-
PDF
- Split View
-
Views
-
Cite
Cite
Kirsteen M. MacKenzie, Clive N. Trueman, Martin R. Palmer, Andy Moore, Anton T. Ibbotson, William R. C. Beaumont, Ian C. Davidson, Stable isotopes reveal age-dependent trophic level and spatial segregation during adult marine feeding in populations of salmon, ICES Journal of Marine Science, Volume 69, Issue 9, November 2012, Pages 1637–1645, https://doi.org/10.1093/icesjms/fss074
Close - Share Icon Share
Abstract
Locating and differentiating the marine feeding areas used by adult salmon (Salmo salar) is essential to stock-based management and conservation, but traditional tagging studies are limited and influenced by the uneven distribution of the fisheries or research vessel surveys. Here, a novel approach is used, based on the observation that the isotopic composition of animal tissues is intrinsically linked to the environmental conditions during tissue growth, which allows for the distinction of pelagic fish feeding in different locations. This isotopic approach is applied using archived collections of salmon scales and shows that (i) salmon act as size-structured pelagic predators, (ii) adult salmon from different natal origins within the UK (and hence components of the southern European stock complex) feed in different oceanic regions before their return, (iii) one-sea-winter (1SW) and multi-sea-winter salmon returning to some rivers in the UK are separated in their marine feeding areas, whereas those from others are not, and (iv) salmon from the rivers sampled are not feeding in regions of the Northwest Atlantic used by 1SW salmon returning to rivers in Newfoundland. Therefore, the stable isotope approach allows for retrospective investigations of marine diet, location, and migration at stock- and cohort-specific levels.MacKenzie, K. M., Trueman, C. N., Palmer, M. R., Moore, A., Ibbotson, A. T., Beaumont, W. R. C., and Davidson, I. C. 2012. Stable isotopes reveal age-dependent trophic level and spatial segregation during adult marine feeding in populations of salmon from the UK. – ICES Journal of Marine Science, 69: 1637–1645.
Introduction
Many factors have been implicated in the decline in the abundance of wild Atlantic salmon (Salmo salar) in recent decades, but there is a strong evidence of an increase in mortality during the marine phase (Cairns, 2002; Jonsson and Jonsson, 2004; Friedland et al., 2005, 2009; Peyronnet et al., 2007), despite effort reductions in, or closure of, many marine fisheries for salmon (ICES, 2009). Significant gaps remain in our understanding of the life of salmon at sea (Friedland, 1998; Vøllestad et al., 2009; Dadswell et al., 2010), particularly after the initial post-smolt migration, and these knowledge gaps are a major obstacle to stock-based management and conservation.
Ocean climate variables strongly influence salmon growth and survival, either directly through temperature effects (Friedland, 1998; Friedland et al., 2000; Peyronnet et al., 2008; Todd et al., 2008), or through resultant changes in the abundance, composition, and distribution of prey species (Beaugrand and Reid, 2003). Because these factors are spatially heterogeneous, it is important to identify populations and age cohorts that share common marine feeding locations, because this would allow for a more effective study of the interplay between climatic and environmental variability and responses in salmon populations.
Current understanding of the distribution of Atlantic salmon at sea is based largely on radioisotope research (Tucker et al., 1999; Spares et al., 2007), scale reading (Lear and Sandeman, 1980; Reddin, 1986; Reddin et al., 1988; Friedland et al., 1999; Holst et al., 1999), and tagging data (ICES, 2007, 2008, 2009). However, recent research efforts have focused on identifying the migration routes and distribution of post-smolt salmon in their first year at sea (Holst et al., 1999; Friedland et al., 2000, 2009). Such studies are beginning to yield a clearer picture of the initial migration routes of some regional populations, but relatively little is known of the distribution and movements of salmon after their first winter at sea other than from areas where there are distant-water fisheries.
Two main hypotheses have been developed concerning the distribution and migration of salmon at sea. In the first hypothesis, European salmon from rivers north of 62°N migrate to feeding areas around the Faroe Islands and in the Norwegian and Barents Seas, whereas multi-sea-winter (MSW) salmon originating in rivers south of 62°N in Europe and from rivers in North America feed in the Labrador Sea (Reddin et al., 1988; Holst et al., 1999; ICES, 2008). Fish returning to some southern European rivers as one-sea-winter (1SW) salmon are known to feed in the Norwegian Sea, but it is not known if MSW salmon returning to southern European rivers spend their first winter in the Norwegian Sea or migrate to the Labrador Sea. This hypothesis is supported by tag returns showing that a large proportion of the European-origin salmon caught at West Greenland are from the southern European stock complex, and conversely, that a large proportion of tagged fish caught in the Faroes or Norwegian Sea fisheries are from the northern European stock complex (Holst et al., 1999; Hansen and Jacobsen, 2003).
The second, “Merry-Go-Round”, hypothesis proposes that all Atlantic salmon undertake transoceanic migrations using surface currents of the North Atlantic Subpolar Gyre (Dadswell et al., 2010). Under that hypothesis, salmon enter the surface current system on their respective sides of the Atlantic, then feed, grow, and, crucially, mix until they mature and return to their homewaters. The hypothesis suggests that all fish undergo continuous, transoceanic movements within the Subpolar Gyre, but that the position of fish within the gyre depends on their latitude of origin, their age, and seawater temperature. The “Merry-Go-Round” hypothesis is supported by the location of Faroese fishery captures throughout the year (Jacobsen et al., 2001), the movement of post-smolt fish in the eastern North Atlantic, the presence of tagged fish of mixed continent of origin in all distant-water fisheries (Reddin et al., 1984; Hansen and Jacobsen, 2003; Dadswell et al., 2010), and the few incidences of such fish caught in fisheries other than those at West Greenland, Faroes, and in the Norwegian Sea (Hansen and Jacobsen, 2003).
The limitations of fishery-dependent data, however, mean that neither hypothesis can be supported adequately, despite there having been decades of research (Dadswell et al., 2010). Studies combining tag–recapture data with salmon swimming speeds and oceanographic variables do, nevertheless, suggest that post-smolt salmon use the prevailing shelf edge currents in their initial migrations (Jonsson et al., 1993; Booker et al., 2008), a hypothesis that is supported by the results of post-smolt surveys (Holm et al., 2000).
Nitrogen isotope composition (expressed here as δ15N values) in tissues of surface-dwelling marine fish is strongly linked to the trophic level and, therefore, to the total size of the fish (Jennings et al., 2008a, b). Tissue-diet fractionation, i.e. the enrichment of a predator's tissues relative to its prey, has been measured at ∼3–4‰ in the muscle of fish (Sweeting et al., 2007a); the measured value approximates the oft-used ca. 3‰ per trophic level (DeNiro and Epstein, 1981). This is considerably higher than tissue-diet fractionation for carbon isotopes in fish muscle, which has been measured at around 1.5‰ (Sweeting et al., 2007b). The amount of isotopic fractionation between predator and prey depends, however, on the growth rate of the organism (Trueman et al., 2005). The nitrogen isotopic composition of tissues also depends on the isotopic compositions at the base of the foodweb, which are controlled by ambient environmental conditions and primary production taxonomy (detailed below; e.g. Lara et al., 2010). The nitrogen isotope composition of pelagic predators such as Atlantic salmon that frequently show size-dependent changes in the trophic level of their prey is, however, likely to be progressively enriched in 15N with body size.
The isotopic composition of carbon (expressed here as δ13C values) in pelagic fish tissues depends more strongly on that of the phytoplankton community at the base of the marine foodweb (Vander Zanden and Rasmussen, 1999) than on the trophic level of the fish. The fractionation of carbon isotopes during photosynthesis is controlled by several factors, particularly the ratio of cell growth rate to the concentration of aqueous carbon dioxide in seawater (Popp et al., 1989; Rau et al., 1996; Vander Zanden and Rasmussen, 1999; Hofmann et al., 2000). Additionally, photosynthetic carbon isotope fractionation differs between major phytoplankton groups (Popp et al., 1989; Rau et al., 1996; Graham et al., 2010), so the taxonomic composition of the phytoplankton community present in a particular area is also important. Cell growth rates, dissolved carbonate concentrations, and plankton community compositions vary spatially and temporally across ocean basins (Richardson and Schoeman, 2004), leading to great spatio-temporal differences in the isotope compositions of phytoplankton (Goericke and Fry, 1994; Hofmann et al., 2000; Tagliabue and Bopp, 2008; Lara et al., 2010) that are transferred up the food chain. Subsequent trophic interactions cause minor additional fractionation of carbon isotopes relative to the initial variability of primary production (Vander Zanden and Rasmussen, 1999), so variations in intermediate food sources (e.g. different zooplankton species) have relatively little influence on the carbon isotope composition of pelagic predators. Consequently, the carbon isotope composition of marine predators varies according to their feeding location and can be used to discriminate between animals feeding in different areas or to identify specific foraging locations (Schell et al., 1989; Cherel and Hobson, 2007; Phillips et al., 2009; Graham et al., 2010; Jaeger et al., 2010; Newsome et al., 2010).
Previous studies have demonstrated that the carbon isotope composition of Atlantic salmon is highly variable both among river stocks and through time, suggesting that salmon occupy a wide range of foraging areas (Trueman and Moore, 2007; Sinnatamby et al., 2009), and that salmon originating in Canada and Ireland must feed in different, if unspecified, areas (Dempson et al., 2010). Here, we use a stable isotope approach to test four hypotheses concerning marine diet and feeding areas during summer (i.e. non-winter months) growth season after the first winter at sea for Atlantic salmon from the southern European stock complex (ICES, 2009) returning to rivers in the UK. These hypotheses were:
salmon are opportunistic, size-structured pelagic predators with a cosmopolitan diet;
salmon returning to different rivers in the UK feed in a common area during the time sampled before return;
both 1SW and two-sea-winter (2SW) salmon returning to rivers in the UK feed in the same regions in the time sampled before their return;
both 1SW and 2SW salmon returning to rivers in the UK feed in the same area as 1SW salmon from Newfoundland (North American stock complex) in the time sampled before their return.
Material and methods
Salmon scales were obtained from collections taken routinely for age studies from fish returning to: (i) the River Frome, Dorset, England (Centre for Ecology and Hydrology, CEH, archive, 1980–2002); (ii) the English northeast coast mixed-stock salmon fishery (Cefas driftnet archive, 1985–2001) incorporating salmon returning to northeast English and eastern Scottish rivers (from the Yorkshire Humber to the Aberdeenshire Dee; Potter and Swain, 1982); and (iii) the River Dee, Wales (UK Environment Agency archive, 1984–2008; Figure 1). ICES assigns all of sampled stocks to the southern European stock complex (ICES, 2009). For each archive, we determined δ13C and δ15N values of scales from approximately ten 1SW and ten 2SW salmon for each available year (MacKenzie et al., 2011), except for the River Dee, where isotope values were only determined for 1SW fish. The values measured represent the isotopic composition of scale tissues averaged over the time sampled and do not reflect feeding during the post-smolt migration before the first winter at sea. Mass data (kilogramme whole wet weight) were available for most of the fish sampled.
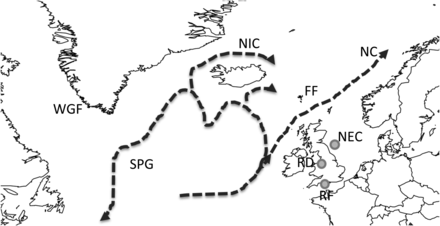
Location of sample populations and a schematic diagram of the main warm surface currents in the North Atlantic (NC, North Atlantic Current; SPG, Subpolar Gyre; NIC, North Icelandic Current); RF, River Frome, NEC, northeast coast, RD, River Dee. Locations of distant-water salmon fisheries are indicated as follows: FF, Faroese fishery; WGF, West Greenland fishery.
Scales are composite bioapatite-collagen structures. The mineralized component contains a small amount of inorganic carbonate, but acid pretreatment of scales does not change the bulk isotopic composition significantly (Sinnatamby et al., 2007). The collagen component of fish scales is consistently depleted in 13C relative to muscle values, reflecting the large proportion of glycine in collagen (McMahon et al., 2010). Scale δ13C and δ15N values are, however, linearly related to the isotopic composition of other body tissues across multiple genera, under differing diet conditions and growth rates (Satterfield and Finney, 2002; Sinnatamby et al., 2009). A range of intrinsic and extrinsic factors can influence δ13C fractionation at successive levels in foodwebs (Post, 2002; Trueman et al., 2005), but these variations are small compared with the variations in fractionation during photosynthesis (Rau et al., 1996; Lara et al., 2010), and are further dampened by the integration time of several months associated with the scale growth. Hence, whereas δ13C values in scale collagen are enriched relative to the diet, there is a clear, linear relationship between scale collagen and muscle δ13C values. Muscle δ13C values are themselves tightly coupled to diet (Satterfield and Finney, 2002), and, therefore, to environmental conditions (Schell et al., 1989).
Scales were read initially under a transmitted-light binocular dissecting microscope to determine the age of the fish. They were then dissected to sample the portion corresponding to the period of marine growth between the last winter at sea until return to homewaters for 1SW fish, and the last full period of growth between the first and second winters at sea for 2SW fish. This dissection is essential when sampling fish scales for isotopic analysis, to avoid mixing collagen of different ages in unknown proportions (Hutchinson and Trueman, 2006; MacKenzie et al., 2011). The sampled marine sections of scales were then analysed by continuous-flow isotope ratio mass spectrometry (EA-IRMS) to determine their carbon and nitrogen isotopic ratios (see MacKenzie, 2010, for a full description of the analytical procedure).
Statistical analyses
Relationships between the size and the trophic level [hypothesis (i)] were investigated graphically and with basic descriptive statistics. Similarities in feeding areas within and among groups [hypothesis (ii)] were tested by a simple time-by-time analysis (Diggle et al., 2002), using two-tailed t-tests to assess similarity in δ13C values in all years with sufficient sample numbers. The influence of regional origin and/or sea age on isotopic composition and, therefore, feeding location [hypothesis (iii)] was tested using generalized additive models (GAMs), which were applied to the time-series of δ13C values. Models were fitted using the R package “mgcv” with a cubic spline smoothing function, where optimal smoothing parameters were estimated by cross-validation. To test whether the isotopic compositions recorded in fish sampled from the UK were comparable with those of 1SW salmon from Newfoundland [hypothesis (iv)], pooled data from the River Frome, northeast coast, and River Dee were compared with data for 1SW fish returning to various rivers in Newfoundland (Sinnatamby et al., 2009). For display purposes, yearly averaged values of isotope compositions were estimated by fitting LOWESS smoothers through the time-series data for each sea age and river-origin group. All statistical calculations were performed using the R statistical language (R Development Core Team, 2008).
Results
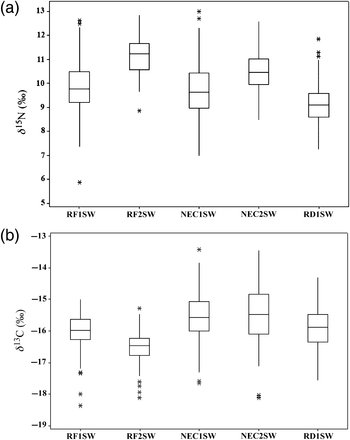
To test hypothesis (i), the values of δ15N for all salmon scale samples were plotted according to their sea age at return (Figure 2a). For both 1SW and 2SW salmon, the portion of the scale sampled reflects feeding after the first winter at sea, but the duration of marine feeding is longer (and the incremental increase in scale size is generally larger) in the scale section sampled for 2SW fish. The variance in δ15N values in 2SW salmon is less than that for 1SW fish (River Frome 1SW, s.d. = 1.04; River Frome 2SW, s.d. = 0.73; northeast coast 1SW, s.d. = 1.11; northeast coast 2SW, s.d. = 0.76); F-test (δ15N) River Frome 1SW, River Frome 2SW: n = 179, 140, F = 2.15, p < 0.001; northeast coast 1SW, northeast coast 2SW: n = 150, 139, F = 3.96, p < 0.001). This is consistent with the size-dependent trophic fractionation of N strong enough to overprint any potential temporal fluctuations in baseline nitrogen isotopes within each age class and spatial variations in baseline nitrogen isotopes between feeding areas. Based on these data, 2SW salmon are likely to occupy a higher trophic level during marine feeding than the smaller 1SW salmon. Pelagic marine ecosystems are strongly size-structured, with prey δ15N values increasing systematically with size (Jennings et al., 2002). Gape size likely limits the upper δ15N value for all fish. Lower δ15N values are controlled by both primary production at the site of feeding and the trophic level of feeding, and the range in δ15N values between these two limits is controlled by the extent of prey selectivity. The observed δ15N values therefore imply that returning 1SW salmon have a more opportunistic diet across a wide range of trophic levels, whereas 2SW salmon may feed more selectively, concentrating on higher trophic level prey items. This finding supports hypothesis (i) because it suggests that Atlantic salmon are size-structured predators, but that their diet becomes less cosmopolitan and more focused on higher trophic level prey as they increase in size.
Boxplots of (a) δ15N values and (b) δ13C values for River Frome 1SW and 2SW salmon, northeast coast 1SW and 2SW salmon, and River Dee 1SW salmon. Boxes represent lower (Q1) to upper (Q3) quartiles, with median values denoted by lines within the boxes. The whiskers extend to the minimum and maximum values excluding outliers, where the datapoint is no more than the range multiplied by the inter-quartile range from the box. Outliers are indicated by asterisks.
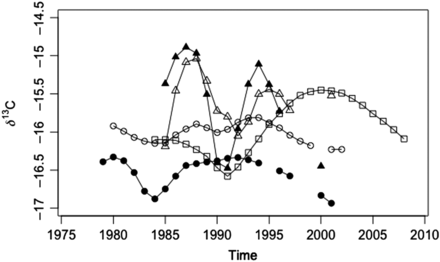
Temporal patterns of LOWESS-smoothed values and variation in carbon isotope values for each sea age group and origin are presented in Table 1 and Figures 2b and 3. The values of δ13C in River Frome salmon show less variation than those of northeast coast fish (Figures 2b and 3), suggesting more stable environmental conditions throughout the time covered by these analyses. The variation seen in the River Dee 1SW fish appears intermediate between that of the River Frome and northeast coast salmon (Figures 2b and 3). The different levels of variation suggest that hypothesis (ii), i.e. that salmon returning to different rivers in the UK feed in a common area during the time sampled before return, may be incorrect.
Time-series of carbon isotope ratios (‰) in scale collagen, showing each term as the value for a LOWESS smoother fitted to the full dataset.
| Year . | RF 1SW . | n . | RF 2SW . | n . | NEC 1SW . | n . | NEC 2SW . | n . | RD 1SW . | n . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1979 | n.d. | −16.39 | 7 | n.d. | n.d. | n.d. | ||||
| 1980 | −15.92 | 3 | −16.33 | 10 | n.d. | n.d. | n.d. | |||
| 1981 | −15.99 | 9 | −16.38 | 5 | n.d. | n.d. | n.d. | |||
| 1982 | −16.07 | 2 | −16.53 | 10 | n.d. | n.d. | n.d. | |||
| 1983 | −16.12 | 10 | −16.78 | 9 | n.d. | n.d. | n.d. | |||
| 1984 | −16.15 | 10 | −16.88 | 10 | n.d. | n.d. | −16.10 | 12 | ||
| 1985 | −16.14 | 10 | −16.75 | 10 | −16.19 | 10 | −15.37 | 8 | −16.10 | 10 |
| 1986 | −16.04 | 10 | −16.58 | 10 | −15.46 | 2 | −15.02 | 8 | −16.11 | 10 |
| 1987 | −15.96 | 10 | −16.44 | 10 | −15.08 | 12 | −14.89 | 24 | −16.16 | 9 |
| 1988 | −15.90 | 10 | −16.41 | 10 | −15.04 | 14 | −14.97 | 9 | −16.23 | 10 |
| 1989 | −15.94 | 10 | −16.39 | 1 | −15.33 | 16 | −15.51 | 16 | −16.32 | 10 |
| 1990 | −16.01 | 8 | −16.38 | 6 | −15.72 | 10 | −16.40 | 9 | −16.47 | 9 |
| 1991 | −15.97 | 1 | −16.35 | 5 | −15.80 | 10 | −16.48 | 10 | −16.58 | 10 |
| 1992 | −15.87 | 6 | −16.33 | 6 | −16.05 | 10 | −15.96 | 11 | −16.46 | 9 |
| 1993 | −15.81 | 9 | −16.36 | 1 | −15.87 | 10 | −15.37 | 5 | −16.27 | 10 |
| 1994 | −15.81 | 8 | −16.41 | 7 | −15.50 | 9 | −15.11 | 6 | −16.09 | 10 |
| 1995 | −15.88 | 10 | n.d. | −15.44 | 10 | −15.38 | 20 | −15.90 | 10 | |
| 1996 | −15.95 | 12 | −16.51 | 8 | −15.50 | 9 | −15.73 | 10 | −15.76 | 10 |
| 1997 | −16.04 | 7 | −16.58 | 5 | −15.72 | 10 | n.d. | −15.62 | 10 | |
| 1998 | −16.12 | 12 | n.d. | n.d. | n.d. | −15.52 | 10 | |||
| 1999 | −16.18 | 7 | n.d. | n.d. | n.d. | −15.47 | 10 | |||
| 2000 | n.d. | −16.83 | 8 | n.d. | −16.45 | 3 | −15.45 | 10 | ||
| 2001 | −16.23 | 10 | −16.93 | 2 | −15.52 | 18 | n.d. | −15.46 | 10 | |
| 2002 | −16.23 | 5 | n.d. | n.d. | n.d. | −15.49 | 10 | |||
| 2003 | n.d. | n.d. | n.d. | n.d. | −15.56 | 10 | ||||
| 2004 | n.d. | n.d. | n.d. | n.d. | −15.65 | 10 | ||||
| 2005 | n.d. | n.d. | n.d. | n.d. | −15.76 | 10 | ||||
| 2006 | n.d. | n.d. | n.d. | n.d. | −15.86 | 10 | ||||
| 2007 | n.d. | n.d. | n.d. | n.d. | −15.97 | 10 | ||||
| 2008 | n.d. | n.d. | n.d. | n.d. | −16.09 | 10 | ||||
| Total n | 179 | 140 | 150 | 139 | 249 |
| Year . | RF 1SW . | n . | RF 2SW . | n . | NEC 1SW . | n . | NEC 2SW . | n . | RD 1SW . | n . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1979 | n.d. | −16.39 | 7 | n.d. | n.d. | n.d. | ||||
| 1980 | −15.92 | 3 | −16.33 | 10 | n.d. | n.d. | n.d. | |||
| 1981 | −15.99 | 9 | −16.38 | 5 | n.d. | n.d. | n.d. | |||
| 1982 | −16.07 | 2 | −16.53 | 10 | n.d. | n.d. | n.d. | |||
| 1983 | −16.12 | 10 | −16.78 | 9 | n.d. | n.d. | n.d. | |||
| 1984 | −16.15 | 10 | −16.88 | 10 | n.d. | n.d. | −16.10 | 12 | ||
| 1985 | −16.14 | 10 | −16.75 | 10 | −16.19 | 10 | −15.37 | 8 | −16.10 | 10 |
| 1986 | −16.04 | 10 | −16.58 | 10 | −15.46 | 2 | −15.02 | 8 | −16.11 | 10 |
| 1987 | −15.96 | 10 | −16.44 | 10 | −15.08 | 12 | −14.89 | 24 | −16.16 | 9 |
| 1988 | −15.90 | 10 | −16.41 | 10 | −15.04 | 14 | −14.97 | 9 | −16.23 | 10 |
| 1989 | −15.94 | 10 | −16.39 | 1 | −15.33 | 16 | −15.51 | 16 | −16.32 | 10 |
| 1990 | −16.01 | 8 | −16.38 | 6 | −15.72 | 10 | −16.40 | 9 | −16.47 | 9 |
| 1991 | −15.97 | 1 | −16.35 | 5 | −15.80 | 10 | −16.48 | 10 | −16.58 | 10 |
| 1992 | −15.87 | 6 | −16.33 | 6 | −16.05 | 10 | −15.96 | 11 | −16.46 | 9 |
| 1993 | −15.81 | 9 | −16.36 | 1 | −15.87 | 10 | −15.37 | 5 | −16.27 | 10 |
| 1994 | −15.81 | 8 | −16.41 | 7 | −15.50 | 9 | −15.11 | 6 | −16.09 | 10 |
| 1995 | −15.88 | 10 | n.d. | −15.44 | 10 | −15.38 | 20 | −15.90 | 10 | |
| 1996 | −15.95 | 12 | −16.51 | 8 | −15.50 | 9 | −15.73 | 10 | −15.76 | 10 |
| 1997 | −16.04 | 7 | −16.58 | 5 | −15.72 | 10 | n.d. | −15.62 | 10 | |
| 1998 | −16.12 | 12 | n.d. | n.d. | n.d. | −15.52 | 10 | |||
| 1999 | −16.18 | 7 | n.d. | n.d. | n.d. | −15.47 | 10 | |||
| 2000 | n.d. | −16.83 | 8 | n.d. | −16.45 | 3 | −15.45 | 10 | ||
| 2001 | −16.23 | 10 | −16.93 | 2 | −15.52 | 18 | n.d. | −15.46 | 10 | |
| 2002 | −16.23 | 5 | n.d. | n.d. | n.d. | −15.49 | 10 | |||
| 2003 | n.d. | n.d. | n.d. | n.d. | −15.56 | 10 | ||||
| 2004 | n.d. | n.d. | n.d. | n.d. | −15.65 | 10 | ||||
| 2005 | n.d. | n.d. | n.d. | n.d. | −15.76 | 10 | ||||
| 2006 | n.d. | n.d. | n.d. | n.d. | −15.86 | 10 | ||||
| 2007 | n.d. | n.d. | n.d. | n.d. | −15.97 | 10 | ||||
| 2008 | n.d. | n.d. | n.d. | n.d. | −16.09 | 10 | ||||
| Total n | 179 | 140 | 150 | 139 | 249 |
RF, River Frome; NEC, northeast coast; n.d., no data (so n = 0).
Time-series of carbon isotope ratios (‰) in scale collagen, showing each term as the value for a LOWESS smoother fitted to the full dataset.
| Year . | RF 1SW . | n . | RF 2SW . | n . | NEC 1SW . | n . | NEC 2SW . | n . | RD 1SW . | n . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1979 | n.d. | −16.39 | 7 | n.d. | n.d. | n.d. | ||||
| 1980 | −15.92 | 3 | −16.33 | 10 | n.d. | n.d. | n.d. | |||
| 1981 | −15.99 | 9 | −16.38 | 5 | n.d. | n.d. | n.d. | |||
| 1982 | −16.07 | 2 | −16.53 | 10 | n.d. | n.d. | n.d. | |||
| 1983 | −16.12 | 10 | −16.78 | 9 | n.d. | n.d. | n.d. | |||
| 1984 | −16.15 | 10 | −16.88 | 10 | n.d. | n.d. | −16.10 | 12 | ||
| 1985 | −16.14 | 10 | −16.75 | 10 | −16.19 | 10 | −15.37 | 8 | −16.10 | 10 |
| 1986 | −16.04 | 10 | −16.58 | 10 | −15.46 | 2 | −15.02 | 8 | −16.11 | 10 |
| 1987 | −15.96 | 10 | −16.44 | 10 | −15.08 | 12 | −14.89 | 24 | −16.16 | 9 |
| 1988 | −15.90 | 10 | −16.41 | 10 | −15.04 | 14 | −14.97 | 9 | −16.23 | 10 |
| 1989 | −15.94 | 10 | −16.39 | 1 | −15.33 | 16 | −15.51 | 16 | −16.32 | 10 |
| 1990 | −16.01 | 8 | −16.38 | 6 | −15.72 | 10 | −16.40 | 9 | −16.47 | 9 |
| 1991 | −15.97 | 1 | −16.35 | 5 | −15.80 | 10 | −16.48 | 10 | −16.58 | 10 |
| 1992 | −15.87 | 6 | −16.33 | 6 | −16.05 | 10 | −15.96 | 11 | −16.46 | 9 |
| 1993 | −15.81 | 9 | −16.36 | 1 | −15.87 | 10 | −15.37 | 5 | −16.27 | 10 |
| 1994 | −15.81 | 8 | −16.41 | 7 | −15.50 | 9 | −15.11 | 6 | −16.09 | 10 |
| 1995 | −15.88 | 10 | n.d. | −15.44 | 10 | −15.38 | 20 | −15.90 | 10 | |
| 1996 | −15.95 | 12 | −16.51 | 8 | −15.50 | 9 | −15.73 | 10 | −15.76 | 10 |
| 1997 | −16.04 | 7 | −16.58 | 5 | −15.72 | 10 | n.d. | −15.62 | 10 | |
| 1998 | −16.12 | 12 | n.d. | n.d. | n.d. | −15.52 | 10 | |||
| 1999 | −16.18 | 7 | n.d. | n.d. | n.d. | −15.47 | 10 | |||
| 2000 | n.d. | −16.83 | 8 | n.d. | −16.45 | 3 | −15.45 | 10 | ||
| 2001 | −16.23 | 10 | −16.93 | 2 | −15.52 | 18 | n.d. | −15.46 | 10 | |
| 2002 | −16.23 | 5 | n.d. | n.d. | n.d. | −15.49 | 10 | |||
| 2003 | n.d. | n.d. | n.d. | n.d. | −15.56 | 10 | ||||
| 2004 | n.d. | n.d. | n.d. | n.d. | −15.65 | 10 | ||||
| 2005 | n.d. | n.d. | n.d. | n.d. | −15.76 | 10 | ||||
| 2006 | n.d. | n.d. | n.d. | n.d. | −15.86 | 10 | ||||
| 2007 | n.d. | n.d. | n.d. | n.d. | −15.97 | 10 | ||||
| 2008 | n.d. | n.d. | n.d. | n.d. | −16.09 | 10 | ||||
| Total n | 179 | 140 | 150 | 139 | 249 |
| Year . | RF 1SW . | n . | RF 2SW . | n . | NEC 1SW . | n . | NEC 2SW . | n . | RD 1SW . | n . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1979 | n.d. | −16.39 | 7 | n.d. | n.d. | n.d. | ||||
| 1980 | −15.92 | 3 | −16.33 | 10 | n.d. | n.d. | n.d. | |||
| 1981 | −15.99 | 9 | −16.38 | 5 | n.d. | n.d. | n.d. | |||
| 1982 | −16.07 | 2 | −16.53 | 10 | n.d. | n.d. | n.d. | |||
| 1983 | −16.12 | 10 | −16.78 | 9 | n.d. | n.d. | n.d. | |||
| 1984 | −16.15 | 10 | −16.88 | 10 | n.d. | n.d. | −16.10 | 12 | ||
| 1985 | −16.14 | 10 | −16.75 | 10 | −16.19 | 10 | −15.37 | 8 | −16.10 | 10 |
| 1986 | −16.04 | 10 | −16.58 | 10 | −15.46 | 2 | −15.02 | 8 | −16.11 | 10 |
| 1987 | −15.96 | 10 | −16.44 | 10 | −15.08 | 12 | −14.89 | 24 | −16.16 | 9 |
| 1988 | −15.90 | 10 | −16.41 | 10 | −15.04 | 14 | −14.97 | 9 | −16.23 | 10 |
| 1989 | −15.94 | 10 | −16.39 | 1 | −15.33 | 16 | −15.51 | 16 | −16.32 | 10 |
| 1990 | −16.01 | 8 | −16.38 | 6 | −15.72 | 10 | −16.40 | 9 | −16.47 | 9 |
| 1991 | −15.97 | 1 | −16.35 | 5 | −15.80 | 10 | −16.48 | 10 | −16.58 | 10 |
| 1992 | −15.87 | 6 | −16.33 | 6 | −16.05 | 10 | −15.96 | 11 | −16.46 | 9 |
| 1993 | −15.81 | 9 | −16.36 | 1 | −15.87 | 10 | −15.37 | 5 | −16.27 | 10 |
| 1994 | −15.81 | 8 | −16.41 | 7 | −15.50 | 9 | −15.11 | 6 | −16.09 | 10 |
| 1995 | −15.88 | 10 | n.d. | −15.44 | 10 | −15.38 | 20 | −15.90 | 10 | |
| 1996 | −15.95 | 12 | −16.51 | 8 | −15.50 | 9 | −15.73 | 10 | −15.76 | 10 |
| 1997 | −16.04 | 7 | −16.58 | 5 | −15.72 | 10 | n.d. | −15.62 | 10 | |
| 1998 | −16.12 | 12 | n.d. | n.d. | n.d. | −15.52 | 10 | |||
| 1999 | −16.18 | 7 | n.d. | n.d. | n.d. | −15.47 | 10 | |||
| 2000 | n.d. | −16.83 | 8 | n.d. | −16.45 | 3 | −15.45 | 10 | ||
| 2001 | −16.23 | 10 | −16.93 | 2 | −15.52 | 18 | n.d. | −15.46 | 10 | |
| 2002 | −16.23 | 5 | n.d. | n.d. | n.d. | −15.49 | 10 | |||
| 2003 | n.d. | n.d. | n.d. | n.d. | −15.56 | 10 | ||||
| 2004 | n.d. | n.d. | n.d. | n.d. | −15.65 | 10 | ||||
| 2005 | n.d. | n.d. | n.d. | n.d. | −15.76 | 10 | ||||
| 2006 | n.d. | n.d. | n.d. | n.d. | −15.86 | 10 | ||||
| 2007 | n.d. | n.d. | n.d. | n.d. | −15.97 | 10 | ||||
| 2008 | n.d. | n.d. | n.d. | n.d. | −16.09 | 10 | ||||
| Total n | 179 | 140 | 150 | 139 | 249 |
RF, River Frome; NEC, northeast coast; n.d., no data (so n = 0).
Temporal records of carbon isotopes recorded in salmon-scale collagen for River Frome 1SW (open circles), River Frome 2SW (dots), northeast coast 1SW (open triangles), northeast coast 2SW (closed triangles), and River Dee 1SW (open squares). Lines represent LOWESS smoothers through the full dataset.
Hypothesis (ii) was further tested by comparing δ13C values in scales of 1SW and 2SW salmon within and among samples from the River Frome, northeast coast, and River Dee. There were no significant differences in δ13C values between 1SW and 2SW salmon in about one-third of all years for salmon returning to the River Frome and in half of all years for salmon returning to the northeast coast (Table 2). These data imply that spatio-temporal variations in carbon isotopes at the base of the foodweb are sufficient to mask any differences in δ13C values related to the trophic level. 1SW salmon from the River Frome and the northeast coast had more similar δ13C values, with significant differences in only 4 of the 13 years (ca. 30%). The δ13C values for 1SW salmon from the River Dee are relatively similar to those from the River Frome, with significant differences in 7 of the 17 years (41%), but are more distinct isotopically from northeast coast salmon, with significant differences in 9 of the 14 years (65%). For 2SW salmon from the River Frome and the northeast coast, there were significant differences in δ13C values in 6 of the 9 years (67%) sampled. These results suggest some separation in feeding locations between 1SW and 2SW salmon, refuting hypothesis (iii), and between fish returning to different areas within the UK component of the southern European stock complex, refuting hypothesis (ii).
Probability (p) values for two-tailed t-tests with unequal variance.
| Year . | Frome . | NEC . | Frome–NEC . | Frome–NEC . | Frome–Dee . | Dee–NEC . |
|---|---|---|---|---|---|---|
| 1SW–2SW . | 1SW–2SW . | 1SW . | 2SW . | 1SW . | 1SW . | |
| 1981 | 0.39 | – | – | – | – | – |
| 1982 | – | – | – | – | – | – |
| 1983 | 0.03 | – | – | – | – | – |
| 1984 | 0.01 | – | – | – | 0.75 | – |
| 1985 | 0.004 | 0.041 | 0.9 | <0.001 | 0.37 | 0.48 |
| 1986 | 0.1 | 0.27 | 0.44 | 0.005 | 0.91 | 0.01 |
| 1987 | 0.05 | 0.75 | 0.07 | <0.001 | 0.54 | 0.0001 |
| 1988 | <0.001 | 0.62 | <0.001 | <0.001 | 0.03 | 0.0001 |
| 1989 | – | 0.47 | 0.01 | – | 0.28 | 0.04 |
| 1990 | 0.003 | <0.001 | 0.36 | 0.85 | 0.002 | 0.0001 |
| 1991 | – | 0.001 | – | 0.48 | – | 0.0002 |
| 1992 | 0.78 | 0.313 | 0.77 | 0.66 | 0.49 | 0.74 |
| 1993 | – | <0.001 | 0.88 | – | 0.05 | 0.0008 |
| 1994 | 0.006 | 0.006 | 0.47 | <0.001 | 0.002 | <0.0001 |
| 1995 | – | <0.001 | <0.001 | – | 0.17 | 0.03 |
| 1996 | <0.001 | 0.96 | 0.9 | <0.001 | 0.08 | 0.2 |
| 1997 | 0.59 | – | 0.15 | – | 0.27 | 0.72 |
| 1998 | – | – | – | – | 0.02 | – |
| 1999 | – | – | – | – | 0.01 | – |
| 2000 | – | – | – | – | – | – |
| 2001 | – | – | 0.001 | – | 0.001 | 0.65 |
| 2002 | – | – | – | – | 0.48 | – |
| % difference | 67 | 50 | 30 | 67 | 41 | 64 |
| Year . | Frome . | NEC . | Frome–NEC . | Frome–NEC . | Frome–Dee . | Dee–NEC . |
|---|---|---|---|---|---|---|
| 1SW–2SW . | 1SW–2SW . | 1SW . | 2SW . | 1SW . | 1SW . | |
| 1981 | 0.39 | – | – | – | – | – |
| 1982 | – | – | – | – | – | – |
| 1983 | 0.03 | – | – | – | – | – |
| 1984 | 0.01 | – | – | – | 0.75 | – |
| 1985 | 0.004 | 0.041 | 0.9 | <0.001 | 0.37 | 0.48 |
| 1986 | 0.1 | 0.27 | 0.44 | 0.005 | 0.91 | 0.01 |
| 1987 | 0.05 | 0.75 | 0.07 | <0.001 | 0.54 | 0.0001 |
| 1988 | <0.001 | 0.62 | <0.001 | <0.001 | 0.03 | 0.0001 |
| 1989 | – | 0.47 | 0.01 | – | 0.28 | 0.04 |
| 1990 | 0.003 | <0.001 | 0.36 | 0.85 | 0.002 | 0.0001 |
| 1991 | – | 0.001 | – | 0.48 | – | 0.0002 |
| 1992 | 0.78 | 0.313 | 0.77 | 0.66 | 0.49 | 0.74 |
| 1993 | – | <0.001 | 0.88 | – | 0.05 | 0.0008 |
| 1994 | 0.006 | 0.006 | 0.47 | <0.001 | 0.002 | <0.0001 |
| 1995 | – | <0.001 | <0.001 | – | 0.17 | 0.03 |
| 1996 | <0.001 | 0.96 | 0.9 | <0.001 | 0.08 | 0.2 |
| 1997 | 0.59 | – | 0.15 | – | 0.27 | 0.72 |
| 1998 | – | – | – | – | 0.02 | – |
| 1999 | – | – | – | – | 0.01 | – |
| 2000 | – | – | – | – | – | – |
| 2001 | – | – | 0.001 | – | 0.001 | 0.65 |
| 2002 | – | – | – | – | 0.48 | – |
| % difference | 67 | 50 | 30 | 67 | 41 | 64 |
Significant values are shown emboldened.
Probability (p) values for two-tailed t-tests with unequal variance.
| Year . | Frome . | NEC . | Frome–NEC . | Frome–NEC . | Frome–Dee . | Dee–NEC . |
|---|---|---|---|---|---|---|
| 1SW–2SW . | 1SW–2SW . | 1SW . | 2SW . | 1SW . | 1SW . | |
| 1981 | 0.39 | – | – | – | – | – |
| 1982 | – | – | – | – | – | – |
| 1983 | 0.03 | – | – | – | – | – |
| 1984 | 0.01 | – | – | – | 0.75 | – |
| 1985 | 0.004 | 0.041 | 0.9 | <0.001 | 0.37 | 0.48 |
| 1986 | 0.1 | 0.27 | 0.44 | 0.005 | 0.91 | 0.01 |
| 1987 | 0.05 | 0.75 | 0.07 | <0.001 | 0.54 | 0.0001 |
| 1988 | <0.001 | 0.62 | <0.001 | <0.001 | 0.03 | 0.0001 |
| 1989 | – | 0.47 | 0.01 | – | 0.28 | 0.04 |
| 1990 | 0.003 | <0.001 | 0.36 | 0.85 | 0.002 | 0.0001 |
| 1991 | – | 0.001 | – | 0.48 | – | 0.0002 |
| 1992 | 0.78 | 0.313 | 0.77 | 0.66 | 0.49 | 0.74 |
| 1993 | – | <0.001 | 0.88 | – | 0.05 | 0.0008 |
| 1994 | 0.006 | 0.006 | 0.47 | <0.001 | 0.002 | <0.0001 |
| 1995 | – | <0.001 | <0.001 | – | 0.17 | 0.03 |
| 1996 | <0.001 | 0.96 | 0.9 | <0.001 | 0.08 | 0.2 |
| 1997 | 0.59 | – | 0.15 | – | 0.27 | 0.72 |
| 1998 | – | – | – | – | 0.02 | – |
| 1999 | – | – | – | – | 0.01 | – |
| 2000 | – | – | – | – | – | – |
| 2001 | – | – | 0.001 | – | 0.001 | 0.65 |
| 2002 | – | – | – | – | 0.48 | – |
| % difference | 67 | 50 | 30 | 67 | 41 | 64 |
| Year . | Frome . | NEC . | Frome–NEC . | Frome–NEC . | Frome–Dee . | Dee–NEC . |
|---|---|---|---|---|---|---|
| 1SW–2SW . | 1SW–2SW . | 1SW . | 2SW . | 1SW . | 1SW . | |
| 1981 | 0.39 | – | – | – | – | – |
| 1982 | – | – | – | – | – | – |
| 1983 | 0.03 | – | – | – | – | – |
| 1984 | 0.01 | – | – | – | 0.75 | – |
| 1985 | 0.004 | 0.041 | 0.9 | <0.001 | 0.37 | 0.48 |
| 1986 | 0.1 | 0.27 | 0.44 | 0.005 | 0.91 | 0.01 |
| 1987 | 0.05 | 0.75 | 0.07 | <0.001 | 0.54 | 0.0001 |
| 1988 | <0.001 | 0.62 | <0.001 | <0.001 | 0.03 | 0.0001 |
| 1989 | – | 0.47 | 0.01 | – | 0.28 | 0.04 |
| 1990 | 0.003 | <0.001 | 0.36 | 0.85 | 0.002 | 0.0001 |
| 1991 | – | 0.001 | – | 0.48 | – | 0.0002 |
| 1992 | 0.78 | 0.313 | 0.77 | 0.66 | 0.49 | 0.74 |
| 1993 | – | <0.001 | 0.88 | – | 0.05 | 0.0008 |
| 1994 | 0.006 | 0.006 | 0.47 | <0.001 | 0.002 | <0.0001 |
| 1995 | – | <0.001 | <0.001 | – | 0.17 | 0.03 |
| 1996 | <0.001 | 0.96 | 0.9 | <0.001 | 0.08 | 0.2 |
| 1997 | 0.59 | – | 0.15 | – | 0.27 | 0.72 |
| 1998 | – | – | – | – | 0.02 | – |
| 1999 | – | – | – | – | 0.01 | – |
| 2000 | – | – | – | – | – | – |
| 2001 | – | – | 0.001 | – | 0.001 | 0.65 |
| 2002 | – | – | – | – | 0.48 | – |
| % difference | 67 | 50 | 30 | 67 | 41 | 64 |
Significant values are shown emboldened.
The year of sampling contributed significantly to the GAM in all analyses. However, the contribution was weaker in River Frome samples (p < 0.05) than in those from the northeast coast and the River Dee (p < 0.001). In River Frome samples, sea age contributed significantly to the model (p < 0.001), but larger 2SW fish had lower δ13C values, indicating that this difference is regional rather than trophic in origin. Sea age was not a significant component of the model fit for the northeast coast samples, again demonstrating that variation in δ13C values at the base of the foodweb is sufficient to mask any trophic effects. Natal origin (Frome, northeast coast) contributed significantly to modelled δ13C time-series, but this effect was weaker for 1SW (p < 0.05) than for 2SW (p < 0.001) salmon. When River Dee 1SW fish are included, natal origin distinguishes the northeast coast population (p < 0.001), but natal origin does not contribute significantly to models containing River Dee and River Frome 1SW fish.
The salmon sampled were from rivers within the southern European stock complex used by ICES to provide management advice (ICES, 2009). Hypothesis (ii) states that salmon returning to different rivers in the UK feed in a common area during the time sampled before their return. In a large number of the years sampled, δ13C values differed among River Frome, northeast coast, and River Dee samples, and origin had a significant influence on the modelled δ13C time-series. As we found significant geographic variation in marine feeding locations within a small component of the southern European stock complex in the UK, we conclude that salmon of southern European origin are not mixed in a common feeding area in summer feeding season before return. The differences in δ13C values between 1SW and 2SW fish from the River Frome, but not from the northeast coast samples, also imply that salmon of different sea ages from some rivers may be segregated during summer feeding, but salmon from other rivers may not be, i.e. a qualified rejection of hypothesis (iii).
The carbon isotope compositions of both 1SW and 2SW salmon of UK origin were significantly different from 1SW salmon of Newfoundland origin (ANOVA, all UK vs. Newfoundland 1SW, n=1410, d.f. = 1, F =60.86, p<0.001; UK 1SW vs. Newfoundland 1SW, n = 1129, d.f. = 1, F = 96.49, p < 0.001; UK 2SW vs. Newfoundland 1SW, n=827, d.f.=1, F=13.24, p<0.001). Consequently, the hypothesis that these UK-origin fish are feeding in the same area as 1SW salmon from rivers in Newfoundland [hypothesis (iv)] is rejected.
Discussion
The stable isotope composition in collagen obtained from archived scales provides information on diet and location of salmon after their first winter at sea. The differences in δ15N values and ranges observed between returning 1SW and 2SW salmon suggest that 1SW fish feed as opportunistic pelagic predators, consistent with hypothesis (i), and that the extent of selectivity towards higher trophic level prey increases with growth and age of return. There may be some components of spatial and temporal variation in baseline 15N within these patterns, but the increase in δ15N values with size is, as discussed above, consistent with size-dependent trophic fractionation of nitrogen stable isotopes. This suggests that changes in the trophic level with size constitute the majority of the differences in δ15N values between 1SW and 2SW fish in the populations studied. The similarities in patterns of δ15N shown by River Frome and northeast coast salmon suggest a marked difference in dietary behaviour between fish returning as 1SW salmon, generally weighing <4–5 kg, and fish returning as larger 2SW salmon, with the fish probably becoming more piscivorous with age.
Stable isotope data allow the rejection of the hypothesis that salmon from different UK rivers share a common feeding area in the period sampled before return. Rather, there is strong evidence that fish from different natal-origin stocks are consistently separated in their marine feeding areas.
Salmon returning as 2SW fish to the River Frome show the most similar carbon isotope values to 1SW Newfoundland fish, but when the full time-series is considered, all sampled European salmon can be differentiated statistically from 1SW Newfoundland fish. We therefore reject hypothesis (iv) that the sampled UK origin salmon fed primarily in the same area as 1SW Newfoundland fish in the Labrador Sea in summer before returning to their natal river, or if they did, there is little evidence of successful return of these fish. A similar isotopic separation and, therefore, separation in feeding locations between North American and Irish salmon were found by Dempson et al. (2010), who reported, as with the populations analysed here, that the Irish fish had more positive δ13C and more negative δ15N values than North American salmon. Modelled plankton isotope data (Hofmann et al., 2000) suggest that Labrador Sea δ13C values of particulate organic matter are more negative than elsewhere in the northern North Atlantic, suggesting, therefore, that the 2SW salmon returning to the UK are less likely to be feeding in the Labrador Sea. Taken together, these results do not support a model of salmon migration with UK origin 1SW or 2SW salmon feeding as a mixed stock in the Labrador Sea. However, genetic studies indicate that during the period 2002–2010, non-maturing 1SW (i.e. potential MSW) salmon of European origin, predominantly from the southern European stock complex, comprised on average 22.7% (9–32%) of the salmon taken in the West Greenland fishery (ICES, 2011). Furthermore, tagged fish of UK origin (including one fish originating in the River Frome) have been recovered in this fishery, so clearly salmon of southern European origin do feed in these waters. However, stable isotope analyses suggest that most of the salmon returning to the rivers sampled in the UK do not feed in the same area of the Labrador Sea as 1SW salmon from Newfoundland.
Natal origin contributed significantly to models of salmon scale collagen δ13C values. Migration through multiple isotopically distinct regions, as implied by the “Merry-Go-Round” hypothesis (Dadswell et al., 2010), would tend to homogenize the isotopic composition of scale collagen between populations over the growth period sampled from scales. Instead, the stable isotope data support the suggestion that salmon migrate to discrete regions and remain in these broad areas during marine feeding. Graham et al. (2010) reported similar behaviour for yellowfin and bigeye tuna.
The large multiyear variations in δ13C scale values seen in salmon from the northeast coast fishery (Figures 2b and 3) suggest either fluctuations in the location of the feeding location or climatically driven changes in primary production within the same feeding area. The smooth cyclic nature of the fluctuations strongly suggests a climatic control on ecosystem conditions rather than disparate migration routes. Therefore, it appears that salmon returning to the northeast coast feed in areas subject to larger interannual variability in oceanic conditions than those experienced by River Frome fish. This in turn implies that variations in ocean climate have a greater potential to influence growth or return rates for northeast coast salmon than for River Frome salmon. Hence, differences in marine feeding location may underpin the variation in relationships between growth indices, return rates, and ocean climate indices observed between salmon populations (Vøllestad et al., 2009). It should be emphasized that the northeast coast fish are from a mixed-stock fishery and as such may take fish from rivers extending from the Yorkshire Humber to the Aberdeenshire Dee (Potter and Swain, 1982). Despite this mixed stock, however, the observed cyclic pattern is coherent throughout the δ13C scale values for these fish.
As well as testing existing hypotheses concerning salmon migrations, stable isotope data can be used to generate new hypotheses concerning feeding location. MacKenzie et al. (2011) identified probable feeding areas for River Frome and northeast coast salmon using long-term records of carbon isotopes incorporated into scales in conjunction with coeval, fine-scale records of sea surface temperature. As carbon isotope composition of primary production is intrinsically linked to temperature and passed relatively conservatively up the trophic chain to salmon (Goericke and Fry, 1994; Vander Zanden and Rasmussen, 1999; Hofmann et al., 2000), these coeval records of isotopes and temperature can be matched to determine the likely areas where the salmon grew the scale tissue (MacKenzie et al., 2011). The results presented by MacKenzie et al. (2011) suggested that salmon returning to the northeast coast of the UK migrate to feeding areas in the Norwegian Sea, with 2SW fish feeding in more northern areas than 1SW salmon. Salmon returning to the River Frome were identified as feeding farther west, around Iceland. In this study, we show that the isotopic distinction between River Frome and northeast coast salmon is greater in 2SW salmon than in 1SW fish, and that 1SW salmon returning to the River Dee have a relatively similar isotope composition to those returning to the River Frome. This implies possible mixing between 1SW fish of UK origin while feeding in the Iceland–Faroes–southern Norwegian Sea area in the period sampled before return. Isotopic separation in the 2SW returning component from these natal stocks, however, suggests that the distinct migration trajectories for these populations are fixed during the post-smolt migration before feeding after the first winter at sea. Fish from the River Frome and River Dee, presumably entering the Atlantic between 50 and 55°N, potentially encounter western branches of the Subpolar Gyre and consequently may follow a more westerly migration route than those from the northeast coast (Hátún et al., 2009; MacKenzie et al., 2011). Salmon exiting rivers on the UK northeast coast presumably enter the Atlantic north of Scotland, around 60°N, and encounter the shelf edge North Atlantic current, directing a northward migration into the Norwegian Sea. All proposed feeding areas lie in regions where relatively warm, saline waters from the Subtropical Gyre are advected north and west and mix with relatively cold, freshwaters of the polar seas. One branch of this mixed water flows through the Faroes–Shetland Channel into the Norwegian Sea, and a second branch is pulled west by the Subpolar Gyre and also splits to flow clockwise around Iceland (Figure 1). The strength and location of the easterly branch of the Subpolar Gyre varies over decadal time-scales, influencing the extent of mixing between subtropical and subpolar waters (Hátún et al., 2005, 2009). It is possible that variations in Subpolar Gyre properties can lead to variations in the absolute location of feeding areas and the extent of mixing of natal-origin populations during oceanic feeding, but we cannot test this hypothesis with our current data. It is important to note that our samples are derived from fish returning to the UK, so perhaps fish feed in other areas but have lower return rates. These results highlight the need for river- or subpopulation-specific management policies that reflect the ocean conditions experienced by each stock in their feeding areas.
Conclusions
Stable isotope compositions of archived scale collagen allow the following ecological conclusions to be drawn: The genetic results from the SALSEA project (J. Gilbey, pers. comm.) are likely to be instrumental in determining the marine locations of post-smolt salmon from specific populations. This information is vital when assessing the threats to salmon during their early lives at sea, when factors such as predation, unfavourable temperatures, and food availability are thought to pose significant problems (Friedland et al., 2000, 2005; Peyronnet et al., 2007). Studies of the location of adult salmon at sea will also be valuable in helping to determine migratory pathways and adding to data obtained from genetic analysis of post-smolts caught at sea.
salmon feed opportunistically at sea, but become more selective towards prey of a higher trophic level as they grow;
there is little isotopic evidence to support extensive mixing of stocks either in the Labrador Sea or within the Subpolar Gyre, and the relatively close geographic association between feeding locations for 1SW and 2SW fish for the populations in which both age groups were sampled argues against constant motion within the Subpolar Gyre;
salmon originating from three regions within the UK feed in distinct areas, arguing against common migration routes and/or feeding locations for these southern European origin salmon.
Acknowledgements
This research was funded by Defra projects SF0250 and SF0246 to CNT. We thank the reviewers and the guest editor Peter Hutchinson for helpful and insightful comments and suggestions. Cefas provided access to samples from their northeast coast archive, and advice on scale-reading and salmon biology, particularly through Barry Bendall and Bill Riley. CEH (now GWCT) provided access to the River Frome archive and additional advice on salmon population ecology. The UK's Environment Agency provided access to the River Dee archive and advice on the population. Finally, we thank the University of Southampton's Catherine Cole (for sample preparation) and Mike Bolshaw (for mass spectrometry), and David Poulter for help with the physical data.