-
PDF
- Split View
-
Views
-
Cite
Cite
Corey B. Wakefield, Stephen J. Newman, Ross J. Marriott, Dion K. Boddington, David V. Fairclough, Contrasting life history characteristics of the eightbar grouper Hyporthodus octofasciatus (Pisces: Epinephelidae) over a large latitudinal range reveals spawning omission at higher latitudes, ICES Journal of Marine Science, Volume 70, Issue 3, April 2013, Pages 485–497, https://doi.org/10.1093/icesjms/fst020
Close - Share Icon Share
Abstract
Demographic data on deep-water groupers are limited despite them being highly exploited throughout the Indo-Pacific. In Western Australia, the continuous distribution of the eightbar grouper, Hyporthodus octofasciatus, spans tropical to temperate waters over ∼3500 km from 12°S–35°S. The maximum age was markedly higher in the northern tropical waters than in southern temperate waters, i.e. 47 vs 20 years. Females attained a significantly larger length-at-age in southern temperate waters. Macroscopic and microscopic examination of gonads and annual trends in mean monthly gonadosomatic indices (GSIs) were used to determine that this monandric protogynous hermaphrodite spawns from late spring to summer (October–February) in northwestern Australia. In the temperate waters of WA, there was no evidence of reproduction and no males were observed south of ∼30°S latitude. The lengths at which 50% of female H. octofasciatus matured and changed sex were estimated from northern tropical populations at 560 mm (6.1 years) and 1022 mm (≥11 years). Although the population connectivity of H. octofasciatus is unknown, the spawning omission in temperate waters suggests recruitment from the northern tropical areas and highlights the importance of preserving spawning stocks in those northern waters.Wakefield, C. B., Newman, S. J., Marriott, R. J., Boddington, D. K., and Fairclough, D. V. 2013. Contrasting life history characteristics of the eightbar grouper, Hyporthodus octofasciatus (Pisces: Epinephelidae), over a large latitudinal range reveals spawning omission at higher latitudes. – ICES Journal of Marine Science, 70: 485–497.
Introduction
The eightbar grouper, Hyporthodus octofasciatus (Griffin, 1926), of the family Epinephelidae, is a large (∼150 cm total length) and valuable deep-water demersal fish (Smith and Craig, 2007; Craig et al., 2011). The monophyletic family Epinephelidae was resurrected based on the molecular analyses by Smith and Craig (2007) utilizing mitochondrial and nuclear DNA sequence data to resolve the relationships among serranid and percid fishes. Previously, H. octofasciatus had been assigned to the genus Epinephelus (Randall and Heemstra, 1991; Heemstra and Randall, 1993). Craig and Hastings (2007) resurrected the genus Hyporthodus based on genetic data from two nuclear and two mitochondrial genes as part of their examination of the molecular phylogeny of the groupers of the subfamily Epinephelinae (Serranidae). Hyporthodus is characterized by having a much deeper body than species in the genus Epinephelus (this is particularly apparent in juveniles) and a drab brown or olive coloration that may or may not include several dark bars along the body (Craig and Hastings, 2007). A total of 14 species of groupers, including H. octofasciatus, have been accorded the genus Hyporthodus (Craig et al., 2011).
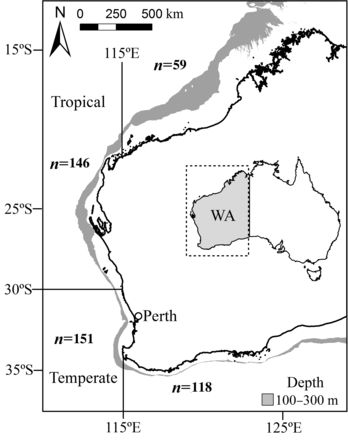
Hyporthodus octofasciatus occurs in tropical to temperate waters and has a widespread Indo-Pacific distribution from east Africa (Somalia and South Africa) to the Marquesas Islands, extending north to Japan and south to Australia and New Zealand (Craig et al., 2011). H. octofasciatus closely resembles H. septemfasciatus (Randall and Heemstra, 1991; Heemstra and Randall, 1993; Craig et al., 2011). However, H. septemfasciatus has only been reported with certainty from Korea, China and Japan (Craig et al., 2011) and those records of H. septemfasciatus outside these localities appear to be based on misidentifications of H. octofasciatus. Furthermore, Randall and Heemstra (1991) determined that H. octofasciatus in Western Australia differs from all other localities in its distributional range by having a slightly rounded to truncate caudal fin with a distinctive white posterior border as an adult. In Western Australia, H. octofasciatus is distributed across almost the entire state from the north coast (12°S), south and east along the south coast as far as ca 35°S and 119°E (Figure 1).
Map showing the tropical and temperate regions (relative to an arbitrary latitude of 30°S) and sample sizes of Hyporthodus octofasciatus collected from the north, upper west, lower west and south coasts of Western Australia (WA). The common depth range of this species is 100–300 m (grey shading).
The large size of H. octofasciatus and the high quality of its flesh, make this species highly valued by commercial and recreational fishers (Heemstra and Randall, 1993; Craig et al., 2011). The commercial landings of H. octofasciatus were first reported in Western Australia in 1986 (derived from compulsory catch statistics provided by commercial fishers to the Department of Fisheries, Government of Western Australia). Since then, annual statewide commercial catches have gradually increased, peaking at 83 tonnes in 2004, with an average annual catch of ca 26 t in the period from 1999–2009. In general, like most demersal fisheries in Western Australia, these annual commercial catches are low relative to fisheries elsewhere and may be indicative of a low productivity environment (Molony et al., 2011). This is of concern in Western Australia, given that there has been a possible shift in fishing effort by both recreational and commercial fishers to deeper offshore waters, particularly in the Perth metropolitan region (31–33°S) following a marked decline in the abundances of many inshore demersal fish species (Wise et al., 2007).
At present, there is insufficient life history information on which to base a management strategy and stock assessment for this species along the coast of Western Australia or elsewhere, despite its broad distribution. Biological data on H. octofasciatus, and the Hyporthodus clade in general, is limited. The maximum age recorded for this genus was 85 years for Hyporthodus flavolimbatus from the Gulf of Mexico (Cook et al., 2009). Andrews et al. (2011) analysed the bomb radiocarbon composition of otoliths of H. octofasciatus and confirmed that estimates of fish age derived from growth zones in thin otolith sections are valid. Of the 14 species of Hyporthodus, five have been found to be protogynous hermaphrodites (e.g. Keener, 1984; Wyanski et al., 2000). The fact that H. octofasciatus is commonly misidentified and relatively rarely encountered throughout its range has led to a lack of reliable quantitative catch and effort statistics and thus a “Data Deficient” status according to the International Union for Conservation of Nature (IUCN) Red List classifications (To and Pollard, 2008). Knowledge of age, growth and reproduction are critical inputs to determine stock status and risks to sustainability from extractive fishing. Reliable biological parameter estimates will better inform fishery managers regarding sustainable management strategies for fishing this species, and for the offshore demersal scalefish resource in Western Australia, for which this species is used as an indicator (Department of Fisheries, 2011).
This study is the first detailed investigation of the age, growth and reproductive biology of H. octofasciatus. Specifically, we sought to determine the age, growth and reproductive parameters of relevance to satisfy base level information requirements for fisheries management of H. octofasciatus populations. Considering the very large latitudinal range that this species occupies along the Western Australian coast, samples were divided into those from generally tropical or temperate regions, with an arbitrary divide at 30°S. This was considered appropriate given the contrasting demographic characteristics exhibited by other demersal teleosts with similar widespread distributions along this coast (e.g. Wakefield, 2006). Thus, we compared biological parameters between tropical and temperate regions in order to evaluate the relative production potential of this species in different areas. Furthermore, we compared the results obtained from this study with available age-based demographic data for all 14 species of deep-bodied groupers in the genus Hyporthodus (Craig et al., 2011), including those listed as incertae sedis by Craig and Hastings (2007).
Methods
Sampling and somatic measurements
Samples of H. octofasciatus were collected from commercial (using lines, fish traps and fish trawls) and recreational catches (using lines only) from 1995–2012 across almost the entire north, west and south coasts of Western Australia (12–35°S, Figure 1). The total length (LT) of each H. octofasciatus was measured to the nearest 1 mm, and when possible, the wet weight (Ww) of each whole individual was measured to the nearest 0.1 g. An allometric length (LT) versus weight (Ww) relationship for females and males combined was derived from the log-transformed data and was used to estimate the weights of specimens that were unable to be sampled whole (Quinn and Deriso, 1999).
Age and growth
The sagittae of each H. octofasciatus were removed, cleaned and stored dry. The right sagitta of each fish was embedded in epoxy resin and sectioned transversely through the primordium in a direction perpendicular to the sulcus acusticus, using a low speed saw with a diamond tipped blade. Otolith sections were cut thinly, i.e. 0.11–0.15 mm, to improve growth zone clarity. Sections were mounted on glass slides with a cover slip using casting resin. The opaque zones on each otolith section were counted along an axis from the primordium to the crista superior (dorsal rim of the sulcus acusticus) under reflected light at x20 to x40 magnification, without any knowledge of the size of the fish from which they had been derived.
Bomb radiocarbon dating has been used to validate that a single opaque zone is deposited annually, and thus opaque zone counts from otolith sections can be used for determining ages of H. octofasciatus (Andrews et al., 2011). The primary reader (CBW) examined each otolith section (n = 418), while a secondary reader (SJN) examined a subsample of these sections (n = 108). To establish the level of confidence that can be placed in the interpretation of the otolith structure, the precision of counts between readers was assessed using the Index of Average Percent Error (IAPE, Beamish and Fournier, 1981).
The age of each H. octofasciatus was estimated using a combination of the birth date, the time of year when the opaque zones on the otoliths of the majority of H. octofasciatus became delineated, and the number of opaque zones. The birth date of H. octofasciatus was considered to be the approximate onset of peak spawning (1 November), as determined from the annual trends in gonadosomatic indices (GSIs) for the tropical region.
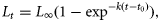
where Lt = the predicted mean total length (mm) of fish at age t (years), L∞ = asymptotic mean length (mm), k = the growth coefficient (year−1), t = estimated age (years), and to = the hypothetical age (years) at which fish would have zero length. The von Bertalanffy growth curves of females were compared between the tropical and temperate regions using a likelihood ratio test (Cerrato, 1990). The mean length of males (only recorded in the tropical region) was not positively correlated with age, and the distributions of their lengths at each age were comparable with those of females across the same age range. It is therefore likely that the growth of males is a continuation from that of females, and thus a von Bertalanffy growth curve was also fitted to the lengths-at-age of both sexes combined in the tropical region. A growth equation that combines data for both sexes would theoretically provide a better description of the length-at-age of older age classes and be more suitable for population model assessments. A comparison of available log-transformed weight–length data between tropical and temperate H. octofasciatus was also undertaken using analysis of covariance (ANCOVA) to evaluate if there was sufficient evidence to reject the null hypothesis of there being no difference in relative condition between regions. Mean-square error terms derived from simple linear regressions were compared using Cochran's C test for homogeneity of error variances. Owing to heterogenous residual variances (Cochran's C = 0.70 with 124 degrees of freedom), comparisons of groups with uneven sample sizes were made using Type III sums of squares.
Reproduction
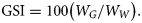
Data for GSIs were pooled for each calendar month for tropical and temperate regions separately. Spawning periods of female H. octofasciatus were assessed for the two regions using annual trends in mean monthly GSIs and macroscopically staged ovaries.
Freshly dissected gonads were preserved in pH neutral 10% buffered formalin for investigating histological characteristics relating to ovarian development and suspected protogynous hermaphroditism for a subsample of specimens (n = 61), which included many of the gonads categorized as indeterminate. This was done to confirm the sex and developmental stage assigned to gonads macroscopically (n = 500). Medial transverse sections of the preserved gonads were embedded in paraffin wax, sectioned at 5 µm, mounted on slides, and stained with Mayer's haematoxylin and eosin. Ovarian developmental stages were assessed microscopically from the identification of the most developed oocytes (Yamamoto et al., 1965; Wallace and Selman, 1981) and other relevant structures, such as the structural appearance of lamellae, thickness of the ovarian wall, intra-lamellar stromal tissue, atretic oocytes, brown bodies and postovulatory follicles, consistent with Burton et al. (1997), Bean et al. (2003) and Marriott et al. (2010). Gonads were classified as “transitional” sensuSadovy de Mitcheson and Liu (2008) if they contained degenerating functional ovarian tissue (yolk granule or later stage oocytes and/or brown bodies) and developing testicular tissue (crypts comprising sperm cell stages earlier than spermatozoa) (Sadovy and Shapiro, 1987; Grier and Taylor, 1998; Sadovy de Mitcheson and Liu, 2008; Marriott et al., 2010). This was consistent with an assumption that the presence of spermatozoa would indicate a functional testis, i.e. a male was ready to spawn or in the act of spawning and thus had completed the sex transition. Gonads were categorized as “bisexual” sensu Sadovy de Mitcheson and Liu (2008) if they contained both immature ovarian (previtellogenic oocytes) and testicular tissue (sperm cell stages earlier than spermatozoa), which does not indicate function of either sex. Additional microscopic evidence of suspected protogynous sex transition was the detection of vestigial ovarian structure and/or sperm sinuses in testes (Sadovy and Shapiro, 1987).
Estimates of sexual maturity and sex change
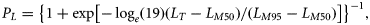
Results
Length and age compositions, ageing precision and growth
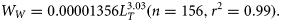
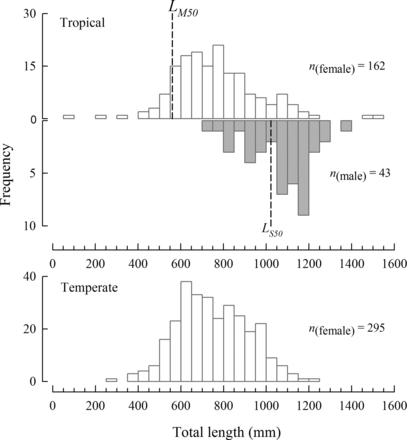
Length-frequency histograms for females (white bars) and males (grey bars) of Hyporthodus octofasciatus from the tropical region (above) and females only from the temperate region (below) of Western Australia (sample sizes shown, n). The dashed lines represent the corresponding lengths at 50% maturity for females (560 mm, LM50, see Figure 7) and sex change to males (1022 mm, LS50, see Figure 8) for the tropical region.
Growth increments were visible as alternating translucent and opaque bands in sectioned otoliths when viewed under reflected light. One translucent followed by one opaque band was considered to represent growth over 12 months following age validation results from bomb radiocarbon analysis (Andrews et al., 2011). The IAPE between the counts of annuli from two readers was 4.9%. Annuli counts by the secondary reader for the randomly selected subset of otoliths concurred with 40% of the counts made by the primary reader. No annuli counts by the secondary reader differed by more than two from the primary reader.
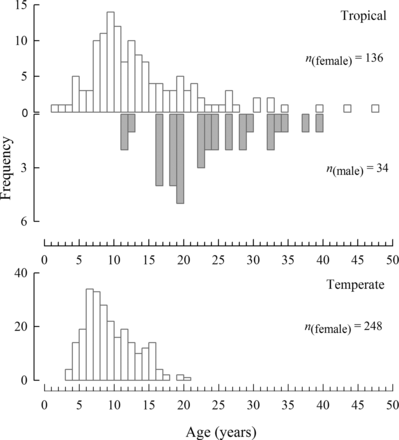
The age of H. octofasciatus from the tropical region ranged from 0.7–46.6 years for females and 11.2–38.6 years for males (Figure 3). The age composition of females from this region increased from one individual in the 1+ age class to a maximum of 14 fish in the 9+ age class, before gradually declining in frequency through older ages (Figure 3). The maximum frequency of male H. octofasciatus in this region occurred at an older age with five fish recorded in the 19+ age class. In comparison, the age-frequency distribution of females from the temperate region peaked at a younger age (6+ age class) and frequencies in older age groups declined faster to a longevity of less than half that of the tropical region (i.e. 20.4 years, Figure 3). The youngest recorded female from the temperate region was 3.2 years. A smaller female at 262 mm LT was sampled from the temperate region, however otoliths were not obtained and thus an age of ∼16 months was estimated from the von Bertalanffy growth equation.
Age–frequency histograms for females (white bars) and males (grey bars) of Hyporthodus octofasciatus from the tropical region (above) and females only from the temperate region (below) of Western Australia (sample sizes shown, n).
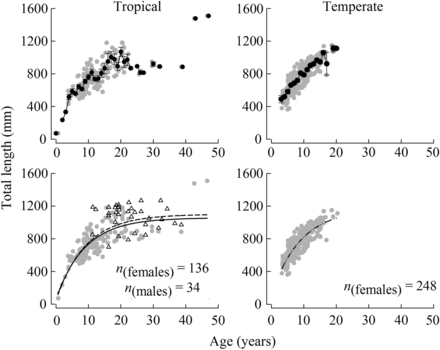
The mean total length of females from the tropical region increased from 517 mm at five years to 952 mm at 16 years (39.5 mm year−1), with very little increase, typical of an asymptote, displayed after this age, e.g. mean LT of 922 mm at 30 years (Figure 4). The mean length of females from the temperate region increased relatively linearly with age from 491 mm at four years to 1059 mm at 16 years (47.3 mm year−1, Figure 4). As only three individual females > 17 years were recorded in that region, the trend in the mean length above that age was uncertain. The von Bertalanffy growth curves of females from the tropical and temperate regions were significantly different (P < 0.001, Figure 5, Table 1). However, there was insufficient evidence to reject the null hypothesis of there being no difference in the relative condition of H. octofasciatus between regions, from comparing the slopes (F1,155 = 0.56, p = 0.46) and intercepts (F1,155 = 0.93, p = 0.34) of the log-transformed linear weight on length relationships. The estimated LT from the von Bertalanffy equations attained by females at ages 5, 10 and 15 years, from the tropical region were 502, 744 and 868 mm, compared with 569, 825 and 964 mm, respectively, from the temperate region. On average, the difference in the lengths of females from the temperate region, estimated from those curves, was always ≥ 9% larger than that of the tropical region between the ages of two and 17 years, which were the ages best represented in the samples (Figure 5).
Mean length-at-age (±1 s.e., above) and age–length relationships (below) fitted with von Bertalanffy growth curves for females (grey circles) of Hyporthodus octofasciatus from the tropical (left, solid line) and temperate (right) regions of Western Australia (sample sizes shown, n). Males (triangles, bottom left) were only recorded in the tropical region and have been combined with females in a second von Bertalanffy growth curve (dashed line).
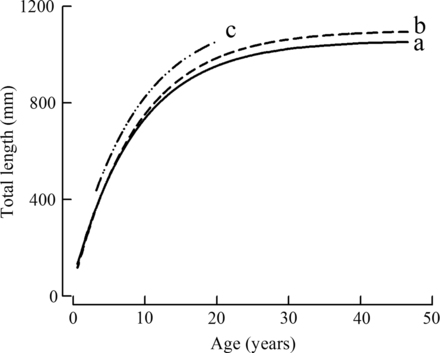
Comparison of von Bertalanffy growth curves of female Hyporthodus octofasciatus from the tropical region (a, solid line), females and males combined from the tropical region (b, dashed line) and females from the temperate region (c, dash-dot line) of Western Australia.
Comparison of von Bertalanffy growth parameters (L∞,k, t0), mortality estimates (Z, M, F), longevity and method of growth determination for the 14 species that comprise the monophyletic clade of the deep bodied grouper genus Hyporthodus in the Epinephelidae (Craig et al., 2011).
| Species . | Growth parameters . | Mortality estimates . | Max. Longevity (year) . | Method of Ageing . | Reproductive mode . | Locality . | Reference . | IUCN Red List Classification . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L∞ (mm) . | k (yr–1) . | to (yr) . | Z . | M . | F . | |||||||
| Hyporthodus acanthistius (Gilbert, 1892) | Least concern | |||||||||||
| Hyporthodus darwinensis (Randall and Heemstra, 1991) | Data deficient | |||||||||||
| Hyporthodus ergastularius (Whitley, 1930) | Least concern | |||||||||||
| Hyporthodus exsul (Fowler, 1944) | Data deficient | |||||||||||
| Hyporthodus flavolimbatus (Poey, 1865) | Vulnerable | |||||||||||
| 891 | 0.163 | 15 | Prot. H. | South Carolina | (Keener, 1984) | |||||||
| 831 | 0.191 | 27 | TS | E Gulf of Mexico | (Bullock and Godcharles, 1984) | |||||||
| Prot. H. | (Bullock et al., 1996) | |||||||||||
| 963 | 0.099 | –0.08 | 35 | TS | Trinidad & Tobago | (Manickchand-Heileman and Phillip, 2000) | ||||||
| Prot. H. | (Musick et al., 2000) | |||||||||||
| 85 | TS (B) | Gulf of Mexico | (Cook et al., 2009) | |||||||||
| Hyporthodus haifensis (Ben-Tuvia, 1953) | Data deficient | |||||||||||
| Hyporthodus mystacinus (Poey, 1852) | Least concern | |||||||||||
| 0.14 | (Craig et al., 2011) | |||||||||||
| Hyporthodus nigritus (Holbrook, 1855) | Critically endangered | |||||||||||
| 2394 | 0.1156 | –0.927 | 41 | TS | (Manooch and Mason, 1987) | |||||||
| Prot. H. | (Parker and Mays, 1998) | |||||||||||
| Prot. H. | (Musick et al., 2000) | |||||||||||
| Hyporthodus niphobles (Gilbert and Starks, 1897) | Data deficient | |||||||||||
| Hyporthodus niveatus (Valenciennes, 1828) | Vulnerable | |||||||||||
| 1320 | 0.087 | –1.013 | 0.175a | 27 | TS | Prot. H. | Florida Keys | (Moore and Labisky, 1984) | ||||
| 1255 | 0.074 | –1.92 | 0.15 | 25 | TS | S Atlantic Bight | (Matheson and Huntsman, 1984) | |||||
| 1993–1994 (com. SR) | 1201 | 0.103 | –1.149 | 29 | TS | Prot. H. | N & S Carolina | (Wyanski et al., 2000) | ||||
| 1982–1985 (res.) | 948 | 0.122 | –0.668 | TS | N & S Carolina | (Wyanski et al., 2000) | ||||||
| 1993–1996 (com. LL) | 1117 | 0.119 | –1.1409 | TS | N & S Carolina | (Wyanski et al., 2000) | ||||||
| Prot. H. | (Musick et al., 2000) | |||||||||||
| Hyporthodus octofasciatus (Griffin, 1926) | Data deficient | |||||||||||
| Tropical (♀) | 1057 | 0.11 | –0.59 | 0.09 | 47 | TS | Prot. H. | Western Australia | This study | |||
| Tropical (comb) | 1100 | 0.11 | –0.41 | 0.09 | 47 | TS | Western Australia | This study | ||||
| Temperate (♀) | 1166 | 0.11 | –1.00 | 20 | TS | Western Australia | This study | |||||
| 43 | TS (B) | Western Australia | (Andrews et al., 2011) | |||||||||
| Hyporthodus perplexus (Randall, Hoese & Last, 1991) | Data deficient | |||||||||||
| Hyporthodus quernus (Seale, 1901) | Near threatened | |||||||||||
| Prot. H. | (Craig et al., 2011) | |||||||||||
| 1080–1186 | 0.119–0.126 | 0.233–0.253b | Hawaii | (Moffitt, 2006) | ||||||||
| 823 | 0.157 | –1.45 | 34 | TS | (Nichols and DeMartini, 2008) | |||||||
| Hyporthodus septemfasciatus (Thunberg, 1793) | Least concern | |||||||||||
| Prot. H. | (Craig et al., 2011) | |||||||||||
| Species . | Growth parameters . | Mortality estimates . | Max. Longevity (year) . | Method of Ageing . | Reproductive mode . | Locality . | Reference . | IUCN Red List Classification . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L∞ (mm) . | k (yr–1) . | to (yr) . | Z . | M . | F . | |||||||
| Hyporthodus acanthistius (Gilbert, 1892) | Least concern | |||||||||||
| Hyporthodus darwinensis (Randall and Heemstra, 1991) | Data deficient | |||||||||||
| Hyporthodus ergastularius (Whitley, 1930) | Least concern | |||||||||||
| Hyporthodus exsul (Fowler, 1944) | Data deficient | |||||||||||
| Hyporthodus flavolimbatus (Poey, 1865) | Vulnerable | |||||||||||
| 891 | 0.163 | 15 | Prot. H. | South Carolina | (Keener, 1984) | |||||||
| 831 | 0.191 | 27 | TS | E Gulf of Mexico | (Bullock and Godcharles, 1984) | |||||||
| Prot. H. | (Bullock et al., 1996) | |||||||||||
| 963 | 0.099 | –0.08 | 35 | TS | Trinidad & Tobago | (Manickchand-Heileman and Phillip, 2000) | ||||||
| Prot. H. | (Musick et al., 2000) | |||||||||||
| 85 | TS (B) | Gulf of Mexico | (Cook et al., 2009) | |||||||||
| Hyporthodus haifensis (Ben-Tuvia, 1953) | Data deficient | |||||||||||
| Hyporthodus mystacinus (Poey, 1852) | Least concern | |||||||||||
| 0.14 | (Craig et al., 2011) | |||||||||||
| Hyporthodus nigritus (Holbrook, 1855) | Critically endangered | |||||||||||
| 2394 | 0.1156 | –0.927 | 41 | TS | (Manooch and Mason, 1987) | |||||||
| Prot. H. | (Parker and Mays, 1998) | |||||||||||
| Prot. H. | (Musick et al., 2000) | |||||||||||
| Hyporthodus niphobles (Gilbert and Starks, 1897) | Data deficient | |||||||||||
| Hyporthodus niveatus (Valenciennes, 1828) | Vulnerable | |||||||||||
| 1320 | 0.087 | –1.013 | 0.175a | 27 | TS | Prot. H. | Florida Keys | (Moore and Labisky, 1984) | ||||
| 1255 | 0.074 | –1.92 | 0.15 | 25 | TS | S Atlantic Bight | (Matheson and Huntsman, 1984) | |||||
| 1993–1994 (com. SR) | 1201 | 0.103 | –1.149 | 29 | TS | Prot. H. | N & S Carolina | (Wyanski et al., 2000) | ||||
| 1982–1985 (res.) | 948 | 0.122 | –0.668 | TS | N & S Carolina | (Wyanski et al., 2000) | ||||||
| 1993–1996 (com. LL) | 1117 | 0.119 | –1.1409 | TS | N & S Carolina | (Wyanski et al., 2000) | ||||||
| Prot. H. | (Musick et al., 2000) | |||||||||||
| Hyporthodus octofasciatus (Griffin, 1926) | Data deficient | |||||||||||
| Tropical (♀) | 1057 | 0.11 | –0.59 | 0.09 | 47 | TS | Prot. H. | Western Australia | This study | |||
| Tropical (comb) | 1100 | 0.11 | –0.41 | 0.09 | 47 | TS | Western Australia | This study | ||||
| Temperate (♀) | 1166 | 0.11 | –1.00 | 20 | TS | Western Australia | This study | |||||
| 43 | TS (B) | Western Australia | (Andrews et al., 2011) | |||||||||
| Hyporthodus perplexus (Randall, Hoese & Last, 1991) | Data deficient | |||||||||||
| Hyporthodus quernus (Seale, 1901) | Near threatened | |||||||||||
| Prot. H. | (Craig et al., 2011) | |||||||||||
| 1080–1186 | 0.119–0.126 | 0.233–0.253b | Hawaii | (Moffitt, 2006) | ||||||||
| 823 | 0.157 | –1.45 | 34 | TS | (Nichols and DeMartini, 2008) | |||||||
| Hyporthodus septemfasciatus (Thunberg, 1793) | Least concern | |||||||||||
| Prot. H. | (Craig et al., 2011) | |||||||||||
TS = transverse sections, B = bomb radiocarbon dating used to validate age estimates derived from transverse sections of otoliths, comb = sexes combined, ♀ = female, Prot. H. = protogynous hermaphrodite, a = estimates of total mortality Z, derived from catch curves, b = estimates of natural mortality, M, derived mainly from the equation of Ralston (1987), com. SR = commercial snapper reels, res. = research surveys, comm. LL = commercial longline.
Comparison of von Bertalanffy growth parameters (L∞,k, t0), mortality estimates (Z, M, F), longevity and method of growth determination for the 14 species that comprise the monophyletic clade of the deep bodied grouper genus Hyporthodus in the Epinephelidae (Craig et al., 2011).
| Species . | Growth parameters . | Mortality estimates . | Max. Longevity (year) . | Method of Ageing . | Reproductive mode . | Locality . | Reference . | IUCN Red List Classification . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L∞ (mm) . | k (yr–1) . | to (yr) . | Z . | M . | F . | |||||||
| Hyporthodus acanthistius (Gilbert, 1892) | Least concern | |||||||||||
| Hyporthodus darwinensis (Randall and Heemstra, 1991) | Data deficient | |||||||||||
| Hyporthodus ergastularius (Whitley, 1930) | Least concern | |||||||||||
| Hyporthodus exsul (Fowler, 1944) | Data deficient | |||||||||||
| Hyporthodus flavolimbatus (Poey, 1865) | Vulnerable | |||||||||||
| 891 | 0.163 | 15 | Prot. H. | South Carolina | (Keener, 1984) | |||||||
| 831 | 0.191 | 27 | TS | E Gulf of Mexico | (Bullock and Godcharles, 1984) | |||||||
| Prot. H. | (Bullock et al., 1996) | |||||||||||
| 963 | 0.099 | –0.08 | 35 | TS | Trinidad & Tobago | (Manickchand-Heileman and Phillip, 2000) | ||||||
| Prot. H. | (Musick et al., 2000) | |||||||||||
| 85 | TS (B) | Gulf of Mexico | (Cook et al., 2009) | |||||||||
| Hyporthodus haifensis (Ben-Tuvia, 1953) | Data deficient | |||||||||||
| Hyporthodus mystacinus (Poey, 1852) | Least concern | |||||||||||
| 0.14 | (Craig et al., 2011) | |||||||||||
| Hyporthodus nigritus (Holbrook, 1855) | Critically endangered | |||||||||||
| 2394 | 0.1156 | –0.927 | 41 | TS | (Manooch and Mason, 1987) | |||||||
| Prot. H. | (Parker and Mays, 1998) | |||||||||||
| Prot. H. | (Musick et al., 2000) | |||||||||||
| Hyporthodus niphobles (Gilbert and Starks, 1897) | Data deficient | |||||||||||
| Hyporthodus niveatus (Valenciennes, 1828) | Vulnerable | |||||||||||
| 1320 | 0.087 | –1.013 | 0.175a | 27 | TS | Prot. H. | Florida Keys | (Moore and Labisky, 1984) | ||||
| 1255 | 0.074 | –1.92 | 0.15 | 25 | TS | S Atlantic Bight | (Matheson and Huntsman, 1984) | |||||
| 1993–1994 (com. SR) | 1201 | 0.103 | –1.149 | 29 | TS | Prot. H. | N & S Carolina | (Wyanski et al., 2000) | ||||
| 1982–1985 (res.) | 948 | 0.122 | –0.668 | TS | N & S Carolina | (Wyanski et al., 2000) | ||||||
| 1993–1996 (com. LL) | 1117 | 0.119 | –1.1409 | TS | N & S Carolina | (Wyanski et al., 2000) | ||||||
| Prot. H. | (Musick et al., 2000) | |||||||||||
| Hyporthodus octofasciatus (Griffin, 1926) | Data deficient | |||||||||||
| Tropical (♀) | 1057 | 0.11 | –0.59 | 0.09 | 47 | TS | Prot. H. | Western Australia | This study | |||
| Tropical (comb) | 1100 | 0.11 | –0.41 | 0.09 | 47 | TS | Western Australia | This study | ||||
| Temperate (♀) | 1166 | 0.11 | –1.00 | 20 | TS | Western Australia | This study | |||||
| 43 | TS (B) | Western Australia | (Andrews et al., 2011) | |||||||||
| Hyporthodus perplexus (Randall, Hoese & Last, 1991) | Data deficient | |||||||||||
| Hyporthodus quernus (Seale, 1901) | Near threatened | |||||||||||
| Prot. H. | (Craig et al., 2011) | |||||||||||
| 1080–1186 | 0.119–0.126 | 0.233–0.253b | Hawaii | (Moffitt, 2006) | ||||||||
| 823 | 0.157 | –1.45 | 34 | TS | (Nichols and DeMartini, 2008) | |||||||
| Hyporthodus septemfasciatus (Thunberg, 1793) | Least concern | |||||||||||
| Prot. H. | (Craig et al., 2011) | |||||||||||
| Species . | Growth parameters . | Mortality estimates . | Max. Longevity (year) . | Method of Ageing . | Reproductive mode . | Locality . | Reference . | IUCN Red List Classification . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L∞ (mm) . | k (yr–1) . | to (yr) . | Z . | M . | F . | |||||||
| Hyporthodus acanthistius (Gilbert, 1892) | Least concern | |||||||||||
| Hyporthodus darwinensis (Randall and Heemstra, 1991) | Data deficient | |||||||||||
| Hyporthodus ergastularius (Whitley, 1930) | Least concern | |||||||||||
| Hyporthodus exsul (Fowler, 1944) | Data deficient | |||||||||||
| Hyporthodus flavolimbatus (Poey, 1865) | Vulnerable | |||||||||||
| 891 | 0.163 | 15 | Prot. H. | South Carolina | (Keener, 1984) | |||||||
| 831 | 0.191 | 27 | TS | E Gulf of Mexico | (Bullock and Godcharles, 1984) | |||||||
| Prot. H. | (Bullock et al., 1996) | |||||||||||
| 963 | 0.099 | –0.08 | 35 | TS | Trinidad & Tobago | (Manickchand-Heileman and Phillip, 2000) | ||||||
| Prot. H. | (Musick et al., 2000) | |||||||||||
| 85 | TS (B) | Gulf of Mexico | (Cook et al., 2009) | |||||||||
| Hyporthodus haifensis (Ben-Tuvia, 1953) | Data deficient | |||||||||||
| Hyporthodus mystacinus (Poey, 1852) | Least concern | |||||||||||
| 0.14 | (Craig et al., 2011) | |||||||||||
| Hyporthodus nigritus (Holbrook, 1855) | Critically endangered | |||||||||||
| 2394 | 0.1156 | –0.927 | 41 | TS | (Manooch and Mason, 1987) | |||||||
| Prot. H. | (Parker and Mays, 1998) | |||||||||||
| Prot. H. | (Musick et al., 2000) | |||||||||||
| Hyporthodus niphobles (Gilbert and Starks, 1897) | Data deficient | |||||||||||
| Hyporthodus niveatus (Valenciennes, 1828) | Vulnerable | |||||||||||
| 1320 | 0.087 | –1.013 | 0.175a | 27 | TS | Prot. H. | Florida Keys | (Moore and Labisky, 1984) | ||||
| 1255 | 0.074 | –1.92 | 0.15 | 25 | TS | S Atlantic Bight | (Matheson and Huntsman, 1984) | |||||
| 1993–1994 (com. SR) | 1201 | 0.103 | –1.149 | 29 | TS | Prot. H. | N & S Carolina | (Wyanski et al., 2000) | ||||
| 1982–1985 (res.) | 948 | 0.122 | –0.668 | TS | N & S Carolina | (Wyanski et al., 2000) | ||||||
| 1993–1996 (com. LL) | 1117 | 0.119 | –1.1409 | TS | N & S Carolina | (Wyanski et al., 2000) | ||||||
| Prot. H. | (Musick et al., 2000) | |||||||||||
| Hyporthodus octofasciatus (Griffin, 1926) | Data deficient | |||||||||||
| Tropical (♀) | 1057 | 0.11 | –0.59 | 0.09 | 47 | TS | Prot. H. | Western Australia | This study | |||
| Tropical (comb) | 1100 | 0.11 | –0.41 | 0.09 | 47 | TS | Western Australia | This study | ||||
| Temperate (♀) | 1166 | 0.11 | –1.00 | 20 | TS | Western Australia | This study | |||||
| 43 | TS (B) | Western Australia | (Andrews et al., 2011) | |||||||||
| Hyporthodus perplexus (Randall, Hoese & Last, 1991) | Data deficient | |||||||||||
| Hyporthodus quernus (Seale, 1901) | Near threatened | |||||||||||
| Prot. H. | (Craig et al., 2011) | |||||||||||
| 1080–1186 | 0.119–0.126 | 0.233–0.253b | Hawaii | (Moffitt, 2006) | ||||||||
| 823 | 0.157 | –1.45 | 34 | TS | (Nichols and DeMartini, 2008) | |||||||
| Hyporthodus septemfasciatus (Thunberg, 1793) | Least concern | |||||||||||
| Prot. H. | (Craig et al., 2011) | |||||||||||
TS = transverse sections, B = bomb radiocarbon dating used to validate age estimates derived from transverse sections of otoliths, comb = sexes combined, ♀ = female, Prot. H. = protogynous hermaphrodite, a = estimates of total mortality Z, derived from catch curves, b = estimates of natural mortality, M, derived mainly from the equation of Ralston (1987), com. SR = commercial snapper reels, res. = research surveys, comm. LL = commercial longline.
Spawning period
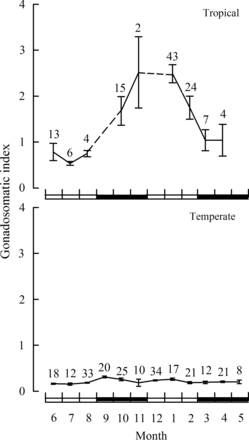
The mean monthly GSIs for female H. octofasciatus ≥ the LM50 from the tropical region of Western Australia was relatively low (<1.0) from June through August (Figure 6). The first appearance of gonadal recrudescence (Stages II and III) occurred in October and was accompanied by an increase in GSI to 1.7 (± 0.3 se, Figure 6). Note, it is possible that gonad development commenced one month earlier in September, but no samples were collected for that month. The highest mean monthly GSIs were recorded during November and January at ca 2.5 before declining to 1.7 (± 0.3 se) in February and to < 1.0 in both March and April (Figure 6). Ovaries containing hydrated oocytes (Stage IV) were recorded from October to February, with gonadal regression (Stage V) most prevalent in February and March. In contrast, mean monthly GSIs of female H. octofasciatus caught in the temperate region of Western Australia were less than 0.31 for all months of the year (Figure 6). Ovaries of all fish from the temperate region were always recorded as immature/resting (Stage I).
Mean monthly gonadosomatic indices (GSI, ± 1 s.e.) of female Hyporthodus octofasciatus from the tropical and temperate regions of Western Australia. The data have been pooled for corresponding months (sample sizes shown) and are limited to fish ≥ the length at 50% maturity of 560 mm (LM50, see Figure 7). On the x-axis, white rectangles represent winter and summer, and black rectangles spring and autumn.
Maturity and sex change
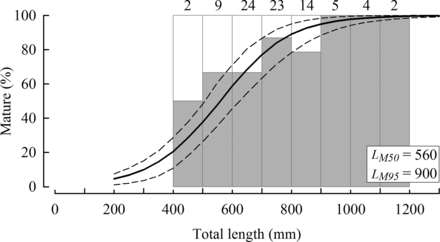
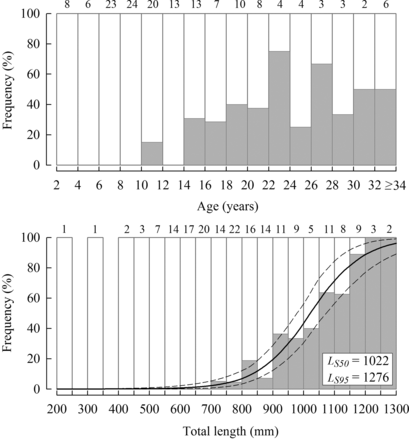
On the basis of their macroscopic appearance, the smallest mature (Stage II–V) female sampled from the tropical region during the spawning season measured 495 mm LT (Figure 7), and the youngest such female was 6 years of age. The proportion of mature females from this region increased from 50% in the 400–499 mm length class, to > 65% in the 500–899 mm length classes and 100% in fish ≥900 mm LT (Figure 7). The estimated length at which 50% and 95% of H. octofasciatus attain maturity in the tropical region was 560 and 900 mm LT, respectively (Figure 7). The estimated ages from the von Bertalanffy growth equation (see below) at which H. octofasciatus reaches these lengths at maturity in the tropical region were 6.1 and 16.4 years, respectively. No mature (Stage II–V) females were recorded in the temperate region.
Percentage-frequency of immature (Stage I, white bars) and mature (Stages II–V, grey bars) females of Hyporthodus octofasciatus in sequential 100-mm length classes (<1300 mm LT) collected during the spawning months (October–February) from the tropical region of Western Australia (sample sizes noted above histograms). The logistic curve (solid line) and its 95% CI (dashed lines) represent the expected percentage of mature females at corresponding lengths.
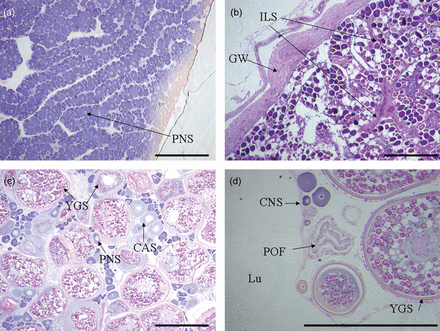
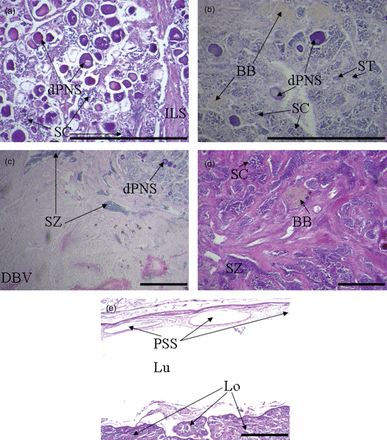
Microscopic examination of gonads was consistent with the conclusions of no males and an absence of evidence for spawning in the temperate region, although the number of specimens analysed from the temperate region was small (n = 6). Only inactive ovaries were observed, which were staged as either immature (n = 2) or inactive (n = 4). Immature ovaries were characterized by long thin lamellae that were densely packed with previtellogenic oocytes and relatively thin ovarian walls (Figure 8a). Inactive ovaries were characterized by a marked thickening in the ovarian wall, thicker and more prevalent eosinophilic intra-lamellar stromal strands, and lamellae that were less densely packed with previtellogenic oocytes and not as long and thin and more irregular than in immature ovaries (Figure 8b). Owing to the small number of samples examined histologically for the temperate region and the absence of definitive characteristics indicating prior spawning (e.g. brown bodies or atretic oocytes), it was not possible to identify with certainty any mature ovaries in that region. For tropical samples, asynchronous oocyte development was observed in developing (n = 2), developed (n = 10, Figure 8c), and spawning (n = 14) ovaries. Spawning ovaries were characterized by the presence of hydrated oocytes and/or postovulatory follicles (Figure 8d).
Histological features of ovarian development of Hyporthodus octofasciatus from tropical (a, c, d) and temperate (b) regions. a) Immature ovary from an individual measuring 559 mm in total length; b) inactive ovary from an individual measuring 817 mm and 10 years of age; c) developed ovary from an individual measuring 562 mm and 10 years of age; d) spawning ovary from an individual measuring 1180 mm and 15 years of age. CAS = cortical alveoli stage oocytes, CNS = chromatin nucleolus stage oocytes, GW = gonad wall, ILS = intra-lamellar stromal strands, Lu = lumen, PNS = perinucleolus stage oocytes, POF = postovulatory follicle, YGS = yolk globule stage oocytes (scale bar: 500 µm).
The proportion of males in samples from the tropical region increased with length and age (Figure 9). Given the observed length (72–1509 mm LT) and age range (0.7–46.6 years) for the tropical region in this study, the fact that males were not sampled until 700 mm LT and 11.2 years of age, which were greater than the LM50 of 560 mm and derived age at maturity of 6.1 years, respectively, is a good indication of monandric protogyny. The estimated lengths at which 50% and 95% of H. octofasciatus transitioned to male in the tropical region were 1022 and 1276 mm LT, respectively (Figure 9).
Percentage-frequency of female (white bars) and male (grey bars) Hyporthodus octofasciatus in two-year age-class categories (above) and sequential 50-mm length classes (<1300 mm LT, below) from the tropical region of Western Australia (sample sizes noted above histograms). The logistic curve (solid line) and its 95% CI (dashed lines) represent the expected percentage of males at corresponding lengths.
Gonads of two individuals that were sectioned histologically contained both ovarian and testicular tissue that were intermixed on lamellar structures and were thus of the undelimited Type Two arrangement (Sadovy and Shapiro, 1987). The lamellae of one of those gonads comprised (degenerating) previtellogenic oocytes and both spermatogonia and spermatocytes in crypts, i.e. immature testicular tissue sensuGrier and Taylor (1998, Figure 10a). This individual was thus classified as bisexual. The lamellae of the other gonad were primarily comprised of testicular tissue, i.e. spermatocytes and spermatids, in conjunction with degenerating previtellogenic oocytes and also brown bodies (Figure 10b). Brown bodies provided evidence of previous ovarian function. This gonad was presumably at a late stage of functional protogynous sex change and was categorized as transitional. Degenerating previtellogenic oocytes (Figure 10c) and brown bodies (Figure 10d) were also observed in functional testes, which exhibited spermatozoa located in sperm sinuses in the outer gonad wall, indicating further evidence in support of protogyny. All of the testes examined histologically contained a vestigial ventral in situ ovarian lumen, while mature testes also contained peripheral sperm sinuses in their gonad wall (Figure 10e).
Histological evidence for protogynous sex change. a) Bisexual gonad comprised primarily of ovarian tissue with developing crypts of spermatocytes from an individual measuring 1177 mm and 20 years of age; b) transitional gonad showing signs of previous reproductive activity as a female by the presence of brown bodies, degenerating previtellogenic oocytes from an individual measuring 847 mm and 27 years of age; c) functional testis with small number of degenerating previtellogenic oocytes from an individual measuring 1082 mm and 16 years of age; d) testis containing brown bodies from an individual measuring 905 mm and 21 years of age; e) testis in (d) showing vestigial ovarian lumen with peripheral sperm sinus on the ventral side of the gonad. BB = brown body, CAS = cortical alveoli stage oocytes, DBV = dorsal blood vessel, dPNS = degenerating perinucleolus stage oocytes, ILS = intra-lamellar stromal strands, Lo = lobular tissue containing developing sperm, Lu = lumen, PSS = peripheral sperm sinus, SC = spermatocytes, ST = spermatids, SZ = spermatozoa (scale bar: 500 µm).
Discussion
Rarely do studies on the age, growth and reproduction of a teleost span such a large latitudinal distribution as that covered in this study of H. octofasciatus. This species was sampled from the upper continental slope at depths of 105–480 m, over an exceptionally large latitudinal range (12–35°S) spanning tropical and temperate waters along the Western Australian coast (ca 3500 km). This is the first study investigating the life history characteristics of this species and thus could contribute towards the revision of its IUCN red list status from “Data Deficient” (To and Pollard, 2008). Further, the results of this study, in association with those from other species of the genus Hyporthodus, allow us to characterize the likely population parameters for this data-limited group and to construct a general risk profile for this species in light of the potential for ongoing and increased rates of exploitation. Individuals of H. octofasciatus in samples reached a relatively large size (maximum 1509 mm LT, estimated weight 66.5 kg), which combined with an excellent palatability increases their value among commercial and recreational anglers. Results on the life history characteristics of this species, and observed latitudinal variation throughout its range will therefore add to critical knowledge requirements for managing its harvest by all fishing sectors.
Age, growth and longevity
Ageing precision of H. octofasciatus based on counts of opaque zones from thin otolith sections were found to be within acceptable bounds, i.e. IAPE < 5.5% (Campana, 2001), but slightly less precise than other deep-water species (e.g. Wakefield et al., 2010). Both the mean length at each age and estimated lengths-at-age of females from the von Bertalanffy equation were markedly higher (>9%) in the temperate region. It is uncertain whether this larger length-at-age at higher latitudes represents the temperature-size relationship expressed by ectotherms in accordance with certain ecological theories (e.g. Metabolic Theory of Ecology, MTE, Atkinson, 1994; Arendt, 2010), or a result of energy partitioned toward growth in the absence of reproduction. The larger length-at-age of H. octofasciatus at higher latitudes is consistent with the growth patterns expressed by many teleosts along the Western Australian coastline, all of which are reproductively active (e.g. Cossington et al., 2010; Lek et al., 2012). In contrast, a parabolic relationship between length-at-age and water temperature (latitude) is expressed by Pagrus auratus, where a greater length-at-age is attained at the centre of its latitudinal distribution in Australia and New Zealand with reduced performance occurring towards the ends of that range (Wakefield, 2006). It cannot be ruled out that H. octofasciatus achieved a greater length-at-age in the temperate region as a result of energy partitioned toward growth in the absence of reproduction considering the mean length-at-age in the temperate region was conspicuously linear through all ages sampled, which extended past the ages at which this species matured in the tropical region. These varying growth trajectories for H. octofasciatus are consistent with that described by Lester et al. (2004), who postulated that growth of teleosts is best described using two models, reflecting two subsequent life history stages. This growth model proposed that prior to maturation somatic growth is relatively linear, and that the von Bertalanffy growth equation is more appropriate for describing post-maturational growth (Lester et al., 2004).
Longevity estimates were more than twice that observed for the northern tropical than southern temperate region (i.e. 47 vs. 20). This trend contradicts the positive correlations that have been demonstrated between longevity and water temperature in accordance with the MTE (Munch and Salinas, 2009). The latitudinal trend in longevity recorded for H. octofasciatus in Western Australia may be explained by the fact that settlement and survival of recruits in the southern temperate waters are reliant on spawning and transport from northern tropical waters, and thus subject to relatively large variations in environmental conditions. If the age structure of H. octofasciatus in the southern waters of Western Australia is a good indication of recruitment success, their consistent abundance in recent cohorts suggests conditions have been favourable since the early 1980s. It is possible that recent warming trends in water temperatures along the lower west coast of Western Australia since the 1950s, particularly during the austral autumn–winter, may have improved the chances of H. octofasciatus settling and surviving in more southern temperate waters in recent years (Pearce and Feng, 2007; Caputi et al., 2009). The maximum longevity of 47 years for H. octofasciatus in this study is similar to that of other Hyporthodus species (e.g. Manooch and Mason, 1987; Manickchand-Heileman and Phillip, 2000; Wyanski et al., 2000), but only about half that recorded for H. flavolimbatus from the Gulf of Mexico (e.g. 85 years, Table 1, Cook et al., 2009).
Reproductive mode
Multiple macroscopic and microscopic characteristics of sampled gonads were used to determine the reproductive mode of H. octofasciatus (Sadovy and Shapiro, 1987; Sadovy de Mitcheson and Liu, 2008). In the tropical waters of Western Australia all H. octofasciatus < 700 mm LT and 11 years of age were females. The length and age of the smallest and youngest male (700 mm LT and 11.2 years) were much greater than the length at which 50% of females reached maturity (LM50 = 560 mm LT) and the estimated age at which this occurred (6.1 years). The prevalence of males increased with increasing length, to essentially 100% of the largest fish collected (1200–1300 mm LT). Although males were present in almost all age classes above 10 years in tropical waters, females were recorded in some of the oldest age classes, indicating that not all females change to male. Further sampling of the upper age range would help determine such prevalences. Microscopic examination of gonads sampled from the northern tropical waters identified one individual undergoing protogynous sex transition, which was evident from the presence of both degenerating female (previtellogenic oocytes and brown bodies) and developing male (germ cell stages less advanced than spermatozoa) characteristics in the gonads. Further, all testes of this species possessed a central lumen, and mature testes often contained spermatozoa located in sperm sinuses in the gonad wall. The combination of the above lines of evidence is consistent with monandric protogynous hermaphroditism, whereby all males are derived from mature females. This reproductive mode appears to be consistent among the Hyporthodus genus (e.g. Heemstra and Randall, 1993; Wyanski et al., 2000). On this basis, the length at which 50% of H. octofasciatus change sex is ca 1022 mm LT and ≥ 11 years of age.
Reproductive period and spawning omission in the temperate region
The spawning period of H. octofasciatus was determined from trends exhibited throughout the year by the prevalence of stages in gonad development and the mean monthly GSIs. Individuals in spawning condition were only recorded in the northern tropical region, where the majority of spawning occurred during the austral spring and summer from October to February.
Reproductive characteristics of H. octofasciatus in southern temperate waters varied significantly from those observed in northern tropical waters in a manner that suggested no evidence of reproduction south of ca 30°S in WA. Both macroscopic and microscopic examination of gonads revealed all H. octofasciatus sampled in the temperate region were immature or inactive (Stage I) females. For inactive ovaries, there was no definitive histological evidence to indicate that any of these females had previously matured and spawned. In addition, the mean monthly GSIs were always lower than those recorded for any month in the tropical region where a spawning season was clearly identified. Monthly sample sizes of H. octofasciatus (collected throughout all months of the year) included a wide length (262–1230 mm LT) and age range (3.2–20.4 years), and were thus considered to provide an adequate representation of seasonal reproductive activity for the temperate region. That a much smaller number of fish sampled from the tropical region were sufficient to demonstrate a seasonal pattern in reproduction lends further support to the observed lack of seasonal reproductive activity for the temperate region.
Reproductive seasonality in teleosts is common, with the primary environmental cues for spawning (or gonadal recrudescence) suggested to be water temperature and photoperiod (Pankhurst and Porter, 2003; Wakefield, 2010). Given the apparent spawning omission for H. octofasciatus in temperate waters, and considering this species spawns only during the warmer months, i.e. spring and summer, in the tropical region, it is expected that spawning of this species could be temperature-dependent. In which case, the annual maximum water temperatures in the temperate region may be lower than the threshold required for gonad recrudescence. Unfortunately, water temperature data from the depths occupied by this species in the tropical waters of Western Australia are currently unavailable. Therefore, the relationship between water temperature and reproduction in this poikilotherm could not be assessed. Further investigation into correlations between reproduction and environmental variables is required to elucidate the potential mechanisms influencing the spawning omission of H. octofasciatus at higher latitudes.
Management implications
Hyporthodus octofasciatus is part of the deep-water fish complex inhabiting the upper continental slope (typically >200 m depth) across all management Bioregions in Western Australia. Due to its high value, wide distribution and high inherent vulnerability this species has been selected as an indicator species for this suite of offshore demersal scalefish in all marine bioregions of the state (Department of Fisheries, 2011). This species, like others inhabiting relatively deep continental slope waters, and the majority of groupers in general, is long-lived, slow growing and has low rates of natural mortality (Morato et al., 2006; Wakefield et al., 2010; Sadovy de Mitcheson et al., 2012). As such, the inherent vulnerability of this species is relatively high and the sustainable level of fishing exploitation is likely to be relatively low. Considering the depths this species inhabits, traditional management strategies such as bag, boat and size limits for single species within a multi-species fishery are not likely to be effective given the likely high rate of mortality associated with barotrauma. Current management arrangements for recreational fishers in Western Australia set a combined bag limit of two fish from a list of high risk species that occupy the continental slope (offshore demersal), per recreational fishing from boat license. The State-managed commercial fisheries for demersal scalefish in Western Australia are based on limited access and annual restrictions in total catch or fishing effort allocations in the northern and western coasts (Fletcher and Santoro, 2012); however, currently the commercial take of demersal scalefish from the south coast is not subject to such fishery-specific management regulations (Fletcher and Santoro, 2012). Hyporthodus octofasciatus is also harvested by the Commonwealth-managed Great Australian Bight Trawl Fishery and Western Deepwater Trawl Fishery that operates in waters adjacent to, or overlapping, the west and south coast State-managed fisheries in depths between 200 m and the Australian Fishing Zone boundary. Thus, future stock assessments for this species need to consider catch and effort statistics from both State and Commonwealth fisheries, as they are all likely to be harvesting the same stock.
The apparent spawning omission of H. octofasciatus in the temperate waters of Western Australia suggests that recruits to this region are most likely originating from spawning in the northern tropical waters, with progeny transported southward by the dominant poleward-flowing Leeuwin current (Caputi et al., 1996). If this is the case, then fishing exploitation of this species in the temperate region poses little risk to the breeding stock levels of the population. However, a higher emphasis may need to be placed on conserving spawning stocks in the northern tropical region to maintain the recruitment source for the broader Western Australian coastline. Until an improved understanding of the stock structure of H. octofasciatus is formed using more comprehensive methods (e.g. genetics or otolith microchemistry), the evidence provided in this study assumes the extensive distribution of H. octofasciatus in Western Australia represents a single population. Such limited genetic diversity has been found for other demersal species that occur along this coastline, e.g. Glaucosoma hebraicum (Berry et al., 2012). Future monitoring and assessment programs for this species should incorporate sampling in southern waters of the state to determine if the spawning range of H. octofasciatus begins to extend southward, and how this relates with the long-term warming trend in water temperatures occurring in the temperate region of Western Australia (Pearce and Feng, 2007; Caputi et al., 2009).
Funding
We gratefully acknowledge logistical and financial support from the Department of Fisheries, Government of Western Australia.
Acknowledgements
We would like to thank the crews of the RV “Flinders” and the RV “Naturaliste” and all the research staff, fishers and wholesalers across Western Australia who helped in the sourcing and collection of samples. We also thank Brett Molony and Shane O'Donoghue for providing useful comments on earlier versions of this manuscript.
References
Author notes
Handling editor: Francis Juanes