-
PDF
- Split View
-
Views
-
Cite
Cite
Daniela Alemany, Oscar O. Iribarne, Eduardo M. Acha, Effects of a large-scale and offshore marine protected area on the demersal fish assemblage in the Southwest Atlantic, ICES Journal of Marine Science, Volume 70, Issue 1, January 2013, Pages 123–134, https://doi.org/10.1093/icesjms/fss166
Close - Share Icon Share
Abstract
There are few extensive and offshore located marine protected areas (MPAs) in the world oceans and their performance is still being debated. We evaluated the effects of a large-scale offshore MPA located on the Southwest Atlantic Patagonian Shelf (43°S 63°W) on the demersal fish assemblage. Compliance of the Patagonian MPA was assessed by analysing eight years of satellite vessel monitoring system (VMS; 2000–2008) data, which showed compliance and fishing effort concentrated near the protection boundaries. MPA effects were studied by employing a five year database collected by a scientific research vessel in protected and fishing locations, before and after the MPA establishment. We assessed 152 scientific trawling stations using multivariate analysis of fish assemblage structure, fish abundance (discriminating target and non-target species), and mean size and proportion of juveniles of the target species (Argentine hake, Merluccius hubbsi). The identified MPA effects were a trend towards increasing abundance of the demersal fish assemblage, the target and non-target fish species, and hake juvenile size, and a higher proportion of juveniles aged 2+ inside the MPA. These positive trends support the case for offshore, large-scale MPAs.Alemany, D., Iribarne, O. O., and Acha, E. M. 2013. Effects of a large-scale and offshore marine protected area on the demersal fish assemblage in the Southwest Atlantic. – ICES Journal of Marine Science, 70:123–134.
Introduction
The decline of marine resources is of global concern and very restrictive rebuilding measures, such as large-scale fishery closures, are being implemented (Worm et al., 2009). The establishment of marine protected areas (MPAs) seems to be one of the potential solutions to this ecological crisis (e.g. Roberts, 2012). There is global interest in the use of protected areas as a management measure (e.g. Hilborn et al., 2004; Worm et al., 2009), and their performance needs to be assessed. However, MPAs alone are not enough to reduce fishing pressure, as there is a trade-off between recovery in the MPA and decline in the areas to which the fishing effort is displaced (Greenstreet et al., 2009). Many studies have debated the effectiveness and limitations of MPAs for conserving and managing marine resources (e.g. Jones, 2007; Lester et al., 2009).
Most MPAs are relatively small in size, and they are mainly located in tropical near-shore habitats (e.g. Halpern, 2003). Only a few large-scale MPAs have been established in offshore areas (i.e. continental shelves, oceanic environments; see Murawski et al., 2000; Blyth-Skyrme et al., 2006; Game et al., 2009). In particular, MPAs are scarce in the South Atlantic coasts of America, and although there are fishery management areas, there is a lack of comprehensive studies of the existing MPAs.
The Southwest Atlantic includes the Patagonian Shelf Large Marine Ecosystem (PSLME; Sherman, 2005), which is one of the most productive areas in the ocean world and the largest shelf area in the southern hemisphere (e.g. Acha et al., 2004). There are several important fishery resources in the PSLME. Argentine hake (Merluccius hubbsi) sustains one of the most important fisheries over most of the Argentinean Patagonian Shelf. The Argentine hake stocks were overexploited by the mid-1990s (Aubone, 2004) and by the end of 1997 an extensive year-round fishing closure, the Patagonian closed area (PCA), was established in the PSLME to protect Argentine hake juveniles and spawners (Argentinean “Secretaría de Agricultura, Ganadería, Pesca y Alimentación”; SAGyP Res. No. 930/97; Figure 1). In terms of fishery conservation, these year-round closures are considered analogous to MPAs (Fisher and Frank, 2002). The Patagonian MPA is quite large (119000 km2) representing about 12% of the Argentine Continental Shelf (see Figure 1). Since this is one of the few large-scale offshore MPAs, we assessed its effects on the demersal fish assemblage. We are aware that sufficient spatial and temporal replication of a large-scale MPA is challenging and not always feasible but, nevertheless, some useful patterns for understanding MPA effects may emerge. Increases in fishing pressures usually drive population abundance and length distribution to decrease (Rochet et al., 2010). Fishing promotes age truncation, as larger, older fish are removed, even at moderate levels of exploitation (Berkeley et al., 2004). MPAs are not only effective for increasing abundance, but they also induce changes in fish assemblage structure (García-Charton et al., 2008). As the Patagonian Shelf is an intensively fished region (FAO, 2009) positive effects of the MPA on the demersal fish assemblage can be expected.
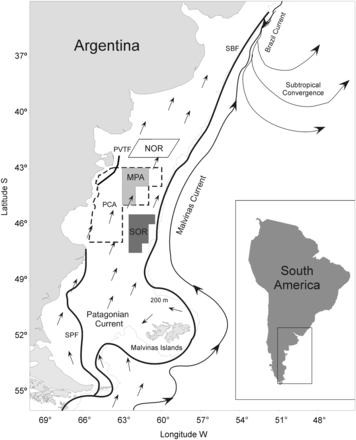
Study area in the Patagonian Shelf Large Marine Ecosystem. The Patagonian closed area (PCA; dotted line) established at the end of 1997 and the three zones analysed are shown: MPA (marine protected area; light grey background), NOR (northern fishing area outside the reserve boundaries; white background), and SOR (southern fishing area outside the reserve limits; dark grey background). Black arrows represent mean water circulation on the Argentine Continental Shelf, and black lines the main frontal systems (SBF =Shelf break front, PVTF =Península Valdés tidal front, SPF =Southern Patagonian front) in a schematic way (adapted from Matano et al., 2010).
We first assessed the compliance of fishing vessels by analysing eight years (2000–2008, 2002 not available) of fishing events (as a proxy for fishing effort) from VMS data. Then, we evaluated the performance of the Patagonian MPA as a fisheries management measure, assessing MPA effects on:
structure and abundance of the demersal fish assemblage, discriminating target and non-target species. We expected changes in fish assemblage structure due to protection and positive effects of the MPA on fish assemblage abundance; as reported for other regions (Babcock et al., 2010), target species should respond more strongly than non-target species to protection;
size of the target species (the Argentine hake), and the proportion of juveniles inside the MPA. We predicted larger fish within the protected area as MPAs protect larger/older individuals from fishing pressure (e.g. García-Charton et al., 2008). Moreover, given that the PCA was implemented to protect juvenile hakes by closing their nursery grounds to fishing, we expected a higher proportion of juveniles inside the PCA.
Methods
Study site
The study site extends from 41°S to 48°S, covering much of the Argentine Continental Shelf, which is included in the Patagonian Shelf Large Marine Ecosystem (Figure 1). The Argentine Continental Shelf varies in width but is regular in topographical features. It is relatively narrow and shallow to the north (38°S) and becomes broader and deeper to the south (51°S; Matano et al., 2010). Argentine Shelf waters are of sub-Antarctic origin and show a north-eastward mean flow (Palma et al., 2008). In the study area three major, highly productive frontal systems occur: the Shelf Break Front, the Península Valdés Tidal Front, and the Southern Patagonian Front (Acha et al., 2004).
The PCA is located in the central portion of the Argentine Continental Shelf, between 43°S and 47°S, covering almost 119 000 km2 in 1998 (approximately 12% of the Argentine Continental Shelf; Figure 1). Although large parts of the area always remain closed to fishing, its shape and extent varied from year to year following the recommendations by the National Institute for Fisheries Research and Development (INIDEP, Argentina) to protect Argentine hake juveniles and spawning adults (Figure 2). Depth ranges between 50 m near the coast and down to 100 m at its offshore boundary. Fishing by trawlers was permanently banned inside the PCA since its legal establishment in December 1997 and, to date, only vessels fishing red shrimp (Pleoticus muelleri) are allowed to operate within certain peripheral regions inside the western edge of the PCA (Figure 2). Therefore, for our purposes, we selected a sub-area (hereafter the Marine Protected Area; MPA) that was never opened to any type of fishing (ca. 28000 km2) to compare with adjacent non-protected zones. The study area was divided into three zones to assess spatial and temporal trends: (i) the MPA, and two unprotected areas: (ii) a fishing area north of the MPA boundaries (NOR), and (iii) a fishing area south of the MPA limits (SOR; Figure 1). The three areas for comparison were equivalent in size and depth range, and located in the same biogeographical region (Angelescu and Prenski, 1987). To avoid border effects, zones were separated at least by 0.5° latitude and/or 0.5° longitude.
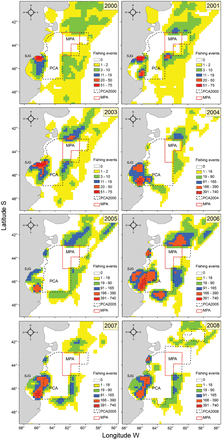
Spatial pattern of the fishing effort per year (2000–2008) on the Patagonian Shelf and MPA surroundings. Data were obtained from vessels using VMS (Vessel Monitoring System); acqusition frequency 1–2 a day up to 2003 and every hour from 2004. Kernel density plots express fishing events per 10' latitude ×10' longitude squares (uncorrected for acqusition frequency), note that are two color scales: 2000–2003 and 2004–2008 (see the text). PCA =Patagonian Closed Area, MPA =Marine Protected Area, SJG =San Jorge Gulf.
Data source
Compliance in the Patagonian MPA
Since 1997 the Argentine administration adopted a satellite vessel monitoring system (VMS) to achieve a comprehensive control over the fleet and to preserve areas closed to fishing on the Continental Shelf. Between 1997 and 2003, 66% of the fishing fleet (ca. 400 fishing vessels) was equipped with GPS and monitored at a low daily rate (i.e. 1–15 times a day) up to 2003. Since 2004, all fishing vessels are required to have GPS and the VMS records vessel position and speed every hour; making it impossible for fishing vessels to get into the MPA without being noticed. The VMS is the only enforcement mechanism to control the fleets. The complete VMS database from 2000 to 2008 was used to estimate a proxy for fishing effort inside each analysed zone.
All records from vessels performing bottom trawling for demersal fish were treated together (jigging vessels specializing in shortfin squid were discarded). Data were filtered to include only records with vessel speeds between 2 and 5 knots (3.7 to 9.3 km h−1), which are typical trawling speeds. To test the hypothesis that fishing effort inside the MPA was lower than outside, we analysed VMS records from 2000 (n = 71041), 2001 (n = 63464), 2003 (n = 78988), 2004 (n = 871224), 2005 (n = 1095985), 2006 (n = 1899706), 2007 (n = 1150012) and 2008 (n = 1159728); 2002 records were not available due to financial constraints during Argentina's economic crisis. All fishing operations recorded in the Argentine Shelf were considered as 100% of fishing effort for each year, and the percentage contribution of each zone (NOR, MPA, SOR and remaining Shelf) was then calculated. We also investigated the spatial pattern of annual fishing effort on the Patagonian Shelf and MPA surroundings. ArcGIS (version 9.3; Environmental Systems Research Institute, Redlands, CA, USA) was used for data management and analysis; VMS positions were converted into a continuous raster using the kernel density estimation function (Spatial Analyst, ArcMap 9.3). Output cell size was 18.5 km (ca. 10'). Six density classes were defined based on Jenks' natural breaks classification method (Jenks, 1967), which determines the best arrangement of values into different classes. This method seeks to reduce the variance within classes and maximizes the variance between classes. The resulting density plots express fishing events per 10' latitude ×10' longitude square, and are uncorrected for differences in acquisition (ping) rate and number of equipped vessels.
Demersal fish assemblage
For demersal fish comparisons we utilized a database of fishery research cruises from the National Institute of Fisheries Research and Development (INIDEP, Argentina). We analysed five cruises focused on demersal fish assessment that covered most of the Patagonian Shelf before (1996) and after (2000, 2001, 2003 and 2005) the creation of the Patagonian MPA (Supplementary Table S1). Given the lack of research cruises covering the study area, no information was available after 2005. Data were collected during the cold season (July to October) by the Argentinean trawler RV “Dr E. Holmberg”. Sampling stations were located following a random sampling scheme and distributed inside and outside the MPA, obtaining large spatial coverage using a bottom trawl (5 m vertical and 30 m horizontal opening, codend mesh size 2.4 cm). Trawls were performed at 4 knots (7.4 km h−1) for 30 minutes. To enable comparison between trawling stations, only the 152 hauls whose swept area ranged between 0.07 and 0.09 km2 were selected for the analyses; as this range is so narrow we analysed absolute catch weights (t) instead of CPUE. Total catch was sampled at each station and all fish species (identified on board to the lowest taxonomic level) were included in the demersal assemblage analyses. For Argentine hake (MPA target species), sex and total length (LT, accurate to 1 cm) of each individual were recorded for all hauls. Demersal hauls were ascribed to the three zones (NOR, MPA or SOR; Supplementary Table S1).
Data analysis
MPA effects on demersal fish assemblage structure and abundance
Since ecological data are mainly multivariate, we used the permutational multivariate analysis of variance (PERMANOVA) to evaluate spatial and inter-annual variations in the demersal fish assemblage structure and in fish abundance. This routine analyses multivariate or univariate data coming from complex and unbalanced designs (Anderson et al., 2008). The two main factors were area and year.
To perform PERMANOVAs, PRIMER 6 software was used (Clarke and Gorley, 2006). To test the multivariate null hypothesis of no differences in assemblage structure among groups (Area and Year), Bray–Curtis abundance similarity matrices, on fourth root transformed data, were constructed to reduce the influence of the most abundant species (Clarke and Warwick, 2001). Two similarity matrices were constructed, at the whole fish assemblage level and for the non-target fish species, i.e. excluding Argentine hake. The Argentine hake is by far the most abundant species, representing more than 70% of catches, so it could mask possible patterns of the non-target and less abundant fish species.
Similarity matrices were used to explore fish assemblage structure with non-metric multidimensional scaling (MDS) ordination, where a stress value below 0.20 gives an adequate representation of the 2D MDS (Clarke and Warwick, 2001).
Fish species most responsible for the multivariate pattern were identified using a similarity percentages (SIMPER) analysis on fish abundance data. This method compares average abundances and examines the contribution of each species to similarities within a given group, or dissimilarities between groups (Clarke and Warwick, 2001).
To evaluate the null hypothesis of no difference in fish abundance among groups (Area and Year), abundances of all fish, Argentine hake, and of non-target fish species were calculated for each haul (expressed in tonnes, t) and tested with PERMANOVAs.
All permutation tests relied on 4999 permutations of residuals under a reduced model to obtain p-values. This permutation method is the more appropriate because it provides the best statistical power and the most accurate Type I error (Anderson et al., 2008). When significant at the 0.05 level, the Area ×Year interactions were compared through a posteriori pair-wise comparisons using 4999 random permutations to obtain p-values.
MPA effects on Argentine hake mean size and age-2 juveniles
To assess temporal changes in size of the target species in each area, mean total lengths (LT, in cm) of Argentine hake were compared using ANOVA, evaluating the null hypothesis of no difference between years in each area. Pair-wise post hoc tests for unequal sample size were used when statistical differences in one-way ANOVAs were detected (Zar, 1999). Argentine hakes were categorized as juveniles (LT < 35 cm) and adults (LT > 35 cm) following Simonazzi (2003). The total length of 68448 Argentine hakes were analysed; the number of hakes is summarized in Supplementary Table S2.
To evaluate temporal changes, age-2 hake were selected as they are well represented in catches. Age estimation of hake was based on otolith ring counting. For the study period, the total length of age-2 hake ranged between 28 and 44 cm (M. Renzi, Ageing Lab, INIDEP, pers. comm.). The abundance of age-2 hake inside the MPA was expressed in proportion to age-2 individuals in the entire survey area in each cruise. To test for differences among years, pair-wise comparisons with 1996 (designated as “control”) were carried out (Zar, 1999).
Results
Compliance in the Patagonian MPA
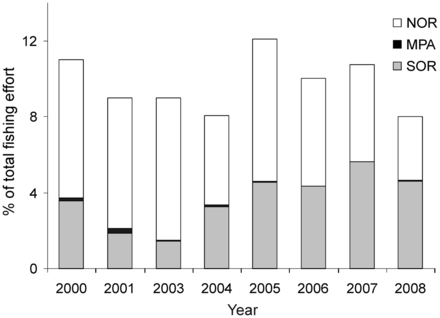
From 2000 to 2008 (except 2002), fishing effort (E) inside the MPA was negligible when compared with adjacent non-protected areas (NOR, SOR and remaining Shelf; Figure 3). Overall, the percentage of E within the MPA was lower than 0.09%, with the highest value registered in 2001 (0.26%) and the lowest in 2007 (0.01%). Inside NOR, the mean fishing effort was 6% while in SOR it was 3.7%. The rest of the Argentine Shelf contributed with 90% of the total effort. Given that the size of the Patagonian MPA and the fishing grounds were very similar, the low percentage of tracks within the protected area reflected true compliance.
Annual percentage of the total fishing effort in the Argentine Shelf in the three zones analysed (NOR, MPA and SOR). Abbreviations as in Figure 1.
Spatial patterns of fishing effort in the Patagonian Shelf and in MPA surroundings varied interannually, but in most the years a border effect is apparent (Figure 2). Note that due to the higher acquisition rate from 2004 more fishing events were recorded. Fishing effort concentrated particularly around the PCA during 2001, 2003 and 2006. Particularly, in 2001, 2003 and 2005, fishing effort was higher along the northern and eastern edges of the PCA. More fishing effort occurred in 2006 along the Patagonian Shelf than in other years, and the VMS data concentrated at the northern, eastern and southern boundaries of the PCA. In 2007 and 2008, fishing effort was higher on the eastern edge of the PCA. For all years, it is clear that there was more effort along the PCA boundaries than in distant areas. Highest values in the San Jorge Gulf corresponded to the red shrimp fishery.
Demersal fish assemblage
A total of 73 fish taxa were identified along the Patagonian Shelf during the cruises from 1996 to 2005. Supplementary Table S3 shows the fish occurrence in each area.
MPA effects on demersal fish assemblage structure and abundance
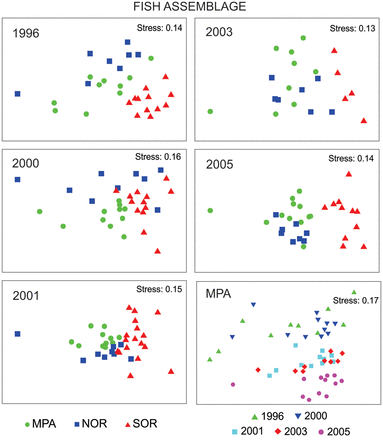
In the case of the structure of the whole demersal fish assemblage and that of the non-target fish species, PERMANOVAs showed a significant Area ×Year interaction (Table 1). The MDS ordination was split by year given the complexity of showing the data (152 hauls) in a single MDS (Figure 4). The ordination of the non-target fish assemblage structure is not shown as it is almost equal to that of the whole fish assemblage.
Non-metric multidimensional scaling (MDS) ordination on fish assemblage structure comparing the three areas (MPA, NOR and SOR) per year. At the lower right corner, the MDS on fish assemblage structure inside the MPA across five years is shown.
Summary of the PERMANOVA main tests to assess differences in the structure of the whole fish assemblage and of the non-target fish species assemblage (excluding the MPA target species, the Argentine hake), between areas (MPA, NOR and SOR) and years (1996, 2000, 2001, 2003 and 2005).
| Source of variation . | df . | MS . | Pseudo-F . | p-value . |
|---|---|---|---|---|
| Fish assemblage | ||||
| Area | 2 | 14 303.0 | 17.6 | 0.0002 |
| Year | 4 | 15 485.0 | 19.0 | 0.0002 |
| Area x Year | 8 | 2 158.2 | 2.7 | 0.0002 |
| Residuals | 137 | 814.2 | ||
| Non-target fish species | ||||
| Area | 2 | 19 239.0 | 17.1 | 0.0002 |
| Year | 4 | 22 928.0 | 20.4 | 0.0002 |
| Area x Year | 8 | 3 338.9 | 3.0 | 0.0002 |
| Residuals | 137 | 1 122.2 |
| Source of variation . | df . | MS . | Pseudo-F . | p-value . |
|---|---|---|---|---|
| Fish assemblage | ||||
| Area | 2 | 14 303.0 | 17.6 | 0.0002 |
| Year | 4 | 15 485.0 | 19.0 | 0.0002 |
| Area x Year | 8 | 2 158.2 | 2.7 | 0.0002 |
| Residuals | 137 | 814.2 | ||
| Non-target fish species | ||||
| Area | 2 | 19 239.0 | 17.1 | 0.0002 |
| Year | 4 | 22 928.0 | 20.4 | 0.0002 |
| Area x Year | 8 | 3 338.9 | 3.0 | 0.0002 |
| Residuals | 137 | 1 122.2 |
Summary of the PERMANOVA main tests to assess differences in the structure of the whole fish assemblage and of the non-target fish species assemblage (excluding the MPA target species, the Argentine hake), between areas (MPA, NOR and SOR) and years (1996, 2000, 2001, 2003 and 2005).
| Source of variation . | df . | MS . | Pseudo-F . | p-value . |
|---|---|---|---|---|
| Fish assemblage | ||||
| Area | 2 | 14 303.0 | 17.6 | 0.0002 |
| Year | 4 | 15 485.0 | 19.0 | 0.0002 |
| Area x Year | 8 | 2 158.2 | 2.7 | 0.0002 |
| Residuals | 137 | 814.2 | ||
| Non-target fish species | ||||
| Area | 2 | 19 239.0 | 17.1 | 0.0002 |
| Year | 4 | 22 928.0 | 20.4 | 0.0002 |
| Area x Year | 8 | 3 338.9 | 3.0 | 0.0002 |
| Residuals | 137 | 1 122.2 |
| Source of variation . | df . | MS . | Pseudo-F . | p-value . |
|---|---|---|---|---|
| Fish assemblage | ||||
| Area | 2 | 14 303.0 | 17.6 | 0.0002 |
| Year | 4 | 15 485.0 | 19.0 | 0.0002 |
| Area x Year | 8 | 2 158.2 | 2.7 | 0.0002 |
| Residuals | 137 | 814.2 | ||
| Non-target fish species | ||||
| Area | 2 | 19 239.0 | 17.1 | 0.0002 |
| Year | 4 | 22 928.0 | 20.4 | 0.0002 |
| Area x Year | 8 | 3 338.9 | 3.0 | 0.0002 |
| Residuals | 137 | 1 122.2 |
A posteriori tests showed that in 1996, fish assemblage structure and that of the non-target fish species did not differ between MPA and NOR, but both differed from SOR. After 1996 fish assemblage structures were different for MPA, NOR and SOR.
The results of SIMPER analysis on the whole fish assemblage showed that the Argentine hake was the fish species that contributed most to dissimilarities among areas (Table 2), its average abundance in the MPA being 3-fold higher than in NOR, and 2-fold higher than in SOR. Other fish species contributed to dissimilarities among areas, but their percentage contributions were less than 10%. In the case of the non-target fish assemblage, eight species contributed to dissimilarities between areas, with Squalus acanthias, Patagonotothen sp. and Macruronus magellanicus making the highest contributions.
SIMPER results showing the fish species that contributed most to the dissimilarity across areas (MPA, NOR and SOR) at the fish assemblage level and for the non-target fish species assemblage.
| . | % Dissimilarity . | ||
|---|---|---|---|
| . | MPA-NOR . | MPA-SOR . | NOR-SOR . |
| Fish assemblage | |||
| Merluccius hubbsi | 60 | 63 | 36 |
| Squalus acanthias | 5 | – | – |
| Macruronus magellanicus | – | 8 | 12 |
| Genypterus blacodes | – | – | 6 |
| Non-target fish species | |||
| Squalus acanthias | 14 | 17 | 9 |
| Patagonotothen sp. | 10 | 10 | 8 |
| Patagonotothen ramsayi | 8 | – | – |
| Congiopodus peruvianus | 6 | 8 | – |
| Genypterus blacodes | 6 | 9 | 7 |
| Acanthistius brasilianus | 5 | 6 | – |
| Macruronus magellanicus | – | 12 | 12 |
| Notothenia sp. | – | 9 | 6 |
| . | % Dissimilarity . | ||
|---|---|---|---|
| . | MPA-NOR . | MPA-SOR . | NOR-SOR . |
| Fish assemblage | |||
| Merluccius hubbsi | 60 | 63 | 36 |
| Squalus acanthias | 5 | – | – |
| Macruronus magellanicus | – | 8 | 12 |
| Genypterus blacodes | – | – | 6 |
| Non-target fish species | |||
| Squalus acanthias | 14 | 17 | 9 |
| Patagonotothen sp. | 10 | 10 | 8 |
| Patagonotothen ramsayi | 8 | – | – |
| Congiopodus peruvianus | 6 | 8 | – |
| Genypterus blacodes | 6 | 9 | 7 |
| Acanthistius brasilianus | 5 | 6 | – |
| Macruronus magellanicus | – | 12 | 12 |
| Notothenia sp. | – | 9 | 6 |
‘–’ = dissimilarity <1%.
SIMPER results showing the fish species that contributed most to the dissimilarity across areas (MPA, NOR and SOR) at the fish assemblage level and for the non-target fish species assemblage.
| . | % Dissimilarity . | ||
|---|---|---|---|
| . | MPA-NOR . | MPA-SOR . | NOR-SOR . |
| Fish assemblage | |||
| Merluccius hubbsi | 60 | 63 | 36 |
| Squalus acanthias | 5 | – | – |
| Macruronus magellanicus | – | 8 | 12 |
| Genypterus blacodes | – | – | 6 |
| Non-target fish species | |||
| Squalus acanthias | 14 | 17 | 9 |
| Patagonotothen sp. | 10 | 10 | 8 |
| Patagonotothen ramsayi | 8 | – | – |
| Congiopodus peruvianus | 6 | 8 | – |
| Genypterus blacodes | 6 | 9 | 7 |
| Acanthistius brasilianus | 5 | 6 | – |
| Macruronus magellanicus | – | 12 | 12 |
| Notothenia sp. | – | 9 | 6 |
| . | % Dissimilarity . | ||
|---|---|---|---|
| . | MPA-NOR . | MPA-SOR . | NOR-SOR . |
| Fish assemblage | |||
| Merluccius hubbsi | 60 | 63 | 36 |
| Squalus acanthias | 5 | – | – |
| Macruronus magellanicus | – | 8 | 12 |
| Genypterus blacodes | – | – | 6 |
| Non-target fish species | |||
| Squalus acanthias | 14 | 17 | 9 |
| Patagonotothen sp. | 10 | 10 | 8 |
| Patagonotothen ramsayi | 8 | – | – |
| Congiopodus peruvianus | 6 | 8 | – |
| Genypterus blacodes | 6 | 9 | 7 |
| Acanthistius brasilianus | 5 | 6 | – |
| Macruronus magellanicus | – | 12 | 12 |
| Notothenia sp. | – | 9 | 6 |
‘–’ = dissimilarity <1%.
Inside the MPA, the fish assemblage structure and that of the non-target fish species changed with years (P perm = 0.0002, in both cases), except between 2001 and 2003 (Figure 4; MDS of the non-target fish species assemblage inside the MPA is not shown as is almost equal to that of the whole assemblage). Again, at the fish assemblage level, the SIMPER analysis revealed that inside the MPA the Argentine hake contributed more than 70% to dissimilarities among years (Table 3), with the most remarkable differences occurring between 1996 and 2003 (average abundance 1.4-fold higher in 2003) and between 1996 and 2005 (average abundance 1.6-fold higher in 2005). Regarding the non-target assemblage inside the MPA, 10 fish species were most responsible for the differences between years. These species, except Squalus acanthias, were more abundant after protection, with Genypterus blacodes, Macruronus magellanicus and Patagonotothen ramsayi showing the most remarkable increases.
SIMPER results showing the fish species that contributed most to the dissimilarity inside the MPA across years, comparing 1996 (year before protection) with years after protection, at the fish assemblage level and for the non-target fish species assemblage.
| . | % Dissimilarity . | |||
|---|---|---|---|---|
| 1996 vs. . | 2000 . | 2001 . | 2003 . | 2005 . |
| Fish assemblage | ||||
| Merluccius hubbsi | 71 | 77 | 79 | 73 |
| Squalus acanthias | 6 | 6 | – | – |
| Congiopodus peruvianus | 5 | – | – | – |
| Acanthistius brasilianus | – | 5 | – | – |
| Patagonotothen sp. | – | – | – | 12 |
| Non-target fish species | ||||
| Congiopodus peruvianus | 22 | 8 | 8 | 8 |
| Squalus acanthias | 19 | 28 | 23 | 12 |
| Raja flavirostris | 12 | – | – | – |
| Genypterus blacodes | 8 | 11 | 6 | – |
| Acanthistius brasilianus | 8 | 17 | 10 | – |
| Stromateus brasiliensis | 5 | – | – | – |
| Xystreuris rasile | 5 | – | 8 | – |
| Dipturus chilensis | – | 8 | – | 12 |
| Macruronus magellanicus | – | – | 10 | – |
| Patagonotothen ramsayi | – | – | 7 | 40 |
| . | % Dissimilarity . | |||
|---|---|---|---|---|
| 1996 vs. . | 2000 . | 2001 . | 2003 . | 2005 . |
| Fish assemblage | ||||
| Merluccius hubbsi | 71 | 77 | 79 | 73 |
| Squalus acanthias | 6 | 6 | – | – |
| Congiopodus peruvianus | 5 | – | – | – |
| Acanthistius brasilianus | – | 5 | – | – |
| Patagonotothen sp. | – | – | – | 12 |
| Non-target fish species | ||||
| Congiopodus peruvianus | 22 | 8 | 8 | 8 |
| Squalus acanthias | 19 | 28 | 23 | 12 |
| Raja flavirostris | 12 | – | – | – |
| Genypterus blacodes | 8 | 11 | 6 | – |
| Acanthistius brasilianus | 8 | 17 | 10 | – |
| Stromateus brasiliensis | 5 | – | – | – |
| Xystreuris rasile | 5 | – | 8 | – |
| Dipturus chilensis | – | 8 | – | 12 |
| Macruronus magellanicus | – | – | 10 | – |
| Patagonotothen ramsayi | – | – | 7 | 40 |
‘–’ = dissimilarity <1%.
SIMPER results showing the fish species that contributed most to the dissimilarity inside the MPA across years, comparing 1996 (year before protection) with years after protection, at the fish assemblage level and for the non-target fish species assemblage.
| . | % Dissimilarity . | |||
|---|---|---|---|---|
| 1996 vs. . | 2000 . | 2001 . | 2003 . | 2005 . |
| Fish assemblage | ||||
| Merluccius hubbsi | 71 | 77 | 79 | 73 |
| Squalus acanthias | 6 | 6 | – | – |
| Congiopodus peruvianus | 5 | – | – | – |
| Acanthistius brasilianus | – | 5 | – | – |
| Patagonotothen sp. | – | – | – | 12 |
| Non-target fish species | ||||
| Congiopodus peruvianus | 22 | 8 | 8 | 8 |
| Squalus acanthias | 19 | 28 | 23 | 12 |
| Raja flavirostris | 12 | – | – | – |
| Genypterus blacodes | 8 | 11 | 6 | – |
| Acanthistius brasilianus | 8 | 17 | 10 | – |
| Stromateus brasiliensis | 5 | – | – | – |
| Xystreuris rasile | 5 | – | 8 | – |
| Dipturus chilensis | – | 8 | – | 12 |
| Macruronus magellanicus | – | – | 10 | – |
| Patagonotothen ramsayi | – | – | 7 | 40 |
| . | % Dissimilarity . | |||
|---|---|---|---|---|
| 1996 vs. . | 2000 . | 2001 . | 2003 . | 2005 . |
| Fish assemblage | ||||
| Merluccius hubbsi | 71 | 77 | 79 | 73 |
| Squalus acanthias | 6 | 6 | – | – |
| Congiopodus peruvianus | 5 | – | – | – |
| Acanthistius brasilianus | – | 5 | – | – |
| Patagonotothen sp. | – | – | – | 12 |
| Non-target fish species | ||||
| Congiopodus peruvianus | 22 | 8 | 8 | 8 |
| Squalus acanthias | 19 | 28 | 23 | 12 |
| Raja flavirostris | 12 | – | – | – |
| Genypterus blacodes | 8 | 11 | 6 | – |
| Acanthistius brasilianus | 8 | 17 | 10 | – |
| Stromateus brasiliensis | 5 | – | – | – |
| Xystreuris rasile | 5 | – | 8 | – |
| Dipturus chilensis | – | 8 | – | 12 |
| Macruronus magellanicus | – | – | 10 | – |
| Patagonotothen ramsayi | – | – | 7 | 40 |
‘–’ = dissimilarity <1%.
PERMANOVAs performed on the abundance of the fish assemblage, Argentine hake, and the non-target fish species showed a significant Area ×Year interaction (Table 4).
Summary of the PERMANOVA main tests to assess differences in the abundance of the fish assemblage, Argentine hake and non-target fish species between areas (MPA, NOR and SOR) and years (1996, 2000, 2001, 2003 and 2005).
| Source of variation . | df . | MS . | Pseudo-F . | p-value . |
|---|---|---|---|---|
| Fish assemblage | ||||
| Area | 2 | 5 082.9 | 5.7 | 0.0002 |
| Year | 4 | 5 088.1 | 5.7 | 0.0002 |
| Area × Year | 8 | 2 939.4 | 3.3 | 0.0002 |
| Residuals | 137 | 885.7 | ||
| Argentine hake | ||||
| Area | 2 | 9 600.8 | 8.2 | 0.0002 |
| Year | 4 | 6 238.1 | 5.3 | 0.0002 |
| Area × Year | 8 | 3 400.6 | 2.9 | 0.0002 |
| Residuals | 137 | 1 168.3 | ||
| Non-target fish species | ||||
| Area | 2 | 10 123.0 | 9.7 | 0.0002 |
| Year | 4 | 7 605.5 | 7.3 | 0.0002 |
| Area × Year | 8 | 3 225.9 | 3.1 | 0.0002 |
| Residuals | 137 | 1 037.4 |
| Source of variation . | df . | MS . | Pseudo-F . | p-value . |
|---|---|---|---|---|
| Fish assemblage | ||||
| Area | 2 | 5 082.9 | 5.7 | 0.0002 |
| Year | 4 | 5 088.1 | 5.7 | 0.0002 |
| Area × Year | 8 | 2 939.4 | 3.3 | 0.0002 |
| Residuals | 137 | 885.7 | ||
| Argentine hake | ||||
| Area | 2 | 9 600.8 | 8.2 | 0.0002 |
| Year | 4 | 6 238.1 | 5.3 | 0.0002 |
| Area × Year | 8 | 3 400.6 | 2.9 | 0.0002 |
| Residuals | 137 | 1 168.3 | ||
| Non-target fish species | ||||
| Area | 2 | 10 123.0 | 9.7 | 0.0002 |
| Year | 4 | 7 605.5 | 7.3 | 0.0002 |
| Area × Year | 8 | 3 225.9 | 3.1 | 0.0002 |
| Residuals | 137 | 1 037.4 |
Summary of the PERMANOVA main tests to assess differences in the abundance of the fish assemblage, Argentine hake and non-target fish species between areas (MPA, NOR and SOR) and years (1996, 2000, 2001, 2003 and 2005).
| Source of variation . | df . | MS . | Pseudo-F . | p-value . |
|---|---|---|---|---|
| Fish assemblage | ||||
| Area | 2 | 5 082.9 | 5.7 | 0.0002 |
| Year | 4 | 5 088.1 | 5.7 | 0.0002 |
| Area × Year | 8 | 2 939.4 | 3.3 | 0.0002 |
| Residuals | 137 | 885.7 | ||
| Argentine hake | ||||
| Area | 2 | 9 600.8 | 8.2 | 0.0002 |
| Year | 4 | 6 238.1 | 5.3 | 0.0002 |
| Area × Year | 8 | 3 400.6 | 2.9 | 0.0002 |
| Residuals | 137 | 1 168.3 | ||
| Non-target fish species | ||||
| Area | 2 | 10 123.0 | 9.7 | 0.0002 |
| Year | 4 | 7 605.5 | 7.3 | 0.0002 |
| Area × Year | 8 | 3 225.9 | 3.1 | 0.0002 |
| Residuals | 137 | 1 037.4 |
| Source of variation . | df . | MS . | Pseudo-F . | p-value . |
|---|---|---|---|---|
| Fish assemblage | ||||
| Area | 2 | 5 082.9 | 5.7 | 0.0002 |
| Year | 4 | 5 088.1 | 5.7 | 0.0002 |
| Area × Year | 8 | 2 939.4 | 3.3 | 0.0002 |
| Residuals | 137 | 885.7 | ||
| Argentine hake | ||||
| Area | 2 | 9 600.8 | 8.2 | 0.0002 |
| Year | 4 | 6 238.1 | 5.3 | 0.0002 |
| Area × Year | 8 | 3 400.6 | 2.9 | 0.0002 |
| Residuals | 137 | 1 168.3 | ||
| Non-target fish species | ||||
| Area | 2 | 10 123.0 | 9.7 | 0.0002 |
| Year | 4 | 7 605.5 | 7.3 | 0.0002 |
| Area × Year | 8 | 3 225.9 | 3.1 | 0.0002 |
| Residuals | 137 | 1 037.4 |
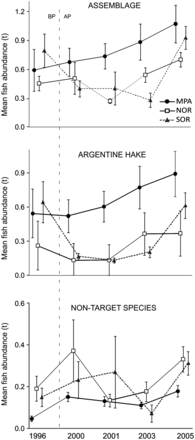
At the fish assemblage level, a posteriori comparisons showed that in 1996, 2000 and 2005, fish abundance within the MPA did not differ from that of NOR and SOR, although there were differences between areas in 2001 and 2003, with fish abundance being higher in the MPA (Figure 5). Inside the MPA, the mean abundance of fish increased across years, being 1.8-fold higher in 2005 than in 1996. In NOR and SOR, a different trend was observed; fish abundance decreased after 1996 but then increased in 2005 (in NOR 1.5-fold higher in 2005 than in 1996; in SOR 1.2-fold higher in 2005 than in 1996).
Abundance of the demersal fish assemblage, the Argentine hake (the MPA target species) and the non-target fish species (mean ± s.e.) at each area (MPA, NOR and SOR) through years. Dotted line indicates before protection period (1996). BP =before protection, AP =after protection. Abbreviations as in Figure 1.
Given that Argentine hake represented, on average, 70% of the catch in each haul, the pattern observed at the assemblage level is similar to that of Argentine hake, the MPA target species (Figure 5). In 1996, Argentine hake abundance did not differ between areas, however, after protection (since 2000 up to 2005) the protected area always showed higher abundance of hake in comparison with the fishing zones (NOR and SOR). Inside the MPA, Argentine hake abundance increased with years, being 1.6-fold higher in 2005 than in 1996. In NOR and SOR, the abundance of Argentine hake decreased after 1996 up to 2001 but then increased up to 2005. In NOR, the abundance was 1.4-fold higher in 2005 than in 1996 but in SOR Argentine hake abundance was slightly lower in 2005 than in 1996.
In reference to non-target fish species, there were no clear trends as their abundance changed between areas and years, although inside the MPA these fluctuations were lower in comparison with NOR and SOR (Figure 5). In 1996, mean abundance of non-target fish was lower in the MPA than in NOR and SOR, but in 2005 there were no differences between areas. In the three studied areas, a higher abundance of non-target species was registered in 2005 than in 1996, with the strongest trend in the MPA (3.8 fold higher; in NOR: 1.7 fold higher; in SOR: 2.1 fold higher).
MPA effects on Argentine hake mean size and age-2 juveniles
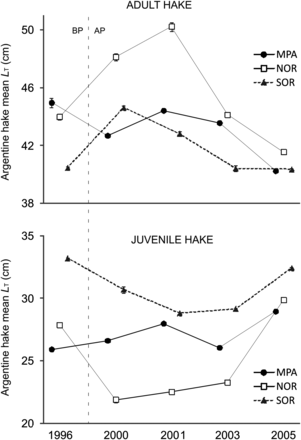
Mean total length (LT) of Argentine hake adults differed among years in the protected area (F4 = 235, p < 0.001) and in the two fished areas (NOR: F4 = 297, p < 0.001; SOR: F4 = 262, p < 0.001). Figure 6 show an overall decreasing trend in the LT of hake in the three zones (MPA, NOR and SOR). Post hoc tests confirmed that at the MPA, mean LT was higher in 1996 and 2001 than in 2000, 2003 and 2005. In 1996, Argentine hake adults were, on average, 4.7 cm larger than in 2005. Within the NOR area, mean LT increased from 1996 to 2001 but then sharply decreased in 2003 and 2005. In 2005, adults were 2.5 cm smaller than in 1996. The trend observed in SOR was similar to that in NOR; the mean LT of adult hakes increased from 1996 to 2000 and decreased up to 2003, however, no differences were detected between 1996 and 2005.
Total length (LT, mean ± s.e.) of adults and juveniles of Argentine hake for each area (MPA, NOR and SOR) through years. Dotted line indicates before protection period (1996). BP =before protection, AP =after protection. Abbreviations as in Figure 1.
Juveniles of Argentine hake differed in their mean LT among years in the three areas (MPA: F4 = 163, p < 0.001; NOR: F4 = 486.5, p < 0.001; SOR: F4 = 588, p < 0.001) showing a different pattern from adults (Figure 6). Within the MPA, the mean LT of juveniles increased from 1996 to 2001, decreased in 2003 and increased again in 2005. In 2005, inside the protected area, juveniles of Argentine hake were on average 3 cm larger (10%) than in 1996. As for adults, individuals in NOR and SOR showed a similar pattern. In NOR, juveniles decreased in size from 1996 to 2000 but then increased up to 2005, being on average 2 cm larger (7%) in comparison with 1996. In SOR, the mean LT of juveniles decreased until 2001 and then increased until 2005, but in this area the size of juveniles in 2005 was slightly smaller (2.5%) than in 1996.
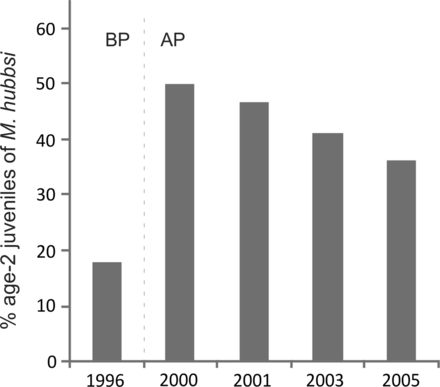
The proportion of age-2 juveniles of Argentine hake was, on average, 2.4-fold higher in 2000, 2001, 2003 and 2005 than in 1996 (p < 0.01; Figure 7). A decreasing trend in the proportion of age-2 juveniles of Argentine hake after 2000 appears in Figure 7. However, in 2005 the proportion of juveniles was still 2-fold higher than in 1996 (36% vs. 18%, respectively).
Percentage of age-2 juveniles of Merluccius hubbsi (Argentine hake) inside the MPA across years. Dotted line indicates before protection period (1996). BP =before protection, AP =after protection.
Discussion
We investigated the effects on demersal fishes of an offshore large-scale MPA, placed in the PSLME. Our results are in general agreement with the scarce contributions on the topic. MPA effects did not vary consistently through time but general trends show that the highest abundance of the fish assemblage, and that of the Argentine hake, occurs inside the MPA. An increasing trend in non-target fish species abundance and in hake juvenile size was also registered inside the protected area. Moreover, inside the MPA, the proportion of age-2 Argentine hake at least doubled compared with the proportion registered before protection.
Compliance in the Patagonian MPA
Analysis of hake landings showed that the region now occupied by the MPA was highly exploited before its implementation at the end of 1997; south of 41°S catches continuously increased from 300 000 t in 1987 to 600000 t in 1997 (Irusta et al., 2001). Our analysis of satellite monitoring data showed that fishing effort within the protected area was negligible, but the MPA was not completely intangible. Nevertheless, the results indicate that fishes may have benefited from the protection. This agrees with results from the Western Indian Ocean (McClanahan et al., 2009) that showed positive fish responses to protection, even though closure regulations were not fully respected in some MPAs.
It is generally assumed that protection should enhance catch rates in non-protected areas through the net export of fish, and “fishing the line” is nowadays a common tactic in which fishing effort is concentrated on the edge of a protected area (Kellner et al., 2007). In that sense, the spatial analysis of VMS data showed that fishing pressure is higher near the boundaries of the PCA, which suggest better catches in their surroundings than in distant areas. Similarly, high concentrations of trawling effort were found around the boundaries of the large offshore Georges Bank MPAs (Murawski et al., 2005); they were attributed to the apparent “spill-over” or seasonal movement of certain species out of the closed area. Although the high fishing effort around the PCA could be attributed to protection effects, it is important to note that before the PCA was implemented the Patagonian area was already one of the best fishing grounds of the Argentine Continental Shelf. Thus, it would be expected that after implementing protection measures, fishing effort displaced to the PCA boundaries, as occurred in closed areas off the northeast USA (Murawski et al., 2005). Hence, a similar pattern of fishing effort concentration is expected due to “spill over” effects and/or because the area was an important fishing zone before protection. Finally, our results indicate that the boundaries of the PCA are preferred by fishers, since fishing activities concentrate around the protected area (presumably because they get better catches). This suggests that the MPA continues to be the area of greatest concentration of hake.
MPA effects on demersal fish assemblage structure and abundance
At the community level, changes in assemblage structure are some of the expected ecological effects of MPAs (García-Charton et al., 2008). In the study area, demersal fish assemblage structure changed with time, suggesting some effect of protection, the most remarkable changes being attributed to changes in Argentine hake abundance, the MPA target species. Regarding the non-target species responsible for the pattern observed, the information analysed did not allow us to discern the processes that could explain changes in their abundances. However, we detected changes in non-target fish assemblage structure after protection. In that sense, our findings agree with studies that propose that MPAs induce shifts in fish assemblage structure, as they protect large long-lived predators that are vulnerable to fishing (García-Charton et al., 2008).
As expected, the abundance pattern of the fish assemblage showed higher average abundances in the MPA than in fished areas. This is mostly due to the fact that the Argentine hake is by far the most abundant species of this assemblage, significantly affecting its abundance pattern. Our results are in agreement with studies on Mediterranean MPAs, showing higher fish abundance within protected areas, not only for target species but for fish assemblages as a whole (e.g. García-Charton et al., 2008).
The highest abundance of the Argentine hake was observed inside the protected area, suggesting positive effects of protection on this exploited target species. This is in agreement with results in multiple marine reserves in both tropical and temperate habitats (Babcock et al., 2010) and in the Channel Islands reserves network in the Pacific Ocean (Hamilton et al., 2010). Both studies reported that the most important target species showed a more evident response to protection than the rest. Protection affects individual species differently (Lester et al., 2009), and non-target species show no overall response to it (Micheli et al., 2004). However, our results showed that, although abundance of non-target species is at least one order of magnitude lower than that of the Argentine hake, their abundance increased with time inside the MPA.
Thus, at the fish assemblage level, as for the target and non-target fish species, the increasing trend of abundance inside the MPA with years suggests a positive relationship with the Patagonian MPA implementation.
MPA effects on Argentine hake mean size and age-2 juveniles
Since fish mobility and home range size increase with body size and age (Grüss et al., 2011), and considering part of the Argentine hake nursery ground is located inside the MPA, we predicted a stronger positive effect of the MPA on juveniles than on adults. In 1993 and 1994, in the area of the Patagonian MPA the mean total length of hake juveniles (x = 25.6, s.e. = 0.18; Author, unpublished data) was similar to that in 1996. As expected, the mean total lengths of juveniles increased (3 cm, 11%) inside the protected area after MPA implementation since protection may allow them to attain larger sizes, preventing the increase of early-maturing and small-sized individuals that negatively affects the reproductive output of the stock (Rochet, 2009). Although a decreasing trend of size at maturity of Argentine hake has been reported in the Patagonian Sea, given that from 1989–1994, length at first maturity was 34.7 cm for males and 36.2 cm for females (Ruiz and Fondacaro, 1997) and in 2001 decreased to 28.6 cm for males and 32.5 cm for females (Pájaro et al., 2005), the mean total length of hake juveniles increased within the MPA. Moreover, the Argentine hake age-2 juvenile proportion increased inside the MPA after protection. Thus, the Patagonian MPA could be contributing to restoring the Argentine hake stock.
On the other hand, the size of adults varied between years, but on average, mean total lengths were smaller at the end of the study period. Analysis of adult hake sizes in the area of the MPA before its implementation (performed in 1993, 1994; author, unpublished data) showed a mean total length of 44.8 cm (s.e. = 0.21), similar to that reported for 1996. As a result of the high fishing pressure, a decreasing trend in mean total length has been reported in hake for all the Argentine Sea between 1986 and 1997 (Aubone et al., 2004); and since 1997 older fish are less represented in catches (Renzi et al., 2009). So our results could be explained by the general trend of hake size diminishing in the region as a consequence of the high fishing pressure outside the MPA, since fishing activities remove the largest and oldest fish (Berkeley et al., 2004). In the case of species that exhibit a large home-range, such as the Argentine hake, closed areas may not be too effective as a primary protection tool for adults (e.g. Murawski et al., 2000). This could be the situation in the Patagonian MPA regarding spatial patterns of fishing effort (Figure 3). Although MPAs are considered to be one management method that helps to protect older fish (Berkeley et al., 2004), our results show that Argentine hake adults, regularly moving large distances, inside/outside the MPA, are smaller after eight years of protection. However, all evidence suggests that the Patagonian MPA, implemented to protect the nursery ground of Argentine hake, positively affects juveniles by limiting their exploitation, resulting in larger size and a higher proportion of them inside the MPA.
Global assessment of the Patagonian MPA
MPAs seem to be more effective for species with a limited home range and strong site fidelity (i.e. coral reef or benthic communities) than for migratory or far-ranging species (Blyth-Skyrme et al., 2006; West et al., 2009). The most abundant fish species inhabiting the Patagonian Shelf (Merluccius hubbsi, Macruronus magellanicus, Micromesistius australis, Salilota australis) are highly mobile and they migrate hundreds to thousands of kilometres to feed and/or reproduce (e.g. Podestá, 1990; Giussi et al., 2002). Although all these migratory movements have much larger spatial scale than the Patagonian MPA, we found some positive effects.
The time framework for analysis is crucial for detecting positive effects of protected areas (Claudet et al., 2008). Since fish population recovery is a cumulative process (Babcock et al., 2010) decades could be needed to produce noticeable effects (Micheli et al., 2004). The Patagonian MPA was implemented by the end of 1997 and, since we only evaluated it up to 2005, it could be possible that it was not long enough to show sound benefits.
Lack of time after MPA implementation, some degree of non-compliance with full protection, and the large home range of most of the Patagonian species, could reduce or prevent positive results being found for the protected area (e.g. Pelletier et al., 2008). Despite these disadvantageous features of the Patagonian MPA, the increasing trend of abundance of the demersal fish assemblage, of abundance of the target and non-target fish species, of hake juvenile size, and the higher proportion of age-2 juveniles inside the protected area, provide promising results regarding the benefits of offshore, large-scale MPAs.
Supplementary data
Acknowledgements
The authors thank G. Navarro (Subsecretaría de Pesca y Acuicultura de la Nación, Gestión de Pesquerías), F. Castañeda and D. Hernández (INIDEP) for their help with data management, A. M. Freggiaro for assistance in Argentine fishery legislation, and A. Parma, J. Claudet and two anonymous reviewers for very constructive comments on an early version of this manuscript. This work was part of the doctoral thesis of DA at the UNMdP. This is INIDEP contribution No. 1755.
Funding
This project was partially supported by Universidad Nacional de Mar del Plata (UNMdP) EXA 470/10 and 504/10, CONICET (PIP 5669, granted to OI; and PIP 5009, granted to EMA), PICT 2007-02200 and the Inter-American Institute for Global Change Research (IAI) CRN 2076, which is supported by the US National Science Foundation (grant GEO-0452325). DA was supported by scholarships from CONICET.
References
Handling editor: Verena Trenkel