-
PDF
- Split View
-
Views
-
Cite
Cite
Alicia S. Miller, Timothy F. Sheehan, Mark D. Renkawitz, Alfred L. Meister, Timothy J. Miller, Revisiting the marine migration of US Atlantic salmon using historical Carlin tag data, ICES Journal of Marine Science, Volume 69, Issue 9, November 2012, Pages 1609–1615, https://doi.org/10.1093/icesjms/fss039
Close - Share Icon Share
Abstract
The development of a fishery for Atlantic salmon (Salmo salar) in the sea at West Greenland in the early 1960s prompted the start of a US tagging programme in 1962. Between 1962 and 1996, more than 1.5 million salmon from New England rivers, primarily hatchery-reared smolts, were tagged and released. Overall, the rate of tag recovery was 0.55%, with 23.2% of the tags recovered from Canada, 26.0% from Greenland, and 50.8% from the United States. A generalized additive model was used to analyse marine survival based on returns of tagged salmon to the Penobscot River. The month and year of release, sea age, smolt age, and environmental variables, such as the North Atlantic Oscillation (NAO) and Atlantic Multidecadal Oscillation (AMO) indices and local sea surface temperatures (SSTs), were assessed to explain the variability in the return rate. The AMO and NAO indices, SST, sea age, and time across years all affected survival assessed in terms of returns to the Penobscot River. The results provide information to support the management of Atlantic salmon stocks on a spatial and temporal scale in US rivers and the fishery at West Greenland.Miller, A. S., Sheehan, T. F., Renkawitz, M. D., Meister, A. L., and Miller, T. J. 2012. Revisiting the marine migration of US Atlantic salmon using historical Carlin tag data. – ICES Journal of Marine Science, 69: 1609–1615.
Introduction
The abundance of the Atlantic salmon (Salmon salar) has declined throughout its North Atlantic range. Mortality during the marine phase has been identified as a critical factor driving overall abundance (Dieperink et al., 2002; Potter et al., 2003). In 1962, a Carlin tagging programme was implemented in ME, USA, to investigate large-scale migrations and marine survival of salmon through tag recoveries in North Atlantic interception fisheries and from adult returns to rivers (Meister and Cutting, 1966). The preliminary results provided the first documentation of US-origin Atlantic salmon migrating to Greenland (Meister, 1984), where they were exploited in a fishery that developed in the 1960s. This justified US participation in international salmon management fora and led to the US ratifying the Convention for the Conservation of Salmon in the North Atlantic Ocean in 1982.
Commercial salmon fishing in the United States ceased in 1948. Although stocks rebuilt somewhat during the period 1969–1985, continued declines resulted in the closure of the recreational fishery in 1999 (Fay et al., 2006) and listing of the Atlantic salmon under the US Endangered Species Act in 2000 (74 Federal Register 29 344, 19 June 2009; www.access.gpo.gov/fr).
The influence of climate variability on marine survival has been the focus of salmon research across the North Atlantic (Friedland et al., 1998, 2000, 2003). Studies have linked survival to environmental conditions in the ocean (Reddin and Shearer, 1987; Friedland et al., 1993), and large-scale mortality is hypothesized to occur early in the post-smolt migration (Potter et al., 2003; Kallio-Nyberg et al., 2004; Jutila et al., 2005). Friedland et al. (2000) reported a positive correlation between warm water in the North Sea in May, when salmon entered the sea, and return rates of one-sea-winter (1SW) adults. Holm et al. (2000) found that Norwegian post-smolts occupied warmer, more-saline waters, and improved post-smolt survival is associated with a positive North Atlantic Oscillation (NAO) index characterized by a warmer ocean climate (Kallio-Nyberg et al., 2004). Conversely, Condron et al. (2005) found that a negative Atlantic Multidecadal Oscillation (AMO) index (associated with cooler temperature phases) was correlated with increased abundance of North American Atlantic salmon.
Early evaluations of the US Carlin tagging programme focused on using recaptures to evaluate movement rates (Meister and Cutting, 1966; Meister, 1984). Given the linkages between marine survival and large-scale, climate-forcing mechanisms and a documented phase shift in productivity for Atlantic salmon (Chaput et al., 2005), the goal of this study was to reanalyse the historical tagging data and to examine the influence of biological and environmental variables on return rates.
Methods
Tagging programme
The US Atlantic salmon Carlin tagging programme began in Maine in 1962. Initially, only returning adults and kelts were tagged in natal rivers. Large-scale tagging of hatchery-reared smolts began in 1966, mainly on salmon released in the Penobscot River (ME, USA, 42°29′N 68°47′W). Releases of tagged adults and smolts expanded to other rivers in the United States and continued until 1996, when alternative tagging methods (e.g. coded-wire tags) were implemented. Tag recoveries were reported from marine and freshwater environments from the United States north to Greenland. Miller et al. (in press) provide a detailed description of the tagging programme and associated database. Ocean recoveries were heavily reliant on fishing effort, and the relationship between distant-water tag recoveries and Canadian and Greenland salmon landings was analysed using a Kendall's tau-test.
Penobscot River return-rate model
The return rate to US homewaters, an indicator of marine survival, was investigated using recovery data from Carlin-tagged hatchery-reared smolts released at various locations in the Penobscot River. This subset of the database was selected because it had the largest sample size (n = 3549) over the longest release period (1969–1994). The proportion of adult returns (offset by the natural logarithm of the total number of tags released) was modelled using a generalized additive model with a negative binomial distribution. Negative binomial distributions are useful when data are overdispersed (i.e. when variances are greater than the means) to avoid violations of the standard Poisson model assumptions (e.g. McCullagh and Nelder, 1989; White and Bennetts, 1996).
The following variables were considered and incorporated in the model categorically: Model selection was made using a stepwise selection process based on Akaike's Information Criterion corrected for sample size (AICc), starting with the most complex model and ending with the most parsimonious. Thin-plate regression-spline smoothers were applied to continuous variables, such as release year, the NAO and AMO indices, and surface temperature, and the remaining variables were considered factors. Model fitting and selection were performed using R (R Development Core Team, 2011) in conjunction with the “mgcv” package (Wood, 2006).
life-history characteristics (smolt age, 1–3 years, at release and sea age, 1SW–4SW, of returning salmon);
environmental covariates during the critical early marine post-smolt phase [mean monthly sea surface temperature (SST) from ∼55 km southwest of Penobscot Bay from the Boothbay Harbor Sea Water Temperature Record (available at http://www.gomoos.org/dmr/index.php)];
the smoothed, monthly AMO index (available at http://www.esrl.noaa.gov/psd/data/timeseries/AMO/);
the monthly mean NAO index (available for the month of release at http://www.cpc.ncep.noaa.gov/products/precip/CWlink/pna/nao.shtml);
stocking descriptors (release month and year).
Results
Tagging programme
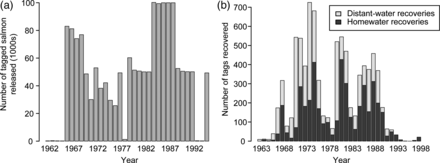
During the period 1962–1996, 1 548 296 tagged Atlantic salmon (adults and smolts from all rivers) were released (Figure 1a) and 8540 tags were recovered (Figure 1b). Distant-water recoveries (Greenland and Canada) accounted for nearly half (4229) of all recoveries, with the remaining tags recovered from adult salmon that had returned to rivers in the United States (4311). Most tag recoveries in US rivers (82.7%) were from maiden 2SW adults (3567), with maiden 1SW (460) and 3SW adults (49) accounting for 10.7 and 1.1% of the homewater tag recoveries, respectively. The remaining 235 (5.4%) tag recoveries were from repeat spawning salmon, the majority (94.0%) originating from adult releases.
Number of tagged salmon (a) released and (b) recovered during the Carlin tag programme.
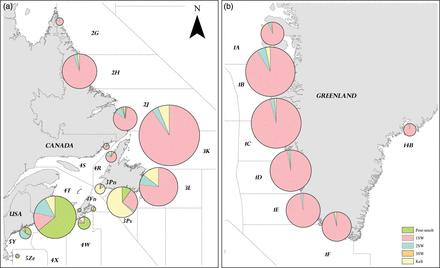
Distant-water tag recoveries were obtained mainly from nearshore gillnet fisheries for salmon, with occasional recoveries from salmon taken as bycatch in fisheries for other species [e.g. cod traps (13), mackerel traps (75), and other saltwater weirs (180)], recreational angling, fishway traps, and research surveys, although more than one-third of the records (39.2%) did not specify how the fish were captured. Tag recoveries from post-smolts (290) accounted for 6.8% of the distant-water tag recoveries, primarily those from the Bay of Fundy and along the Atlantic coasts of Nova Scotia and Newfoundland (Figure 2). A few tagged post-smolts were reported from Labrador, >3000 km from the release site. The majority of distant-water tag recoveries were from 1SW salmon (3301, 78.1%), with 2SW and 3SW salmon accounting for 215 (5.1%) and 15 (0.4%) of the tag recoveries, respectively. There were 295 (7.0%) tags recovered from kelts, undertaking their second marine migration, and 113 tags (2.6%) from salmon that had no reported sea age. Although Carlin tagging continued until 1996, distant-water recoveries declined in the late 1980s as fishing effort was reduced as a result of management restrictions and closures. No Carlin tags were recovered in the distant-water fisheries from salmon released after 1991.
Geographic distribution of Carlin tag recoveries of US-origin Atlantic salmon by sea age (a) in US and Canadian waters and (b) at Greenland. The areas shown are NAFO Divisions, except at East Greenland where the area shown is ICES Statistical Area XIV. For scale, the smallest sample size (n= 2) came from NAFO Division 4T in the Gulf of St Lawrence and the largest sample size (n= 712) from NAFO Division 3K off Newfoundland.
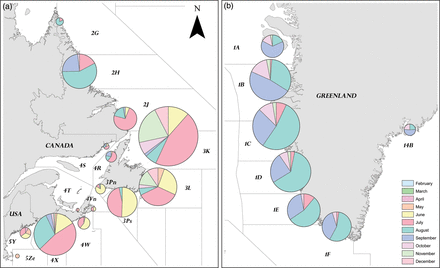
The geographic distribution of distant-water tag recoveries was correlated with salmon landings in Canada (tau = 0.21) and Greenland (tau = 0.32, p = 0.01) over time. Most tag recoveries at sea came from Greenland (52.4% of all distant-water recoveries). The location of tag recoveries over time provides valuable information on the distribution of US salmon at sea (Figure 3). The calculated number of days at large indicates that the earliest tag recoveries in Nova Scotia, Newfoundland, Labrador, and Greenland were 25, 47, 82, and 421 d, respectively, after the tagged smolts were released. A salmon tagged as an adult, however, was recovered at Greenland after 409 d. Apart from distinct patterns attributable to the timing of the fishery, annual patterns between time of release and recapture were not evident.
Geographic distribution of Carlin tag recoveries of US-origin Atlantic salmon by month of the year (a) in US and Canadian waters and (b) at Greenland. The areas shown are NAFO Divisions, except at East Greenland where the area shown is ICES Statistical Area XIV. For scale, the smallest sample size (n= 2) came from NAFO Division 4T in the Gulf of St Lawrence and the largest sample size (n= 712) from NAFO Division 3K off Newfoundland.
Penobscot River return-rate model
Homewater returns were influenced by life history, all environmental covariates, and year. The final model included sea age, mean monthly SST, monthly NAO and AMO indices, and year of release as covariates (Table 1), with an AICc value of 1083, much lower than the null model (1475). Smolt age at release and release month were not retained in the model because they did not substantially improve the model fit. The final model explained 89.2% of the deviance, with an adjusted r2 value of 0.65.
Parameters and estimated factorial and smoother coefficients of the final Penobscot River return-rate model, where ΔAICc is the difference in model AICc if that parameter is excluded from the model, and the Z-value is the ratio of the estimated coefficient to the standard error.
| Factorial coefficient . | Estimated coefficient . | Standard error . | Z-value . | ΔAICc . |
|---|---|---|---|---|
| Intercept | −8.636 | 0.259 | −33.37 | – |
| Sea age 2SW | 2.151 | 0.123 | 17.45 | 386.13 |
| Sea age 3SW | −2.419 | 0.217 | −11.16 | – |
| Sea age 4SW | −4.632 | 0.529 | −8.76 | – |
| Smoother coefficient | Estimated d.f. | χ2 | ΔAICc | |
| s(AMO index) | 1 | 2.02 | 5.17 | |
| s(temperature) | 9.937 | 39.05 | 2.81 | |
| s(NAO index) | 6.375 | 18.02 | 1.84 | |
| s(release year) | 7.17 | 30.19 | 4.41 |
| Factorial coefficient . | Estimated coefficient . | Standard error . | Z-value . | ΔAICc . |
|---|---|---|---|---|
| Intercept | −8.636 | 0.259 | −33.37 | – |
| Sea age 2SW | 2.151 | 0.123 | 17.45 | 386.13 |
| Sea age 3SW | −2.419 | 0.217 | −11.16 | – |
| Sea age 4SW | −4.632 | 0.529 | −8.76 | – |
| Smoother coefficient | Estimated d.f. | χ2 | ΔAICc | |
| s(AMO index) | 1 | 2.02 | 5.17 | |
| s(temperature) | 9.937 | 39.05 | 2.81 | |
| s(NAO index) | 6.375 | 18.02 | 1.84 | |
| s(release year) | 7.17 | 30.19 | 4.41 |
Final model diagnostics: dispersion parameter (theta) = 2.97; 2 × log likelihood = −1020.38; AICc = 1083.29.
Parameters and estimated factorial and smoother coefficients of the final Penobscot River return-rate model, where ΔAICc is the difference in model AICc if that parameter is excluded from the model, and the Z-value is the ratio of the estimated coefficient to the standard error.
| Factorial coefficient . | Estimated coefficient . | Standard error . | Z-value . | ΔAICc . |
|---|---|---|---|---|
| Intercept | −8.636 | 0.259 | −33.37 | – |
| Sea age 2SW | 2.151 | 0.123 | 17.45 | 386.13 |
| Sea age 3SW | −2.419 | 0.217 | −11.16 | – |
| Sea age 4SW | −4.632 | 0.529 | −8.76 | – |
| Smoother coefficient | Estimated d.f. | χ2 | ΔAICc | |
| s(AMO index) | 1 | 2.02 | 5.17 | |
| s(temperature) | 9.937 | 39.05 | 2.81 | |
| s(NAO index) | 6.375 | 18.02 | 1.84 | |
| s(release year) | 7.17 | 30.19 | 4.41 |
| Factorial coefficient . | Estimated coefficient . | Standard error . | Z-value . | ΔAICc . |
|---|---|---|---|---|
| Intercept | −8.636 | 0.259 | −33.37 | – |
| Sea age 2SW | 2.151 | 0.123 | 17.45 | 386.13 |
| Sea age 3SW | −2.419 | 0.217 | −11.16 | – |
| Sea age 4SW | −4.632 | 0.529 | −8.76 | – |
| Smoother coefficient | Estimated d.f. | χ2 | ΔAICc | |
| s(AMO index) | 1 | 2.02 | 5.17 | |
| s(temperature) | 9.937 | 39.05 | 2.81 | |
| s(NAO index) | 6.375 | 18.02 | 1.84 | |
| s(release year) | 7.17 | 30.19 | 4.41 |
Final model diagnostics: dispersion parameter (theta) = 2.97; 2 × log likelihood = −1020.38; AICc = 1083.29.
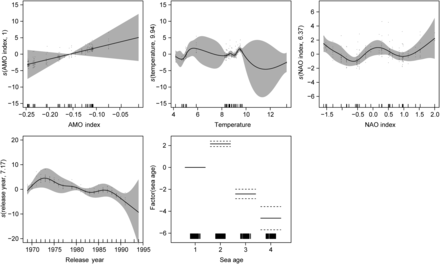
The results from the model indicate that return rates decreased over time (Figure 4). There was a negative relationship between sea age and return rate for all years, except for 2SW salmon. The effect of SST varied mainly because of temperature anomalies outside the typical range experienced during the period of the releases. Apart from these anomalies, an overall increase in temperature was associated with greater survival. An increasing AMO index (generally characterized by warming temperatures) also had a positive effect on survival. The NAO index had varying effects on Penobscot River returns. More extreme negative and positive values of the NAO index as well as those centred on a stable zero index had a positive effect on the river return rate, whereas moderate values (both positive and negative) were associated with reduced river returns.
Estimated smoother and factorial variable effects on the Penobscot River return rate of Carlin-tagged Atlantic salmon. Black dots indicate raw datapoints, and shaded areas and dashed lines are the 2 SE confidence intervals. Rugplots along the x-axes are indicative of samples available for each variable.
Discussion
Tagging programme
As new tagging technologies become available and more research is directed towards investigating the distribution and migration of salmon at sea, exploring historical information is important in providing reference points (Righton et al., 2007) and in developing hypotheses. The Carlin tagging programme in Maine, the primary source of historical data on marine migrations of US-origin Atlantic salmon, was reanalysed with the goal of informing current and future tagging programmes and in understanding the biological and environmental factors affecting the return rate.
US-origin salmon are believed to overwinter in the Labrador Sea (Reddin and Short, 1991; Friedland et al., 1993) and are not expected at West Greenland until after spending a full winter at sea. The earliest tag recoveries indicate that US-origin salmon had spent 14 months at sea before capture in the fishery at West Greenland. However, because of the timing of the Greenland fishery (late July to early November), perhaps 1SW salmon arrived in Greenlandic waters before the fishery and were undetected. Monthly patterns of tag recoveries at Greenland are also likely to reflect the dynamics of the fishery, but may also represent the response of fishers to the arrival of salmon (Figure 3). Salmon arriving in Greenlandic waters become susceptible to fishing in the south 1 or 2 months earlier than in the north.
As reported by Munro and Swain (1980), the Canadian and Greenland fisheries at that time probably harvested mainly 1SW salmon (as indicated by the high percentage of 1SW tags recovered in the fisheries) that would otherwise have returned to homewater rivers the following spring as 2SW adults. 2SW salmon in the Gulf of Maine accounted for >50% of tag recoveries (Figure 3). Post-smolts were the second largest source of tag recoveries in the Gulf of Maine. Tags were recovered from post-smolts as late as July, many from the Bay of Fundy (NAFO Division 4X), indicating a different migration pattern from post-smolts heading directly for Greenland.
The migration patterns reported here build on earlier analyses of the data derived from the US Carlin tagging programme. Meister (1984) used Carlin tagging data to estimate the distance travelled and time spent at large by US-origin salmon. However, these findings should be viewed with caution owing to the reliance on fisheries for tag recoveries (Reddin et al., 2012). The timing of the fishery, the gear type deployed, and other factors may influence the capture of tagged fish, and the lack of detailed formal reporting procedures (e.g. details of catch coordinates, date of capture) will affect the utility of the information obtained. In this analysis, the estimated times taken for post-smolts to reach landmark locations en route to the Labrador Sea were consistent with those determined in recent studies using other methods. For example, in 2008, the mean time taken for post-smolts tagged with ultrasonic transmitters to reach Nova Scotia was 30 d (J. Kocik, pers. comm.), only 5 d more than the earliest reported recovery from the US Carlin tagging programme.
Penobscot River return-rate model
The variables with the strongest influence in the model (i.e. sea age, SST, AMO and NAO indices, and release year) were not unexpected in view of the phase shift in marine productivity in recent decades (Chaput et al., 2005). The model indicates a reduction in the return rate to the Penobscot River throughout the tagging programme, with the decline being most marked for 2SW adults (USASAC, 2011). These findings for tagged salmon are consistent with the general trends in abundance for US salmon populations (USASAC, 2011). Perhaps, sea-age plasticity has been reduced because of the combined effect of reduced genetic diversity through marked population declines and the release of more hatchery-reared salmon to supplement wild populations (Bigler et al., 1996; Thorpe et al., 1998; Jonsson et al., 2003). Lower return rates of salmon are to be expected as sea age increases, because older fish are exposed to additional years of natural mortality and potentially fishing pressure.
Overall, warmer coastal temperatures at the time of release had a positive effect on salmon returns. The orientation of the Rugplots shows two clear temperature ranges at the time of release that correspond to the temperature data collected from each of the release months. A substantial amount of variance exists from temperature anomalies.
There was a slight increase in river returns with an SST increase from 4–6 to 8–10°C, consistent with the findings of others (Friedland et al., 2000; Kallio-Nyberg et al., 2006; Hvidsten et al., 2009).
The influence of the AMO index in the model is consistent with SST results, with warmer temperatures typically associated with an increasing AMO index. However, these results are in contrast to the findings of Condron et al. (2005), who found that a negative AMO was associated with greater salmon abundance. Because the AMO covers such a broad geographic area and temporal range, it may be difficult to detect finer-scale influences on post-smolt salmon during early migration. The longer time-series used by Condron et al. (2005) may be more amenable to the detection of long-term responses than that used in this study.
The influence of the NAO index in the Penobscot River return-rate model probably reflects the spatial and temporal component of the index. A negative NAO is typically associated with cooler conditions in the Gulf of Maine and warmer conditions off Labrador, Newfoundland, and West Greenland (Petrie, 2007), which might explain the varying effects of the NAO index on the river return rate. Additionally, temperature-related changes reflected by both the NAO and AMO indices are much more pronounced during winter (Peyronnet et al., 2008), and salmon smolts are released in late spring.
Survival of early marine-phase post-smolts may depend on a small temporal and thermal window of optimal migration as salmon migrate out of rivers and enter the sea. Climate shifts cause a mismatch in optimal riverine and oceanic environmental conditions that may have negative impacts on survival in nearshore waters (Winder and Schindler, 2004) and have severe impacts on a smolt cohort. It is also important to note that factors operating in the river before the marine migration (i.e. dam passage survival, bird and fish predators, river temperatures) and fishing effort may influence the return rate, but these factors are not taken into account in the model.
Management strategies and future work
This retrospective analysis of the historical US Carlin tagging programme data has generated information relevant to managers regarding environmental influences on river return rates during a multidecadal period (i.e. 1960s–1990s) of declining salmon abundance. Managers may be able to improve marine survival by adapting the timing of smolt releases so as to target optimal conditions (e.g. timing smolt releases based on temperature preference). In river systems with large-scale hatchery releases (e.g. the Penobscot River), altering stocking strategies to improve survival may be simple to implement and could be based on seasonal weather and climate projections.
Reddin et al. (2012) discuss the spatial patterns of North American-origin Atlantic salmon in the fishery at Greenland. By combining the temporal migration information from telemetry tagging studies with spatial patterns of the North American harvest, management measures could be developed to adjust fishing effort in time and space, to reduce the probability of harvesting endangered US-origin stocks.
Modelling temporal and spatially explicit environmental variables throughout the entire marine migration with distant-water fishing effort and exploitation rates would allow for a more advanced survival analysis. The use of winter indices before release events (Peyronnet et al., 2008) may benefit future analyses. More advanced modelling techniques for conventional tag-recovery experiments could provide a more complete estimate of salmon survival during the marine migration, and incorporating other datasets [e.g. the Northwest Atlantic Salmon Tagging Database (Reddin et al., 2012)] may improve the statistical power of the current model and assist in understanding differences and similarities between US and Canadian stocks.
Acknowledgements
We thank the many individuals from different countries and states who had various levels of participation in this large-scale project over several decades. Thanks are also due to John Kocik and two anonymous reviewers for their constructive reviews.
References
Author notes
Handling editor: Emory Anderson