. (2011) suggests that during future warming periods, with no summer ice in the Arctic Ocean, the distribution of C. finmarchicus will be confined to the BS and that C. glacialis will almost disappear from the BS. Observations from that modeling study indicate declining trends for the Arctic species C. glacialis and C. hyperboreus in the western BS, especially since 2004 and onwards. This is potentially serious because the BS ecosystem is home to one of the largest concentrations of seabirds in the world, and it holds an abundant and diverse assemblage of marine mammals.
A schematic representation of the possible effects of warming on the major zooplankton species/groups in the diets of capelin, young cod, herring, 0-group cod/herring/haddock, and polar cod in the BS. Warming is anticipated mainly to affect Arctic zooplankton negatively (shown by minus signs) and zooplankton that reaches its northern distribution limit in the BS positively (shown by plus signs). The polygons and arrows show the main zooplankton prey types for each fish group, and indirectly, the anticipated changes in prey availabilities. Note that “Amphipods” here refers to the Arctic species T. libellula.
Cod and capelin in the BS currently constitute the largest stocks of their species in the world. This is due to successful management strategies (in particular for cod) as well as the present favourable “warm state” of the ecosystem (ICES, 2011). To further improve fisheries and ecosystem management policies for the BS, the effects of climate variability and associated ecological impacts, as partly demonstrated in this study, should be taken into consideration to a larger extent than they are today.
Acknowledgements
The work resulting in this article was partly financed by the Norwegian Research Council through ADMAR (Adaptive management of living marine resources by integrating different data sources and key ecological processes, grant 200497/130), a collaborative project between the IMR, Bergen, and CEES, University of Oslo. The work is a contribution to the “Ecosystem and Population Dynamics” and the “Barents Sea Ecosystem” research programmes at the IMR. We are grateful to the production modelling group at the IMR for providing us initial estimates on secondary production, E. Orlova and PINRO for allowing us to use published krill dataseries, and two anonymous referees and the editor for their constructive comments on the manuscript.
References
Author notes
Handling editor: Audrey Geffen