-
PDF
- Split View
-
Views
-
Cite
Cite
Lucía López-López, Izaskun Preciado, Begoña Villamor, Francisco Velasco, Magdalena Iglesias, Enrique Nogueira, Jose Luis Gutierrez-Zabala, Ignacio Olaso, Is juvenile anchovy a feeding resource for the demersal community in the Bay of Biscay? On the availability of pelagic prey to demersal predators, ICES Journal of Marine Science, Volume 69, Issue 8, September 2012, Pages 1394–1402, https://doi.org/10.1093/icesjms/fss117
Close - Share Icon Share
Abstract
The role that juvenile anchovy (Engraulis encrasicolus) play as a food resource for the demersal community in the southern Bay of Biscay is assessed using 21 years of anchovy abundance data and demersal predator diets. During the study period, a total of 26 fish and elasmobranch species preyed on anchovy either frequently or occasionally. Predators with a crustacean-based diet targeted the smaller anchovy individuals. The size range of anchovy juveniles (centred at 7.5–8.9 cm) was comparable to that of the largest nektonic–benthic crustaceans, but generally smaller than other demersal and pelagic fish prey. Hake (Merluccius merluccius) and megrim (Lepidorhombus whiffiagonis) were the predators that consumed the highest number of anchovy, one of the main prey items driving the variability of their diets. Anchovy consumption conformed only partially to the abundance of anchovy in the southern Bay of Biscay, suggesting that factors other than abundance might condition its availability to demersal predators. Prey size could be one of them, as the size of the anchovy preyed on proved to be significantly smaller than the individuals collected with bottom trawls. However, other factors, such as the vertical position of the shoals of anchovy juveniles, could also constrain anchovy availability to demersal predators.López-López, L., Preciado, I., Villamor, B., Velasco, F., Iglesias, M., Nogueira, E. Gutierrez-Zabala, J. L., and Olaso, I. 2012. Is juvenile anchovy a feeding resource for the demersal community in the Bay of Biscay? On the availability of pelagic prey to demersal predators. – ICES Journal of Marine Science, 69: .
Introduction
Much effort has been devoted during recent decades to understanding how trophic interactions shape population dynamics. Cascading effects in marine ecosystems following the collapse of large predators are well documented (Frank et al., 2005; Casini et al., 2009; Baum and Worm, 2009), and the bottom–up effect that fluctuations in mid-trophic fish can exert in the top predator guilds has also been proven (Frederiksen et al., 2006; Luczak et al., 2011). However, most foodweb studies obviate the dynamism of the interactions, and thus the adaptability of the foodweb to changing conditions, since they are based either on a snapshot of the interactions at particular moments or on integrations over certain time-periods (Moloney et al., 2011).
In coastal environments, small pelagic fish play a fundamental role in the benthic–pelagic coupling since they link plankton production and demersal predators (Palomera et al., 2007). Anchovy species are recognized keystone species in upwelling regions (Libralato et al., 2006) and in highly productive temperate seas (Somarakis and Nikolioudakis, 2007). Their key role is achieved through their importance as a food resource for a great variety of organisms, from seabirds (Okes et al., 2009; Luczak et al., 2011) to large pelagic fish (Lezama-Ochoa et al., 2010), demersal fish (Preciado et al., 2008; Byron and Link, 2010), and jellyfish (Sabates et al., 2010), which feed on different anchovy life stages.
In the Bay of Biscay, the European anchovy (Engraulis encrasicolus) not only plays an important role as a forage fish in structuring the ecosystem, but also constitutes an important fishery resource. Recent years have seen fluctuations of the stock, related to both changes in environmental variables and fishing pressure (Borja et al., 2008). The anchovy fishery remained closed between 2006 and 2009 due to the low biomass level and the failure of the fishery (ICES, 2010). However, there is high variability in the annual anchovy stock biomass due to natural recruitment variability (Uriarte et al., 1996).
The distribution of European anchovy in the Bay of Biscay spans from shelf to oceanic areas. The southern part of the Bay is characterized by a narrow continental shelf and marked environmental gradients. It has been suggested that the complex oceanography of the region favours anchovy recruitment success through a spatial loophole, where early stages are advected off the shelf, thus avoiding high predation pressure, while juveniles return to the shelf during autumn, withstanding countercurrents, once they have achieved a sufficient size (Uriarte et al., 2001; Irigoien et al., 2007). During this cross-shelf migration, young-of-the-year anchovy might be exposed to demersal predators living on the continental shelf. The degree to which juvenile anchovy contribute to the diet of the demersal fish community in the Bay of Biscay is, however, still unclear (Olaso, 1990; Velasco and Olaso, 1998; Preciado et al., 2008).
In this study, we focus on young-of-the-year European anchovy in the southern Bay of Biscay (Cantabrian Sea) aiming to determine their role as forage fish for the demersal fish community, and whether the strong anchovy fluctuations in recent decades have had an effect on the diet of demersal predators. To this end, we investigate the relationship between anchovy abundance and its availability to the demersal community, hypothesizing on possible factors affecting this relationship.
Methods
Data sources
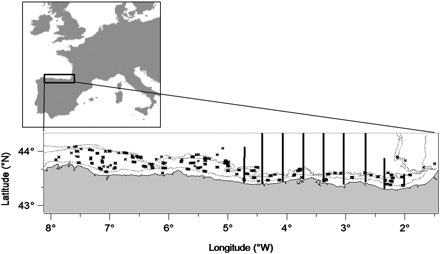
Bottom trawl surveys for the assessment of demersal and benthic stocks (“Demersales” surveys) were conducted every autumn from mid- September to October between 1990 and 2010 on soft bottoms of the Cantabrian Sea continental shelf, between 1 and 9°W (Figure 1). Trawling operations were carried out during the daytime using a “Baka” 44/60 net with 20 mm mesh in the codend. The tows lasted for ca. 30 min at a constant speed of 3 knots. The survey design followed a stratified random sampling scheme covering the depth range 70–500 m. For this work, we focused on the depth strata 70–120 m and 120–200 m, where anchovies are normally found. A few special tows were also performed at shallower depths (<70 m) and considered in this study. After each haul, the catch was separated into species. Anchovy specimens were measured (total length to the lower centimetre), and the total weight of the anchovy catch was recorded. From each potential predator species, a maximum of ten specimens were randomly selected from each haul and set aside for extended biological sampling. The sampling effort was higher for Merluccius merluccius, Lepidorhombus whiffiagonis, and L. boscii, from which a maximum of ten individuals per size category were sampled (categories comprised size ranges of 5 cm).
Location of the study site. The locations of bottom trawls are marked with crosses (“Demersales” survey), while the vertical lines indicate the position of the acoustic transects (“Pelacus10” surveys).
As part of the extended biological sampling, quantitative diet estimates were obtained by measuring the stomach contents using a trophometer (Olaso, 1990), which consists of a set of graduated half-cylinders of different diameters for measuring the volume of the stomach pellet. For each stomach, prey were separated, identified to the lowest taxonomic level, and measured (to the lower millimetre) whenever possible. In the case of partly-digested prey, hard structures, such as exoskeletons for crustaceans, or otoliths for fish, were used for identification; however, some specimens had to be grouped as unidentified fish (UNID <10% of the total prey). The volume percentage occupied by each prey item in the total stomach content volume was visually estimated. All dissections and identification of stomach contents were carried out on board. Fresh prey or any items presumably consumed in the net were excluded from the analysis.
The one-year-old anchovy abundance and recruitment series were obtained from the analytical assessment of the total stock (anchovy of the Bay of Biscay) for the period 1991–2011 (ICES, 2011). Additionally, acoustic surveys were carried out on board R/V “Thalassa” in the Bay of Biscay (between 5°W and 2°W) every autumn (September–October) between 2006 and 2009 to estimate the biomass of small pelagic fish, with anchovy (young-of-the-year plus adults) as the target species (“Pelacus10” surveys). Acoustic transects were run perpendicular to the coast between 30 and 2000 m depth, with an intertransect distance of 15 nautical miles. Pelagic trawls were conducted in those areas where singular echotraces were recorded using a “Pelágico” 72/76 net with 20 mm mesh in the codend. These fishing operations aimed to identify the species composition and size structure of the observed shoals in order to divide the recorded acoustic energy for the biomass assessment of the pelagic fish community. The catch was sorted by species, weighed, and all specimens were measured to the lower 0.5 cm (total length).
Age–length keys estimated from these surveys (Demersales and Pelacus10) were applied to anchovy length distributions and stomach contents to obtain age distributions (data not shown). Similar information to Pelacus10 for autumn (September) of 2003–2005 and 2010 was obtained from the reports of the acoustic Juvena surveys, which aimed to estimate the biomass of anchovy (young-of-the-year plus adults) (Boyra et al., 2005, 2006, 2007, 2008).
Statistical analysis
We determined the number of anchovy consumed per predator and year to obtain a clear picture of the changes in anchovy consumed during the study period (1990–2010). Differences in the size of consumed anchovy between piscivorous predators and predators with a crustacean-based diet were assessed using a Kruskal Wallis test. To identify changes in the predation on anchovy during the predators' lifespans, we used predator size ranges from the literature (Velasco, 2007) and ontogenetic dietary shifts previously determined by clustering predator lengths based on their diet. Preliminary analysis showed that predator diet was not density-dependent, specifically regarding predation on anchovy, and thus we did not account for predator density in our analyses. The size distribution of the items in the diet was calculated per predator and ontogenetic group in order to compare it with the size of anchovy juveniles. Using the first and third quartiles of the anchovy size distribution, we identified those prey items with similar size that could occupy the same trophic guild as anchovy, and thus act as anchovy surrogates. The habitats for these species were extracted from the online databases SeaLifeBase (SeaLifeBase, 2011) and FishBase (FishBase, 2011), while their maximum recorded lengths were obtained from the bottom trawl “Demersales” surveys database.
To assess the role of anchovy in triggering shifts in the predator diets, years were classified by their prey assemblage for the species with a higher consumption of anchovy (Lepidorhombus whiffiagonis and Merluccius merluccius). As prey volume is highly dependent on predator size, we standardized the volume of each prey species by predator weight (partial fullness index), thus eliminating the overrepresentation of the larger fish diet in the prey matrix. Subsequently, this standardized volume was annually averaged by the number of predators (with no zero repletion), to eliminate the effect of annual differences in predator abundance. In order to eliminate noise in the prey matrix, those prey species whose relative abundance was lower than 0.01% in all years or which occurred only during one year were eliminated. The number of dimensions of the prey assemblage was further reduced by applying a principal component analysis, from which only the two first components were retained. Species contributing more than 0.05 (in absolute values) to the loadings were identified as the main drivers of diet variability.
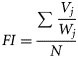
The relationship between consumed anchovy and the abundance of anchovy juveniles was assessed using the total abundance of spring recruits in the Bay of Biscay, the abundance of juveniles estimated from the bottom trawls, and the anchovy consumption by the demersal community. We also computed, using the Pearson correlation test, the relationship between anchovy in the diet of demersal predators and the abundance of anchovy in hauls at each sampling station from the bottom trawl survey.
As anchovy in the southern Bay of Biscay show high abundance variability along a zonal gradient in the Cantabrian Sea (Uriarte et al., 2001), we analysed the interannual variation and the accumulated frequency of anchovy consumption for the entire study period along this zonal gradient. Since the size of juvenile anchovy could influence its availability as prey for the demersal community, we computed the zonal differences in the size of juvenile anchovy using data from the acoustic surveys (2003–2010), the demersal trawling (1990–2010), and the fish diet analysis (1990–2010) along the zonal gradient in the Cantabrian Sea.
All statistical analysis was performed with the software R for mathematical and statistical computing R 2.13.2 (R Development Core Team, 2011).
Results
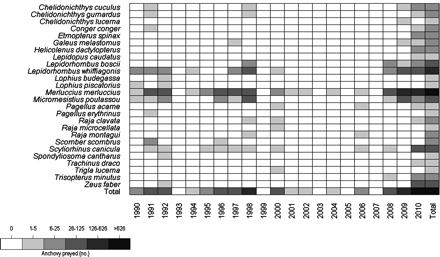
A total of 26 fish and elasmobranch predators consumed anchovy either frequently or occasionally during the study period (Figure 2). Nevertheless, its importance as a food resource for demersal predators showed remarkable annual variability, with high consumption in 1991–1992, 1996–1998, and 2000, and exceptionally high consumption in 2010. The proportion of predators that consumed anchovy also increased during these periods. Hake (Merluccius merluccius) and megrim (Lepidorhombus whiffiagonis) consumed the highest number of anchovy (Figure 2). Anchovy consumption in the diet of these two main predators took place across ontogenetic categories, with the exception of juvenile megrim (<16 cm in length) that did not consume anchovy. The relative importance of juvenile anchovy in hake juveniles (<18 cm), medium hake (18–35 cm), and large hake (>35 cm) was 34.15, 7.49, and 21.03%, respectively. In the case of megrim, the importance of anchovy in the different ontogenetic categories was 15.88 (16–23 cm), 30.57 (24–36 cm), and 34.00% (>36 cm). The volumetric importance of juvenile anchovy in the diet of these species ranged annually between 0 and 15% in hake and between 0 and 31% in megrim.
Annual and decadal consumption of anchovy (number of specimens) by demersal predators.
Regardless of the predator, the bulk of anchovy consumption (>75%) was found to occur between sunrise (approximately 06:00 UTC) and 08:00 UTC independently of the digestion state, pointing to a time-constrained consumption of anchovy, preferentially during night and early morning.
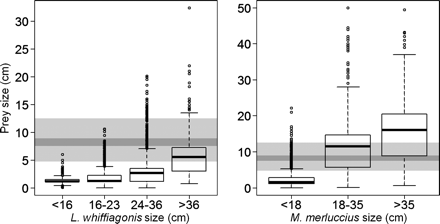
The size of anchovy preyed upon in this study corresponded to age 0, as identified with the age–length keys. Its size distribution ranged between 2.0 and 15.0 cm, with the first and third quartiles of the distribution being 7.5 and 8.9 cm, respectively. Comparing the size of the anchovy juveniles with the size of other items in the predator diets, clear differences between hake and megrim arose. In the case of megrim, the size of anchovy juveniles was larger than the average size of other prey items (Figure 3). Only the group of large megrim adults (≥35 cm) consumed other prey items in a size range similar to the size range of anchovy juveniles. On the other hand, hake juveniles (<18 cm) generally consumed prey smaller than anchovy, while in the two categories of larger hake (≥18 cm), anchovy juveniles taken as prey were generally smaller than other prey items consumed (Figure 3). On expanding this size analysis to the entire predator community, piscivorous predators (i.e. M. merluccius, L. whiffiagonis, Zeus faber) consumed larger anchovy juveniles than the predators that generally fed on crustaceans (i.e. Lepidorhombus boscii, Raja montagui, Chelidonichthys cuculus), with an average difference of 5 cm (d.f. = 159.212; p < 0.01).
Size distribution of all prey items consumed by the different ontogenetic groups of the main anchovy predators (Lepidorhombus whiffiagonis and Merluccius merluccius). The size of the young-of-the-year anchovy preyed upon is represented as a grey band in the background of the plots; the light grey band indicates the total range of the consumed anchovy size, and the dark grey indicates the area between the first and third quartiles in this anchovy size distribution.
Only a limited number of prey in the demersal community had a size range overlapping the size range of anchovy juveniles, making them a suitable substitute in the absence of anchovy. They consisted mostly of small demersal fish, such as Argentina sphyraena, Callionymus spp., Gaidropsaurus macrophthalmus, and small pelagic fish, such as Gadiculus argenteus or small Trachurus trachurus. The size distribution of the largest demersal shrimp, such as Solenocera membranacea or Chlorotocus crassicornis, also overlapped with the size of anchovy juveniles. Most of the fish species which were also consumed by demersal predators in the size range 7.5–8.9 cm were small individuals of the prey species, as their maximum length was generally much larger (FishBase, 2011; SeaLifeBase, 2011; F. Velasco, pers. comm.).
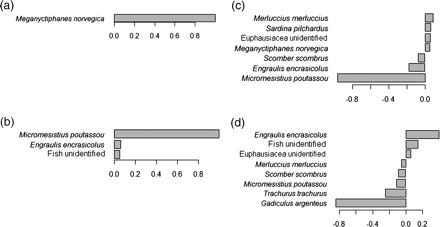
The PCA analysis identified the main prey species responsible for the variability in the predator diets during the study period (Figure 4). The first PCA of the Lepidorhombus whiffiagonis diet, which explained 88.0% of the diet variability, was almost uniquely driven by the euphausiid Meganyctiphanes norvegica, and the second PCA (11.2% of the variability) by blue whiting. Although these two prey items controlled the variability in the L. whiffiagonis diet, anchovy was the third prey item in order of importance (Figure 4). In the diet of Merluccius merluccius, the number of contributors to the diet variability was much higher, and anchovy was well represented in both the first and the second PCA (explaining 55.4 and 14.6%, respectively, of the diet variability), occupying the second position in both components after Micromesistius poutaussou and Gadiculus argenteus, respectively.
Prey species with the highest contribution (loadings >0.05) to the diet variability of Lepidorhombus whiffiagonis as resulting from the first component (a) and second component (b) of the principal component analysis of the species diet. Similarly, prey species which contribute to the diet variability of Merluccius merluccius as resulting from the first (c) and second (d) components of the principal component analysis of the species diet.
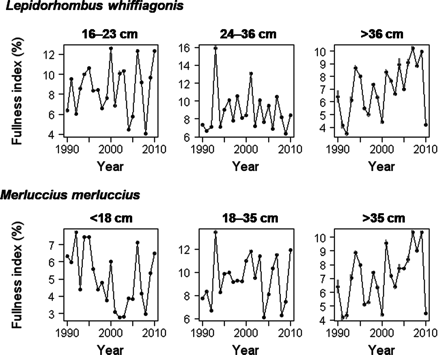
The fullness index of M. merluccius and L. whiffiagonis showed high variability during the study period (Figure 5), ranging from 3 to 16%. Although trends during the two decades could not be recognized, there was a remarkable resemblance in the variability of the fullness index between the largest ontogenetic group of megrim and of hake (Figure 5). High correlations were found between the annual fullness index of the last ontogenetic group in hake and megrim (r = 0.96; p < 0.01). In these two groups, anchovy consumption seems to have coincided with periods when the fullness index decreased, such as in 1991, 2000, and 2010 (Figure 5). Between the second group of hake and the third group of megrim, a similar pattern occurred (r = 0.69, p < 0.01). The time-series of fullness index of the smaller size classes were, however, not significantly correlated. In the smaller size classes, positive peaks occurred in 1991, 2000, and 2010 (Figure 5), suggesting opposite trends between the fullness of large and small predators in years of high anchovy consumption.
Average annual fullness index of the ontogenetic groups of Lepidorhombus whiffiagonis and Merluccius merluccius which prey on juvenile anchovy. The standard error of the mean is represented by a grey bar behind each individual value.
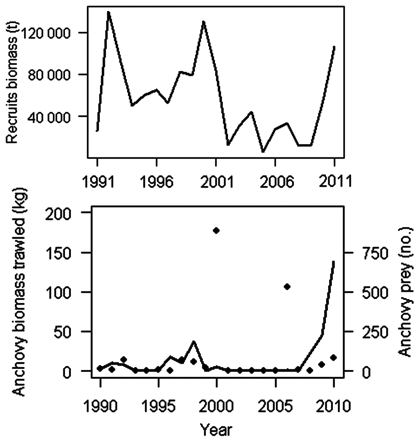
The occurrence of anchovy in predator diets conforms to its abundance in bottom trawl hauls (Pearson correlation = 0.66; p = 0.002), except in 2000 and 2006 when abundance of anchovy in the hauls was remarkably high in comparison to its presence in the diet of the demersal fish community (Figure 6). For each sampling station, there was, however, no significant relationship between anchovies consumed and anchovies trawled (r2 = 0.001). Although the peaks in recruitment were related to increased consumption of anchovy juveniles in the previous year, as happened in 1991–1992, 2000–2001, and 2010–2011 (Figure 6), the general resemblance between anchovy consumption and Bay of Biscay recruits from the analytical assessment (one-year-old anchovy the following year) was low (Figure 6). This suggests that factors other than anchovy abundance might be controlling anchovy consumption by demersal predators.
(a) Estimated abundance of anchovy recruits in the Bay of Biscay, and (b) abundance of anchovy juveniles in the Bay of Biscay estimated in biomass by demersal trawls (points) and number of anchovy juveniles found as part of the diet of demersal predators (solid line).
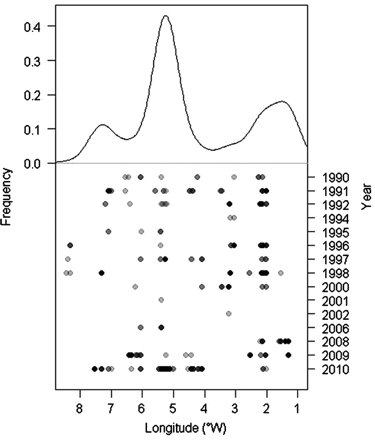
Anchovy consumption varied longitudinally in the Cantabrian Sea, with two relative maxima at ca. 5 and 2°W (Figure 7). The major contribution to these maxima corresponded mainly to anchovy consumed during the last three years of the series (2008–2010) (Figure 7). During the study period, anchovy consumption was noted preferentially between 2 and 7°W, although important interannual variability occurred (Figure 6).
Frequency of occurrence of anchovy juveniles in the diet of demersal predators integrated over the 21 years of study on the Cantabrian shelf (between 9 and 1°W) (upper panel). Interannual variation of the frequency in anchovy predation on the Cantabrian shelf (lower panel). The greyscale of the points reflects the number of anchovy found as prey in a particular location and year.
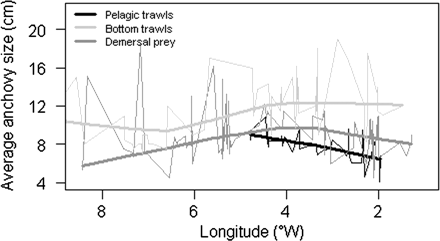
Comparison of the size of anchovy juveniles in the Cantabrian Sea highlighted the significant differences between sampling methods and focus of the surveys used (i.e. bottom trawl vs. acoustic surveys). Although there was relatively high variability among fishing locations for a given sampling method, the general trend was that the largest anchovies were caught with bottom trawl, and that anchovies found in the demersal predator diets were generally smaller. However, juvenile anchovies sampled in the pelagic surveys were still smaller than those sampled by the other two methods (Figure 8).
Spatial differences in size of anchovy juveniles in the Cantabrian Sea estimated from pelagic hauls (black line), bottom trawls (light grey line), and anchovy prey found during the stomach content determination of demersal predators (dark grey line). The thin lines correspond to average values for a particular location, while the thick lines correspond to a lower pass filter applied to the previous in order to identify the general tendency.
Discussion
Anchovy species account for a substantial portion of the diet of demersal predators (Byron and Link, 2010). Specifically, European anchovy is recognized as an important food resource for hake throughout European basins (Bozzano et al., 1997; Carpentieri et al., 2005; Mahe et al., 2007). Our study shows the importance of juvenile anchovy as a food resource for hake in the southern Bay of Biscay. In the Celtic Sea and the northern Bay of Biscay, hake preferentially consumed conspecifics and small clupeid fish, such as Engraulis encrasicolus, Sardina pilchardus, and Argentina sphyraena, while selecting negatively Trachurus trachurus and Micromesistius poutassou, despite their high occurrence in the environment (Mahe et al., 2007). A preference for anchovy over other suitable prey species could stem from the higher energy content that clupeids have relative to gadoids, as suggested by Pinnegar et al. (2003). However, some other factors should not be overlooked; for instance, the abundance of clupeid fishes might be underestimated when sampling with a bottom trawl net due to their primarily pelagic life style (Preciado et al., 2008). In addition, size is an important variable characterizing predator diets (Preciado et al., 2008), as our study also shows.
Seasonal cycles can also exist for consumption. Different size groups of hake change their preferences toward anchovy seasonally. Large hake feed on anchovy during spring, while during autumn, both small and large hake have a significant proportion of anchovy in their diets (Olaso, 1990; Bozzano et al., 1997). Anchovy, therefore, might not have the same significance for the demersal community diet during the entire year. In early autumn, the particular size of anchovy juveniles allows other demersal predators, such as megrim and blue whiting, to consume it. However, in spring, anchovy might have already achieved a size too large to be eaten by megrim, blue whiting, or small hake. In the central Mediterranean Sea, anchovy was found in the diets of hake ranging in length from 11 to 90 cm, attaining greater relevance in the diet of 11–15.9 cm hake (Carpentieri et al., 2005). This suggests that some of the anchovies consumed consisted of young-of-the-year individuals.
Some other studies on the diet of European hake have not identified anchovy as an important food item (Velasco and Olaso, 1998; Cartes et al., 2004). Nevertheless, these studies were based on a different approach, with intensive sampling effort during shorter time-periods. Due to the high variability both in anchovy abundance and anchovy consumption, it is perfectly feasible that anchovy did not occur during a particular year in predator diets, notwithstanding their importance as a food resource when considering longer time-periods. Cartes et al. (2004) did not find anchovy in the diet of hake during late spring, when sardine was an important prey item instead. As sardine and anchovy have temporally segregated spawning periods in the Gulf of Lion (Olivar et al., 2001), the consumption of sardine in late spring could respond to a size constraint, and be replaced by anchovy consumption later in the year.
Regarding megrim, the second-most important predator of anchovy in our study area, studies on its feeding ecology are scarce. Santic et al. (2009) studied megrim diet in the eastern-central Adriatic, but did not observe any anchovy. In the Adriatic Sea, however, anchovy spawning sites are located in the northern and western continental shelves (Regner, 1996), and, therefore, the study site of Santic et al. (2009) was out of the migration area of anchovy juveniles. In our study site, megrim fed exclusively on suprabenthic and demersal prey, except anchovy. This suggests that megrim are not highly mobile in the water column.
Similarities concerning the fullness index of hake and megrim did not stem directly from their predation on anchovy, but possibly were influenced by other common prey, such as blue whiting and other small demersal and pelagic fish. The role of anchovy in the diet differed for large and small predators. Years with strong anchovy consumption seemed to decrease the stomach fullness of large hake (whose prey are generally larger than juvenile anchovy), while increasing the fullness in small megrim and hake (which generally prey on smaller items). However, we cannot rule out the importance of other major prey (such as blue whiting) in driving the variability in the predator fullness indices.
Several factors support the importance of anchovy juveniles as a food resource for demersal predators, among which we can highlight their suitable size, high energy content in comparison with other small fish (Spitz et al., 2010), and short reaction distance in the presence of a predator (Scharf et al., 2003). The latter characterizes clupeid fish, which allow predators to get close before initiating an escape response (Scharf et al., 2003). As predators tend to target the largest fish they can handle (Pinnegar et al., 2003), the low reaction distance of anchovy could favour its consumption by predators that generally prey on smaller items. Nevertheless, selection by predators might rely on physiological traits other than size. Takasuka et al. (2007) showed that some predators specifically target slow-growing larvae independently of their actual size. Slow-growing individuals might have lower potential for antipredator behaviour and might be isolated from the shoals due to their poorer condition, thus increasing the risk of predation. Larger individuals would also have better swimming condition and a faster response in the presence of a predator.
Our study demonstrated that not only did piscivorous fish benefit from juvenile anchovy, but also fish that generally fed on crustaceans. The fact that anchovy consumption simultaneously increased during certain years by predators with different diets speaks against considering anchovy as a substitute in times of shortage of other prey, and points to a higher availability of anchovy to the demersal community during these years. However, anchovy consumption by demersal predators did not totally comply with anchovy abundance in the southern Bay of Biscay. Prey consumption is known to be both a function of prey abundance and prey–predator overlap both in space and time. In the case of pelagic species, not only the geographical overlap, but also the vertical positioning of predator and prey can affect the relationship. In our study, several predators with benthic and benthic–demersal diets (such as the four-spotted megrim, Lepidorhombus boscii) consumed anchovy with a relatively high frequency during the study period. This suggests that juvenile anchovy can get sufficiently close to the bottom to become suitable prey for these predators. Uriarte et al. (2001) studied juvenile anchovy aggregations during 1998 and 1999 and found the majority of the shoals to be in the upper 25 m of the water column, mainly associated with less saline water off the shelf. Although daily vertical migration of juvenile anchovy shoals occurred to some degree, with a shallower and more dispersed distribution at night, they were not found under 40 m depth in any case. Once over the continental shelf, anchovy start displaying circadian migrations, aggregating in tight shoals close to the sea bottom during the day then dispersing and ascending towards the surface at night. Massé et al. (1996) showed significant percentages of anchovy in shoals that were <10 m from the bottom during 1989–1994. The dispersion at night could increase anchovy vulnerability to predation (Hoare et al., 2000 and references therein), concurring with the higher anchovy consumption we found in early morning. However, interannual variability in the vertical position of the shoals could also be a factor limiting anchovy availability to demersal predators. Variability in the depth of the shoals could depend on the size of the aggregations and also on climate conditions, as they define the seasonal thermocline (Muiño et al., 2003). In the southern Bay of Biscay, there is high interannual variability in the upwelling index (Alvarez et al., 2011), which causes differential distribution of temperature in the water column. The upwelling intensity in the southern Bay of Biscay has been suggested as affecting anchovy recruitment success (Borja et al., 2008), and could also be one of the factors affecting the depth positioning of E. encrasicolus juvenile shoals.
We found significant differences between the size of juvenile anchovy obtained from the acoustic surveys, bottom trawling, and stomach content determination. The acoustic surveys returned a lower size for juvenile anchovy than the other two methods; however, while bottom trawling and thus diet determinations took place strictly on the shelf, the acoustic transects extended to the continental slope. We cannot rule out the possibility that the size differences stem from this spatial mismatch, since acoustic estimates corresponded mainly to the outer edge of the continental shelf (data not shown), and thus to anchovies that had just started their cross-shelf migration from the oceanic area and outer shelf to the inner shelf. Differences in size were, however, exceptional between anchovies preyed upon and those caught with the bottom trawl. While anchovies in the hauls were close to the 10-cm size reported by Uriarte et al. (2001) as the size at which juvenile anchovies would start their cross-shelf migration, the size of the prey was smaller. Size-selective mortality can be due to different constraints, such as mouth gape-size limitation, behavioural selection, or reduced escape capability of smaller prey (Sogard, 1997). In addition, smaller individuals within a shoal might experience stronger foraging competition due to their higher metabolic requirements and lower competing ability, compromising at times their antipredator behaviour in favour of an increasing foraging prospect (reviewed in Hoare et al., 2000).
Interannual variation in juvenile anchovy size (Villamor et al., 2011) could thus condition anchovy consumption by demersal predators. These size fluctuations could respond to variability in peak spawning due to water temperature (Bellier et al., 2007), upwelling intensity, or interspecific competition when there is a high abundance of juvenile anchovies. In addition to annual fluctuations in size, the spatial distribution of anchovy juveniles in the Cantabrian Sea also exhibits high interannual variability (Uriarte et al., 2001). Our results demonstrate agreement between extension of the area in which predation on anchovy was found and total abundance of anchovy recruits the following year (recruitment). This association could reflect the spatial shrinkage that occurs at small population levels in pelagic fish (Bertrand et al., 2004). Thus, in years of high recruitment, the population would be likely to be distributed over the entire continental shelf in the Cantabrian Sea.
Advection of larvae and cross-shelf migration of juveniles seem to be widespread in Engraulis encrasicolus in European waters. In the Gulf of Lion, larvae are advected offshore following the low salinity plume of the River Rhone outflow and a southward track of eddies originating in the Gulf of Lion (Sabates et al., 2007). Similarly, in the northern Adriatic Sea, there is a transport of post-larvae offshore influenced by the outflow of the River Po (Santojanni et al., 2006). The migration of juveniles to coastal areas seems thus to be a common feature across anchovy spawning sites (Agostini and Bakun, 2002), and the consumption of these potential prey by demersal predators might be similarly widespread in European waters.
Interannual variability in anchovy abundance and availability might not strongly affect predator diets, as most demersal predators have a wide trophic niche and demonstrate high adaptability to environmental prey abundance (Scharf et al., 2000). Nevertheless, predation on anchovy juveniles by demersal fish contributes to the natural mortality of the species, together with predation on all anchovy life stages that is well studied in the pelagic realm (Okes et al., 2009; Lezama-Ochoa et al., 2010; Sabates et al., 2010; Luczak et al., 2011).
Acknowledgements
The authors are grateful to all the scientific staff and crew participating in the “Demersales” and “Pelacus 10” surveys, especially all the researchers who participated in the trophic ecology team during the study period. We thankfully acknowledge the work of Pilar Pereda, who was responsible for the Fisheries area of the IEO (2003–2011) and who made possible the participation of LL-L in the “Demersales” surveys. We thank the researchers involved in the development and maintenance of the online databases SeaLifeBase and FishBase. We are grateful to Jorge Landa for providing guidance and information regarding megrim (L. whiffiagonis) ecology, and to Sylvain Lenoir for useful comments on an early version of the manuscript.
References
Author notes
†Present Address: IEO Centro Oceanográfico de Santander, Promontorio San Martín s/n, PO Box 240, 39080 Santander, Spain
Handling Editor: Emory Anderson