-
PDF
- Split View
-
Views
-
Cite
Cite
A. Le Port, S. Lavery, J. C. Montgomery, Conservation of coastal stingrays: seasonal abundance and population structure of the short-tailed stingray Dasyatis brevicaudata at a Marine Protected Area, ICES Journal of Marine Science, Volume 69, Issue 8, September 2012, Pages 1427–1435, https://doi.org/10.1093/icesjms/fss120
Close - Share Icon Share
Abstract
Elasmobranch (shark, ray, and skate) populations around the world are in decline, and effective conservation measures are urgently needed. Marine Protected Areas (MPA) placed in locations important for key life-history stages may form part of an effective conservation strategy. In this context, we examined the seasonal abundance and population structure of the short-tailed stingray (Dasyatis brevicaudata) at an offshore MPA in northeastern New Zealand, and the reported use of this location as a mating ground. Diver surveys were conducted from 2004 to 2007 at the Poor Knights Islands Marine Reserve (PKIMR). During this time, we observed: (i) a substantial increase in adult and subadult numbers, particularly females during the suggested breeding season, and a corresponding increase in females bearing fresh mating scars; and (ii) large numbers of smaller (probably immature) D. brevicaudata individuals of both sexes from spring to autumn. These results suggest that the PKIMR acts as both a mating aggregation location and a nursery for this species. We suggest that for coastal stingrays such as D. brevicaudata, small MPAs may be effective at protecting key life-history stages, but that as movements outside of reserve boundaries also occur, additional management tools may also be necessary.Le Port, A., Lavery, S., and Montgomery, J. C. 2012. Conservation of coastal stingrays: seasonal abundance and population structure of the short-tailed stingray Dasyatis brevicaudata at a Marine Protected Area. – ICES Journal of Marine Science, 69: .
Introduction
Marine Protected Areas (MPAs) have the potential to elevate the biodiversity, abundance, and size of exploited species within their boundaries (Denny et al., 2004; Russ et al., 2004; Lamb and Johnson, 2010), as well as offering benefits to adjacent fisheries through spillover and recruitment subsidies (Roberts et al., 2005; Jones et al., 2009). Although elasmobranch fishes (sharks, rays, and skates) are increasingly threatened by overexploitation and habitat degradation around the world (Speed et al., 2010; White and Kyne, 2010), little has been done to address the effectiveness of MPAs as a tool for their conservation (Chapman et al., 2005; Carraro and Gladstone, 2006; Robbins et al., 2006; Heupel et al., 2010; Wiegand et al., 2011; Knip et al., 2012). This is mostly due to the commonly held perception that elasmobranchs, and especially sharks, are long-distance roamers that would receive little benefit from the often limited size of MPAs. However, in actuality, there is a paucity of long-term species-specific movement and habitat use data (Bonfil, 1999).
Studies that have evaluated the conservation benefits of MPAs for elasmobranchs have focused on sharks and suggest that currently, MPAs have varying levels of success in protecting exploited populations within their boundaries (Bonfil, 1999; Chapman et al., 2005; Carraro and Gladstone, 2006; Robbins et al., 2006; Heupel et al., 2010; Knip et al., 2012). It is possible, however, that MPAs can be effective management tools for sharks if the spatial and temporal dynamics of targeted species are taken into account when designing MPAs. The first step towards achieving these goals is to increase our understanding of the biological characteristics (i.e. scale and timing of movements, key life-history areas, and level of site attachment) of threatened elasmobranchs. For rays, most of these aspects remain poorly understood, despite an increasing number of ray species being recognized by the IUCN Red List of Threatened species as ‘Vulnerable’ or ‘Near threatened’ worldwide (IUCN, 2011).
Little is known of the movement patterns of rays, but available data suggest that similar to coastal-dwelling sharks, they exhibit some degree of repeatability of movements to and from specific areas, although the scale of these movements may be smaller (Vaudo and Lowe, 2006). These movements, and the areas used, have been linked to reproductive behaviours (Gray et al., 1997; Hoisington and Lowe, 2005; Vaudo and Lowe, 2006), foraging (Dewar et al., 2008), predator refuges (Vaudo and Heithaus, 2009), and seasonal changes in water temperature (Hopkins and Cech, 2003; Vaudo and Lowe, 2006; Dewar et al., 2008; Vaudo and Heithaus, 2009). Ray species, especially those resident in coastal areas, may therefore be best protected by ‘targeted’ MPAs (Grüss et al., 2011), whereby the location of MPAs coincides with critical life stages, especially that of breeding aggregations which are particularly vulnerable to fishery exploitation (Kinney and Simpfendorfer, 2009).
However, what ultimately determines the success of spatially fixed MPAs in protecting elasmobranchs will likely not only be the knowledge of the location of key life-history areas (e.g. breeding and nursery grounds), but also the understanding of the patterns of movement and site fidelity of targeted species, as high rates of movement would reduce the effectiveness of an MPA (Kramer and Chapman, 1999). These patterns often vary considerably between the sexes and life-history stages in shark and ray species (Struhsaker, 1969; Snelson and Williams, 1981; Smith and Merriner, 1987; Gray et al., 1997; Yokota and Lessa, 2006). Therefore, the conservation value of MPAs for these species needs to be considered across a range of life-history stages (Kinney and Simpfendorfer, 2009; Grüss et al., 2011). Ontogenetic differences in the scale of movements to and from core areas have been reported in sharks, whereby older and larger species have been shown to range over much wider areas (Speed et al., 2010). This has also been suggested to occur in rays (Struhsaker, 1969) and skates (Wearmouth and Sims, 2009). A further critical factor is the level of fidelity shown by these species to key areas (site fidelity). Site fidelity is known to occur at certain stages of the life cycle of a range of shark species (e.g. Garla et al., 2006; DeAngelis et al., 2008; Chapman et al., 2009; Anderson et al., 2011). It has been shown less often in rays (Vaudo and Lowe, 2006), but this is likely due to both a paucity of information on the location of nursery, mating, and foraging grounds in rays, as well as a lack of studies focusing on this group.
Each austral summer, hundreds of short-tailed stingrays (Dasyatis brevicaudata) gather at the Poor Knights Island Marine Reserve (PKIMR), providing a world-renowned display for marine ecotourists and a unique opportunity for scientific study (A. Le Port, pers. obs.). This ‘gathering’ is the only one of its kind that has been documented throughout the range (New Zealand, South Africa, and southern Australia) of this species. It has been suggested that the main purpose of the aggregation may be reproduction. Nothing, however, is known of the timing and population structure of this seasonal grouping, or whether the seasonal occurrence of D. brevicaudata at the PKIMR is in response to biological factors (e.g. reproduction, feeding, or predator avoidance), is the result of seasonal physical changes (e.g. sea temperature, photoperiod, or currents), or is a combination of both. Although, D. brevicaudata is prohibited as a commercial target species throughout most of its range, it is still caught as bycatch and regularly taken by recreational fishers (IUCN, 2011, A. Le Port, pers. obs.). Dasyatis brevicaudata exhibit the slow growth, late maturity, and low reproductive output typical of viviparous elasmobranchs, which is likely to make this species susceptible to overfishing. The PKIMR offers a unique opportunity to gather baseline data on the seasonal occurrence, abundance, and population structure of a common coastal stingray under minimal to no exploitation.
This study aimed to document the seasonal abundance and population structure (sex and size) of D. brevicaudata at the PKIMR. Based on previous studies of elasmobranch aggregations, it was hypothesized that abundance would be highest during summer and lowest in winter. We also wished to investigate the anecdotal observations that the PKIMR served as a mating aggregation. If this aggregation was indeed for breeding purposes, we would expect a significant seasonal increase in large, mature males and/or females. An increase solely in mature females could be an indication of the PKIMR being used as a pupping ground or as a gestation site (with or without pupping). In the former case (pupping ground), an increase in the sightings of pregnant females followed by an increase in pup/juvenile numbers would also be expected. Finally, we aimed to discuss, using the information available, whether an MPA the size of the PKIMR could be effective in the conservation of a coastal stingray, such as D. brevicaudata, and related or ecologically similar batoids.
Material and methods
Study site
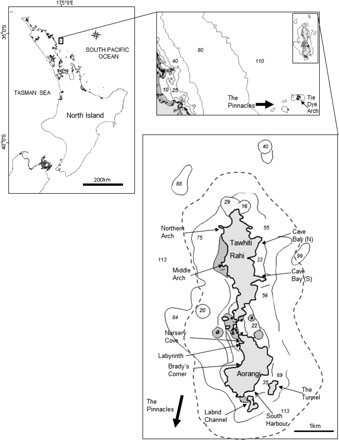
The PKIMR is located 24 km offshore of the northeast coast of the North Island of New Zealand (35°30′S, 174°45'E; Figure 1), and extends 800 m from the islands, covering a total of 24 km2. This group of islands is characterized by: (i) the East Auckland Current (EAUC), a warm subtropical current which brings water from the north that is clearer and often warmer (∼2°C; temperature range from 2005 to 2007: 14.5–22.0°C; A. Le Port, unpublished data) than nearby coastal areas; and (ii) a bottom topography which is steep and deep (depth range: <5 m to ≥ 100 m), with unique geological features consisting of deep underwater caves and tunnels, archways, and chimneys (Edney, 2001). The PKIMR which were given no-take marine reserve status in 1998, provide a unique opportunity to study animals in a ‘natural’ setting largely removed from anthropogenic effects.
Study location, the Poor Knights Islands Marine Reserve (PKIMR), and distribution of 11 survey sites (shown with thin arrows). Note: Tie Dye Arch is contained within The Pinnacles at the southern boundary of the PKIMR (2.4 nm southwest from the two main Islands) and is shown with a thick arrow. The dashed line indicates the PKIMR boundary.
Seasonal changes in relative abundance and ecological surveys
This study was conducted at 11 sites distributed throughout the PKIMR (Figure 1). Diver visual surveys (30 min timed swims) were conducted during daylight hours (0730–1800) from November 2004 to April 2007. Surveys were carried out monthly where possible, or every 2 months (depending on logistics). Transects were not fixed, but the same area was sampled at each site using fixed starting points and compass bearings. Divers only recorded stingrays up to 20 m on either side, above and below the path swum. Typically, visibility ranged between 15 and 30 m year-round. Counts, sex, and size class of the short-tailed stingray (D. brevicaudata) were recorded. Sex was easily determined underwater by the presence of paired genital claspers visible on either side of the tail in males. Ray sizes were split into three categories: juveniles [<1.0 m disc width (DW)], subadults (1.0–1.5 m DW), and mature individuals (>1.5 m DW). As size at maturity data were not available, these size classes and corresponding life stages were adapted from Struhsaker (1969) for the thorny stingray (Dasyatis centroura). Dasyatis centroura has a size at birth of 34.0–37.0 cm and a maximum DW of ∼2.0 m, which are comparable with those of D. brevicaudata (Last and Stevens, 2009). The presence of mating wounds on the dorsal surface of both female and male stingrays was also recorded to ascertain the occurrence and timing of potential mating activity at the PKIMR. Wounds were only recorded when a white dermal layer was clearly visible (indicative of recently inflicted tissue trauma; Kajiura et al., 2000).
Ambient seafloor temperatures (±0.2°C) were collected continuously every 30 min at Nursery Cove (see Figure 1), between 13 December 2004 and 19 April 2007. These were recorded using a temperature data logger (Onset Computer Corporation, Bourne, MA, USA) attached to a permanent mooring on the seafloor at 18 m depth. The logger was retrieved every 4–6 months, and its data were downloaded using Boxcar Pro software (v 4.3.1.1) and re-deployed immediately.
A critical assumption of the present study is that underwater surveys effectively measured stingray abundance and that any seasonal fluctuations in numbers were not merely a reflection of the movements of animals out of the study area into neighbouring sites within or outside of the PKIMR. The present study benefited from local conditions at the PKIMR, including clear visibility (15–30 m) year-round, as well as D. brevicaudata being easy to locate, approach, and identify in the majority of cases, thus enabling sex determination and behavioural observations of individuals. There were, however, instances when individuals could not be sexed or sized reliably due to the distance and activity of individuals (e.g. swimming away). Also, small rays ( ≤ 1.0 m) were sometimes challenging to sex due to the difficulty in determining the presence of small-sized claspers, resulting in several individuals of this size class with undetermined sex (Table 1).
Yearly sampling effort, total abundance, and sex differences at 11 Poor Knights Islands Marine Reserve sites surveyed from November 2004 to April 2007 for short-tailed stingrays (Dasyatis brevicaudata).
| Year . | Total surveys . | Total counts . | Females . | Males . | Unknown sex . |
|---|---|---|---|---|---|
| 2005 | 72 | 189 | 119 | 65 | 5 |
| 2006 | 67 | 84 | 42 | 38 | 4 |
| 2007 | 43 | 139 | 82 | 49 | 8 |
| Total | 182 | 412 | 243 | 152 | 17 |
| Year . | Total surveys . | Total counts . | Females . | Males . | Unknown sex . |
|---|---|---|---|---|---|
| 2005 | 72 | 189 | 119 | 65 | 5 |
| 2006 | 67 | 84 | 42 | 38 | 4 |
| 2007 | 43 | 139 | 82 | 49 | 8 |
| Total | 182 | 412 | 243 | 152 | 17 |
Yearly sampling effort, total abundance, and sex differences at 11 Poor Knights Islands Marine Reserve sites surveyed from November 2004 to April 2007 for short-tailed stingrays (Dasyatis brevicaudata).
| Year . | Total surveys . | Total counts . | Females . | Males . | Unknown sex . |
|---|---|---|---|---|---|
| 2005 | 72 | 189 | 119 | 65 | 5 |
| 2006 | 67 | 84 | 42 | 38 | 4 |
| 2007 | 43 | 139 | 82 | 49 | 8 |
| Total | 182 | 412 | 243 | 152 | 17 |
| Year . | Total surveys . | Total counts . | Females . | Males . | Unknown sex . |
|---|---|---|---|---|---|
| 2005 | 72 | 189 | 119 | 65 | 5 |
| 2006 | 67 | 84 | 42 | 38 | 4 |
| 2007 | 43 | 139 | 82 | 49 | 8 |
| Total | 182 | 412 | 243 | 152 | 17 |
Data analysis
Abundance data were analysed with a mixed generalized linear model (GLMM). Data were fit to a Poisson distribution with log link function. The fixed effects factors used were season and sex, whereas year was chosen as a random effect factor as years sampled (2005–2007) were considered a random sample from a larger population of values and we were not interested in looking at specific contrasts between years. Seasons were defined as follows: spring (September–November), summer (December–February), autumn (March–May), and winter (June–August). Seasonal changes in size frequency were analysed with a chi2 contingency table. Differences within groups were tested using a Holm–Sidak test for pairwise comparisons. Departures of sex ratios from equality (1:1) were tested with a z-test, whereas seasonal differences in sex ratios were analysed with a χ2 contingency table. Mating activity was recorded as presence of ‘fresh’ mating wounds in both males and females. The (%) occurrence of mating wounds in females was pooled by season and size class to assess seasonal and size-related trends in mating activity. Males were not included in the analysis. The effects of sea temperature and daylength (photoperiod) on seasonal abundance were analysed using regression analyses. All analyses were performed in SigmaStat 3.10 (Jandel Scientific Software, San Rafael, CA, USA), with the exception of GLMM analyses which were run in SPSS version 20.0 (SPSS, Inc., Chicago, IL, USA). The alpha (α)value for all analyses was set to 0.05.
Results
Seasonal abundance
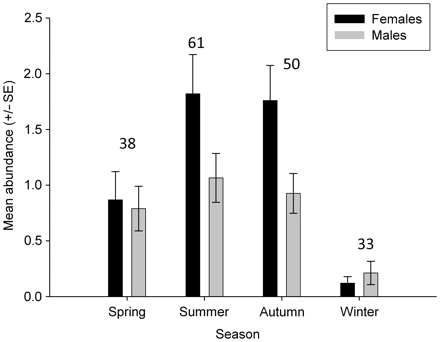
A total of 182 visual surveys were conducted at 11 sites around the PKIMR between November 2004 and April 2007 (Table 1). Rays were observed at all survey sites, with a total of 412 D. brevicaudata recorded (Table 1). Due to logistical and weather constraints, the number and frequency of surveys varied slightly between years, with the highest sampling effort conducted in the 2005 sampling season (Table 1). Mean D. brevicaudata abundances were significantly different between seasons (GLMM: F = 18.86, d.f. = 3, p < 0.001) and the sexes (GLMM: F = 20.97, d.f. = 1, p < 0.001). The overall seasonal abundance trend peaked in summer and autumn and was lowest in winter (Figure 2). Stingray numbers were significantly different between the sexes in summer (Holm–Sidak: p = 0.02) and autumn (Holm–Sidak: p = 0.01). This was due to an increase in female numbers. There was no significant difference between the sexes in spring and winter, and male numbers did not vary significantly seasonally (Figure 2).
Mean seasonal abundance of D. brevicaudata males and females averaged across all years sampled. Numbers above the bars represent the number of surveys from which means were derived.
Size distribution
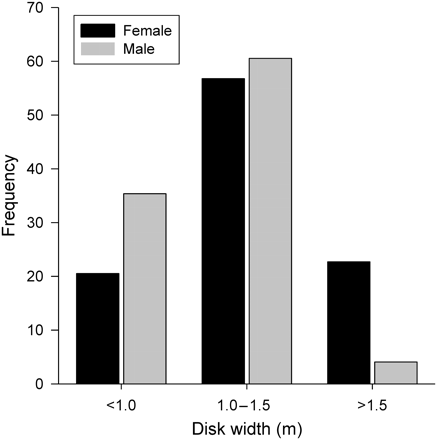
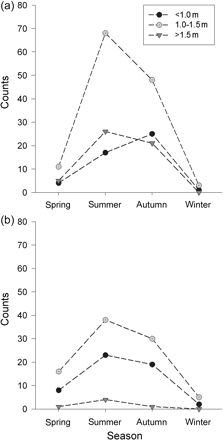
The majority of D. brevicaudata had DWs between 1.0 and 1.5 m (Figure 3). This pattern did not differ between the sexes (Holm–Sidak: p > 0.05). Large D. brevicaudata (>1.5 m) were mostly females (Figure 3). The size frequency distribution of D. brevicaudata was not significantly different seasonally (χ2 = 5.95, p = 0.43). Seasonal abundances of each size class varied considerably between the sexes (Figure 4a and b). Ray numbers within the 1.0–1.5 m category peaked sharply in summer driven by a sixfold increase in females (Figure 4a) and a twofold increase in males (Figure 4b). Larger individuals (>1.5 m) also peaked in summer for both females and males, but to a lesser degree (Figure 4b). Small (<1.0 m) female numbers increased steadily from spring, peaking in autumn with a sixfold increase (Figure 4a), while males of that size class peaked in summer (Figure 4b).
Size frequency distribution for D. brevicaudata males (n = 147) and females (n = 229).
Seasonal abundance of D. brevicaudata by size class for (a) females and (b) males.
Sex ratios
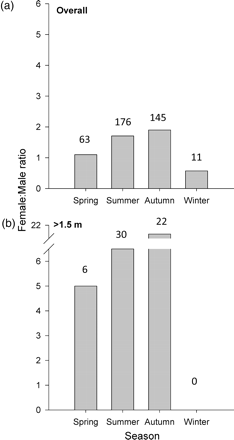
The overall sex ratio in D. brevicaudata was dominated by females (1.6:1; z = 4.54, p < 0.001). Female to male ratios varied seasonally, although this was not statistically significant (chi2 = 6.32, p = 0.09). Females were more numerous than males (2:1) in summer (2:1; Holm–Sidak: t = 2.63, p = 0.01) and autumn (2:1; Holm–Sidak: t = 2.46, p = 0.02; Figure 5a), while males were twice as abundant as females (0.5:1) in winter, although this was not statistically significant. Seasonal sex ratios were not found to change according to size. However, although seasonal sex ratios for rays <1.0 m and rays 1.0–1.5 m DW were comparable with the overall (pooled) pattern described for Figure 5a (above), a substantial increase in large (>1.5 m DW) females in summer and autumn months resulted in very high female to male ratios for this size class, particularly in autumn (21:1). Large rays of both sexes were absent in winter (Figure 5b).
Dasyatis brevicaudata female to male sex ratios (F:M) between seasons and size classes recorded for (a) all size classes, and (b) mature individuals (>1.5 m DW). Numbers above the bars indicate the total number of rays per season per size class. Note: values >1 indicate (F > M), values = 1 (F = M), values <1 (F < M).
Mating activity
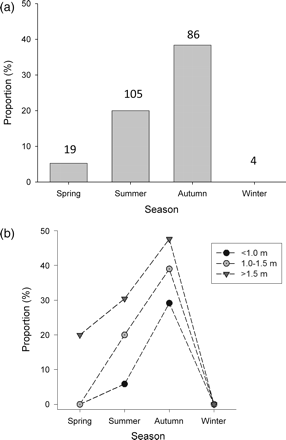
The presence of mating wounds on the dorsal surface of female stingrays increased during summer and autumn (Figure 6a). Mating wounds were absent in individuals during winter and present only in a minority of D. brevicaudata (5%) in spring. However, females with mating wounds increased fourfold in summer, that number doubling once more in autumn (38% of females; Figure 6a). Occurrence of mating wounds clearly increased with increasing female size (Figure 6b), even though many ( >50%) D. brevicaudata with mating wounds were juvenile or subadult (Figure 6b).
Percentage of D. brevicaudata females with mating wounds for (a) each season and (b) each size class between seasons. Numbers above the bars indicate the total numbers of females recorded in each season.
Environmental cues: temperature and daylength
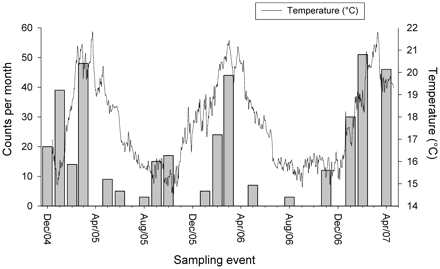
The overall abundance of D. brevicaudata was positively correlated with temperature (R2 = 0.44, p = 0.003), but not with daylength (R2 = 0.07, p = 0.26). Maximum abundance occurred at temperatures >20°C, while <19°C resulted in decreases, with minimum numbers at temperatures <16°C (Figure 7).
Monthly D. brevicaudata counts and corresponding sea temperatures. Note: in none of the monthly surveys was D. brevicaudata absent, thus ‘0’ values correspond to months when surveys were not conducted.
Discussion
This study is a first attempt at addressing questions of seasonal patterns of abundance and behavioural ecology in a coastal stingray to assess the value of an offshore marine reserve to elasmobranch conservation. Data presented here are supportive of the idea that the PKIMR is a mating ground for D. brevicaudata, and possibly also a nursery area for juvenile and subadult D. brevicaudata. There is no indication though that the area is being used as a pupping ground or gestation site.
As expected from anecdotal reports, D. brevicaudata followed a seasonal pattern of abundance, their numbers being highest from spring to autumn and lowest in winter. Similar seasonal patterns of occurrence have been reported in many ray studies [e.g. bat rays (Myliobatis californica; Gray et al., 1997), cownose rays (Rhinoptera bonasus; Smith and Merriner, 1987), Atlantic stingrays (Dasyatis sabina; Snelson and Williams, 1981; Snelson et al., 1988), and round stingrays (Urobatis halleri; Hoisington and Lowe, 2005)]. This study differs from previous ray population studies, however, in that the seasonal aggregation seen at the PKIMR occurs at a relatively deep offshore location (depth range ∼5–100 m) as opposed to shallower coastal areas. It is possible that ray offshore aggregation locations are more common than reported, but, due to the logistical difficulties in studying them, are seldom described. Regardless, if these seasonal aggregations are predictable and repeatable, and are a common occurrence in coastal stingrays, these key locations should be the ones investigated and considered for MPA implementation for exploited ray species.
Peak D. brevicaudata abundances observed during summer–autumn were driven largely by an increase in female numbers. Summer–autumn is the presumed breeding season for D. brevicaudata based on reproductive activity of similar species (Snelson et al., 1988; Ebert and Cowley, 2009). The observed pattern in abundance combined with an increased frequency of large females with fresh mating wounds over the same period suggests that the PKIMR is a mating ground. This has important conservation implications for this species, as this is the first and only report of a mating ground for D. brevicaudata. Until further breeding aggregations are reported for this species, this site is unique and should be considered critical for their reproductive success. As such, the PKIMR fits the criteria for protection of essential life-history habitat areas (Grüss et al., 2011).
Of interest in relation to the mating behaviour of D. brevicaudata was the presence of fresh mating scars and the observation of presumed courtship behaviour (‘close following’ and ‘pre-copulatory biting’; Chapman et al., 2003) on females considered as immature ( ≤ 1.0–1.5 m DW; A. Le Port, pers. obs.). Dasyatis brevicaudata may therefore be sexually mature at a smaller size (i.e. closer to 1.0 m than the 1.5 m DW used in this study) than we had originally anticipated based on maturity in similar sized species (D. centroura; Struhsaker 1969). Alternatively, ‘play-mating’ behaviour may be taking place between immature D. brevicaudata individuals. Our size classes were based on size at maturity parameters for the Western Atlantic thorny stingray (D. centroura; Struhsaker 1969), which attains a maximum size similar to D. brevicaudata ( ≥ 2.0 m DW; Last and Stevens, 2009). However, size at maturity may be different for D. brevicaudata, as not only are these values likely to differ interspecifically, but life-history parameters such as size at maturity are also known to vary regionally within a single species (Struhsaker, 1969; Capapé, 1993). Data on sexual maturity and mating behaviour for this species are required to distinguish between the two alternatives (‘play-mating’ behaviour vs. smaller size at maturity).
Juveniles and subadults (<1.5 m DW) of both sexes made up the majority of the rays recorded at the PKIMR over three consecutive years. Heupel et al. (2007) outlined three criteria for an area to be identified as a nursery for sharks: (i) sharks are more commonly encountered in the area than other areas; (ii) sharks have a tendency to remain or return for extended periods; and (iii) the area or habitat is repeatedly used across years. Given that these criteria can be applied for the designation of ray nurseries, the PKIMR fulfils all three criteria: (i) while sampling and surveying several New Zealand coastal and offshore islands, juvenile and subadult (<1.5 m DW) D. brevicaudata were encountered more predictably and in larger numbers at the PKIMR (A. Le Port, pers. obs.); (ii) although additional data are required, PSAT (Pop-up Satellite Archival Tag) data suggest that rays of that size class may only move short distances away ( ≤ 25 km) in winter, and return to the PKIMR as sea temperatures increase (Le Port et al., 2008); and (iii) our surveys run over three consecutive years support the PKIMR being used by juveniles and subadults on a yearly basis. Thus, although additional work focusing on D. brevicaudata juveniles is required, data presented here support the use of the PKIMR as a nursery area for this species. Dasyatis brevicaudata are born at a size (DW) of ∼0.36 m (Last and Stevens, 2009). The absence of neonates (individuals <0.7 m DW) in our surveys, however, suggests that D. brevicaudata do not use the PKIMR as a pupping ground. This is further supported by the absence of pregnant females at the PKIMR year-round (advanced pregnancy is easily identifiable in this species; A. Le Port, pers. obs.). Although most shark and ray nursery areas described to date have been located in shallow, protected areas (e.g. Castro, 1993), other species [thorny stingrays (D. centroura) and southern stingrays (D. americana)] use coastal or deeper water areas as nurseries (Struhsaker, 1969; Yokota and Lessa, 2006). Thus, D. brevicaudata pups (∼45 cm DW) which have been observed in large shallow, sheltered harbours during summer (A. Le Port, pers. obs.) may move to deeper offshore waters, including the PKIMR, where they remain until sexual maturity. The PKIMR may be all the more attractive because its complex system of caves and tunnels may offer protection from predators (killer whale, Orcinus orca; Visser, 1999). Furthermore, the warmer temperatures (∼1–2°C) at the PKIMR may prove to be an additional draw for both immature and mature stingrays alike.
Ray abundance was highest in summer and therefore correlated with sea temperature at the PKIMR, suggesting that temperature could play a part in this ray aggregation. Although temperature is widely recognized as a key environmental factor affecting reproduction (Wearmouth and Sims, 2008) and feeding in ectotherms such as elasmobranch fishes (Wallman and Bennett, 2006), its role has been poorly documented. Exploitation of high temperatures for the purpose of increasing embryonic developmental rate or achieving faster growth rates has been suggested in grey reef sharks (Carcharhinus amblyrhynchos; Economakis and Lobel, 1998). Pregnant D. brevicaudata females were not observed at the PKIMR, suggesting that it is unlikely that the warmer waters of this location are being specifically used for this purpose. Nevertheless, it is possible that immature stingrays may benefit from higher temperatures with increased core body temperatures, leading to faster growth rates (e.g. Hight and Lowe, 2007). Indirectly, higher temperatures found at the PKIMR may also lead to stingrays reaching sexual maturity at a smaller size than in the cooler coastal waters. This could explain the mating wounds and ‘play-mating’ behaviour in smaller, supposedly immature individuals, and needs to be further investigated by comparing size at sexual maturity between the PKIMR and coastal individuals.
Marine reserves such as the PKIMR may protect elasmobranchs when they are within their boundaries, but daily and seasonal movements are likely to expose individuals to fishery extraction, making the full extent of their protection difficult to gauge. Commercial longliners and trawlers operate outside the PKIMR boundaries, and D. brevicaudata are commonly caught as bycatch. No information is available on the daily movements of D. brevicaudata; however, preliminary data from pop-up satellite tags attached to immature (∼1.0 m DW) D. brevicaudata suggest that small-scale ( ≤ 25 km) seasonal movements outside of reserve boundaries are likely during winter (Le Port et al., 2008). Furthermore, rays were observed at greater depths during winter (>100 m; Le Port et al., 2008) in the current study, and an immature female ray tagged within the PKIMR was recaptured outside of the reserve (in waters of 130–180 m depth) by a commercial fisher during winter (A. Le Port, unpublished data), within 25 km of the reserve boundary. These data support short-distance movements of some D. brevicaudata to deeper waters outside of the PKIMR boundary in winter, at least for immature females. Similar seasonal movements to deeper waters have been reported previously in several rays [e.g. Atlantic stingrays (D. sabina; Snelson and Williams, 1981) and thornback rays (Raja clavata; Walker et al., 1997)]. The lack of genetic differentiation of adult D. brevicaudata from the PKIMR and coastal populations up to 600 km apart (Le Port and Lavery, 2012) further implies that adults may undertake large enough migrations between offshore aggregations and coastal areas to homogenize populations. However, the same study also revealed population structuring among several more distant New Zealand coastal locations, suggesting that adult movements may be limited by specific habitat requirements and/or by site fidelity to localized coastal areas. Long-term tagging of D. brevicaudata adults of both sexes is necessary to explore this further, as the results would have direct implications for the conservation of coastal batoids. Interestingly, the re-sighting of a large mature (>2.0 m) female 2 years after original observations at the PKIMR (B. Doak, pers. comm.) suggests that some adult D. brevicaudata may show a degree of site fidelity to the PKIMR. Site fidelity or residential behaviour has also been suggested for immature females (Le Port et al., 2008). However, we are currently limited in our conclusions by the small amount of data on small-scale movements of this species.
This study suggests that even a small sized fixed MPA co-located with a breeding aggregation and/or nursery site could still have a significant conservation benefit for coastal rays, and other elasmobranchs. Even though D. brevicaudata of all size classes are likely to move into fished waters during their seasonal migrations, the MPA may provide significant protection from fishing during a period of increased vulnerability at a crucial life-history stage. The extent of the conservation benefit provided by small MPAs, such as the one in this study, will depend on a suite of characteristics, including: the size and location of the MPA; the location of key areas (e.g. breeding, nursery grounds) for the species; which life-history stages use these areas; their degree of site fidelity; and their movements and relative vulnerabilities to adjacent targeted harvest and bycatch. The majority of coastal rays with populations at risk or declining worldwide are caught as a bycatch of more lucrative fisheries, making full MPAs difficult and/or impractical to implement (Wiegand et al., 2011). In the case of the declining North Sea thornback ray (Raja clavata), minimum length landing restrictions have been suggested as the best compromise between skate recovery and the multispecies trawl fisheries operating in the area, despite the greater effectiveness of full MPAs (Wiegand et al., 2011). Thus, although it is clear that MPAs can have a positive role to play in protecting vulnerable stingrays and other coastal elasmobranchs, in some cases they also need to go hand in hand with other fisheries management approaches for effective conservation. Further research on locations and daily/seasonal use of key life-history areas (e.g. breeding and nursery grounds), as well as on the movement patterns of different ontogenetic stages of endangered ray species would enable MPAs to be assessed, and designed, to improve their contribution to elasmobranch conservation.
Acknowledgements
All procedures were conducted under the animal care protocols approved by the University of Auckland Animal Ethics Committee (AEC Permit R240), and research within the Poor Knights Islands Marine Reserve was conducted under Department of Conservation Permit no. NO-14234-RES. Many thanks to the skippers of R/V Hawere, B. Doak and M. Birch, and to the Leigh Marine Lab students (D. Egli, C. Radford, N. Usmar, K. Yopak, E. Newcombe, and J. Saunders) and numerous volunteers for their invaluable help in the field. Many thanks also to two anonymous reviewers for their constructive reviews of this manuscript.
References
Author notes
Handling editor: Bill Turrell