-
PDF
- Split View
-
Views
-
Cite
Cite
Tor Arne Øigård, Anne Kirstine Frie, Kjell Tormod Nilssen, Mike Osborne Hammill, Modelling the abundance of grey seals (Halichoerus grypus) along the Norwegian coast, ICES Journal of Marine Science, Volume 69, Issue 8, September 2012, Pages 1436–1447, https://doi.org/10.1093/icesjms/fss103
Close - Share Icon Share
Abstract
An age-structured population dynamics model of the Norwegian grey seal (Halichoerus grypus) population has been developed. The model is of a Bayesian character in the sense that priors for various parameters were used. Model runs indicated an increase in the abundance of the total Norwegian grey seal population during the last 30 years, suggesting a total of 8740 (95% confidence interval: 7320–10 170) animals in 2011. A total catch of 707 (95% confidence interval: 532–882) grey seals would maintain the population size at the 2011 level. Model runs suggest that current catch levels will likely result in a reduction in the population size in Sør-Trøndelag and Nord-Trøndelag counties, and an increase in the population size in Rogaland, Nordland, Troms, and Finnmark counties. The model runs assumed that 80% of the seals taken in Rogaland came from the UK and that 50 and 55% of the catches in Troms and Finnmark, respectively, were immigrants from Russia.Øigård, T. A., Frie, A. K., Nilssen, K. T., and Hammill, M. O. 2012. Modelling the abundance of grey seals (Halichoerus grypus) along the Norwegian coast. – ICES Journal of Marine Science, 69: .
Introduction
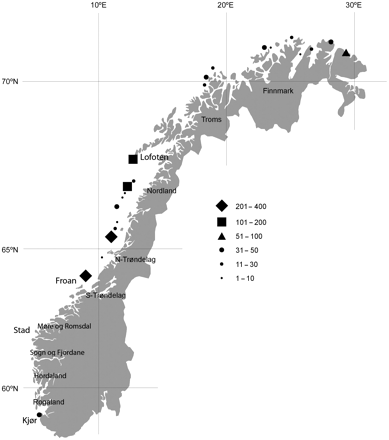
The grey seal (Halichoerus grypus) is widespread on both sides of the North Atlantic, with at least three evolutionary significant units (ESU; Boskovic et al., 1996). One ESU is found along the east coast of the United States and Canada from Massachusetts in the south to Labrador in the north (Hammill et al., 2007; Wood et al., 2007). A second ESU occurs in Europe, where it is distributed from Brittany, France, in the south to the coast of the Kola Peninsula in the north, and including the British Isles, the Faroe Islands, and Iceland (Duck and Thompson, 2007; Härkönen et al., 2007; Hauksson, 2007a; Mikkelsen, 2007; Nilssen and Haug, 2007). The third ESU occurs in the Baltic Sea (Harding et al., 2007). The species is resident in Norwegian coastal waters, where Wiig (1986) suggested a discontinuous distribution, with most animals being seen in mid-Norway (between 63 and 68°N; Figure 1).
Norwegian grey seal breeding sites with indicated number of pups.
Early investigations on grey seals during the 1960s, based on interviews with fishers, lighthouse crews, and seal hunters suggested that no animals were born south of Stad, while an estimated 660 pups were born annually in the areas north of Stad (62°N; Figure 1; Øynes, 1964, 1966). The Froan area in Sør-Trøndelag County was described as the most important breeding area, with ∼300 pups born annually. Very few animals were recorded in the northernmost parts of the country (Troms and Finnmark counties). Since then, several visual surveys carried out over the years have indicated that the population has increased and that the range has expanded. Wiig (1986) reviewed data from aerial and boat surveys carried out between Rogaland and Finnmark during 1974–1988, which resulted in a minimum estimate of around 3100 grey seals. Grey seals had extended their range southwards based on the discovery of pups born at the Kjør Islands in Rogaland. From 1987 to 1992, visual surveys covering the same areas along the coast indicated that the population had increased to around 4000–5000 animals (Wiig, 1987a, b, c, 1988, 1989; Wiig and Øritsland, 1987; Wiig et al., 1990; Haug et al., 1994; Bakke and Lorentsen, 1999).
During the period 1996–1999, aerial photo surveys to estimate grey seal pup production were flown in the area from Froan (64°N) to Lofoten (67°N), and the number of moulting grey seals were registered in Troms and Finnmark counties (69–71°N). These investigations resulted in a total population estimate of around 4400 grey seals along the Norwegian coast from Froan northwards (Bjørge and Øien, 1999). Rogaland County was not included in these surveys.
In 2001–2003, boat-based surveys along the entire coast from eastern Finnmark to southern parts of Rogaland provided estimates of annual pup production of nearly 1200. This corresponded to an estimated population of 4600–5500 age 1 and older grey seals (1+; Nilssen and Haug, 2007), based on conversion factors of 4.0–4.7 between pups and 1+ seals (Wickens and Shelton, 1992). This result was slightly higher than that obtained in the period 1996–1999, but the methods were different.
Combined boat and aerial photo surveys were performed during September–December 2006–2008 covering the same areas as in 2001–2003. The surveys estimated a minimum pup production of 1275, corresponding to an estimate of 5100–6000 (1+) grey seals in Norwegian waters, based on conversion factors of 4.0–4.7.
Grey seals are harvested in Norway, but the hunt was not regulated (except for protection in some nature reserves) until 1973, when seals were protected throughout the year south of Stad and during the period 1 May to 31 September north of Stad. The regulations did not include restrictions on the number of seals taken (Anon., 1990). Starting in 1997, a new management regime was implemented for grey seals in Norway. The major management objective was to ensure viable stocks within their natural ranges, but consideration would be given to conflicts between seals and fisheries. In areas where the seal population was proven to sustain a harvest, hunting was recommended as a means to control seal abundance.
An implication of the new management regime was that quotas should restrict the hunt. Although the population structure of grey seals along the Norwegian coast was unresolved, quotas were given separately for three management areas: (i) a southwest Norwegian area (Rogaland–Stad) including the counties of Rogaland, Hordaland, and Sogn og Fjordane; (ii) a mid-Norwegian area (Stad–Lofoten) including the counties Møre og Romsdal, Sør-Trøndelag, Nord-Trøndelag, and Nordland; and (iii) a northern area, including the counties Troms and Finnmark (Figure 1). Tagging studies of grey seals in Norwegian waters (Bjørge et al., 2002) and differences in timing of breeding (Nilssen and Haug, 2007) support population differentiation between the three management areas.
The introduction of quotas required updated information on seal abundance, and it was recommended that a monitoring programme (Anon, 1990) should survey the grey seal population every 5 years. Initially, quota recommendations given by the Institute of Marine Research (IMR) were set at 5% of the current abundance estimates, which was assumed to be sustainable. In some areas, quota recommendations were increased due to the assumed immigration of grey seals from large neighbouring colonies, e.g. from the UK in the south (Bjørge and McConnell, 1986; Wiig, 1986, 1987b) and from the Murman coast in Russia in the north (Haug et al., 1994; Henriksen et al., 2007). In areas with particular conflicts between grey seals and fisheries, the recommended quotas could be increased by 30%. In 2003, this approach was taken a large step further by the Directorate of Fisheries when the quotas in most areas were set to 25% of current population estimates. Also, a bounty was introduced for each grey seal documented as being killed. Relatively high quotas and the bounty regime were continued until 2010 (Nilssen, 2011).
Hunters must report the number of animals taken (Nilssen and Haug, 2007). This improved the catch statistics from 1999 onwards. The increased quotas and the bounty system starting in 2003 resulted in increased catches of 353–516 seals taken annually in the period 2003–2010 (Nilssen, 2011). In addition to the direct harvest, grey seals along the Norwegian coast are also subjected to some bycatch mortality. In a mark–recapture experiment, where 3571 grey seal pups were tagged, 7% of the tags were returned (Bjørge et al., 2002). Incidental mortality, mainly in bottom-set gillnets, accounted for the majority of deaths (79%). The seals were most vulnerable during the first 3 months after birth, although high incidental mortality prevailed during the first 8–10 months. Preliminary estimates suggest that 100–200 grey seals are taken annually as bycatch in the fisheries (Nilssen and Bjørge, 2009). Interactions with fish farms by grey seals are known to occur, and fish farmers are allowed to kill nuisance seals. These takes must be reported (Nilssen and Haug, 2007).
In this study, we use a population model to describe the dynamics of the Norwegian grey seal population, based on empirical data from pup counts covering the entire distribution area of Norwegian grey seals in the period 1979–2008, as well as empirical data on hunting and estimated bycatch mortality. The model requires estimates of natural mortality and female reproductive rates, but since empirical data on these parameters are outdated or absent, they are estimated by the model using a Bayesian approach. Female reproductive rates are estimated using priors from data on Northwest Atlantic (NWA) grey seals, and available datasets from other grey seal populations are used to assess the general variability in parity curves in grey seal females. Natural mortality rates are estimated using uninformative priors. Estimated values for both reproductive and mortality rates are compared with available empirical data for grey seals as part of the model validation procedure.
Material and methods
Data
Pup production estimates
Surveys aimed at estimating pup production have mainly covered whelping sites in different parts of the Norwegian coast each year. Available grey seal pup production estimates from each county are given in Table 1, based on the results from surveys conducted in the periods 1979–1989 (Wiig, 1986, 1987a, b; Røv et al., 1990), 1990–1998 (Haug et al., 1994, 1998; Lorentsen and Bakke, 1995; Bjørge and Øien, 1999), 2001–2003 (Nilssen and Haug, 2007), and 2006–2008 (present study). The surveys during the periods 1996–1999, 2001–2003, and 2006–2008 covered the entire grey seal distribution area along the coast, except the Kjør Islands in Rogaland in the first period. In the 2001–2003 and 2006–2008 surveys, pupping sites were visited two or three times. The surveys were timed to cover the peak of the pupping season. All islands were surveyed on each visit, and all pups were tagged (Dalton Rototags) when possible during the two first visits to ensure that pups were not double counted. Also, their developmental stages (age) based on Kovacs and Lavigne (1986) were recorded. In 2006, all whelping sites in Troms and Finnmark were surveyed twice using a boat, except for only one survey in the easternmost site in Finnmark. In 2007, the whelping sites in Sør-Trøndelag (Froan) and Nord-Trøndelag were covered simultaneously by boat and aerial photo surveys during the first visit. Due to difficult weather conditions, it was not possible to cover the whelping sites more than twice. Aerial photo surveys were carried out a second time to cover the Froan area and one small whelping site in Nordland. To avoid double counts, only pup stages 1–3 (Kovacs and Lavigne, 1986) were used on the photos from the second surveys. In 2008, the most abundant whelping sites in the Lofoten area in Nordland were surveyed three times by boat, whereas the other whelping sites in Lofoten and the Kjør Islands in Rogaland were covered twice. The population model is fitted to the survey pup production estimates. No uncertainties in the pup production estimates are available for the survey data. However, the population model estimates a mean coefficient of variation (CV) for each county.
Survey counts of grey seal pup production for all management areas along the Norwegian coast (Wiig, 1986, 1987a; Røv et al., 1990; Haug et al., 1994, 1998; Lorentsen and Bakke, 1995; Bjørge and Øien, 1999; Nilssen and Haug, 2007; present study for 2006–2008).
| Year . | Rogaland . | Sør-Trøndelag . | Nord-Trøndelag . | Nordland . | Troms . | Finnmark . |
|---|---|---|---|---|---|---|
| 1979 | – | 228 | 47 | 140 | – | – |
| 1985 | – | 200 | – | – | – | – |
| 1989 | – | 230 | – | – | – | – |
| 1990 | – | – | – | – | – | 39 |
| 1991 | – | – | – | 171 | 17 | – |
| 1992 | – | – | – | – | – | – |
| 1993 | – | 226 | – | – | – | – |
| 1994 | – | – | – | – | – | – |
| 1995 | – | – | – | – | – | – |
| 1996 | – | 262 | – | – | – | – |
| 1997 | – | – | – | – | – | – |
| 1998 | – | – | 67 | 399 | – | 119 |
| 1999 | – | – | – | – | – | – |
| 2000 | 30 | – | – | – | – | – |
| 2001 | 30 | – | 84 | – | – | 142 |
| 2002 | 28 | 283 | – | 573 | – | – |
| 2003 | 35 | – | – | – | 41 | 143 |
| 2004 | – | – | – | – | – | – |
| 2005 | 31 | – | – | – | – | – |
| 2006 | – | – | – | – | 76 | 232 |
| 2007 | – | 189 | 135 | 619a | – | – |
| 2008 | 43 | – | – | –b | – | – |
| Year . | Rogaland . | Sør-Trøndelag . | Nord-Trøndelag . | Nordland . | Troms . | Finnmark . |
|---|---|---|---|---|---|---|
| 1979 | – | 228 | 47 | 140 | – | – |
| 1985 | – | 200 | – | – | – | – |
| 1989 | – | 230 | – | – | – | – |
| 1990 | – | – | – | – | – | 39 |
| 1991 | – | – | – | 171 | 17 | – |
| 1992 | – | – | – | – | – | – |
| 1993 | – | 226 | – | – | – | – |
| 1994 | – | – | – | – | – | – |
| 1995 | – | – | – | – | – | – |
| 1996 | – | 262 | – | – | – | – |
| 1997 | – | – | – | – | – | – |
| 1998 | – | – | 67 | 399 | – | 119 |
| 1999 | – | – | – | – | – | – |
| 2000 | 30 | – | – | – | – | – |
| 2001 | 30 | – | 84 | – | – | 142 |
| 2002 | 28 | 283 | – | 573 | – | – |
| 2003 | 35 | – | – | – | 41 | 143 |
| 2004 | – | – | – | – | – | – |
| 2005 | 31 | – | – | – | – | – |
| 2006 | – | – | – | – | 76 | 232 |
| 2007 | – | 189 | 135 | 619a | – | – |
| 2008 | 43 | – | – | –b | – | – |
aFour hundred and eighty-one pups counted along the mainland coast in Nordland.
bOne hundred and thirty-eight pups counted at the Lofoten area.
Survey counts of grey seal pup production for all management areas along the Norwegian coast (Wiig, 1986, 1987a; Røv et al., 1990; Haug et al., 1994, 1998; Lorentsen and Bakke, 1995; Bjørge and Øien, 1999; Nilssen and Haug, 2007; present study for 2006–2008).
| Year . | Rogaland . | Sør-Trøndelag . | Nord-Trøndelag . | Nordland . | Troms . | Finnmark . |
|---|---|---|---|---|---|---|
| 1979 | – | 228 | 47 | 140 | – | – |
| 1985 | – | 200 | – | – | – | – |
| 1989 | – | 230 | – | – | – | – |
| 1990 | – | – | – | – | – | 39 |
| 1991 | – | – | – | 171 | 17 | – |
| 1992 | – | – | – | – | – | – |
| 1993 | – | 226 | – | – | – | – |
| 1994 | – | – | – | – | – | – |
| 1995 | – | – | – | – | – | – |
| 1996 | – | 262 | – | – | – | – |
| 1997 | – | – | – | – | – | – |
| 1998 | – | – | 67 | 399 | – | 119 |
| 1999 | – | – | – | – | – | – |
| 2000 | 30 | – | – | – | – | – |
| 2001 | 30 | – | 84 | – | – | 142 |
| 2002 | 28 | 283 | – | 573 | – | – |
| 2003 | 35 | – | – | – | 41 | 143 |
| 2004 | – | – | – | – | – | – |
| 2005 | 31 | – | – | – | – | – |
| 2006 | – | – | – | – | 76 | 232 |
| 2007 | – | 189 | 135 | 619a | – | – |
| 2008 | 43 | – | – | –b | – | – |
| Year . | Rogaland . | Sør-Trøndelag . | Nord-Trøndelag . | Nordland . | Troms . | Finnmark . |
|---|---|---|---|---|---|---|
| 1979 | – | 228 | 47 | 140 | – | – |
| 1985 | – | 200 | – | – | – | – |
| 1989 | – | 230 | – | – | – | – |
| 1990 | – | – | – | – | – | 39 |
| 1991 | – | – | – | 171 | 17 | – |
| 1992 | – | – | – | – | – | – |
| 1993 | – | 226 | – | – | – | – |
| 1994 | – | – | – | – | – | – |
| 1995 | – | – | – | – | – | – |
| 1996 | – | 262 | – | – | – | – |
| 1997 | – | – | – | – | – | – |
| 1998 | – | – | 67 | 399 | – | 119 |
| 1999 | – | – | – | – | – | – |
| 2000 | 30 | – | – | – | – | – |
| 2001 | 30 | – | 84 | – | – | 142 |
| 2002 | 28 | 283 | – | 573 | – | – |
| 2003 | 35 | – | – | – | 41 | 143 |
| 2004 | – | – | – | – | – | – |
| 2005 | 31 | – | – | – | – | – |
| 2006 | – | – | – | – | 76 | 232 |
| 2007 | – | 189 | 135 | 619a | – | – |
| 2008 | 43 | – | – | –b | – | – |
aFour hundred and eighty-one pups counted along the mainland coast in Nordland.
bOne hundred and thirty-eight pups counted at the Lofoten area.
Removals
In a culling programme during the period 1980–1990, 15 and 948 grey seals were shot in the Rogaland-Stad and Stad-Lofoten regions, respectively (Anon., 1990). In Finnmark, 670 grey seals were shot during the same decade, based on interviews with all local hunters known to hunt grey seals (Haug et al., 1994). Some catch numbers before 1999 (in statistics from the Directorate of Fisheries) occur as total numbers in each area over a given period of years and were subsequently averaged over years (Table 2). No other quantitative information is available on grey seals taken in regular hunting activities in Norway before 1997.
Grey seal catch data for all management areas along the Norwegian coast in the years 1980–2009.
| Year . | Rogaland . | Sør-Trøndelag . | Nord-Trøndelag . | Nordland . | Troms . | Finnmark . |
|---|---|---|---|---|---|---|
| 1979 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1980 | 0 | 0 | 14 | 8 | 3 | 55 |
| 1981 | 0 | 0 | 31 | 20 | 3 | 55 |
| 1982 | 0 | 80 | 10 | 65 | 3 | 55 |
| 1983 | 0 | 55 | 0 | 78 | 3 | 55 |
| 1984 | 15 | 200 | 8 | 146 | 3 | 55 |
| 1985 | 5 | 32 | 0 | 0 | 3 | 55 |
| 1986 | 5 | 10 | 0 | 16 | 3 | 68 |
| 1987 | 5 | 10 | 22 | 38 | 3 | 68 |
| 1988 | 5 | 10 | 5 | 20 | 3 | 68 |
| 1989 | 5 | 10 | 5 | 20 | 3 | 68 |
| 1990 | 5 | 10 | 5 | 20 | 3 | 68 |
| 1991 | 5 | 10 | 5 | 3 | 3 | 5 |
| 1992 | 5 | 10 | 5 | 3 | 3 | 5 |
| 1993 | 5 | 10 | 5 | 3 | 3 | 5 |
| 1994 | 5 | 10 | 5 | 3 | 3 | 5 |
| 1995 | 5 | 10 | 5 | 3 | 3 | 5 |
| 1996 | 5 | 10 | 5 | 3 | 3 | 5 |
| 1997 | 5 | 10 | 5 | 3 | 3 | 5 |
| 1998 | 5 | 10 | 5 | 3 | 3 | 5 |
| 1999 | 9 | 44 | 14 | 7 | 3 | 53 |
| 2000 | 70 | 45 | 5 | 31 | 3 | 22 |
| 2001 | 27 | 20 | 12 | 34 | 12 | 0 |
| 2002 | 23 | 24 | 19 | 20 | 5 | 19 |
| 2003 | 44 | 96 | 46 | 120 | 9 | 50 |
| 2004 | 30 | 67 | 51 | 94 | 42 | 54 |
| 2005 | 51 | 48 | 34 | 105 | 14 | 127 |
| 2006 | 60 | 51 | 27 | 69 | 39 | 129 |
| 2007 | 60 | 40 | 23 | 134 | 35 | 174 |
| 2008 | 60 | 40 | 72 | 103 | 37 | 203 |
| 2009 | 67 | 31 | 62 | 119 | 4 | 235 |
| 2010 | 38 | 19 | 38 | 41 | 20 | 208 |
| Year . | Rogaland . | Sør-Trøndelag . | Nord-Trøndelag . | Nordland . | Troms . | Finnmark . |
|---|---|---|---|---|---|---|
| 1979 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1980 | 0 | 0 | 14 | 8 | 3 | 55 |
| 1981 | 0 | 0 | 31 | 20 | 3 | 55 |
| 1982 | 0 | 80 | 10 | 65 | 3 | 55 |
| 1983 | 0 | 55 | 0 | 78 | 3 | 55 |
| 1984 | 15 | 200 | 8 | 146 | 3 | 55 |
| 1985 | 5 | 32 | 0 | 0 | 3 | 55 |
| 1986 | 5 | 10 | 0 | 16 | 3 | 68 |
| 1987 | 5 | 10 | 22 | 38 | 3 | 68 |
| 1988 | 5 | 10 | 5 | 20 | 3 | 68 |
| 1989 | 5 | 10 | 5 | 20 | 3 | 68 |
| 1990 | 5 | 10 | 5 | 20 | 3 | 68 |
| 1991 | 5 | 10 | 5 | 3 | 3 | 5 |
| 1992 | 5 | 10 | 5 | 3 | 3 | 5 |
| 1993 | 5 | 10 | 5 | 3 | 3 | 5 |
| 1994 | 5 | 10 | 5 | 3 | 3 | 5 |
| 1995 | 5 | 10 | 5 | 3 | 3 | 5 |
| 1996 | 5 | 10 | 5 | 3 | 3 | 5 |
| 1997 | 5 | 10 | 5 | 3 | 3 | 5 |
| 1998 | 5 | 10 | 5 | 3 | 3 | 5 |
| 1999 | 9 | 44 | 14 | 7 | 3 | 53 |
| 2000 | 70 | 45 | 5 | 31 | 3 | 22 |
| 2001 | 27 | 20 | 12 | 34 | 12 | 0 |
| 2002 | 23 | 24 | 19 | 20 | 5 | 19 |
| 2003 | 44 | 96 | 46 | 120 | 9 | 50 |
| 2004 | 30 | 67 | 51 | 94 | 42 | 54 |
| 2005 | 51 | 48 | 34 | 105 | 14 | 127 |
| 2006 | 60 | 51 | 27 | 69 | 39 | 129 |
| 2007 | 60 | 40 | 23 | 134 | 35 | 174 |
| 2008 | 60 | 40 | 72 | 103 | 37 | 203 |
| 2009 | 67 | 31 | 62 | 119 | 4 | 235 |
| 2010 | 38 | 19 | 38 | 41 | 20 | 208 |
Catches in Hordaland and Sogn og Fjordane counties are included in the Rogaland numbers, and catches in Møre og Romsdal are included in the Sør-Trøndelag numbers.
Grey seal catch data for all management areas along the Norwegian coast in the years 1980–2009.
| Year . | Rogaland . | Sør-Trøndelag . | Nord-Trøndelag . | Nordland . | Troms . | Finnmark . |
|---|---|---|---|---|---|---|
| 1979 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1980 | 0 | 0 | 14 | 8 | 3 | 55 |
| 1981 | 0 | 0 | 31 | 20 | 3 | 55 |
| 1982 | 0 | 80 | 10 | 65 | 3 | 55 |
| 1983 | 0 | 55 | 0 | 78 | 3 | 55 |
| 1984 | 15 | 200 | 8 | 146 | 3 | 55 |
| 1985 | 5 | 32 | 0 | 0 | 3 | 55 |
| 1986 | 5 | 10 | 0 | 16 | 3 | 68 |
| 1987 | 5 | 10 | 22 | 38 | 3 | 68 |
| 1988 | 5 | 10 | 5 | 20 | 3 | 68 |
| 1989 | 5 | 10 | 5 | 20 | 3 | 68 |
| 1990 | 5 | 10 | 5 | 20 | 3 | 68 |
| 1991 | 5 | 10 | 5 | 3 | 3 | 5 |
| 1992 | 5 | 10 | 5 | 3 | 3 | 5 |
| 1993 | 5 | 10 | 5 | 3 | 3 | 5 |
| 1994 | 5 | 10 | 5 | 3 | 3 | 5 |
| 1995 | 5 | 10 | 5 | 3 | 3 | 5 |
| 1996 | 5 | 10 | 5 | 3 | 3 | 5 |
| 1997 | 5 | 10 | 5 | 3 | 3 | 5 |
| 1998 | 5 | 10 | 5 | 3 | 3 | 5 |
| 1999 | 9 | 44 | 14 | 7 | 3 | 53 |
| 2000 | 70 | 45 | 5 | 31 | 3 | 22 |
| 2001 | 27 | 20 | 12 | 34 | 12 | 0 |
| 2002 | 23 | 24 | 19 | 20 | 5 | 19 |
| 2003 | 44 | 96 | 46 | 120 | 9 | 50 |
| 2004 | 30 | 67 | 51 | 94 | 42 | 54 |
| 2005 | 51 | 48 | 34 | 105 | 14 | 127 |
| 2006 | 60 | 51 | 27 | 69 | 39 | 129 |
| 2007 | 60 | 40 | 23 | 134 | 35 | 174 |
| 2008 | 60 | 40 | 72 | 103 | 37 | 203 |
| 2009 | 67 | 31 | 62 | 119 | 4 | 235 |
| 2010 | 38 | 19 | 38 | 41 | 20 | 208 |
| Year . | Rogaland . | Sør-Trøndelag . | Nord-Trøndelag . | Nordland . | Troms . | Finnmark . |
|---|---|---|---|---|---|---|
| 1979 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1980 | 0 | 0 | 14 | 8 | 3 | 55 |
| 1981 | 0 | 0 | 31 | 20 | 3 | 55 |
| 1982 | 0 | 80 | 10 | 65 | 3 | 55 |
| 1983 | 0 | 55 | 0 | 78 | 3 | 55 |
| 1984 | 15 | 200 | 8 | 146 | 3 | 55 |
| 1985 | 5 | 32 | 0 | 0 | 3 | 55 |
| 1986 | 5 | 10 | 0 | 16 | 3 | 68 |
| 1987 | 5 | 10 | 22 | 38 | 3 | 68 |
| 1988 | 5 | 10 | 5 | 20 | 3 | 68 |
| 1989 | 5 | 10 | 5 | 20 | 3 | 68 |
| 1990 | 5 | 10 | 5 | 20 | 3 | 68 |
| 1991 | 5 | 10 | 5 | 3 | 3 | 5 |
| 1992 | 5 | 10 | 5 | 3 | 3 | 5 |
| 1993 | 5 | 10 | 5 | 3 | 3 | 5 |
| 1994 | 5 | 10 | 5 | 3 | 3 | 5 |
| 1995 | 5 | 10 | 5 | 3 | 3 | 5 |
| 1996 | 5 | 10 | 5 | 3 | 3 | 5 |
| 1997 | 5 | 10 | 5 | 3 | 3 | 5 |
| 1998 | 5 | 10 | 5 | 3 | 3 | 5 |
| 1999 | 9 | 44 | 14 | 7 | 3 | 53 |
| 2000 | 70 | 45 | 5 | 31 | 3 | 22 |
| 2001 | 27 | 20 | 12 | 34 | 12 | 0 |
| 2002 | 23 | 24 | 19 | 20 | 5 | 19 |
| 2003 | 44 | 96 | 46 | 120 | 9 | 50 |
| 2004 | 30 | 67 | 51 | 94 | 42 | 54 |
| 2005 | 51 | 48 | 34 | 105 | 14 | 127 |
| 2006 | 60 | 51 | 27 | 69 | 39 | 129 |
| 2007 | 60 | 40 | 23 | 134 | 35 | 174 |
| 2008 | 60 | 40 | 72 | 103 | 37 | 203 |
| 2009 | 67 | 31 | 62 | 119 | 4 | 235 |
| 2010 | 38 | 19 | 38 | 41 | 20 | 208 |
Catches in Hordaland and Sogn og Fjordane counties are included in the Rogaland numbers, and catches in Møre og Romsdal are included in the Sør-Trøndelag numbers.
Reproductive rates
Wiig (1991) backcalculated pregnancy rates for Norwegian grey seals based on the presence or the absence of a Corpus albicans (CA) in breeding females sampled between Froan and Lofoten during the period 1982–1984. Due to the low presence of non-reproducing females in breeding patches, these samples may be biased towards highly productive females. Unfortunately, samples from other periods in the year were not obtained. As an alternative, we used foetus-based pregnancy rates estimated for 772 grey seals sampled between implantation and late gestation in the Gulf of St Lawrence in 1969–2008 (Hammill and Gosselin, 1995; present study). For this dataset, reproductive tracts were removed and processed as described in Hammill and Gosselin (1995). Age was determined by counting the annual growth layer groups in the cementum of a lower canine tooth following Mansfield (1991). Pregnancy rates were assigned to age classes based on the estimated age in the upcoming whelping season. No significant differences in age-specific pregnancy rates were found between years (Hammill and Stenson, 2011).
To explore the general variability in age-specific pregnancy rates in grey seals, we plotted age-specific pregnancy rates for the Canadian dataset with published data for grey seals in Norway (Wiig, 1991), the UK (Boyd, 1985), and Iceland (Hauksson, 2007b; Table 3). The first study is based on the registration of CAs in breeding females, the second study uses a mixture of CA and foetus-based pregnancy rates, and the third study uses only foetus-based pregnancy rates. The timing of data collection for the estimation of foetus-based pregnancy rates is not explicitly stated by Boyd (1985) and Hauksson (2007b), but is assumed to have occurred between implantation and birth as needed for reliable estimation.
Estimates of proportion of mature grey seal females (P) at ages 3–10.
| Age . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | 10 . | MAP (s.e) . |
|---|---|---|---|---|---|---|---|---|---|
| PCanada (fitted) | 0 (0.02) | 0.167 (0.17) | 0.607 (0.61) | 0.849 (0.85) | 0.906 (0.89) | 0.888 (0.90) | 0.888 (0.90) | 0.888 (0.90) | 5.2 (0.2) |
| n | 88 | 73 | 77 | 80 | 55 | 399a | – | – | |
| PUK (fitted) | 0.045 (0.04) | 0.154 (0.16) | 0.565 (0.56) | 0.920 (0.88) | 0.933 (0.88) | 0.714 (0.88) | 0.906 (0.88) | 0.867 (0.88) | 5.3 (0.3) |
| PIceland (fitted) | 0.050 (0.07) | 0.270 (0.21) | 0.330 (0.46) | 0.760 (0.71) | 0.830 (0.86) | 0.960 (0.93) | 0.920 (0.95) | 0.960 (0.96) | 5.2 (0.4) |
| PNorway (fitted) | 0 (0.00) | 0.400 (0.40) | 0.880 (0.81) | 0.800 (0.81) | 0.800 (0.81) | 0.800 (0.81) | 0.800 (0.81) | 0.800 (0.81) | 4.7 (NA) |
| Age . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | 10 . | MAP (s.e) . |
|---|---|---|---|---|---|---|---|---|---|
| PCanada (fitted) | 0 (0.02) | 0.167 (0.17) | 0.607 (0.61) | 0.849 (0.85) | 0.906 (0.89) | 0.888 (0.90) | 0.888 (0.90) | 0.888 (0.90) | 5.2 (0.2) |
| n | 88 | 73 | 77 | 80 | 55 | 399a | – | – | |
| PUK (fitted) | 0.045 (0.04) | 0.154 (0.16) | 0.565 (0.56) | 0.920 (0.88) | 0.933 (0.88) | 0.714 (0.88) | 0.906 (0.88) | 0.867 (0.88) | 5.3 (0.3) |
| PIceland (fitted) | 0.050 (0.07) | 0.270 (0.21) | 0.330 (0.46) | 0.760 (0.71) | 0.830 (0.86) | 0.960 (0.93) | 0.920 (0.95) | 0.960 (0.96) | 5.2 (0.4) |
| PNorway (fitted) | 0 (0.00) | 0.400 (0.40) | 0.880 (0.81) | 0.800 (0.81) | 0.800 (0.81) | 0.800 (0.81) | 0.800 (0.81) | 0.800 (0.81) | 4.7 (NA) |
The reproductive rates PUK are from Boyd (1985), the reproductive rates PIceland are from Hauksson (2007b), and the reproductive rates PNorway are from Wiig (1991). Values obtained from fitted quasi-parity curves are shown in parentheses. Mean age-at-primiparity (MAP) values are based on the fitted curves and 95% CI are given for all datasets except Wiig (1991) (see Figure 3 for a visual illustration of these age-specific reproductive rates). Sample size n is given for the present Canadian data.
aData for Canadian animals ages 8 and older were available as a pooled 8+ age class.
Estimates of proportion of mature grey seal females (P) at ages 3–10.
| Age . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | 10 . | MAP (s.e) . |
|---|---|---|---|---|---|---|---|---|---|
| PCanada (fitted) | 0 (0.02) | 0.167 (0.17) | 0.607 (0.61) | 0.849 (0.85) | 0.906 (0.89) | 0.888 (0.90) | 0.888 (0.90) | 0.888 (0.90) | 5.2 (0.2) |
| n | 88 | 73 | 77 | 80 | 55 | 399a | – | – | |
| PUK (fitted) | 0.045 (0.04) | 0.154 (0.16) | 0.565 (0.56) | 0.920 (0.88) | 0.933 (0.88) | 0.714 (0.88) | 0.906 (0.88) | 0.867 (0.88) | 5.3 (0.3) |
| PIceland (fitted) | 0.050 (0.07) | 0.270 (0.21) | 0.330 (0.46) | 0.760 (0.71) | 0.830 (0.86) | 0.960 (0.93) | 0.920 (0.95) | 0.960 (0.96) | 5.2 (0.4) |
| PNorway (fitted) | 0 (0.00) | 0.400 (0.40) | 0.880 (0.81) | 0.800 (0.81) | 0.800 (0.81) | 0.800 (0.81) | 0.800 (0.81) | 0.800 (0.81) | 4.7 (NA) |
| Age . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | 10 . | MAP (s.e) . |
|---|---|---|---|---|---|---|---|---|---|
| PCanada (fitted) | 0 (0.02) | 0.167 (0.17) | 0.607 (0.61) | 0.849 (0.85) | 0.906 (0.89) | 0.888 (0.90) | 0.888 (0.90) | 0.888 (0.90) | 5.2 (0.2) |
| n | 88 | 73 | 77 | 80 | 55 | 399a | – | – | |
| PUK (fitted) | 0.045 (0.04) | 0.154 (0.16) | 0.565 (0.56) | 0.920 (0.88) | 0.933 (0.88) | 0.714 (0.88) | 0.906 (0.88) | 0.867 (0.88) | 5.3 (0.3) |
| PIceland (fitted) | 0.050 (0.07) | 0.270 (0.21) | 0.330 (0.46) | 0.760 (0.71) | 0.830 (0.86) | 0.960 (0.93) | 0.920 (0.95) | 0.960 (0.96) | 5.2 (0.4) |
| PNorway (fitted) | 0 (0.00) | 0.400 (0.40) | 0.880 (0.81) | 0.800 (0.81) | 0.800 (0.81) | 0.800 (0.81) | 0.800 (0.81) | 0.800 (0.81) | 4.7 (NA) |
The reproductive rates PUK are from Boyd (1985), the reproductive rates PIceland are from Hauksson (2007b), and the reproductive rates PNorway are from Wiig (1991). Values obtained from fitted quasi-parity curves are shown in parentheses. Mean age-at-primiparity (MAP) values are based on the fitted curves and 95% CI are given for all datasets except Wiig (1991) (see Figure 3 for a visual illustration of these age-specific reproductive rates). Sample size n is given for the present Canadian data.
aData for Canadian animals ages 8 and older were available as a pooled 8+ age class.
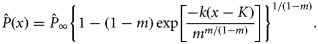
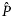 is the estimated proportion pregnant at age x, x the integer age (years) at parturition,
is the estimated proportion pregnant at age x, x the integer age (years) at parturition, 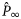 the asymptotic value of the curve, K the age (years) at the point of inflection, k the slope of the curve at the point of inflection, and m the shape parameter related to
the asymptotic value of the curve, K the age (years) at the point of inflection, k the slope of the curve at the point of inflection, and m the shape parameter related to 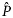 at the point of inflection as
at the point of inflection as 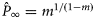 . Full age-class resolution was available for all datasets except Wiig (1991), who reported aggregated estimates for seals older than 6 years. In this case, we assumed homogeneus pregnancy rates and a uniform age distribution in females aged 6–10 years. No uncertainty was estimated for MAP in this dataset. For the other datasets, 95% maximum likelihood-based support intervals of MAP were estimated as outlined in Frie et al. (2003).
. Full age-class resolution was available for all datasets except Wiig (1991), who reported aggregated estimates for seals older than 6 years. In this case, we assumed homogeneus pregnancy rates and a uniform age distribution in females aged 6–10 years. No uncertainty was estimated for MAP in this dataset. For the other datasets, 95% maximum likelihood-based support intervals of MAP were estimated as outlined in Frie et al. (2003).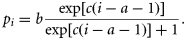
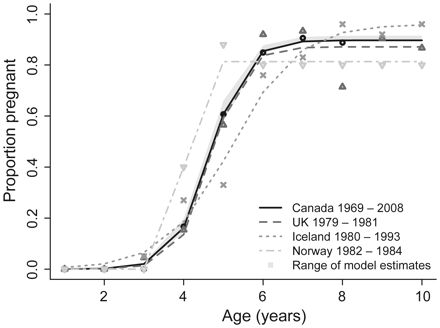
Figure 2 shows the reproductive data found in Table 3, along with the model fit for all datasets using Equation (2).
Observed age-specific pregnancy rates and fitted Richards curves for four different grey seal datasets (see Table 3 for data). The reproductive rates are from Canada (present study), Boyd (1985), Hauksson (2007b), and Wiig (1991). The shaded area shows the range of the model estimates of the age-specific grey seal reproductive rates, pi, for the entire Norwegian coast.
Curves derived from Equations (1) and (2) are referred to as quasi-parity curves, because they, in fact, show age-specific pregnancy rates and not proportions parous as such (i.e. some parous females may be barren in the year of census).
The population model
The population model is an age-structured population dynamics model that uses historical catch data and pup production counts to estimate the current total population. A similar model is used to assess the abundance of the Northeast Atlantic harp seal (Pagophilus groenlandicus) and hooded seal (Cystophora cristata) populations (ICES, 2006) and for assessing the historical population of Barents Sea harp seals (Skaug et al., 2007).
The natural mortality rate M determines the survival probabilities s1+ = exp(−M) and s0 = exp(−γM), which are the quantities that appear in the population dynamics equations. The “1+” denotes all ages ≥1 year. The assumption that the mortality rate is age-independent within the 1+ is because available data do not allow for a more detailed age-dependence to be estimated. The γ parameter scales the mortality in the first year and is set to γ = 3 (threefold higher than 1+ mortality).
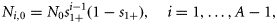
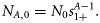
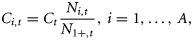
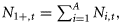 Ni,t is the number of individuals at age i in year t, and Ct is the catch level in year t.
Ni,t is the number of individuals at age i in year t, and Ct is the catch level in year t.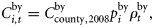
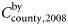 is the bycatch level in 2008 for a given county and
is the bycatch level in 2008 for a given county and  is the total bycatch level in 2008 for all counties. The bycatch levels for each county depend on the population size in each county, i.e. Nordland County has ∼48% of the total population. Therefore, the bycatch level in Nordland is 48% of the total bycatch level. Further,
is the total bycatch level in 2008 for all counties. The bycatch levels for each county depend on the population size in each county, i.e. Nordland County has ∼48% of the total population. Therefore, the bycatch level in Nordland is 48% of the total bycatch level. Further, 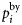 is the age-specific distribution of animals in the bycatch, and
is the age-specific distribution of animals in the bycatch, and 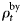 scales the annual bycatch so that it is proportional to population size. The
scales the annual bycatch so that it is proportional to population size. The 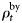 is found by running the model without bycatch included, then scaling the obtained population trajectory such that
is found by running the model without bycatch included, then scaling the obtained population trajectory such that 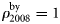 .
.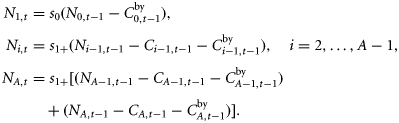
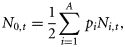
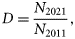
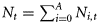 . Using the depletion coefficient, the equilibrium catch levels are estimated. The equilibrium catch level is defined as the catch level that maintains the population size at the 2011 level, i.e. the catch level that gives D = 1. The equilibrium catch level is found by solving the equation D = 1 numerically using Newton's method.
. Using the depletion coefficient, the equilibrium catch levels are estimated. The equilibrium catch level is defined as the catch level that maintains the population size at the 2011 level, i.e. the catch level that gives D = 1. The equilibrium catch level is found by solving the equation D = 1 numerically using Newton's method.Parameter estimation
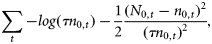
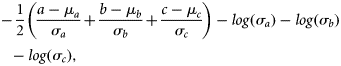
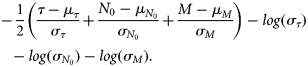
Mean values and standard deviations of normal priors used in the grey seal population model.
| Parameter . | μ . | σ . |
|---|---|---|
| a | 3.67 | 0.50 |
| b | 0.89 | 0.15 |
| c | 2.26 | 0.50 |
| τ | 0.10 | 0.10 |
| N0 | 500 | 500 |
| M | 0.1 | 0.05 |
| Parameter . | μ . | σ . |
|---|---|---|
| a | 3.67 | 0.50 |
| b | 0.89 | 0.15 |
| c | 2.26 | 0.50 |
| τ | 0.10 | 0.10 |
| N0 | 500 | 500 |
| M | 0.1 | 0.05 |
Mean values and standard deviations of normal priors used in the grey seal population model.
| Parameter . | μ . | σ . |
|---|---|---|
| a | 3.67 | 0.50 |
| b | 0.89 | 0.15 |
| c | 2.26 | 0.50 |
| τ | 0.10 | 0.10 |
| N0 | 500 | 500 |
| M | 0.1 | 0.05 |
| Parameter . | μ . | σ . |
|---|---|---|
| a | 3.67 | 0.50 |
| b | 0.89 | 0.15 |
| c | 2.26 | 0.50 |
| τ | 0.10 | 0.10 |
| N0 | 500 | 500 |
| M | 0.1 | 0.05 |
All parameter estimates are found by minimizing the likelihood function using the statistical software AD Model Builder (Fournier et al., 2012). AD Model Builder calculates standard errors (s.e.) for the model parameters, as well as the derived parameters such as present population size, D, and the equilibrium catch level. AD Model Builder uses a quasi-Newton optimization algorithm with bounds on the parameters and calculates the estimates of standard errors of model parameters using the “delta-method” (Skaug et al., 2007).
The catch data enter the model through Equation (5), but do not otherwise contribute to the objective function. No mechanisms for density-dependence were considered due to the low population levels. The model was run for each county separately.
Results
For the Rogaland area, the catch level is very high compared with pup production estimates. Based on Scottish tagging experiments, it is believed that a large fraction of the animals caught are from the UK (Bjørge and McConnell, 1986). It is also assumed, based on earlier tagging experiments in Russia (Henriksen et al., 2007), that the hunt in Troms and Finnmark includes animals from the Kola coast, Russia.
Running the model using the original catch data (Table 2) causes the population to collapse in Rogaland and a strong decrease in population size in Troms and Finnmark. This does not fit with the observed pup production, with increasing number of pups in those areas. To explore the impact on potential immigration from the UK and Russia, the catch data were scaled to remove the effect of animals coming from other areas.
No quantitative data on immigration of animals from outside areas are available for Rogaland, Troms, or Finnmark. To provide crude estimates of the fraction of animals from outside areas in the catches, we inspected the ratio between the catch level of 1+ animals and the survey pup production counts for areas where no immigration from other areas is assumed, e.g. Sør-Trøndelag, Nord-Trøndelag, and Nordland. The catch-to-pup ratios in these areas were 0.21, 0.27, and 0.22, respectively, using the catch level for the year the latest pup count exists. For Rogaland, Troms, and Finnmark, these ratios were 1.40, 0.51, and 0.56, respectively. From this, we estimated how much the catch levels in Rogaland, Troms, and Finnmark had to be scaled to obtain a similar fraction as the average value of the catch-to-pup ratio in the areas where no immigration was assumed. We estimated that 84% of the animals caught in Rogaland belonged to the UK population. For Troms and Finnmark, we estimated that 55 and 59%, respectively, of animals caught belonged to the Russian population. Slightly more conservative values were chosen when running the population model, i.e. 80% for Rogaland, 50% for Troms, and 55% for Finnmark.
Estimates of MAP were virtually identical for Canadian, British, and Icelandic grey seals (5.2–5.3 years, Table 3). The estimated MAP for the Norwegian dataset was slightly lower (4.7 years) due to a more abrupt recruitment pattern and the stabilization of pregnancy rates at a lower level (0.81) in the Norwegian dataset than in the other datasets (0.87–0.96; Table 3, Figure 2). The highest asymptotic pregnancy rate was observed for the Icelandic dataset.
Not much variability was observed in the parity curves derived by the population dynamics model. As in the data-based curves, the youngest parous females were estimated to be 3–4 years old, and full parity was reached at 6–7 years of age. Model-derived estimates of asymptotic pregnancy rates were also within the range of the empirical values (Figure 2).
The adult natural mortality rate was estimated at 5–8% in five of six subareas and at 13% in Sør-Trøndelag. Standard deviations of these estimates ranged from 0.01 to 0.02, and the mortality rate estimated for Sør-Trøndelag was thus significantly higher than for most of the other subareas.
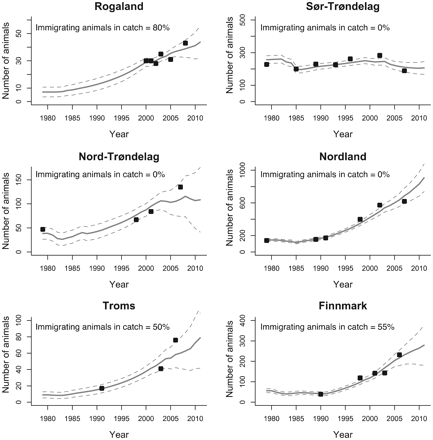
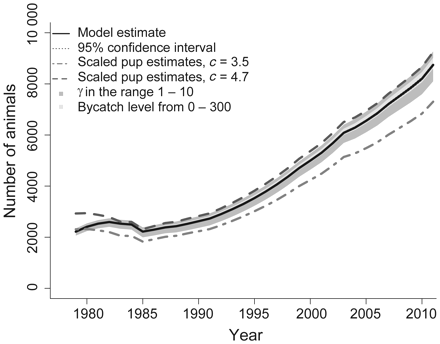
The model fit for the pup production estimates corresponds well with the survey data (Figure 3). The modelled population trajectory for all areas combined (full lines) is shown in Figure 4, along with scaled estimates (dashed and dashed-dotted lines) from the pup production previously used to obtain the estimates of the abundance of 1+-year-old animals (Nilssen and Haug, 2007). A bycatch level of 200 animals and a γ = 3 were chosen for this population trajectory. The model estimate corresponds very well with the abundance estimates obtained from scaling the pup production estimates and appears to be somewhere between the lower and the upper scaling limits of c = 3.5 and c = 4.7, respectively. The dark-grey shaded area shows the range of estimated population trajectories when using γ in the range of 1–10, and the light-grey shaded area shows the range of estimated population trajectories when using bycatch levels from 0 to 300 animals in 2008. All model estimates are found in Table 5.
Estimates and estimated standard errors of the grey seal model parameters for all management areas along the coast of Norway.
| Parameters . | Rogaland . | Sør-Trøndelag . | Nord-Trøndelag . | |||
|---|---|---|---|---|---|---|
| . | Mean . | s.e. . | Mean . | s.e. . | Mean . | s.e. . |
| N0 | 21 | 7.8 | 974 | 119 | 117 | 32 |
| M | 0.08 | 0.02 | 0.13 | 0.01 | 0.07 | 0.02 |
| N0,2010 | 44 | 6 | 206 | 18 | 108 | 34 |
| N1+,2010 | 202 | 37 | 863 | 129 | 491 | 169 |
| Ntotal,2010 | 246 | 38 | 1069 | 130 | 599 | 173 |
| D | 1.72 | 0.40 | 0.94 | 0.08 | 0.69 | 0.87 |
| τ | 0.09 | 0.03 | 0.09 | 0.02 | 0.15 | 0.04 |
| Ceq | 20 | 5.4 | 25 | 6.2 | 48 | 24.4 |
| Nordland | Troms | Finnmark | ||||
| Mean | s.e. | Mean | s.e. | Mean | s.e. | |
| N0 | 432 | 46 | 26 | 8 | 152 | 18 |
| M | 0.08 | 0.01 | 0.07 | 0.02 | 0.05 | 0.02 |
| N0,2010 | 907 | 86 | 79 | 19 | 279 | 50 |
| N1+,2010 | 3 921 | 606 | 353 | 100 | 1290 | 299 |
| Ntotal,2010 | 4 828 | 612 | 432 | 101 | 1569 | 303 |
| D | 2.33 | 0.16 | 2.39 | 0.52 | 2.05 | 0.53 |
| τ | 0.09 | 0.03 | 0.16 | 0.05 | 0.12 | 0.04 |
| Ceq | 407 | 69.5 | 39 | 15.5 | 168 | 47.0 |
| Parameters . | Rogaland . | Sør-Trøndelag . | Nord-Trøndelag . | |||
|---|---|---|---|---|---|---|
| . | Mean . | s.e. . | Mean . | s.e. . | Mean . | s.e. . |
| N0 | 21 | 7.8 | 974 | 119 | 117 | 32 |
| M | 0.08 | 0.02 | 0.13 | 0.01 | 0.07 | 0.02 |
| N0,2010 | 44 | 6 | 206 | 18 | 108 | 34 |
| N1+,2010 | 202 | 37 | 863 | 129 | 491 | 169 |
| Ntotal,2010 | 246 | 38 | 1069 | 130 | 599 | 173 |
| D | 1.72 | 0.40 | 0.94 | 0.08 | 0.69 | 0.87 |
| τ | 0.09 | 0.03 | 0.09 | 0.02 | 0.15 | 0.04 |
| Ceq | 20 | 5.4 | 25 | 6.2 | 48 | 24.4 |
| Nordland | Troms | Finnmark | ||||
| Mean | s.e. | Mean | s.e. | Mean | s.e. | |
| N0 | 432 | 46 | 26 | 8 | 152 | 18 |
| M | 0.08 | 0.01 | 0.07 | 0.02 | 0.05 | 0.02 |
| N0,2010 | 907 | 86 | 79 | 19 | 279 | 50 |
| N1+,2010 | 3 921 | 606 | 353 | 100 | 1290 | 299 |
| Ntotal,2010 | 4 828 | 612 | 432 | 101 | 1569 | 303 |
| D | 2.33 | 0.16 | 2.39 | 0.52 | 2.05 | 0.53 |
| τ | 0.09 | 0.03 | 0.16 | 0.05 | 0.12 | 0.04 |
| Ceq | 407 | 69.5 | 39 | 15.5 | 168 | 47.0 |
Current catch levels (average over last 3 years) are used when estimating D.
Estimates and estimated standard errors of the grey seal model parameters for all management areas along the coast of Norway.
| Parameters . | Rogaland . | Sør-Trøndelag . | Nord-Trøndelag . | |||
|---|---|---|---|---|---|---|
| . | Mean . | s.e. . | Mean . | s.e. . | Mean . | s.e. . |
| N0 | 21 | 7.8 | 974 | 119 | 117 | 32 |
| M | 0.08 | 0.02 | 0.13 | 0.01 | 0.07 | 0.02 |
| N0,2010 | 44 | 6 | 206 | 18 | 108 | 34 |
| N1+,2010 | 202 | 37 | 863 | 129 | 491 | 169 |
| Ntotal,2010 | 246 | 38 | 1069 | 130 | 599 | 173 |
| D | 1.72 | 0.40 | 0.94 | 0.08 | 0.69 | 0.87 |
| τ | 0.09 | 0.03 | 0.09 | 0.02 | 0.15 | 0.04 |
| Ceq | 20 | 5.4 | 25 | 6.2 | 48 | 24.4 |
| Nordland | Troms | Finnmark | ||||
| Mean | s.e. | Mean | s.e. | Mean | s.e. | |
| N0 | 432 | 46 | 26 | 8 | 152 | 18 |
| M | 0.08 | 0.01 | 0.07 | 0.02 | 0.05 | 0.02 |
| N0,2010 | 907 | 86 | 79 | 19 | 279 | 50 |
| N1+,2010 | 3 921 | 606 | 353 | 100 | 1290 | 299 |
| Ntotal,2010 | 4 828 | 612 | 432 | 101 | 1569 | 303 |
| D | 2.33 | 0.16 | 2.39 | 0.52 | 2.05 | 0.53 |
| τ | 0.09 | 0.03 | 0.16 | 0.05 | 0.12 | 0.04 |
| Ceq | 407 | 69.5 | 39 | 15.5 | 168 | 47.0 |
| Parameters . | Rogaland . | Sør-Trøndelag . | Nord-Trøndelag . | |||
|---|---|---|---|---|---|---|
| . | Mean . | s.e. . | Mean . | s.e. . | Mean . | s.e. . |
| N0 | 21 | 7.8 | 974 | 119 | 117 | 32 |
| M | 0.08 | 0.02 | 0.13 | 0.01 | 0.07 | 0.02 |
| N0,2010 | 44 | 6 | 206 | 18 | 108 | 34 |
| N1+,2010 | 202 | 37 | 863 | 129 | 491 | 169 |
| Ntotal,2010 | 246 | 38 | 1069 | 130 | 599 | 173 |
| D | 1.72 | 0.40 | 0.94 | 0.08 | 0.69 | 0.87 |
| τ | 0.09 | 0.03 | 0.09 | 0.02 | 0.15 | 0.04 |
| Ceq | 20 | 5.4 | 25 | 6.2 | 48 | 24.4 |
| Nordland | Troms | Finnmark | ||||
| Mean | s.e. | Mean | s.e. | Mean | s.e. | |
| N0 | 432 | 46 | 26 | 8 | 152 | 18 |
| M | 0.08 | 0.01 | 0.07 | 0.02 | 0.05 | 0.02 |
| N0,2010 | 907 | 86 | 79 | 19 | 279 | 50 |
| N1+,2010 | 3 921 | 606 | 353 | 100 | 1290 | 299 |
| Ntotal,2010 | 4 828 | 612 | 432 | 101 | 1569 | 303 |
| D | 2.33 | 0.16 | 2.39 | 0.52 | 2.05 | 0.53 |
| τ | 0.09 | 0.03 | 0.16 | 0.05 | 0.12 | 0.04 |
| Ceq | 407 | 69.5 | 39 | 15.5 | 168 | 47.0 |
Current catch levels (average over last 3 years) are used when estimating D.
Modelled grey seal pup production (full lines) for all areas and 95% confidence intervals (dashed lines). Black dots show pup production estimates obtained from surveys. The proportion of immigrating grey seals in the catch is shown for each area.
Modelled total grey seal population trajectory for all areas combined (full) with 95% confidence intervals (dotted). Total grey seal population estimates obtained from scaling the pup production estimates using scaling factors of 3.5 (dashed dotted) and 4.7 (dashed). Dark grey shaded area shows the range of the population trajectory obtained using γ in the range 1–10, and light grey shaded area shows the range of the population trajectory obtained using the bycatch level in 2008 in the range of 0–300 animals.
The 2011 estimate (with 95% confidence intervals) of the 1+ grey seal abundance along the coast of Norway was 7120 (5710–8540), and the pup production was estimated to be 1620 (1410–3050), making a total of 8740 (7320–10 170) animals.
The total annual equilibrium catch level of Norwegian grey seals was estimated to be 707 (95% confidence interval: 532–882) animals. Annual equilibrium catches (with 95% confidence intervals) for the various counties along the coast of Norway were estimated to be: Rogaland, 20 (9–32); Sør-Trøndelag, 25 (13–37); Nord-Trøndelag, 48 (0–96); Nordland, 407 (271–543); Troms, 39 (9–69); and Finnmark, 168 (76–260). The equilibrium catch estimates for Rogaland, Troms, and Finnmark do not take into account the immigration of seals from outside areas. The catch levels assume constant natural mortalities and reproductive parameters. Bycatches have already been removed from the population before estimating these catch levels.
Discussion
Based on interviews in the early 1960s by Øynes (1964, 1966), the annual grey seal pup production north of Stad (62°N) was ∼660. Since then, this number appears to have almost doubled. However, in the Froan area in Sør-Trøndelag, which was the most important breeding area in the 1960s (∼300 pups born annually), pup production changed little until 2003. Results from 2007 suggest a decrease in pup production, with only 189 born. From Nord-Trøndelag and farther north, grey seal abundance has increased significantly. However, the low pup production numbers before 1996 could partly be due to lower coverage in the breeding areas compared with the surveys carried out later. In Lofoten, Troms, and Finnmark, grey seal abundance has increased significantly over the last 20 years. In Lofoten, pup production has tripled over the last two decades, from 46 pups observed in 1989 (Haug et al., 1994) to 138 pups in 2008. In Troms, pup production was more than fourfold higher in 2006 than in 1991. Pup production seems to have doubled during the last decade in Finnmark. In Rogaland, annual pup production has increased from only 5 pups observed during the mid-1980s (Wiig, 1987b) to more than 40 pups born in 2008. High hunting pressure on grey seals after World War II and during the 1950s kept the population at a low level and almost exterminated grey seals in some areas. A subsequent reduction in hunting pressure appears to be the most likely reason for the recent increase in grey seal abundance in Norway.
According to the present model, the total population of grey seals along the coast of Norway numbered ∼8740 animals in 2011. The model runs indicate an increase in pup production in Rogaland, Nordland, Troms, and Finnmark. Pup production in Sør-Trøndelag appears to have been stable, but with a slight reduction during the last 20 years. Pup production in Nord-Trøndelag seems to have stabilized in recent years. The total population trajectory for all counties combined indicates an increase in abundance between 1985 and 2011. The rate of increase appears to have slowed since 2005, possibly due to higher catch levels. The mean annual rate of increase was ∼6.5% before 2005, but between 2005 and 2011, the rate was 3.2%.
For the areas Rogaland, Troms, and Finnmark, the catch levels in Table 2 had to be adjusted. Continuation of the current catch level will cause the population to be seriously depleted in Rogaland, Troms, and Finnmark, if no immigration is assumed. No quantitative data exist on immigration. Crude estimates of immigration rates were obtained by inspecting the catch-to-pup ratios, and comparing these with areas where no immigration was assumed. For Rogaland, it was assumed that 80% of the animals in the catches came from the UK. The modelled pup production closely follows the increasing trend in the survey pup count data. The model was also run assuming 70% of immigrant animals in the catch. Under this scenario, the model indicated a stabilization in pup production. For Troms, it was assumed that 50% of the hunted animals were immigrants from Russian waters. Also, in this case, the modelled pup production followed the increasing trend in the survey data. Without corrections for immigrant animals in the catches, the modelled pup production trajectory showed a slightly decreasing trend over the years 2005–2009, when catches were high, and an increase in total abundance from 2009 to 2011, consistent with reductions in catch levels. For Finnmark, it was assumed that 55% of the animals in the catches were Russian seals, and again there is a close correspondence between the modelled pup production and the survey data. The model was also run assuming 30% immigrant animals in the catches. Under this scenario, the modelled pup production showed a weak decline between 2005 and 2011.
The population model was not sensitive to changes in prior distributions or changes in initial values of the M or N0 parameters. Changing the mean value of the prior distribution for the CV from 0.10 to 0.20 caused a 0.5% reduction in the estimated population size.
Due to lack of data on temporal and spatial variability in reproductive rates of Norwegian grey seals, age-specific pregnancy rates were assumed to be constant over time and identical for all subareas. This is consistent with the low level of variability in reproductive rates previously observed both within grey seal populations (Hammill and Gosselin, 1995) and between grey seal populations (Harding et al., 2007; present study). It should be noted that values of MAP and asymptotic pregnancy rates based on Wiig (1991) in the present study differ somewhat from results for the same dataset in Harding et al. (2007). This is mainly due to differences in the interpretation of the backcalculation method used by Wiig (1991), but also to the fact that analyses in the present study are based on fitted parity curves.
Nevertheless, the present analyses reinforce the main conclusions by Harding et al. (2007) that observed grey seal reproductive rates show very low variability compared with some other seal species like harp seals (Kjellqwist et al., 1995; Sjare and Stenson, 2010), hooded seals (Frie et al., 2012), and ringed seals (Phoca hispida; Krafft et al., 2006). One reason for this could be that available cross-sectional datasets for grey seals are from populations that are at relatively low abundance levels compared with the environmental carrying capacity. Bowen and McMillan (2007) reported an increase in age at primiparity for the 1998–2000 cohorts at Sable Island by comparison with the mid-late 1980s. This change coincided with a slow-down in the population growth rate and is hypothesized to reflect density-dependent changes associated with a change in total abundance from ∼10 000 to 200 000 from the mid-1980s to 2000. The abundance of grey seals in the Gulf of St Lawrence has also increased considerably from ∼7300 in 1960 to 72 000 in 2009 (Hammill and Stenson, 2011). This stock is, however, still much smaller than at Sable Island and has not shown signs of density-dependent responses on vital rates since the low density situation in the 1960s. It, therefore, seems reasonable that reproductive data from the Gulf of St Lawrence are appropriate for the low-density Norwegian grey seal population.
Information on age-specific mortality rates of grey seals is not available. Adult mortality rates of long-lived vertebrates are expected to be rather constant within populations (Gaillard et al., 1998; Gaillard and Yoccoz, 2003) and among populations with otherwise similar life-history characteristics (Promislow and Harvey, 1990). The general similarity in estimated adult mortality rates among almost all subareas in the present model is thus consistent with expectations based on the life-history theory. For all but one subarea, adult mortality rates were estimated at 5–8% and were thus similar to the adult mortality rate of 6% estimated for British grey seals by Harwood and Prime (1978). Our estimates of adult mortality rates also fall within the range of 2–12% obtained from longitudinal mark–recapture studies on Sable Island (Manske et al., 2002; Schwarz and Stobo, 2000).
In the present study, an atypically high adult mortality rate of 13% (s.e. = 0.01) was estimated for Sør-Trøndelag compared with the other subareas. Possible explanations for this could be the permanent emigration of seals or unreported removals. The latter appears unlikely since hunters were paid a considerable bounty for each grey seal reported taken in the period 2003–2010. Movements between breeding areas, on the other hand, have been observed in the UK (Pomeroy et al., 2000) and the NWA (Wood et al., 2007). Conventional tagging studies have estimated a median dispersal distance of 86 km for grey seals tagged in Sør-Trøndelag (Bjørge et al., 2002), but no maximum dispersal distances were reported, and possible dispersal rates to neighboring counties are, therefore, unknown. Also, tagging data in Bjørge et al. (2002) mainly included recaptures of seals aged 0–1 year and are, therefore, not representative of older animals. The observed increase in pup production in northern parts of Nordland, including the Lofoten area, could be at least partly due to immigration from Sør-Trøndelag. Based on the uncertainty regarding levels of natural adult mortality in grey seals, it is not clear whether the adult mortality rates estimated for the northern counties support the hypothesis of northward migration of grey seals from Sør-Trøndelag.
In contrast to adult mortality rates, first-year mortality rates of long-lived vertebrates are expected to be highly sensitive to both density-dependent factors and environmental variability (Eberhardt, 1977; Gaillard et al., 1998). In the absence of information on first-year mortality rates, we set first-year mortality rates as a fixed multiple of adult mortality rates, which were then estimated by the model. Hall et al. (2001) estimated first-year mortality for average-weight pups at 49% for females and 81% for males in a high-density grey seal population in Scotland. This is far higher than estimates in the present study based on scaling factors (γ-values). However, first-year mortality may be significantly lower in Norwegian grey seals living at low density in highly productive waters (Agnalt et al., 2011). Based on adult mortality rates of 5–6%, scaling factors of 2–5 have previously been used for NWA grey seals (Trzcinski et al., 2006). Using γ = 3 was originally proposed in Roff and Bowen (1983). Increasing γ from 1 to 3 showed a modest effect on overall abundance (reduction of 5.7%). Increasing γ from 3 to 10, which would give first-year mortality rates similar to those observed by Hall et al. (2001), resulted in an overall 7.3% decline in the population. Since estimated total population abundance declines as γ-values increase, a γ-value in the high end may be considered conservative.
For the entire Norwegian population, a catch of 707 (532–882) grey seals would maintain the population size at the 2011 level. Model runs suggest that current catch levels will likely result in a reduction in population size in Sør-Trøndelag and Nord-Trøndelag, and an increase in population size in Nordland. In Rogaland, Nordland, and Troms, the unscaled current catch levels will result in a reduction in these populations. However, the scaled current catch levels will result in an increase in the populations in these areas, which means that the estimated annual equilibrium catches most likely could be increased due to immigration of seals from the UK in Rogaland and from Russia in Troms and Finnmark.
Acknowledgements
Special thanks go to H. J. Skaug for useful discussions and comments during the development of the population model. We also thank all persons who took part in the surveys aimed at estimating pup production, particularly N. E. Skavberg and M. Poltermann. Ø. Wiig is acknowledged for commenting on the manuscript and S. Hartvedt for graphic help.
References
Author notes
Handling editor: Emory Anderson