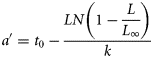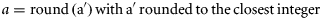-
PDF
- Split View
-
Views
-
Cite
Cite
E. Prigge, L. Marohn, R. Oeberst, R. Hanel, Model prediction vs. reality—testing the predictions of a European eel (Anguilla anguilla) stock dynamics model against the in situ observation of silver eel escapement in compliance with the European eel regulation, ICES Journal of Marine Science, Volume 70, Issue 2, March 2013, Pages 309–318, https://doi.org/10.1093/icesjms/fss188
Close - Share Icon Share
Abstract
A direct monitoring of European silver eel (Anguilla anguilla, L) escapement from rivers and estuaries has been proven to be challenging, and a Europe-wide documentation of escaping silver eel numbers therefore hardly seems realistic. To reinforce management decisions, policy-makers are thus widely reliant on the accuracy of escapement models. A 3-year programme of silver eel escapement monitoring was undertaken to compile model input data and revise an eel population model (German Eel Model II; GEM II) already used in the decision-making process of management authorities. By compiling necessary input data and analysing vital system-specific population characteristics, it was possible to compare the documented silver eel escapement with the modelled potential silver eel escapement. Resulting model predictions were close to actually monitored escapement numbers, which were distinctly lower than reference escapement values for the same freshwater system given in the implementation report of the German Eel Management Plans. Applying different commercial and recreational catch scenarios revealed the sensitivity of the model. The results show the potential of the GEM II and highlight the importance of high-quality input data to use model predictions as the basis for management measures.Prigge, E., Marohn, L., Oeberst R., and Hanel, R. 2013. Model prediction vs. reality—testing the predictions of a European eel (Anguilla anguilla) stock dynamics model against the in situ observation of silver eel escapement in compliance with the European eel regulation – ICES Journal of Marine Science, 70: 309–318.
Introduction
For centuries, the European eel (Anguilla anguilla, L) has been an important target species for fishers all over Europe (Tesch, 2003). However, since the 1980s, the stock has been in a steep decline, and alarmingly low recruitment numbers are documented in virtually every time-series available, as well as reflected in landing numbers all over Europe (Dekker, 2003; ICES, 2010). Nowadays, the European eel stock is considered to be out of safe biological limits and the species is listed in Annex II of the Convention on International Trade in Endangered Species (CITES; ICES, 2010). To stop the downward trend, the European Council (EC) issued regulation No.1100 (European Union, 2007), establishing measures for the recovery of the stock of European eel. One of its main objectives is to guarantee a minimum of a 40% spawner escapement to the sea in relation to the potential spawner escapement under pristine conditions. Each EU member state was obliged to elaborate Eel Management Plans (EMPs) on a river basin level to reach the given escapement target. A rejection of the EMP would entail strict reductions and eventually the closure of the national eel fisheries.
Therefore, a proper evaluation of any management measure implies the assessment of silver eel spawner escapement, which is methodologically challenging, especially for large river systems. Recent attempts have been largely based on commercial landings (Charrier et al., 2012), sonar-based quantification (Bilotta et al., 2011), mark–recapture studies, or estimations derived from tagging experiments (Feunteun et al., 2000; Amilhat et al., 2008) as well as the application of stock and escapement models (Aprahamian et al., 2007; Bevacqua et al., 2007; Fenske et al., 2011; reviewed by De Leo et al., 2009).
As a panmictic species, the European eel lacks a well-defined population structure (Als et al., 2011). Fenske et al. (2011) pointed out for Anguilla rostrata that a common spawning ground and overlapping generations probably lead to a situation where local recruitment is not directly related to overall stock size or local spawner abundance. This also holds true for the closely related European eel. Most common fish population models have been developed for iteroparous species with well-defined population structures. To take this into account, models estimating population dynamics in eel are often trying to cover large geographical scales and are therefore largely dependent on input data compiled from literature sources (Aprahamian et al., 2007; Åström and Dekker, 2007; Lambert and Rochard, 2007). Thus, in the absence of system-specific input data, it is often necessary to convert and generalize vital population characteristics onto a larger scale and/or simplify the model assumptions (Aprahamian et al., 2007; Åström and Dekker, 2007). It has, however, been shown for several Anguillidae species (including the European eel) that vital population characteristics differ with system and/or habitat (Vøllestad, 1992; Poole and Reynolds, 1998; Melia et al., 2006; Lin et al., 2007; Jessop, 2010). Therefore, generalizations can significantly bias the model outcome and limit the conclusiveness of model-based management measures.
Factors influencing the actual number of escaping silver eels from freshwater systems into coastal waters are numerous and widely discussed (Aprahamian et al., 2007; ICES, 2010). They range from commercial and recreational fishing, to predation by birds (e.g. cormorants), to migration barriers (ICES, 2010). Additionally, to evaluate the influence of each factor, initial recruitment numbers, e.g. stocking and/or natural immigration of juveniles to a freshwater system, are of high importance. In order to estimate the dynamics of the European eel stock with respect to immigration, restocking, natural mortality, mortality caused by commercial and recreational fisheries, predators (such as cormorants), and hydropower turbines, Oeberst and Fladung (2012) developed an age-structured eel population model for the River Elbe system (Germany)—the GEM II (German Eel Model II). One of the main output parameters of this model is the number of silver eels that are potentially able to migrate out of a specific system. The main advantage of the model is the possibility to account for uncertainties of the source data, such as, for example, the fact that data on immigrating eels are often of only poor quality.
In order to evaluate models directly used in the decision-making process of management authorities, it is important to ensure detailed and continuous system-specific data time-series. The advantage of the Schwentine River system is the absence of any natural immigration of eels (eliminating an input factor often hard to grasp), the relatively high quality of model input data due to their limited size, and a relatively small number of stakeholders, together with the possibility to directly monitor the actual silver eel escapement, thereby allowing a comparison of different management scenarios.
The study objective was to compare predicted silver eel escapement of the eel population model described by Oeberst and Fladung (2012) with the direct count of escaping silver eels. Thereby, the potential of eel population modelling in supporting the decision-making process of management authorities could be evaluated.
Material and methods
Study area
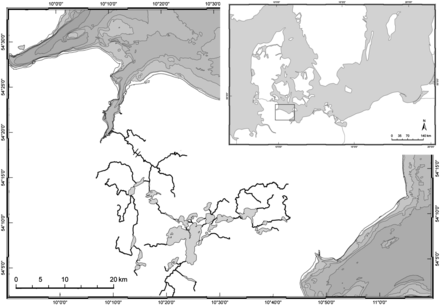
The Schwentine River system (part of the Schlei–Trave river basin district) close to Kiel was chosen as an exemplary freshwater system to monitor actual silver eel escapement and collect necessary model input data (Figure 1). The system consists of several freshwater lakes connected by the Schwentine River and has a total area of ∼7500 ha (total area of lakes: 5984 ha). It discharges into the Kiel Fjord (54°19′39''N, 10°11'11″E), with an average discharge of 3.9 m3 s−1 and ranging from ∼0.6 to 13.4 m3 s−1. It has been blocked for any upstream fish migration for >100 years by two consecutive hydropower stations ∼7 and 9 km upstream of the river mouth. Since 2005, the upper hydropower station (“Raisdorf II”) has been equipped with a fish pass combined with trash racks (light spacing: 20 mm) in front of the turbine entries. All silver eels leaving the system are forced to use the fish pass and end up in a fish trap. To date, the lower hydropower station is still not equipped with a fish pass and, thus, no natural ascent of juvenile eels into the system is possible. Therefore, the entire local eel population depends on stocking conducted by commercial fishers, angling societies, and government authorities.
Study area: Schwentine River system in northern Germany, discharging into the Baltic Sea. The inset shows the location of the study area in the Baltic Sea area. Black bars in the main map indicate hydropower stations.
Model description
The GEM II, developed by Oeberst and Fladung (2012), is an age-structured population model describing eel stock dynamics and estimating the potential silver eel escapement both in numbers and in total weight. Escapement is predicted based on vital population characteristics, immigration (including stocking), natural mortality, and mortality caused by recreational/commercial fisheries, cormorants, and hydropower (Oeberst and Fladung, 2012). Cormorant predation was considered separately to better incorporate its highly variable effects in distinct freshwater systems (Oeberst and Fladung, 2012). Even though this model has been developed to document eel stock dynamics in the Elbe river, it is not limited to it and can be transferred to other freshwater systems. Developed in 2008, the model was subsequently adapted according to new data and literature information to meet the requirements of the implementation report of the German EMPs in 2012 (Oeberst and Fladung, 2012), and was part of the EU project “Pilot projects to estimate potential and actual escapement of silver eel” (POSE; Walker et al., 2011).
The GEM II used here was restricted to 20 age classes, because age reading resulted in only a negligible number of eels older than 20 years. Therefore, it was assumed that eels rarely stay longer than 20 years in the Schwentine River system. Availability of data dramatically decreased prior to the early 1990s, and the time horizon for the model had to be set to 17 years (1993–2010). This time frame, however, was considered to minimize sufficiently the bias introduced by using a dummy start population for a comparison calculated with actually monitored escapement in the years 2009 and 2010. It was therefore necessary to compile model input data stretching back to the year 1993. For a detailed description of the GEM II, see Oeberst and Fladung (2012).
The GEM II allows integration of mortality caused by hydropower plants. In the Schwentine River system, two hydropower plants impede the escapement of silver eels (Figure 1). However, since escapement numbers were assessed at the upper hydropower plant and trash racks were regularly checked for eels, hydropower mortality was set to be zero in the present study. Furthermore, the model was restricted to a total wetted area of 5984 ha. The often small rivers connecting the systems' lakes were negligible, because fishing as well as stocking activities are mainly restricted to lake habitats.
Data collection
A 3-year silver eel monitoring programme was initiated to collect system-specific model input data and escapement numbers of the Schwentine River system. Monitoring of silver eel escapement out of the Schwentine River in the years 2009 and 2010 was performed by regular inspection of a trap blocking the fish pass that bypasses the upper hydropower station. Total length, wet mass, eye diameter, and pectoral fin length of every single eel caught were documented, and eels were assigned to a maturity stage according to Durif et al. (2005). To reinforce data collected at the fish trap and prevent biasing the assessment of system-specific vital population characteristics (e.g. length–weight relationships) towards migrating eels leaving the system, commercial catches were analysed for their length–weight relationship and proportion of silver eels (subsample) once a month in each summer. In total, 147 eels (total length spectrum: 20.2–102.5 cm) were measured to determine the length-at-age relationship for the Schwentine River system. Otoliths were prepared and read according to the manual published by the “ICES Workshop on Age Reading of European and American Eel” (ICES, 2009).
Since natural recruitment was considered to be zero in the Schwentine River system, recruitment numbers equal stocking numbers. Stocking with eel is decentralized in Germany, and the respective data (1993–2010) were collected from local state authorities (State Agency for Agriculture, Environment and Rural Areas, LLUR) as well as fishers and angling societies operating in the Schwentine River system. The numbers of stocked eels were provided in total weight (kg) per year and respective water body within the system and (when possible) subclassified into “glass eels”, “elvers”, and “yellow eels”. Total stocking in kg was converted into numbers based on the average weight of different estimated age groups, and varied between ∼238 000 and 13 500 individuals per year.
Commercial catch data were provided by the LLUR. Data were cross-checked and, if possible, amended by data from the individual fishers. In cases where no catch data were available for individual lakes and single years, gaps were filled according to yearly overall average catch per hectare. Catch data were provided in total weight (kg) per year and respective water body. The minimum legal size of eels in the Schwentine River system is 35 cm for both commercial and recreational fishers. However, local fishers communicated that eels of this size are usually too small to be put on the market, and the actual minimal landing size was assumed to be 50 cm. Since recreational catch data were not collected in a centralized database, historical catch data of two of the biggest angling societies located in the Schwentine area were analysed to estimate an average catch (kg) of eel per angler and year. The average catch was then multiplied by the total number of recreational fishers in the Schwentine River system per year.
As the cormorant (Phalacrocorax carbo) is often regarded as one of the major predators on eel in European freshwater systems, bird predation was assessed separately by stomach content analysis and analysis of regurgitant samples collected in the central colony of the Schwentine River system. Cormorants shot by authorized fishers were collected and stored frozen at –40°C for subsequent stomach content analysis. Stomach contents were screened for otoliths and other remains of A. anguilla. Regurgitant samples were collected during the daytime once a month and stored frozen at –20°C prior to analysis. In addition, total cormorant numbers (residents and migrants) were counted. Retrospective cormorant predation on eel was modelled using a time-series of cormorant numbers (2000–2010) for the Schwentine River system and an energetic consumption model according to Tasker and Furness (1996; N. Sonntag, unpublished data). Cormorant predation for the years 2000–2010 was estimated to range between 700 and 1800 kg. Due to a lack of information on the cormorant numbers prior to the year 2000, the cormorant predation assed by Worthmann and Spratte (1987) for the year 1987 (1187 kg) was used as a constant for 1993–1999.
Vital population characteristics
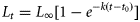
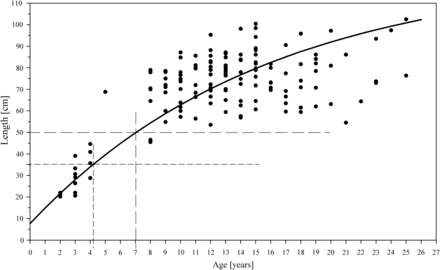
von Bertalanffy growth function (VBGF) for European eel in the Schwentine River system. The horizontal lines indicates the legal minimal landing size (35 cm) of A. anguilla for commercial fishers and angling societies, and actual minimal landing size (50 cm) after personal communication with fishers.
System-specific mean weight by age group was estimated based on the weight-at-length relationship W = a ×Lb (a = 0.0012 and b = 3.1106) in combination with Equations (2) and (3). The regression parameters a and b were estimated using weight and length data of 518 eels sampled in the fish pass and from commercial catches (length range, 32.5–100.7 cm; weight range, 62.4–2249.7 g). All length-based data were then converted into age-based data by combining Equations (2) and (3).
System-specific sexual maturity (fraction of silver eels) was assessed as the proportion of mature females per age class. The fraction of silver eels per age class was described by fitting a logistic model to maturity data (silver stage according to Durif et al., 2005) from female eels (n = 640) collected in the Schwentine River system.
Natural mortality was included according to the model of Bevacqua et al. (2011). The model estimates the proportion of eels that died from natural causes per age class based on the mean water temperature of the system, the density of the eels (three possible density levels), and the system-specific length–weight relationship. As small changes in temperature led to significant changes in natural mortality, a temperature time-series (provided by the LLUR) was used to model natural mortality on a yearly basis retrospective to 1993. With respect to the stocking numbers assessed for the model period, system-specific eel density was considered to be high.
Parameter fitting and model scenarios
While most of the parameters were directly assessed by sampling data on eels in the Schwentine River system (length-at-age, age-at-maturity, etc.), it was necessary to estimate the dummy start population artificially. The age distribution of the start population (1993) was estimated based on the years 2002–2004. It strongly influences the age distribution and stock abundance of the first model years, with decreasing effects during the following model period (Oeberst and Fladung, 2012). The start population can be further adjusted to presumable stocking activities prior to 1993 by introducing an additional factor (“factor for year one”). The “factor for year one” influences the age distribution of the first model year compared with the reference period for the dummy start population. Setting this factor to >1 indicates a higher stocking prior to 1993 compared with the following years. In contrast, values <1 indicate lower stocking prior to 1993. In addition, the fraction of silver eels per age class can be corrected for large yellow eels otherwise not accounted for by the logistic model. This can be done by varying the maximum proportion of yellow eels that become silver eels (FB ≤1). Changing FB strongly influences the age distribution of potentially emigrating silver eels. Values approaching 1 for FB will result in relatively young silver eels leaving the system, while decreasing FB increases the number of older silver eels (Oeberst and Fladung, 2012). These two parameters can be varied to achieve the best possible structural agreement between model outcome and field data, and thereby to identify the best performing model variant. To avoid the unintentional levelling of predicted and documented escapement numbers, parameter fitting was based on the comparison of modelled and documented relative age distribution instead of absolute numbers. To achieve frequencies >5 necessary for the statistical analysis, the 20 existing age classes were grouped as four age groups. Absolute numbers of potentially escaping silver eels (based on the GEM II) were normalized to monitoring numbers. Goodness-of-fit between observed and predicted relative age distributions of silver eels was tested by χ2 test (MS EXCEL).
To document the sensitivity of the model, several model scenarios were run, once the best performing model variant was identified. Scenarios included the reduction and increase of commercial and recreational fisheries by 10% and the reduction and increase of stocking by 10%. In all scenarios, only the respective input parameter was changed, while the remaining set of input parameters stayed the same as in the best performing model variant.
Results
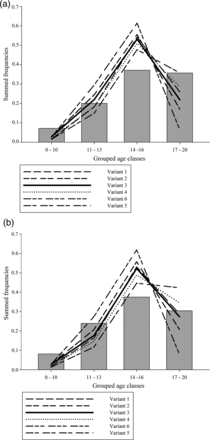
Relative age distribution (based on the GEM II) of potentially escaping silver eels from the Schwentine River system was strongly influenced by changes in FB and “factor for year one”, while resulting changes in absolute numbers were relatively small. It was not possible to adjust the parameters in such a way that relative age distribution of GEM II variant and monitoring results matched beyond doubt. The χ2 goodness-of-fit analysis resulted in significant differences between the observed and expected relative age distribution of escaping silver eels for 2009 and 2010 in all model variants. The χ2 test results of varying FB and “factor for year one” are given in Table 1. Observed and predicted relative age distributions for the years 2009 and 2010 and varying GEM II variants are given in Figure 3a and b. To compare absolute numbers of actually escaping silver eels (monitoring 2009 and 2010) and potentially escaping silver eels estimated by the GEM II, the model variant with the smallest sum of χ2 test values (variant 3; FB =0.3 and “factor for year one” = 0.4) was used.
(a) Relative frequency distribution of grouped age classes (2009) of actually escaping silver eels (grey bars) and potentially escaping silver eels from different model variants lines). (b) Relative frequency distribution of grouped age classes (2010) of actually escaping silver eels (grey bars) and potentially escaping silver eels from different model variants (lines).
Model variants tested to identify the best performing setting of FB and “factor for year one”.
| . | Variant 1 . | Variant 2 . | Variant 3 . | Variant 4 . | Variant 5 . | Variant 6 . |
|---|---|---|---|---|---|---|
| Factor for year one | 1 | 0.5 | 0.4 | 0.4 | 0.4 | 2 |
| FB | 1 | 0.5 | 0.3 | 0.2 | 0.1 | 1 |
| d.f. | 3 | 3 | 3 | 3 | 3 | 3 |
| χ2 test (year 2009) | 20.40 | 23.54 | 14.06 | 12.54 | 17.41 | 109.90 |
| χ2 test (year 2010) | 60.89 | 38.13 | 32.26 | 54.26 | 96.53 | 155.64 |
| Critical value χ2 (d.f. = 3, p = 0.05) | 7.81 | 7.81 | 7.81 | 7.81 | 7.81 | 7.81 |
| . | Variant 1 . | Variant 2 . | Variant 3 . | Variant 4 . | Variant 5 . | Variant 6 . |
|---|---|---|---|---|---|---|
| Factor for year one | 1 | 0.5 | 0.4 | 0.4 | 0.4 | 2 |
| FB | 1 | 0.5 | 0.3 | 0.2 | 0.1 | 1 |
| d.f. | 3 | 3 | 3 | 3 | 3 | 3 |
| χ2 test (year 2009) | 20.40 | 23.54 | 14.06 | 12.54 | 17.41 | 109.90 |
| χ2 test (year 2010) | 60.89 | 38.13 | 32.26 | 54.26 | 96.53 | 155.64 |
| Critical value χ2 (d.f. = 3, p = 0.05) | 7.81 | 7.81 | 7.81 | 7.81 | 7.81 | 7.81 |
All variants were run with the same set of assessed input parameters while changing FB and “factor for year one” according to the values given here. Goodness-of-fit analysis (χ2 test) was done comparing the relative age distribution of predicted and documented silver eel escapement. The χ2 test indentified Variant 3 (highlighted) as representing the best fit.
Model variants tested to identify the best performing setting of FB and “factor for year one”.
| . | Variant 1 . | Variant 2 . | Variant 3 . | Variant 4 . | Variant 5 . | Variant 6 . |
|---|---|---|---|---|---|---|
| Factor for year one | 1 | 0.5 | 0.4 | 0.4 | 0.4 | 2 |
| FB | 1 | 0.5 | 0.3 | 0.2 | 0.1 | 1 |
| d.f. | 3 | 3 | 3 | 3 | 3 | 3 |
| χ2 test (year 2009) | 20.40 | 23.54 | 14.06 | 12.54 | 17.41 | 109.90 |
| χ2 test (year 2010) | 60.89 | 38.13 | 32.26 | 54.26 | 96.53 | 155.64 |
| Critical value χ2 (d.f. = 3, p = 0.05) | 7.81 | 7.81 | 7.81 | 7.81 | 7.81 | 7.81 |
| . | Variant 1 . | Variant 2 . | Variant 3 . | Variant 4 . | Variant 5 . | Variant 6 . |
|---|---|---|---|---|---|---|
| Factor for year one | 1 | 0.5 | 0.4 | 0.4 | 0.4 | 2 |
| FB | 1 | 0.5 | 0.3 | 0.2 | 0.1 | 1 |
| d.f. | 3 | 3 | 3 | 3 | 3 | 3 |
| χ2 test (year 2009) | 20.40 | 23.54 | 14.06 | 12.54 | 17.41 | 109.90 |
| χ2 test (year 2010) | 60.89 | 38.13 | 32.26 | 54.26 | 96.53 | 155.64 |
| Critical value χ2 (d.f. = 3, p = 0.05) | 7.81 | 7.81 | 7.81 | 7.81 | 7.81 | 7.81 |
All variants were run with the same set of assessed input parameters while changing FB and “factor for year one” according to the values given here. Goodness-of-fit analysis (χ2 test) was done comparing the relative age distribution of predicted and documented silver eel escapement. The χ2 test indentified Variant 3 (highlighted) as representing the best fit.
Yearly stocking with A. anguilla of different age classes ranged from ∼13 500 (2008) to 238 000 (1993) individuals. The local eel biomass in the Schwentine River system was estimated (GEM II) at 24 981 kg (85 695 eels) in 2009 and 21 123 kg (119 633 eels) in 2010 (Table 2). Directly assessed commercial landings summed up to a total of 5233 kg in 2009 and 6610 kg in 2010. The GEM II estimated the corresponding absolute numbers to be 9486 eels in 2009 and 13 690 eels in 2010 (Table 2). Calculated recreational landings amounted to 830 kg in both 2009 and 2010. Cormorant predation was assessed to be 1100 kg in 2009 and 1200 kg in 2010. The GEM II estimated the corresponding absolute numbers to be 10 680 eels in 2009 and 11 650 eels in 2010 (Table 2).
Angulla anguilla stock size, mortality, and potentially escaping silver eels for the Schwentine River system for 2009 and 2010, estimated by the GEM II (variant 3). Numbers in parentheses indicate the percentage with respect to the total stock size of the same year.
| . | 2009 . | . | 2010 . | . |
|---|---|---|---|---|
| . | Weight (kg) . | No. of eels . | Weight (kg) . | No. of eels . |
| Stock size | 24 981 | 85 695 | 21 123 | 119 633 |
| Natural mortality | 2267 (9.1) | 12 193 (14.2) | 2120 (10.0) | 21 648 (18.1) |
| Commercial fishers | 5233 (20.9) | 9486 (11.1) | 6610 (31.3) | 13 690 (11.4) |
| Recreational fishers | 830 (3.3) | 1505 (1.8) | 830 (3.9) | 1720 (1.4) |
| Cormorants | 1100 (4.4) | 10 680 (12.5) | 1200 (5.7) | 11 650 (9.7) |
| Silver eels | 748 (3.0) | 728 (0.8) | 385 (1.8) | 363 (0.3) |
| Weight (kg) ha−1 | No. of eels ha−1 | Weight (kg) ha−1 | No. of eels ha−1 | |
| Commercial fishers | 0.9 | 1.6 | 1.1 | 2.3 |
| Recreational fishers | 0.1 | 0.3 | 0.1 | 0.3 |
| Cormorants | 0.2 | 1.8 | 0.2 | 1.9 |
| Silver eels | 0.13 | 0.12 | 0.06 | 0.06 |
| . | 2009 . | . | 2010 . | . |
|---|---|---|---|---|
| . | Weight (kg) . | No. of eels . | Weight (kg) . | No. of eels . |
| Stock size | 24 981 | 85 695 | 21 123 | 119 633 |
| Natural mortality | 2267 (9.1) | 12 193 (14.2) | 2120 (10.0) | 21 648 (18.1) |
| Commercial fishers | 5233 (20.9) | 9486 (11.1) | 6610 (31.3) | 13 690 (11.4) |
| Recreational fishers | 830 (3.3) | 1505 (1.8) | 830 (3.9) | 1720 (1.4) |
| Cormorants | 1100 (4.4) | 10 680 (12.5) | 1200 (5.7) | 11 650 (9.7) |
| Silver eels | 748 (3.0) | 728 (0.8) | 385 (1.8) | 363 (0.3) |
| Weight (kg) ha−1 | No. of eels ha−1 | Weight (kg) ha−1 | No. of eels ha−1 | |
| Commercial fishers | 0.9 | 1.6 | 1.1 | 2.3 |
| Recreational fishers | 0.1 | 0.3 | 0.1 | 0.3 |
| Cormorants | 0.2 | 1.8 | 0.2 | 1.9 |
| Silver eels | 0.13 | 0.12 | 0.06 | 0.06 |
Angulla anguilla stock size, mortality, and potentially escaping silver eels for the Schwentine River system for 2009 and 2010, estimated by the GEM II (variant 3). Numbers in parentheses indicate the percentage with respect to the total stock size of the same year.
| . | 2009 . | . | 2010 . | . |
|---|---|---|---|---|
| . | Weight (kg) . | No. of eels . | Weight (kg) . | No. of eels . |
| Stock size | 24 981 | 85 695 | 21 123 | 119 633 |
| Natural mortality | 2267 (9.1) | 12 193 (14.2) | 2120 (10.0) | 21 648 (18.1) |
| Commercial fishers | 5233 (20.9) | 9486 (11.1) | 6610 (31.3) | 13 690 (11.4) |
| Recreational fishers | 830 (3.3) | 1505 (1.8) | 830 (3.9) | 1720 (1.4) |
| Cormorants | 1100 (4.4) | 10 680 (12.5) | 1200 (5.7) | 11 650 (9.7) |
| Silver eels | 748 (3.0) | 728 (0.8) | 385 (1.8) | 363 (0.3) |
| Weight (kg) ha−1 | No. of eels ha−1 | Weight (kg) ha−1 | No. of eels ha−1 | |
| Commercial fishers | 0.9 | 1.6 | 1.1 | 2.3 |
| Recreational fishers | 0.1 | 0.3 | 0.1 | 0.3 |
| Cormorants | 0.2 | 1.8 | 0.2 | 1.9 |
| Silver eels | 0.13 | 0.12 | 0.06 | 0.06 |
| . | 2009 . | . | 2010 . | . |
|---|---|---|---|---|
| . | Weight (kg) . | No. of eels . | Weight (kg) . | No. of eels . |
| Stock size | 24 981 | 85 695 | 21 123 | 119 633 |
| Natural mortality | 2267 (9.1) | 12 193 (14.2) | 2120 (10.0) | 21 648 (18.1) |
| Commercial fishers | 5233 (20.9) | 9486 (11.1) | 6610 (31.3) | 13 690 (11.4) |
| Recreational fishers | 830 (3.3) | 1505 (1.8) | 830 (3.9) | 1720 (1.4) |
| Cormorants | 1100 (4.4) | 10 680 (12.5) | 1200 (5.7) | 11 650 (9.7) |
| Silver eels | 748 (3.0) | 728 (0.8) | 385 (1.8) | 363 (0.3) |
| Weight (kg) ha−1 | No. of eels ha−1 | Weight (kg) ha−1 | No. of eels ha−1 | |
| Commercial fishers | 0.9 | 1.6 | 1.1 | 2.3 |
| Recreational fishers | 0.1 | 0.3 | 0.1 | 0.3 |
| Cormorants | 0.2 | 1.8 | 0.2 | 1.9 |
| Silver eels | 0.13 | 0.12 | 0.06 | 0.06 |
Silver eel monitoring at the hydropower station (2009 and 2010) resulted in unexpectedly low escapement numbers of female silver eels. In 2009, only 133 silver eels were documented, with a total weight of 144 kg, resulting in 0.02 kg ha−1 year−1 (total area accounted for =5984 ha). In 2010, the total number of emigrating silver eels was 683, with a total weight of 691 kg, resulting in 0.12 kg ha−1 year−1 (total area accounted for =5984 ha). Potential silver eel escapement numbers estimated by the GEM II differed slightly from monitoring results. The model calculated 728 silver eels (748 kg) for 2009 and 363 silver eels (385 kg) for 2010, corresponding to 0.13 kg ha−1 year−1 in 2009 and 0.06 kg ha−1 year−1 in 2010 (Tables 2 and 3).
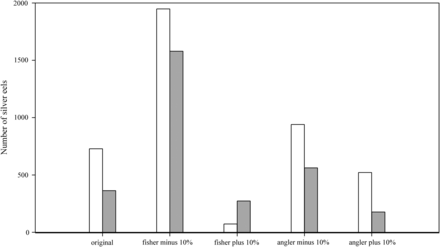
Reducing the catch numbers of the commercial fisheries by 10% (“fisher minus 10%” scenario) resulted in 1948 potentially escaping silver eels (2044 kg) in 2009 and 1579 silver eels (1709 kg) in 2010 (Figure 4). The “fisher minus 10%” scenario resulted in an ∼168% increase in the number of escaping silver eels for 2009 and an ∼335% increase for 2010. Increasing the catch numbers of the commercial fisheries by 10% (“fisher plus 10%” scenario) resulted in 72 silver eels (63 kg) in 2009 and 273 silver eels (259 kg) in 2010 (Figure 4). The “fisher plus 10%” scenario resulted in an ∼90% decrease in silver eel numbers for 2009 and an ∼25% decrease for 2010. Reducing the catch numbers of the recreational fishery by 10% (“angler minus 10%” scenario) resulted in 940 potentially escaping silver eels (970 kg) in 2009 and 562 silver eels (599 kg) in 2010 (Figure 4). The “angler minus 10%” scenario resulted in an ∼29% increase in silver eels numbers for 2009 and an ∼45% increase for 2010. Increasing the catch numbers of the recreational fishery by 10% (“angler plus 10%” scenario) resulted in 521 silver eels (533 kg) in 2009 and 177 silver eels (188 kg) in 2010 (Figure 4). The “angler plus 10%” scenario resulted in an ∼28% decrease in silver eel numbers for 2009 and an ∼51% decrease for 2010. Silver eel output for different scenarios is summarized in Table 3.
Number of potentially escaping silver eels for 2009 (white bars) and 2010 (grey bars) with different fishery scenarios.
Silver eel output for fisher/angler scenarios, reference estimate, and 40% aim for the Schwentine River system.
| Scenario . | 2009 . | 2010 . | ||
|---|---|---|---|---|
| . | kg ha−1 year−1 . | no. of eels ha−1 year−1 . | kg ha−1 year−1 . | no. of eels ha−1 year−1 . |
| Reference estimatea | 1.5–3.8 kg ha−1 year−1 | |||
| 40% aimb | 0.6–1.52 kg ha−1 year−1 | |||
| Original | 0.13 | 0.12 | 0.06 | 0.06 |
| Fisher –10% | 0.34 | 0.33 | 0.29 | 0.26 |
| Fisher +10% | 0.01 | 0.01 | 0.04 | 0.05 |
| Angler –10% | 0.16 | 0.16 | 0.10 | 0.09 |
| Angler +10% | 0.09 | 0.09 | 0.03 | 0.03 |
| Scenario . | 2009 . | 2010 . | ||
|---|---|---|---|---|
| . | kg ha−1 year−1 . | no. of eels ha−1 year−1 . | kg ha−1 year−1 . | no. of eels ha−1 year−1 . |
| Reference estimatea | 1.5–3.8 kg ha−1 year−1 | |||
| 40% aimb | 0.6–1.52 kg ha−1 year−1 | |||
| Original | 0.13 | 0.12 | 0.06 | 0.06 |
| Fisher –10% | 0.34 | 0.33 | 0.29 | 0.26 |
| Fisher +10% | 0.01 | 0.01 | 0.04 | 0.05 |
| Angler –10% | 0.16 | 0.16 | 0.10 | 0.09 |
| Angler +10% | 0.09 | 0.09 | 0.03 | 0.03 |
bEscapement aim formulated by the EU.
Silver eel output for fisher/angler scenarios, reference estimate, and 40% aim for the Schwentine River system.
| Scenario . | 2009 . | 2010 . | ||
|---|---|---|---|---|
| . | kg ha−1 year−1 . | no. of eels ha−1 year−1 . | kg ha−1 year−1 . | no. of eels ha−1 year−1 . |
| Reference estimatea | 1.5–3.8 kg ha−1 year−1 | |||
| 40% aimb | 0.6–1.52 kg ha−1 year−1 | |||
| Original | 0.13 | 0.12 | 0.06 | 0.06 |
| Fisher –10% | 0.34 | 0.33 | 0.29 | 0.26 |
| Fisher +10% | 0.01 | 0.01 | 0.04 | 0.05 |
| Angler –10% | 0.16 | 0.16 | 0.10 | 0.09 |
| Angler +10% | 0.09 | 0.09 | 0.03 | 0.03 |
| Scenario . | 2009 . | 2010 . | ||
|---|---|---|---|---|
| . | kg ha−1 year−1 . | no. of eels ha−1 year−1 . | kg ha−1 year−1 . | no. of eels ha−1 year−1 . |
| Reference estimatea | 1.5–3.8 kg ha−1 year−1 | |||
| 40% aimb | 0.6–1.52 kg ha−1 year−1 | |||
| Original | 0.13 | 0.12 | 0.06 | 0.06 |
| Fisher –10% | 0.34 | 0.33 | 0.29 | 0.26 |
| Fisher +10% | 0.01 | 0.01 | 0.04 | 0.05 |
| Angler –10% | 0.16 | 0.16 | 0.10 | 0.09 |
| Angler +10% | 0.09 | 0.09 | 0.03 | 0.03 |
bEscapement aim formulated by the EU.
Discussion
Estimated potential silver eel escapement based on the GEM II output was at about the same level as the directly monitored escapement of silver eels out of the Schwentine River system. However, it has to be highlighted that the GEM II, besides describing the dynamics of the eel stock, estimates the potential silver eel escapement, namely the number of eels that become silver and potentially could start their migration out of the system. It has, however, been shown that the actual number of silver eels starting their migration is strongly influenced by environmental parameters and obstacles (Vøllestad et al., 1986; Feunteun et al., 2000; Breteler et al., 2007; Acou et al., 2008), indicating that silver eels, despite an advanced maturity stage, might postpone the onset of migration and even revert to the yellow stage when facing unfavourable conditions (Durif et al., 2003, 2005).
The age-structured GEM II requires length-based data to be converted into age-based data. Therefore, the growth function used to convert length-based data into age-based data strongly influenced the necessary input data and thus had a large influence on the model outcome. Eel growth is highly dependent on habitat (Svedäng et al., 1996; Melia et al., 2006), and published growth functions for female A. anguilla vary significantly (De Leo and Gatto, 1995; Poole and Reynolds, 1996; Holmgren et al., 1997; Simon, 2007). Describing eel growth, in general, has been proven to be challenging, and Sparre (1979) even suggested abandoning the use of VBGF. Nevertheless, VBGF was used to describe the growth of eels in the original GEM II (Oeberst and Fladung, 2012) and to document the model's potential also in the present model variant. In the present study, fixing the L∞ at 1.5 times the average size of escaping silver eels accounted for system-specific maturation patterns. The resulting VBGF closely resembled both the documented system-specific length-at-age relationship and the VBGF (L∞ = 114 cm, k = 0.058, and t0 = –0.81) for eels in the Schwentine River system previously given by Worthmann and Spratte (1987). The high plasticity of growth in eels causes enormous variation not only between habitats, but also between sexes (Poole and Reynolds, 1996), and a sensible growth description usually requires two individual VGBFs for females and males, respectively (Poole and Reynolds, 1996; Melia et al., 2006). As it was not possible to catch enough males in the Schwentine River system to allow a sound analysis, the present VBGF and the GEM II were restricted to females. Sex determination in freshwater eels is believed to be environmentally induced, and eels enter freshwater as sexually undifferentiated glass eels (Wiberg, 1983; Holmgren and Mosegaard, 1996; Davey and Jellyman, 2005). Besides population density and habitat quality, geographical habitat differences have been proposed to influence the sex ratio of European eels (Tesch, 2003; Kettle et al., 2011). Tesch (2003) and Kettle et al. (2011) described northern European waters to favour predominantly female-dominated populations, while southern European waters may support eel populations strongly skewed towards males. Stocking numbers and available habitat area in the Schwentine River system implicated a relatively high population density, and natural mortality was calculated appropriately. It has been proposed that high population densities relative to available habitat area and quality result in male-dominated sex ratios in A. rostrata (Krueger and Oliveira, 1999). Due to the relatively small size of male European eel (Tesch, 2003), the monitoring in the present study was probably biased towards females. However, the legal minimum catch size for the European eel and corresponding mesh sizes in the commercial fishery should have been able to document a male-dominated population in the Schwentine River system. In addition, Wickström et al. (1996) showed that juvenile eels stocked into northern European freshwater systems with limited natural recruitment predominantly develop into females. They estimated that in a lake with even higher stocking densities than in the Schwentine River system, only ∼3.4% of stocked elvers and glass eels developed into males (Wickström et al., 1996). The present catch composition of commercial fishers and escapement monitoring results confirmed an eel population in the Schwentine River system dominated by females, and the male bias was considered to be negligible.
In spite of the absence of a Baltic Sea glass eel time-series, natural glass eel recruitment to Baltic Sea tributaries is commonly believed to be low (Westerberg, 1998; ICES, 2011). Generally, low recruitment combined with the still existent blockage of any upstream migration into the Schwentine River system by two consecutive hydropower stations support the idea of a local occurrence of eel being entirely dependent on stocking. Additionally, Prigge et al. (in press) showed that at least 62.5% of silver eels emigrating from the Schwentine River system had otolith strontium to calcium ratio profiles consistent with an individual stocking history. Stocking numbers provided by commercial fishers, recreational angling societies, and state authorities were therefore considered to represent the entire recruitment of juveniles to the Schwentine River eel population.
While the cumulated catch data for the commercial fishery were directly provided by commercial fishers and legal authorities, the effect of recreational fishers had to be estimated from average catch and total number of anglers in the Schwentine River system. The generalization of individual preferences and target species in angling most probably led to a loss in data quality. In addition, it was not possible to account for anglers who are not organized in angling societies in the Schwentine River system, and total recreational catch numbers used in the current GEM II variant are most probably underestimated. It has previously been reported that on average recreational fishers in Germany land ∼0.65 kg of eel per year (Anonymous, 2012). Calculated average catch in the Schwentine River system was slightly higher (∼0.9 kg per recreational fisher and year) (L. Marohn, unpublished data).
The milestone report of the implementation of the German EMP estimates the pristine reference silver eel escapement of the Schlei–Trave river basin (discharging into the Baltic Sea) at between 1.5 and 3.8 kg ha−1 year−1. The 40% minimum escapement postulated by the EU (Anonymous, 2012) would therefore require an escapement between 0.6 and 1.52 kg ha−1 year−1. Potential escapement based on the GEM II for 2009 and 2010 was thus at ∼10–21% (0.06 and 0.13 kg ha−1 year−1) of the lower 40% escapement aim.
Connecting rivers in the Schwentine River system are usually rather shallow and fast flowing, whereas Laffaille et al. (2003) showed that larger eels tend to prefer deeper freshwater habitats. In addition, stocking and eel fisheries in the Schwentine River system outside the lake habitats are negligible. Consequently, uncertainties in estimated eel population dynamics due to area restriction of the GEM II were also considered to be negligible.
Different eel fishery scenarios showed that estimates of potential escapement are strongly dependent on input data. Changing the number of eels taken by commercial and recreational fisheries by only 10% significantly influenced the number of potentially escaping silver eels. Legal restrictions of the commercial/recreational eel fisheries, however, seem to be one of the possible measures showing instant short-term improvement of escapement numbers, but concurrently are often the subject of great public controversies (Dorow and Arlinghaus, 2012). Due to the retrospective construction of the different scenarios, however, implications for system-specific management decision are limited.
The present study constitutes one of the first actually to compare the silver eel escapement of a small freshwater system with the potential escapement predicted by an escapement model. Even though the Schwentine River system has to be considered a small to medium freshwater system and the number of involved stakeholders is limited, compiling the necessary input data and characterizing the vital population characteristics was challenging. Nevertheless, results document the potential of the GEM II when estimating escaping silver eels in numbers and weight. At the same time, the present results highlight the importance of high-quality databases to secure a sound model-based management and question the compliance of regional management strategies with the minimum criteria defined by the European eel regulation.
Acknowledgements
We would like to thank Roland Lemcke and Siegfried Spratte from the State Agency for Agriculture, Environment and Rural Areas Schleswig-Holstein (LLUR), Dieter Bohn and Martin Purps from the State Angling Federation Schleswig-Holstein (LSFV), Sabine Schwarten, head of the Federation of Commercial Freshwater Fishermen e.V., and all involved commercial fishers and angling societies for providing catch and stocking data. We also thank Elisabeth Wesseler and Gudrun Plambeck (LLUR) for providing the water temperature time series, and the three anonymous reviewers for providing insightful comments on the manuscript. This study was founded by the Federal Ministry of Food, Agriculture and Consumer Protection of Germany (Project 2807HS010–“Quantification of eel mortalities in German inland waters”).
References
Author notes
Handling editor: Howard Browman