-
PDF
- Split View
-
Views
-
Cite
Cite
Mitchell T. Zischke, Shane P. Griffiths, Ian R. Tibbetts, Robert J. G. Lester, Stock identification of wahoo (Acanthocybium solandri) in the Pacific and Indian Oceans using morphometrics and parasites, ICES Journal of Marine Science, Volume 70, Issue 1, January 2013, Pages 164–172, https://doi.org/10.1093/icesjms/fss164
Close - Share Icon Share
Abstract
The wahoo (Acanthocybium solandri) is an increasingly important by-product species of tropical pelagic fisheries worldwide. However, specific management of the species is currently hindered by a dearth of information on basic biology and stock structure. This study examined the stock structure of wahoo using morphometric characters and parasite fauna from fish collected in three regions of the western Pacific, and one region in each of the eastern Pacific and eastern Indian Oceans. Similar morphometric measurements and parasite abundance of wahoo collected off eastern Australia suggest they may form part of a single phenotypic stock in the western Pacific Ocean. Morphometric measurements and parasite fauna were significantly different among wahoo from the western Pacific and eastern Pacific Oceans, suggesting multiple discrete phenotypic stocks despite genetic homogeneity. Assessing fish from a range of regions throughout the Pacific Ocean may help discriminate stock boundaries in this region. Future research using complementary techniques, such as otolith microchemistry and genetic microsatellites, may improve our understanding of the global stock structure of wahoo to suitably inform regional fishery management organizations.Zischke, M. T., Griffiths, S. P., Tibbetts, I. R., and Lester, R. J. G. 2013. Stock identification of wahoo (Acanthocybium solandri) in the Pacific and Indian Oceans using morphometrics and parasites. – ICES Journal of Marine Science, 70:164–172.
Introduction
A “stock” may be defined by certain characteristics that are homogeneous among fish units of interest to fishery management (Begg et al., 1999; Cadrin et al., 2005). These characteristics include genetic markers and phenotypic characters, such as morphometrics, life history traits, isotope ratios, and parasite occurrence. Depending on the characteristics analysed, stocks may represent time-scales from single to multiple generations. As such, adopting a holistic approach that examines a suite of these characteristics provides the most robust method for identifying stock units (Begg and Waldman, 1999). Stock identification is essential for applying an appropriate spatial scale for biological monitoring, stock assessment, and sustainable management of exploited species (Quinn and Deriso, 1999). Inadequately identifying the stock structure of species may cause depletion of localized populations and a reduction in the genetic diversity of a species. This can occur if highly structured populations, such as the seamount-associated orange roughy (Hoplostethus atlanticus), are managed at an inappropriately large spatial scale, or if wide-ranging species, such as Atlantic bluefin tuna (Thunnus thynnus), are not jointly managed by all stakeholder nations (Thresher and Proctor, 2007; Viñas et al., 2011).
The wahoo (Acanthocybium solandri) is a tuna-like pelagic species that is distributed in tropical and subtropical oceanic waters worldwide. It is globally exploited as a by-product species in commercial fisheries targeting tuna and billfishes, with annual catches increasing tenfold over the last 15 years (FAO, 2011). In addition, wahoo is important to recreational, subsistence, and artisanal fisheries where a largely unquantified catch may equal, or even exceed, the commercial catch in some regions (Zischke, 2012 and references therein). Despite its importance to global fisheries, research on basic biology, stock structure, and movement is preliminary in some regions and absent in others, which currently hinders stock assessment and management.
Conventional and electronic tagging of wahoo has revealed both small- and large-scale movements that may be influenced by the local oceanography of the study region. For example, Sepulveda et al. (2011) reported high site fidelity in the vicinity of a seamount in the eastern Pacific Ocean, whereas Theisen (2007) posited that wahoo in the Atlantic undertake extensive movement in association with the Gulf Stream. Genetic analyses at regional and global scales suggest that wahoo exists as a single genetically homogeneous population throughout its circumtropical distribution (Garber et al., 2005; Theisen et al., 2008). Global genetic similarity is rare for marine teleosts, with populations of other pelagic species often segregated by ocean basin or hemisphere (Chow and Ushiama, 1995; Bromhead et al., 2004). However, migrations of only a few individuals per generation might be sufficient for traditional genetic techniques to fail to identify separate phenotypic stocks. Additionally, these techniques examine genetic variability across multiple generations; therefore, they may not be appropriate for defining suitable stock boundaries for fishery management (Moore et al., 2003). Management and future assessment of wahoo populations may benefit from alternative stock identification methods that investigate fish movement within shorter time-scales (Zischke, 2012).
The two stock identification methods used in this study, morphometrics and parasites, were chosen over other methods, such as otolith microchemistry, due to their low cost and relative ease of application. Morphometrics can be used to discriminate differences in species or populations by analysing variance in animal body shape (Cadrin et al., 2005). Fish body shape is a phenotypic trait influenced by a number of factors including inherited genes, fitness obtained through selection and environmental conditions, particularly those experienced during early life stages (Cadrin, 2000). The relative influence of each of these factors on morphometric characters is unknown (Barlow, 1961). Therefore, while differences in phenotypic traits cannot support genetic variation, they may indicate prolonged separation of individuals in different environmental conditions (Begg and Waldman, 1999). Morphometrics have been extensively used for stock identification in small and large pelagic species across a range of spatial scales (e.g. Schaefer, 1991; Murta et al., 2008; Erguden et al., 2009).
Parasites can be used as biological markers of fish to elucidate stock structure, as parasites often have different distributions to that of their host (MacKenzie and Abaunza, 1998). As fish move within a parasite's distribution, thus enabling transmission, the parasitic fauna of the fish provides a legacy of their movements (Zischke et al., 2009; Moore et al., 2011). The time-scale for host movement history ultimately depends on the infection duration of the parasite examined (Lester and MacKenzie, 2009). Temporary parasites yield short-term movement information (e.g. <12 months), while permanent parasites provide long-term movement information (e.g. entire life span since transmission). Host–parasite ecology has been used to discriminate the stock structure of pelagic species globally, and often produces results at a resolution appropriate for fishery management (Lester et al., 1985; MacKenzie, 1990).
The increasing importance of wahoo to fisheries highlights the need for formal assessments and management arrangements. To provide input to such assessments, this study used morphometrics and parasites to identify putative phenotypic stocks of wahoo in the Pacific and Indian Oceans, given that the species has been suggested to consist of a single circumtropical genetic population (Theisen et al., 2008).
Material and methods
Sample collection
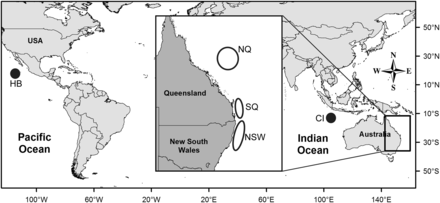
Wahoo were collected from commercial, charter (i.e. for-hire), and recreational fishers in five regions between June 2008 and September 2011 (Figure 1; Table 1). Three regions in the western Pacific Ocean (north Queensland – NQ, south Queensland – SQ, and New South Wales – NSW) were used to investigate localized fish movement off eastern Australia. Two other sampling regions (Hurricane Bank – HB and Christmas Island – CI) provided a large-scale comparison among western Pacific, eastern Pacific, and eastern Indian Oceans. Whole fish, or fish skeletons with viscera intact, were collected as either fresh or frozen samples. For each sample, location of capture, sex, total length (LT), fork length (LF), and weight of the left sagittal otolith (WO) were recorded.
Map of tropical and subtropical waters of the Pacific and Indian Oceans, with eastern Australian waters shown in the insert. The five sampling regions are HB =Hurricane Bank in the eastern Pacific Ocean, CI =Christmas Island in the eastern Indian Ocean, NQ =northern Queensland, SQ =southern Queensland, NSW =New South Wales in the western Pacific Ocean.
Sample size, mean (range in parentheses) fork length (LF), and otolith weight (WO) of wahoo sampled from five regions in the Pacific and Indian Oceans.
| Ocean . | Eastern Pacific Ocean . | Western Pacific Ocean . | . | . | Eastern Indian Ocean . |
|---|---|---|---|---|---|
| Sample region | HB | NQ | SQ | NSW | CI |
| Sample collection period | Jan 2011 | Dec 2008–Sep 2011 | June 2008–May 2011 | Jan 2009–Mar 2011 | Mar–Dec 2010 |
| Sample size (morphometrics) | 44 | 57 | 126 | 37 | 26 |
| Sample size (parasites) | 55 | 83 | 103 | 37 | 26 |
| Mean LF (mm) | 1 259 (915–1 515) | 1 374 (965–1 765) | 1 143 (790–1 570) | 1 265 (1 000–1 680) | 1 005 (800–1 340) |
| Mean WO (g)a | 0.019 14 | 0.025 83 | 0.016 27 | 0.018 14 | – |
| Temporary parasites | |||||
| 1. Brachiella thynnib | 2.4 (98) | 3.1 (98) | 2.2 (95) | 2.1 (92) | – |
| 2. Nematobothrium spinneribc | - | 3.3 (72) | 2.6 (81) | 2.6 (79) | 2.0 (46) |
| 3. Didymocystis acanthocybii | 36.3 (86) | 22.3 (79) | 25.2 (87) | 22.4 (76) | 22.8 (85) |
| Permanent parasites | |||||
| 4. Anisakis sp. Type I | 0.0 (2) | 74.2 (61) | 23.1 (53) | 51.1 (68) | 2.7 (23) |
| 5. Terranova sp. Type II | 0.0 (0) | 25.2 (47) | 5.0 (28) | 7.7 (43) | 0.0 (0) |
| 6. Tentacularia coryphaenae | 0.8 (40) | 1.0 (32) | 0.4 (15) | 0.7 (22) | 0.5 (27) |
| 7. Floriceps sp. | 0.0 (0) | 0.8 (21) | 0.2 (5) | 0.1 (11) | 0.0 (0) |
| Ocean . | Eastern Pacific Ocean . | Western Pacific Ocean . | . | . | Eastern Indian Ocean . |
|---|---|---|---|---|---|
| Sample region | HB | NQ | SQ | NSW | CI |
| Sample collection period | Jan 2011 | Dec 2008–Sep 2011 | June 2008–May 2011 | Jan 2009–Mar 2011 | Mar–Dec 2010 |
| Sample size (morphometrics) | 44 | 57 | 126 | 37 | 26 |
| Sample size (parasites) | 55 | 83 | 103 | 37 | 26 |
| Mean LF (mm) | 1 259 (915–1 515) | 1 374 (965–1 765) | 1 143 (790–1 570) | 1 265 (1 000–1 680) | 1 005 (800–1 340) |
| Mean WO (g)a | 0.019 14 | 0.025 83 | 0.016 27 | 0.018 14 | – |
| Temporary parasites | |||||
| 1. Brachiella thynnib | 2.4 (98) | 3.1 (98) | 2.2 (95) | 2.1 (92) | – |
| 2. Nematobothrium spinneribc | - | 3.3 (72) | 2.6 (81) | 2.6 (79) | 2.0 (46) |
| 3. Didymocystis acanthocybii | 36.3 (86) | 22.3 (79) | 25.2 (87) | 22.4 (76) | 22.8 (85) |
| Permanent parasites | |||||
| 4. Anisakis sp. Type I | 0.0 (2) | 74.2 (61) | 23.1 (53) | 51.1 (68) | 2.7 (23) |
| 5. Terranova sp. Type II | 0.0 (0) | 25.2 (47) | 5.0 (28) | 7.7 (43) | 0.0 (0) |
| 6. Tentacularia coryphaenae | 0.8 (40) | 1.0 (32) | 0.4 (15) | 0.7 (22) | 0.5 (27) |
| 7. Floriceps sp. | 0.0 (0) | 0.8 (21) | 0.2 (5) | 0.1 (11) | 0.0 (0) |
Untransformed mean parasite abundance and prevalence (in parentheses) are given for temporary and permanent parasites. “–” = missing data.
aOtoliths were not collected for wahoo from CI; therefore, no WO was recorded.
bDue to restrictions with sample collection, counts of B. thynni and N. spinneri were not taken from CI and HB, respectively.
cCounts of cysts were taken for N. spinneri. Cysts commonly contain one to three parasites (Lester, 1979).
Sample size, mean (range in parentheses) fork length (LF), and otolith weight (WO) of wahoo sampled from five regions in the Pacific and Indian Oceans.
| Ocean . | Eastern Pacific Ocean . | Western Pacific Ocean . | . | . | Eastern Indian Ocean . |
|---|---|---|---|---|---|
| Sample region | HB | NQ | SQ | NSW | CI |
| Sample collection period | Jan 2011 | Dec 2008–Sep 2011 | June 2008–May 2011 | Jan 2009–Mar 2011 | Mar–Dec 2010 |
| Sample size (morphometrics) | 44 | 57 | 126 | 37 | 26 |
| Sample size (parasites) | 55 | 83 | 103 | 37 | 26 |
| Mean LF (mm) | 1 259 (915–1 515) | 1 374 (965–1 765) | 1 143 (790–1 570) | 1 265 (1 000–1 680) | 1 005 (800–1 340) |
| Mean WO (g)a | 0.019 14 | 0.025 83 | 0.016 27 | 0.018 14 | – |
| Temporary parasites | |||||
| 1. Brachiella thynnib | 2.4 (98) | 3.1 (98) | 2.2 (95) | 2.1 (92) | – |
| 2. Nematobothrium spinneribc | - | 3.3 (72) | 2.6 (81) | 2.6 (79) | 2.0 (46) |
| 3. Didymocystis acanthocybii | 36.3 (86) | 22.3 (79) | 25.2 (87) | 22.4 (76) | 22.8 (85) |
| Permanent parasites | |||||
| 4. Anisakis sp. Type I | 0.0 (2) | 74.2 (61) | 23.1 (53) | 51.1 (68) | 2.7 (23) |
| 5. Terranova sp. Type II | 0.0 (0) | 25.2 (47) | 5.0 (28) | 7.7 (43) | 0.0 (0) |
| 6. Tentacularia coryphaenae | 0.8 (40) | 1.0 (32) | 0.4 (15) | 0.7 (22) | 0.5 (27) |
| 7. Floriceps sp. | 0.0 (0) | 0.8 (21) | 0.2 (5) | 0.1 (11) | 0.0 (0) |
| Ocean . | Eastern Pacific Ocean . | Western Pacific Ocean . | . | . | Eastern Indian Ocean . |
|---|---|---|---|---|---|
| Sample region | HB | NQ | SQ | NSW | CI |
| Sample collection period | Jan 2011 | Dec 2008–Sep 2011 | June 2008–May 2011 | Jan 2009–Mar 2011 | Mar–Dec 2010 |
| Sample size (morphometrics) | 44 | 57 | 126 | 37 | 26 |
| Sample size (parasites) | 55 | 83 | 103 | 37 | 26 |
| Mean LF (mm) | 1 259 (915–1 515) | 1 374 (965–1 765) | 1 143 (790–1 570) | 1 265 (1 000–1 680) | 1 005 (800–1 340) |
| Mean WO (g)a | 0.019 14 | 0.025 83 | 0.016 27 | 0.018 14 | – |
| Temporary parasites | |||||
| 1. Brachiella thynnib | 2.4 (98) | 3.1 (98) | 2.2 (95) | 2.1 (92) | – |
| 2. Nematobothrium spinneribc | - | 3.3 (72) | 2.6 (81) | 2.6 (79) | 2.0 (46) |
| 3. Didymocystis acanthocybii | 36.3 (86) | 22.3 (79) | 25.2 (87) | 22.4 (76) | 22.8 (85) |
| Permanent parasites | |||||
| 4. Anisakis sp. Type I | 0.0 (2) | 74.2 (61) | 23.1 (53) | 51.1 (68) | 2.7 (23) |
| 5. Terranova sp. Type II | 0.0 (0) | 25.2 (47) | 5.0 (28) | 7.7 (43) | 0.0 (0) |
| 6. Tentacularia coryphaenae | 0.8 (40) | 1.0 (32) | 0.4 (15) | 0.7 (22) | 0.5 (27) |
| 7. Floriceps sp. | 0.0 (0) | 0.8 (21) | 0.2 (5) | 0.1 (11) | 0.0 (0) |
Untransformed mean parasite abundance and prevalence (in parentheses) are given for temporary and permanent parasites. “–” = missing data.
aOtoliths were not collected for wahoo from CI; therefore, no WO was recorded.
bDue to restrictions with sample collection, counts of B. thynni and N. spinneri were not taken from CI and HB, respectively.
cCounts of cysts were taken for N. spinneri. Cysts commonly contain one to three parasites (Lester, 1979).
Morphometrics
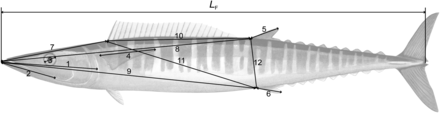
Due to the large size of wahoo, linear measurement between fixed anatomical features (e.g. fin insertion point) was favoured over digital shape analysis methods (see Cadrin, 2000). Because anatomical features are associated with the skeletal structure of the fish, morphometric measurements were considered comparable between whole and skeletal specimens. Fish were placed left side up and 12 morphometric characters (Figure 2) were measured linearly using small (0.0–150.0 mm) or large (150–1000 mm) callipers, similar to those methods described for yellowfin tuna (Thunnus albacares) (Schaefer, 1991). If fish were damaged in a manner that affected consistent and accurate measurement of morphometric characters, and right-side measurements could not be substituted (i.e. measurements 1–4), they were excluded from the analysis.
The 12 morphometric measurements taken from wahoo. 1 =head length, 2 =maxilla length, 3 =eye diameter, 4 =pectoral fin length, 5 =second dorsal fin length, 6 =anal fin length, 7 =snout to insertion of first dorsal fin, 8 =snout to insertion of second dorsal fin, 9 =snout to insertion of anal fin, 10 =insertion of first dorsal fin to insertion of second dorsal fin, 11 =insertion of first dorsal fin to insertion of anal fin, 12 =insertion of second dorsal fin to insertion of anal fin.
Parasites
Initially, ten fish from each study region were comprehensively examined for all parasite fauna to identify species that may be appropriate for use as biological markers. Key characteristics in determining candidate parasite species were (i) ease of macroscopic detection and identification, (ii) frequency of occurrence, and (iii) longevity on the host (MacKenzie and Abaunza, 1998). Parasite specimens were preserved in 10% neutral buffered formalin and processed to aid microscopic identification. Trematodes were stained with haematoxylin and eosin, nematodes were cleared using methyl salicylate, and live trypanorhynchs were placed in freshwater to induce tentacle eversion (Zischke et al., 2009; Moore et al., 2011). Once identified, three species of temporary parasites and four species of permanent parasites were selected as biological markers (Table 1). Parasite markers were identified macroscopically in all subsequent samples, and their presence and abundance was recorded for each fish examined.
Statistical analysis
Univariate and multivariate analyses were used to test for differences in morphometric measurements and parasite abundance of wahoo in the five study regions. Prior to univariate analyses, Shapiro–Wilk's test and the Brown–Forsythe test were used to test for normality and homoscedasticity of variances, respectively. The results of these tests dictated whether any transformations were required prior to analyses, and which univariate and multivariate analyses were appropriate for each dataset.
Morphometric data
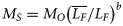
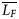 is the mean fork length of all fish from all regions, LF is fork length, and b was estimated for each morphometric character as the slope of the regression between log MO and log LF (Elliot et al., 1995). Six of the 12 morphometric measurements had non-normal distributions (p < 0.05), so all measurements were log transformed for subsequent analyses. All morphometric data were homoscedastic (p > 0.05).
is the mean fork length of all fish from all regions, LF is fork length, and b was estimated for each morphometric character as the slope of the regression between log MO and log LF (Elliot et al., 1995). Six of the 12 morphometric measurements had non-normal distributions (p < 0.05), so all measurements were log transformed for subsequent analyses. All morphometric data were homoscedastic (p > 0.05).Parasite data
Long-lived parasites can accumulate with host age and, therefore, obscure potential differences in parasite abundance where the age of sampled fish differs among regions (Rohde, 1982). McBride et al. (2008) showed that age of wahoo closely correlates with otolith weight in the Atlantic Ocean; therefore, WO was considered to be a suitable proxy of age in the present study. Similarly, for the closely related Spanish mackerel (Scomberomorus commerson), WO was shown to be better than host age for determining parasite accumulation (Lester et al., 2001). To investigate parasite accumulation for wahoo, regressions between WO and parasite abundance were performed for each sample region. Mean parasite abundance estimates for WO groups were also plotted. Where parasite accumulation was evident, abundance data were adjusted accordingly (see Results).
Summary statistics, including mean abundance and prevalence, were compiled for each parasite species in each region (Zischke et al., 2009; Moore et al., 2011). Differences in parasite prevalence among regions were examined using a Pearson chi-squared test. Parasite abundance data distributions were non-normal (p < 0.001) and were ln (x + 1) transformed prior to multivariate analysis. Temporary parasite abundance data were homoscedastic (p > 0.05), while distributions of permanent parasite abundance data were heteroscedastic (p < 0.001).
Univariate analysis
One-way analysis of variance (ANOVA) was performed on standardized and transformed morphometric data. Parasite abundance data were non-normal, even after transformation; therefore, parametric univariate analyses were not appropriate. Instead, a generalized linear model (GLM), which allows data to have distributions other than normal, was used to compare individual parasite abundance among regions. Untransformed parasite abundance data were overdispersed due to a large zero component and closely represented a negative binomial distribution. Therefore, untransformed parasite abundance data were analysed using a GLM that assumed a negative binomial probability distribution. Summary statistics and univariate analyses were completed using R software (version 2.13.0).
Multivariate analysis
Non-metric multidimensional scaling (nMDS) was used to conduct multivariate analyses on log-transformed morphometric and parasite abundance data. Similarity matrices were constructed based on resemblance measures that were appropriate for each dataset. Euclidean distances were used for standardized morphometric data, while transformed parasite data were analysed using Bray–Curtis similarity, a measure that is often used for complex and heterogeneous community assemblage data (Clarke and Gorley, 2006).
Pair-wise, one-way analysis of similarity (ANOSIM) was used to compare morphometric and parasite assemblage data among regions. This was done by generating a pseudo F-statistic using 5000 random permutations of the data to determine the probability that the structure of our sample data could arise by chance. ANOSIM has been previously used to delineate fish stocks using morphometric and parasite data (Garraffoni and Camargo, 2007; Moore et al., 2011), and is suitable where data diverge from assumptions of normality and homogeneous dispersion (Clarke, 1993). Similarity percentages (SIMPER) were calculated to determine which morphometric characters and parasite species contributed most to the within-region similarity and among-region dissimilarity. Multivariate analyses were completed using PRIMER (version 6.0.11).
Results
Morphometrics
Morphometric data were collected for a total of 290 wahoo (Table 1). There was no significant correlation between standardized morphometric measurements and LF (p > 0.05), indicating that the effect of fish size was successfully removed from the data. Univariate pair-wise comparisons revealed that fish from NQ and HB had the highest number of morphometric measurements that were significantly different, while no measurements were significantly different between fish from SQ and NSW (Table 2).
Pair-wise comparison of 12 morphometric measurements of wahoo from five regions in the Pacific and Indian Oceans (see Figure 1).
| Region . | HB . | NQ . | SQ . | NSW . | CI . |
|---|---|---|---|---|---|
| HB | 1–4, 7, 9, 10–12 | 2, 3, 4, 9 | 3, 4, 7, 9, 12 | 1, 3, 7 | |
| NQ | 1, 3, 7, 10 | 1, 2, 10, 11 | 4, 9 | ||
| SQ | - | 1, 7, 9, 10 | |||
| NSW | 1, 9 | ||||
| CI |
| Region . | HB . | NQ . | SQ . | NSW . | CI . |
|---|---|---|---|---|---|
| HB | 1–4, 7, 9, 10–12 | 2, 3, 4, 9 | 3, 4, 7, 9, 12 | 1, 3, 7 | |
| NQ | 1, 3, 7, 10 | 1, 2, 10, 11 | 4, 9 | ||
| SQ | - | 1, 7, 9, 10 | |||
| NSW | 1, 9 | ||||
| CI |
Numbers in the table represent the morphometric measurements (see Figure 2) that were significantly different (p < 0.05) using a one-way analysis of variance.
Pair-wise comparison of 12 morphometric measurements of wahoo from five regions in the Pacific and Indian Oceans (see Figure 1).
| Region . | HB . | NQ . | SQ . | NSW . | CI . |
|---|---|---|---|---|---|
| HB | 1–4, 7, 9, 10–12 | 2, 3, 4, 9 | 3, 4, 7, 9, 12 | 1, 3, 7 | |
| NQ | 1, 3, 7, 10 | 1, 2, 10, 11 | 4, 9 | ||
| SQ | - | 1, 7, 9, 10 | |||
| NSW | 1, 9 | ||||
| CI |
| Region . | HB . | NQ . | SQ . | NSW . | CI . |
|---|---|---|---|---|---|
| HB | 1–4, 7, 9, 10–12 | 2, 3, 4, 9 | 3, 4, 7, 9, 12 | 1, 3, 7 | |
| NQ | 1, 3, 7, 10 | 1, 2, 10, 11 | 4, 9 | ||
| SQ | - | 1, 7, 9, 10 | |||
| NSW | 1, 9 | ||||
| CI |
Numbers in the table represent the morphometric measurements (see Figure 2) that were significantly different (p < 0.05) using a one-way analysis of variance.
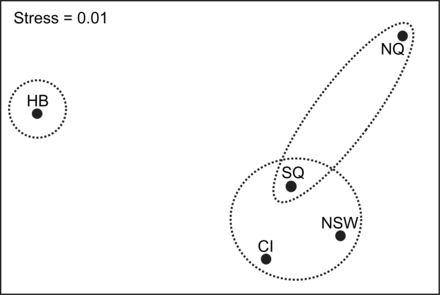
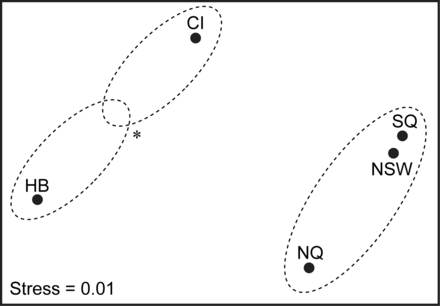
An ordination plot of nMDS results based on transformed morphometric measurements revealed separation between some regions (Figure 3). ANOSIM confirmed that there was a significant statistical difference (Global r = 0.072, p = 0.001) in morphometric measurements of fish among the five sampling regions. Pair-wise comparisons reveal that morphometrics of fish from HB were significantly different from all other regions, NQ were significantly different from all regions except SQ, while SQ, NSW, and CI did not differ from each other (Figure 3).
Ordination plot of non-metric multidimensional scaling of mean morphometric measurements of wahoo from five regions in the Pacific and Indian Oceans (see Figure 1; stress = 0.01). Dashed circles represent groupings defined by ANOSIM at p < 0.05.
SIMPER revealed that second dorsal fin length, anal fin length, pectoral fin length, and eye diameter made the highest contribution to differences in the composition of morphometric characters among regions.
Parasites
Seven parasite species were deemed suitable as biological markers for wahoo (Table 1). The copepod, Brachiella thynni Cuvier, 1830, and two didymozoids, Didymocystis acanthocybii Yamaguti, 1938, and Nematobothrium spinneriLester, 1979, are likely to have short resident times (<12 months) on wahoo – given their transmission strategy and life cycle – and were assigned as temporary parasite markers. Individual B. thynni were found at the base of the pectoral fin. D. acanthocybii formed cysts in the muscle of the palate and at the base off the gills of wahoo, and while they often occurred in pairs, each individual parasite was counted. N. spinneri were found to form large black cysts in the abdominal muscles, containing one to three parasites each (Lester, 1979). These cysts occurred in two forms: large “soft” cysts containing live adult worms thought to be short-lived and smaller “hard” cysts containing what appeared to be dead worms and eggs. Counts of all cysts (soft and hard combined) were used in univariate and multivariate analyses, but comparisons between the prevalence of both cyst types were examined for seasonality in parasite infection.
Two larval nematodes, Anisakis sp. Type I and Terranova sp. Type II (Cannon, 1977) and two larval cestodes, Tentacularia coryphaenae Bosc, 1802, and Floriceps sp. (Palm, 2004), were found in wahoo specimens. These parasites infect wahoo as an intermediate host in their life cycle, awaiting ingestion of the wahoo (and larvae) by a definitive host (e.g. sharks or marine mammals). As such, they are likely to permanently infect wahoo, making excellent permanent parasite markers (Zischke et al., 2009; Moore et al., 2011). All permanent parasite species were found encapsulated throughout the body cavity, particularly on the outside of the stomach wall.
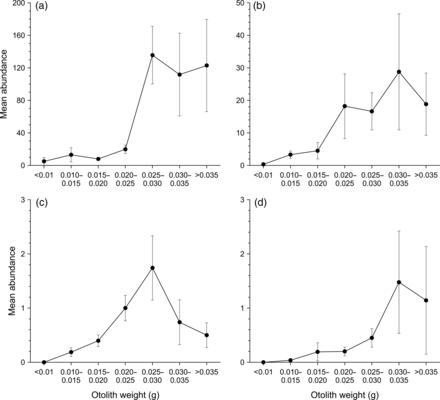
There were no linear correlations between parasite abundance and WO in any sampling region (r2 = 0.00–0.29). However, there was a distinct increase in mean permanent parasite abundance in fish with a WO >0.025 g (Figure 4). This suggests that permanent parasites may accumulate with host age, which could affect analysis of parasite abundance among regions where host age differs. Therefore, samples for all regions were restricted to fish with WO < 0.025 g to minimize the effect of parasite accumulation and ensure comparable analysis among regions. Linear correlation between WO and LF was high (WO = 3e−5LF - 0.023, n = 408, r2 = 0.73); therefore, in regions where WO was not recorded (i.e. Christmas Island), LF was converted to WO to enable correction of samples. The adjusted sample sizes for each region were: NQ = 47, SQ = 98, NSW = 32, CI = 26, and HB = 38.
Mean permanent parasite abundance (± s.e.) of (a) Anisakis sp. Type I, (b) Terranova sp. Type II, (c) Tentacularia coryphaenae, and (d) Floriceps sp. for seven otolith weight groups of wahoo in the Pacific and Indian Oceans.
Chi-squared tests indicated that the prevalence of N. spinneri was the only temporary parasite to significantly differ among the five regions (χ2 = 12.99, d.f. = 3, p = 0.004). Soft and hard cysts of N. spinneri were found in fish from all three regions in the western Pacific Ocean, with both cyst types simultaneously occurring in 19.8% of infected fish. The monthly prevalence of hard cysts was high (>80%), but peaked close to 100% between May and October. Conversely, the prevalence of soft cysts was below 10% during the same period, but peaked above 40% between November and March. All permanent parasites had significantly different prevalence among study regions (p < 0.001).
The p-values obtained from a GLM for each parasite species were examined for significant differences among regions using pair-wise comparisons (Table 3). The only temporary parasite that statistically differed between regions was B. thynni, whose abundance was significantly different between NQ and SQ. Permanent parasites, in particular, the abundance of Anisakis sp. Type I and Terranova sp. Type II, were significantly different in most pair-wise comparisons. NQ and SQ, NQ and HB, and SQ and HB had three permanent parasites species significantly differing in abundance. In contrast, comparisons between SQ and NSW, NSW and CI, and CI and HB only had one parasite species differing significantly (Table 3).
Pair-wise comparison of seven parasite species infecting wahoo from five regions in the Pacific and Indian Oceans (see Figure 1).
| Region . | HB . | NQ . | SQ . | NSW . | CI . |
|---|---|---|---|---|---|
| HB | 4, 5, 7 | 4, 5, 6 | 4, 5 | 4 | |
| NQ | 1, 4, 5, 6 | 5 | 4, 5, 7 | ||
| SQ | 4 | 4, 5 | |||
| NSW | 4, 5 | ||||
| CI |
| Region . | HB . | NQ . | SQ . | NSW . | CI . |
|---|---|---|---|---|---|
| HB | 4, 5, 7 | 4, 5, 6 | 4, 5 | 4 | |
| NQ | 1, 4, 5, 6 | 5 | 4, 5, 7 | ||
| SQ | 4 | 4, 5 | |||
| NSW | 4, 5 | ||||
| CI |
Numbers in the table represent the parasite species (see Table 1) that were significantly different (p < 0.05) using a generalized linear model.
Pair-wise comparison of seven parasite species infecting wahoo from five regions in the Pacific and Indian Oceans (see Figure 1).
| Region . | HB . | NQ . | SQ . | NSW . | CI . |
|---|---|---|---|---|---|
| HB | 4, 5, 7 | 4, 5, 6 | 4, 5 | 4 | |
| NQ | 1, 4, 5, 6 | 5 | 4, 5, 7 | ||
| SQ | 4 | 4, 5 | |||
| NSW | 4, 5 | ||||
| CI |
| Region . | HB . | NQ . | SQ . | NSW . | CI . |
|---|---|---|---|---|---|
| HB | 4, 5, 7 | 4, 5, 6 | 4, 5 | 4 | |
| NQ | 1, 4, 5, 6 | 5 | 4, 5, 7 | ||
| SQ | 4 | 4, 5 | |||
| NSW | 4, 5 | ||||
| CI |
Numbers in the table represent the parasite species (see Table 1) that were significantly different (p < 0.05) using a generalized linear model.
Abundance data for B. thynni and N. spinneri were not obtained from CI and HB, respectively. Therefore, multivariate analysis of temporary parasites was only conducted for the three regions off eastern Australia. ANOSIM (Global r = 0.003, p = 0.188) failed to detect a significant difference in temporary parasite abundance among the three regions in the western Pacific Ocean. An ordination plot of nMDS results suggested differences in the permanent parasite fauna of wahoo from HB and CI to that of the three regions in the western Pacific (Figure 5). ANOSIM confirmed that the assemblage structure of the parasite fauna was statistically significant among the five sampling regions (Global r = 0.096, p < 0.001). Pair-wise comparisons revealed that parasite assemblages of fish from CI and HB were significantly different from parasite assemblages of fish from all western Pacific regions, but not to each other (p = 0.05). Pair-wise comparisons failed to detect a significant difference in the permanent parasite assemblages of fish from NQ, SQ and NSW (Figure 5).
Ordination plot of non-metric multidimensional scaling of mean permanent parasite abundance of wahoo from five regions in the Pacific and Indian Oceans (see Figure 1; stress = 0.01). Dashed circles represent groupings defined by ANOSIM at p < 0.05. *Indicates that grouping is tentative as p = 0.05.
For multivariate analysis of parasite data, SIMPER determined that Anisakis sp. Type I and Terranova sp. Type II contributed the most dissimilarity in parasite assemblages among regions.
Discussion
Analyses of morphometric measurements and parasite assemblage data provided insight into the phenotypic stock structure of wahoo in the Pacific and Indian Oceans. Our results complement previous research on their global genetic population (Theisen et al., 2008), providing information at a shorter time-scale that may be useful to fisheries assessment and management.
Western Pacific Ocean
Parasite abundance data suggests that wahoo off eastern Australia belong to a single stock. While univariate analyses revealed that some parasite species differed in abundance between regions, multivariate analysis did not detect a significant difference in the overall parasite assemblages of fish from the three regions. In contrast, analyses of morphometric measurements suggest that the body shape of wahoo significantly differed between the two most geographically separated regions, NQ and NSW, but was similar between neighbouring regions (i.e. between NQ and SQ, and between SQ and NSW). While Euclidean distance can be overly sensitive to small differences in morphometric measurements (Wang et al., 2005), we conducted ANOSIM on morphometric data using alternative similarity measures (e.g. Bray–Curtis), and the results were identical to those using Euclidean distance. Given these morphometric results, the phenotypic stock structure of wahoo off eastern Australia is uncertain.
The Coral Sea, off northern Queensland, encompasses a wide variety of bathymetric features including coral reefs and deep oceanic regions, and experiences stable sea surface temperatures (SSTs) ranging between 24 and 28°C annually (BOM, 2012). These conditions provide suitable habitat for wahoo year-round (Zischke, 2012), which is highlighted by the consistency of wahoo in commercial catches off northern Queensland (AFMA, 2011). The flow of the East Australian Current (EAC) strengthens during the Austral summer, moving warm water from the Coral Sea to temperate regions of southeastern Australia (Ridgway and Godfrey, 1997). The seasonal movements of several large pelagic species such as tunas and billfishes are closely linked with this seasonal southward expansion of warm water (Gunn et al., 2003; Young et al., 2011).
Seasonal expansion of the EAC may facilitate movement of wahoo from NQ to SQ and NSW. This may explain the similar parasite assemblages in fish from all three regions. Also, this conclusion is supported by the “temporary” parasites, particularly the didymozoids. Though the life cycles are not known, didymozoids are generally a tropical or subtropical parasite group (Lester et al., 1985). Soft cysts containing live N. spinneri were predominant for approximately six months through summer, suggesting an adult lifespan of a similar duration. Therefore, the occurrence of live N. spinneri in fish from NSW suggests that fish may acquire these worms in the warmer waters of NQ prior to moving southward. Parasite assemblages of the closely related S. commerson suggest similar seasonal movement and phenotypic stock homogeneity off eastern Australia (Williams and Lester, 2006).
While parasite data suggest a homogeneous stock at a single generation time-scale, morphometric data appear to suggest separation between NQ and NSW over a longer time-scale. Some morphometric measurements (e.g. measurements across the body) may be influenced by fish condition at the time of collection. The majority of fish from NSW (∼92%) were collected in March, which is immediately after a protracted spawning season (Zischke et al., in press) and approximately coincides with a weakening of the EAC and subsequent decline in local SST (Ridgway and Godfrey, 1997). Therefore, if these fish had recently finished spawning and had reached the end of their southward migration, their body condition may be poorer than during other times of the year. This may have resulted in significant morphometric differences between fish from NQ and NSW, while fish collected from SQ were similar to both other regions as it represents a midpoint along the migration. Given the similarity in parasite fauna, we conclude that wahoo off eastern Australia appear to belong to a single phenotypic stock. However, further research using complementary techniques, particularly electronic tagging, may elucidate potential seasonal migration of wahoo and clarify their stock structure off eastern Australia.
The Eastern Tuna and Billfish Fishery (ETBF) is a federally managed fishery spaning the entire Exclusive Economic Zone (EEZ) of eastern Australia. While this study suggests that wahoo in this region belong to a single phenotypic stock, if this stock extends throughout the south Pacific Ocean, collaborative regional management strategies, similar to those in place for other large pelagic species, will be required (WCPFC, 2000). Currently, each state government individually manages recreational fisheries off eastern Australia. Given the potential phenotypic homogeneity of wahoo in this region, collaboration between states and the federal government, and neighbouring countries, may be necessary for monitoring, assessment, and management of the species.
Eastern and western Pacific Ocean
All analyses highlighted that the body shape and parasite assemblages of wahoo from HB in the eastern Pacific Ocean were significantly different from fish from the three regions in the western Pacific Ocean. These results indicate that wahoo may consist of at least two discrete phenotypic stocks in the Pacific Ocean. The large geographic distance across the Pacific Ocean, combined with the fast growth rates and short lifespan of wahoo (McBride et al., 2008), suggests that adult fish may not be capable of making large-scale migrations in sufficient numbers to cause phenotypic homogeneity. Also, wahoo do not appear to have specific spawning areas, but spawn throughout their distribution during spawning season (Zischke et al., in press; Jenkins and McBride, 2009). Therefore, if suitable habitat and food availability exist throughout tropical and subtropical waters of the Pacific, adult wahoo may have no requirement to undertake large-scale migrations. Other large pelagic species, such as yellowfin tuna and striped marlin (Kajikia audax), have been shown to have stock heterogeneity throughout the Pacific Ocean, as indicated by morphometrics and electronic tagging (Schaefer, 1991; Domeier, 2006).
While wahoo may exist as a single circumtropical genetic population (Theisen et al., 2008), differences in morphometrics and parasite fauna between fish from the western and eastern Pacific Ocean suggest that wahoo may form multiple phenotypic stocks in these regions. Theisen et al. (2008) suggested that rare transoceanic migrations by adult wahoo could be sufficient to cause global genetic homogeneity. Apart from highly mobile adults, wahoo also have buoyant eggs and pelagic larvae (Wollam, 1969; Collette et al., 1984). Therefore, dispersal during preadult stages by ocean currents may also facilitate genetic homogeneity. Discontinuity between genetic and phenotypic stocks has been shown for other pelagic species. Horse mackerel (Trachurus trachurus) have low levels of genetic structuring, but display significant differences in the morphometrics and parasite fauna of fish in the Northeast Atlantic and Mediterranean Sea, suggesting discrete phenotypic stocks in these regions (Abaunza et al., 2008). Future research examining wahoo from a wide range of regions in the western, central, and eastern Pacific may help to discriminate the boundaries of these stocks to assist fishery agencies in determining an appropriate scale for regional management.
Eastern Indian Ocean
The evidence for identifying a discrete phenotypic stock of wahoo in the eastern Indian Ocean using morphometric characters and parasite assemblages was less clear than for the Pacific Ocean regions. Differences in the parasite assemblage of fish from CI and those from the three regions in the western Pacific Ocean suggests separation over a single generation, while morphometric measurements only differ between fish from CI and NQ. Similarly, morphometrics of fish differed between CI and HB, but the parasite fauna was not significantly different (p = 0.05) between fish from these two regions.
Christmas Island is located approximately 1600 km northwest of Australia within the Indian Ocean Gyre. This region is separated from the other regions sampled in this study by vast geographic distances (i.e. >5000 km). The closest migration routes between the southern Pacific and Indian Oceans are via the Timor Sea around northern Australia, or via the Southern Ocean off southern Australia. Shallow, turbid waters in the north and cold SSTs in the south may create unfavourable conditions for wahoo. Based on these potential barriers to extensive adult migrations, the hypothesis that wahoo in the eastern Indian Ocean represent a separate phenotypic stock to fish in the Pacific Ocean is valid, although the results from this study cannot confidently support this. Our smallest sample size for both morphometric and parasite analysis was for fish collected from CI, which may have affected the ability of these analyses to detect a significant difference between some regions.
Conclusions
Wahoo are a highly mobile pelagic species that are valuable to commercial and recreational fisheries worldwide. While previous research suggests they may form a single circumglobal genetic population, it was unknown whether this translates to a single phenotypic stock. Morphometrics and parasite fauna examined in this study suggest that wahoo in the western and eastern Pacific Ocean belong to separate phenotypic stocks. Results from this study could not confidently determine the stock identity of wahoo in the eastern Indian Ocean; however, considering the substantial oceanographic boundaries to migration around Australia, it is unlikely that many individuals migrate between the Pacific and Indian Oceans. Future research, utilizing complimentary tools such as otolith microchemistry and genetic microsatellites, is needed to compare and validate current research, thereby providing a comprehensive account of the stock structure of wahoo worldwide.
Funding
This research was funded by the Winifred Violet Scott Estate and an Ethel Mary Reid award from the Royal Zoological Society of NSW. MTZ was funded by a University of Queensland Research Scholarship, with support provided by the CSIRO Division of Marine and Atmospheric Research. This research was approved by The University of Queensland Animal Ethics Committee (approval number CMS/884/08/FRDC/URG).
Acknowledgements
We thank the numerous commercial, charter, and recreational fishers who donated samples for this research. We also thank S. Hall, W. Hagedoorn, J. Cavallaro, B. Lamason, D. Fuller, O. Snodgrass, C. Skepper, M. Rochfort, and M. Hourston for assisting with the logistics of sample collection, storage, and freight. B. Gilby, B. Lockett, J. Lerner, M. O'Haire, A. Heemsoth, and O. Snodgrass assisted with laboratory dissections. B. Moore and E. Lawrence are thanked for statistical assistance. L. Marshall of Stickfigurefish is thanked for the wahoo illustration in Figure 2. Finally, we thank two anonymous reviewers whose comments greatly improved the manuscript.
References
Handling editor: Emory Anderson