-
PDF
- Split View
-
Views
-
Cite
Cite
Ruairí MacNamara, T. Kieran McCarthy, Size-related variation in fecundity of European eel (Anguilla anguilla), ICES Journal of Marine Science, Volume 69, Issue 8, September 2012, Pages 1333–1337, https://doi.org/10.1093/icesjms/fss123
Close - Share Icon Share
Abstract
Declining European eel (Anguilla anguilla) recruitment has focused attention on conservation of potential spawners leaving continental waters. Fecundity of wild, seaward-migrating silver-phase eels was shown to be size-related and higher than previously reported from artificial maturation experiments. Reliable information on fecundity is essential for stock modelling and future development of eel management policies.MacNamara, R., and McCarthy, T. K. 2012. Size-related variation in fecundity of European eel (Anguilla anguilla). – ICES Journal of Marine Science, 69: .
Introduction
Since the 1980s, the European eel has undergone a serious population collapse throughout its range, and a variety of causes have been proposed (Stone, 2003). In response, the Council of the European Union (2007) has established a legislative framework (Council Regulation No. 1100/2007) to restore spawner escapement biomass from river basins to levels comparable (at least >40%) to those that occurred when pristine environmental conditions existed. Accordingly, European eel spawning dynamics have become a priority research area. Recent satellite tracking of the oceanic migration route (Aarestrup et al., 2009) and swim trial experiments (e.g. Palstra and van den Thillart, 2010) have contributed to our understanding of the reproductive migration. The artificial completion of the Japanese eel Anguilla japonica lifecycle (Ijiri et al., 2011) is encouraging for European researchers (PRO-EEL, 2011), although a complete understanding of the lifecycle and causes of the collapse of A. anguilla are necessary before artificial propagation will become a viable conservation action.
Current European eel stock recovery plans are almost entirely focused on increasing European eel escapement biomass. However, to determine what proportion of eels successfully migrate and reproduce, information on the health and quality status of potential spawners (e.g. Belpaire et al., 2009; Clevestam et al., 2011) is essential. In particular, knowledge of the reproductive ecology, including fecundity, would enable estimation of the egg numbers required to maintain the standing stock, and could also facilitate future development of eel management policies.
A small number of published fecundity estimates of wild eels exist: for American eel A. rostrata (Wenner and Musick, 1974; Barbin and McCleave, 1997; Tremblay, 2009); New Zealand shortfin eel A. australis, and longfin eel A. dieffenbachii (Todd, 1981); A. japonica (Matsui, 1952); and the tropical giant mottled eel A.marmorata (Aoyama and Miller, 2003). It appears that European eel fecundity estimates are exclusively of artificially-matured eels (Kokhnenko et al., 1977; Boetius and Boetius, 1980; van Ginneken et al., 2005). Therefore, the aim of the present study was to estimate the fecundity of wild silver-phase European eels, captured undergoing their seaward spawning migration, and to relate this to body size.
Material and methods
Sampling area and collection
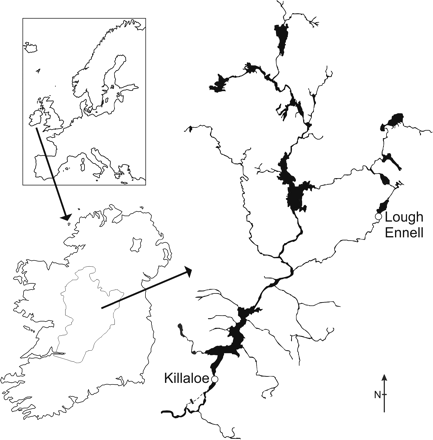
The Shannon International River Basin District, defined according to the Water Framework Directive (European Commission, 2010), occupies an area of 18 000 km2. The River Shannon predominantly drains the central lowlands of Ireland and discharges through a 97 km estuary into the North Atlantic (Figure 1). It is regulated for hydroelectric generation, and the mean annual discharge is 186 m3 s−1 (McCarthy and Cullen, 2000). During the 2007–2008 migration season, 25 silver-phase eels were randomly subsampled from the catch of a commercial fishing crew operating a winged stow net at the outlet of Lough Ennell (53°27′N 7°23′W). This 14.3 km2 mesotrophic lowland lake in the upper Shannon catchment forms part of the River Brosna tributary. The limited size range of Lough Ennell eels (84% were 630–750 mm) precluded analysis of the complete River Shannon female size range (McCarthy and Cullen, 2000) from this location. Therefore, during the 2008–2009 migration season, supplementary silver-phase eels were obtained at Killaloe eel weir (52°48′N 8°27′W) on the lower River Shannon. Thirteen eels were selected from the catch, to represent the entire River Shannon female size range. The fishing gears at both locations captured all sizes of female silver-phase eels (McCarthy and Cullen, 2000; Tesch, 2003).
Silver eel sampling locations (Killaloe eel weir and Lough Ennell outlet) in the River Shannon catchment, Ireland.
Treatment and analysis
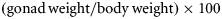
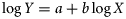
Differences between the intercept and slope of the Killaloe and Lough Ennell fecundity–length regression equations were tested using the General Linear Test Method (Neter et al., 1996).
Results
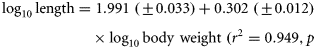
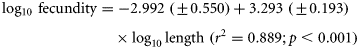
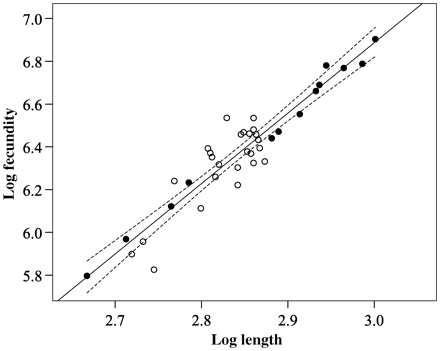
The log10-transformed fecundity–length regression (and associated 95% confidence intervals) is illustrated (Figure 2). Fecundity estimates for the eels examined ranged from 626 000 to 8 006 667 for individuals of 465 mm (211 g) to 1003 mm (2472 g). Based on the fecundity–length regression equation, these eels would have estimated fecundities of 619 331 to 7 785 455. The relative fecundity (eggs/kg) was estimated to be 3 591 699. The morphological characteristics and fecundity of the silver-phase eels examined (by location and pooled data) are presented in Table 1.
Log10-transformed fecundity–length regression of pooled River Shannon (Killaloe; closed circles, Lough Ennell; open circles) silver-phase eels, with 95% confidence intervals.
Summary of the morphological characteristics and fecundity of the silver-phase eels examined.
| . | L. Ennell (n = 25) . | Killaloe (n = 13) . | Pooled (n = 38) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | Mean . | ± S.E. . | Min–max . | Mean . | ± S.E. . | Min–max . | Mean . | ± S.E. . | Min– max . |
| Length (mm) | 673 | 13 | 524–747 | 771 | 49 | 465–1003 | 707 | 20 | 465–1003 |
| Weight (g) | 587 | 31 | 267–798 | 1121 | 188 | 211–2472 | 770 | 78 | 211–2472 |
| GSI (%) | 1.75 | 0.06 | 1.24–2.57 | 1.66 | 0.05 | 1.44–1.99 | 1.72 | 0.04 | 1.24–2.57 |
| Eye index | 7.13 | 0.30 | 6.54–10.57 | ||||||
| Fecundity (millions) | 2.23 | 0.15 | 0.67–3.43 | 3.80 | 0.65 | 0.63–8.01 | 2.76 | 0.27 | 0.63–8.01 |
| . | L. Ennell (n = 25) . | Killaloe (n = 13) . | Pooled (n = 38) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | Mean . | ± S.E. . | Min–max . | Mean . | ± S.E. . | Min–max . | Mean . | ± S.E. . | Min– max . |
| Length (mm) | 673 | 13 | 524–747 | 771 | 49 | 465–1003 | 707 | 20 | 465–1003 |
| Weight (g) | 587 | 31 | 267–798 | 1121 | 188 | 211–2472 | 770 | 78 | 211–2472 |
| GSI (%) | 1.75 | 0.06 | 1.24–2.57 | 1.66 | 0.05 | 1.44–1.99 | 1.72 | 0.04 | 1.24–2.57 |
| Eye index | 7.13 | 0.30 | 6.54–10.57 | ||||||
| Fecundity (millions) | 2.23 | 0.15 | 0.67–3.43 | 3.80 | 0.65 | 0.63–8.01 | 2.76 | 0.27 | 0.63–8.01 |
Summary of the morphological characteristics and fecundity of the silver-phase eels examined.
| . | L. Ennell (n = 25) . | Killaloe (n = 13) . | Pooled (n = 38) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | Mean . | ± S.E. . | Min–max . | Mean . | ± S.E. . | Min–max . | Mean . | ± S.E. . | Min– max . |
| Length (mm) | 673 | 13 | 524–747 | 771 | 49 | 465–1003 | 707 | 20 | 465–1003 |
| Weight (g) | 587 | 31 | 267–798 | 1121 | 188 | 211–2472 | 770 | 78 | 211–2472 |
| GSI (%) | 1.75 | 0.06 | 1.24–2.57 | 1.66 | 0.05 | 1.44–1.99 | 1.72 | 0.04 | 1.24–2.57 |
| Eye index | 7.13 | 0.30 | 6.54–10.57 | ||||||
| Fecundity (millions) | 2.23 | 0.15 | 0.67–3.43 | 3.80 | 0.65 | 0.63–8.01 | 2.76 | 0.27 | 0.63–8.01 |
| . | L. Ennell (n = 25) . | Killaloe (n = 13) . | Pooled (n = 38) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | Mean . | ± S.E. . | Min–max . | Mean . | ± S.E. . | Min–max . | Mean . | ± S.E. . | Min– max . |
| Length (mm) | 673 | 13 | 524–747 | 771 | 49 | 465–1003 | 707 | 20 | 465–1003 |
| Weight (g) | 587 | 31 | 267–798 | 1121 | 188 | 211–2472 | 770 | 78 | 211–2472 |
| GSI (%) | 1.75 | 0.06 | 1.24–2.57 | 1.66 | 0.05 | 1.44–1.99 | 1.72 | 0.04 | 1.24–2.57 |
| Eye index | 7.13 | 0.30 | 6.54–10.57 | ||||||
| Fecundity (millions) | 2.23 | 0.15 | 0.67–3.43 | 3.80 | 0.65 | 0.63–8.01 | 2.76 | 0.27 | 0.63–8.01 |
Discussion
Fecundity in the temperate eels A. rostrata (Wenner and Musick, 1974; Barbin and McCleave, 1997; Tremblay, 2009), A. australis and A. dieffenbachii (Todd, 1981) has been shown to increase exponentially with increasing body size (Table 2). In the present study, which provides the first fecundity estimates of wild A. anguilla, fecundity was also shown to be size-related in this species. No difference in fecundity–size relationship was observed between the upper and lower River Shannon sampling locations. Tremblay (2009) did find differences in fecundity between five subpopulations of A. rostrata in a large North American catchment (Saint Lawrence River), but concluded that this was not related to migration distance. However, considerable variation in A. rostrata fecundity estimates from Chesapeake (37°N) (Wenner and Musick, 1974), Maine (45°N) (Barbin and McCleave, 1997) and the Saint Lawrence (44–49°N) (Tremblay, 2009) suggest differences may exist on a larger spatial (or temporal) scale (Table 2). No such data is available for A. anguilla at present, but possible geographical variation in reproductive potential, reflecting energy requirements and migration distance, has been suggested by Belpaire et al. (2009).
Summary of anguillid fecundity studies.
| Species . | Study . | Size range . | Min–max fecundity (millions of eggs) . | Relative fecundity (millions of eggs/kg) . |
|---|---|---|---|---|
| A. anguilla | Kokhnenko et al. (1977)§ | N/a | N/a | 3.0 |
| Boetius and Boetius (1980)§ | 640–920 mm | 0.7–2.6 | 1.6 | |
| van Ginneken et al. (2005)§ | 690–870 mm | 0.8–4.0 | 1.8 | |
| This study | 465–1003 mm | 0.6–8.0 | 3.6 | |
| A. rostrata | Wenner and Musick (1974) | 490–724 mm | 0.5–2.6 | 3.8† |
| Barbin and McCleave (1997) | 452–1133 mm | 1.7–20.7 | 8.1† | |
| Tremblay (2009) | 532–1159 mm | 3.4–22.0 | 6.5–10.0 | |
| A. australis | Todd (1981) | 516–933 mm | 0.5–3.1 | 1.9 |
| A. dieffenbachii | Todd (1981) | 711–1452 mm | 1.1–20.8 | 2.0 |
| A. japonica | Matsui (1952) | 357–924 mm | 7.2–12.7 | N/a |
| A. marmorata | Aoyama and Miller (2003) | 2400 g | 34.8 | N/a |
| Species . | Study . | Size range . | Min–max fecundity (millions of eggs) . | Relative fecundity (millions of eggs/kg) . |
|---|---|---|---|---|
| A. anguilla | Kokhnenko et al. (1977)§ | N/a | N/a | 3.0 |
| Boetius and Boetius (1980)§ | 640–920 mm | 0.7–2.6 | 1.6 | |
| van Ginneken et al. (2005)§ | 690–870 mm | 0.8–4.0 | 1.8 | |
| This study | 465–1003 mm | 0.6–8.0 | 3.6 | |
| A. rostrata | Wenner and Musick (1974) | 490–724 mm | 0.5–2.6 | 3.8† |
| Barbin and McCleave (1997) | 452–1133 mm | 1.7–20.7 | 8.1† | |
| Tremblay (2009) | 532–1159 mm | 3.4–22.0 | 6.5–10.0 | |
| A. australis | Todd (1981) | 516–933 mm | 0.5–3.1 | 1.9 |
| A. dieffenbachii | Todd (1981) | 711–1452 mm | 1.1–20.8 | 2.0 |
| A. japonica | Matsui (1952) | 357–924 mm | 7.2–12.7 | N/a |
| A. marmorata | Aoyama and Miller (2003) | 2400 g | 34.8 | N/a |
§Eels were artificially-matured.
†Estimated from the regression equation for a 1 kg eel.
Summary of anguillid fecundity studies.
| Species . | Study . | Size range . | Min–max fecundity (millions of eggs) . | Relative fecundity (millions of eggs/kg) . |
|---|---|---|---|---|
| A. anguilla | Kokhnenko et al. (1977)§ | N/a | N/a | 3.0 |
| Boetius and Boetius (1980)§ | 640–920 mm | 0.7–2.6 | 1.6 | |
| van Ginneken et al. (2005)§ | 690–870 mm | 0.8–4.0 | 1.8 | |
| This study | 465–1003 mm | 0.6–8.0 | 3.6 | |
| A. rostrata | Wenner and Musick (1974) | 490–724 mm | 0.5–2.6 | 3.8† |
| Barbin and McCleave (1997) | 452–1133 mm | 1.7–20.7 | 8.1† | |
| Tremblay (2009) | 532–1159 mm | 3.4–22.0 | 6.5–10.0 | |
| A. australis | Todd (1981) | 516–933 mm | 0.5–3.1 | 1.9 |
| A. dieffenbachii | Todd (1981) | 711–1452 mm | 1.1–20.8 | 2.0 |
| A. japonica | Matsui (1952) | 357–924 mm | 7.2–12.7 | N/a |
| A. marmorata | Aoyama and Miller (2003) | 2400 g | 34.8 | N/a |
| Species . | Study . | Size range . | Min–max fecundity (millions of eggs) . | Relative fecundity (millions of eggs/kg) . |
|---|---|---|---|---|
| A. anguilla | Kokhnenko et al. (1977)§ | N/a | N/a | 3.0 |
| Boetius and Boetius (1980)§ | 640–920 mm | 0.7–2.6 | 1.6 | |
| van Ginneken et al. (2005)§ | 690–870 mm | 0.8–4.0 | 1.8 | |
| This study | 465–1003 mm | 0.6–8.0 | 3.6 | |
| A. rostrata | Wenner and Musick (1974) | 490–724 mm | 0.5–2.6 | 3.8† |
| Barbin and McCleave (1997) | 452–1133 mm | 1.7–20.7 | 8.1† | |
| Tremblay (2009) | 532–1159 mm | 3.4–22.0 | 6.5–10.0 | |
| A. australis | Todd (1981) | 516–933 mm | 0.5–3.1 | 1.9 |
| A. dieffenbachii | Todd (1981) | 711–1452 mm | 1.1–20.8 | 2.0 |
| A. japonica | Matsui (1952) | 357–924 mm | 7.2–12.7 | N/a |
| A. marmorata | Aoyama and Miller (2003) | 2400 g | 34.8 | N/a |
§Eels were artificially-matured.
†Estimated from the regression equation for a 1 kg eel.
Factors other than spatial variation may also affect fecundity. The accumulation of lipophilic compounds in gonads may reduce egg production and development (e.g. Belpaire et al., 2009). The water quality of the River Shannon is mainly classified as unpolluted (Lucey, 2009) and in general, contaminant levels in Irish eels are low compared to other European countries (McHugh et al., 2010). Habitat use and migratory type may also affect fecundity, as observed in relation to other biological characteristics (e.g. Svedäng et al., 1996; Arai et al., 2006). Otolith microchemical analysis of upper and lower River Shannon eels indicates that the eel populations are composed almost entirely of freshwater residents (Arai et al., 2006). It seems that further eel fecundity studies from a range of locations may be necessary, particularly when significant differences in biological characteristics can occur even at the scale of neighbouring catchments (Acou et al., 2009). Furthermore, silver-phase eel quality and maturation status within a river system may also change during the downstream migration season, and this should be incorporated in a more comprehensive sampling programme.
Differences between wild and artificially-matured A. anguilla fecundity estimates (Table 2) may be due to the methodology used, or may reflect changes in the gonads accompanying the maturation process. The treatment of ovaries with 2% acetic acid and subsequent volumetric subsampling has successfully been used in previous anguillid fecundity studies (Barbin and McCleave, 1997; Tremblay, 2009). However, A. anguilla fecundity estimates obtained by Boetius and Boetius (1980) by counting eggs retained by a 0.224 mm mesh, were underestimated, as only eggs which had responded to hormonal treatment were counted, and the relative fecundity (eggs/kg) reported (1.6 million) is less than half that obtained in the present study (3.6 million). Likewise, the relative fecundity of artificially-matured A. anguilla by van Ginneken et al. (2005) is considerably lower (1.8 million) than in the present study, possibly reflecting the counting method (gravimetric subsampling) or an effect of the artificial maturation process. Russian maturation experiments conducted in the 1970s quote a relative fecundity of 3 million (Kokhnenko et al., 1977), although no details of counting method are described.
The application of fecundity estimates derived from artificial maturation experiments to wild eel populations may not be appropriate, given the issues discussed above. Similarly, fecundity estimates derived from wild eels captured in continental waters (i.e. early vitellogenic stage) may differ from the actual fecundity at the spawning grounds. Ideally, eels in advanced spawning condition (mid-/late-vitellogenic stage) should be examined. However, to date only female eels of A. japonica and A. marmorata have been captured at their spawning grounds (Iriji et al., 2011). Analysis of changes in the number of eggs in the gonads during the maturation process may be possible using artificially-matured eels (e.g. Durif et al., 2006), although the extent to which hormonal treatment reflects the natural maturation of eels at sea will need to be verified. The limited reproductive success of artificial maturation experiments with A. anguilla (Pedersen, 2004; Palstra et al., 2005) suggests that hormonal treatment results in certain artifactual outcomes (e.g. poor egg quality, delayed hatching and abnormal morphology), which may bias fecundity estimates.
Different growth strategies have been proposed for female eels during the continental phase of the lifecycle i.e. size-maximizing, with its associated higher pre-reproductive mortality rates, to achieve maximum fecundity (e.g. Davey and Jellyman, 2005) or time-minimizing (Svedäng et al., 1996). Laboratory observations of the spawning behaviour of artificially-matured eels suggest batch spawning by females (Boetius and Boetius, 1980), and that a single male may be capable of fertilizing several egg batches (van Ginneken et al., 2005). If this is typical of natural spawning events, female eels could be considered of greater reproductive value than males. Therefore, stock recovery plans should prioritize the protection of large, highly fecund, female eels. Clevestam et al. (2011) proposed similar protection measures for large females in the Baltic Sea, as their large size and high lipid content would make them most likely to successfully migrate and spawn (Belpaire et al. 2009; Palstra and van den Thillart, 2010).
Modelling of A. anguilla population dynamics has been attempted using fisheries data (Dekker, 2000), demographics (van der Meer et al., 2011), and integrated genetic and demographic models (Pujolar et al., 2010; Andrello et al., 2011). Such models often involve numerous input variables, including fecundity. However, the use of differing fecundity estimates [e.g. Boetius and Boetius (1980) in Andrello et al. (2011); Barbin and McCleave (1997) in Pujolar et al. (2010)] has a knock-on effect on subsequent calculations, and it would seem that neither estimate is appropriate, as respectively, they relate to artificially-matured eels (underestimate) and A. rostrata (overestimate) (see Table 2). Van der Meer et al. (2011) reject both of these estimates and instead use a notional figure of 2 million eggs for a 560 g eel, which is very similar to our presented data (1.98 million).
Andrello et al. (2011) divided the continental eel stock into three production units (North, north of 50°N; Atlantic, between 35°N and 50°N; Mediterranean, south of 35°N), with a sex ratio of 0.34 females in the breeding stock and mean female silver-phase eel sizes of 663 mm, 664 mm and 572 mm in each unit respectively. Applying this scenario to Dekker's (2000) estimate of silver-phase eel escapement (8.8 million), we calculate an A. anguilla population fecundity of 5.21 ×1012 million eggs based on the mean size of eels in each production unit, and the fecundity–length relationship presented in Figure 2
The currently available models do not take into account the complexities associated with, for example, the impact of pollution on gonadal development and egg production (e.g. Belpaire et al., 2009), or the possibility of multiple spawning events (Boetius and Boetius, 1980; Ijiri et al., 2011). However, as illustrated by the present study, the need for reliable knowledge of eel fecundity and other population parameters is important for population modelling and spawner stock management. Integration of data on various aspects of European eel biology (fecundity, mating dynamics, larval survival, recruitment, escapement, spawning migration, demographics etc.) may enable estimation of total spawner numbers required in the Sargasso Sea to maintain the standing stock. If possible, this would represent a major step in the conservation of this endangered species.
Acknowledgements
This project was undertaken as part of a study of River Shannon eel populations that was funded by the Electricity Supply Board (Fisheries Conservation). Advice given by Jerome Sheahan and Anne Bateman is gratefully acknowledged.
References
Author notes
Handling Editor: Rochelle Seitz