-
PDF
- Split View
-
Views
-
Cite
Cite
Richard D. M. Nash, Peter J. Wright, Iveta Matejusova, Stefan Petev Dimitrov, Martha O'Sullivan, Julian Augley, Hannes Höffle, Spawning location of Norway pout (Trisopterus esmarkii Nilsson) in the North Sea, ICES Journal of Marine Science, Volume 69, Issue 8, September 2012, Pages 1338–1346, https://doi.org/10.1093/icesjms/fss130
Close - Share Icon Share
Abstract
The northern region of the North Sea (56–62°N) was sampled in February/March 2009 for eggs and in May 2010 for larvae. To aid in the identification of Norway pout stage I eggs and distinguish them from other ‘cod-like’ eggs, a Taq-Man probe was designed for this species and used here. Stage I Norway pout egg diameters collected from the field were in the range 1.03–1.28 mm and largely overlapped with the size range determined for whiting (Merlangius merlangus). The distribution of Norway pout stage I eggs in 2009 revealed the distribution of spawning in the North Sea and showed that it was similar to the distribution of 2 + Norway pout taken during the International Bottom Trawl Surveys (IBTS) over the same period covering the whole North Sea. The larvae sampled in 2010 were largely in the same area; however, larger larvae occurred to the south-east of the survey area, suggesting advection of young stages from the principal spawning areas in the north-western North Sea to the south-east and toward the Skagerrak.Nash, R. D. M., Wright, P. J., Matejusova, I., Dimitrov, S. P., O'Sullivan, M., Augley, J., and Höffle, H. 2012. Spawning location of Norway pout (Trisopterus esmarkii Nilsson) in the North Sea. – ICES Journal of Marine Science, 69: .
Introduction
Norway pout (Trisopterus esmarkii) is a relatively abundant gadoid that occurs mainly from the North Sea to the Barents Sea, between depths of 50 and 300 m (Poulsen, 1968; Wheeler, 1969; Albert, 1994; Sparholt et al., 2002a, b). It is also a small, relatively short-lived (4–5 years) species whose abundance is closely linked to recruitment, which has varied considerably over the last decade (ICES, 2011). While Norway pout may mature at age 1, they are typically mature at age 2 (Raitt, 1968; Albert, 1994). Norway pout spawn in the North Sea between January and March mainly over the deeper parts of the northern North Sea (>100 m), with a peak in spawning occurring between March and April (Ehrenbaum, 1905–09 cited in Russell, 1976; Hislop, 1984). with the more northern populations (off the north-west coast of Norway) starting to spawn in late March and continuing through to June (Baranenkova and Khokhlina 1968). Spawning between Norway and Shetland in the North Sea is reported to occur between mid February/March and April (Raitt and Mason, 1968; Albert, 1994). Lambert et al. (2009) suggest that the eggs and larvae drift away from the western spawning grounds generally towards the south and east; however, this species is not considered to have specific nursery grounds.
Both ichthyoplankton and trawl survey data have been used to map the distribution of spawning aggregations, although the latter is restricted by bottom type (ICES, 2006, 2007; Wieland et al., 2009). In addition, the distribution of fish in spawning condition sometimes does not match the distribution of active spawning sites as indicated by the presence of recently spawned eggs (Fox et al., 2008). As Norway pout spawn in the water column and there is no clear evidence of large spawning aggregations, ichthyoplankton surveys may offer a more reliable approach for mapping spawning locations. However, as eggs and larvae can be dispersed by currents, the identification of spawning areas is reliant on the correct identification of early stage eggs (Fox et al., 2008; Goodsir et al., 2008). For example, cod (Gadus morhua) eggs take ∼2–4 d to develop through the stage I phase in typical North Sea temperatures (4–8°C; Geffen et al., 2006; Geffen and Nash, 2012). Egg identification of such early developmental stages has been traditionally made using egg size ranges and morphological features, such as presence of oil globules, segmentation of the yolk, and patterns of pigmentation in the embryos (Russell, 1976; Materese and Sandknop, 1984). However, many gadoid eggs, including cod and Norway pout, can only be confidently identified to species using such an approach when they have reached later stages of development (Munk and Nielsen, 2005). A number of molecular techniques have been developed to overcome the problem of misidentification of fish eggs and used in the field (e.g. Fox et al., 2008; Bui et al., 2011; Maxwell et al., 2012). Taylor et al. (2002) developed a quantitative polymerase chain reaction (qPCR) TaqMan assay which allows the identification of cod, haddock (Melanogrammus aeglefinus), and whiting (Merlangius merlangus), but unfortunately no such probe was developed for Norway pout.
In this study, a species-specific TaqMan probe for distinguishing Norway pout eggs that can be run together with the probes of Taylor et al. (2002) was developed. By apportioning the number of Norway pout eggs as determined through molecular analyses to the total number of stage I gadoid eggs subsampled at plankton stations, an estimate of the distribution of Norway pout spawning locations in 2009 was produced. These data were compared with the distribution of age 2 + (potential spawning) Norway pout in January/March of the same year to assess whether trawl surveys do give a true indication of spawning extent. The links between adult and larval distribution were examined in 2010 based on the distribution of age 2 + Norway pout in January/March and then larvae were sampled in May.
Methods
Field sampling
Egg sampling in 2009
Sampling took place in the northern North Sea, between 56 and 61°N and 7°E to 1°W, during the 2009 winter/spring ICES PGEGGS ichthyoplankton survey (ICES, 2009). Sampling was carried out by two research vessels using different types of gear: a bongo net (Wiebe and Benfield, 2003) and a Gulf VII high-speed plankton sampler (see Nash et al., 1998 for a general description of the equipment); Table 1. In both cases double oblique hauls were undertaken. RV ‘G.O. Sars‘ (Bergen, Norway) focused on the eastern area of the region while FRV ‘Scotia‘ (Aberdeen, Scotland) focused on the western area. The timing of the survey (Table 1) was planned to coincide with the spawning activity of cod based on historical information (Hislop, 1984). In total, 112 hauls were undertaken over this period, covering 99 ICES rectangles (ICES, 2009).
Summary of the two surveys undertaken for mapping the distribution of fish eggs in the northern North Sea (see Figure 4b for station locations).
| Country . | Ship . | Cruise type . | Start . | End . | Hauls made . | Gear . | Vertical profile monitored . |
|---|---|---|---|---|---|---|---|
| Norway | G.O. Sars | IBTS | 08/02/09 | 21/02/09 | 59 | 76 cm Gulf VII, 40cm opening, 280 µm mesh net | SCANMAR |
| Scotland | Scotia | PLACES | 20/02/09 | 06/03/09 | 53 | Bongo, 60 cm opening, 330 µm mesh nets | SCANMAR |
| Country . | Ship . | Cruise type . | Start . | End . | Hauls made . | Gear . | Vertical profile monitored . |
|---|---|---|---|---|---|---|---|
| Norway | G.O. Sars | IBTS | 08/02/09 | 21/02/09 | 59 | 76 cm Gulf VII, 40cm opening, 280 µm mesh net | SCANMAR |
| Scotland | Scotia | PLACES | 20/02/09 | 06/03/09 | 53 | Bongo, 60 cm opening, 330 µm mesh nets | SCANMAR |
The types of sampling equipment are also given.
Summary of the two surveys undertaken for mapping the distribution of fish eggs in the northern North Sea (see Figure 4b for station locations).
| Country . | Ship . | Cruise type . | Start . | End . | Hauls made . | Gear . | Vertical profile monitored . |
|---|---|---|---|---|---|---|---|
| Norway | G.O. Sars | IBTS | 08/02/09 | 21/02/09 | 59 | 76 cm Gulf VII, 40cm opening, 280 µm mesh net | SCANMAR |
| Scotland | Scotia | PLACES | 20/02/09 | 06/03/09 | 53 | Bongo, 60 cm opening, 330 µm mesh nets | SCANMAR |
| Country . | Ship . | Cruise type . | Start . | End . | Hauls made . | Gear . | Vertical profile monitored . |
|---|---|---|---|---|---|---|---|
| Norway | G.O. Sars | IBTS | 08/02/09 | 21/02/09 | 59 | 76 cm Gulf VII, 40cm opening, 280 µm mesh net | SCANMAR |
| Scotland | Scotia | PLACES | 20/02/09 | 06/03/09 | 53 | Bongo, 60 cm opening, 330 µm mesh nets | SCANMAR |
The types of sampling equipment are also given.
Following ICES guidelines (ICES, 2008), plankton samples were taken at a speed of 2.5 knots to within 5 m of the bottom, except in areas with depths >100 m where the maximum depth sampled was 100 m. In shallower situations, multiple oblique tows were made to ensure that enough water was filtered. Sampling depth profiles were also measured during deployment of the actual sampling gear by means of a minilogger attached to the plankton gear (bongo nets) and via SCANMAR (Scanmar AS, 3167 Åsgårdstrand, Norway) sensor (Gulf VII).
All fish eggs were pre-sorted from the total sample at sea, and the ‘cod-like’ eggs (lacking oil globules and segmented yolk sac) were staged, measured for egg diameter (ED), and up to 50 ‘cod-like’ eggs within the diameter range of 0.9–1.7 mm were placed in 100% ethanol in individual vials for subsequent molecular identification. Only stage I eggs were sampled for molecular identification at stations where sufficient numbers were available, otherwise other stages were also sampled. The average number of stage I ‘cod-like’ eggs was 27 per haul, but the range was 0–268. Gill tissue from adult cod, haddock, Norway pout, and whiting was taken in the Scottish survey (from RV ‘Scotia‘ in the north-western North Sea) to act as TaqMan controls and, in the case of Norway pout, to develop a species-specific primer.
Larvae sampling in 2010
Sampling was undertaken by the RV ‘G.O. Sars‘ in the northern North Sea between 25 April and 5 May 2010 during a routine survey covering standard oceanographic transects (stations across the North Sea at 57°, 58°, 59°17′, and 60°45’N). Two types of sampling equipment were used to obtain the larvae. First, a multiple opening/closing net and environmental sensing system (MOCNESS; Wiebe et al., 1985) with a 1 m2 opening and up to eight separate nets (each with 180 µm mesh) was used in a stepwise fashion from low in the water column up to the surface. The net system was equipped with flow meters and environmental sensors. The volume of water (m3) filtered was estimated for each sampling depth covered by each of the nets, with the number of nets used being dependent on water column depth. Second, samples were collected with a 76 cm diameter Gulf VII high-speed plankton sampler (see Table 1). In this case the volume of water filtered was estimated from a mechanical flow meter (General Oceanics, USA) mounted in the mouth of the nosecone. Water temperature readings throughout the haul were provided by the SCANMAR depth/temperature sensor. All larvae were sorted from the samples at sea and stored in buffered 4% formalin for later identification.
Laboratory studies
Eggs: species TaqMan probes/extraction and sequencing of Norway pout probe
Unlike in Taylor et al. (2002), the Chelex™ method of Estoup et al. (1996) was used to extract genomic DNA as this was found to give a higher quantity and quality of template. Genomic DNA was extracted from the adult gill tissues (cod, whiting, haddock, Norway pout) using a DNeasy Blood and Tissue Kit (Qiagen) following the manufacturer's instructions, and re-suspended in 100 µl of DNase/RNase-free dH2O (Sigma). During the real-time PCR, 1 µl of adult DNA for each species was run on each plate as a positive control.
In order to develop a specific marker for Norway pout, genomic DNA was extracted from gill tissue and used as a template in a PCR containing: 2 µl of DNA, 250 µM dNTPs, 1× PCR reaction buffer (Bioline), 1.5 mM MgCl2, 0.5 µM of each primer (Invitrogen), and 1.5 U of Taq DNA polymerase (Bioline), in a final volume of 20 µl. PCR amplification was performed on a Thermo Hybaid Multiblock MBS 0.2G thermal cycler using 8.2_L8331 and CO3.2_H9236 primers published in Taylor et al. (2002). The PCR conditions were as follows: 95°C for 2 min, followed by 35 cycles of 95°C for 1 min, 50°C for 1 min, and 72°C for 1 min 30 s, and one cycle of 72°C for 2 min. Product was visualized on a 2% agarose gel stained with ethidium bromide (Sigma) and purified using a MinElute® gel purification kit (Qiagen). Approximately 25–30 ng of purified PCR product (low DNA mass ladder, Invitrogen) was used for sequencing in both directions using a Genome Lab DTCS Quick Start kit (Beckman Coulter) and an automated CEQTM 8800 DNA Sequencer (Beckman Coulter). The PCR mix contained 4 µl of Quickstart Master Mix, 1.5 µl of sequencing buffer, 8 µl of betaine (Sigma), 0.5 µM primer (8.2_L8331 or CO3.2_H9236), and DNase/RNase-free water (Sigma) to a final volume of 20 µl.
The specific TaqMan-MGB probe for identification of Norway pout and the universal TaqMan primer (GAD-RII) was designed using Primer Express Software (Applied Biosystems). In order to guarantee the specificity of the designed assay, sequences of the primers and probes were subjected to a BLAST search in the GenBank database to check for any potential cross-reactions. Primer sets and probes used in this study are summarized in Table 2.
Probes and primers designed for species-specific identification using real-time PCR.
| Primer or probe . | Primer or probe sequence 5’–3’ . | Reporter . | Quencher . | Reference . |
|---|---|---|---|---|
| Sequencing primer | ||||
| Forward (8.2_L8331) | AAA GCR TYR GCC TTT TAA GC | – | – | Taylor et al. (2002) |
| Reverse (CO3.2_H9236) | GTT AGT GGT CAK GGG CTT GGR TC | – | – | Taylor et al. (2002) |
| TaqMan primer | ||||
| GAD-F | GCA ATC GAG TYG TAT CYC TWC AAG GAT | – | – | Taylor et al. (2002) |
| GAD-R II | GCA AGW AGY GGH GCR CAT TTG TG | – | – | This study |
| TaqMan probe | ||||
| Cod | CTT TTT ACC TCT AAA TGT GGG AGG | FAM | Non-fluorescent | Taylor et al. (2002) |
| Haddock | CTT TCT TCC TTT AAA CGT TGG AGG | NED | Non-fluorescent | Taylor et al. (2002) |
| Northern pout | CGC TCG TTT TAC CAA CCA GCT | VIC | Non-fluorescent | This study |
| Whiting | GTT TAT YCC TCT AAA CGT AGG AGG | VIC | Non-fluorescent | Taylor et al. (2002) |
| Primer or probe . | Primer or probe sequence 5’–3’ . | Reporter . | Quencher . | Reference . |
|---|---|---|---|---|
| Sequencing primer | ||||
| Forward (8.2_L8331) | AAA GCR TYR GCC TTT TAA GC | – | – | Taylor et al. (2002) |
| Reverse (CO3.2_H9236) | GTT AGT GGT CAK GGG CTT GGR TC | – | – | Taylor et al. (2002) |
| TaqMan primer | ||||
| GAD-F | GCA ATC GAG TYG TAT CYC TWC AAG GAT | – | – | Taylor et al. (2002) |
| GAD-R II | GCA AGW AGY GGH GCR CAT TTG TG | – | – | This study |
| TaqMan probe | ||||
| Cod | CTT TTT ACC TCT AAA TGT GGG AGG | FAM | Non-fluorescent | Taylor et al. (2002) |
| Haddock | CTT TCT TCC TTT AAA CGT TGG AGG | NED | Non-fluorescent | Taylor et al. (2002) |
| Northern pout | CGC TCG TTT TAC CAA CCA GCT | VIC | Non-fluorescent | This study |
| Whiting | GTT TAT YCC TCT AAA CGT AGG AGG | VIC | Non-fluorescent | Taylor et al. (2002) |
R = AG; Y = CT; K = GT; W = AT; H = AGT.
Probes and primers designed for species-specific identification using real-time PCR.
| Primer or probe . | Primer or probe sequence 5’–3’ . | Reporter . | Quencher . | Reference . |
|---|---|---|---|---|
| Sequencing primer | ||||
| Forward (8.2_L8331) | AAA GCR TYR GCC TTT TAA GC | – | – | Taylor et al. (2002) |
| Reverse (CO3.2_H9236) | GTT AGT GGT CAK GGG CTT GGR TC | – | – | Taylor et al. (2002) |
| TaqMan primer | ||||
| GAD-F | GCA ATC GAG TYG TAT CYC TWC AAG GAT | – | – | Taylor et al. (2002) |
| GAD-R II | GCA AGW AGY GGH GCR CAT TTG TG | – | – | This study |
| TaqMan probe | ||||
| Cod | CTT TTT ACC TCT AAA TGT GGG AGG | FAM | Non-fluorescent | Taylor et al. (2002) |
| Haddock | CTT TCT TCC TTT AAA CGT TGG AGG | NED | Non-fluorescent | Taylor et al. (2002) |
| Northern pout | CGC TCG TTT TAC CAA CCA GCT | VIC | Non-fluorescent | This study |
| Whiting | GTT TAT YCC TCT AAA CGT AGG AGG | VIC | Non-fluorescent | Taylor et al. (2002) |
| Primer or probe . | Primer or probe sequence 5’–3’ . | Reporter . | Quencher . | Reference . |
|---|---|---|---|---|
| Sequencing primer | ||||
| Forward (8.2_L8331) | AAA GCR TYR GCC TTT TAA GC | – | – | Taylor et al. (2002) |
| Reverse (CO3.2_H9236) | GTT AGT GGT CAK GGG CTT GGR TC | – | – | Taylor et al. (2002) |
| TaqMan primer | ||||
| GAD-F | GCA ATC GAG TYG TAT CYC TWC AAG GAT | – | – | Taylor et al. (2002) |
| GAD-R II | GCA AGW AGY GGH GCR CAT TTG TG | – | – | This study |
| TaqMan probe | ||||
| Cod | CTT TTT ACC TCT AAA TGT GGG AGG | FAM | Non-fluorescent | Taylor et al. (2002) |
| Haddock | CTT TCT TCC TTT AAA CGT TGG AGG | NED | Non-fluorescent | Taylor et al. (2002) |
| Northern pout | CGC TCG TTT TAC CAA CCA GCT | VIC | Non-fluorescent | This study |
| Whiting | GTT TAT YCC TCT AAA CGT AGG AGG | VIC | Non-fluorescent | Taylor et al. (2002) |
R = AG; Y = CT; K = GT; W = AT; H = AGT.
Species-specific Taqman qPCR assays for identification of cod, haddock, and whiting previously published by Taylor et al. (2002) were utilized in the present study. Duplex assays, one for cod/whiting and other for haddock/Norway pout, were run on an ABI Prism 7000® sequence detection system (Applied Biosystems) using a single cycle of 37°C for 10 min and 95°C for 10 min, to allow uracil N-glycosylase digestion of previously amplified potential amplicons, followed by 45 cycles of 95°C for 15 s and 60°C for 60 s. All real-time PCRs contained 1 µl of template DNA, 1× SensiMix® (Quantase), 900 nM each primer, 250 nM probe, and DNase/RNase-free water (Sigma) in a final volume of 20 µl. The results of the real-time PCR run were set to read above a default threshold value of 0.2 standard deviations above the mean fluorescence, under passive reference (ROX). A pre-optimized internal positive control (IPC) assay (Taqman Exogenous Internal Positive Control Reagents, Applied Biosystems) was used to check for the presence of inhibitors in the DNA extracted from individual eggs and to distinguish true target negatives from PCR inhibition (Matejusova et al., 2008).
Eggs: data treatment
The proportion of each species identified from the probes was used to apportion the total catch per haul of gadoid eggs to species. Differences in ED frequency composition were examined using Kolmogorov–Smirnov test. The results were mapped using SURFER v9 (Golden Software, Inc., Golden, CO, USA).
Larval distributions
All larvae in all samples were identified to the lowest taxonomic level possible using either Russell (1976) or Munk and Nielsen (2005). Each larva was measured to the nearest 0.1 mm standard length (i.e. from the tip of snout to the end of the notochord) using an ocular micrometer on a binocular microscope. The density of larvae per haul was estimated from the numbers of larvae caught and the volume of water filtered. All densities were subsequently reported as the numbers of larvae per square metre (m−2) by multiplying the density by the maximum depth of the sample.
Potential spawning distributions
The abundance of Norway pout by age class and ICES statistical rectangle for the first quarter (January–March) International Bottom Trawl Surveys (IBTS) of the North Sea for the years 2009 and 2010 was extracted from the ICES database. These data are given as numbers of individuals per 1 h haul. Analyses were limited to age 2 + to represent potential spawning aggregations. The protocols for the IBTS are given in ICES (2010). Syrjala's modification of the Cramér–von Mises test (Syrjala, 1996), performed with the R package ecespa (de la Cruz, 2008), was used to compare the density distributions of Norway pout eggs and age 2 + . The Syrjala (1996) test compares spatial distributions in a way that is insensitive to differences in sample size and has been used previously on aggregated or binned data (e.g. Wright and Begg, 1997; de la Cruz, 2008). As plankton and trawl stations were not taken on identical locations, data were averaged into ICES statistical rectangles (1° of longitude by 0.5° latitude).
Results
Egg identification
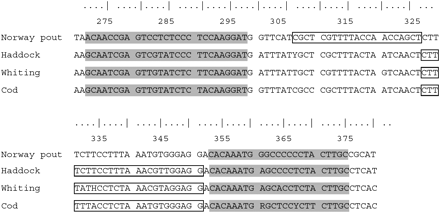
The ATPase sequence obtained from Norway pout was aligned with sequences of 29 other fish species. Figure 1 illustrates the positions of four species-specific real-time PCR primers and probes within a partial multiple alignment of the ATPase 6 subunit at 275–385 nucleotides from the origin of the ATPase gene (accession no. AY091663 used in the comparison). To ensure specificity of the Norway pout probe, it was designed in the region containing four variable positions in comparison with cod, haddock, and whiting, 5‘ upstream from the probes designed by Taylor et al. (2002).
Multiple alignments of the studied species with the positions within ATPase 6 at nucleotides 275–385 from the origin of the ATPase gene. In grey are the positions of the primers, while the positions of the probes are represented by the bordered areas.
Egg survey
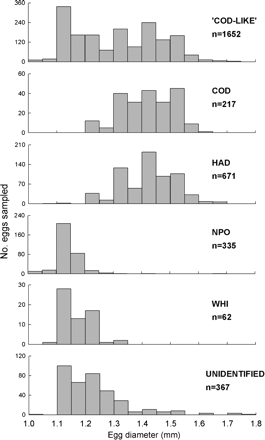
A total of 15 357 pelagic fish eggs were collected over the two separate ichthyoplankton surveys (RV ‘Scotia‘ collected 11 073; ‘G.O. Sars‘ collected 4284) in 2009, out of which 2026 were identified as stage I ‘cod-like’ eggs. A total of 1696 of these ‘cod-like’ stage I eggs were screened using four TaqMan qPCR assays to identify cod, haddock, Norway pout, and whiting. Of the whole number of screened eggs, 1278 (75%) were identified as belonging to one of the four study species. The remaining 418 eggs were classified as ‘unidentified’, as TaqMan assays failed to identify a signal from a probe of one of the four studied species even following checks for extraction and amplification bias across species. Such negative results were due to either a failure in the DNA extraction method or the presence of another species for which specific probes have not been developed. Of the 1696 ‘cod-like’ stage I eggs screened, 12.9% were cod, 39.5% were haddock, 19.4% were Norway pout, 3.7% were whiting, and 24.5% remained ‘unidentified‘ eggs.
The size frequency distribution of eggs identified as Norway pout was 1.03–1.28 mm ED, but was skewed to the higher level of the size range (1.07–1.23 mm; 95% percentiles). The observed ED range for whiting largely overlapped with that of Norway pout eggs screened, ranging from 1.07 to 1.31 mm (1.10–1.28 mm; 95% confidence interval; Figure 2). Despite the overlap in size ranges, there was a significant difference in size distributions between the species (Kolmogorov–Smirnov tests; p < 0.0001), except between cod and haddock (Kolmogorov–Smirnov; p = 0.2, D = 0.09).
Size distribution of stage I ‘cod-like’ eggs from the northern North Sea in February/March 2009.
The mean temperature in the water column where the Norway pout eggs were caught was between 6 and 8°C. Friðgeirsson (1978) gives 3.25 d development time from fertilization to the end of stage 4 at 7.2°C. Using the end of stage 4 as the equivalent of the end of stage I (see Geffen et al., 2006; Geffen and Nash, 2012) used in this study, the eggs identified from DNA were therefore most probably <3.5 d old.
Larvae survey
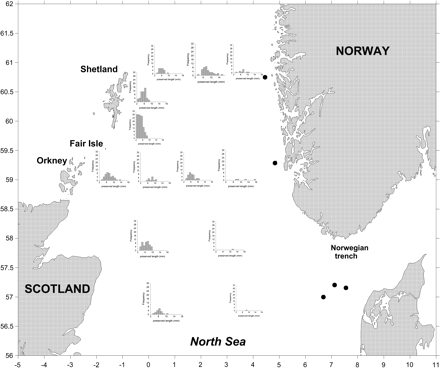
A total of 571 larvae were caught in the northern North Sea survey in April/May, with maximum concentrations of 215 m−2 in the Gulf VII samples and 31 m−2 in the MOCNESS samples. The size range of preserved standard larval lengths for Norway pout was 2.1–17.5 mm for the Gulf VII and 3.7–12.0 mm for the MOCNESS. Where larvae occurred, the general trend was for the smallest mean lengths to occur closer to Shetland with an increase in mean length to the south-east (Figure 3).
Length frequencies (mm, preserved in formalin) of Norway pout larvae in April/May 2010 in the northern North Sea. Black dots indicate stations where no Norway pout larvae were caught.
Comparison of egg distributions with adult spawning and larvae abundances
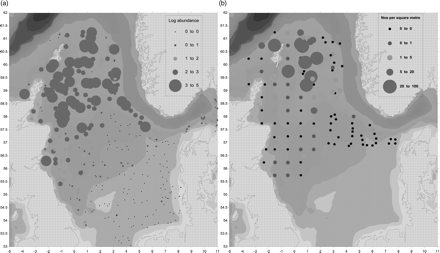
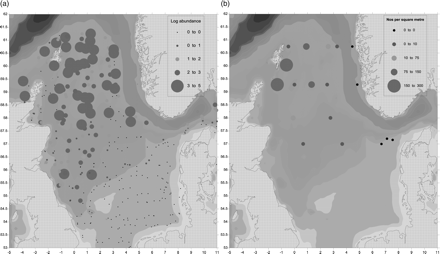
The highest concentrations of adult Norway pout in January/March 2009 were to the east and south-east of the Shetland Isles, with additional elevated concentrations occurring on the edge of the Norwegian trench to the south-west of Norway (see Figure 4a). The highest concentrations of eggs matched the distribution of adults to the east and south-east of Shetland (Figure 4b). Eggs occurred along the western slope of the Norwegian trench; however, the distribution to the east is unknown as no sampling was carried out over the deep water along the Norwegian coast. The Syrjala test indicated that the egg and age 2 + density distributions were similar at the ICES rectangle scale (Ψ = 0.072; p = 0.73).
The distribution of Norway pout (Trisopterus esmarkii). (a) Adults (age 2 + ) and (b)stage I eggs in the northern North Sea in January/March 2009. The size of the dot reflects the abundance on a logarithmic scale. Smallest (black) dots represent a complete absence. Depth contours are shaded.
The pattern of adult Norway pout in January/March 2010 was similar to that of the previous year, again with elevated concentrations to the east and south-east of Shetland and to the south-west of Norway (Figure 5a). Similar to the egg distributions in 2009, the higher concentrations of larvae in 2010 were to the north and west of the survey region in the south and east vicinity of the Shetland and the Orkney Isles (Figure 5b). No larvae occurred in samples in the eastern section toward the entrance to the Skagerrak.
The distribution of Norway pout (Trispoterus esmarkii). (a) Adults (age 2 + ) in January/March 2010 and (b) larvae in April/May 2010 in the northern North Sea. The size of the dot reflects the abundance on a logarithmic scale. Smallest (black) dots represent a complete absence. Depth contours are shaded.
Discussion
The large overlap in gadoid spawning times (Hislop, 1984) together with the inability to distinguish stage I gadoid eggs visually (Russell, 1976) has hampered attempts to monitor spawning distribution and estimate egg production numbers of these species for sometime (see Armstrong et al., 2001; Fox et al., 2005; ICES, 2006). Baranenkova and Khokhlina (1968) specifically report the distribution of Norway pout stage I and II eggs off the north-western coast of Norway; however, they acknowledge that there may be some doubt as to their identification. As such, considerable effort has been undertaken to find other methods for identifying these morphologically identical eggs, including this present study.
Of the five species examined, Norway pout, whiting, and saithe (Pollachius virens) have a similar ED range (0.99–1.17 mm), with cod and haddock being slightly larger (1.32–1.49 mm ED; Friðgeirsson, 1978). The application of development of a TaqMan assay for the identification of Norway pout, whiting cod, and haddock stage I eggs along with probes already developed by Taylor et al. (2002) improves our ability to distinguish between gadoid eggs, avoiding a reliance on the interpretation of morphological features.
The other two species from the same genus as Norway pout, poor cod (Trisopterus minutus) and bib (T. luscus), have similar EDs (0.95–1.03 mm and 0.90–1.23 mm, respectively; Russell, 1976; Alonso-Fernández et al., 2010), occur in the northern North Sea, and spawn approximately over the same period (Wheeler, 1969; Russell, 1976). There is a possibility that these two species, along with saithe, could have made up a large proportion of the ‘cod-like’ eggs in the size range 1.0–1.25 mm that were unidentified by TaqMan.
The areas with the highest Norway pout egg abundance were found to be at Fair Isle and east of Shetland. Since only gadoid eggs between 1.00 and 1.70 mm ED were collected during the 2009 ichthyoplankton survey, and the diameter of Norway pout eggs ranges from 0.92 to 1.20 mm (Munk and Nielsen, 2005), the survey is likely to have underestimated Norway pout egg abundance. However, the importance of being able to identify these eggs was illustrated by the relatively large numbers of the stage I gadoid eggs being positively identified as Norway pout.
Since the stage I eggs were <3.5 d old (development time given in Friðgeirsson, 1978), the distribution of eggs should be consistent with the distribution of spawning. ‘The maximum monthly mean residual currents in the area are approximately 0.15 m s−1, the highest velocities occurring in the surface waters, which would result in a maximum displacement of approximately 13 km per day‘ (M. Skogen IMR Bergen, Norway, pers. comm.). However, the actual displacement may be considerably less since the velocities vary considerably through the water column, and displacement will depend on the depth of the eggs. The distribution of age 2 + Norway pout from the IBTS ( ICES, 2009) was consistent with the concentration of eggs in the ichthyoplankton survey. Accepting that the age 2 + distribution provides information on the probable spawning location of Norway pout, the distribution in 2009 was remarkably similar to that reported in 1981 (Dann, 1983). There were differences in spatial distribution between 2009 and 2010. Although the spatial distribution of age 2 + appeared to contract between 2009 and 2010, the biomass actually doubled (ICES, 2011). Moreover, while 2009 egg production gave rise to one of the largest year classes on record, that in 2010 was close to the lowest (ICES, 2011). While the spatial distribution of spawning and hence egg production may have an influence on recruitment, there are many other potential factors that can affect survival between the two life history stages.
Distribution of larvae in April/May 2010 was consistent with the distribution in 2004 (see ICES, 2007). The distribution of larvae, along with the tendency for larger larvae to be on the eastern side of the distribution, was also consistent with a south and eastward advection from the relatively broad spawning area off Fair Isle and east of Shetland. In 2004, the densities of Norway pout larvae over large parts of the northern North Sea in March ranged from 30 to 146 m−2 (ICES, 2007). These larvae were sampled with a Gulf III high-speed sampler. Given the similarity to the Gulf VII, these densities were comparable with the results from sampling in April/May 2010 where the maximum density was slightly higher (215 m−2). However, it is unknown whether the higher densities reflect interannual differences in abundance, spatial differences related to ‘patchiness’, or simply the time of sampling, with 2010 sampling being later in the year and thus a greater proportion of spawning and hatching had occurred.
There was quite a large range in lengths of larvae (1.3–17.5 mm) in the north-western North Sea in April/May 2010. Friðgeirsson (1978), using development rates for 7.2°C, gives first feeding larvae at 4.5 mm (fresh standard length, assumed since there is no specific mention otherwise) and at 8–9 d after hatching. Therefore, many of the larvae are older than 16 d since spawning (7 d to hatch then 9 d to first feeding, from Friðgeirsson, 1978); however, some larvae were newly hatched and so spawning must have occurred within the last 7 d, suggesting that spawning can occur in this region through to the end of April. In the north-eastern North Sea and in the Skagerrak area over the period 1991–1994, Norway pout larvae were found to have a preserved (in 96% ethanol) standard mean length of 15.4–17.2 mm (Munk et al., 1999). The predominance of smaller larvae in the north-western area in the present study probably reflects a combination of an earlier sampling period and support for the notion that the majority of the spawning is to the north and west, and eggs and larvae drift into the Skaggerak region (see Lambert et al., 2009). The study off the north-western coast of Norway indicated a mean length of Norway pout larvae in April and May of 3.9 and 4.6 mm, respectively (Baranenkova and Khokhlina 1968). These larvae could reflect the later spawning period or cooler water temperatures at these higher latitudes. However, in one June sample, larvae were up to 20 mm in length, which is comparable with larvae lengths seen in May in the North Sea (Munk et al., 1999; this study).
The 1991–1994 study in the North Sea indicated considerable variations in abundance of larvae in the region (Munk et al., 1999) and this could reflect either large variations in survival of eggs and/or larvae or considerable changes in transport. It is unclear which of these is more prevalent. Large interannual variations in Norway pout larvae abundance were also seen to the north-west of Norway (Baranenkova and Khokhlina 1968).
Presumably larvae which are entrained into the Norwegian coastal current, along the Norwegian coastline, will be transported northward. There is little information on the distribution of Norway pout larvae in the area. The studies in the entrance of the Skagerrak (Munk et al., 1999) indicated Norway pout distributions where individuals were more likely to be carried into the Skagerrak rather than transported northward by the Norwegian coastal current. There are populations (although there is no evidence of subpopulations in Lambert et al., 2009) to the north all along the Norwegian coast, through the Lofoten area to the Barents Sea. Eggs and larvae spawned off the north-western coast of Norway are generally transported in toward the Barents Sea (Baranenkova and Khokhlina 1968). Norway pout also occurs around the Faeroe Islands (Lambert et al., 2009). The dynamics and connectivities of these northern populations are less well known than those to the south in the northern North Sea, Skagerrak, and Kattegat.
In conclusion, it is apparent that the principal spawning area of Norway pout in relation to the North Sea is in the north-western region. The distribution of 2 + Norway pout gives an indication of the spawning locations; however, stage I egg productions provide clear details on where the fish are spawning. In addition, this spawning area could be the principal source of individuals that occur in the Skagerrak.
Acknowledgements
The authors thank the skippers and crews of FRV ‘Scotia‘ (Marine Scotland, Science) and RV ‘G.O. Sars‘ (IMR) for their assistance in the collection of samples. Members of the Plankton group at IMR (Julio Erices, Lena Omli, Jon Rønning, and Monica Martinussen) undertook sorting of eggs and larvae from the samples while ‘at-sea’. R.D.M.N. was supported by the IMR North Sea Research Programme.
References
Author notes
Present address: BMT Cordah Ltd., Scotstown Road, Bridge of Don, Aberdeen AB23 8HG, UK.
Present address: Genesis Oil & Gas Consultants Ltd, 6 Albyn Place, Aberdeen AB10 1YH, UK.
Handling editor: Claire Paris