-
PDF
- Split View
-
Views
-
Cite
Cite
Kjell Arne Mork, John Gilbey, Lars Petter Hansen, Arne J. Jensen, Jan Arge Jacobsen, Marianne Holm, Jens Christian Holst, Niall Ó Maoiléidigh, Frode Vikebø, Philip McGinnity, Webjørn Melle, Katie Thomas, Eric Verspoor, Vidar Wennevik, Modelling the migration of post-smolt Atlantic salmon (Salmo salar) in the Northeast Atlantic, ICES Journal of Marine Science, Volume 69, Issue 9, November 2012, Pages 1616–1624, https://doi.org/10.1093/icesjms/fss108
Close - Share Icon Share
Abstract
The migration of post-smolt Atlantic salmon (Salmo salar) during their first 4 months at sea in the Northeast Atlantic was simulated using an individual-based model that combined a particle-tracking scheme with growth and behaviour routines. The migration was decomposed into both passive pelagic drift with the surface currents, provided by an ocean model, and active horizontal swimming behaviour. The active swimming direction was aligned with the surface current. Swimming speed was a function of body length and calculated from recaptured tagged salmon. Releases of particles in the model were made to the west of Ireland and to the southwest of Norway. The modelled post-smolt distributions were compared with the observed distributions, and a sensitivity analysis using different swimming speeds was performed. The strength and direction of the flow can transport the post-smolts towards areas with favourable feeding conditions. However, in some areas, the direction of migration was sensitive to interannual changes in the windforcing, leading the post-smolts to areas with a different environment and prey. Inclusion in the swimming behaviour of a preference for water with higher temperature and salinity displaced the northward migration more offshore, away from coastal areas.Mork, K. A., Gilbey, J., Hansen, L. P., Jensen, A. J., Jacobsen, J. A., Holm, M., Holst, J. C., Ó Maoiléidigh, N., Vikebø, F., McGinnity, P., Melle, W., Thomas, K., Verspoor, E., and Wennevik, V. 2012. Modelling the migration of post-smolt Atlantic salmon (Salmo salar) in the Northeast Atlantic. – ICES Journal of Marine Science, 69: 1616–1624.
Introduction
The Atlantic salmon (Salmo salar) undertakes long migrations to feeding areas in the ocean, including in the northern Norwegian Sea and around the Faroe Islands (Jacobsen et al., 2012). Information on the migration and distribution of Atlantic salmon during their first months at sea is available from the systematic sampling of post-smolts at sea (Holst et al., 1993, 2000; Holm et al., 2000). Less is known about the swimming behaviour of post-smolts, but new technologies such as data storage tags and acoustic tracking, are being used to increase the understanding of the behaviour of salmon at sea (Reddin et al., 2004; Rikardsen et al., 2007; Hedger et al., 2008; Martin et al., 2009). Salmon smolts do not just drift passively with the current, and active swimming can account for two-thirds of the total migration speed (Martin et al., 2009). After Atlantic salmon smolts enter the ocean, they migrate as post-smolts to feeding areas during late spring and summer (Thorpe, 1988; Mills, 1989) and are distributed over large areas of the North Atlantic (Holm et al., 2004).
At the population level, the distribution of Atlantic salmon at sea is poorly understood, but tagged fish originating from several rivers are present in the same areas (Hansen and Jacobsen, 2000, 2003). The distribution at sea probably depends on a combination of factors such as abiotic environmental conditions, prey availability, and stock-specific migration patterns (Hansen and Quinn, 1998; Jacobsen and Hansen, 2001; Holm et al., 2004). In the Northeast Atlantic, post-smolts mainly occupy the surface layer (Holm et al., 2004), and they prefer water temperatures between 8 and 12°C (Friedland et al., 2000; Holm et al., 2004) and with salinities >35 psu (Holm et al., 2003). Post-smolts originating in southern European rivers move north with the currents (Shelton et al., 1997), possibly to minimize energy expenditure and maximize growth during migration (Hansen et al., 1993).
There have been few attempts to model the migration trajectories and temporal distribution of post-smolts, probably because of the lack of knowledge of the behavioural processes that affect migration. In the only study of the migration patterns of salmon in the Northeast Atlantic, Booker et al. (2008) modelled the trajectories of 15 fish emanating from the west of Ireland, using random, current-directed, and temperature-directed swimming. They concluded that either current or temperature, or a combination of both, is an important factor influencing the direction of migration.
In this study, the migration of salmon post-smolts during their first 4 months at sea in the Northeast Atlantic was investigated using a high-resolution numerical ocean model driven by atmospheric hindcast data. The velocity fields from the ocean model, together with different post-smolt swimming behaviours, were incorporated into a Lagrangian particle-tracking model (Ramsden and Holloway, 1991) to simulate the movements of particles, representing post-smolts, in space and time. The sensitivity of the post-smolt migratory response was modelled in relation to different swimming speeds of the post-smolts, interannual differences in windforcing, and a range of possible swimming behaviours. An evaluation of the model was conducted by comparing the modelled and observed distributions of two stock groups representing northern and southern European stocks.
Material and methods
The ocean model and the Lagrangian particle-tracking model
Ocean current fields were simulated with the three-dimensional numerical Regional Ocean Modelling System (ROMS; www.myroms.org; Haidvogel et al., 2008). The model domain covers the Northeast Atlantic and the region to the north, including part of the Arctic Ocean. It has a spatial resolution of 4 km and 30 terrain-following vertical levels. The lateral boundary conditions were taken from a global version of the ROMS with a resolution of ∼20 km in the North Atlantic and Arctic. Both regional and global models were driven with six-hourly ERA40 interim atmospheric data (sea surface air pressure, windstress, latent, sensible, downward shortwave radiative, and net longwave radiative heat flux) and tidal forces. Details of the regional model setup and comparison with observations were described by Vikebø et al. (2010). The model reproduced the hydrographic and circulation features in the southern Norwegian Sea (Vikebø et al., 2010), where the modelled annual mean volume flux of the inner branch of the Norwegian Atlantic Current (NwAC), the Norwegian Atlantic Slope Current (NwASC), equalled the values obtained by Orvik et al. (2001). The modelled NwASC was seen as a narrow (∼50 km wide), topographic trapped current over the shelf break with a strong seasonal signal as reported by Orvik et al. (2001). On the shelf, the modelled Norwegian Coastal Current (NCC) was along the 200-m isoline, with less unidirectional flow above topographic features, such as banks and troughs, as observed by Sætre (1999) using satellite-tracked drifter buoys.
Daily averages from the regional model were used in an offline particle-tracking model (Ådlandsvik and Sundby, 1994) to simulate the transport of particles from release locations. The model has also been used in several other studies in the same area, such as those on the drift of fish larvae (Vikebø et al., 2010; Opdal et al., 2011) and the dispersal of radionuclides (Heldal et al., 2012). Additionally, the particle-tracking model has a built-in behavioural module that was modified for individual post-smolt growth and active swimming behaviour. Because the post-smolts mainly migrate close to the surface, within the uppermost 1 m (Holm et al., 2004), no vertical migration was assumed and only surface currents were used to calculate the movement of each post-smolt on an hourly basis.
Migration speed from recaptured tagged fish
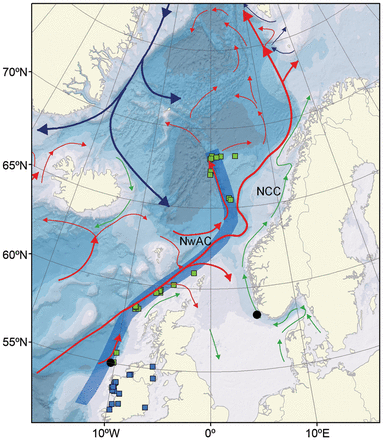
As the migration of post-smolts is the sum of drift with the ocean currents and active swimming, both components were included in the particle-tracking model. The former component is taken from the ocean model, but the latter needed to be defined. The swimming component has two elements: direction and speed. The swimming speed was estimated using information from 86 tagged hatchery-reared smolts of Irish origin released between 1996 and 2009 and recaptured within the same year as their release (Figure 1). It was assumed that hatchery-reared fish would undertake the same migration and would have the same swimming speed as wild fish. Based on the locations of the recaptured post-smolts, the most likely migration path, associated with the main current, was defined (Figure 1). An estimated migration speed of 20 cm s−1 (Booker et al., 2008) was used for the post-smolts to swim from the release point to the nearest location in the defined path. For each tagged fish, the average migration speed along the path was calculated to match the time and location of each recapture. The surface velocity component along the direction of the migration path was estimated for May and June for the period 1996−2009 using the ocean model ROMS. Both mean and maximum velocities from the model were calculated in grid boxes along the path, where each grid box was 125 km wide and 25 km long. By excluding the mean and the maximum modelled velocity, averaged over the same route as the tagged fish, from the migration speed, the respective mean and minimum active swimming speeds of each post-smolt could be calculated.
Schematic overview of the main surface currents in the Northeast Atlantic and the Nordic Seas. Red, blue, and green vectors indicate surface currents with relatively warm/saline (Atlantic) water, cold/fresh (Arctic) water, and fresh (coastal) water, respectively. Release and recapture locations of tagged Atlantic salmon are shown as blue and green boxes, respectively, and the defined migration route for the recaptured tagged post-smolts is indicated as a thick blue line. The two locations of released particles (black dots) in the model are also shown.
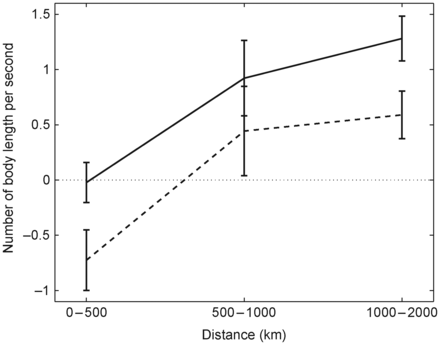
Using the average of the body length at release and at recapture for each individual fish, the active swimming speed, as a function of body length, was calculated for each fish. The swimming speeds of all post-smolts were estimated for different distances travelled and averaged over three different intervals: 0–500, 500–1000, and 1000–2000 km. The corresponding averaged body lengths within these intervals were 18, 19, and 22 cm, respectively. The calculated averaged swimming speeds were ≤1.5 body length s−1, with greater speeds over longer swimming distances (Figure 2). A lower swimming speed was needed (<1.0 body length s−1) to match the time and location of the recaptures if the fish stayed in the area with the strongest current. However, we expected the post-smolts to be more randomly distributed in the current branch, and because we were interested mainly in the migration for the longer distances travelled, a swimming speed of 1.5 body length s−1 was used as the active swimming speed in the particle-tracking model. This value is close to the constant value (20 cm s−1) used by Booker et al. (2008) and the optimum swimming speed, defined as the minimum energy expenditure per metre, for post-smolt Oncorhynchus nerka of 1.6 body length s−1 (Videler, 1993). The active swimming direction of the post-smolts was defined as being in the same direction as the local current, i.e. a rheotactic behavioural response, in accord with the results from recaptured tagged fish and the published literature (Shelton et al., 1997; Booker et al., 2008).
Averaged active swimming speeds for migrating post-smolts (body length s−1) with error bars within different ranges of migration distance. The migration distance is the distance along the defined migration path (Figure 1) between the release and recapture locations. The minimum (dashed line) and averaged (solid line) active swimming speeds were calculated by removing the maximum and the averaged modelled current speed, respectively, from the mean migration speeds along the migration path.
Growth model
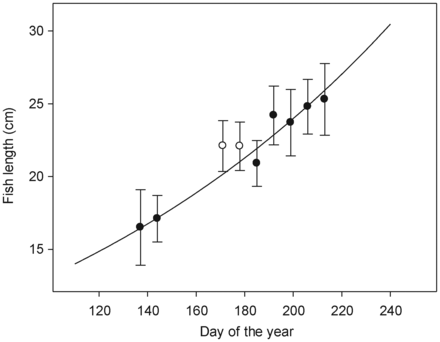
A simple post-smolt growth model was added to the particle-tracking model, using the mean lengths (L, cm) of 2-year-old post-smolts with time (t, day of the year) captured for each week by surface trawling in the Northeast Atlantic in 2008 and 2009. An exponential line was fitted to these data (L = 7.34 e0.0059t, r2 = 0.969, p < 0.001; Figure 3). Hence, the body length was estimated in the model to increase by 0.6% per day. At the release point, the body length was set to 16 cm (Kennedy and Crozier, 2010).
Observed lengths of 2-year-old post-smolt Atlantic salmon captured in the Northeast Atlantic by surface trawling in 2008 and 2009 (black dots with s.d. bars), and the exponential line fitted (black line). Observed data for 2-year-old post-smolts collected in 2002 are also shown as open circles. Observations are mean values for 13–274 individuals.
Oceanographic data for temperature and salinity preferences
In some experiments, the swimming behaviour was allowed to vary according to temperature and salinity. When the post-smolts were outside the preferred temperature (8–12°C) and salinity (≥35 psu) ranges (Friedland et al., 2000; Holm et al., 2003), swimming direction and speed were added towards areas with the preferred temperature and salinity ranges using horizontal temperature and salinity gradients. An increase in swimming speed has been observed in smolts when actively swimming towards higher salinities (Hedger et al., 2008). The added swimming speed increased linearly from 0 to a maximum of 0.5 body length s−1 when the difference between the ambient temperature/salinity and the preferred temperature/salinity ranges increased from 0 to 1°C/psu. For differences >1°C/psu, the added speed was 0.5 body length s−1. As it is not clear currently what levels of temporal and spatial variation in temperature or salinity affect the behaviour of post-smolts, we chose to use the monthly mean climatology of temperature and salinity (Engedahl et al., 1998).
Genetic assignment of post-smolts for comparison with modelled distribution
Post-smolts caught during surveys conducted in 2008 and 2009 under the EU-funded SALSEA-Merge project were used for comparison with the modelled post-smolt distribution. The surveys took place in May off Northwest Ireland, in June/July in the central Norwegian Sea, and in July/August in the northern Norwegian Sea. The post-smolts caught were genetically allocated to different groups at different assignment levels using the SALSEA-Merge Genetically-based Regional Assignment of Atlantic Salmon Protocol (GRAASP) database (J. Gilbey, pers. comm.). The first assignment level assigned the salmon into three regional groups: southern European, northern European, and Icelandic, and higher levels gave more detailed information on the locations of origin (J. Gilbey, pers. comm.). To have a large number of observations for comparison, the data from both 2008 and 2009 at the first assignment level were compiled for comparison with the modelled distribution for 1 year.
The modelled distribution of post-smolts for 2008 was compared with the post-smolt catches for 2008/2009 in the SALSEA-Merge surveys. At each location with a trawl station, with or without the presence of post-smolts, the modelled distribution was checked for particles within a radius of 25 km of that location and ±1 week in time.
Simulations
The simulations were performed for 2008, a year with SALSEA-Merge marine surveys, and 2002. The latter year was chosen because the post-smolt growth (Jensen et al., 2012) and the windforcing (and consequently the surface currents) were considerably different from those in 2008. The locations of the released particles were west of Ireland and southwest of Norway, to represent both a southern and a northern European salmon stock, respectively (Figure 1). For each simulation, 50 000 particles were released at one location, randomly distributed within a 25-km radius from the centre of the location. Within each year and location, the particles were released over a 3-week period, with a Gaussian distribution, centred on 1 May for the Irish stock (Kennedy and Crozier, 2010) and 15 May for the Norwegian stock (Hvidsten et al., 1998). These dates represented the peak post-smolt migration periods for the respective stocks. The simulations were run to the end of August. The following experiments were conducted: (a) post-smolt migration from west of Ireland in 2008; (b) post-smolt migration from west of Ireland in 2002; (c) post-smolt migration from west of Ireland in 2002 including temperature/salinity influenced swimming behaviour; and (d) post-smolt migration from southwest Norway in 2008.
The sensitivity of the model results was estimated by analysing the modelled distributions using different swimming speeds. For two experiments, the post-smolt migration from west of Ireland in 2008 (a) and southwest of Norway in 2008 (d), additional simulations were performed with different swimming speeds ranging from 0 to 2.5 body length s−1 at intervals of 0.5 body length s−1. A swimming speed of 0 body length s−1 corresponds to passive drift with the currents. The modelled distribution for each experiment was compared with the observed post-smolt distribution by counting the number of overlaps where the modelled post-smolt distribution was within ±1 week in time and a radius of 25 km of the observed post-smolt location. The comparison with observed distributions was made for both 2008 only and 2008/2009 combined. In addition, the minimum distance between the modelled distribution locations and the captured post-smolt location for each post-smolt, on the date of capture, was calculated, and averaged for all observations in each experiment.
Results
For each experiment, a particle-concentration map was developed presenting the total number of particles passing each grid point during the entire period, where a particle only counts once at each grid point. These maps allow the main migration routes and the distribution of post-smolts to be visualized over the entire period.
Post-smolt migration from west of Ireland
2008
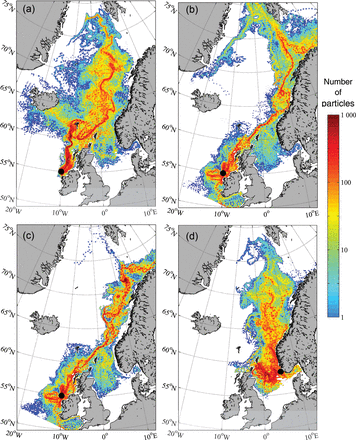
The map generated for the simulated migration in 2008 indicates that the post-smolts released west of Ireland (southern stock) were present throughout most of the Norwegian Sea, but particularly towards topographic features such as the edge of the Norwegian and Faroese continental shelves (Figure 4a). There were also some particles in the Irminger, Iceland, and Greenland Seas. The main migration direction was northwards, but several alternative migration routes were evident before the particles eventually entered the Norwegian Sea; clockwise around the Faroes, through the Faroe–Shetland Channel, and a minor route towards Iceland.
Concentration plots of the simulated migration of Atlantic salmon post-smolts (a) for the southern stock in 2008, (b) for the southern stock in 2002, (c) for the southern stock in 2002 with temperature and salinity preferences included in the model, and (d) for the northern stock in 2008.
2002
The simulated migration of post-smolts in 2002 resulted in a very different distribution pattern and migration routes from those of 2008 (Figure 4b). The distribution in 2002 was shifted to the east, with particles located closer to the Norwegian coast and also distributed into the Barents Sea and the Arctic Ocean. In 2002, no particles were located in the west towards Iceland, and just a few particles moved around the Faroes; many of the particles travelled a greater distance than in 2008. In 2002, the northward migration route predominantly followed the inner branch of the NwAC (Figure 1), whereas in 2008, the migration route in the Norwegian Sea followed the outer (offshore) branch of the NwAC. Migration routes changed in two key areas between the 2 years. One of these areas was south of the Faroes, where the direction was either northeastwards through the Faroe–Shetland Channel or northwestwards. The other area was in the southern Norwegian Sea, where the direction of particle movement was either along the inner or outer branch of the NwAC.
2002 including temperature/salinity-influenced swimming behaviour
When the post-smolt preferences for different temperature and salinity ranges were included in the simulations, migration routes of Irish post-smolts in 2002 were concentrated more to the west relative to simulations without such preferences (Figure 4c). The main reason for this is the avoidance of the coastal water over the Norwegian shelf and farther offshore. The distribution was also less to the north, mainly because the post-smolts were displaced farther west and were, therefore, less likely to follow the inner branch of the NwAC. Relative to the output for 2002 and 2008 from the model with no temperature/salinity preference, there was a distinct change in direction to the south in the Norwegian Sea (west from Møre, Norway, at 63°N) in one of the key areas described previously.
Post-smolt migration from southwest Norway in 2008
The release of particles to the southwest of Norway (northern stock) in 2008 revealed a northward migration into the same area of the Norwegian Sea as observed for the Irish release in 2008 (Figure 4d). Hence, there was considerable overlap between the northern and southern stocks in the Norwegian Sea. Some particles moved into the North Sea, and several of these continued northwards into the Norwegian Sea after a period. This implies that there were two different pathways, both ending in the same area, but with different environmental conditions such as light availability, prey, and predators.
Comparison with observations
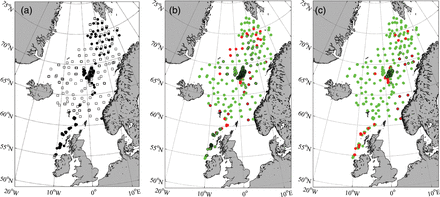
Generally, there was close agreement between the modelled (2008) and the observed (2008/2009) distributions for the southern stock (Figure 5a and b). In fact, 85 and 75% of the modelled post-smolts were within a 2-week interval of when post-smolts were observed in the surveys in 2008 and 2008/2009 combined, respectively. However, modelled and observed distributions differed in some regions, such as close to the Faroes and in the central and northern Norwegian Sea. The difference in the timing of modelled and observed smolt occurrence was usually less than a few days, but at some locations north of 72°N in 2009, the modelled distribution was delayed by 1 month (KAM, pers. obs.). A similar comparison was made for the northern stock, for which there was also a good agreement between modelled and observed distributions (Figure 5c), but as for the southern stock, the modelled distribution missed or delayed some of the most northern post-smolts and those in the central Norwegian Sea. Moreover, the model missed the post-smolts caught west of Ireland. There, only 32 and 43% of the modelled distribution was within a 2-week interval centred on the time of the observations for 2008 and 2008/2009 combined, respectively.
(a) Locations of trawl stations in 2008 (black dots) and 2009 (squares). The area of overlap between observed (2008/2009) and modelled (2008) distributions of post-smolts for (b) the southern and (c) the northern stock. The modelled distribution is only presented if it is within a radius of 25 km of the observed location and with less than 1-week difference in time. Green points indicate where the modelled and observed concentrations of post-smolts are consistent, i.e. post-smolt are present in either both or neither of them. Red points are where observations of post-smolts are available but are inconsistent with the model, i.e. post-smolts are present in either the observations but not in the model or the opposite. A black ring around a green or a red point indicates that modelled post-smolts are present.
The sensitivity of the model output to different swimming speeds showed that faster swimming speeds gave better overlap between the observed and modelled distributions for both stocks (Table 1). The modelled distribution of the southern stock was in much closer agreement with the observations than that of the northern stock in all experiments. The largest increase in the percentage overlap was when the swimming speed increased from 1.0 to 1.5 body length s−1 (e.g. from 46 to 85% overlap for the southern stock using only 2008 observations). Swimming speeds >1.5 body length s−1 resulted in only slightly better overlap, particularly for the southern stock. In addition, the averaged minimum distance between the observations and the modelled distributions showed the greatest reduction when the swimming speed increased from 1.0 to 1.5 body length s−1. Swimming speeds >2.0 body length s−1 did not increase the overlap or reduce the minimum distance between the observations and the modelled distributions for the southern stock, whereas a small improvement was seen for the northern stock.
Comparison of observed and modelled distributions in 2008 for southern and northern post-smolt stocks using different swimming speeds (body length s−1).
| Swimming speed (body length s−1) . | Southern stock . | Northern stock . | ||
|---|---|---|---|---|
| . | Overlap (%) . | Distance (km) . | Overlap (%) . | Distance (km) . |
| 0.0 | 22 (11) | 875 ± 702 (1 111 ± 571) | 0 (0) | 636 ± 164 (626 ± 175) |
| 0.5 | 29 (15) | 328 ± 280 (420 ± 252) | 1 (2) | 458 ± 209 (415 ± 200) |
| 1.0 | 46 (34) | 164 ± 160 (223 ± 172) | 12 (17) | 373 ± 263 (310 ± 236) |
| 1.5 | 85 (75) | 50 ± 74 (77 ± 99) | 32 (43) | 307 ± 283 (233 ± 238) |
| 2.0 | 97 (96) | 27 ± 36 (35 ± 47) | 47 (60) | 260 ± 287 (170 ± 233) |
| Swimming speed (body length s−1) . | Southern stock . | Northern stock . | ||
|---|---|---|---|---|
| . | Overlap (%) . | Distance (km) . | Overlap (%) . | Distance (km) . |
| 0.0 | 22 (11) | 875 ± 702 (1 111 ± 571) | 0 (0) | 636 ± 164 (626 ± 175) |
| 0.5 | 29 (15) | 328 ± 280 (420 ± 252) | 1 (2) | 458 ± 209 (415 ± 200) |
| 1.0 | 46 (34) | 164 ± 160 (223 ± 172) | 12 (17) | 373 ± 263 (310 ± 236) |
| 1.5 | 85 (75) | 50 ± 74 (77 ± 99) | 32 (43) | 307 ± 283 (233 ± 238) |
| 2.0 | 97 (96) | 27 ± 36 (35 ± 47) | 47 (60) | 260 ± 287 (170 ± 233) |
Columns show the percentage overlap between modelled post-smolt occurrence within a 2-week interval and a radius of 25 km of the locations of observed post-smolts, and the minimum distances (mean ± 1 s.d.) of the nearest modelled particles to observed post-smolts.
The numbers in parenthesis in the overlap (%) and distance (km) columns show the combined 2008/2009 data for comparison with 2008 data.
Comparison of observed and modelled distributions in 2008 for southern and northern post-smolt stocks using different swimming speeds (body length s−1).
| Swimming speed (body length s−1) . | Southern stock . | Northern stock . | ||
|---|---|---|---|---|
| . | Overlap (%) . | Distance (km) . | Overlap (%) . | Distance (km) . |
| 0.0 | 22 (11) | 875 ± 702 (1 111 ± 571) | 0 (0) | 636 ± 164 (626 ± 175) |
| 0.5 | 29 (15) | 328 ± 280 (420 ± 252) | 1 (2) | 458 ± 209 (415 ± 200) |
| 1.0 | 46 (34) | 164 ± 160 (223 ± 172) | 12 (17) | 373 ± 263 (310 ± 236) |
| 1.5 | 85 (75) | 50 ± 74 (77 ± 99) | 32 (43) | 307 ± 283 (233 ± 238) |
| 2.0 | 97 (96) | 27 ± 36 (35 ± 47) | 47 (60) | 260 ± 287 (170 ± 233) |
| Swimming speed (body length s−1) . | Southern stock . | Northern stock . | ||
|---|---|---|---|---|
| . | Overlap (%) . | Distance (km) . | Overlap (%) . | Distance (km) . |
| 0.0 | 22 (11) | 875 ± 702 (1 111 ± 571) | 0 (0) | 636 ± 164 (626 ± 175) |
| 0.5 | 29 (15) | 328 ± 280 (420 ± 252) | 1 (2) | 458 ± 209 (415 ± 200) |
| 1.0 | 46 (34) | 164 ± 160 (223 ± 172) | 12 (17) | 373 ± 263 (310 ± 236) |
| 1.5 | 85 (75) | 50 ± 74 (77 ± 99) | 32 (43) | 307 ± 283 (233 ± 238) |
| 2.0 | 97 (96) | 27 ± 36 (35 ± 47) | 47 (60) | 260 ± 287 (170 ± 233) |
Columns show the percentage overlap between modelled post-smolt occurrence within a 2-week interval and a radius of 25 km of the locations of observed post-smolts, and the minimum distances (mean ± 1 s.d.) of the nearest modelled particles to observed post-smolts.
The numbers in parenthesis in the overlap (%) and distance (km) columns show the combined 2008/2009 data for comparison with 2008 data.
Discussion
Migration routes
The model presented here is useful in identifying the likely migration routes of post-smolts in different years and assuming different patterns of swimming behaviour. Consistent with earlier studies (Shelton et al., 1997; Booker et al., 2008), our results suggest that the strength and direction of ocean currents can guide migrating post-smolts towards areas favourable for feeding. In addition, our results indicate that interannual variations in the direction (and strength) of the surface currents alter post-smolt migration routes. The surface current is strongly dependent on surface winds, and there was a considerable difference in the wind strength in 2002 and 2008. Whereas the North Atlantic Oscillation (NAO) index was highly positive (characterized by strong westerly winds) in the period from April to July 2002, it was very negative (characterized by weak westerly winds) in the same months in 2008 and slightly negative in 2009 (e.g. updated NAO index time-series from Hurrell, 1995). During periods with a high NAO index, the current system in the subpolar North Atlantic shifts southeastwards, and the current along the edge of the Irish and Norwegian continental shelf intensifies (Orvik et al., 2001; Flatau et al., 2003). Hence, the weaker westerly winds in 2008 probably caused the more westerly simulated northward migration route in 2008 relative to 2002.
Changes in migratory pathways have been suggested as a factor affecting the survival of North American post-smolts (Friedland et al., 1999), and they may also affect the survival of European post-smolts. Migratory species change migration routes in response to changing currents, temperature, and salinity (Minns et al., 1995; Ottersen et al., 2004). The more coastal migration route in 2002 may have had consequences for the post-smolts in terms of food availability and other factors influencing behaviour and physiology and could be one possible explanation for the observed higher growth rates in 2002 relative to 2008 (Jensen et al., 2012; see also Figure 3). However, the different growth rate of post-smolts in the 2 years was controlled by food availability rather than sea temperature (Jensen et al., 2012). However, a growth model dependent on prey abundance would be needed to consider the effects of different growth rates in the simulations, and defining such a complex growth model was outside the scope of this work.
When post-smolts were allowed to respond to ambient temperature and salinity and move towards what might be presumed to be preferred environments, they were displaced in a more westerly direction in 2002. As we do not know in detail the response of post-smolts to horizontal gradients in temperature and salinity, the post-smolts in the model were guided by a filtered hydrographic signal represented by climatological monthly mean temperatures and salinities. Nevertheless, this significantly affected the swimming behaviour, and further studies on this topic should be undertaken to improve individual-based models of salmon migration.
The model indicated that the ocean conditions in 2008 could result in some post-smolts from the southern stock migrating into Icelandic waters, a finding that has been confirmed by genetic assignment to southern European rivers of post-smolts caught by Icelandic pelagic fishing boats in the same year (J. Gilbey, pers. comm.).
Key areas of direction changes
The simulations identified at least two key areas along the migration routes where the subsequent drift is particularly sensitive to horizontal perturbations, either because of variability in ocean currents or modelled parameters of post-smolt behaviour. One key area was south of the Faroes, where the routes were either through the Faroe–Shetland Channel or farther west, to the south of the Faroes. The other was in the southern Norwegian Sea, where there are two branches of the NwAC.
The effect of salinity on post-smolt swimming behaviour has received less attention than temperature. However, salinity in estuarine systems has an important effect on swimming behaviour, with smolts actively swimming towards areas with more saline water (Hedger et al., 2008; Martin et al., 2009). Our results also indicate that salinity may be important in post-smolt orientation in the open sea. This is particularly important in the southern Norwegian Sea, where a thin coastal layer with relatively freshwater, originating from the NCC, is present during spring and summer. Post-smolts have a preference for waters of higher salinity and will move offshore to the outer branch of the current rather than migrating north with the stronger inner branch. This delays the northward drift but ensures continued off-shelf transport without the risk of being trapped in the NCC flowing towards the southeastern Barents Sea. In addition, the avoidance of the continental shelf might be more favourable because the predation pressure on post-smolts is probably higher over the continental shelf where predatory fish and birds are more abundant.
Modelled distribution vs. observations
Although there was a good agreement between the modelled and observed spatio-temporal distributions of post-smolts, there were some differences. For example, the model missed or delayed the migration timing of several post-smolts in the northern Norwegian Sea. This might have been due to an underestimation of the swimming speed, a false assumption that tagged hatchery-origin fish undertake the same migrations as wild fish, and/or weak modelled surface currents. It is also possible that the southern stock group included post-smolts that originate from regions other than the west of Ireland. Also, contrary to expectations based on free pelagic drift, some post-smolts caught west of Ireland were genetically assigned to the northern stock. However, it is most likely that those post-smolts were escaped farmed salmon (J. Gilbey, pers. comm.). Further, the recaptured tagged fish released in Ireland were assumed to have migrated through the Faroe−Shetland Channel, but for 2008, most of the fish migrated around the Faroes, and a faster swimming speed should probably have been assumed in the model for comparisons with the observations.
The sensitivity analysis indicated that faster swimming speed gave better overlap between modelled and observed distributions. There was a considerable difference in the model output using swimming speeds of 1.0 and 1.5 body length s−1, but swimming speeds >1.5 body length s−1 only improved the model results slightly. Swimming speeds >2.0 body length s−1, particularly for the southern stock, did not improve the model results. Hence, the use of a swimming speed of >1.5 body length s−1 would make the overlap between the modelled and observed distributions less sensitive to, for example, uncertainties or perturbations in the swimming speeds or in the speeds of ocean currents. A value between 1.5 and 2.0 body length s−1 is also close to the optimum swimming speed of 1.6 body length s−1 for post-smolt O. nerka (Videler, 1993). Such swimming speeds could be faster than those calculated from the tagged fish because the trajectory of the tagged fish might be longer than the defined migration path (Figure 1). This might be caused by the relatively high-resolution ocean current fields, in space (4 km) and time (daily), used in the particle-tracking model resolving mesoscale variability in the currents, and hence causing a delay in the northward transport. The simulated distributions of the southern and northern stock groups also indicate that the different stocks overlap both spatially and temporally, although they enter the sea at different locations and times of the year.
The results from this study suggest that any climate-driven change in windforcing patterns would affect the migration routes of post-smolts. The potential for salmon to adapt to changes depends on the magnitude, rate, and duration of the change (Ottersen et al., 2004). Simulated migration routes can be used to optimize the planning of marine surveys and salmon management through predictions of abundances. For instance, there have been no (or few) surveys to the south and west of the Faroes, but the model suggests that in some years, this area is a potential migration route, though minor in terms of post-smolt numbers. Pepin and Helbig (2012) and Stenevik et al. (in press) combined model outputs and observations for survey design purposes, and Vikebø et al. (2011) made real-time predictions of ichthyoplankton distributions for operational survey planning.
The two key areas, south of the Faroes and in the southern Norwegian Sea, where there may be substantial changes in the direction of post-smolt migration, merit further study.
Acknowledgements
We thank the reviewers, Myron Peck, and Hans-Harald Hinrichsen for constructive comments and helpful suggestions that improved the manuscript and guest editor Peter Hutchinson for his efficient handling of the manuscript. The study was partly financed by the European Commission under the 7th Framework Programme, Grant Agreement no 212529 (SALSEA-Merge). It was also co-sponsored by the Atlantic Salmon Trust and the Total Foundation, who we thank for financial support. Tag information for the Irish tag recovery samples used in the model was made available from the Marine Institute's National Coded Wire Tagging and Tag Recovery Programme, with special thanks to Anne Cullen, Deirdre Cotter, and Russell Poole of the Marine Institute. PM was partly supported by the Beaufort Marine Research Award in Fish Population Genetics funded by the Irish Government under the Sea Change Programme.
References
Author notes
Handling editor: Emory Anderson