-
PDF
- Split View
-
Views
-
Cite
Cite
Timothy F. Sheehan, David G. Reddin, Gérald Chaput, Mark D. Renkawitz, SALSEA North America: a pelagic ecosystem survey targeting Atlantic salmon in the Northwest Atlantic, ICES Journal of Marine Science, Volume 69, Issue 9, November 2012, Pages 1580–1588, https://doi.org/10.1093/icesjms/fss052
Close - Share Icon Share
Abstract
Pelagic ecosystem surveys were conducted in the Labrador Sea during 2008 and 2009 as part of SALSEA North America. In total, 107 Atlantic salmon (Salmo salar) were captured using a pelagic surface trawl and multipanel surface gillnets. Surface trawling provided a broad spatial sampling of the fish and macroinvertebrate communities in the upper 10 m of the water column, but caught few salmon (23). Gillnetting was more effective at capturing post-smolt (60) and adult (24) salmon. Multiple smolt cohorts were captured, indicating that post-smolts and returning adults from different rivers in North America have similar autumnal habitat requirements. Post-smolts were caught at night and in water temperatures exceeding 10°C, both novel results. Post-smolts and adults consumed similar and diverse prey species, although Themisto compressa was the most important prey item. Intestinal macroparasite loads were substantial and could be a significant source of mortality. Concurrent planktonic assemblage and oceanographic conditions were also quantified. A full exploration of these data, historical datasets, and parallel data collected during SALSEA Greenland and SALSEA-Merge will further understanding of the ecology of marine-phase Atlantic salmon and inform investigations into stock-specific differences in marine productivity.Sheehan, T. F., Reddin, D. G., Chaput, G., and Renkawitz, M. D. 2012. SALSEA North America: a pelagic ecosystem survey targeting Atlantic salmon in the Northwest Atlantic. – ICES Journal of Marine Science, 69: 1580–1588.
Introduction
Declines in Atlantic salmon (Salmo salar) abundance during the late 1980s and 1990s were unprecedented in magnitude (ICES, 2011). Population dynamics are initially set by events in freshwater (recruits), but survival through the marine phase establishes the initial state (spawners) for the next generation (Hansen and Quinn, 1998). Despite decades of research, the mechanisms driving the marine survival of Atlantic salmon remain elusive. Our current understanding of Atlantic salmon ocean ecology stems from sampling the distant-water fisheries and a limited number of dedicated research surveys. However, these efforts are limited in time and space by fishery dynamics and logistical constraints on research surveys.
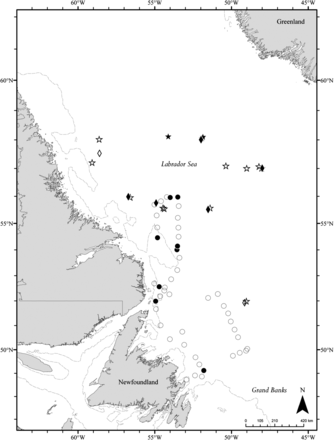
Atlantic salmon marine mortality is high and variable within and among stocks (ICES, 2011), and is attributable to man-made (Fairchild et al., 1999) and natural factors (Ritter, 1989; Reddin and Friedland, 1993; Jonsson and Jonsson, 2004). Detailed information on stock-specific migration routes and distributions is lacking, although various life stages and stocks overlap (Ritter, 1989). North American post-smolts are found north and east of Newfoundland (Figure 1) during summer (Montevecchi et al., 2002) and in much of the Labrador Sea during autumn (Reddin and Short, 1991), probably overwintering in the southern Labrador Sea and Grand Banks (Reddin, 1985, 1988; Ritter, 1989). In spring, salmon have been documented from the southern edge of the Grand Banks to slightly south of Greenland in surface waters ranging from 3 to 8°C (Reddin and Shearer, 1987; Reddin, 1988). Non-maturing one-sea-winter (1SW) salmon are assumed to have overwintered in the Labrador Sea (Idler et al., 1981) and are found along the coast of Greenland in the following summer and autumn (ICES, 2011). This information allows definition of the areas occupied by salmon at sea and elaboration of general migration routes (Reddin, 1988; Dadswell et al., 2010).
SALSEA North America station locations and the 200 m isobath. Map symbols represent surface trawl stations (2008, circles; 2009, stars), gillnet sets (diamonds), and stations where Atlantic salmon were caught (filled symbols) and not caught (open symbols).
The Salmon at Sea programme (SALSEA) has facilitated the sharing of resources among countries, to conduct a comprehensive marine research survey for salmon focusing on areas with many cohabiting stocks. A component of this programme, SALSEA North America, was designed as a comprehensive research initiative involving marine surveys, tracking studies, index-river monitoring, and analysis of historical datasets to address questions related to the marine-phase dynamics and ecology of North American salmon populations. Pelagic ecosystem surveys were conducted in the Labrador Sea during August 2008 and September 2009, to provide information on the position of salmon within the pelagic ecosystem by characterizing their relative abundance and distribution in relation to the co-occurring fish species' complex and oceanographic conditions.
Methods
Single-vessel trawling was conducted to sample the upper 10 m of the water column. The trawl was 123 m long with a circumference of 237 m at the mouth. It had extra-long wings (59 m along the headrope) and was fished with Thyboron Type 8 (3 m2) pelagic doors to maximize net spread. The headrope was fitted with 100 m of 70-mm polyrope to provide extra floatation and ensure contact with the surface. An aluminium aquarium codend was intermittently used in 2009. A full description of the trawl gear is provided by Lacroix and Knox (2005).
Each station was trawled for 1 h in 2008 and for 2 h in 2009. A rudder setting of 5–10° port or starboard kept the trawl away from the propeller wash. The objective was to obtain a ground speed of 3.5–4.0 knots for all tows. Adjustments to boat speed, warp deployed (generally 285 m), and bearing were made to keep the pelagic doors below the surface and to maintain headrope visibility at the surface. During 2009, gillnet sets of 750 (1372 m), 1050 (1920 m), and 1200 (2195 m) fathoms in length (1, 4, and 2 sets, respectively) were fished at the surface. Six mesh sizes were deployed in each set (64, 76, 89, 102, 114, and 127 mm). The nets were 3 m deep and constructed of monofilament. Gillnets were fished for 2.5–33.5 h, depending on weather conditions. Mechanical issues prevented gillnetting in 2008.
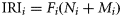
Catch per unit effort (cpue) was calculated for all gear sets. Gillnet cpue was expressed as the number of salmon caught per nautical mile–hour of gear fished. Mile–hour is the product of the total length of the nets fished and hours fished. Surface-trawl cpue is expressed as the number of salmon caught per hour trawled. These two cpue measures, although not directly comparable because of different fishing gear and methods, provide a relative measure of abundance.
Oceanographic data were collected at selected stations using Sea-Bird 19 SEACAT (2008) and SEACAT 25 (2009) conductivity, temperature, and depth profilers (CTDs). CTDs were deployed either to the bottom or to a maximum depth of 300 m. Vertical plankton samples were collected from 100 m to the surface using a net of 200-μm mesh retrieved at a rate of 1 m s–1. Samples were preserved in formalin and processed in the laboratory. Vemco© 8-bit minilog dataloggers were attached to the trawl footrope and gillnet leadline to record temperature and depth.
Results
Surveys were conducted from 8 to 21 August 2008 (CCGS “Wilfred Templeman”) and from 11 to 26 September 2009 (CCGS “Alfred Needler”). Mechanical issues prevented sampling from 11 to 14 August 2008, and severe weather and operational logistics severely hindered operations throughout the 2009 survey. In all, 46 and 14 surface trawls were conducted in 2008 and 2009, respectively, and 7 gillnet sets were made in 2009. Sampling took place from just south of 49°N to 56°N and from 49°W to 55°W in 2008, and from 52°N to 58°N and from 48°W to 59°W in 2009 (Figure 1). Bottom depths ranged from 100 m to 3700+ m, and water temperatures (∼10 m depth) ranged from 1.9 to 14.4°C. The mean depth of the trawl footrope was 9.0 m (6.4–10.9 m) in 2008 and 14.3 m (8.8–19.9 m) in 2009.
In total, 107 salmon were captured (15 in 2008 and 92 in 2009). In 2008, all salmon caught were post-smolts, all salmon catches were made during daylight, and all but one salmon was caught north of 52°N (Figure 1). In 2009, eight post-smolts were caught with the surface trawl (all with the aquarium attached), five of which were captured during two night tows. Compared with 2008, salmon in 2009 were caught at locations with cooler water temperatures at footrope depth (5.0–7.5°C in 2009 vs. 9.5–13.4°C in 2008) and at greater depth (1000–3700+ m in 2009 vs. 187–3000+ m in 2008). In total, 84 salmon were captured with gillnets: 60 post-smolts and 24 adults. Six post-smolts and one adult dropped out of the net during retrieval and so yielded no biological data. Two surface trawls and one gillnet set were conducted in the colder water of the Labrador Current, but no salmon were caught. In all, 100 salmon were sampled for biological, morphometric, and biochemical data.
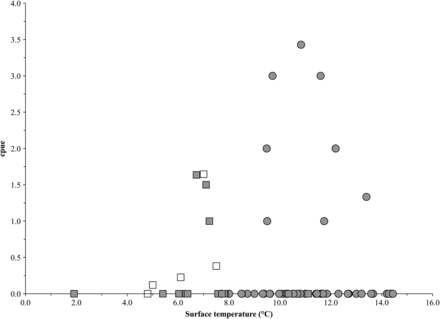
No salmon were caught in 76% of the sets (83 and 79% of the surface trawls in 2008 and 2009, respectively, and 29% of the gillnet sets). Salmon catches were made at surface water temperatures ranging from 5.0 to 13.4°C (Figure 2). The overall mean surface temperature, weighted by the number of salmon caught, was 6.7°C (±2.9). The mean surface temperature for trawls that caught salmon was 10.9°C (±1.3) in 2008 and 7.0°C (±0.2) in 2009, and 5.9°C (±2.6) for the gillnets.
Atlantic salmon cpue for the 2008 surface trawl (filled grey circles), 2009 surface trawl (filled grey squares), and 2009 gillnet sets (open squares), by surface temperature. For two gillnet sets (cpue 0.0 and 1.194), the temperature was not recorded.
The relative abundance of Atlantic salmon was low in both trawl and gillnet catches. In 2008, the mean trawl cpue for post-smolts was 0.36 (±0.89), slightly greater than the 2009 trawl mean (0.30 ± 0.60). No adult salmon were captured with the surface trawl in 2008 or 2009. Gillnet cpue (adult and post-smolt) was 0.51 (±0.65). Mesh sizes of 64, 76, and 89 mm caught 98% of the post-smolts, and 88% of the adult catches came from mesh sizes of 102, 114, and 127 mm.
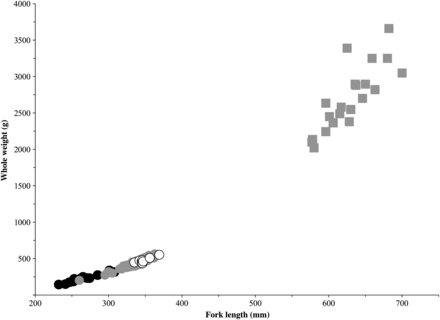
Trawl-caught post-smolts ranged in length from 232 to 369 mm, and those from gillnets from 260 to 363 mm (Figure 3). The mean post-smolt fork lengths and whole weights were 267.6 mm (±23.7 s.d.) and 231.6 g (±59.7) in 2008, and 335.8 mm (±19.6, gillnet), 435.5 g (±74.2, gillnet), 348.3 mm (±10.2, trawl), and 478.0 g (±37.4, trawl) in 2009. For adults, the mean fork lengths and whole weights were 628.7 mm (±35.3) and 1006.1 g (±444.9), respectively. Surface trawl-caught post-smolts were ∼50% female, gillnet-caught post-smolts were 64% female, and adults were 70% female. Salmon with a wide range of river ages were caught (1–5 years, n= 83), but 84% of the fish had spent 2–4 years in freshwater. Two adults were previous spawners and 21 were 1SW fish. Scale samples were not available in 2008 because of net-induced descaling in the absence of the aquarium codend.
Atlantic salmon adult and post-smolt length–weight data, with symbols representing 2008 trawl-captured post-smolts (black circles), 2009 gillnet-captured post-smolts (grey circles), 2009 trawl-captured post-smolts (open circles), and 2009 gillnet-caught adults (grey squares).
A single attached sea louse (Caligidae) was recorded in 2008, and 5.3% of the salmon captured in 2009 had an average of 2.3 sea lice. In both years, ∼60% of the post-smolts and adults were infested with intestinal macroparasites (Table 1). Intestinal macroparasite prevalence was generally higher in 2009 and highest in the adult samples. Overall, 16% of the salmon had more than one type of parasite, and 19% were heavily infested. “Heavily infested” was a subjective determination made during sample processing based on an assessment of the parasite load relative to the size of the intestine. Generally speaking, it amounted to either >0.1 g of the parasite (Acanthocephala or Cestoda) or 0.2 individual parasites (Nematoda) per centimetre length of the fish. The high 2009 post-smolt Acanthocephala mean intensity measure was influenced by a few individuals with high parasite loads.
Macroparasites identified from Atlantic salmon intestines collected during the 2008 and 2009 surveys, giving detailed information on the sample size, number of samples containing 0–3 different parasite types, prevalence, mean intensity (I)±standard deviation, and intensity range for each parasite type across all samples for each life stage in each year.
| Parameter . | 2008 post-smolts . | 2009 post-smolts . | 2009 adults . |
|---|---|---|---|
| Sample size | 15 | 63 | 19 |
| Number of parasite types | |||
| 0 | 10 | 28 | 1 |
| 1 | 3 | 26 | 13 |
| 2 | 2 | 8 | 5 |
| 3 | 0 | 1 | 0 |
| Acanthocephala | |||
| Prevalence | 33.3% | 20.6% | 21.1% |
| I (g) | 0.03 ± 0.02 | 8.03 ± 13.12 | 1.50 ± 0.58 |
| Range | 0.01–0.05 | 1.0–41.0 | 1.0–2.0 |
| Cestoda | |||
| Prevalence | 6.7% | 9.5% | 15.8% |
| I (g) | 0.06a | 0.02 ± 0.02 | 2.56 ± 3.58 |
| Range | a | 0.01–0.06 | 0.36–6.69 |
| Nematoda | |||
| Prevalence | 6.7% | 41.3% | 84.2% |
| I (number) | 1.0a | 1.9 ± 1.26 | 15.8 ± 18.38 |
| Range | a | 1.0–5.0 | 1.0–61.0 |
| Parameter . | 2008 post-smolts . | 2009 post-smolts . | 2009 adults . |
|---|---|---|---|
| Sample size | 15 | 63 | 19 |
| Number of parasite types | |||
| 0 | 10 | 28 | 1 |
| 1 | 3 | 26 | 13 |
| 2 | 2 | 8 | 5 |
| 3 | 0 | 1 | 0 |
| Acanthocephala | |||
| Prevalence | 33.3% | 20.6% | 21.1% |
| I (g) | 0.03 ± 0.02 | 8.03 ± 13.12 | 1.50 ± 0.58 |
| Range | 0.01–0.05 | 1.0–41.0 | 1.0–2.0 |
| Cestoda | |||
| Prevalence | 6.7% | 9.5% | 15.8% |
| I (g) | 0.06a | 0.02 ± 0.02 | 2.56 ± 3.58 |
| Range | a | 0.01–0.06 | 0.36–6.69 |
| Nematoda | |||
| Prevalence | 6.7% | 41.3% | 84.2% |
| I (number) | 1.0a | 1.9 ± 1.26 | 15.8 ± 18.38 |
| Range | a | 1.0–5.0 | 1.0–61.0 |
aIdentified in one sample only.
Macroparasites identified from Atlantic salmon intestines collected during the 2008 and 2009 surveys, giving detailed information on the sample size, number of samples containing 0–3 different parasite types, prevalence, mean intensity (I)±standard deviation, and intensity range for each parasite type across all samples for each life stage in each year.
| Parameter . | 2008 post-smolts . | 2009 post-smolts . | 2009 adults . |
|---|---|---|---|
| Sample size | 15 | 63 | 19 |
| Number of parasite types | |||
| 0 | 10 | 28 | 1 |
| 1 | 3 | 26 | 13 |
| 2 | 2 | 8 | 5 |
| 3 | 0 | 1 | 0 |
| Acanthocephala | |||
| Prevalence | 33.3% | 20.6% | 21.1% |
| I (g) | 0.03 ± 0.02 | 8.03 ± 13.12 | 1.50 ± 0.58 |
| Range | 0.01–0.05 | 1.0–41.0 | 1.0–2.0 |
| Cestoda | |||
| Prevalence | 6.7% | 9.5% | 15.8% |
| I (g) | 0.06a | 0.02 ± 0.02 | 2.56 ± 3.58 |
| Range | a | 0.01–0.06 | 0.36–6.69 |
| Nematoda | |||
| Prevalence | 6.7% | 41.3% | 84.2% |
| I (number) | 1.0a | 1.9 ± 1.26 | 15.8 ± 18.38 |
| Range | a | 1.0–5.0 | 1.0–61.0 |
| Parameter . | 2008 post-smolts . | 2009 post-smolts . | 2009 adults . |
|---|---|---|---|
| Sample size | 15 | 63 | 19 |
| Number of parasite types | |||
| 0 | 10 | 28 | 1 |
| 1 | 3 | 26 | 13 |
| 2 | 2 | 8 | 5 |
| 3 | 0 | 1 | 0 |
| Acanthocephala | |||
| Prevalence | 33.3% | 20.6% | 21.1% |
| I (g) | 0.03 ± 0.02 | 8.03 ± 13.12 | 1.50 ± 0.58 |
| Range | 0.01–0.05 | 1.0–41.0 | 1.0–2.0 |
| Cestoda | |||
| Prevalence | 6.7% | 9.5% | 15.8% |
| I (g) | 0.06a | 0.02 ± 0.02 | 2.56 ± 3.58 |
| Range | a | 0.01–0.06 | 0.36–6.69 |
| Nematoda | |||
| Prevalence | 6.7% | 41.3% | 84.2% |
| I (number) | 1.0a | 1.9 ± 1.26 | 15.8 ± 18.38 |
| Range | a | 1.0–5.0 | 1.0–61.0 |
aIdentified in one sample only.
Prey consumption by weight varied annually (Table 2). In 2008, post-smolts had consumed primarily Themisto compressa (67.8%), fish (44.8%, Leptoclinus maculatus, Helicolenus dactylopterus, miscellaneous remains, and Leptagonus decagonus), and Cephalopoda (4.7%, Gonatus fabricii). In 2009, although the diversity of prey in the post-smolt diet increased, T. compressa (37.0%) remained the primary prey, with fish (11.6%, Ammodytes americanus, miscellaneous remains, and Myctophidae sp.), Cephalopoda (19.3%, G. fabricii, Teuthida and Octopoda), and Meganyctiphanes norvegica (4.3%) also consumed. Adults consumed similar items to the post-smolts, with the addition of Arctozenus risso (12.9%) and Mollusca (2.4%). The diversity of prey consumed is of note. However, the IRI values of 93.2, 94.0, and 97.2%, respectively, highlight the importance of T. compressa to Atlantic salmon diets in the Labrador Sea.
Prey items identified from Atlantic salmon stomachs collected during the 2008 and 2009 surveys (in all, 15 post-smolt stomachs were collected in 2008 and 19 post-smolt and 55 adult stomachs in 2009), giving detailed information on each prey item's percent occurrence, mass, and IRI across all samples for each life stage for each year.
| Prey item . | 2008 post-smolts . | 2009 post-smolts . | 2009 adults . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Occurrence (%) . | Mass (%) . | IRI (%) . | Occurrence (%) . | Mass (%) . | IRI (%) . | Occurrence (%) . | Mass (%) . | IRI (%) . | |
| Animal remains | – | – | – | 28.1 | 25.3 | – | 33.3 | 13.1 | – |
| Pisces | 14.3 | 10.3 | – | 3.1 | 4.2 | – | 11.9 | 19.6 | – |
| Mollusca | – | – | – | – | – | – | 2.4 | 0.6 | <0.1 |
| Decapoda | 3.6 | 6.0 | 0.2 | 1.6 | 1.1 | <0.1 | – | – | – |
| Octopoda | – | – | – | 0.8 | 0.3 | <0.1 | 2.4 | 1.7 | 0.2 |
| Teuthida | – | – | – | 1.6 | 1.2 | <0.1 | 2.4 | 1.1 | <0.1 |
| Cancer spp. | 10.7 | 24.5 | 3.2 | – | – | – | – | – | – |
| Ammodytes americanus | – | – | – | 3.1 | 4.3 | 0.3 | 4.8 | 4.7 | 0.8 |
| Arctozenus risso | – | – | – | – | – | – | 2.4 | 12.9 | 1.0 |
| Helicolenus dactylopterus | 3.6 | 12.8 | 0.5 | – | – | – | – | – | – |
| Leptoclinus maculatus | 10.7 | 17.8 | 2.5 | – | – | – | – | – | – |
| Leptagonus decagonus | 3.6 | 3.9 | 0.2 | – | – | – | – | – | – |
| Myctophidae | – | – | – | 2.3 | 3.1 | 0.2 | – | – | – |
| Meganyctiphanes norvegica | – | – | – | 5.5 | 4.3 | 0.5 | 2.4 | 12.9 | <0.1 |
| Gonatus fabricii | 3.6 | 4.7 | 0.2 | 11.7 | 19.0 | 4.9 | 4.8 | 2.8 | 0.6 |
| Themisto compressa | 50.0 | 67.8 | 93.2 | 42.2 | 37.0 | 94.0 | 33.3 | 30.6 | 97.2 |
| Prey item . | 2008 post-smolts . | 2009 post-smolts . | 2009 adults . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Occurrence (%) . | Mass (%) . | IRI (%) . | Occurrence (%) . | Mass (%) . | IRI (%) . | Occurrence (%) . | Mass (%) . | IRI (%) . | |
| Animal remains | – | – | – | 28.1 | 25.3 | – | 33.3 | 13.1 | – |
| Pisces | 14.3 | 10.3 | – | 3.1 | 4.2 | – | 11.9 | 19.6 | – |
| Mollusca | – | – | – | – | – | – | 2.4 | 0.6 | <0.1 |
| Decapoda | 3.6 | 6.0 | 0.2 | 1.6 | 1.1 | <0.1 | – | – | – |
| Octopoda | – | – | – | 0.8 | 0.3 | <0.1 | 2.4 | 1.7 | 0.2 |
| Teuthida | – | – | – | 1.6 | 1.2 | <0.1 | 2.4 | 1.1 | <0.1 |
| Cancer spp. | 10.7 | 24.5 | 3.2 | – | – | – | – | – | – |
| Ammodytes americanus | – | – | – | 3.1 | 4.3 | 0.3 | 4.8 | 4.7 | 0.8 |
| Arctozenus risso | – | – | – | – | – | – | 2.4 | 12.9 | 1.0 |
| Helicolenus dactylopterus | 3.6 | 12.8 | 0.5 | – | – | – | – | – | – |
| Leptoclinus maculatus | 10.7 | 17.8 | 2.5 | – | – | – | – | – | – |
| Leptagonus decagonus | 3.6 | 3.9 | 0.2 | – | – | – | – | – | – |
| Myctophidae | – | – | – | 2.3 | 3.1 | 0.2 | – | – | – |
| Meganyctiphanes norvegica | – | – | – | 5.5 | 4.3 | 0.5 | 2.4 | 12.9 | <0.1 |
| Gonatus fabricii | 3.6 | 4.7 | 0.2 | 11.7 | 19.0 | 4.9 | 4.8 | 2.8 | 0.6 |
| Themisto compressa | 50.0 | 67.8 | 93.2 | 42.2 | 37.0 | 94.0 | 33.3 | 30.6 | 97.2 |
Prey items identified from Atlantic salmon stomachs collected during the 2008 and 2009 surveys (in all, 15 post-smolt stomachs were collected in 2008 and 19 post-smolt and 55 adult stomachs in 2009), giving detailed information on each prey item's percent occurrence, mass, and IRI across all samples for each life stage for each year.
| Prey item . | 2008 post-smolts . | 2009 post-smolts . | 2009 adults . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Occurrence (%) . | Mass (%) . | IRI (%) . | Occurrence (%) . | Mass (%) . | IRI (%) . | Occurrence (%) . | Mass (%) . | IRI (%) . | |
| Animal remains | – | – | – | 28.1 | 25.3 | – | 33.3 | 13.1 | – |
| Pisces | 14.3 | 10.3 | – | 3.1 | 4.2 | – | 11.9 | 19.6 | – |
| Mollusca | – | – | – | – | – | – | 2.4 | 0.6 | <0.1 |
| Decapoda | 3.6 | 6.0 | 0.2 | 1.6 | 1.1 | <0.1 | – | – | – |
| Octopoda | – | – | – | 0.8 | 0.3 | <0.1 | 2.4 | 1.7 | 0.2 |
| Teuthida | – | – | – | 1.6 | 1.2 | <0.1 | 2.4 | 1.1 | <0.1 |
| Cancer spp. | 10.7 | 24.5 | 3.2 | – | – | – | – | – | – |
| Ammodytes americanus | – | – | – | 3.1 | 4.3 | 0.3 | 4.8 | 4.7 | 0.8 |
| Arctozenus risso | – | – | – | – | – | – | 2.4 | 12.9 | 1.0 |
| Helicolenus dactylopterus | 3.6 | 12.8 | 0.5 | – | – | – | – | – | – |
| Leptoclinus maculatus | 10.7 | 17.8 | 2.5 | – | – | – | – | – | – |
| Leptagonus decagonus | 3.6 | 3.9 | 0.2 | – | – | – | – | – | – |
| Myctophidae | – | – | – | 2.3 | 3.1 | 0.2 | – | – | – |
| Meganyctiphanes norvegica | – | – | – | 5.5 | 4.3 | 0.5 | 2.4 | 12.9 | <0.1 |
| Gonatus fabricii | 3.6 | 4.7 | 0.2 | 11.7 | 19.0 | 4.9 | 4.8 | 2.8 | 0.6 |
| Themisto compressa | 50.0 | 67.8 | 93.2 | 42.2 | 37.0 | 94.0 | 33.3 | 30.6 | 97.2 |
| Prey item . | 2008 post-smolts . | 2009 post-smolts . | 2009 adults . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Occurrence (%) . | Mass (%) . | IRI (%) . | Occurrence (%) . | Mass (%) . | IRI (%) . | Occurrence (%) . | Mass (%) . | IRI (%) . | |
| Animal remains | – | – | – | 28.1 | 25.3 | – | 33.3 | 13.1 | – |
| Pisces | 14.3 | 10.3 | – | 3.1 | 4.2 | – | 11.9 | 19.6 | – |
| Mollusca | – | – | – | – | – | – | 2.4 | 0.6 | <0.1 |
| Decapoda | 3.6 | 6.0 | 0.2 | 1.6 | 1.1 | <0.1 | – | – | – |
| Octopoda | – | – | – | 0.8 | 0.3 | <0.1 | 2.4 | 1.7 | 0.2 |
| Teuthida | – | – | – | 1.6 | 1.2 | <0.1 | 2.4 | 1.1 | <0.1 |
| Cancer spp. | 10.7 | 24.5 | 3.2 | – | – | – | – | – | – |
| Ammodytes americanus | – | – | – | 3.1 | 4.3 | 0.3 | 4.8 | 4.7 | 0.8 |
| Arctozenus risso | – | – | – | – | – | – | 2.4 | 12.9 | 1.0 |
| Helicolenus dactylopterus | 3.6 | 12.8 | 0.5 | – | – | – | – | – | – |
| Leptoclinus maculatus | 10.7 | 17.8 | 2.5 | – | – | – | – | – | – |
| Leptagonus decagonus | 3.6 | 3.9 | 0.2 | – | – | – | – | – | – |
| Myctophidae | – | – | – | 2.3 | 3.1 | 0.2 | – | – | – |
| Meganyctiphanes norvegica | – | – | – | 5.5 | 4.3 | 0.5 | 2.4 | 12.9 | <0.1 |
| Gonatus fabricii | 3.6 | 4.7 | 0.2 | 11.7 | 19.0 | 4.9 | 4.8 | 2.8 | 0.6 |
| Themisto compressa | 50.0 | 67.8 | 93.2 | 42.2 | 37.0 | 94.0 | 33.3 | 30.6 | 97.2 |
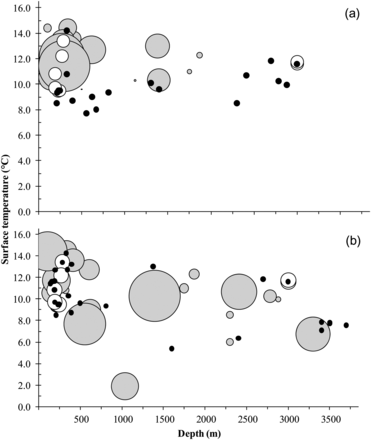
Gillnets were highly selective, catching only Atlantic salmon, with some seabird and marine mammal bycatch. The surface trawl, although specifically designed to catch Atlantic salmon, was effective at sampling other species present in the upper part of the pelagic ecosystem (Table 3). In both years, lumpfish (Cyclopterus lumpus) was the most common species captured, at 63 and 86% of the stations sampled in 2008 and 2009, respectively. Atlantic saury (Scomberesox saurus) was abundant in the 2008 survey (59%), but not in 2009 (7%). A number of species that were captured in 2008 were not captured in 2009, and barracudina (Paralepididae sp.) was the only new species captured in 2009. Juvenile Atlantic wolfish (Anarhichas lupus), a species of Special Concern in Canada (Species at Risk Act, SC 2002, c. 29), was captured at 17% of the stations sampled in 2008. Other commercially important juvenile species, Greenland halibut (Reinhardtius hippoglossoides) and redfish (Sebastidae), were also captured. Potential Atlantic salmon prey were captured, Atlantic sandlance (A. americanus), capelin (Mallotus villosus), Arthropoda, and Cephalopoda spp., as well as other species of interest (Figure 4). Lanternfish (Myctophidae) were predictably captured at night and in water depths of 500+ m.
Percentage occurrence at the surface trawl stations, mean weight (kg) caught, and the mean number of each species caught for all non-salmon species taken during the 2008 and 2009 surveys (mean number captured has been estimated for some groups).
| Species . | 2008 . | 2009 . | ||||
|---|---|---|---|---|---|---|
| Occurrence (%) . | Mean weight captured (kg) . | Mean number captured . | Occurrence (%) . | Mean weight captured (kg) . | Mean number captured . | |
| Ammodytes americanus | 17.4 | 0.03 | 5.3 | 14.3 | 0.01 | 1.5 |
| Anarhichas lupus | 17.4 | 0.02 | 10.9 | – | – | – |
| Arthropoda spp.a | 41.3 | 0.38 | 57.6 | 50.0 | 0.06 | 235.7 |
| Cephalopoda spp.b | 67.4 | 0.18 | 30.5 | 50.0 | 0.16 | 16.7 |
| Clupea harengus | 6.5 | 0.17 | 1.0 | – | – | – |
| Cnidaria spp.c | 45.7 | 0.89 | 16.5 | 64.3 | 6.33 | 328.4 |
| Cottoidea sp. | 17.4 | 0.00 | 5.9 | – | – | – |
| Cyclopterus lumpus | 63.0 | 11.12 | 13.3 | 85.7 | 21.82 | 17.1 |
| Gadus morhua | 8.7 | 0.01 | 14.8 | – | – | – |
| Gaidropsarus argentatus | 23.9 | 0.02 | 6.6 | 14.3 | 0.01 | 1.0 |
| Gasterosteus aculeatus | 8.7 | 0.00 | 1.0 | 7.1 | 0.01 | 4.0 |
| Leptoclinus maculatus | 8.7 | 0.02 | 37.3 | – | – | – |
| Mallotus villosus | 13.0 | 0.02 | 2.5 | – | – | – |
| Myctophidae sp. | 19.6 | 8.14 | 1 364.9 | 28.6 | 22.47 | 2 442.5 |
| Paralepididae sp. | – | – | – | 7.1 | 4.20 | 78.0 |
| Reinhardtius hippoglossoides | 21.7 | 0.01 | 7.0 | 14.3 | 0.02 | 5.0 |
| Scomberesox saurus | 58.7 | 47.31 | 567.8 | 7.1 | 14.95 | 255.0 |
| Sebastidae sp. | 30.4 | 0.06 | 20.1 | 42.9 | 0.06 | 33.5 |
| Ulcina olrikii | 6.5 | 0.00 | 2.3 | – | – | – |
| Unidentified fish | 32.6 | 0.00 | 3.3 | – | – | – |
| Species . | 2008 . | 2009 . | ||||
|---|---|---|---|---|---|---|
| Occurrence (%) . | Mean weight captured (kg) . | Mean number captured . | Occurrence (%) . | Mean weight captured (kg) . | Mean number captured . | |
| Ammodytes americanus | 17.4 | 0.03 | 5.3 | 14.3 | 0.01 | 1.5 |
| Anarhichas lupus | 17.4 | 0.02 | 10.9 | – | – | – |
| Arthropoda spp.a | 41.3 | 0.38 | 57.6 | 50.0 | 0.06 | 235.7 |
| Cephalopoda spp.b | 67.4 | 0.18 | 30.5 | 50.0 | 0.16 | 16.7 |
| Clupea harengus | 6.5 | 0.17 | 1.0 | – | – | – |
| Cnidaria spp.c | 45.7 | 0.89 | 16.5 | 64.3 | 6.33 | 328.4 |
| Cottoidea sp. | 17.4 | 0.00 | 5.9 | – | – | – |
| Cyclopterus lumpus | 63.0 | 11.12 | 13.3 | 85.7 | 21.82 | 17.1 |
| Gadus morhua | 8.7 | 0.01 | 14.8 | – | – | – |
| Gaidropsarus argentatus | 23.9 | 0.02 | 6.6 | 14.3 | 0.01 | 1.0 |
| Gasterosteus aculeatus | 8.7 | 0.00 | 1.0 | 7.1 | 0.01 | 4.0 |
| Leptoclinus maculatus | 8.7 | 0.02 | 37.3 | – | – | – |
| Mallotus villosus | 13.0 | 0.02 | 2.5 | – | – | – |
| Myctophidae sp. | 19.6 | 8.14 | 1 364.9 | 28.6 | 22.47 | 2 442.5 |
| Paralepididae sp. | – | – | – | 7.1 | 4.20 | 78.0 |
| Reinhardtius hippoglossoides | 21.7 | 0.01 | 7.0 | 14.3 | 0.02 | 5.0 |
| Scomberesox saurus | 58.7 | 47.31 | 567.8 | 7.1 | 14.95 | 255.0 |
| Sebastidae sp. | 30.4 | 0.06 | 20.1 | 42.9 | 0.06 | 33.5 |
| Ulcina olrikii | 6.5 | 0.00 | 2.3 | – | – | – |
| Unidentified fish | 32.6 | 0.00 | 3.3 | – | – | – |
A single Porbeagle shark (Lamna nasus) and a sea snail (Gastropoda) were captured in 2008.
aArthropoda spp. represents euphausiids, amphipods (Themisto sp.), and various shrimp species.
bCephalopoda spp. represents Illex illecebrosus and G. fabricii, determined via stomach content analysis of captured Atlantic salmon.
cCnidaria spp. represents Clionoideas (sea angles), Ctenophores (comb jellies), and Scyphozoans (true jellies).
Percentage occurrence at the surface trawl stations, mean weight (kg) caught, and the mean number of each species caught for all non-salmon species taken during the 2008 and 2009 surveys (mean number captured has been estimated for some groups).
| Species . | 2008 . | 2009 . | ||||
|---|---|---|---|---|---|---|
| Occurrence (%) . | Mean weight captured (kg) . | Mean number captured . | Occurrence (%) . | Mean weight captured (kg) . | Mean number captured . | |
| Ammodytes americanus | 17.4 | 0.03 | 5.3 | 14.3 | 0.01 | 1.5 |
| Anarhichas lupus | 17.4 | 0.02 | 10.9 | – | – | – |
| Arthropoda spp.a | 41.3 | 0.38 | 57.6 | 50.0 | 0.06 | 235.7 |
| Cephalopoda spp.b | 67.4 | 0.18 | 30.5 | 50.0 | 0.16 | 16.7 |
| Clupea harengus | 6.5 | 0.17 | 1.0 | – | – | – |
| Cnidaria spp.c | 45.7 | 0.89 | 16.5 | 64.3 | 6.33 | 328.4 |
| Cottoidea sp. | 17.4 | 0.00 | 5.9 | – | – | – |
| Cyclopterus lumpus | 63.0 | 11.12 | 13.3 | 85.7 | 21.82 | 17.1 |
| Gadus morhua | 8.7 | 0.01 | 14.8 | – | – | – |
| Gaidropsarus argentatus | 23.9 | 0.02 | 6.6 | 14.3 | 0.01 | 1.0 |
| Gasterosteus aculeatus | 8.7 | 0.00 | 1.0 | 7.1 | 0.01 | 4.0 |
| Leptoclinus maculatus | 8.7 | 0.02 | 37.3 | – | – | – |
| Mallotus villosus | 13.0 | 0.02 | 2.5 | – | – | – |
| Myctophidae sp. | 19.6 | 8.14 | 1 364.9 | 28.6 | 22.47 | 2 442.5 |
| Paralepididae sp. | – | – | – | 7.1 | 4.20 | 78.0 |
| Reinhardtius hippoglossoides | 21.7 | 0.01 | 7.0 | 14.3 | 0.02 | 5.0 |
| Scomberesox saurus | 58.7 | 47.31 | 567.8 | 7.1 | 14.95 | 255.0 |
| Sebastidae sp. | 30.4 | 0.06 | 20.1 | 42.9 | 0.06 | 33.5 |
| Ulcina olrikii | 6.5 | 0.00 | 2.3 | – | – | – |
| Unidentified fish | 32.6 | 0.00 | 3.3 | – | – | – |
| Species . | 2008 . | 2009 . | ||||
|---|---|---|---|---|---|---|
| Occurrence (%) . | Mean weight captured (kg) . | Mean number captured . | Occurrence (%) . | Mean weight captured (kg) . | Mean number captured . | |
| Ammodytes americanus | 17.4 | 0.03 | 5.3 | 14.3 | 0.01 | 1.5 |
| Anarhichas lupus | 17.4 | 0.02 | 10.9 | – | – | – |
| Arthropoda spp.a | 41.3 | 0.38 | 57.6 | 50.0 | 0.06 | 235.7 |
| Cephalopoda spp.b | 67.4 | 0.18 | 30.5 | 50.0 | 0.16 | 16.7 |
| Clupea harengus | 6.5 | 0.17 | 1.0 | – | – | – |
| Cnidaria spp.c | 45.7 | 0.89 | 16.5 | 64.3 | 6.33 | 328.4 |
| Cottoidea sp. | 17.4 | 0.00 | 5.9 | – | – | – |
| Cyclopterus lumpus | 63.0 | 11.12 | 13.3 | 85.7 | 21.82 | 17.1 |
| Gadus morhua | 8.7 | 0.01 | 14.8 | – | – | – |
| Gaidropsarus argentatus | 23.9 | 0.02 | 6.6 | 14.3 | 0.01 | 1.0 |
| Gasterosteus aculeatus | 8.7 | 0.00 | 1.0 | 7.1 | 0.01 | 4.0 |
| Leptoclinus maculatus | 8.7 | 0.02 | 37.3 | – | – | – |
| Mallotus villosus | 13.0 | 0.02 | 2.5 | – | – | – |
| Myctophidae sp. | 19.6 | 8.14 | 1 364.9 | 28.6 | 22.47 | 2 442.5 |
| Paralepididae sp. | – | – | – | 7.1 | 4.20 | 78.0 |
| Reinhardtius hippoglossoides | 21.7 | 0.01 | 7.0 | 14.3 | 0.02 | 5.0 |
| Scomberesox saurus | 58.7 | 47.31 | 567.8 | 7.1 | 14.95 | 255.0 |
| Sebastidae sp. | 30.4 | 0.06 | 20.1 | 42.9 | 0.06 | 33.5 |
| Ulcina olrikii | 6.5 | 0.00 | 2.3 | – | – | – |
| Unidentified fish | 32.6 | 0.00 | 3.3 | – | – | – |
A single Porbeagle shark (Lamna nasus) and a sea snail (Gastropoda) were captured in 2008.
aArthropoda spp. represents euphausiids, amphipods (Themisto sp.), and various shrimp species.
bCephalopoda spp. represents Illex illecebrosus and G. fabricii, determined via stomach content analysis of captured Atlantic salmon.
cCnidaria spp. represents Clionoideas (sea angles), Ctenophores (comb jellies), and Scyphozoans (true jellies).
Catches of (a) Scomberesox saurus and (b) cephalopod species by station depth and surface temperature for 2008 and 2009 combined. Filled grey circles represent scaled catch weight per station, and open and black circles represent stations where Atlantic salmon were caught and not caught, respectively. Salmon symbols are not scaled to the weight of the salmon catch.
Equipment failure in both years reduced the ability to collect temperature and salinity data. Only eight CTD casts were conducted in 2008 (during the first half of the survey), and the 2009 data were unreliable because of a cracked unit housing. Available data show a distinct thermohalocline between 10 and 20 m depth characterized by a significant decrease in temperature and a slight increase in salinity. At some stations, salinity measurements actually decreased with depth, likely as a result of local currents. Across all stations sampled, temperatures ranged from 19°C at the surface to –1.3°C at a depth of 300 m, within a range of salinities (27.5–34.9). Salinity differences within individual profiles averaged 3.2 between minimum and maximum values.
In total, 11 and 6 plankton samples were collected in 2008 and 2009, respectively (Table 4). Overall, samples were dominated by individuals from the Phylum Arthropoda (∼93% of all individuals in the samples). In 2008, the warmer southern waters were dominated by small-bodied Oithona nana (63.2%) and much larger Calanidae spp. (28.8%), probably Calanus finmarchicus. The cooler northern waters sampled in 2009 were dominated by the same two copepod species, but at different levels (17.9 and 67.2%, respectively). The 2008 samples also contained Gastropoda (3.3%) and tunicates (Oikipleura sp., 2.9%). Chaetognatha (Sagitta sp.), Polychaeta, and anthropods (Hyperiidae, Ostracoda, Mysida, Cladocera, and Malacostraca) made up 1.3% of the community. The 2009 samples contained ostracods (Proceroecia vitjazi, 3.4%) and tunicates (Oikopleura sp., 2.7%) and arthropods [i.e. Hyperiidae (1.2%), Mysiidae (2.7%)]. Chaetognatha (Sagitta sp.), Polychaeta (Tomopteris sp.), and gastropods collectively made up 3.2% of the community. Ctenophores accounted for 0.1 and 0.7% of all individuals in the samples in 2008 and 2009, respectively. Larval fish and eggs were documented at three stations in 2008 (13 Tautogolabrus adspersus and 1 Argyropelecus sp. Larvae, and 5 Glyptocephalus cynoglossus, 1 Hippoglossoides platessoides, and 1 Enchelyopus cimbrius eggs). No larval fish or eggs were documented in 2009.
Plankton sample results by lowest taxonomic level identified for the 2008 and 2009 surveys, with percentages representing counts of individuals, not mass.
| Phylum . | Group (species) . | 2008 (%) . | 2009 (%) . |
|---|---|---|---|
| Annelida | Polychaeta (Tomopteris sp.) | 0.03 | 0.14 |
| Arthropoda | – | 0.07 | |
| Calanidae (Calanus finmarchicus) | – | 67.24 | |
| Calanidae | 28.85 | – | |
| Cladocera (Pseudevadne tergestina) | 0.38 | – | |
| Cladocera (Pleopis polyphaemoides) | 0.00 | – | |
| Hyperiidea | 0.22 | 1.21 | |
| Malacostraca | 0.01 | 0.95 | |
| Mysida | 0.13 | 2.72 | |
| Oithonidae (Oithona nana) | 63.18 | 17.88 | |
| Ostracoda | 0.16 | – | |
| Ostracoda (Proceroecia vitjazi) | – | 3.37 | |
| Chaetognatha | (Sagitta sp.) | 0.41 | 1.82 |
| Chordata | Tunicata (Oikopleura sp.) | 2.90 | 2.72 |
| Ctenophora | 0.14 | 0.56 | |
| (Aglantha sp.) | – | 0.10 | |
| (Bolinopsis infundibulum) | – | 0.03 | |
| Foraminifera | 0.23 | – | |
| Mollusca | Gastropoda | 3.35% | 1.21 |
| Phylum . | Group (species) . | 2008 (%) . | 2009 (%) . |
|---|---|---|---|
| Annelida | Polychaeta (Tomopteris sp.) | 0.03 | 0.14 |
| Arthropoda | – | 0.07 | |
| Calanidae (Calanus finmarchicus) | – | 67.24 | |
| Calanidae | 28.85 | – | |
| Cladocera (Pseudevadne tergestina) | 0.38 | – | |
| Cladocera (Pleopis polyphaemoides) | 0.00 | – | |
| Hyperiidea | 0.22 | 1.21 | |
| Malacostraca | 0.01 | 0.95 | |
| Mysida | 0.13 | 2.72 | |
| Oithonidae (Oithona nana) | 63.18 | 17.88 | |
| Ostracoda | 0.16 | – | |
| Ostracoda (Proceroecia vitjazi) | – | 3.37 | |
| Chaetognatha | (Sagitta sp.) | 0.41 | 1.82 |
| Chordata | Tunicata (Oikopleura sp.) | 2.90 | 2.72 |
| Ctenophora | 0.14 | 0.56 | |
| (Aglantha sp.) | – | 0.10 | |
| (Bolinopsis infundibulum) | – | 0.03 | |
| Foraminifera | 0.23 | – | |
| Mollusca | Gastropoda | 3.35% | 1.21 |
The 2008 Calanidae samples probably represent C. finmarchicus, although the samples were not identified to species.
Plankton sample results by lowest taxonomic level identified for the 2008 and 2009 surveys, with percentages representing counts of individuals, not mass.
| Phylum . | Group (species) . | 2008 (%) . | 2009 (%) . |
|---|---|---|---|
| Annelida | Polychaeta (Tomopteris sp.) | 0.03 | 0.14 |
| Arthropoda | – | 0.07 | |
| Calanidae (Calanus finmarchicus) | – | 67.24 | |
| Calanidae | 28.85 | – | |
| Cladocera (Pseudevadne tergestina) | 0.38 | – | |
| Cladocera (Pleopis polyphaemoides) | 0.00 | – | |
| Hyperiidea | 0.22 | 1.21 | |
| Malacostraca | 0.01 | 0.95 | |
| Mysida | 0.13 | 2.72 | |
| Oithonidae (Oithona nana) | 63.18 | 17.88 | |
| Ostracoda | 0.16 | – | |
| Ostracoda (Proceroecia vitjazi) | – | 3.37 | |
| Chaetognatha | (Sagitta sp.) | 0.41 | 1.82 |
| Chordata | Tunicata (Oikopleura sp.) | 2.90 | 2.72 |
| Ctenophora | 0.14 | 0.56 | |
| (Aglantha sp.) | – | 0.10 | |
| (Bolinopsis infundibulum) | – | 0.03 | |
| Foraminifera | 0.23 | – | |
| Mollusca | Gastropoda | 3.35% | 1.21 |
| Phylum . | Group (species) . | 2008 (%) . | 2009 (%) . |
|---|---|---|---|
| Annelida | Polychaeta (Tomopteris sp.) | 0.03 | 0.14 |
| Arthropoda | – | 0.07 | |
| Calanidae (Calanus finmarchicus) | – | 67.24 | |
| Calanidae | 28.85 | – | |
| Cladocera (Pseudevadne tergestina) | 0.38 | – | |
| Cladocera (Pleopis polyphaemoides) | 0.00 | – | |
| Hyperiidea | 0.22 | 1.21 | |
| Malacostraca | 0.01 | 0.95 | |
| Mysida | 0.13 | 2.72 | |
| Oithonidae (Oithona nana) | 63.18 | 17.88 | |
| Ostracoda | 0.16 | – | |
| Ostracoda (Proceroecia vitjazi) | – | 3.37 | |
| Chaetognatha | (Sagitta sp.) | 0.41 | 1.82 |
| Chordata | Tunicata (Oikopleura sp.) | 2.90 | 2.72 |
| Ctenophora | 0.14 | 0.56 | |
| (Aglantha sp.) | – | 0.10 | |
| (Bolinopsis infundibulum) | – | 0.03 | |
| Foraminifera | 0.23 | – | |
| Mollusca | Gastropoda | 3.35% | 1.21 |
The 2008 Calanidae samples probably represent C. finmarchicus, although the samples were not identified to species.
Discussion
The objective of the SALSEA North America marine surveys was to sample Atlantic salmon in the nearshore and offshore areas of the Northwest Atlantic and to determine their role within the pelagic ecosystem. Despite a number of problems related to weather, sampling gear, and logistical issues, a considerable amount of new information was collected that, when combined with historical datasets and data collected from the other SALSEA initiatives, will contribute significantly to improved understanding of the marine-phase ecology of Atlantic salmon. Salmon were caught near previously surveyed offshore areas (Reddin and Short, 1991) and in previously unsurveyed inshore areas. Because the marine phase of Atlantic salmon is very dynamic and characterized by fast growth across variable environments (Jonsson and Jonsson, 2004), spatial and temporal differences between surveys renders year-to-year comparisons speculative. Sampling farther north, 1 month later, with a different vessel and gear in 2009 resulted in a vastly different composition and biological characteristics of the catch.
Gillnet cpue was comparable with previous estimates (Reddin and Short, 1991), and surface-trawl cpue was similar between years. Surface trawling allows sampling of a larger area and a broader species assemblage than gillnetting. Surface trawling proved successful at catching post-smolts, although typically in lower numbers than gillnets. The surface trawl appeared to be size-selective, capturing the upper end of the post-smolt size range (Figure 3). This was contrary to expectations that the trawl would be more efficient at capturing smaller-bodied fish with slower swimming speeds than larger conspecifics. It is unlikely that smaller post-smolts escaped through the 20-mm mesh in front of the aquarium codend. However, smaller post-smolts may have escaped through the larger 800 mm upper section behind the headrope. Installing a smaller mesh liner along this section may help prevent this phenomenon (Lacroix and Knox, 2005).
Salmon have previously been caught at temperatures between 5 and 10°C with gillnets (Reddin and Shearer, 1987; Reddin and Short, 1991), but the highest trawl cpue estimates in this study were at temperatures >10°C (Figure 2). However, spatial and temporal differences between the 2008 and 2009 surveys may have contributed to this discrepancy, because the 2008 catches occurred at a time and in an area not well represented in the historical databases. Post-smolts were also captured at night, contrary to previous findings (Shelton et al., 1997). Trawl catchability could be improved by using a vessel with increased horsepower or by pair-trawling (Sheehan et al., 2011). However, the efficacy for capturing adults is unknown (Skilbrei and Jørgensen, 2010). These results will enable researchers to evaluate the utility of different gear types to meet future research objectives.
The age distribution of the catches suggests that salmon of different life stages from across their North American range (ICES, 2011) were present in the Labrador Sea in August and September. This overlap suggests similar habitat requirements. Post-smolts captured in their first year at sea are expected to overwinter in the southern Labrador Sea (Reddin, 1985, 1988; Ritter, 1989) and return to their natal rivers to spawn as grilse (1SW salmon) or continue to feeding grounds at Greenland, returning home to spawn the following year as multi-sea-winter (MSW) salmon (Parrish and Horsted, 1980). Given the large proportion of male grilse in North American rivers (Chaput et al., 2006), it was not unexpected to have an approximately equal male to female post-smolt sex ratio with a skewed adult female proportion among the MSW salmon.
Marine parasitic infestations can reduce survival in salmonid populations directly and indirectly (Bakke and Harris, 1998). Small numbers of sea lice were expected, given that the gear deployed probably removed attached sea lice during sampling. Preliminary results suggest that internal macroparasite loads can be substantial for some salmon in the Labrador Sea and could result in increased mortality via heart disease (Rahkonen et al., 1996), intestinal rupture, or starvation. Even minor infestations can influence adult returns (Bakke and Harris, 1998), and the effects could be more severe at low temperatures (Hurst, 2007).
As growth may influence survival (Friedland et al., 1993; Peyronnet et al., 2007), information on post-smolt foraging conditions is critical when evaluating marine conditions experienced before the first winter at sea. Themisto sp. was the most abundant food item consumed by Atlantic salmon in both years, but different fish species were consumed between years. The observed annual variation in prey consumption and diversity may have been the result of spatial or temporal differences in catch locations between surveys (Lacroix and Knox, 2005). Information on the feeding ecology of post-smolts can be used to evaluate the quality of foraging conditions experienced by post-smolts in the marine phase, given that prey items vary in nutritional and energetic content (Renkawitz and Sheehan, 2011).
The two SALSEA North America surveys provide new information on the ichthyofaunal and planktonic assemblages associated with salmon in the Labrador Sea. Atlantic salmon and Atlantic saury (Table 3 and Figure 4) were two of the few longer-bodied pelagic fish present in considerable numbers in the upper portion of the water column. The biomass of Atlantic saury was more than an order of magnitude greater than that of Atlantic salmon, and the size distribution of the two species overlapped significantly. Atlantic saury could be a competitor with post-smolts for prey resources or could serve as a predation buffer (Hall and Rudstam, 1999).
The capture of Cephalopoda, Paralepididae, and Myctophidae spp. (Table 3 and Figure 4) in trawls and Arthropoda spp. in plankton samples provides some information on prey availability. However, considering the stomach composition of the salmon caught, the two capture methods employed during these surveys (i.e. plankton net and surface trawl) provided a size-biased view of the available forage base. More effective methods designed specifically for sampling smaller pelagic fish and invertebrates will provide a more accurate description of foraging conditions (Brodeur et al., 2008) and greatly add to our knowledge of Atlantic salmon marine ecology. These data are also important when evaluating potential effects of trophic changes on salmon (Mills, 2001; Peyronnet et al., 2008), especially in the light of future climate scenarios (Beaugrand and Reid, 2003).
The SALSEA North America marine surveys met the objectives of sampling the surface layer ecosystem in the Labrador Sea. Although comparatively few Atlantic salmon were captured, those sampled were studied intensively, and the information gained on the surface layer ecosystem is valuable. The surveys provided insights into the marine ecology of salmon at multiple life stages. The additional samples collected for genetic stock identification, disease, stable isotope, and lipid analyses will reveal the status of the Labrador Sea salmon stock as surveyed in 2008 and 2009. A full exploration of these data, historical datasets, and the parallel data collected during SALSEA Greenland and SALSEA-Merge will allow the investigation of stock-specific differences in marine productivity.
Management implications
The experience gained in surface trawling should aid the development of future monitoring programmes. Many national jurisdictions engage in fishery-independent annual sampling programmes to gather data on economically and ecologically important species and the oceanographic and planktonic conditions that influence the health and status of these marine resources. Fish and macroinvertebrate communities in the upper water column are rarely targeted during these surveys, and Atlantic salmon are rarely captured, given the gear types used and the limited abundance of salmon in the ocean (ICES, 2011). However, coupling surface trawling with these existing monitoring programmes/platforms could provide an opportunity to obtain additional data at relatively low cost. As an example, incorporating surface trawling into the Fisheries and Oceans Canada Atlantic Zone Monitoring Programme (Therriault et al., 1998) could provide pelagic ecosystem data from the 11 transects surveyed from southern Nova Scotia through southern Labrador. Demonstration of the quick deployment and retrieval of the surface trawl could help justify the incorporation of this sampling into this existing monitoring programme.
Acknowledgements
This paper is in memory of Bruce Short, a dedicated technician with Fisheries and Oceans Canada for 35 years. Without his efforts, there would be far less information available on salmon in the Labrador Sea. SALSEA North America was a multijurisdictional project supported by Fisheries and Oceans Canada, NOAA National Marine Fisheries Service (USA), Torngat Wildlife, Plants and Fisheries Secretariat Labrador, and Quebec Ministère des Ressouces Naturelles et de la Faune. The paper benefited greatly from reviews by Sean Hayes (National Marine Fisheries Service, Southwest Fisheries Science Center) and Joan Trial (State of Maine, Department of Marine Resources).
References
Author notes
Handling editor: Emory Anderson