-
PDF
- Split View
-
Views
-
Cite
Cite
Eneko Bachiller, Xabier Irigoien, Allometric relations and consequences for feeding in small pelagic fish in the Bay of Biscay, ICES Journal of Marine Science, Volume 70, Issue 1, January 2013, Pages 232–243, https://doi.org/10.1093/icesjms/fss171
Close - Share Icon Share
Abstract
The body size of fish is an important factor in determining their biology and ecology, as predators eat prey smaller than themselves. Predator mouth size restricts the availability of possible prey. In this paper we provide the allometric relationships of eight common, small pelagic fish species in the Bay of Biscay. In addition, we describe the predator-prey size ratios for different species, and we determine changes in their ratio-based trophic-niche breadth with increasing body size. Results suggest that gape size does not totally determine the predator-prey size ratio distribution, but predators use the entire available prey size range, including the smallest. As they grow they simply incorporate larger prey as their increased gape size permits. Accordingly, a large degree of overlap was found in the diet composition in terms of size and predator-prey ratios, even between fish of different sizes. Of the species studied, only horse mackerels seem to be clearly specialized in relatively large prey.Bachiller, E. and Irigoien, X. 2013. Allometric relations and consequences for feeding in small pelagic fish in the Bay of Biscay. – ICES Journal of Marine Science, 70:232–243.
Introduction
Body size has an important influence on the biology and ecology of any animal (Peters, 1986; Brown et al., 2004; Barnes et al., 2010). Similarly, prey size can affect the predator's feeding success (Eggers, 1982; Luo et al., 1996; Pepin and Penney, 1997) and prey selection (Eggers, 1977, 1982; Schmitt, 1986; Pepin and Penney, 1997; Barnes et al., 2010), although the variability in prey selection could be related to interspecific morphological differences depending on the species (Sabatés and Saiz, 2000).
Many studies have shown a general trend for the food spectrum of fish to widen as they grow (Peterson and Ausubel, 1984; Mahé et al., 2007; Bacha and Amara, 2009). Due to allometric relationships small increases in prey length can result in large increases in the energy intake (Conway et al., 1999). According to the prey size spectrum of small pelagic fish, relatively large prey that are only caught occasionally by the fish, but which make an important contribution to their diet, are difficult to quantify. For example, several studies have shown that krill is important in terms of ingested biomass for pelagic fish such as anchovy (Plounevez and Champalbert, 1999; Espinoza and Bertrand, 2008), mackerel (Olaso et al., 2005) and horse mackerel (Olaso et al., 1999).
Other pelagic fish species overlap in size during growth and share the same environment. Little is known about the relative influence of the size of different species on their diet composition or interspecies competition. In the Bay of Biscay several small pelagic fish species share the environment (sardines, anchovies, mackerels, horse mackerels, sprats and bogues). It has been suggested that intraguild predation (Polis et al., 1989) could be an important factor regulating the population dynamics of these populations (Irigoien et al., 2008; Irigoien and De Roos, 2011). Intraguild predation can be divided into two aspects: competition for food and predation on each other (Polis et al. 1989). In this paper we focus on the competition aspect of this mechanism by determining predator-prey sizes and their contribution to the diet of the different small pelagic species present in the Bay of Biscay.
Therefore, the objective of this study is to contribute allometric information about pelagic fish, prey sizes, and predator-prey ratios in the Bay of Biscay.
Material and methods
Small pelagic fish were caught with pelagic trawls in 2008 and 2009 during four different oceanographic surveys and in different sampling areas around the Bay of Biscay (Figure 1). Samples of European anchovy (Engraulis encrasicolus), sardine (Sardina pilchardus), Atlantic horse mackerel (Trachurus trachurus), Mediterranean horse mackerel (Trachurus mediterraneus), Atlantic mackerel (Scomber scombrus), Atlantic Chub mackerel (Scomber colias), bogue (Boops boops) and sprat (Sprattus sprattus) were caught and immediately frozen on board for later biological sampling in the laboratory.
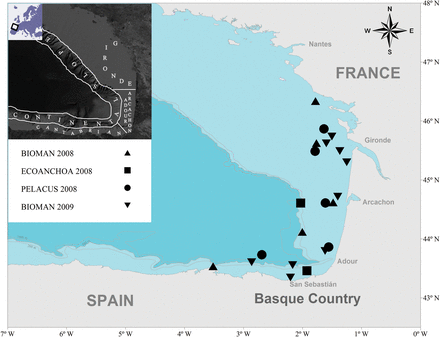
Study area and location of samples used in the study in relation to the survey. Predefined geographical areas (Cotano et al., 2008) are shown in the small map in the top left of the figure. Pelagic trawl was used to catch fish samples during BIOMAN 2008 (May 6–May 26) and BIOMAN 2009 (May 5–May 25) surveys aboard the R/V Emma Bardán (Vigo) and aboard the R/V Thalassa (IFREMER) during the PELACUS 2008 survey (Sept 17–Oct 16). Purse seine was used to catch samples during ECOANCHOA 2008 survey (June 27–July 13) aboard the F/V Ama Antiguakoa (Ondarroa).
Morphological measurements of gape height (GH) and gape width (GW) were obtained with a calliper; according to Scharf et al. (2000), gape height was defined as the maximum linear distance between the upper and lower jaws with the mouth stretched open, and gape width as the linear distance between the left and right corners of the open mouth. Total length (using an ichtyometer model SCANTROL FishMeter 100) and weight (using an electronic balance model SCANVAEGT Marine Scale 8400 Series) were measured on board, and stomach weight was measured later in the laboratory (with a balance model METTLER TOLEDO PB1502). Collected stomachs (Figure 1) were preserved in ph7 buffered formaldehyde (4%), according to Harris et al. (2000).
Diet characterization
Prey size
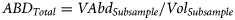
The total length of the first whole 50 individuals was measured in each stomach with an upper limit of 30 measurements per prey species and stomach; i.e. when the first 30 individuals corresponded to the same species, we continued measuring the rest of the prey species in order to obtain a total of 50 prey length measurements per stomach. Broken zooplankton was not measured.
Prey length (TLPrey) was determined by the average length obtained from measurements in each of the prey species and predator species (i.e. one TLPrey mean value per prey species or taxonomic group and fish species).
We tested whether prey lengths varied significantly depending on survey, area or predator species. Areas were defined according to Figure 1 (Cotano et al., 2008): the “Cantabrian area” is the area within the Cantabrian continental shelf, the “Adour-Arcachon area” is the area within the French continental shelf under the influence of the Adour River and the Arcachon estuary, the “Gironde area” is the area within the French continental shelf that is under the influence of the Gironde River input, and the “Continental Slope area” is the area that delimitates the shelf break (>250 m depth).
Since the data on prey length averages were not normally distributed (Shapiro-Wilkinson normality test, p values >0.05), multiple range (homogeneous groups) and Kruskal-Wallis tests were performed in order to test differences in prey lengths.
Predator-prey ratio
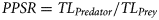
Trophic-niche breadth
Trophic-niche breadth was examined on a ratio scale by determining changes in the range of relative prey sizes with increasing predator size (Scharf et al., 2000). PPSRs versus predator size regression quantiles (90th and 10th) were generated to estimate the extremes of the ratio scale data for each predator species. Slope comparisons were made between upper and lower bounds, with significant differences indicating an increase (divergent slopes) or decrease (convergent slopes) in PPSR-based trophic-niche breadth with increasing predator size. The difference between predicted values of upper and lower bound regressions at any given predator size represented the trophic-niche breadth (Scharf et al., 2000).
Results
Allometric relationships
Total length and weight
In order to simplify comparisons between predator fish species, we established 130 and 230 mm as the limits for separating small, medium and large individuals. Therefore, all species would be represented in at least two of the three size ranges and a balanced n would be obtained for all ranges. All sampled species showed a significant length–weight relationship. Descriptive statistics, the sample size and the length–weight relationship parameters are presented in Table 1. The length–weight relationships of the different species were significantly different (F-tests for both the slopes and intercepts, p < 0.0001). Anchovy and sprat had the highest slopes, and the difference remained significant when the other fish data used were restricted to the maximum length of anchovy and sprat.
Sample size (ranged by size), descriptive statistics and weight-length relationship parameters for the eight small pelagic fish species sampled.
| . | Sample size (n) . | Length (mm) . | Weight (g) . | Regression parameters . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Species . | <130mm . | 130–230mm . | >230mm . | Min . | Max . | Min . | Max . | a . | b . | s.e. (b) . | r2 . |
| E. encrasicolus | 246 | 359 | – | 65 | 194 | 1.22 | 68.7 | 8.47 (10−7) | 3.42 | 18 (10−3) | 0.98 |
| S. pilchardus | 48 | 450 | 28 | 100 | 247 | 5.9 | 119.6 | 3.97 (10−6) | 3.13 | 22 (10−3) | 0.98 |
| T. trachurus | 343 | 176 | 179 | 45 | 370 | 0.77 | 376.2 | 5.74 (10−6) | 3.06 | 8 (10−3) | 0.99 |
| T. mediterraneus | 137 | 85 | 62 | 69 | 391 | 2.37 | 420 | 6.22 (10−6) | 3.04 | 13 (10−3) | 0.99 |
| S. scombrus | 8 | 241 | 150 | 108 | 420 | 7.9 | 551.1 | 4.80 (10−6) | 3.07 | 15 (10−3) | 0.99 |
| S. colias | – | 33 | 64 | 137 | 405 | 16.58 | 660.7 | 1.49 (10−6) | 3.30 | 27 (10−3) | 0.99 |
| B. boops | 2 | 58 | 69 | 122 | 360 | 17 | 453.4 | 7.25 (10−6) | 3.05 | 26 (10−3) | 0.99 |
| S. sprattus | 161 | 4 | – | 50 | 143 | 0.56 | 23.6 | 2.71 (10−7) | 3.75 | 43 (10−3) | 0.98 |
| . | Sample size (n) . | Length (mm) . | Weight (g) . | Regression parameters . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Species . | <130mm . | 130–230mm . | >230mm . | Min . | Max . | Min . | Max . | a . | b . | s.e. (b) . | r2 . |
| E. encrasicolus | 246 | 359 | – | 65 | 194 | 1.22 | 68.7 | 8.47 (10−7) | 3.42 | 18 (10−3) | 0.98 |
| S. pilchardus | 48 | 450 | 28 | 100 | 247 | 5.9 | 119.6 | 3.97 (10−6) | 3.13 | 22 (10−3) | 0.98 |
| T. trachurus | 343 | 176 | 179 | 45 | 370 | 0.77 | 376.2 | 5.74 (10−6) | 3.06 | 8 (10−3) | 0.99 |
| T. mediterraneus | 137 | 85 | 62 | 69 | 391 | 2.37 | 420 | 6.22 (10−6) | 3.04 | 13 (10−3) | 0.99 |
| S. scombrus | 8 | 241 | 150 | 108 | 420 | 7.9 | 551.1 | 4.80 (10−6) | 3.07 | 15 (10−3) | 0.99 |
| S. colias | – | 33 | 64 | 137 | 405 | 16.58 | 660.7 | 1.49 (10−6) | 3.30 | 27 (10−3) | 0.99 |
| B. boops | 2 | 58 | 69 | 122 | 360 | 17 | 453.4 | 7.25 (10−6) | 3.05 | 26 (10−3) | 0.99 |
| S. sprattus | 161 | 4 | – | 50 | 143 | 0.56 | 23.6 | 2.71 (10−7) | 3.75 | 43 (10−3) | 0.98 |
n = sample size corresponding to each of the predefined length ranges, Min and Max = minimum and maximum length (mm) and weight (g) recorded, a and b = parameters of the weight–length relationship TW = a TLb, s.e. (b) = standard error of b, r2 =coefficient of determination.
Sample size (ranged by size), descriptive statistics and weight-length relationship parameters for the eight small pelagic fish species sampled.
| . | Sample size (n) . | Length (mm) . | Weight (g) . | Regression parameters . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Species . | <130mm . | 130–230mm . | >230mm . | Min . | Max . | Min . | Max . | a . | b . | s.e. (b) . | r2 . |
| E. encrasicolus | 246 | 359 | – | 65 | 194 | 1.22 | 68.7 | 8.47 (10−7) | 3.42 | 18 (10−3) | 0.98 |
| S. pilchardus | 48 | 450 | 28 | 100 | 247 | 5.9 | 119.6 | 3.97 (10−6) | 3.13 | 22 (10−3) | 0.98 |
| T. trachurus | 343 | 176 | 179 | 45 | 370 | 0.77 | 376.2 | 5.74 (10−6) | 3.06 | 8 (10−3) | 0.99 |
| T. mediterraneus | 137 | 85 | 62 | 69 | 391 | 2.37 | 420 | 6.22 (10−6) | 3.04 | 13 (10−3) | 0.99 |
| S. scombrus | 8 | 241 | 150 | 108 | 420 | 7.9 | 551.1 | 4.80 (10−6) | 3.07 | 15 (10−3) | 0.99 |
| S. colias | – | 33 | 64 | 137 | 405 | 16.58 | 660.7 | 1.49 (10−6) | 3.30 | 27 (10−3) | 0.99 |
| B. boops | 2 | 58 | 69 | 122 | 360 | 17 | 453.4 | 7.25 (10−6) | 3.05 | 26 (10−3) | 0.99 |
| S. sprattus | 161 | 4 | – | 50 | 143 | 0.56 | 23.6 | 2.71 (10−7) | 3.75 | 43 (10−3) | 0.98 |
| . | Sample size (n) . | Length (mm) . | Weight (g) . | Regression parameters . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Species . | <130mm . | 130–230mm . | >230mm . | Min . | Max . | Min . | Max . | a . | b . | s.e. (b) . | r2 . |
| E. encrasicolus | 246 | 359 | – | 65 | 194 | 1.22 | 68.7 | 8.47 (10−7) | 3.42 | 18 (10−3) | 0.98 |
| S. pilchardus | 48 | 450 | 28 | 100 | 247 | 5.9 | 119.6 | 3.97 (10−6) | 3.13 | 22 (10−3) | 0.98 |
| T. trachurus | 343 | 176 | 179 | 45 | 370 | 0.77 | 376.2 | 5.74 (10−6) | 3.06 | 8 (10−3) | 0.99 |
| T. mediterraneus | 137 | 85 | 62 | 69 | 391 | 2.37 | 420 | 6.22 (10−6) | 3.04 | 13 (10−3) | 0.99 |
| S. scombrus | 8 | 241 | 150 | 108 | 420 | 7.9 | 551.1 | 4.80 (10−6) | 3.07 | 15 (10−3) | 0.99 |
| S. colias | – | 33 | 64 | 137 | 405 | 16.58 | 660.7 | 1.49 (10−6) | 3.30 | 27 (10−3) | 0.99 |
| B. boops | 2 | 58 | 69 | 122 | 360 | 17 | 453.4 | 7.25 (10−6) | 3.05 | 26 (10−3) | 0.99 |
| S. sprattus | 161 | 4 | – | 50 | 143 | 0.56 | 23.6 | 2.71 (10−7) | 3.75 | 43 (10−3) | 0.98 |
n = sample size corresponding to each of the predefined length ranges, Min and Max = minimum and maximum length (mm) and weight (g) recorded, a and b = parameters of the weight–length relationship TW = a TLb, s.e. (b) = standard error of b, r2 =coefficient of determination.
Gape size vs. total length
The gape height and width were found to be related to the total length; however, this relationship differed significantly (F-tests for slopes, p < 0.0005 for gape height and p < 0.0001 for gape width; F-tests for intercepts, p < 0.0001 both for gape height and width) depending on the predator species (Table 2): all sampled predator species showed a similar polynomial increase in gape size with total length, except anchovies and bogues. Anchovies had the largest gape size compared with the other species at the same size. Bogues had the smallest gape height and width (Table 2).
Allometric relationships between the gape size (mm) and total length of fish (mm) for the eight small pelagic fish species sampled.
| . | . | Gape width (GW) . | Gape height (GH) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Species . | n . | a . | b . | c . | s.e. (c) . | r2 . | a . | b . | c . | s.e. (c) . | r2 . |
| E. encrasicolus | 118 | –1.25 (10−3) | 0.57 | –29.77 | 4.81 | 0.94 | –8.90 (10−4) | 0.43 | –16.84 | 4.72 | 0.93 |
| S. pilchardus | 98 | –2.66 (10−4) | 0.20 | –6.42 | 4.50 | 0.85 | –1.61 (10−4) | 0.17 | –3.34 | 2.37 | 0.96 |
| T. trachurus | 129 | –3.36 (10−4) | 0.27 | –12.17 | 1.67 | 0.98 | –3.01 (10−4) | 0.25 | –9.56 | 1.88 | 0.97 |
| T. mediterraneus | 32 | –3.22 (10−4) | 0.20 | –2.43 | 5.28 | 0.91 | –3.90 (10−4) | 0.22 | –3.66 | 4.84 | 0.92 |
| S. scombrus | 70 | –3.43 (10−4) | 0.31 | –24.43 | 5.41 | 0.94 | –2.59 (10−4) | 0.26 | –16.33 | 5.29 | 0.94 |
| S. colias | 9 | –1.12 (10−4) | 0.18 | –4.73 | 77.48 | 0.93 | –9.26 (10−4) | 0.70 | –81.77 | 41.62 | 0.95 |
| B. boops | 22 | –9.35 (10−5) | 0.08 | –0.99 | 7.48 | 0.87 | –2.12 (10−4) | 0.13 | –4.87 | 7.90 | 0.90 |
| S. sprattus | 9 | –3.3 (10−3) | 0.75 | –32.01 | 38.90 | 0.74 | –1.05 (10−3) | 0.31 | –10.14 | 11.15 | 0.86 |
| . | . | Gape width (GW) . | Gape height (GH) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Species . | n . | a . | b . | c . | s.e. (c) . | r2 . | a . | b . | c . | s.e. (c) . | r2 . |
| E. encrasicolus | 118 | –1.25 (10−3) | 0.57 | –29.77 | 4.81 | 0.94 | –8.90 (10−4) | 0.43 | –16.84 | 4.72 | 0.93 |
| S. pilchardus | 98 | –2.66 (10−4) | 0.20 | –6.42 | 4.50 | 0.85 | –1.61 (10−4) | 0.17 | –3.34 | 2.37 | 0.96 |
| T. trachurus | 129 | –3.36 (10−4) | 0.27 | –12.17 | 1.67 | 0.98 | –3.01 (10−4) | 0.25 | –9.56 | 1.88 | 0.97 |
| T. mediterraneus | 32 | –3.22 (10−4) | 0.20 | –2.43 | 5.28 | 0.91 | –3.90 (10−4) | 0.22 | –3.66 | 4.84 | 0.92 |
| S. scombrus | 70 | –3.43 (10−4) | 0.31 | –24.43 | 5.41 | 0.94 | –2.59 (10−4) | 0.26 | –16.33 | 5.29 | 0.94 |
| S. colias | 9 | –1.12 (10−4) | 0.18 | –4.73 | 77.48 | 0.93 | –9.26 (10−4) | 0.70 | –81.77 | 41.62 | 0.95 |
| B. boops | 22 | –9.35 (10−5) | 0.08 | –0.99 | 7.48 | 0.87 | –2.12 (10−4) | 0.13 | –4.87 | 7.90 | 0.90 |
| S. sprattus | 9 | –3.3 (10−3) | 0.75 | –32.01 | 38.90 | 0.74 | –1.05 (10−3) | 0.31 | –10.14 | 11.15 | 0.86 |
n = sample size, a and b = parameters of the TL–gape size relationship GH or GW = aTL2+ bTL + c; s.e. (c) = standard error of constant c; r2= coefficient of determination.
Allometric relationships between the gape size (mm) and total length of fish (mm) for the eight small pelagic fish species sampled.
| . | . | Gape width (GW) . | Gape height (GH) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Species . | n . | a . | b . | c . | s.e. (c) . | r2 . | a . | b . | c . | s.e. (c) . | r2 . |
| E. encrasicolus | 118 | –1.25 (10−3) | 0.57 | –29.77 | 4.81 | 0.94 | –8.90 (10−4) | 0.43 | –16.84 | 4.72 | 0.93 |
| S. pilchardus | 98 | –2.66 (10−4) | 0.20 | –6.42 | 4.50 | 0.85 | –1.61 (10−4) | 0.17 | –3.34 | 2.37 | 0.96 |
| T. trachurus | 129 | –3.36 (10−4) | 0.27 | –12.17 | 1.67 | 0.98 | –3.01 (10−4) | 0.25 | –9.56 | 1.88 | 0.97 |
| T. mediterraneus | 32 | –3.22 (10−4) | 0.20 | –2.43 | 5.28 | 0.91 | –3.90 (10−4) | 0.22 | –3.66 | 4.84 | 0.92 |
| S. scombrus | 70 | –3.43 (10−4) | 0.31 | –24.43 | 5.41 | 0.94 | –2.59 (10−4) | 0.26 | –16.33 | 5.29 | 0.94 |
| S. colias | 9 | –1.12 (10−4) | 0.18 | –4.73 | 77.48 | 0.93 | –9.26 (10−4) | 0.70 | –81.77 | 41.62 | 0.95 |
| B. boops | 22 | –9.35 (10−5) | 0.08 | –0.99 | 7.48 | 0.87 | –2.12 (10−4) | 0.13 | –4.87 | 7.90 | 0.90 |
| S. sprattus | 9 | –3.3 (10−3) | 0.75 | –32.01 | 38.90 | 0.74 | –1.05 (10−3) | 0.31 | –10.14 | 11.15 | 0.86 |
| . | . | Gape width (GW) . | Gape height (GH) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Species . | n . | a . | b . | c . | s.e. (c) . | r2 . | a . | b . | c . | s.e. (c) . | r2 . |
| E. encrasicolus | 118 | –1.25 (10−3) | 0.57 | –29.77 | 4.81 | 0.94 | –8.90 (10−4) | 0.43 | –16.84 | 4.72 | 0.93 |
| S. pilchardus | 98 | –2.66 (10−4) | 0.20 | –6.42 | 4.50 | 0.85 | –1.61 (10−4) | 0.17 | –3.34 | 2.37 | 0.96 |
| T. trachurus | 129 | –3.36 (10−4) | 0.27 | –12.17 | 1.67 | 0.98 | –3.01 (10−4) | 0.25 | –9.56 | 1.88 | 0.97 |
| T. mediterraneus | 32 | –3.22 (10−4) | 0.20 | –2.43 | 5.28 | 0.91 | –3.90 (10−4) | 0.22 | –3.66 | 4.84 | 0.92 |
| S. scombrus | 70 | –3.43 (10−4) | 0.31 | –24.43 | 5.41 | 0.94 | –2.59 (10−4) | 0.26 | –16.33 | 5.29 | 0.94 |
| S. colias | 9 | –1.12 (10−4) | 0.18 | –4.73 | 77.48 | 0.93 | –9.26 (10−4) | 0.70 | –81.77 | 41.62 | 0.95 |
| B. boops | 22 | –9.35 (10−5) | 0.08 | –0.99 | 7.48 | 0.87 | –2.12 (10−4) | 0.13 | –4.87 | 7.90 | 0.90 |
| S. sprattus | 9 | –3.3 (10−3) | 0.75 | –32.01 | 38.90 | 0.74 | –1.05 (10−3) | 0.31 | –10.14 | 11.15 | 0.86 |
n = sample size, a and b = parameters of the TL–gape size relationship GH or GW = aTL2+ bTL + c; s.e. (c) = standard error of constant c; r2= coefficient of determination.
Stomach contents
Stomach weight
Stomach weights (SW) were related to the total weight (TW) of fish (Table 3); however, slopes and intercepts of that relationship differed significantly between predator species (F-tests, p < 0.0001). For the small sizes, the data from different species overlapped. However, Atlantic and Atlantic Chub mackerels, which are larger fish, had heavier stomach weights than Atlantic and Mediterranean horse mackerels of the same size.
Descriptive statistics and the total weight (TW)–stomach weight (SW) relationship parameters for the eight small pelagic fish species sampled.
| . | . | Total weight (g) . | Stomach weight (g) . | Regression parameters . | |||||
|---|---|---|---|---|---|---|---|---|---|
| Species . | n . | Min . | Max . | Min . | Max . | a . | b . | s.e. (b) . | r2 . |
| E. encrasicolus | 533 | 1.22 | 68.70 | 0.10 | 4.70 | 24 (10−3) | -41 (10−3) | 2 (10−3) | 0.64 |
| S. pilchardus | 497 | 5.90 | 119.60 | 0.01 | 4.30 | 19 (10−3) | 113 (10−3) | 1 (10−3) | 0.58 |
| T. trachurus | 659 | 0.77 | 376.20 | 0.10 | 5.20 | 9 (10−3) | 117 (10−3) | 2 (10−4) | 0.78 |
| T. mediterraneus | 267 | 2.37 | 420.00 | 0.02 | 5.90 | 9 (10−3) | 79 (10−3) | 2 (10−4) | 0.84 |
| S. scombrus | 342 | 7.90 | 551.10 | 0.30 | 13.60 | 14 (10−3) | 662 (10−3) | 1 (10−3) | 0.71 |
| S. colias | 80 | 16.58 | 660.70 | 0.40 | 16.80 | 15 (10−3) | 531 (10−3) | 2 (10−3) | 0.65 |
| B. boops | 108 | 17.00 | 453.40 | 0.10 | 16.20 | 26 (10−3) | -333 (10−3) | 2 (10−3) | 0.81 |
| S. sprattus | 155 | 0.56 | 23.60 | 0.01 | 0.80 | 17 (10−3) | 13 (10−3) | 2 (10−3) | 0.50 |
| . | . | Total weight (g) . | Stomach weight (g) . | Regression parameters . | |||||
|---|---|---|---|---|---|---|---|---|---|
| Species . | n . | Min . | Max . | Min . | Max . | a . | b . | s.e. (b) . | r2 . |
| E. encrasicolus | 533 | 1.22 | 68.70 | 0.10 | 4.70 | 24 (10−3) | -41 (10−3) | 2 (10−3) | 0.64 |
| S. pilchardus | 497 | 5.90 | 119.60 | 0.01 | 4.30 | 19 (10−3) | 113 (10−3) | 1 (10−3) | 0.58 |
| T. trachurus | 659 | 0.77 | 376.20 | 0.10 | 5.20 | 9 (10−3) | 117 (10−3) | 2 (10−4) | 0.78 |
| T. mediterraneus | 267 | 2.37 | 420.00 | 0.02 | 5.90 | 9 (10−3) | 79 (10−3) | 2 (10−4) | 0.84 |
| S. scombrus | 342 | 7.90 | 551.10 | 0.30 | 13.60 | 14 (10−3) | 662 (10−3) | 1 (10−3) | 0.71 |
| S. colias | 80 | 16.58 | 660.70 | 0.40 | 16.80 | 15 (10−3) | 531 (10−3) | 2 (10−3) | 0.65 |
| B. boops | 108 | 17.00 | 453.40 | 0.10 | 16.20 | 26 (10−3) | -333 (10−3) | 2 (10−3) | 0.81 |
| S. sprattus | 155 | 0.56 | 23.60 | 0.01 | 0.80 | 17 (10−3) | 13 (10−3) | 2 (10−3) | 0.50 |
n = sample size, Min and Max = minimum and maximum total weight and stomach weight (g) recorded, a and b = parameters of the TW–SW relationship SW = aTW + b, s.e. (b) = standard error of b, r2 is the coefficient of determination. Note that the linear regression parameters are for the log-transformed data.
Descriptive statistics and the total weight (TW)–stomach weight (SW) relationship parameters for the eight small pelagic fish species sampled.
| . | . | Total weight (g) . | Stomach weight (g) . | Regression parameters . | |||||
|---|---|---|---|---|---|---|---|---|---|
| Species . | n . | Min . | Max . | Min . | Max . | a . | b . | s.e. (b) . | r2 . |
| E. encrasicolus | 533 | 1.22 | 68.70 | 0.10 | 4.70 | 24 (10−3) | -41 (10−3) | 2 (10−3) | 0.64 |
| S. pilchardus | 497 | 5.90 | 119.60 | 0.01 | 4.30 | 19 (10−3) | 113 (10−3) | 1 (10−3) | 0.58 |
| T. trachurus | 659 | 0.77 | 376.20 | 0.10 | 5.20 | 9 (10−3) | 117 (10−3) | 2 (10−4) | 0.78 |
| T. mediterraneus | 267 | 2.37 | 420.00 | 0.02 | 5.90 | 9 (10−3) | 79 (10−3) | 2 (10−4) | 0.84 |
| S. scombrus | 342 | 7.90 | 551.10 | 0.30 | 13.60 | 14 (10−3) | 662 (10−3) | 1 (10−3) | 0.71 |
| S. colias | 80 | 16.58 | 660.70 | 0.40 | 16.80 | 15 (10−3) | 531 (10−3) | 2 (10−3) | 0.65 |
| B. boops | 108 | 17.00 | 453.40 | 0.10 | 16.20 | 26 (10−3) | -333 (10−3) | 2 (10−3) | 0.81 |
| S. sprattus | 155 | 0.56 | 23.60 | 0.01 | 0.80 | 17 (10−3) | 13 (10−3) | 2 (10−3) | 0.50 |
| . | . | Total weight (g) . | Stomach weight (g) . | Regression parameters . | |||||
|---|---|---|---|---|---|---|---|---|---|
| Species . | n . | Min . | Max . | Min . | Max . | a . | b . | s.e. (b) . | r2 . |
| E. encrasicolus | 533 | 1.22 | 68.70 | 0.10 | 4.70 | 24 (10−3) | -41 (10−3) | 2 (10−3) | 0.64 |
| S. pilchardus | 497 | 5.90 | 119.60 | 0.01 | 4.30 | 19 (10−3) | 113 (10−3) | 1 (10−3) | 0.58 |
| T. trachurus | 659 | 0.77 | 376.20 | 0.10 | 5.20 | 9 (10−3) | 117 (10−3) | 2 (10−4) | 0.78 |
| T. mediterraneus | 267 | 2.37 | 420.00 | 0.02 | 5.90 | 9 (10−3) | 79 (10−3) | 2 (10−4) | 0.84 |
| S. scombrus | 342 | 7.90 | 551.10 | 0.30 | 13.60 | 14 (10−3) | 662 (10−3) | 1 (10−3) | 0.71 |
| S. colias | 80 | 16.58 | 660.70 | 0.40 | 16.80 | 15 (10−3) | 531 (10−3) | 2 (10−3) | 0.65 |
| B. boops | 108 | 17.00 | 453.40 | 0.10 | 16.20 | 26 (10−3) | -333 (10−3) | 2 (10−3) | 0.81 |
| S. sprattus | 155 | 0.56 | 23.60 | 0.01 | 0.80 | 17 (10−3) | 13 (10−3) | 2 (10−3) | 0.50 |
n = sample size, Min and Max = minimum and maximum total weight and stomach weight (g) recorded, a and b = parameters of the TW–SW relationship SW = aTW + b, s.e. (b) = standard error of b, r2 is the coefficient of determination. Note that the linear regression parameters are for the log-transformed data.
Prey size
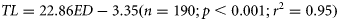
The median prey size (i.e. total length) was not significantly different between surveys (Kruskal-Wallis test, p = 0.75) or geographical areas (Kruskal-Wallis test, p = 0.52). In contrast, significant differences were found between predator species (Kruskal-Wallis test, p = 0.0002).
Since the lack of enough detail for prey is often cited as a problem (e.g. Barnes et al., 2008, 2010), the average and extreme prey-size values for the different predator species ranged by size (i.e. TL < 130 mm, TL 130–230 mm, TL >230 mm) are presented as supplementary data (Table A1).
While large predators (e.g. Atlantic mackerels) were able to predate on the largest prey, they also used small zooplankton as a resource. Likewise, small predators (e.g. anchovies) could also prey on many large particles as they had a relatively wide prey size spectrum. These results were also observed when predator size was compared with the maximum and minimum prey sizes. For example, the prey size range of anchovies was nearly as wide as that of Atlantic and Atlantic Chub mackerels, in spite of being much smaller predators, whereas predators like sardines and bogues showed a narrow prey-size range. Atlantic and Atlantic Chub mackerels of all sizes were also able to predate on large prey. Descriptive statistics are presented in Table 4.
Average minimum, mean and maximum prey lengths (TL) obtained in stomach contents of different predators according to size, as well as for the entire size range (“ALL”).
| Predator species . | npredators . | nprey . | Prey Average Min. TL (mm) . | Prey Average TL (mm) . | Prey Average Max. TL (mm) . | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <130 . | 130–230 . | >230 . | ALL . | <130 . | 130–230 . | >230 . | ALL . | <130 . | 130–230 . | >230 . | ALL . | <130 . | 130–230 . | >230 . | ALL . | <130 . | 130–230 . | >230 . | ALL . | |
| E. encrasicolus | 49 | 101 | 0 | 150 | 5257 | 27 774 | 0 | 33031 | 0.89 | 0.55 | 0.63 | 1.17 | 1.89 | 1.73 | 2.25 | 7.61 | 6.41 | |||
| S. pilchardus | 6 | 114 | 10 | 130 | 1916 | 77 071 | 11216 | 90203 | 0.35 | 0.37 | 0.41 | 0.38 | 0.92 | 0.99 | 1.29 | 1.02 | 1.55 | 2.54 | 5.05 | 2.75 |
| T. trachurus | 64 | 65 | 43 | 172 | 2108 | 14 233 | 919 | 17260 | 0.88 | 0.89 | 1.48 | 1.01 | 1.60 | 1.99 | 4.34 | 2.34 | 3.49 | 4.26 | 11.18 | 5.43 |
| T. mediterraneus | 26 | 0 | 25 | 51 | 4163 | 0 | 72 | 4235 | 0.96 | 0.91 | 0.94 | 1.87 | 1.35 | 1.71 | 14.36 | 1.56 | 10.43 | |||
| S. scombrus | 3 | 41 | 101 | 145 | 20 | 1107 | 449 988 | 451115 | 1.00 | 2.37 | 9.05 | 7.29 | 8.66 | 4.71 | 13.41 | 11.18 | 33.12 | 13.65 | 22.89 | 20.69 |
| S. colias | 0 | 15 | 14 | 29 | 0 | 1818 | 325 | 2143 | 0.36 | 0.61 | 0.47 | 1.82 | 5.24 | 3.32 | 6.94 | 34.26 | 18.96 | |||
| B. boops | 0 | 21 | 32 | 53 | 0 | 164 | 2456 | 2620 | 0.80 | 0.62 | 0.64 | 1.08 | 1.46 | 1.42 | 2.55 | 7.05 | 6.60 | |||
| S. sprattus | 56 | 4 | 0 | 60 | 18468 | 719 | 0 | 19187 | 0.52 | 0.44 | 0.50 | 1.42 | 1.02 | 1.33 | 3.02 | 1.86 | 2.78 | |||
| Predator species . | npredators . | nprey . | Prey Average Min. TL (mm) . | Prey Average TL (mm) . | Prey Average Max. TL (mm) . | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <130 . | 130–230 . | >230 . | ALL . | <130 . | 130–230 . | >230 . | ALL . | <130 . | 130–230 . | >230 . | ALL . | <130 . | 130–230 . | >230 . | ALL . | <130 . | 130–230 . | >230 . | ALL . | |
| E. encrasicolus | 49 | 101 | 0 | 150 | 5257 | 27 774 | 0 | 33031 | 0.89 | 0.55 | 0.63 | 1.17 | 1.89 | 1.73 | 2.25 | 7.61 | 6.41 | |||
| S. pilchardus | 6 | 114 | 10 | 130 | 1916 | 77 071 | 11216 | 90203 | 0.35 | 0.37 | 0.41 | 0.38 | 0.92 | 0.99 | 1.29 | 1.02 | 1.55 | 2.54 | 5.05 | 2.75 |
| T. trachurus | 64 | 65 | 43 | 172 | 2108 | 14 233 | 919 | 17260 | 0.88 | 0.89 | 1.48 | 1.01 | 1.60 | 1.99 | 4.34 | 2.34 | 3.49 | 4.26 | 11.18 | 5.43 |
| T. mediterraneus | 26 | 0 | 25 | 51 | 4163 | 0 | 72 | 4235 | 0.96 | 0.91 | 0.94 | 1.87 | 1.35 | 1.71 | 14.36 | 1.56 | 10.43 | |||
| S. scombrus | 3 | 41 | 101 | 145 | 20 | 1107 | 449 988 | 451115 | 1.00 | 2.37 | 9.05 | 7.29 | 8.66 | 4.71 | 13.41 | 11.18 | 33.12 | 13.65 | 22.89 | 20.69 |
| S. colias | 0 | 15 | 14 | 29 | 0 | 1818 | 325 | 2143 | 0.36 | 0.61 | 0.47 | 1.82 | 5.24 | 3.32 | 6.94 | 34.26 | 18.96 | |||
| B. boops | 0 | 21 | 32 | 53 | 0 | 164 | 2456 | 2620 | 0.80 | 0.62 | 0.64 | 1.08 | 1.46 | 1.42 | 2.55 | 7.05 | 6.60 | |||
| S. sprattus | 56 | 4 | 0 | 60 | 18468 | 719 | 0 | 19187 | 0.52 | 0.44 | 0.50 | 1.42 | 1.02 | 1.33 | 3.02 | 1.86 | 2.78 | |||
n = sample size. The prey number (nprey) has been extrapolated to the total number estimated according to the subsampling ratio.
Average minimum, mean and maximum prey lengths (TL) obtained in stomach contents of different predators according to size, as well as for the entire size range (“ALL”).
| Predator species . | npredators . | nprey . | Prey Average Min. TL (mm) . | Prey Average TL (mm) . | Prey Average Max. TL (mm) . | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <130 . | 130–230 . | >230 . | ALL . | <130 . | 130–230 . | >230 . | ALL . | <130 . | 130–230 . | >230 . | ALL . | <130 . | 130–230 . | >230 . | ALL . | <130 . | 130–230 . | >230 . | ALL . | |
| E. encrasicolus | 49 | 101 | 0 | 150 | 5257 | 27 774 | 0 | 33031 | 0.89 | 0.55 | 0.63 | 1.17 | 1.89 | 1.73 | 2.25 | 7.61 | 6.41 | |||
| S. pilchardus | 6 | 114 | 10 | 130 | 1916 | 77 071 | 11216 | 90203 | 0.35 | 0.37 | 0.41 | 0.38 | 0.92 | 0.99 | 1.29 | 1.02 | 1.55 | 2.54 | 5.05 | 2.75 |
| T. trachurus | 64 | 65 | 43 | 172 | 2108 | 14 233 | 919 | 17260 | 0.88 | 0.89 | 1.48 | 1.01 | 1.60 | 1.99 | 4.34 | 2.34 | 3.49 | 4.26 | 11.18 | 5.43 |
| T. mediterraneus | 26 | 0 | 25 | 51 | 4163 | 0 | 72 | 4235 | 0.96 | 0.91 | 0.94 | 1.87 | 1.35 | 1.71 | 14.36 | 1.56 | 10.43 | |||
| S. scombrus | 3 | 41 | 101 | 145 | 20 | 1107 | 449 988 | 451115 | 1.00 | 2.37 | 9.05 | 7.29 | 8.66 | 4.71 | 13.41 | 11.18 | 33.12 | 13.65 | 22.89 | 20.69 |
| S. colias | 0 | 15 | 14 | 29 | 0 | 1818 | 325 | 2143 | 0.36 | 0.61 | 0.47 | 1.82 | 5.24 | 3.32 | 6.94 | 34.26 | 18.96 | |||
| B. boops | 0 | 21 | 32 | 53 | 0 | 164 | 2456 | 2620 | 0.80 | 0.62 | 0.64 | 1.08 | 1.46 | 1.42 | 2.55 | 7.05 | 6.60 | |||
| S. sprattus | 56 | 4 | 0 | 60 | 18468 | 719 | 0 | 19187 | 0.52 | 0.44 | 0.50 | 1.42 | 1.02 | 1.33 | 3.02 | 1.86 | 2.78 | |||
| Predator species . | npredators . | nprey . | Prey Average Min. TL (mm) . | Prey Average TL (mm) . | Prey Average Max. TL (mm) . | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <130 . | 130–230 . | >230 . | ALL . | <130 . | 130–230 . | >230 . | ALL . | <130 . | 130–230 . | >230 . | ALL . | <130 . | 130–230 . | >230 . | ALL . | <130 . | 130–230 . | >230 . | ALL . | |
| E. encrasicolus | 49 | 101 | 0 | 150 | 5257 | 27 774 | 0 | 33031 | 0.89 | 0.55 | 0.63 | 1.17 | 1.89 | 1.73 | 2.25 | 7.61 | 6.41 | |||
| S. pilchardus | 6 | 114 | 10 | 130 | 1916 | 77 071 | 11216 | 90203 | 0.35 | 0.37 | 0.41 | 0.38 | 0.92 | 0.99 | 1.29 | 1.02 | 1.55 | 2.54 | 5.05 | 2.75 |
| T. trachurus | 64 | 65 | 43 | 172 | 2108 | 14 233 | 919 | 17260 | 0.88 | 0.89 | 1.48 | 1.01 | 1.60 | 1.99 | 4.34 | 2.34 | 3.49 | 4.26 | 11.18 | 5.43 |
| T. mediterraneus | 26 | 0 | 25 | 51 | 4163 | 0 | 72 | 4235 | 0.96 | 0.91 | 0.94 | 1.87 | 1.35 | 1.71 | 14.36 | 1.56 | 10.43 | |||
| S. scombrus | 3 | 41 | 101 | 145 | 20 | 1107 | 449 988 | 451115 | 1.00 | 2.37 | 9.05 | 7.29 | 8.66 | 4.71 | 13.41 | 11.18 | 33.12 | 13.65 | 22.89 | 20.69 |
| S. colias | 0 | 15 | 14 | 29 | 0 | 1818 | 325 | 2143 | 0.36 | 0.61 | 0.47 | 1.82 | 5.24 | 3.32 | 6.94 | 34.26 | 18.96 | |||
| B. boops | 0 | 21 | 32 | 53 | 0 | 164 | 2456 | 2620 | 0.80 | 0.62 | 0.64 | 1.08 | 1.46 | 1.42 | 2.55 | 7.05 | 6.60 | |||
| S. sprattus | 56 | 4 | 0 | 60 | 18468 | 719 | 0 | 19187 | 0.52 | 0.44 | 0.50 | 1.42 | 1.02 | 1.33 | 3.02 | 1.86 | 2.78 | |||
n = sample size. The prey number (nprey) has been extrapolated to the total number estimated according to the subsampling ratio.
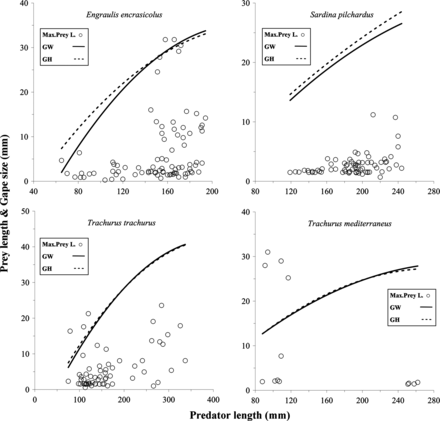
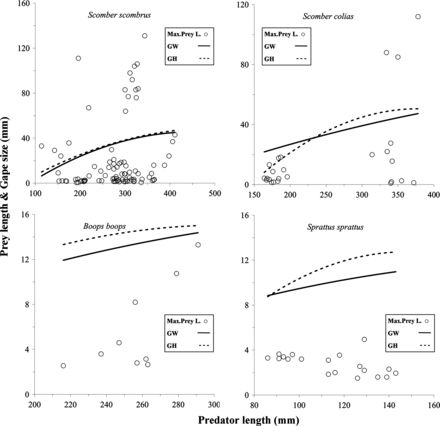
The maximum prey length increased with predator body size. In addition, the maximum prey size measurements were found in predators with large gape size in relation to body size. However, Atlantic and Mediterranean horse mackerels and some small Atlantic and Atlantic Chub mackerels with small mouth apertures were also able to ingest prey with lengths larger than their gape size. This was also observed for anchovies, given that they had a relatively larger gape size than the other predators in relation to their total length (Figure 2; Tables 2 and 4).
Predator length–maximum length of prey scatter diagrams for eight small pelagic fish predators. Each open circle represents the maximum value of the average prey sizes consumed by a predator. For each scatter diagram, thick continuous lines represent gape width vs. predator size regressions, and thick dashed lines represent gape height vs. predator size regressions. Regression equations are presented in Table 2.
Predator-prey size ratios
Predator-prey size ratio frequencies (i.e. frequency of occurrence) were analysed in terms of abundance in the stomach contents of different sized predators. Relative frequency distributions (%) of PPSR, and cumulative frequencies corresponding to different predator species ranged by size (i.e. TL < 130 mm, TL 130–230 mm, TL > 230 mm) are presented as supplementary material (Figure A1).
Atlantic and Atlantic Chub mackerels had the widest prey size range in their diet (1–33.12 mm and 0.36–34.26 mm, respectively) (Table 4). Atlantic mackerels had the widest PPSR range, showing the lowest minimum and the highest maximum PPSR values (PPSR 2–1197); however, they also showed the highest mode, Q1 and Q2, compared to the other predators. Atlantic Chub mackerels had a similar range in ratios (PPSR 4–826), but in general values were lower in relation to the other predators (the minimum mode) as they ingested large prey more frequently (Tables 5 and 6).
Descriptive statistics of the predator-prey size ratio (PPSR) percentages in terms of prey abundances found in the stomach contents of the sampled predators according to size (i.e. small-medium-large).
| Predator species . | PPSRmax . | PPSRmin . | Mode . | Q1 . | Q2 . | Q3 . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <130 . | 130–230 . | >230 . | <130 . | 130–230 . | >230 . | <130 . | 130–230 . | >230 . | <130 . | 130–230 . | >230 . | <130 . | 130–230 . | >230 . | <130 . | 130–230 . | >230 . | ||
| E. encrasicolus | 375 | 547 | 12 | 48 | 245 | 108 | 101 | 105 | 201 | 113 | 245 | 229 | |||||||
| S. pilchardus | 304 | 687 | 773 | 78 | 37 | 23 | 225 | 85 | 178 | 91 | 88 | 175 | 222 | 145 | 182 | 280 | 187 | 370 | |
| T. trachurus | 245 | 518 | 488 | 9 | 16 | 26 | 80 | 64 | 55 | 76 | 67 | 38 | 84 | 98 | 59 | 98 | 129 | 94 | |
| T. mediterraneus | 173 | 437 | 3 | 146 | 66 | 184 | 62 | 181 | 68 | 185 | 76 | 188 | |||||||
| S. scombrus | 63 | 592 | 1197 | 4 | 2 | 3 | 64 | 175 | 265 | 8 | 79 | 198 | 53 | 139 | 258 | 64 | 177 | 266 | |
| S. colias | 742 | 826 | 10 | 4 | 48 | 225 | 51 | 123 | 87 | 226 | 125 | 327 | |||||||
| B. boops | 275 | 525 | 62 | 19 | 114 | 111 | 86 | 107 | 112 | 118 | 184 | 273 | |||||||
| S. sprattus | 367 | 330 | 4 | 61 | 67 | 105 | 63 | 107 | 70 | 139 | 82 | 223 | |||||||
| Predator species . | PPSRmax . | PPSRmin . | Mode . | Q1 . | Q2 . | Q3 . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <130 . | 130–230 . | >230 . | <130 . | 130–230 . | >230 . | <130 . | 130–230 . | >230 . | <130 . | 130–230 . | >230 . | <130 . | 130–230 . | >230 . | <130 . | 130–230 . | >230 . | ||
| E. encrasicolus | 375 | 547 | 12 | 48 | 245 | 108 | 101 | 105 | 201 | 113 | 245 | 229 | |||||||
| S. pilchardus | 304 | 687 | 773 | 78 | 37 | 23 | 225 | 85 | 178 | 91 | 88 | 175 | 222 | 145 | 182 | 280 | 187 | 370 | |
| T. trachurus | 245 | 518 | 488 | 9 | 16 | 26 | 80 | 64 | 55 | 76 | 67 | 38 | 84 | 98 | 59 | 98 | 129 | 94 | |
| T. mediterraneus | 173 | 437 | 3 | 146 | 66 | 184 | 62 | 181 | 68 | 185 | 76 | 188 | |||||||
| S. scombrus | 63 | 592 | 1197 | 4 | 2 | 3 | 64 | 175 | 265 | 8 | 79 | 198 | 53 | 139 | 258 | 64 | 177 | 266 | |
| S. colias | 742 | 826 | 10 | 4 | 48 | 225 | 51 | 123 | 87 | 226 | 125 | 327 | |||||||
| B. boops | 275 | 525 | 62 | 19 | 114 | 111 | 86 | 107 | 112 | 118 | 184 | 273 | |||||||
| S. sprattus | 367 | 330 | 4 | 61 | 67 | 105 | 63 | 107 | 70 | 139 | 82 | 223 | |||||||
PPSRmax and PPSRmin = maximum and minimum PPSRs. For the PPSR frequencies in terms of abundance (%), mode and the 1st (Q1), 2nd (Q2) and 3rd (Q3) quartiles of the ratios are presented. The sample size is the same as described in Table 4. A PPSR value of 800 has been used as the cut-off point in the calculations, which covers ≥99.7% of the accumulated frequency.
Descriptive statistics of the predator-prey size ratio (PPSR) percentages in terms of prey abundances found in the stomach contents of the sampled predators according to size (i.e. small-medium-large).
| Predator species . | PPSRmax . | PPSRmin . | Mode . | Q1 . | Q2 . | Q3 . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <130 . | 130–230 . | >230 . | <130 . | 130–230 . | >230 . | <130 . | 130–230 . | >230 . | <130 . | 130–230 . | >230 . | <130 . | 130–230 . | >230 . | <130 . | 130–230 . | >230 . | ||
| E. encrasicolus | 375 | 547 | 12 | 48 | 245 | 108 | 101 | 105 | 201 | 113 | 245 | 229 | |||||||
| S. pilchardus | 304 | 687 | 773 | 78 | 37 | 23 | 225 | 85 | 178 | 91 | 88 | 175 | 222 | 145 | 182 | 280 | 187 | 370 | |
| T. trachurus | 245 | 518 | 488 | 9 | 16 | 26 | 80 | 64 | 55 | 76 | 67 | 38 | 84 | 98 | 59 | 98 | 129 | 94 | |
| T. mediterraneus | 173 | 437 | 3 | 146 | 66 | 184 | 62 | 181 | 68 | 185 | 76 | 188 | |||||||
| S. scombrus | 63 | 592 | 1197 | 4 | 2 | 3 | 64 | 175 | 265 | 8 | 79 | 198 | 53 | 139 | 258 | 64 | 177 | 266 | |
| S. colias | 742 | 826 | 10 | 4 | 48 | 225 | 51 | 123 | 87 | 226 | 125 | 327 | |||||||
| B. boops | 275 | 525 | 62 | 19 | 114 | 111 | 86 | 107 | 112 | 118 | 184 | 273 | |||||||
| S. sprattus | 367 | 330 | 4 | 61 | 67 | 105 | 63 | 107 | 70 | 139 | 82 | 223 | |||||||
| Predator species . | PPSRmax . | PPSRmin . | Mode . | Q1 . | Q2 . | Q3 . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <130 . | 130–230 . | >230 . | <130 . | 130–230 . | >230 . | <130 . | 130–230 . | >230 . | <130 . | 130–230 . | >230 . | <130 . | 130–230 . | >230 . | <130 . | 130–230 . | >230 . | ||
| E. encrasicolus | 375 | 547 | 12 | 48 | 245 | 108 | 101 | 105 | 201 | 113 | 245 | 229 | |||||||
| S. pilchardus | 304 | 687 | 773 | 78 | 37 | 23 | 225 | 85 | 178 | 91 | 88 | 175 | 222 | 145 | 182 | 280 | 187 | 370 | |
| T. trachurus | 245 | 518 | 488 | 9 | 16 | 26 | 80 | 64 | 55 | 76 | 67 | 38 | 84 | 98 | 59 | 98 | 129 | 94 | |
| T. mediterraneus | 173 | 437 | 3 | 146 | 66 | 184 | 62 | 181 | 68 | 185 | 76 | 188 | |||||||
| S. scombrus | 63 | 592 | 1197 | 4 | 2 | 3 | 64 | 175 | 265 | 8 | 79 | 198 | 53 | 139 | 258 | 64 | 177 | 266 | |
| S. colias | 742 | 826 | 10 | 4 | 48 | 225 | 51 | 123 | 87 | 226 | 125 | 327 | |||||||
| B. boops | 275 | 525 | 62 | 19 | 114 | 111 | 86 | 107 | 112 | 118 | 184 | 273 | |||||||
| S. sprattus | 367 | 330 | 4 | 61 | 67 | 105 | 63 | 107 | 70 | 139 | 82 | 223 | |||||||
PPSRmax and PPSRmin = maximum and minimum PPSRs. For the PPSR frequencies in terms of abundance (%), mode and the 1st (Q1), 2nd (Q2) and 3rd (Q3) quartiles of the ratios are presented. The sample size is the same as described in Table 4. A PPSR value of 800 has been used as the cut-off point in the calculations, which covers ≥99.7% of the accumulated frequency.
Comparison of gape height (GH) and width (GW) as a percentage of the total length and descriptive statistics of the PPSR percentages in terms of prey abundances found in the stomach contents for all size ranges.
| . | GW . | GH . | . | . | Freq. Distrib. of PPSR . | |||
|---|---|---|---|---|---|---|---|---|
| Species . | (% of TL) . | (% of TL) . | PPSRmax . | PPSRmin . | Mode . | Q1 . | Q2 . | Q3 . |
| E. encrasicolus | 17 | 19 | 547 | 12 | 108 | 105 | 115 | 232 |
| S. colias | 13 | 14 | 826 | 4 | 48 | 52 | 112 | 144 |
| T. mediterraneus | 13 | 14 | 437 | 3 | 66 | 63 | 68 | 77 |
| T. trachurus | 13 | 13 | 518 | 9 | 65 | 67 | 94 | 126 |
| S. scombrus | 12 | 13 | 1197 | 2 | 265 | 198 | 258 | 266 |
| S. pilchardus | 12 | 12 | 773 | 23 | 85 | 91 | 163 | 212 |
| S. sprattus | 10 | 10 | 367 | 4 | 67 | 63 | 70 | 85 |
| B. boops | 6 | 6 | 525 | 19 | 111 | 17 | 117 | 264 |
| . | GW . | GH . | . | . | Freq. Distrib. of PPSR . | |||
|---|---|---|---|---|---|---|---|---|
| Species . | (% of TL) . | (% of TL) . | PPSRmax . | PPSRmin . | Mode . | Q1 . | Q2 . | Q3 . |
| E. encrasicolus | 17 | 19 | 547 | 12 | 108 | 105 | 115 | 232 |
| S. colias | 13 | 14 | 826 | 4 | 48 | 52 | 112 | 144 |
| T. mediterraneus | 13 | 14 | 437 | 3 | 66 | 63 | 68 | 77 |
| T. trachurus | 13 | 13 | 518 | 9 | 65 | 67 | 94 | 126 |
| S. scombrus | 12 | 13 | 1197 | 2 | 265 | 198 | 258 | 266 |
| S. pilchardus | 12 | 12 | 773 | 23 | 85 | 91 | 163 | 212 |
| S. sprattus | 10 | 10 | 367 | 4 | 67 | 63 | 70 | 85 |
| B. boops | 6 | 6 | 525 | 19 | 111 | 17 | 117 | 264 |
The sample size is the same as that described in Table 4. A PPSR value of 800 has been used as the cut-off point in the calculations, which covers ≥99.7% of the accumulated frequency. Predators are ordered by relative buccal apertures, from the widest to the smallest.
Comparison of gape height (GH) and width (GW) as a percentage of the total length and descriptive statistics of the PPSR percentages in terms of prey abundances found in the stomach contents for all size ranges.
| . | GW . | GH . | . | . | Freq. Distrib. of PPSR . | |||
|---|---|---|---|---|---|---|---|---|
| Species . | (% of TL) . | (% of TL) . | PPSRmax . | PPSRmin . | Mode . | Q1 . | Q2 . | Q3 . |
| E. encrasicolus | 17 | 19 | 547 | 12 | 108 | 105 | 115 | 232 |
| S. colias | 13 | 14 | 826 | 4 | 48 | 52 | 112 | 144 |
| T. mediterraneus | 13 | 14 | 437 | 3 | 66 | 63 | 68 | 77 |
| T. trachurus | 13 | 13 | 518 | 9 | 65 | 67 | 94 | 126 |
| S. scombrus | 12 | 13 | 1197 | 2 | 265 | 198 | 258 | 266 |
| S. pilchardus | 12 | 12 | 773 | 23 | 85 | 91 | 163 | 212 |
| S. sprattus | 10 | 10 | 367 | 4 | 67 | 63 | 70 | 85 |
| B. boops | 6 | 6 | 525 | 19 | 111 | 17 | 117 | 264 |
| . | GW . | GH . | . | . | Freq. Distrib. of PPSR . | |||
|---|---|---|---|---|---|---|---|---|
| Species . | (% of TL) . | (% of TL) . | PPSRmax . | PPSRmin . | Mode . | Q1 . | Q2 . | Q3 . |
| E. encrasicolus | 17 | 19 | 547 | 12 | 108 | 105 | 115 | 232 |
| S. colias | 13 | 14 | 826 | 4 | 48 | 52 | 112 | 144 |
| T. mediterraneus | 13 | 14 | 437 | 3 | 66 | 63 | 68 | 77 |
| T. trachurus | 13 | 13 | 518 | 9 | 65 | 67 | 94 | 126 |
| S. scombrus | 12 | 13 | 1197 | 2 | 265 | 198 | 258 | 266 |
| S. pilchardus | 12 | 12 | 773 | 23 | 85 | 91 | 163 | 212 |
| S. sprattus | 10 | 10 | 367 | 4 | 67 | 63 | 70 | 85 |
| B. boops | 6 | 6 | 525 | 19 | 111 | 17 | 117 | 264 |
The sample size is the same as that described in Table 4. A PPSR value of 800 has been used as the cut-off point in the calculations, which covers ≥99.7% of the accumulated frequency. Predators are ordered by relative buccal apertures, from the widest to the smallest.
Bogues had relatively high ratio values, showing that they prey more frequently on small prey relative to their size. In fact, after Atlantic mackerels, they showed the highest relative ratios, and 25% of the total prey abundance was composed of relatively large plankton (Tables 5 and 6).
Atlantic and Mediterranean horse mackerels preyed frequently on relatively large prey, and had the lowest mode after Atlantic Chub mackerels. Moreover, Mediterranean horse mackerels showed lower values for the median and the third quartile, which indicates that they ingested larger prey relative to their size more frequently than the rest of the predators. This was also observed in sprats, which showed the lowest maximum PPSR (Tables 5 and 6).
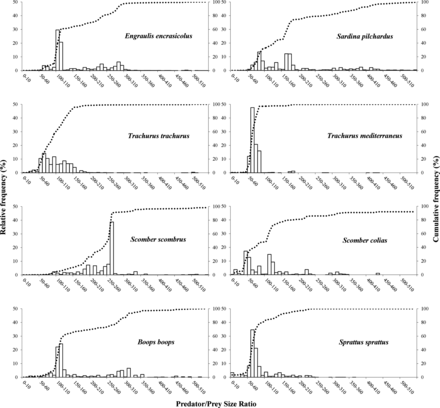
Anchovies and sardines had diets based on relatively small prey and, compared to the other species, they did not show any extreme values except the minimum PPSR value, which was highest for sardines (Tables 5 and 6). However, although both predator species ingested relatively small prey at any size (i.e. relatively high PPSR values were frequently obtained), larger prey were also often found in the gut contents of both predators, especially in fish smaller than 230 mm (Tables 5 and 6).
The cumulated size-frequency curves in Figure 3 show the differences between species in terms of the prey size ratios that made up their diets (in numbers). Sprat and Atlantic and Mediterranean horse mackerels obtained 100% of their diet at predator–prey size ratios below 200. The rest of the species showed a wider food range. However, with the exception of Atlantic mackerels, all of them obtained >60% of their diet at predator-prey size ratios <200. Moreover, 75% of the diet of fish <130 mm, except for anchovies and sardines, consisted of relatively larger prey, i.e. PPSR < 100 (Tables 5 and 6).
Relative frequency distribution of abundances of prey consumed by small pelagic predators. Cumulative PPSR frequencies are indicated by discontinuous lines. A PPSR value of 550 has been used as the cut-off point in graphs, which covers ≥91.93% of the accumulated frequency.
Trophic-niche breadth
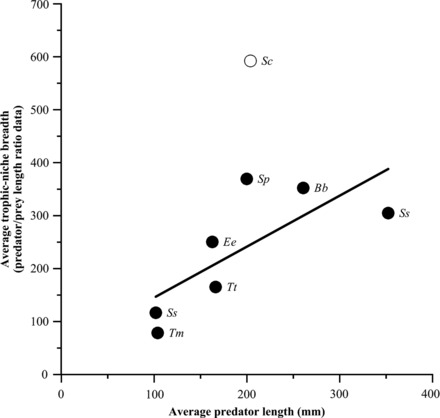
All examined predators demonstrated a significant change in PPSR-based trophic-niche breadth with increasing body size (Table 7). In case of Clupeids, anchovies and sardines tended to show a decrease in PPSR-based trophic-niche breadth with increasing body size, whereas sprats showed an increase. Regarding larger predators, a decrease in the trophic-niche breadth with increasing body size was detected in bogues and Atlantic horse mackerels; in contrast, Mediterranean horse mackerels, as well as Atlantic and Atlantic Chub mackerels, tended to show an increase in the trophic-niche breadth with increasing body size (Table 7). Average PPSR-based trophic-niche breadth demonstrated an increasing trend with increasing average predator size across the range of predators examined (Figure 4). However, the regression equation was significant only when excluding Atlantic Chub mackerels (with much higher average trophic-niche breadth than the rest) from the analysis (Trophic-niche breadth = 0.9627*Predator length + 48.961; r2 = 0.56; p = 0.055).
Average trophic-niche breadth plotted against average predator size for trophic-niche breadths calculated using PPSRs. Error bars (± 1 s.e.) are not visible since they are all <1. Ee = E. encrasicolus, Sp = S. pilchardus, Tt = T. trachurus, Tm = T. mediterraneus, Ss = S. scombrus, Sc = S. colias, Bb = B. boops, Ss = S. sprattus. Open circle indicates that it has been excluded from the regression fit (Sc).
Change in ratio-based trophic-niche breadth with increasing predator size for the eight small pelagic species.
| Predator-prey size ratio vs. Predator size . | |||
|---|---|---|---|
| Predator species . | Upper bound slope (± s.e.) . | Lower bound slope (± s.e.) . | Change in trophic-niche breadth . |
| E. encrasicolus | –0.0782 (±0.0417)' | 0.0476 (±0.0068)*** | decrease |
| S. pilchardus | –0.0825 (±0.0328)* | 0.1256 (±0.0036)*** | decrease |
| T. trachurus | –0.3616 (±0.0571)*** | –0.0442 (±0.0045)*** | decrease |
| T. mediterraneus | 0.4905 (±0.0499)*** | –0.8756 (±0.0545)*** | increase |
| S. scombrus | 1.0083 (±0.0216)*** | 0.0377 (±0.0021)*** | increase |
| S. colias | 0.3881 (±0.1202)** | –0.0871 (±0.0094)*** | increase |
| B. boops | –0.992 (±0.1447)*** | –0.1722 (±0.0251)*** | decrease |
| S. sprattus | 1.7836 (±0.0358)*** | 0.4675 (±0.0063)*** | increase |
| Predator-prey size ratio vs. Predator size . | |||
|---|---|---|---|
| Predator species . | Upper bound slope (± s.e.) . | Lower bound slope (± s.e.) . | Change in trophic-niche breadth . |
| E. encrasicolus | –0.0782 (±0.0417)' | 0.0476 (±0.0068)*** | decrease |
| S. pilchardus | –0.0825 (±0.0328)* | 0.1256 (±0.0036)*** | decrease |
| T. trachurus | –0.3616 (±0.0571)*** | –0.0442 (±0.0045)*** | decrease |
| T. mediterraneus | 0.4905 (±0.0499)*** | –0.8756 (±0.0545)*** | increase |
| S. scombrus | 1.0083 (±0.0216)*** | 0.0377 (±0.0021)*** | increase |
| S. colias | 0.3881 (±0.1202)** | –0.0871 (±0.0094)*** | increase |
| B. boops | –0.992 (±0.1447)*** | –0.1722 (±0.0251)*** | decrease |
| S. sprattus | 1.7836 (±0.0358)*** | 0.4675 (±0.0063)*** | increase |
Upper- and lower-bound slopes are quantile regressions estimating 90th (upper) and 10th (lower) quantiles of PPSR vs. predator length comparisons (***p < 0.0001, **p < 0.001, *p < 0.01, 'p < 0.1). s.e. is the standard error of each quantile regression. Decreases or increases in trophic-niche width are based on statistically significant differences between upper- and lower-bound slopes (p < 0.001 in all species).
Change in ratio-based trophic-niche breadth with increasing predator size for the eight small pelagic species.
| Predator-prey size ratio vs. Predator size . | |||
|---|---|---|---|
| Predator species . | Upper bound slope (± s.e.) . | Lower bound slope (± s.e.) . | Change in trophic-niche breadth . |
| E. encrasicolus | –0.0782 (±0.0417)' | 0.0476 (±0.0068)*** | decrease |
| S. pilchardus | –0.0825 (±0.0328)* | 0.1256 (±0.0036)*** | decrease |
| T. trachurus | –0.3616 (±0.0571)*** | –0.0442 (±0.0045)*** | decrease |
| T. mediterraneus | 0.4905 (±0.0499)*** | –0.8756 (±0.0545)*** | increase |
| S. scombrus | 1.0083 (±0.0216)*** | 0.0377 (±0.0021)*** | increase |
| S. colias | 0.3881 (±0.1202)** | –0.0871 (±0.0094)*** | increase |
| B. boops | –0.992 (±0.1447)*** | –0.1722 (±0.0251)*** | decrease |
| S. sprattus | 1.7836 (±0.0358)*** | 0.4675 (±0.0063)*** | increase |
| Predator-prey size ratio vs. Predator size . | |||
|---|---|---|---|
| Predator species . | Upper bound slope (± s.e.) . | Lower bound slope (± s.e.) . | Change in trophic-niche breadth . |
| E. encrasicolus | –0.0782 (±0.0417)' | 0.0476 (±0.0068)*** | decrease |
| S. pilchardus | –0.0825 (±0.0328)* | 0.1256 (±0.0036)*** | decrease |
| T. trachurus | –0.3616 (±0.0571)*** | –0.0442 (±0.0045)*** | decrease |
| T. mediterraneus | 0.4905 (±0.0499)*** | –0.8756 (±0.0545)*** | increase |
| S. scombrus | 1.0083 (±0.0216)*** | 0.0377 (±0.0021)*** | increase |
| S. colias | 0.3881 (±0.1202)** | –0.0871 (±0.0094)*** | increase |
| B. boops | –0.992 (±0.1447)*** | –0.1722 (±0.0251)*** | decrease |
| S. sprattus | 1.7836 (±0.0358)*** | 0.4675 (±0.0063)*** | increase |
Upper- and lower-bound slopes are quantile regressions estimating 90th (upper) and 10th (lower) quantiles of PPSR vs. predator length comparisons (***p < 0.0001, **p < 0.001, *p < 0.01, 'p < 0.1). s.e. is the standard error of each quantile regression. Decreases or increases in trophic-niche width are based on statistically significant differences between upper- and lower-bound slopes (p < 0.001 in all species).
Discussion
All sampled species showed a length-weight relationship in accordance with the general allometric pattern of small pelagic species (Lucio and Martin, 1989; Lucio, 1997; Mendes et al., 2004; Cicek et al., 2006; Ozaydin and Taskavak, 2006).
Also in accordance with the gravimetric results (Hyslop, 1980), the stomach weight of all sampled predators was strongly related to the total weight of the fish—e.g. Lucio (1997)—although the stomach weights of large Atlantic and Mediterranean horse mackerels were lighter.
Previous literature shows that in general the average prey size increases with the size of the predator (Peterson and Ausubel, 1984; Dahl and Kirkegaard, 1986; Hansen et al., 1994; Olaso et al., 1998; Velasco and Olaso, 1998a, b; Scharf et al., 2000; Dörner and Wagner, 2003; Barnes et al., 2008, 2010). In this study, a clear positive relationship was also found between the average and maximum prey sizes and the predator size. According to the trophic-niche breadth changes in the range of PPSR with increasing predator size (Scharf et al., 2000; this study), differences are observed between species. However, predators showing rapid ontogenetic increases in maximum prey size do not necessarily increase the minimum prey size rapidly; for example, Atlantic Chub mackerels were able to predate on relatively larger prey with increasing body size but they also ingested the smallest prey, showing an increase in trophic-niche breadth. In addition, the observed increasing trend for average trophic-niche breadths with ontogeny (Figure 4) was also observed in the Northwest Atlantic by Pepin and Penney (1997) for many larval fish species, but not by Scharf et al. (2000) for adults, and may indicate that small prey could also be bulked in with larger prey items when selective feeding occurs. This suggests the ability of large competitors to eat a wider range of prey sizes than small ones, large fish being able to use essentially all of the prey size spectrum available to small ones, plus particles too large for the small ones (Brooks and Dodson, 1965; Pearre, 1986). Thus, expansion with length of absolute prey size range for most predators could indicate increases in behavioural and morphological capabilities for capturing and swallowing large prey, combined with high encounter rates and susceptibility of small prey (Scharf et al., 2000).
Only two of the predator species have a gape size in relation to their body size that is significantly different from the other species: anchovies have the largest and bogues have the smallest. However, while bogues prey on smaller prey, the diet of anchovies does not reflect this relation in prey size or in predator–prey size ratios. In fact, although they have the largest gape size in relation to their body size, anchovies show high predator-prey size ratios, and thus ingest relatively small prey, especially when they are <130 mm in length. These results suggest that relatively large gape size (in relation to body size), while making it possible to capture large prey that make a high biomass contribution, does not determine the PPSR distribution. Accordingly, in contrast with what Pepin and Penney (1997) and Sabatés and Saiz (2000) observed for fish larvae, in small pelagic fish in the Bay of Biscay, large gape sizes are not always related to a diet dominated by larger prey; on the other hand, relatively small gape size, observed for example in sprats, does not prevent an increase in trophic-niche breadth with increasing body size. In addition, if the largest predators swallow other large prey (e.g. smaller fish) longitudinally (extreme cases), the limiting size of prey would be more determined by width rather than by total length, and in this case the gape size would not be a good limiting factor of the maximum prey size consumed (Scharf et al., 2000). That could explain larger maximum prey lengths than the estimated gape size of predator observed in some cases (e.g. Atlantic and Atlantic Chub mackerel) in which prey dorsoventral body depth measurements (Scharf et al., 2000) could be more appropriate. Moreover, studies with other species have shown that prey-evasive behaviours and differences in prey availability can also limit the consumption of large prey (Hambright, 1991; Keeley and Grant, 1997; Scharf et al., 2000).
If food is in sufficient amount, one might expect a filtering feeding, by which the smaller fraction would be over-represented (the larger prey would escape when the predator is seen). If food is scarce, one would expect the predator to select larger prey as it shifts to biting behaviour. For example, the plastic feeding behaviour of the anchovy allows them to shift from filtering to biting feeding, and hence they might not necessarily shift to larger prey, even if they are present, if capturing this large prey is energetically too demanding. Hence, the presence of relatively large organisms in stomach contents would indicate active opportunistic predation, which only occurs under favourable conditions, as previously described for various species such as anchovies (Tudela and Palomera, 1997; Plounevez and Champalbert, 1999, 2000; Bacha and Amara, 2009; Borme et al., 2009) and, less frequently, sardines (Van der Lingen, 1994; Garrido et al., 2007). Similarly, bogues also obtained 60% of the total ingested abundance from relatively large prey (PPSR < 150). Surprisingly, sprats mainly eat large prey in relation to their body size (they obtained 100% of their diet in numbers from a PPSR <200). More than 80% of the diet of Atlantic and Mediterranean horse mackerels was comprised of relatively large prey (PPSR < 150), which is in accordance with previous observations (Olaso et al., 1999). More than 40% of the diet in numbers of Atlantic Chub mackerels was comprised of large plankton, and it had lower PPSR values than the other predators (i.e. large prey). In contrast, Atlantic mackerels, with both maximum and minimum PPSR modes, showed the widest prey size spectrum. The diet of Atlantic mackerel is known to be limited mainly by the composition of zooplankton in the area (Castro, 1993; Cabral and Murta, 2002; Olaso et al., 2005) as well as ontogeny (Conway et al., 1999). However, the incorporation of a considerable number of intermediate-sized prey in the diet of large predators, while they continue to feed on small vulnerable prey, has also been observed in previous studies with other species, and the interspecific variability has been attributed to several morphological and behavioural characteristics (Scharf et al., 2000). The differences between Atlantic and Atlantic Chub mackerels may actually be due to the differences in the number of sampling stations where they were found as well as in the number of individuals analysed. In the same way, the stomach contents are a snapshot in time of the diet, and we cannot determine where the prey was captured in the vertical range, or even the horizontal. This limits the value of the comparison with zooplankton samples at the same time of fishing.
All the results suggest that the contribution made by the different prey sizes to fish diets is determined more by the available plankton sizes in relation to predator size (with sprats and mackerels being the extremes in predator size) than the behaviour or morphology of the fish. The minimum ingested prey size shows more similarity between species than the maximum size, which increases with predator size. This indicates that predators use the entire available size range, including the smallest sizes, and as they grow, simply incorporate larger prey as they become capable of catching it. As a result the PPSRs and trophic-niche breadth are dependent on changes in predator size. In addition, most species have similar stomach weights in relation to size, and the percentage of stomachs containing prey is similar. The exception seems to be Atlantic and Mediterranean horse mackerels, which show intra-specific differences when the general trends of their trophic-niche breadth with increasing body size are observed (i.e. opposite trends, Table 7). However, the minimum prey size of both horse mackerel species is larger than that of the other species, and, although they are as large as Atlantic and Atlantic Chub mackerels, most of their diet has low PPSR values, their stomach weight in relation to size is lighter than that of the other species, and the percentage of empty stomachs is much higher (Olaso et al., 1999; this study). All these observations agree with a real specialization in large prey, and therefore ingestion will be less frequent (leading to a lower average stomach weight and a higher frequency of empty stomachs). Nevertheless, it could also be a consequence of higher regurgitation rates during capture and differences in regurgitation depending on the stomach contents. In any case, Atlantic and Mediterranean horse mackerels offer an interesting model for making comparisons with the diets of other small pelagic fish.
In conclusion, our data indicate that, except for horse mackerels, the diets of the different species show a large degree of overlap in terms of prey size, even between fish of different sizes.
Supplementary data
Supplementary data are available at ICES Journal of Marine Science online.
Funding
This research was supported by the project Ecoanchoa promoted by the Department of Agriculture, Fisheries and Food of the Basque Country Government, and the EU FP7 FACTS (Forage Fish Interactions) project, Grant Agreement No. 244966. EB was supported by a doctoral fellowship from the Iñaki Goenaga Zentru Teknologikoen Fundazioa (IG-ZTF).
Acknowledgements
Thanks are due to two anonymous reviewers for their valuable comments. This is contribution No. 599 from AZTI-Tecnalia (Marine Research Division).
References
Author notes
Handling editor: Francis Juanes