-
PDF
- Split View
-
Views
-
Cite
Cite
Lis Lindal Jørgensen, Pavel Ljubin, Hein Rune Skjoldal, Randi B. Ingvaldsen, Natalia Anisimova, Igor Manushin, Distribution of benthic megafauna in the Barents Sea: baseline for an ecosystem approach to management, ICES Journal of Marine Science, Volume 72, Issue 2, January/February 2015, Pages 595–613, https://doi.org/10.1093/icesjms/fsu106
Close - Share Icon Share
Abstract
Benthos plays a significant role as substrate, refuge from predation and food for a wide variety of fish and invertebrates of all life stages and should therefore be considered in the ecosystem approach (EA) to management. Epibenthos from trawl catches, used in annual assessments of commercial fish stocks, was identified and measured on-board. The 2011 dataset present the baseline mapping for monitoring and included 354 taxa (218 to species level) analysed with multivariate statistical methods. This revealed four main megafaunal regions: southwestern (SW), banks/slopes in southeast and west (SEW), northwestern (NW), and northeastern (NE) which were significantly related to depth, temperature, salinity, and number of ice-days. The SW region was dominated by filter-feeders (sponges) in the inflow area of warm Atlantic water while the deeper trenches had a detritivorous fauna (echinoderms). In the SEW region, predators (sea stars, anemones and snow crabs) prevailed together with filtrating species (sea cucumber and bivalves) within a mosaic of banks and slopes. Plankton-feeding brittlestars were common in the NW and NE region, but with increasing snow crab population in NE. Climate change, potentially expanding trawling activity, and increasing snow and king crab populations might all have impacts on the benthos. Benthos should therefore be a part of an integrated assessment of a changing sea, and national agencies might consider adding benthic taxonomic expertise on-board scientific research vessels to identify the invertebrate “by-catch” as part of routine trawl surveys.
Introduction
On Arctic continental shelves, a significant portion of the primary production passes through the epibenthos, which thus plays a significant role for energy flow and trophodynamics (e.g. Piepenburg et al., 1995; Piepenburg and Schmid, 1996a; Ambrose et al., 2001; Grebmeier et al., 2006). Many epibenthic organisms are ecologically important, providing substrate and structure to the benthic habitat (Brodeur, 2001; Tissot et al., 2006), refuges from predation (Malecha et al., 2005), and food for a wide variety of fish and invertebrates of all life stages. Our current understanding of the functional roles of many of the larger-bodied, long-lived species (e.g. as structure forming benthic communities) is limited and should be addressed to predict the outcome of continued fishing disturbances in areas where these animals occur (Collie et al., 2000). Generally, water column productivity is inversely related to ice cover (reviewed in Wassmann et al., 2006), and the benthic fauna exhibits a strong association with the overlying primary productivity regime (Piepenburg et al., 1997; Tremblay et al., 2011). In particular, the often dominant echinoderms on Arctic shelves play an important role in the redistribution and remineraliztion of the organic carbon reaching the seabed (Renaud et al., 2007; Bluhm et al., 2009; Blicher and Sejr, 2011). These organisms contribute significantly to the overall benthic biomass of the Arctic shelves despite their patchy occurrence (Piepenburg, 2000; Ambrose et al., 2001).
Climate change (Denisenko, 2001; Wassmann et al., 2006) has been suggested to cause a northward shift of biogeographic boundaries as a consequence of warming (Blacker, 1965; Dyer et al., 1984; Galkin, 1998; Denisenko, 2007). In addition, bottom trawling (Hiddink et al., 2006; Puig et al., 2012), ocean acidification (Wood et al., 2011), and invasive species (Strayer, 2012) may also affect biodiversity and the functioning of benthic systems. It is therefore important to document temporal and spatial changes of the benthic part of the ecosystem. Several studies of benthic megafauna have been performed in the Chukchi Sea (Bluhm et al., 2009), the Northeast Atlantic (Billett et al., 2001), East Greenland (Mayer and Piepenburg, 1996), the Northwest Atlantic (Beazley et al., 2013), the Arctic deep-sea of the Canadian Basin (MacConald et al., 2010), the southeastern Bering Sea (Yeung and McConnaughey, 2008), and the East Siberian Sea (Sirenko and Denisenko, 2010). The macrobenthic infauna of the Barents Sea has also been studied over the last decade (Zenkevitch, 1963; Galkin, 1987; Kiyko and Pogrebov, 1997; Frolova et al., 2007; Cochrane et al., 2009), and large-scale mapping of macrobenthic fauna in the 1930s, late 1960s, and the early 1990s (Anisimova et al., 2011) revealed long-term changes that could reflect climate variability and fishing activities (Wassmann et al., 2006; Denisenko, 2007). However, similar description of a comprehensive dataset for benthic megafauna is lacking.
The ecosystem approach (EA) to management is an important strategy for sustainable use and conservation of natural resources and biodiversity (CBD, 2004; http://www.cbd.int/sp/targets/). The EA is adaptive and relates to the dynamic conditions of marine ecosystems by implementing an integrated assessment of all relevant ecosystem components, and consequently the overall state of the ecosystem (Misund and Skjoldal, 2005; Skjoldal and Misund, 2008). This includes the effects from natural climate variability and climate change, anthropogenic impacts from fisheries (on targeted stocks, bycatch species, and benthic habitats), effects of introduced species, and effects of other human activities including pollution. Megabenthos in the Barents Sea, and globally, is important and relevant in the context of EA for two main reasons. First, it serves ecologically important functions including redistribution and remineraliztion of organic carbon reaching the seabed, and provision of food, habitat and shelter for many species. Second, at the levels of habitat, communities, and species, epifauna is impacted by bottom trawling, which is one of the most extensive of the human activities that directly affect the seabed. A long-term monitoring programme aimed at recording the faunal composition within the Barents Sea is needed as a point of reference to reveal and document subsequent changes, possible due to oceanographic variability, impacts from fishing activities and oil exploitation, and predation from the growing and spreading populations of the two new species: the snow crab (Chionoecetes opilio) and king crab (Paralithodes camtschaticus). As part of joint Norwegian–Russian ecosystem surveys (Michalsen et al., 2013), benthic experts have since 2006 identified the invertebrate (megafauna) collected by bottom trawls during annual assessments of commercial stocks such as Atlantic cod (Gadus morhua) and northern shrimp (Pandalus borealis). While a standard bottom trawl may not be a traditional benthic sampling device, it effectively collects larger organisms such as corals, sea pens, sponges, sea stars, and crabs that are patchily distributed on the seabed. Since the trawl is used as a standard sampling gear to describe and estimate the distribution and abundance of fish in annual scientific fish assessment surveys, information on benthic megafauna can be obtained at low extra cost and time by adding sufficient taxonomic expertise on-board the vessel.
The annual benthic megafauna records of bycatch in trawl have now resulted in a cost-effective benthic dataset based on sampling with four scientific vessels during August and September since 2006. The 2011 dataset, which is presented here, is considered the most geographically extensive and best standardized of this time-series and qualifies as a baseline mapping for further monitoring of the Barents Sea. There are three main objectives of this study: (i) describe and correlate spatial distribution of benthic megafauna with geography, topography, and oceanography of the Barents Sea; (ii) establish a baseline map which can be used as a reference for monitoring changes in the Barents Sea; (iii) assess strategic elements for including the benthic component in future monitoring of the Barents Sea ecosystem as part of the EA.
Study area
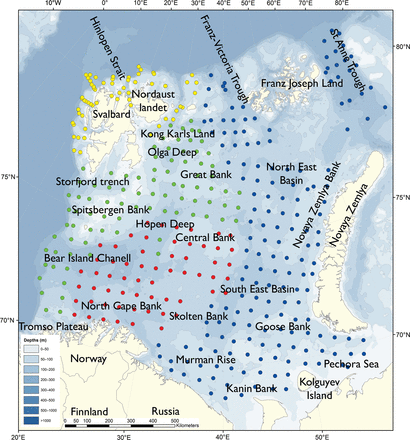
The Barents Sea covers ∼1.6 million km2 (Jakobsson et al., 2004) and is one of the continental shelf-seas surrounding the Arctic Ocean. It is bordered by the Norwegian Sea to the west, the Norwegian and Russian mainland to the south, Novaya Zemlya to the east and the Arctic Ocean to the north (Ozhigin et al., 2011). The average depth is 230 m. There are several bank areas with depths between 50 and 200 m and basins and trenches down to the maximum depth of about 500 m at the western boundary (Figure 1).
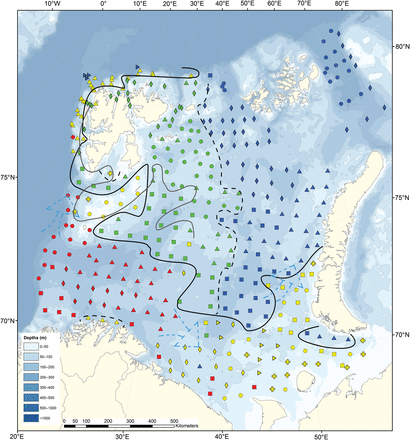
Sampling stations in the Barents Sea map coloured in according to the research vessels: Helmer Hanssen (yellow), Johan Hjort (green), Geo Sars (red), Vilnius (blue).
The general oceanic circulation pattern is strongly influenced by the bottom topography (e.g. Loeng, 1991; Ozhigin et al., 2011), and currents and topography interact to influence the seabed structure and composition of sediments. The bottom sediments change with water depth and relief (slope) of the seabed, with finer mud predominating in deeper areas with slow bottom-water movement, and sandy to stony substrates being common on shallower banks with stronger currents (Klenova, 1960; Vinogradova and Litvin, 1960).
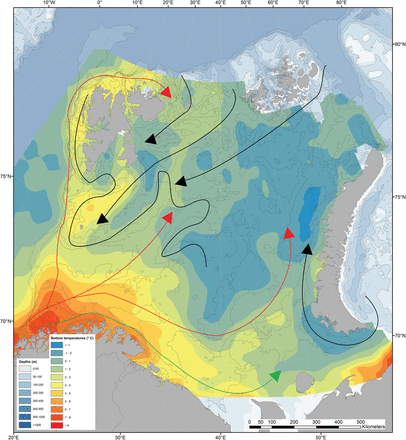
Relatively warm (2–8°C) Atlantic Water of the Norwegian Atlantic Current, and coastal waters of the Norwegian Coastal Current (red and yellow in Figure 2), flow into the Barents Sea from the southwest (Loeng, 1991; Ozhigin et al., 2011). These currents are the main source of heat input to the region and keep the southern Barents Sea relatively warm and ice free. Colder Arctic Water (<0°C) dominates in the northern Barents Sea. The border area between the Atlantic and Arctic water masses forms the oceanographic Polar Front (Figure 2), and is relatively well defined and stable in the western part of the Barents Sea. However, branches of Atlantic water flow northwards below the Arctic water and the Polar Front in the northern Hopen Trench (Figure 2; Loeng, 1991; Ozhigin et al., 2011). Atlantic water also enters the northern Barents Sea from the north in deeper areas between Svalbard and Franz Josef Land (Lind and Ingvaldsen, 2012). The northern parts of the Barents Sea are seasonally ice covered (Figure 3), with maximum ice coverage in March–April and minimum ice coverage in August–September (Vinje, 2009; Ozhigin et al., 2011).
Topography and distribution of near-bottom water temperatures in the Barents Sea obtained averaged over the period 2000–2010 and obtained from vertical casts made with a Seabird CTD. The oceanographic “Polar Front” (adapted from Loeng, 1991) is indicated with a black line. The arrows indicate currents of different waters masses: coastal (green), Atlantic (red) and Arctic (blue).
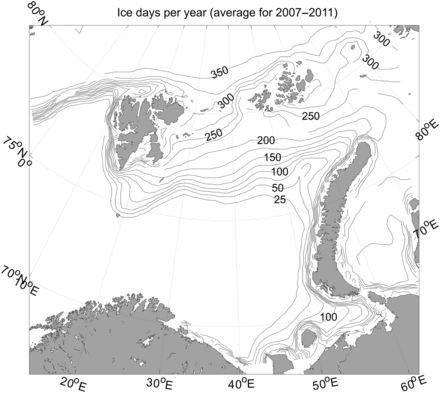
Barents Sea with number of days with ice present in a 25-by-25 km grid resolution where each grid cell was calculated based on average ice concentration data for the period 2007–2011.
Material and methods
Hydrography and sea ice data collection
Bottom-water temperatures and salinities were taken as the lower-most sample (5 m above the seabed) from vertical casts made with a Seabird CTD. The CTD casts were usually performed at the same locations as the bottom trawling. When this was not the case (11 stations), the closest CTD station was used to represent the trawling station.
Monthly averaged sea ice concentrations (Figure 3) were taken from the National Snow and Ice Data Centre in the USA (SMMR and SSM/I passive microwave data; Cavalieri et al., 1996; Maslanik and Stroeve, 1999; Meier et al., 2006). The sea ice data had a 25-by-25 km grid resolution. The number of days with ice absent/present in each grid cell was calculated based on average ice concentration data for the period 2007–2011.
Bottom trawl sampling
The annual joint Norwegian–Russian Ecosystem Survey provides data for assessments of fish stocks and the changing conditions of the Barents Sea ecosystem. Hydrography, plankton, demersal, and pelagic fish stocks, benthos, seabirds, and marine mammals are sampled or observed at more than 400 stations and during extensive cruise tracks covering more or less the whole Barents Sea in August–September (Figure 1; Michalsen et al., 2013). The sampling is based on a regular grid spanning about 1.5 million km2 with fixed positions of stations which make it possible to measure changes in spatial distribution over time. The trawl is a Campelen 1800 bottom trawl rigged with rock-hopper groundgear and towed on double warps (Engås and Godø, 1989). The mesh size is 80 mm (stretched) in the front and 16–22 mm in the cod end, allowing the capture and retention of smaller fish and the largest benthos from the seabed (benthic megafauna). The horizontal opening was 11.7 m, and the vertical opening 4–5 m (Teigsmark and Øynes, 1982). The trawl configuration and bottom contact was monitored remotely by SCANMAR trawl sensors.
The standard distance between trawl stations was 35 nautical miles (65 km), except north and west of Svalbard where a stratified sampling was adapted to the steep continental shelve (Figure 1). The standard procedure was to tow 15 min after the trawl had made contact with the bottom, but the actual tow duration ranged between 5 min and 1 h and data were subsequently standardized to 15 min trawl time. Towing speed was 3 knots, equivalent to a towing distance of 0.75 nautical miles (1.4 km) during a 15 min tow.
The trawl catches were recorded using the same procedures on the Russian research vessel Vilinus and the Norwegian research vessels G.O. Sars, Johan Hjort, and Helmer Hanssen to ensure comparability across Barents Sea regions. The benthic megafauna was separated from the fish and shrimp catch, washed, and sorted to lowest possible taxonomic level, in most cases to species, on-board the vessel. Species identification was carefully standardized between the researcher teams during common workshops in 2006 and 2008, and by annually exchanging the benthic expert's among the vessels and taxon names were fixed each year according to WORMS (http://www.marinespecies.org) when possible. This resulted in an electronic identification manual and photo-compendium as a tool to standardize taxon identifications, in addition to various sources of identification literature. Difficult taxa were photographed and, in some cases, brought back as preserved voucher specimens for further identification.
Wet-weight biomass was recorded with electronic scales (Marel series 1100) in the ship laboratories, and the numbers of individuals were noted for each taxon. For colonial organisms (sponges, colonial ascidians, bryozoans, hydrozoans), only weights were recorded. All individuals were included in subsequent data analysis whether identified to species or to a higher taxonomic level. Only animal fragments with the head-part intact were counted, but as colonial species could not be counted, the abundance values are representing only part of the benthic megafauna taken by the trawl. The biomass determination included all fragments.
Unlike grab and boxcore methods used in traditional benthic sampling programmes, which give quantitative data for faunal abundance and biomass per unit area of seabed, data from trawl samples are semi-quantitative (Eleftheriou and MacIntyre, 2005). However, when carried out consistently over a large number of stations, relative spatial and temporal patterns can be identified. Use of rock-hopper gear and possible differences in rigging of the bottom trawl between the Norwegian and Russian research vessels are expected to create slight compositional differences of the benthic megafauna bycatch and hence the semi-quantitative description of benthos. A fully quantitative account of the macro- and megafauna will only be available when additional sampling gear such as grab and a small epibenthic trawl are used (Jørgensen et al., 2011).
Multivariate analyses of distribution patterns
Fish and Pandalus borealis was excluded from the dataset and all subsequent analyses of the benthic fauna as they most likely are more easily catch by the Campelen bottom trawl compared with the benthos species. The amount of benthos collected was not influenced by the amount of fish and northern shrimp collected in the trawl (R2 = 0.06). In this work, catches from 377 trawl stations sampled in 2011 were analysed.
Taxa identified to species level (∼61% of the taxa) were coded by zoogeographic affinity according to Vasilenko and Petryachov (2009), Buzhinskaja (2010), Stapanjants (2012), Sirenko (2004, 2009), Sirenko and Denisenko (2010) when possible.
Only biomass data were used for the analyses of spatial patterns of species distributions because specimen counts are inapplicable for colonial taxa. The benthic-biomass data were fourth root transformed to compress high values and to spread low values by expressing the values as order of magnitude (McCune and Grace, 2002). The sample stations were clustered into groups by Sorensen (Bray and Curtis) distance measure which is an “unweighted pair-group method with arithmetic mean” (UPGMA) performed in PC-ORD version 6.08 (McCune and Mefford, 2011, http://home.centurytel.net/~mjm/pcordwin.htm. The fourth root transformation contribute to more homogeneous species data, implying that all species are taken into account when comparing assemblages using the Sorensen (Bray and Curtis) distance measure. The most widely used abundance-based measure is the Bray and Curtis measure, due to its strong relationship with ecological distance under varying conditions (Bray and Curtis, 1957; Faith et al., 1987; Minchin, 1987; Clarke, 1993).
Wards method was used as the group linkage method. Ward's linkage has been rarely used in ecology since it is normally used in conjunction with Euclidean distance. However, a previous study has shown that this linkage method performed well with Bray–Curtis distance metrics (Singh et al., 2011). To test the differences among identified clusters of sample stations, a nonparametric method (Multi-Response Permutation Procedures, MRPP in PCord) was used.
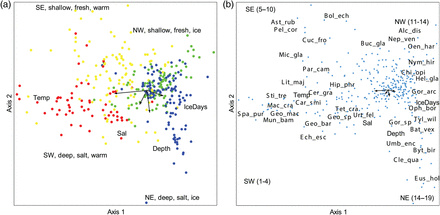
We also used Canonical Correspondence Analysis (CCA) (ter Braak, 1986, 1994) to test for relationships among the faunal community and environmental parameters. This also reveals whether the CCA indicated the same division of stations as the clustering (Figures 4 and 5). To evaluate the statistical significance of species–environment correlations, a randomization test/Monte Carlo test was performed in CCA. This test was applied only to the first axis, because subsequent axes are dependent on the first. Therefore, p values are not reported for axes 2 and 3 because using a simple randomization test for these axes may bias the p values. The “inertia” in the species data (statement of the total amount of variability in the community matrix that could potentially be “explained”), the eigenvalue (representing the variance in the community matrix that is attributed to a particular axis), and the per cent of variance in the community matrix explained by each axis, are given in the figure text of Figure 6.
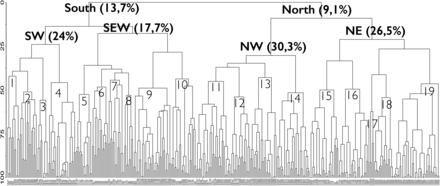
Dendrogram (based on Bray–Curtis clustering) of the bottom trawl stations sampled in the Barents Sea (BS) in 2011 (vertical line) and the level of similarity by which they are linked (horisontal line). From the right part of the dendrogram—South (13.7%): Southern BS and West/North of Svalbard; North (9.1%): Northern BS and Svalbard fjords; SW (24%): South western part of the BS; SE and banks (17.7%): South Eastern part and Banks across the BS; NW (30.3%): Northwest BS and Svalbard; NE (26.5%): Northeast BS and Arctic. 1—Tromsø flake (42.1%). 2—Western slope and other Bear Island channel (BIC) (51.1%). 3—North Cape bank (55.8%). 4—BIC channel and Atlantic inflow region north and east of North Cape Bank (45.8%). 5—Slopes of Banks in East and in Central BS (52.5%). 6—Murmansk Rise and Skolten Bank (48.3%). 7—Pechora Sea, Storfjord trench, other BIC (44.4%). 8—Kanin Bank (53%). 9—Shelf along W and N Svalbard (48.9%). 10—Spitsberg Bank and slopes of south Banks and (43.7%). 11—Hopen Deep, Storfjord trench, south of Central Bank (45.1%). 12 —Central- and GreatBanks and slopes (53.9%). 13—Svalbard coast, fjords and sounds and Nordaustlandet N and E (43%). 14—Great Bank, Olga Deep (51.6%). 15—East Basin (50%). 16—Novaya Zemlya Bank, East of Central Bank, northern Pechora Sea (49.5%). 17—Deep slope of Northern Svalbard (62.3%). 18—Western St Anna Trough. NW of Franz-Victoria Trough (54.3%). 19—Franz-Victoria Trough. NE and E of St Anna Trough (46.5%).
Distribution of the Barents Sea station clusters, based on fauna similarity (Bray–Curtis similarity index and Ward clustering, Figure 4) with the northern (green and blue) and southern (yellow and red) region where the black whole line is the approximate north-south division illustrating the “benthic Polar Front”. The grey full line is the approximate oceanographic Polar Front. The almost vertical dotted line: is partly illustrating a west-east division. Red: South West subregion (SW)—squares, SW 1; circles, SW 2; diamonds, SW 3; triangles, SW 4. Yellow: Southeast, banks and Svalbard coast (SEW)—squares, SE 5; diamonds, SE 6; cross, SE 7; triangles on side SE 8, triangles SE 9, circles, SE 10. Green: Northwest and Svalbard fjords (NW)—squares, NW 11; triangles, NW 12; diamonds, NW 13; circles, NW 14. Blue: Northeast (NE)—squares, NE 15; triangles, NE 16; triangles on side NE 17; circles,, NE 18, diamonds NE 19.
(a and b) Association between stations (left a) and benthos species (right b) and habitat variables in the Barents Sea. The length of the arrows is proportional to the correlation with the CCA1 and CCA2 axes. Total inertia in species data: 11.463. Eigenvalue: 0.361 (Axis 1) and 0.234 (axis 2). Variance explained: 3.2% (axis 1) and 2.0% (axis 2). Kendall Corr., Spp-Envt: 0.667 (axis 1 and 0.653 (axis 2). Randomization test/Monte Carlo test, spp-envt: p = 0.001. Red: SW; yellow: SEW, green: NW: blue: NE (see also Figure 6 for more info). The abbreviation names is only given for the dominating and characteristic species of the local areas 1–19 (see Table 3 and the “Results” section for further explanations): Species abbreviation in alphabetic order: Alc_dis: Alcyonidium disciforme, Car_smi: Caryophyllia smithii, Cer_gra: Ceramaster granularis, Chl_isl: Chlamys islandica, Cle_qua: Cleippides quadricuspis, Bat_vex: Bathybiaster vexillifer, Bri_fra: Brisaster fragilis, Buc_gla: Buccinum glaciale, Byt_bir: Bythocaris biruli, Chi_opi: Chionoecetes opillio, Cuc_fro: Cucumaria frondosa, Ech_esc: Echinus esculentus, Eus_hol: Eusirus holmi, Geo_mac: Geodia macandrewii, Geo_bar: Geodia barretti, Geo_sp: Geodia sp., Gor_arc: Gorgonocephalus arcticus, Gor_sp: Gorgonocephalus sp., Hel_gla: Heliometra glacialis, Lit_maj: Lithodes maja, Mac_cra: Macandrevia cranium, Mic_gla: Microcosmus glacialis, Mun_bam: Munida bamffica, Nep_ven: Neptunea ventricosa, Nym_ser: Nymphon serratum, Oen_har: Oenopota harpa, Oph_bor: Ophiopleura borealis, Par_cam: Paralithodes camtschaticus, Sab_sep: Sabinea septemcarinata, Spa_pur: Spatangus purpureus, Sti_tre: Parastichopus tremulus, Tyl_wil: Tylaster willei, Umb_enc: Umbellula encrinus, Urt_fel: Urticina feline.
Results
Benthic megafauna composition
The 377 trawl stations sampled in 2011 covered the whole Barents Sea except the northeastern most area between Franz Josef Land and Novaya Zemlya, and the shelf north of Franz Josef Land (Figure 1). The stations spanned a wide range of environmental conditions from the shallowest depth of 35 m in the Pechora Sea to a maximum depth of 928 m on the slope north of Svalbard, and from ice-free areas in southwest to areas with about 300 ice-days per year in the northeast (Figure 2). The temperature at the bottom ranged from 6.5°C (Figure 3) in southwest to −1.4°C in northeast, while salinity ranged from 33.4 in the Pechora Sea to 35.6.
A total of 10.6 tons of benthic megafauna biomass were collected and identified, containing 1.07 million individuals (not including colonial taxa). The abundance (number of individuals recorded per 15 min trawl duration) ranged from <10 for some stations in the southeastern Barents Sea, to a maximum of 0.3 million individuals at a station in the northeastern Barents Sea. The recorded biomass spanned a range from 8 g to 1.7 ton per 15 min trawl duration.
Of the total of 354 taxa, 218 were identified to species level. Phyla contributing most to the taxonomic diversity were Mollusca (101 taxa), Crustacea (60 taxa), Echinodermata (60 taxa), Porifera (30 taxa), Polychaeta (23 taxa) and Cnidaria (22 taxa). About one-third (39%) of the taxa were found on <1% of the stations, many of them recorded in only one trawl-haul, while nearly half (45%) of the taxa were found on between 3 and 13% of the stations. Only 11 taxa were widely distributed and found on more than 40% of the stations, seven of them being echinoderms. They included the sea stars Ctenodiscus crispatus and Pontaster tenuispinus, the sea urchin Strongylocentrotus spp., the brittlestars Ophiacantha bidentata, Ophiopholis aculeata, Ophioscolex glacialis and Ophiura sarsi, the sea anemone Hormathia sp., the crangonid crustacean Sabinea septemcarinata, the polychaeta “Polynoidae indet.”, and the group of sponges “Porifera indet.” (Table 1).
Species found at more than 30%, written in bold, of the 377 stations of the Barents Sea (BS) and the south western (SW) stations (no 60), the south eastern, Spitsbergbank and northwestern Svalbard (SEW) stations (no 105), the stations (no 99) in the north western area and fjords of Svalbard (NW), and the north east (NE) stations (no 113). The zoogeographic relations (Zoog) are given as Arctic Boreal (AB), Arctic (A), Boreal (B), or as no information (-). Species among the top 5 most biomass dominant in the BS, SW, SEW, NW, NE (see also table 2) are marked in “Dom”.
| Phylum . | Class . | Taxa . | Zoo . | Dom . | BS . | SW . | SEW . | NW . | NE . |
|---|---|---|---|---|---|---|---|---|---|
| Arthropoda | Pycnogonida | Boreonymphon robustum | AB | 16 | 8 | 4 | 48 | 4 | |
| Colossendeis sp | – | 14 | 0 | 7 | 8 | 35 | |||
| Nymphon hirtipes | A | 10 | 2 | 0 | 35 | 0 | |||
| Nymphon stroemi | AB | 20 | 13 | 23 | 41 | 47 | |||
| Pycnogonida indet | – | 16 | 0 | 5 | 2 | 79 | |||
| Cnidaria | Actiniaria | Actiniaria indet | – | X | 36 | 15 | 20 | 14 | 79 |
| Hormathia sp. | – | 49 | 58 | 49 | 84 | 12 | |||
| Urticina felina | AB | 6 | 30 | 2 | 1 | 0 | |||
| Alcyonacea | Drifa glomerata | AB | 27 | 2 | 29 | 30 | 36 | ||
| Duva florida | B | 11 | 2 | 5 | 34 | 2 | |||
| Gersemia rubiformis | AB | 13 | 5 | 9 | 31 | 4 | |||
| Gersemia sp. | – | 21 | 2 | 7 | 2 | 60 | |||
| Hydroidea | Hydrozoa indet | – | 20 | 10 | 36 | 12 | 15 | ||
| Sertulariidae indet | – | 20 | 7 | 27 | 41 | 1 | |||
| Crustacea | Amphipoda | Anonyx nugax | AB | 12 | 12 | 1 | 35 | 3 | |
| Epimeria loricata | AB | 18 | 43 | 10 | 26 | 4 | |||
| Stegocephalus inflatus | AB | 19 | 3 | 5 | 37 | 23 | |||
| Anomura | Pagurus pubescens | AB | 25 | 22 | 41 | 20 | 11 | ||
| Brachyura | Chionoecetes opilio | AB | X | 21 | 0 | 16 | 8 | 48 | |
| Hyas sp. | – | X | 35 | 33 | 50 | 24 | 22 | ||
| Cirripedia | Balanus sp. | – | X | 14 | 3 | 38 | 9 | 1 | |
| Isopoda | Saduria sabini | A | 18 | 13 | 0 | 14 | 39 | ||
| Natantia | Lebbeus polaris | 32 | 13 | 28 | 39 | 37 | |||
| Pontophilus norvegicus | 15 | 70 | 11 | 3 | 0 | ||||
| Sabinea septemcarinata | AB | X | 69 | 7 | 61 | 99 | 82 | ||
| Sclerocrangon ferox | AB | X | 32 | 2 | 7 | 49 | 54 | ||
| Echinodermata | Asteroidea | Crossaster papposus | AB | 30 | 20 | 31 | 23 | 38 | |
| Ctenodiscus crispatus | AB | 69 | 37 | 60 | 92 | 74 | |||
| Henricia sp. | – | 37 | 62 | 44 | 27 | 21 | |||
| Icasterias panopla | A | X | 37 | 3 | 17 | 60 | 52 | ||
| Pontaster tenuispinus | AB | 51 | 55 | 15 | 67 | 67 | |||
| Urasterias linckii | AB | X | 36 | 3 | 17 | 43 | 62 | ||
| Crinoidea | Heliometra glacialis | AB | X | 24 | 0 | 7 | 35 | 42 | |
| Echinoidea | Strongylocentrotus sp. | – | X | 52 | 22 | 57 | 65 | 49 | |
| Holothuroidea | Molpadia borealis | A | X | 31 | 38 | 6 | 40 | 43 | |
| Ophiuroidea | Gorgonocephalus arcticus | A | X | 29 | 0 | 3 | 37 | 61 | |
| Ophiacantha bidentata | AB | X | 56 | 7 | 26 | 81 | 88 | ||
| Ophiopholis aculeata | AB | 53 | 27 | 57 | 81 | 35 | |||
| Ophiopleura borealis | AB | X | 23 | 0 | 0 | 25 | 53 | ||
| Ophioscolex glacialis | AB | 41 | 10 | 19 | 66 | 54 | |||
| Ophiura sarsi | AB | X | 44 | 22 | 49 | 64 | 30 | ||
| Lophophorata | Bryozoa | Flustridae indet | – | 24 | 13 | 40 | 29 | 10 | |
| Mollusca | Bivalvia | Bathyarca glacialis | AB | 18 | 32 | 9 | 16 | 20 | |
| Chlamys islandica | AB | X | 27 | 3 | 43 | 31 | 19 | ||
| Gastropoda | Buccinum hydrophanum | A | 26 | 5 | 6 | 47 | 35 | ||
| Colus sabini | A | 39 | 10 | 5 | 51 | 76 | |||
| Polychaeta | Polychaeta indet | – | 31 | 10 | 19 | 15 | 65 | ||
| Brada inhabilis | AB | 36 | 28 | 14 | 66 | 31 | |||
| Polynoidae indet | – | 46 | 53 | 41 | 57 | 36 | |||
| Spiochaetopterus typicus | AB | X | 14 | 0 | 12 | 1 | 34 | ||
| Porifera | Porifera indet | – | X | 62 | 57 | 70 | 59 | 57 | |
| Radiella grimaldi | AB | 20 | 32 | 10 | 31 | 15 | |||
| Tetilla polyura | AB | 7 | 33 | 2 | 4 | 1 | |||
| Thenea muricata | AB | 11 | 37 | 6 | 14 | 0 | |||
| Tunicata | Ascidiacea | Ascidia prunum | B | 11 | 32 | 5 | 15 | 1 | |
| Ascidiacea indet | – | 20 | 10 | 31 | 12 | 22 |
| Phylum . | Class . | Taxa . | Zoo . | Dom . | BS . | SW . | SEW . | NW . | NE . |
|---|---|---|---|---|---|---|---|---|---|
| Arthropoda | Pycnogonida | Boreonymphon robustum | AB | 16 | 8 | 4 | 48 | 4 | |
| Colossendeis sp | – | 14 | 0 | 7 | 8 | 35 | |||
| Nymphon hirtipes | A | 10 | 2 | 0 | 35 | 0 | |||
| Nymphon stroemi | AB | 20 | 13 | 23 | 41 | 47 | |||
| Pycnogonida indet | – | 16 | 0 | 5 | 2 | 79 | |||
| Cnidaria | Actiniaria | Actiniaria indet | – | X | 36 | 15 | 20 | 14 | 79 |
| Hormathia sp. | – | 49 | 58 | 49 | 84 | 12 | |||
| Urticina felina | AB | 6 | 30 | 2 | 1 | 0 | |||
| Alcyonacea | Drifa glomerata | AB | 27 | 2 | 29 | 30 | 36 | ||
| Duva florida | B | 11 | 2 | 5 | 34 | 2 | |||
| Gersemia rubiformis | AB | 13 | 5 | 9 | 31 | 4 | |||
| Gersemia sp. | – | 21 | 2 | 7 | 2 | 60 | |||
| Hydroidea | Hydrozoa indet | – | 20 | 10 | 36 | 12 | 15 | ||
| Sertulariidae indet | – | 20 | 7 | 27 | 41 | 1 | |||
| Crustacea | Amphipoda | Anonyx nugax | AB | 12 | 12 | 1 | 35 | 3 | |
| Epimeria loricata | AB | 18 | 43 | 10 | 26 | 4 | |||
| Stegocephalus inflatus | AB | 19 | 3 | 5 | 37 | 23 | |||
| Anomura | Pagurus pubescens | AB | 25 | 22 | 41 | 20 | 11 | ||
| Brachyura | Chionoecetes opilio | AB | X | 21 | 0 | 16 | 8 | 48 | |
| Hyas sp. | – | X | 35 | 33 | 50 | 24 | 22 | ||
| Cirripedia | Balanus sp. | – | X | 14 | 3 | 38 | 9 | 1 | |
| Isopoda | Saduria sabini | A | 18 | 13 | 0 | 14 | 39 | ||
| Natantia | Lebbeus polaris | 32 | 13 | 28 | 39 | 37 | |||
| Pontophilus norvegicus | 15 | 70 | 11 | 3 | 0 | ||||
| Sabinea septemcarinata | AB | X | 69 | 7 | 61 | 99 | 82 | ||
| Sclerocrangon ferox | AB | X | 32 | 2 | 7 | 49 | 54 | ||
| Echinodermata | Asteroidea | Crossaster papposus | AB | 30 | 20 | 31 | 23 | 38 | |
| Ctenodiscus crispatus | AB | 69 | 37 | 60 | 92 | 74 | |||
| Henricia sp. | – | 37 | 62 | 44 | 27 | 21 | |||
| Icasterias panopla | A | X | 37 | 3 | 17 | 60 | 52 | ||
| Pontaster tenuispinus | AB | 51 | 55 | 15 | 67 | 67 | |||
| Urasterias linckii | AB | X | 36 | 3 | 17 | 43 | 62 | ||
| Crinoidea | Heliometra glacialis | AB | X | 24 | 0 | 7 | 35 | 42 | |
| Echinoidea | Strongylocentrotus sp. | – | X | 52 | 22 | 57 | 65 | 49 | |
| Holothuroidea | Molpadia borealis | A | X | 31 | 38 | 6 | 40 | 43 | |
| Ophiuroidea | Gorgonocephalus arcticus | A | X | 29 | 0 | 3 | 37 | 61 | |
| Ophiacantha bidentata | AB | X | 56 | 7 | 26 | 81 | 88 | ||
| Ophiopholis aculeata | AB | 53 | 27 | 57 | 81 | 35 | |||
| Ophiopleura borealis | AB | X | 23 | 0 | 0 | 25 | 53 | ||
| Ophioscolex glacialis | AB | 41 | 10 | 19 | 66 | 54 | |||
| Ophiura sarsi | AB | X | 44 | 22 | 49 | 64 | 30 | ||
| Lophophorata | Bryozoa | Flustridae indet | – | 24 | 13 | 40 | 29 | 10 | |
| Mollusca | Bivalvia | Bathyarca glacialis | AB | 18 | 32 | 9 | 16 | 20 | |
| Chlamys islandica | AB | X | 27 | 3 | 43 | 31 | 19 | ||
| Gastropoda | Buccinum hydrophanum | A | 26 | 5 | 6 | 47 | 35 | ||
| Colus sabini | A | 39 | 10 | 5 | 51 | 76 | |||
| Polychaeta | Polychaeta indet | – | 31 | 10 | 19 | 15 | 65 | ||
| Brada inhabilis | AB | 36 | 28 | 14 | 66 | 31 | |||
| Polynoidae indet | – | 46 | 53 | 41 | 57 | 36 | |||
| Spiochaetopterus typicus | AB | X | 14 | 0 | 12 | 1 | 34 | ||
| Porifera | Porifera indet | – | X | 62 | 57 | 70 | 59 | 57 | |
| Radiella grimaldi | AB | 20 | 32 | 10 | 31 | 15 | |||
| Tetilla polyura | AB | 7 | 33 | 2 | 4 | 1 | |||
| Thenea muricata | AB | 11 | 37 | 6 | 14 | 0 | |||
| Tunicata | Ascidiacea | Ascidia prunum | B | 11 | 32 | 5 | 15 | 1 | |
| Ascidiacea indet | – | 20 | 10 | 31 | 12 | 22 |
Species found at more than 30%, written in bold, of the 377 stations of the Barents Sea (BS) and the south western (SW) stations (no 60), the south eastern, Spitsbergbank and northwestern Svalbard (SEW) stations (no 105), the stations (no 99) in the north western area and fjords of Svalbard (NW), and the north east (NE) stations (no 113). The zoogeographic relations (Zoog) are given as Arctic Boreal (AB), Arctic (A), Boreal (B), or as no information (-). Species among the top 5 most biomass dominant in the BS, SW, SEW, NW, NE (see also table 2) are marked in “Dom”.
| Phylum . | Class . | Taxa . | Zoo . | Dom . | BS . | SW . | SEW . | NW . | NE . |
|---|---|---|---|---|---|---|---|---|---|
| Arthropoda | Pycnogonida | Boreonymphon robustum | AB | 16 | 8 | 4 | 48 | 4 | |
| Colossendeis sp | – | 14 | 0 | 7 | 8 | 35 | |||
| Nymphon hirtipes | A | 10 | 2 | 0 | 35 | 0 | |||
| Nymphon stroemi | AB | 20 | 13 | 23 | 41 | 47 | |||
| Pycnogonida indet | – | 16 | 0 | 5 | 2 | 79 | |||
| Cnidaria | Actiniaria | Actiniaria indet | – | X | 36 | 15 | 20 | 14 | 79 |
| Hormathia sp. | – | 49 | 58 | 49 | 84 | 12 | |||
| Urticina felina | AB | 6 | 30 | 2 | 1 | 0 | |||
| Alcyonacea | Drifa glomerata | AB | 27 | 2 | 29 | 30 | 36 | ||
| Duva florida | B | 11 | 2 | 5 | 34 | 2 | |||
| Gersemia rubiformis | AB | 13 | 5 | 9 | 31 | 4 | |||
| Gersemia sp. | – | 21 | 2 | 7 | 2 | 60 | |||
| Hydroidea | Hydrozoa indet | – | 20 | 10 | 36 | 12 | 15 | ||
| Sertulariidae indet | – | 20 | 7 | 27 | 41 | 1 | |||
| Crustacea | Amphipoda | Anonyx nugax | AB | 12 | 12 | 1 | 35 | 3 | |
| Epimeria loricata | AB | 18 | 43 | 10 | 26 | 4 | |||
| Stegocephalus inflatus | AB | 19 | 3 | 5 | 37 | 23 | |||
| Anomura | Pagurus pubescens | AB | 25 | 22 | 41 | 20 | 11 | ||
| Brachyura | Chionoecetes opilio | AB | X | 21 | 0 | 16 | 8 | 48 | |
| Hyas sp. | – | X | 35 | 33 | 50 | 24 | 22 | ||
| Cirripedia | Balanus sp. | – | X | 14 | 3 | 38 | 9 | 1 | |
| Isopoda | Saduria sabini | A | 18 | 13 | 0 | 14 | 39 | ||
| Natantia | Lebbeus polaris | 32 | 13 | 28 | 39 | 37 | |||
| Pontophilus norvegicus | 15 | 70 | 11 | 3 | 0 | ||||
| Sabinea septemcarinata | AB | X | 69 | 7 | 61 | 99 | 82 | ||
| Sclerocrangon ferox | AB | X | 32 | 2 | 7 | 49 | 54 | ||
| Echinodermata | Asteroidea | Crossaster papposus | AB | 30 | 20 | 31 | 23 | 38 | |
| Ctenodiscus crispatus | AB | 69 | 37 | 60 | 92 | 74 | |||
| Henricia sp. | – | 37 | 62 | 44 | 27 | 21 | |||
| Icasterias panopla | A | X | 37 | 3 | 17 | 60 | 52 | ||
| Pontaster tenuispinus | AB | 51 | 55 | 15 | 67 | 67 | |||
| Urasterias linckii | AB | X | 36 | 3 | 17 | 43 | 62 | ||
| Crinoidea | Heliometra glacialis | AB | X | 24 | 0 | 7 | 35 | 42 | |
| Echinoidea | Strongylocentrotus sp. | – | X | 52 | 22 | 57 | 65 | 49 | |
| Holothuroidea | Molpadia borealis | A | X | 31 | 38 | 6 | 40 | 43 | |
| Ophiuroidea | Gorgonocephalus arcticus | A | X | 29 | 0 | 3 | 37 | 61 | |
| Ophiacantha bidentata | AB | X | 56 | 7 | 26 | 81 | 88 | ||
| Ophiopholis aculeata | AB | 53 | 27 | 57 | 81 | 35 | |||
| Ophiopleura borealis | AB | X | 23 | 0 | 0 | 25 | 53 | ||
| Ophioscolex glacialis | AB | 41 | 10 | 19 | 66 | 54 | |||
| Ophiura sarsi | AB | X | 44 | 22 | 49 | 64 | 30 | ||
| Lophophorata | Bryozoa | Flustridae indet | – | 24 | 13 | 40 | 29 | 10 | |
| Mollusca | Bivalvia | Bathyarca glacialis | AB | 18 | 32 | 9 | 16 | 20 | |
| Chlamys islandica | AB | X | 27 | 3 | 43 | 31 | 19 | ||
| Gastropoda | Buccinum hydrophanum | A | 26 | 5 | 6 | 47 | 35 | ||
| Colus sabini | A | 39 | 10 | 5 | 51 | 76 | |||
| Polychaeta | Polychaeta indet | – | 31 | 10 | 19 | 15 | 65 | ||
| Brada inhabilis | AB | 36 | 28 | 14 | 66 | 31 | |||
| Polynoidae indet | – | 46 | 53 | 41 | 57 | 36 | |||
| Spiochaetopterus typicus | AB | X | 14 | 0 | 12 | 1 | 34 | ||
| Porifera | Porifera indet | – | X | 62 | 57 | 70 | 59 | 57 | |
| Radiella grimaldi | AB | 20 | 32 | 10 | 31 | 15 | |||
| Tetilla polyura | AB | 7 | 33 | 2 | 4 | 1 | |||
| Thenea muricata | AB | 11 | 37 | 6 | 14 | 0 | |||
| Tunicata | Ascidiacea | Ascidia prunum | B | 11 | 32 | 5 | 15 | 1 | |
| Ascidiacea indet | – | 20 | 10 | 31 | 12 | 22 |
| Phylum . | Class . | Taxa . | Zoo . | Dom . | BS . | SW . | SEW . | NW . | NE . |
|---|---|---|---|---|---|---|---|---|---|
| Arthropoda | Pycnogonida | Boreonymphon robustum | AB | 16 | 8 | 4 | 48 | 4 | |
| Colossendeis sp | – | 14 | 0 | 7 | 8 | 35 | |||
| Nymphon hirtipes | A | 10 | 2 | 0 | 35 | 0 | |||
| Nymphon stroemi | AB | 20 | 13 | 23 | 41 | 47 | |||
| Pycnogonida indet | – | 16 | 0 | 5 | 2 | 79 | |||
| Cnidaria | Actiniaria | Actiniaria indet | – | X | 36 | 15 | 20 | 14 | 79 |
| Hormathia sp. | – | 49 | 58 | 49 | 84 | 12 | |||
| Urticina felina | AB | 6 | 30 | 2 | 1 | 0 | |||
| Alcyonacea | Drifa glomerata | AB | 27 | 2 | 29 | 30 | 36 | ||
| Duva florida | B | 11 | 2 | 5 | 34 | 2 | |||
| Gersemia rubiformis | AB | 13 | 5 | 9 | 31 | 4 | |||
| Gersemia sp. | – | 21 | 2 | 7 | 2 | 60 | |||
| Hydroidea | Hydrozoa indet | – | 20 | 10 | 36 | 12 | 15 | ||
| Sertulariidae indet | – | 20 | 7 | 27 | 41 | 1 | |||
| Crustacea | Amphipoda | Anonyx nugax | AB | 12 | 12 | 1 | 35 | 3 | |
| Epimeria loricata | AB | 18 | 43 | 10 | 26 | 4 | |||
| Stegocephalus inflatus | AB | 19 | 3 | 5 | 37 | 23 | |||
| Anomura | Pagurus pubescens | AB | 25 | 22 | 41 | 20 | 11 | ||
| Brachyura | Chionoecetes opilio | AB | X | 21 | 0 | 16 | 8 | 48 | |
| Hyas sp. | – | X | 35 | 33 | 50 | 24 | 22 | ||
| Cirripedia | Balanus sp. | – | X | 14 | 3 | 38 | 9 | 1 | |
| Isopoda | Saduria sabini | A | 18 | 13 | 0 | 14 | 39 | ||
| Natantia | Lebbeus polaris | 32 | 13 | 28 | 39 | 37 | |||
| Pontophilus norvegicus | 15 | 70 | 11 | 3 | 0 | ||||
| Sabinea septemcarinata | AB | X | 69 | 7 | 61 | 99 | 82 | ||
| Sclerocrangon ferox | AB | X | 32 | 2 | 7 | 49 | 54 | ||
| Echinodermata | Asteroidea | Crossaster papposus | AB | 30 | 20 | 31 | 23 | 38 | |
| Ctenodiscus crispatus | AB | 69 | 37 | 60 | 92 | 74 | |||
| Henricia sp. | – | 37 | 62 | 44 | 27 | 21 | |||
| Icasterias panopla | A | X | 37 | 3 | 17 | 60 | 52 | ||
| Pontaster tenuispinus | AB | 51 | 55 | 15 | 67 | 67 | |||
| Urasterias linckii | AB | X | 36 | 3 | 17 | 43 | 62 | ||
| Crinoidea | Heliometra glacialis | AB | X | 24 | 0 | 7 | 35 | 42 | |
| Echinoidea | Strongylocentrotus sp. | – | X | 52 | 22 | 57 | 65 | 49 | |
| Holothuroidea | Molpadia borealis | A | X | 31 | 38 | 6 | 40 | 43 | |
| Ophiuroidea | Gorgonocephalus arcticus | A | X | 29 | 0 | 3 | 37 | 61 | |
| Ophiacantha bidentata | AB | X | 56 | 7 | 26 | 81 | 88 | ||
| Ophiopholis aculeata | AB | 53 | 27 | 57 | 81 | 35 | |||
| Ophiopleura borealis | AB | X | 23 | 0 | 0 | 25 | 53 | ||
| Ophioscolex glacialis | AB | 41 | 10 | 19 | 66 | 54 | |||
| Ophiura sarsi | AB | X | 44 | 22 | 49 | 64 | 30 | ||
| Lophophorata | Bryozoa | Flustridae indet | – | 24 | 13 | 40 | 29 | 10 | |
| Mollusca | Bivalvia | Bathyarca glacialis | AB | 18 | 32 | 9 | 16 | 20 | |
| Chlamys islandica | AB | X | 27 | 3 | 43 | 31 | 19 | ||
| Gastropoda | Buccinum hydrophanum | A | 26 | 5 | 6 | 47 | 35 | ||
| Colus sabini | A | 39 | 10 | 5 | 51 | 76 | |||
| Polychaeta | Polychaeta indet | – | 31 | 10 | 19 | 15 | 65 | ||
| Brada inhabilis | AB | 36 | 28 | 14 | 66 | 31 | |||
| Polynoidae indet | – | 46 | 53 | 41 | 57 | 36 | |||
| Spiochaetopterus typicus | AB | X | 14 | 0 | 12 | 1 | 34 | ||
| Porifera | Porifera indet | – | X | 62 | 57 | 70 | 59 | 57 | |
| Radiella grimaldi | AB | 20 | 32 | 10 | 31 | 15 | |||
| Tetilla polyura | AB | 7 | 33 | 2 | 4 | 1 | |||
| Thenea muricata | AB | 11 | 37 | 6 | 14 | 0 | |||
| Tunicata | Ascidiacea | Ascidia prunum | B | 11 | 32 | 5 | 15 | 1 | |
| Ascidiacea indet | – | 20 | 10 | 31 | 12 | 22 |
Geographic distribution patterns of the benthic megafauna
The cluster dendrogram (Figure 4) revealed a clear and consistent geographical pattern with two main clusters of stations in the southern and northern Barents Sea. These two groups were again subdivided into four station clusters denoted the SW (southwest), SEW (coast, slopes and banks in southeastern Barents Sea, Spitsbergen Bank and west/north of Svalbard), NW (northwest) and NE (northeast) regions shown with different colour symbols on the map in Figure 5. Four stations in the deeper parts of the Pechora Sea (off the southern tip of Novaya Zemlya) were grouped with the NE stations. There were also a few stations of the SW group that extended into the SEW region. The CCA analysis (Figure 6a and b) showed that the four subregions were significantly explained by depth, temperature, salinity, and ice-days [this was also further verified by Non-metric Multidimensional Scaling (NMS) in PCord (not shown)]. The NW and SEW regions included more shallow and low salinity stations, whereas the NE and SW had many deeper and more saline stations. The northern region (NE and NW) had many stations that tended to be colder and with more ice-days compared with the southern region which had most stations with warmer temperatures (Figure 6a). These results were consistent when CCA was performed on “presence–absence” data, which shows the influence of species composition, rather than the biomass of individuals within species (not shown).
The cluster diagram (Figure 4) showed that the four regions (SW, SEW, NW, NE) could be further divided into four to six local areas each (a total of 19 local areas, see also Figure 5) at similarity levels of 42–62%. The Multi-Response Permutation Procedure confirmed that stations were relatively similar within clusters. However, these 19 local areas were not clearly identified by the CCA analysis which might indicate that other environmental variables than those chosen need to be taken into consideration. Speed of bottom current (available from numeric modelling) and sediment structure (Klenova, 1960) are among potential explanatory variables, but these data were not available in a form that gave significant results in CCA and NMS. Acoustic data have not been included in this study, but could advance habitat research for some bottom-associated marine species such as some fish species, the red king crab (Paralithodes camtschaticus), basket star (Gorgonocephalus eucnemis), and sponges (Porifera) (Yeung and McConnaughey, 2008; McConnaughey and Syrjala, 2009).
A summary of information with geographical names and environmental conditions for the 19 local areas is given in Table 2, while Table 3 provides information on the number of taxa, total recorded biomass and abundance, and the five top dominant species in terms of biomass. A short description of environmental conditions and fauna per local area is provided in Supplementary Appendix 1.
The local areas 1–19 within the sub-regions SW, SEW, NW and NE in the Barents Sea (see also figs. 1–6). The number of stations (no st), mean depth (m), temperature (°C), salinity (‰), days of ice-cover (mean of 2007–2011) are given with standard-deviation, minimum and maximum per local area. Largest and smallest mean values in bold.
| Local area . | Geographic area . | No St . | Depth (m) ± SD (min; max) . | Temp (°C) ± SD (min; max) . | Salt (‰) ± SD (min; max) . | Ice-days ± SD (min; max) . |
|---|---|---|---|---|---|---|
| SW 1 | Tromsø flaket | 11 | 242 ± 103.11 (64; 409) | 4.8 ± 1.48 (2.3; 6.5) | 35.11 ± 0.29 (34.4; 35.5) | 0 0 (0) |
| SW 2 | Western slope. Bear Island Channel outer part. | 15 | 383 ± 138.25 (77; 611) | 2.6 ± 0.86 (1.5; 4.3) | 35.03 ± 0.20 (34.1; 35.1) | 4.40 ± 8.32 (0; 26) |
| SW 3 | North Cape Bank. | 10 | 312 ± 40.00 (261; 379) | 4.6 ± 0.31 (4.1; 5.3) | 35.13 ± 0.03 (35.1; 35.2) | 0 ± 0 (0; 0) |
| SW 4 | Bear Island Channel. Atlantic inflow region of North Cape Bank N and E. | 25 | 378 ± 67.43 (240; 479) | 2.4 ± 1.04 (0.1; 4.2) | 35.09 ± 0.01 (34.8; 35.1) | 0.05 ± 0.21 (0; 1) |
| SEW 5 | Slopes of banks (Goose Bank) in SE | 14 | 168 ± 87.63 (38; 330) | 0.8 ± 0.55 (−0.5; 1.5) | 34.84 ± 0.33 (33.5; 35.0) | 61.50 ± 72.96 (0; 215) |
| SEW 6 | Murmansk Rise/Skolpen Bank | 14 | 196 ± 56.07 (107; 309) | 2.4 ± 0.62 (0.1; 3.3) | 34.90 ± 0.14 (34.7; 35.1) | 6.58 ± 13.04 (0; 44) |
| SEW 7 | Slopes of Kolguyev Island, Storfjord trench, outer Bear Island Channel. | 14 | 111 ± 73.28 (35; 287) | 2.0 ± 1.32 (0.4; 4.2) | 34.62 ± 0.51 (33.4; 35.1) | 105.68 ± 57.46 (6; 188) |
| SEW 8 | Kanin Bank and southern slopes of Goose Bank. East Basin. | 8 | 170 ± 104.12 (87; 387) | 1.3 ± 0.80 (0.2; 2.5) | 34.88 ± 0.09 (34.8;35.6) | 16.03 ± 11.93 (0; 45) |
| SEW 9 | Shelf along Svalbard W and N | 33 | 305 ± 156.50 (96; 627) | 2.9 ± 1.26 (0.5; 6.4) | 35.05 ± 0.12 (34.8;35.6) | 150.35 ± 109.28 (12; 349) |
| SEW 10 | Shallow slopes of Kolguyev Island and Nov Zem. Goose-, Kap Kanin-and Svalbard Bank. | 22 | 86 ± 36.42 (43; 179) | 2.5 ± 1.64 (−0.7; 5.5) | 34.46 ± 0.38 (33.6; 34.9) | 78.00 ± 56.34 (0; 189) |
| NW 11 | Hopen Deep. Central Bank S. Storfjord trench | 30 | 264 ± 67.02 (129; 370) | 0.7 ± 0.84 (−0.5; 3.6) | 34.99 ± 0.07 (34.8; 35.1) | 32.92 ± 46.76 (0; 165) |
| NW 12 | Central Bank, slopes of Great Bank, Kong Karls Land, Storfjorden Svalbard E. | 17 | 152 ± 46.51 (92; 226) | −0.01 ± 1.18 (−1.4; 3.4) | 34.68 ± 0.36 (33.9; 35.0) | 135.72 ± 113.01 (0; 165) |
| NW 13 | Svalbard N coast, fjord and sound. Banks of N and E Nordaustlandet. | 23 | 241 ± 83.78 (133; 432) | 1.8 ± 1.04 (−0.8; 3.5) | 34.84 ± 0.15 (34.4; 35.0) | 275.78 ± 40.48 (189; 333) |
| NW 14 | Great Bank. Olga Deep | 29 | 214 ± 55.74 (74; 360) | 0.7 ± 0.68 (−0.8; 1.8) | 34.88 ± 0.16 (34.4; 35.0) | 183.87 ± 74.34 (32; 292) |
| NE 15 | Eastern Basin, slopes of Goose Bank and Nov Zem. | 25 | 298 ± 52.18 (132; 368) | 0.3 ± 0.53 (−0.1; 2.4) | 34.98 ± 0.03 (34.9; 35.0) | 14.96 ± 49.72 (0; 339) |
| NE 16 | Novaya Zemlya (Nov Zem) Bank. Central Bank E flank. Pechora Sea N | 27 | 192 ± 55.34 (114; 310) | 0.2 ± 0.71 (−1.0; 2.4) | 34.92 ± 0.05 (34.9; 35.0) | 63.47 ± 58.28 (0; 249) |
| NE 17 | Slope of Svalbard N | 4 | 795 ± 130.64 (617; 928) | 0.5 ± 1.33 (−0.7; 2.2) | 34.96 ± 0.05 (34.9; 35.0) | 244.73 ± 70.73 (178; 336) |
| NE 18 | St Anna Trough W. Franz-Victoria Trough NW | 17 | 538 ± 54.35 (452; 636) | 0.6 ± 0.17 (0.3; 1.1) | 34.91 ± 0.02 (34.9; 34.9) | 293.40 ± 25.10 (250; 335) |
| NE 19 | Franz-Victoria Trough. Barents Sea NE. St Anna Trough E. Bank B | 40 | 288 ± 72.53 (144; 471) | 0.3 ± 0.51 (−0.9; 2.1) | 34.89 ± 0.07 (34.6; 34.9) | 195.45 ± 69.42 (57; 329) |
| Local area . | Geographic area . | No St . | Depth (m) ± SD (min; max) . | Temp (°C) ± SD (min; max) . | Salt (‰) ± SD (min; max) . | Ice-days ± SD (min; max) . |
|---|---|---|---|---|---|---|
| SW 1 | Tromsø flaket | 11 | 242 ± 103.11 (64; 409) | 4.8 ± 1.48 (2.3; 6.5) | 35.11 ± 0.29 (34.4; 35.5) | 0 0 (0) |
| SW 2 | Western slope. Bear Island Channel outer part. | 15 | 383 ± 138.25 (77; 611) | 2.6 ± 0.86 (1.5; 4.3) | 35.03 ± 0.20 (34.1; 35.1) | 4.40 ± 8.32 (0; 26) |
| SW 3 | North Cape Bank. | 10 | 312 ± 40.00 (261; 379) | 4.6 ± 0.31 (4.1; 5.3) | 35.13 ± 0.03 (35.1; 35.2) | 0 ± 0 (0; 0) |
| SW 4 | Bear Island Channel. Atlantic inflow region of North Cape Bank N and E. | 25 | 378 ± 67.43 (240; 479) | 2.4 ± 1.04 (0.1; 4.2) | 35.09 ± 0.01 (34.8; 35.1) | 0.05 ± 0.21 (0; 1) |
| SEW 5 | Slopes of banks (Goose Bank) in SE | 14 | 168 ± 87.63 (38; 330) | 0.8 ± 0.55 (−0.5; 1.5) | 34.84 ± 0.33 (33.5; 35.0) | 61.50 ± 72.96 (0; 215) |
| SEW 6 | Murmansk Rise/Skolpen Bank | 14 | 196 ± 56.07 (107; 309) | 2.4 ± 0.62 (0.1; 3.3) | 34.90 ± 0.14 (34.7; 35.1) | 6.58 ± 13.04 (0; 44) |
| SEW 7 | Slopes of Kolguyev Island, Storfjord trench, outer Bear Island Channel. | 14 | 111 ± 73.28 (35; 287) | 2.0 ± 1.32 (0.4; 4.2) | 34.62 ± 0.51 (33.4; 35.1) | 105.68 ± 57.46 (6; 188) |
| SEW 8 | Kanin Bank and southern slopes of Goose Bank. East Basin. | 8 | 170 ± 104.12 (87; 387) | 1.3 ± 0.80 (0.2; 2.5) | 34.88 ± 0.09 (34.8;35.6) | 16.03 ± 11.93 (0; 45) |
| SEW 9 | Shelf along Svalbard W and N | 33 | 305 ± 156.50 (96; 627) | 2.9 ± 1.26 (0.5; 6.4) | 35.05 ± 0.12 (34.8;35.6) | 150.35 ± 109.28 (12; 349) |
| SEW 10 | Shallow slopes of Kolguyev Island and Nov Zem. Goose-, Kap Kanin-and Svalbard Bank. | 22 | 86 ± 36.42 (43; 179) | 2.5 ± 1.64 (−0.7; 5.5) | 34.46 ± 0.38 (33.6; 34.9) | 78.00 ± 56.34 (0; 189) |
| NW 11 | Hopen Deep. Central Bank S. Storfjord trench | 30 | 264 ± 67.02 (129; 370) | 0.7 ± 0.84 (−0.5; 3.6) | 34.99 ± 0.07 (34.8; 35.1) | 32.92 ± 46.76 (0; 165) |
| NW 12 | Central Bank, slopes of Great Bank, Kong Karls Land, Storfjorden Svalbard E. | 17 | 152 ± 46.51 (92; 226) | −0.01 ± 1.18 (−1.4; 3.4) | 34.68 ± 0.36 (33.9; 35.0) | 135.72 ± 113.01 (0; 165) |
| NW 13 | Svalbard N coast, fjord and sound. Banks of N and E Nordaustlandet. | 23 | 241 ± 83.78 (133; 432) | 1.8 ± 1.04 (−0.8; 3.5) | 34.84 ± 0.15 (34.4; 35.0) | 275.78 ± 40.48 (189; 333) |
| NW 14 | Great Bank. Olga Deep | 29 | 214 ± 55.74 (74; 360) | 0.7 ± 0.68 (−0.8; 1.8) | 34.88 ± 0.16 (34.4; 35.0) | 183.87 ± 74.34 (32; 292) |
| NE 15 | Eastern Basin, slopes of Goose Bank and Nov Zem. | 25 | 298 ± 52.18 (132; 368) | 0.3 ± 0.53 (−0.1; 2.4) | 34.98 ± 0.03 (34.9; 35.0) | 14.96 ± 49.72 (0; 339) |
| NE 16 | Novaya Zemlya (Nov Zem) Bank. Central Bank E flank. Pechora Sea N | 27 | 192 ± 55.34 (114; 310) | 0.2 ± 0.71 (−1.0; 2.4) | 34.92 ± 0.05 (34.9; 35.0) | 63.47 ± 58.28 (0; 249) |
| NE 17 | Slope of Svalbard N | 4 | 795 ± 130.64 (617; 928) | 0.5 ± 1.33 (−0.7; 2.2) | 34.96 ± 0.05 (34.9; 35.0) | 244.73 ± 70.73 (178; 336) |
| NE 18 | St Anna Trough W. Franz-Victoria Trough NW | 17 | 538 ± 54.35 (452; 636) | 0.6 ± 0.17 (0.3; 1.1) | 34.91 ± 0.02 (34.9; 34.9) | 293.40 ± 25.10 (250; 335) |
| NE 19 | Franz-Victoria Trough. Barents Sea NE. St Anna Trough E. Bank B | 40 | 288 ± 72.53 (144; 471) | 0.3 ± 0.51 (−0.9; 2.1) | 34.89 ± 0.07 (34.6; 34.9) | 195.45 ± 69.42 (57; 329) |
The local areas 1–19 within the sub-regions SW, SEW, NW and NE in the Barents Sea (see also figs. 1–6). The number of stations (no st), mean depth (m), temperature (°C), salinity (‰), days of ice-cover (mean of 2007–2011) are given with standard-deviation, minimum and maximum per local area. Largest and smallest mean values in bold.
| Local area . | Geographic area . | No St . | Depth (m) ± SD (min; max) . | Temp (°C) ± SD (min; max) . | Salt (‰) ± SD (min; max) . | Ice-days ± SD (min; max) . |
|---|---|---|---|---|---|---|
| SW 1 | Tromsø flaket | 11 | 242 ± 103.11 (64; 409) | 4.8 ± 1.48 (2.3; 6.5) | 35.11 ± 0.29 (34.4; 35.5) | 0 0 (0) |
| SW 2 | Western slope. Bear Island Channel outer part. | 15 | 383 ± 138.25 (77; 611) | 2.6 ± 0.86 (1.5; 4.3) | 35.03 ± 0.20 (34.1; 35.1) | 4.40 ± 8.32 (0; 26) |
| SW 3 | North Cape Bank. | 10 | 312 ± 40.00 (261; 379) | 4.6 ± 0.31 (4.1; 5.3) | 35.13 ± 0.03 (35.1; 35.2) | 0 ± 0 (0; 0) |
| SW 4 | Bear Island Channel. Atlantic inflow region of North Cape Bank N and E. | 25 | 378 ± 67.43 (240; 479) | 2.4 ± 1.04 (0.1; 4.2) | 35.09 ± 0.01 (34.8; 35.1) | 0.05 ± 0.21 (0; 1) |
| SEW 5 | Slopes of banks (Goose Bank) in SE | 14 | 168 ± 87.63 (38; 330) | 0.8 ± 0.55 (−0.5; 1.5) | 34.84 ± 0.33 (33.5; 35.0) | 61.50 ± 72.96 (0; 215) |
| SEW 6 | Murmansk Rise/Skolpen Bank | 14 | 196 ± 56.07 (107; 309) | 2.4 ± 0.62 (0.1; 3.3) | 34.90 ± 0.14 (34.7; 35.1) | 6.58 ± 13.04 (0; 44) |
| SEW 7 | Slopes of Kolguyev Island, Storfjord trench, outer Bear Island Channel. | 14 | 111 ± 73.28 (35; 287) | 2.0 ± 1.32 (0.4; 4.2) | 34.62 ± 0.51 (33.4; 35.1) | 105.68 ± 57.46 (6; 188) |
| SEW 8 | Kanin Bank and southern slopes of Goose Bank. East Basin. | 8 | 170 ± 104.12 (87; 387) | 1.3 ± 0.80 (0.2; 2.5) | 34.88 ± 0.09 (34.8;35.6) | 16.03 ± 11.93 (0; 45) |
| SEW 9 | Shelf along Svalbard W and N | 33 | 305 ± 156.50 (96; 627) | 2.9 ± 1.26 (0.5; 6.4) | 35.05 ± 0.12 (34.8;35.6) | 150.35 ± 109.28 (12; 349) |
| SEW 10 | Shallow slopes of Kolguyev Island and Nov Zem. Goose-, Kap Kanin-and Svalbard Bank. | 22 | 86 ± 36.42 (43; 179) | 2.5 ± 1.64 (−0.7; 5.5) | 34.46 ± 0.38 (33.6; 34.9) | 78.00 ± 56.34 (0; 189) |
| NW 11 | Hopen Deep. Central Bank S. Storfjord trench | 30 | 264 ± 67.02 (129; 370) | 0.7 ± 0.84 (−0.5; 3.6) | 34.99 ± 0.07 (34.8; 35.1) | 32.92 ± 46.76 (0; 165) |
| NW 12 | Central Bank, slopes of Great Bank, Kong Karls Land, Storfjorden Svalbard E. | 17 | 152 ± 46.51 (92; 226) | −0.01 ± 1.18 (−1.4; 3.4) | 34.68 ± 0.36 (33.9; 35.0) | 135.72 ± 113.01 (0; 165) |
| NW 13 | Svalbard N coast, fjord and sound. Banks of N and E Nordaustlandet. | 23 | 241 ± 83.78 (133; 432) | 1.8 ± 1.04 (−0.8; 3.5) | 34.84 ± 0.15 (34.4; 35.0) | 275.78 ± 40.48 (189; 333) |
| NW 14 | Great Bank. Olga Deep | 29 | 214 ± 55.74 (74; 360) | 0.7 ± 0.68 (−0.8; 1.8) | 34.88 ± 0.16 (34.4; 35.0) | 183.87 ± 74.34 (32; 292) |
| NE 15 | Eastern Basin, slopes of Goose Bank and Nov Zem. | 25 | 298 ± 52.18 (132; 368) | 0.3 ± 0.53 (−0.1; 2.4) | 34.98 ± 0.03 (34.9; 35.0) | 14.96 ± 49.72 (0; 339) |
| NE 16 | Novaya Zemlya (Nov Zem) Bank. Central Bank E flank. Pechora Sea N | 27 | 192 ± 55.34 (114; 310) | 0.2 ± 0.71 (−1.0; 2.4) | 34.92 ± 0.05 (34.9; 35.0) | 63.47 ± 58.28 (0; 249) |
| NE 17 | Slope of Svalbard N | 4 | 795 ± 130.64 (617; 928) | 0.5 ± 1.33 (−0.7; 2.2) | 34.96 ± 0.05 (34.9; 35.0) | 244.73 ± 70.73 (178; 336) |
| NE 18 | St Anna Trough W. Franz-Victoria Trough NW | 17 | 538 ± 54.35 (452; 636) | 0.6 ± 0.17 (0.3; 1.1) | 34.91 ± 0.02 (34.9; 34.9) | 293.40 ± 25.10 (250; 335) |
| NE 19 | Franz-Victoria Trough. Barents Sea NE. St Anna Trough E. Bank B | 40 | 288 ± 72.53 (144; 471) | 0.3 ± 0.51 (−0.9; 2.1) | 34.89 ± 0.07 (34.6; 34.9) | 195.45 ± 69.42 (57; 329) |
| Local area . | Geographic area . | No St . | Depth (m) ± SD (min; max) . | Temp (°C) ± SD (min; max) . | Salt (‰) ± SD (min; max) . | Ice-days ± SD (min; max) . |
|---|---|---|---|---|---|---|
| SW 1 | Tromsø flaket | 11 | 242 ± 103.11 (64; 409) | 4.8 ± 1.48 (2.3; 6.5) | 35.11 ± 0.29 (34.4; 35.5) | 0 0 (0) |
| SW 2 | Western slope. Bear Island Channel outer part. | 15 | 383 ± 138.25 (77; 611) | 2.6 ± 0.86 (1.5; 4.3) | 35.03 ± 0.20 (34.1; 35.1) | 4.40 ± 8.32 (0; 26) |
| SW 3 | North Cape Bank. | 10 | 312 ± 40.00 (261; 379) | 4.6 ± 0.31 (4.1; 5.3) | 35.13 ± 0.03 (35.1; 35.2) | 0 ± 0 (0; 0) |
| SW 4 | Bear Island Channel. Atlantic inflow region of North Cape Bank N and E. | 25 | 378 ± 67.43 (240; 479) | 2.4 ± 1.04 (0.1; 4.2) | 35.09 ± 0.01 (34.8; 35.1) | 0.05 ± 0.21 (0; 1) |
| SEW 5 | Slopes of banks (Goose Bank) in SE | 14 | 168 ± 87.63 (38; 330) | 0.8 ± 0.55 (−0.5; 1.5) | 34.84 ± 0.33 (33.5; 35.0) | 61.50 ± 72.96 (0; 215) |
| SEW 6 | Murmansk Rise/Skolpen Bank | 14 | 196 ± 56.07 (107; 309) | 2.4 ± 0.62 (0.1; 3.3) | 34.90 ± 0.14 (34.7; 35.1) | 6.58 ± 13.04 (0; 44) |
| SEW 7 | Slopes of Kolguyev Island, Storfjord trench, outer Bear Island Channel. | 14 | 111 ± 73.28 (35; 287) | 2.0 ± 1.32 (0.4; 4.2) | 34.62 ± 0.51 (33.4; 35.1) | 105.68 ± 57.46 (6; 188) |
| SEW 8 | Kanin Bank and southern slopes of Goose Bank. East Basin. | 8 | 170 ± 104.12 (87; 387) | 1.3 ± 0.80 (0.2; 2.5) | 34.88 ± 0.09 (34.8;35.6) | 16.03 ± 11.93 (0; 45) |
| SEW 9 | Shelf along Svalbard W and N | 33 | 305 ± 156.50 (96; 627) | 2.9 ± 1.26 (0.5; 6.4) | 35.05 ± 0.12 (34.8;35.6) | 150.35 ± 109.28 (12; 349) |
| SEW 10 | Shallow slopes of Kolguyev Island and Nov Zem. Goose-, Kap Kanin-and Svalbard Bank. | 22 | 86 ± 36.42 (43; 179) | 2.5 ± 1.64 (−0.7; 5.5) | 34.46 ± 0.38 (33.6; 34.9) | 78.00 ± 56.34 (0; 189) |
| NW 11 | Hopen Deep. Central Bank S. Storfjord trench | 30 | 264 ± 67.02 (129; 370) | 0.7 ± 0.84 (−0.5; 3.6) | 34.99 ± 0.07 (34.8; 35.1) | 32.92 ± 46.76 (0; 165) |
| NW 12 | Central Bank, slopes of Great Bank, Kong Karls Land, Storfjorden Svalbard E. | 17 | 152 ± 46.51 (92; 226) | −0.01 ± 1.18 (−1.4; 3.4) | 34.68 ± 0.36 (33.9; 35.0) | 135.72 ± 113.01 (0; 165) |
| NW 13 | Svalbard N coast, fjord and sound. Banks of N and E Nordaustlandet. | 23 | 241 ± 83.78 (133; 432) | 1.8 ± 1.04 (−0.8; 3.5) | 34.84 ± 0.15 (34.4; 35.0) | 275.78 ± 40.48 (189; 333) |
| NW 14 | Great Bank. Olga Deep | 29 | 214 ± 55.74 (74; 360) | 0.7 ± 0.68 (−0.8; 1.8) | 34.88 ± 0.16 (34.4; 35.0) | 183.87 ± 74.34 (32; 292) |
| NE 15 | Eastern Basin, slopes of Goose Bank and Nov Zem. | 25 | 298 ± 52.18 (132; 368) | 0.3 ± 0.53 (−0.1; 2.4) | 34.98 ± 0.03 (34.9; 35.0) | 14.96 ± 49.72 (0; 339) |
| NE 16 | Novaya Zemlya (Nov Zem) Bank. Central Bank E flank. Pechora Sea N | 27 | 192 ± 55.34 (114; 310) | 0.2 ± 0.71 (−1.0; 2.4) | 34.92 ± 0.05 (34.9; 35.0) | 63.47 ± 58.28 (0; 249) |
| NE 17 | Slope of Svalbard N | 4 | 795 ± 130.64 (617; 928) | 0.5 ± 1.33 (−0.7; 2.2) | 34.96 ± 0.05 (34.9; 35.0) | 244.73 ± 70.73 (178; 336) |
| NE 18 | St Anna Trough W. Franz-Victoria Trough NW | 17 | 538 ± 54.35 (452; 636) | 0.6 ± 0.17 (0.3; 1.1) | 34.91 ± 0.02 (34.9; 34.9) | 293.40 ± 25.10 (250; 335) |
| NE 19 | Franz-Victoria Trough. Barents Sea NE. St Anna Trough E. Bank B | 40 | 288 ± 72.53 (144; 471) | 0.3 ± 0.51 (−0.9; 2.1) | 34.89 ± 0.07 (34.6; 34.9) | 195.45 ± 69.42 (57; 329) |
Station clusters with % cutting level from the clusterdiagram (Fig. 4) for the sub-regions SW, SEW, NW and NE and local areas 1–19 in the Barents Sea presented with number of taxa, mean abundance (excluded colonial taxa), and biomass with standard-deviation, minimum and maximum. The top 5 most dominant species in biomass are given with % of the station biomass and the cumulative biomass. Largest and smallest mean values in bold.
| Sub Reg . | Loc area . | Taxa ± SD (min; max) . | Bio (kg) ± SD (min; max) . | Abu ± SD (min; max) . | Dominant species (% and cumulative % of top 5 in biomass) . |
|---|---|---|---|---|---|
| SW 24% | SW 1 42.1% | 13.72 ± 6.27 (4; 23) | 9.11 ± 13.15 (0.3; 34) | 76.17 ± 71.4 (3; 210) | Geodia Sp (38, 38) Geodia macandrewii (29, 67) Paralithodes camtschaticus (21, 88) Porifera (7, 95) Munida bamffica (1, 96) |
| SW 2 51.1% | 18.86 ± 7.77 (7; 35) | 1.85 ± 5.05 (0.5; 20) | 91.48 ± 105.7 (14; 445) | Geodia macandrewii (71, 71) Geodia barretti (9, 80) Solaster endeca (8, 88) Porifera indet. (2, 90) Polymastia sp. (1, 91) | |
| SW 3 55.8% | 17.00 ± 6.34 (8; 27) | 84.20 ± 130.66 (7; 339) | 95.72 ± 108.8 (21; 338) | Geodia barretti (53, 53) Geodia macandrewii (44, 97) Porifera indet (1, 98) Parastichopus tremulus (1, 99) Thenea muricata (0.4, 99) | |
| SW 4 45.8% | 23.20 ± 8.44 (10; 41) | 3.01 ± 3.7 (0.1; 14) | 214.19 ± 217.5 (18; 734) | Molpadia borealis (23, 23) Geodia barretti (16, 39) Thenea muricata (15, 54) Geodia macandrewii (11, 65) Ctenodiscus crispatus (6, 72) | |
| SEW 17.7% | SEW 5 52.5% | 11.14 ± 4.05 (5; 20) | 1.17 ± 1.5 (0.2; 5) | 127.35 ± 187.7 (13; 743) | Chionoecetes opilio (41, 41) Strongylocentrotus sp. (24, 65) Sabinea septemcarinata (11, 76) Solaster sp. (8, 85) Ctenodiscus crispatus (3, 88) |
| SEW 6 48.3% | 10.78 ± 5.35 (3; 23) | 0.91 ± 1.09 (0.008; 4) | 21.21 ± 16.2 (3; 55) | Suberites sp. (34, 34) Hippasteria phrygiana (21, 55) Actiniaria indet. (14, 70) Porifera indet. (9, 79) Hormathia sp. (5, 84) | |
| SEW 7 44.4% | 7.98 ± 4.61 (2; 16) | 0.21 ± 0.31 (0.01; 1) | 40.44 ± 48.2 (1; 156) | Sabinea septemcarinata (24, 24) Chionoecetes opilio (15, 39) Strongylocentrotus sp. (15, 54) Balanus sp. (8, 62) Sclerocrangon ferox (7, 69) | |
| SEW 8 53% | 7.37 ± 4.30 (4; 17) | 1.21 ± 1.11 (0.2; 3) | 28.89 ± 17.6 (6; 55) | Icasterias panopla (49, 49) Cucumaria frondosa (20, 69) Urasterias linckii (14, 83) Porifera indet. (5, 87) Chlamys islandica (4, 91) | |
| SEW 9 48.9% | 33.51 ± 8.91 (15; 54) | 6.62 ± 8.5 (0.4; 38) | 479.83 ± 502.5 (82; 2592) | Porifera (51, 51) Strongylocentrotus sp. (17, 68) Phakellia sp. (5, 73) Geodia barretti (5, 78) Haliclona sp. (4, 81) | |
| SEW 10 43.7% | 22.68 ± 8.68 (4; 42) | 19.62 ± 33.5 (0.4; 139) | 590.12 ± 1056.8 (5; 4752) | Cucumaria frondosa (44, 44) Chlamys islandica (22, 66) Porifera indet. (7, 73) Hyas spp. (6, 79) Leptasterias sp. (5, 84) | |
| NW 30.3% | NW 11 45.1% | 31.43 ± 8.66 (15; 51) | 1.68 ± 1.60 (0.3; 8) | 514.60 ± 699.2 (72; 3341) | Ctenodiscus crispatus (21, 21) Sabinea septemcarinata (11, 32) Molpadia borealis (8, 40) Icasterias panopla (8, 48) Porifera indet (7, 55) |
| NW 12 53.9% | 38.64 ± 13.11 (19; 70) | 7.81 ± 31.2 (0.9; 32) | 1234.07 ± 1831.0 (282; 7874) | Strongylocentrotus sp. (43, 43) Gorgonocephalus eucnemis (8, 51) Sabinea septemcarinata (8, 59) Heliometra glacialis (6, 64) Urasterias linckii (5, 70) | |
| NW 13 43% | 26.08 ± 9.33 (11; 49) | 11.63 ± 8.60 (0.5; 32) | 2225.96 ± 2040.1 (141; 7623) | Ctenodiscus crispatus (36, 36) Icasterias panopla (8, 44) Gorgonocephalus arcticus (6, 50) Ophiura sarsi (5, 55) Strongylocentrotus sp. (5, 61) | |
| NW 14 51.6% | 32.68 ± 9.13 (20; 48) | 3.43 ± 3.56 (0.3; 15) | 312.09 ± 166.4 (88; 754) | Gorgonocephalus arcticus (38, 38) Gorgonocephalus eucnemis (12, 50) Alcyonidium sp. (6, 57) Ctenodiscus crispatus (6, 62) Molpadia borealis (5, 67) | |
| NE 26.5% | NE 15 50% | 21.96 ± 7.67 (11; 40) | 7.05 ± 8.89 (1.2; 46) | 741.90 ± 645.2 (66; 2615) | Spiochaetopterus typicus (15, 15) Porifera (11, 26) Chionoecetes opilio (10, 37) Molpadia borealis (7, 44) Sabinea septemcarinata (7, 51) |
| NE 16 49.5% | 28.44 ± 8.28 (13; 43) | 70.57 ± 204.24 (6; 1086) | 4854.01 ± 5237.3 (454; 19226) | Porifera (57, 57) Strongylocentrotus sp. (10, 67) Chionoecetes opilio (5, 72) Sabinea septemcarinata (4, 75) Polychaeta indet. (3, 78) | |
| NE 17 62.3% | 31.00 ± 13.73 (15; 46) | 1.67 ± 0.63 (1; 2) | 92.68 ± 53.8 (40; 157) | Gorgonocephalus sp. (57, 57) Gorgonocephalus eucnemis (9, 66) Sclerocrangon ferox (4, 70) Gorgonocephalus arcticus (4, 74) Geodia macandrewii (3, 77) | |
| NE 18 54.3% | 19.17 ± 4.83 (10; 27) | 8.21 ± 9.4 (1; 39) | 1164.49 ± 2702.5 (61; 10997) | Umbellula encrinus (32, 32) Ophiopleura borealis (16, 48) Gorgonocephalus arcticus (10, 59) Porifera (9, 67) Actiniaria (7, 75) | |
| NE 19 46.5% | 29.07 ± 6.56 (14; 45) | 137.56 ± 364.32 (6; 1752) | 19030.73 ± 48022.3 (478; 300285) | Porifera (47, 47) Ophiacantha bidentata (11, 58) Gorgonocephalus arcticus (8, 66) Ophiopleura borealis (7, 73) Bryozoa (7, 80) |
| Sub Reg . | Loc area . | Taxa ± SD (min; max) . | Bio (kg) ± SD (min; max) . | Abu ± SD (min; max) . | Dominant species (% and cumulative % of top 5 in biomass) . |
|---|---|---|---|---|---|
| SW 24% | SW 1 42.1% | 13.72 ± 6.27 (4; 23) | 9.11 ± 13.15 (0.3; 34) | 76.17 ± 71.4 (3; 210) | Geodia Sp (38, 38) Geodia macandrewii (29, 67) Paralithodes camtschaticus (21, 88) Porifera (7, 95) Munida bamffica (1, 96) |
| SW 2 51.1% | 18.86 ± 7.77 (7; 35) | 1.85 ± 5.05 (0.5; 20) | 91.48 ± 105.7 (14; 445) | Geodia macandrewii (71, 71) Geodia barretti (9, 80) Solaster endeca (8, 88) Porifera indet. (2, 90) Polymastia sp. (1, 91) | |
| SW 3 55.8% | 17.00 ± 6.34 (8; 27) | 84.20 ± 130.66 (7; 339) | 95.72 ± 108.8 (21; 338) | Geodia barretti (53, 53) Geodia macandrewii (44, 97) Porifera indet (1, 98) Parastichopus tremulus (1, 99) Thenea muricata (0.4, 99) | |
| SW 4 45.8% | 23.20 ± 8.44 (10; 41) | 3.01 ± 3.7 (0.1; 14) | 214.19 ± 217.5 (18; 734) | Molpadia borealis (23, 23) Geodia barretti (16, 39) Thenea muricata (15, 54) Geodia macandrewii (11, 65) Ctenodiscus crispatus (6, 72) | |
| SEW 17.7% | SEW 5 52.5% | 11.14 ± 4.05 (5; 20) | 1.17 ± 1.5 (0.2; 5) | 127.35 ± 187.7 (13; 743) | Chionoecetes opilio (41, 41) Strongylocentrotus sp. (24, 65) Sabinea septemcarinata (11, 76) Solaster sp. (8, 85) Ctenodiscus crispatus (3, 88) |
| SEW 6 48.3% | 10.78 ± 5.35 (3; 23) | 0.91 ± 1.09 (0.008; 4) | 21.21 ± 16.2 (3; 55) | Suberites sp. (34, 34) Hippasteria phrygiana (21, 55) Actiniaria indet. (14, 70) Porifera indet. (9, 79) Hormathia sp. (5, 84) | |
| SEW 7 44.4% | 7.98 ± 4.61 (2; 16) | 0.21 ± 0.31 (0.01; 1) | 40.44 ± 48.2 (1; 156) | Sabinea septemcarinata (24, 24) Chionoecetes opilio (15, 39) Strongylocentrotus sp. (15, 54) Balanus sp. (8, 62) Sclerocrangon ferox (7, 69) | |
| SEW 8 53% | 7.37 ± 4.30 (4; 17) | 1.21 ± 1.11 (0.2; 3) | 28.89 ± 17.6 (6; 55) | Icasterias panopla (49, 49) Cucumaria frondosa (20, 69) Urasterias linckii (14, 83) Porifera indet. (5, 87) Chlamys islandica (4, 91) | |
| SEW 9 48.9% | 33.51 ± 8.91 (15; 54) | 6.62 ± 8.5 (0.4; 38) | 479.83 ± 502.5 (82; 2592) | Porifera (51, 51) Strongylocentrotus sp. (17, 68) Phakellia sp. (5, 73) Geodia barretti (5, 78) Haliclona sp. (4, 81) | |
| SEW 10 43.7% | 22.68 ± 8.68 (4; 42) | 19.62 ± 33.5 (0.4; 139) | 590.12 ± 1056.8 (5; 4752) | Cucumaria frondosa (44, 44) Chlamys islandica (22, 66) Porifera indet. (7, 73) Hyas spp. (6, 79) Leptasterias sp. (5, 84) | |
| NW 30.3% | NW 11 45.1% | 31.43 ± 8.66 (15; 51) | 1.68 ± 1.60 (0.3; 8) | 514.60 ± 699.2 (72; 3341) | Ctenodiscus crispatus (21, 21) Sabinea septemcarinata (11, 32) Molpadia borealis (8, 40) Icasterias panopla (8, 48) Porifera indet (7, 55) |
| NW 12 53.9% | 38.64 ± 13.11 (19; 70) | 7.81 ± 31.2 (0.9; 32) | 1234.07 ± 1831.0 (282; 7874) | Strongylocentrotus sp. (43, 43) Gorgonocephalus eucnemis (8, 51) Sabinea septemcarinata (8, 59) Heliometra glacialis (6, 64) Urasterias linckii (5, 70) | |
| NW 13 43% | 26.08 ± 9.33 (11; 49) | 11.63 ± 8.60 (0.5; 32) | 2225.96 ± 2040.1 (141; 7623) | Ctenodiscus crispatus (36, 36) Icasterias panopla (8, 44) Gorgonocephalus arcticus (6, 50) Ophiura sarsi (5, 55) Strongylocentrotus sp. (5, 61) | |
| NW 14 51.6% | 32.68 ± 9.13 (20; 48) | 3.43 ± 3.56 (0.3; 15) | 312.09 ± 166.4 (88; 754) | Gorgonocephalus arcticus (38, 38) Gorgonocephalus eucnemis (12, 50) Alcyonidium sp. (6, 57) Ctenodiscus crispatus (6, 62) Molpadia borealis (5, 67) | |
| NE 26.5% | NE 15 50% | 21.96 ± 7.67 (11; 40) | 7.05 ± 8.89 (1.2; 46) | 741.90 ± 645.2 (66; 2615) | Spiochaetopterus typicus (15, 15) Porifera (11, 26) Chionoecetes opilio (10, 37) Molpadia borealis (7, 44) Sabinea septemcarinata (7, 51) |
| NE 16 49.5% | 28.44 ± 8.28 (13; 43) | 70.57 ± 204.24 (6; 1086) | 4854.01 ± 5237.3 (454; 19226) | Porifera (57, 57) Strongylocentrotus sp. (10, 67) Chionoecetes opilio (5, 72) Sabinea septemcarinata (4, 75) Polychaeta indet. (3, 78) | |
| NE 17 62.3% | 31.00 ± 13.73 (15; 46) | 1.67 ± 0.63 (1; 2) | 92.68 ± 53.8 (40; 157) | Gorgonocephalus sp. (57, 57) Gorgonocephalus eucnemis (9, 66) Sclerocrangon ferox (4, 70) Gorgonocephalus arcticus (4, 74) Geodia macandrewii (3, 77) | |
| NE 18 54.3% | 19.17 ± 4.83 (10; 27) | 8.21 ± 9.4 (1; 39) | 1164.49 ± 2702.5 (61; 10997) | Umbellula encrinus (32, 32) Ophiopleura borealis (16, 48) Gorgonocephalus arcticus (10, 59) Porifera (9, 67) Actiniaria (7, 75) | |
| NE 19 46.5% | 29.07 ± 6.56 (14; 45) | 137.56 ± 364.32 (6; 1752) | 19030.73 ± 48022.3 (478; 300285) | Porifera (47, 47) Ophiacantha bidentata (11, 58) Gorgonocephalus arcticus (8, 66) Ophiopleura borealis (7, 73) Bryozoa (7, 80) |
Station clusters with % cutting level from the clusterdiagram (Fig. 4) for the sub-regions SW, SEW, NW and NE and local areas 1–19 in the Barents Sea presented with number of taxa, mean abundance (excluded colonial taxa), and biomass with standard-deviation, minimum and maximum. The top 5 most dominant species in biomass are given with % of the station biomass and the cumulative biomass. Largest and smallest mean values in bold.
| Sub Reg . | Loc area . | Taxa ± SD (min; max) . | Bio (kg) ± SD (min; max) . | Abu ± SD (min; max) . | Dominant species (% and cumulative % of top 5 in biomass) . |
|---|---|---|---|---|---|
| SW 24% | SW 1 42.1% | 13.72 ± 6.27 (4; 23) | 9.11 ± 13.15 (0.3; 34) | 76.17 ± 71.4 (3; 210) | Geodia Sp (38, 38) Geodia macandrewii (29, 67) Paralithodes camtschaticus (21, 88) Porifera (7, 95) Munida bamffica (1, 96) |
| SW 2 51.1% | 18.86 ± 7.77 (7; 35) | 1.85 ± 5.05 (0.5; 20) | 91.48 ± 105.7 (14; 445) | Geodia macandrewii (71, 71) Geodia barretti (9, 80) Solaster endeca (8, 88) Porifera indet. (2, 90) Polymastia sp. (1, 91) | |
| SW 3 55.8% | 17.00 ± 6.34 (8; 27) | 84.20 ± 130.66 (7; 339) | 95.72 ± 108.8 (21; 338) | Geodia barretti (53, 53) Geodia macandrewii (44, 97) Porifera indet (1, 98) Parastichopus tremulus (1, 99) Thenea muricata (0.4, 99) | |
| SW 4 45.8% | 23.20 ± 8.44 (10; 41) | 3.01 ± 3.7 (0.1; 14) | 214.19 ± 217.5 (18; 734) | Molpadia borealis (23, 23) Geodia barretti (16, 39) Thenea muricata (15, 54) Geodia macandrewii (11, 65) Ctenodiscus crispatus (6, 72) | |
| SEW 17.7% | SEW 5 52.5% | 11.14 ± 4.05 (5; 20) | 1.17 ± 1.5 (0.2; 5) | 127.35 ± 187.7 (13; 743) | Chionoecetes opilio (41, 41) Strongylocentrotus sp. (24, 65) Sabinea septemcarinata (11, 76) Solaster sp. (8, 85) Ctenodiscus crispatus (3, 88) |
| SEW 6 48.3% | 10.78 ± 5.35 (3; 23) | 0.91 ± 1.09 (0.008; 4) | 21.21 ± 16.2 (3; 55) | Suberites sp. (34, 34) Hippasteria phrygiana (21, 55) Actiniaria indet. (14, 70) Porifera indet. (9, 79) Hormathia sp. (5, 84) | |
| SEW 7 44.4% | 7.98 ± 4.61 (2; 16) | 0.21 ± 0.31 (0.01; 1) | 40.44 ± 48.2 (1; 156) | Sabinea septemcarinata (24, 24) Chionoecetes opilio (15, 39) Strongylocentrotus sp. (15, 54) Balanus sp. (8, 62) Sclerocrangon ferox (7, 69) | |
| SEW 8 53% | 7.37 ± 4.30 (4; 17) | 1.21 ± 1.11 (0.2; 3) | 28.89 ± 17.6 (6; 55) | Icasterias panopla (49, 49) Cucumaria frondosa (20, 69) Urasterias linckii (14, 83) Porifera indet. (5, 87) Chlamys islandica (4, 91) | |
| SEW 9 48.9% | 33.51 ± 8.91 (15; 54) | 6.62 ± 8.5 (0.4; 38) | 479.83 ± 502.5 (82; 2592) | Porifera (51, 51) Strongylocentrotus sp. (17, 68) Phakellia sp. (5, 73) Geodia barretti (5, 78) Haliclona sp. (4, 81) | |
| SEW 10 43.7% | 22.68 ± 8.68 (4; 42) | 19.62 ± 33.5 (0.4; 139) | 590.12 ± 1056.8 (5; 4752) | Cucumaria frondosa (44, 44) Chlamys islandica (22, 66) Porifera indet. (7, 73) Hyas spp. (6, 79) Leptasterias sp. (5, 84) | |
| NW 30.3% | NW 11 45.1% | 31.43 ± 8.66 (15; 51) | 1.68 ± 1.60 (0.3; 8) | 514.60 ± 699.2 (72; 3341) | Ctenodiscus crispatus (21, 21) Sabinea septemcarinata (11, 32) Molpadia borealis (8, 40) Icasterias panopla (8, 48) Porifera indet (7, 55) |
| NW 12 53.9% | 38.64 ± 13.11 (19; 70) | 7.81 ± 31.2 (0.9; 32) | 1234.07 ± 1831.0 (282; 7874) | Strongylocentrotus sp. (43, 43) Gorgonocephalus eucnemis (8, 51) Sabinea septemcarinata (8, 59) Heliometra glacialis (6, 64) Urasterias linckii (5, 70) | |
| NW 13 43% | 26.08 ± 9.33 (11; 49) | 11.63 ± 8.60 (0.5; 32) | 2225.96 ± 2040.1 (141; 7623) | Ctenodiscus crispatus (36, 36) Icasterias panopla (8, 44) Gorgonocephalus arcticus (6, 50) Ophiura sarsi (5, 55) Strongylocentrotus sp. (5, 61) | |
| NW 14 51.6% | 32.68 ± 9.13 (20; 48) | 3.43 ± 3.56 (0.3; 15) | 312.09 ± 166.4 (88; 754) | Gorgonocephalus arcticus (38, 38) Gorgonocephalus eucnemis (12, 50) Alcyonidium sp. (6, 57) Ctenodiscus crispatus (6, 62) Molpadia borealis (5, 67) | |
| NE 26.5% | NE 15 50% | 21.96 ± 7.67 (11; 40) | 7.05 ± 8.89 (1.2; 46) | 741.90 ± 645.2 (66; 2615) | Spiochaetopterus typicus (15, 15) Porifera (11, 26) Chionoecetes opilio (10, 37) Molpadia borealis (7, 44) Sabinea septemcarinata (7, 51) |
| NE 16 49.5% | 28.44 ± 8.28 (13; 43) | 70.57 ± 204.24 (6; 1086) | 4854.01 ± 5237.3 (454; 19226) | Porifera (57, 57) Strongylocentrotus sp. (10, 67) Chionoecetes opilio (5, 72) Sabinea septemcarinata (4, 75) Polychaeta indet. (3, 78) | |
| NE 17 62.3% | 31.00 ± 13.73 (15; 46) | 1.67 ± 0.63 (1; 2) | 92.68 ± 53.8 (40; 157) | Gorgonocephalus sp. (57, 57) Gorgonocephalus eucnemis (9, 66) Sclerocrangon ferox (4, 70) Gorgonocephalus arcticus (4, 74) Geodia macandrewii (3, 77) | |
| NE 18 54.3% | 19.17 ± 4.83 (10; 27) | 8.21 ± 9.4 (1; 39) | 1164.49 ± 2702.5 (61; 10997) | Umbellula encrinus (32, 32) Ophiopleura borealis (16, 48) Gorgonocephalus arcticus (10, 59) Porifera (9, 67) Actiniaria (7, 75) | |
| NE 19 46.5% | 29.07 ± 6.56 (14; 45) | 137.56 ± 364.32 (6; 1752) | 19030.73 ± 48022.3 (478; 300285) | Porifera (47, 47) Ophiacantha bidentata (11, 58) Gorgonocephalus arcticus (8, 66) Ophiopleura borealis (7, 73) Bryozoa (7, 80) |
| Sub Reg . | Loc area . | Taxa ± SD (min; max) . | Bio (kg) ± SD (min; max) . | Abu ± SD (min; max) . | Dominant species (% and cumulative % of top 5 in biomass) . |
|---|---|---|---|---|---|
| SW 24% | SW 1 42.1% | 13.72 ± 6.27 (4; 23) | 9.11 ± 13.15 (0.3; 34) | 76.17 ± 71.4 (3; 210) | Geodia Sp (38, 38) Geodia macandrewii (29, 67) Paralithodes camtschaticus (21, 88) Porifera (7, 95) Munida bamffica (1, 96) |
| SW 2 51.1% | 18.86 ± 7.77 (7; 35) | 1.85 ± 5.05 (0.5; 20) | 91.48 ± 105.7 (14; 445) | Geodia macandrewii (71, 71) Geodia barretti (9, 80) Solaster endeca (8, 88) Porifera indet. (2, 90) Polymastia sp. (1, 91) | |
| SW 3 55.8% | 17.00 ± 6.34 (8; 27) | 84.20 ± 130.66 (7; 339) | 95.72 ± 108.8 (21; 338) | Geodia barretti (53, 53) Geodia macandrewii (44, 97) Porifera indet (1, 98) Parastichopus tremulus (1, 99) Thenea muricata (0.4, 99) | |
| SW 4 45.8% | 23.20 ± 8.44 (10; 41) | 3.01 ± 3.7 (0.1; 14) | 214.19 ± 217.5 (18; 734) | Molpadia borealis (23, 23) Geodia barretti (16, 39) Thenea muricata (15, 54) Geodia macandrewii (11, 65) Ctenodiscus crispatus (6, 72) | |
| SEW 17.7% | SEW 5 52.5% | 11.14 ± 4.05 (5; 20) | 1.17 ± 1.5 (0.2; 5) | 127.35 ± 187.7 (13; 743) | Chionoecetes opilio (41, 41) Strongylocentrotus sp. (24, 65) Sabinea septemcarinata (11, 76) Solaster sp. (8, 85) Ctenodiscus crispatus (3, 88) |
| SEW 6 48.3% | 10.78 ± 5.35 (3; 23) | 0.91 ± 1.09 (0.008; 4) | 21.21 ± 16.2 (3; 55) | Suberites sp. (34, 34) Hippasteria phrygiana (21, 55) Actiniaria indet. (14, 70) Porifera indet. (9, 79) Hormathia sp. (5, 84) | |
| SEW 7 44.4% | 7.98 ± 4.61 (2; 16) | 0.21 ± 0.31 (0.01; 1) | 40.44 ± 48.2 (1; 156) | Sabinea septemcarinata (24, 24) Chionoecetes opilio (15, 39) Strongylocentrotus sp. (15, 54) Balanus sp. (8, 62) Sclerocrangon ferox (7, 69) | |
| SEW 8 53% | 7.37 ± 4.30 (4; 17) | 1.21 ± 1.11 (0.2; 3) | 28.89 ± 17.6 (6; 55) | Icasterias panopla (49, 49) Cucumaria frondosa (20, 69) Urasterias linckii (14, 83) Porifera indet. (5, 87) Chlamys islandica (4, 91) | |
| SEW 9 48.9% | 33.51 ± 8.91 (15; 54) | 6.62 ± 8.5 (0.4; 38) | 479.83 ± 502.5 (82; 2592) | Porifera (51, 51) Strongylocentrotus sp. (17, 68) Phakellia sp. (5, 73) Geodia barretti (5, 78) Haliclona sp. (4, 81) | |
| SEW 10 43.7% | 22.68 ± 8.68 (4; 42) | 19.62 ± 33.5 (0.4; 139) | 590.12 ± 1056.8 (5; 4752) | Cucumaria frondosa (44, 44) Chlamys islandica (22, 66) Porifera indet. (7, 73) Hyas spp. (6, 79) Leptasterias sp. (5, 84) | |
| NW 30.3% | NW 11 45.1% | 31.43 ± 8.66 (15; 51) | 1.68 ± 1.60 (0.3; 8) | 514.60 ± 699.2 (72; 3341) | Ctenodiscus crispatus (21, 21) Sabinea septemcarinata (11, 32) Molpadia borealis (8, 40) Icasterias panopla (8, 48) Porifera indet (7, 55) |
| NW 12 53.9% | 38.64 ± 13.11 (19; 70) | 7.81 ± 31.2 (0.9; 32) | 1234.07 ± 1831.0 (282; 7874) | Strongylocentrotus sp. (43, 43) Gorgonocephalus eucnemis (8, 51) Sabinea septemcarinata (8, 59) Heliometra glacialis (6, 64) Urasterias linckii (5, 70) | |
| NW 13 43% | 26.08 ± 9.33 (11; 49) | 11.63 ± 8.60 (0.5; 32) | 2225.96 ± 2040.1 (141; 7623) | Ctenodiscus crispatus (36, 36) Icasterias panopla (8, 44) Gorgonocephalus arcticus (6, 50) Ophiura sarsi (5, 55) Strongylocentrotus sp. (5, 61) | |
| NW 14 51.6% | 32.68 ± 9.13 (20; 48) | 3.43 ± 3.56 (0.3; 15) | 312.09 ± 166.4 (88; 754) | Gorgonocephalus arcticus (38, 38) Gorgonocephalus eucnemis (12, 50) Alcyonidium sp. (6, 57) Ctenodiscus crispatus (6, 62) Molpadia borealis (5, 67) | |
| NE 26.5% | NE 15 50% | 21.96 ± 7.67 (11; 40) | 7.05 ± 8.89 (1.2; 46) | 741.90 ± 645.2 (66; 2615) | Spiochaetopterus typicus (15, 15) Porifera (11, 26) Chionoecetes opilio (10, 37) Molpadia borealis (7, 44) Sabinea septemcarinata (7, 51) |
| NE 16 49.5% | 28.44 ± 8.28 (13; 43) | 70.57 ± 204.24 (6; 1086) | 4854.01 ± 5237.3 (454; 19226) | Porifera (57, 57) Strongylocentrotus sp. (10, 67) Chionoecetes opilio (5, 72) Sabinea septemcarinata (4, 75) Polychaeta indet. (3, 78) | |
| NE 17 62.3% | 31.00 ± 13.73 (15; 46) | 1.67 ± 0.63 (1; 2) | 92.68 ± 53.8 (40; 157) | Gorgonocephalus sp. (57, 57) Gorgonocephalus eucnemis (9, 66) Sclerocrangon ferox (4, 70) Gorgonocephalus arcticus (4, 74) Geodia macandrewii (3, 77) | |
| NE 18 54.3% | 19.17 ± 4.83 (10; 27) | 8.21 ± 9.4 (1; 39) | 1164.49 ± 2702.5 (61; 10997) | Umbellula encrinus (32, 32) Ophiopleura borealis (16, 48) Gorgonocephalus arcticus (10, 59) Porifera (9, 67) Actiniaria (7, 75) | |
| NE 19 46.5% | 29.07 ± 6.56 (14; 45) | 137.56 ± 364.32 (6; 1752) | 19030.73 ± 48022.3 (478; 300285) | Porifera (47, 47) Ophiacantha bidentata (11, 58) Gorgonocephalus arcticus (8, 66) Ophiopleura borealis (7, 73) Bryozoa (7, 80) |
The biomass of benthic megafauna collected by trawl showed very large variation both within and between regions and local areas. Generally the northern part of the Barents Sea (NE and NW region) had more taxa than the southern part (290 vs. 268; range of means per station for subareas was 19–39 vs. 7–34), higher biomass (range of means per station for subareas was 2–138 vs. 0.2–84 kg) and higher abundance (range of means per station for subareas was 93–19.000 vs. 21–590).
The SW benthic megafauna region occupies the slope and channels of the entrance in the southwestern Barents Sea, including the relatively deep banks north of the Norwegian coast (Tromsø Plateau, North Cape Bank, Figure 1 and red symbols in Figure 5). This region is, on average, relatively deep (64–611 m), warm (2.5–5°C, Figure 2, Table 2), had the highest salinities of all the regions (34.1–35.5), and is largely ice-free (Figure 3). The mean abundance of specimens in SW was generally lower than in the other regions (range 2–734 individuals per haul). The SW was characterized by the biomass-dominant sponge Geodia spp. Species only recorded in this area included the king crab Paralithodes camtschaticus, boreal species such as the cup coral Caryophyllia smithii, the anomura crustaceans Munida bamffica and Lithodes maja, the sea urchins Echinus acutus and Brisaster fragilis, the sea cucumber Parastichopus tremulus, and the sea star Pseudarchaster parelii (Figure 6b). The boreal-Arctic crangonid crustaceans Sabinea septemcarinata and Sclerocrangon ferox were found in low quantities (<12 g per haul) compared with 0.9–113 kg in the other regions.
The SEW benthic megafauna region is geographically divided into (i) the Pechora Sea and adjacent bank areas (Murmansk Rise, Kanin Bank, Goose Bank, and banks along southwestern Novaya Zemlya) in the southeastern Barents Sea, (ii) the Svalbard Bank and (iii) the shelf along western and northern Svalbard (yellow symbols in Figure 5). It includes a mosaic of shallow banks and slopes (86–196 m), and temperature ranged from −0.7 to 6.4°C while salinity was 33.4–35.1 (Supplementary Appendix 1). Most of the region is ice-covered in winter with the number of ice-days ranging from 0 in some areas in the south to 349 d north of Svalbard.
The benthic megafaunal biomass was the lowest recorded in this study at the slopes of Kolguyev Island (southeastern Barents Sea) and Storfjord trench and outer Bear Island Channel (western Barents Sea) (local area SEW 7 in Tables 2 and 3 and Supplementary Appendix 1). Abundance and species number were also low at the slopes and banks in the southeastern Barents Sea (local areas 6 and 8 in Tables 2 and 3 and Supplementary Appendix 1). At Spitsberg Bank, which also clustered together with stations from the slopes of Kolguyev Island, Novaya Zemlya, and Kap Kanin Bank in the southeastern Barents Sea included the highest biomass recorded in this study (SEW 10 in Tables 2 and 3 and Supplementary Appendix 1). This biomass consisted of the sea cucumber Cucumaria frondosa, the bivalve Chlamys islandica, and undetermined sponges. The snow crab Chionoecetes opilio, the sea urchin Strongylocentrotus spp., and the crangonid crustacean Sabinea septemcarinata dominated at Goose Bank (SEW 5, Tables 2 and 3 and Supplementary Appendix 1) and at the slopes of Kolguyev Islands (SEW 7, Tables 2 and 3 and Supplementary Appendix 1). Less abundant species, but only recorded in the SEW region, was the ascidians Pelonaia corrugate, Microcosmus glacialis, and Boltenia echinata (Figure 6b).
The NW benthic megafauna region is mainly localized east of Svalbard where it includes the Central Bank, the Great Bank, and the Hopen Deep. It also includes stations in Storfjord trench and in the fjords, sounds, and coastal areas of western and northern Svalbard (green symbols in Figure 5). The depths at the stations ranged from 74 to 432 m, the bottom temperature varied from −1.4 to 3.6°C, and salinity varied from 33.9 to 35.1. This region is mostly ice-covered in winter with the number of ice-days ranging from 100 to 300 d. The echinoderms C. crispatus, Strongylocentrotus spp., and Gorgonocephalus arcticus together with the crangonid crustacean Sabinea septemcarinata made up 50% of the biomass on average.
The NE benthic megafauna region covered the eastern Barents Sea from the shelf in the north to the Goose Bank 72°N in the south, including an isolated deeper area south of Novaja Zemlya, and included also most of the Southeast Basin and the St Anna Trough area in the northern Kara Sea (blue symbols in Figure 5). Also included in this group were four deep stations along the slope north of Svalbard. The stations in this region were relatively deep (range 114–928 m) and were characterized by low temperature (−1–2.4°C) and relatively high salinity for most of the stations (34.9–35.0). The region is ice-covered from 0 d in parts of the southeastern area up to 339 d in the northern areas.
The benthic megafauna biomass (up to 1750 kg per haul) and number of specimens (from 40 up to 0.3 million per haul) were of the highest values recorded in this survey. The benthic megafauna biomass in the north was made up of sponges (Porifera) and brittlestars such as Gorgonocephalus arcticus, Ophiopleura borealis, and Ophiocantha bidentata. This region contained the largest snow crab (Chionoecetes opilio) population and also the largest population of the boreal-Arctic species Ophiocten sericeum. Characteristic for this region were a relatively large population of the pennatulacean sea pen Umbellula encrinus (Figure 6b), the isopod Saduria sabini, and the Nephtheidae (octocorallia) Drifa glomerata. Species that were only found in this region included the two Arcto-atlantic bathyal sea stars Bathybiaster vexillifer and Tylaster willei, the sea cucumbers Molpadia arctica (Arctic), the crustaceans Cleippides quadricuspis (Arctic) and Eusirus holmi (Arctic), and the gastropod Propebela sp. (Figure 6b).
Discussion
Broad-scale patterns in benthic megafaunal distribution in relation to oceanography
In this study we present the geographical distribution pattern of the benthic megafauna in the Barents Sea in 2011. The mapping was derived from an extensive sampling of the seabed by bottom trawling, with the primary aim to provide data for annual fish stock assessments as part of the joint Norwegian–Russian Ecosystem Survey (Michalsen et al., 2013). We intend to use the distribution map of 2011 as a baseline reference to detect possible future changes in the benthic megafaunal composition can be detected. The chosen multivariate statistics based on the faunal similarity among the 377 trawl stations, revealed four clear broad-scale patterns of the Barents Sea regions: Southwest (SW), a divided region geographical consisting of the (i) coast of northwestern Svalbard, (ii) the shallow Spitsbergen Bank, and (iii) the shallow banks and slopes in southeastern Barents Sea (SEW), Northwest (NW), and Northeast (NE). These four zoogeographical regions were significantly explained by the environmental variables temperature, depth, salinity, and ice cover, and were consistent with the main oceanographic features of the Barents Sea. This includes the faunal composition of the SW region (red symbols in Figure 5) which can be related to the warm (2–5°C) Atlantic Water of the Norwegian Atlantic Current, and Coastal waters of the Norwegian Coastal Current. From here the water enters the SEW regions (yellow symbols in Figure 5) by flowing (i) northward along the west coast of Svalbard (e.g. Loeng, 1991) or (ii) east to the southeastern Barents Sea. Here the Atlantic Water continues northward into the NE region (blue symbols in Figure 5) where the Atlantic Water is gradually modified by cooling, ice formation, melting and mixing with Arctic Water. In NW region (green symbols in Figure 5) Arctic water masses are dominant. The main flow patterns and transformations of the Atlantic waters determine therefore not only the water mass characteristics but also the large-scale biogeographic pattern where the Barents Sea changes from a boreal region in southwest to mostly Arctic conditions in the northeastern area (Brotskaya and Zenkevitch, 1939; Wassmann et al., 2006; Anisimova et al., 2011). The “flow-through” nature of the oceanography, the shifting topography, and the biology and ecology of species as well as oceanographic variability and human impacts must be taken into account in further analyses of status and trends in species distributions, faunal composition, and biogeography of the Barents Sea.
The four subregions with local areas: establishing a baseline for long-term monitoring
The SW region is characterized by the inflow of warm Atlantic Water is associated with relatively high primary production, strong water currents that resuspend food material, and the presence of hard substrate that supports the sessile filter-feeders observed in this area (Wassmann et al., 2006; Hunt et al., 2013). The total annual primary production for the Barents Sea has been estimated to range from 20 to 200 g C m−2, with an average of about 90 g C m−2 (Sakshaug and Slagstad, 1992; Wassmann et al., 2006), and with high rates found in the Atlantic and Coastal waters of the southwestern entrance area (Wassmann et al., 2006). The inflowing productive Atlantic and Coastal waters might explain that nearly 90% of the biomass on average belonged to the large-bodied Geodia sponge species at the local area “Tromsø Plateau”. The high dominance of Geodia and other sponges (several species) followed the continental slope north towards Svalbard, and also into the Barents Sea in the outer Bear Island Channel and on the North Cape Bank where the biomass was particularly high. Bottom trawling might have an impact on these slow-growing sponges, most likely requiring many years to re-establish themselves in a degraded area. Video recordings (Beazley et al., 2013), which cause no damage to the seabed or sponges, are recommended to be applied to monitor these areas.
At the Tromso Plateau, not only Geodia species but also the king crab Paralithodes camtschaticus dominated in terms of biomass. Although the king crab is a valuable commercial species, the Norwegian government (http://www.fisheries.no/ecosystems-and-stocks/marine_stocks/shellfish/red_king_crab/ as of 19 March 2013) is managing the crab to keep the stock at a minimum at the Tromso Plateau and further south. The crab as intentionally released in the Kolafjord in the 1960s by Russian scientists to create a new and valuable fishing resource (Orlov and Karpevich, 1965; Orlov and Ivanov, 1978) has had impact on the marine ecosystem (Britayev et al., 2010; Oug et al., 2011) and was among the top dominant species in terms of biomass on the Tromso Plateau in 2011. This should encourage an action plan to reduce the king crab population, and more intensive monitoring of possible effects of the king crab in these off shore areas. The king crab is likely to stay along the coastal areas on coarse and mixed sediment (Jørgensen and Nilssen, 2011). Since then, the crab has spread both east along the Kola Peninsula and westwards into the Norwegian zone. The easternmost distribution range includes the Kanin Bank, while the northward distribution is to the Goose Bank. Decapods such as the king crab are known to be macrophagous predators, feeding on organisms on or near the seabed (Jewett and Feder, 1982).
In the deeper Bear Island Channel where currents are most likely calmer, detrivores such as the boreal-Arctic, eurybathic sea star C. crispatus, and the Arctic sea cucumber Molpadia borealis comprise a large standing stock in this area. These two species form dense local populations in areas with soft muddy sediment that is rich in organic matter (Anisimova et al., 2011). The sea cucumber is a head-down conveyor-belt feeder (Miller et al., 2000; http://www.marinespecies.org/index.php, as of 9 May 2014), while C. crispatus is a non-selective subsurface deposit feeder. This sea star has a wide distribution in the Barents Sea. It is known to prefer a stable physical environment and a rich detrital food source. The abundance of the species is correlated with phytoplankton production rather than temperature (Shick et al., 1981). Both C. crispatus and M. borealis are covered with sediment and might be difficult to observe with a video camera. Although the Geodia beds in the west need non-destructive observational sampling, the deeper sediments of these same beds need be sampled by trawl and/or grab.
The relatively shallow, but geographically divided SEW region, has strong seasonal and shorter term temperature variations (Cottier et al., 2007; Tverberg and Nøst, 2009; Ozhigin et al., 2011). In addition, the northwestern Svalbard and the southeastern Barents Sea are located “downstream” in the main current branches of the warm Atlantic waters entering the southwestern Barents Sea, which provides the Svalbard west/north and the southeastern areas (shown as dotted arrows in Figure 5) with cooled and modified waters. The SEW region stretches almost uninterrupted from the northwestern coast of Svalbard, across the Barents Sea and into the southeastern area as a “benthic Polar Front”. This boundary between the northern (NW and NE) and southern (SW and SE) megafaunal regions (shown as whole line in Figure 5), separates the boreal and the Arctic biogeographical regions. This “benthic Polar Front” differs from the traditional Polar Front (see Loeng et al., 1997) in some respects, since the temperature distribution close to the bottom often differs from the upper waters (Ozhigin et al., 2011). The Spitsbergen Bank is included in the southern megafaunal region, although it is north of the oceanographic Polar Front. This possibly reflects the seasonal warming of the water column over the shallow bank. In contrast, the Hopen Deep, south of the Central Bank, and the area south towards the 72°N in the eastern Barents Sea belongs to the northern region, despite frequent inflow of warm Atlantic water. A cold layer of winter bottom water flowing southwards from the adjacent banks (Quadfasel et al., 1992; Årthun et al., 2011) is a possible explanation for this. We assume that the southern position of the “benthic Polar Front”, compared with the oceanographic Polar Front, reflects a “memory” or a “ecological resilience” of the benthos which has been integrated over some ecological time scale, perhaps driven by cold events (when the bottom is covered with water of near-freezing temperature) rather than by average conditions. This is a subject of further research and is of particular relevance to global warming as we now probably are experiencing climate change on top of the natural climate variability of the past (ACIA, 2004; AMAP, 2012). The “benthic Polar Front” may be expected to shift northward, perhaps first over the Central Bank (local area 12 in the NW region, Figure 5), and possibly also into the eastern Barents Sea (local area 16 in the NE) following less winter ice and decreased formation of cold bottom water (Smedsrud et al., 2010, 2013; Årthun et al., 2011). Temperature shifts in the eastern Bering Sea can alter spatial pattern of epibenthic communities, and even small areas, where the epifauna is distinctly different from surrounding fauna were explained by unique local currents conditions and associated water mass properties (Yeung and McConnaughey, 2006). We suggest that part of the monitoring should focus on the transitional areas along the front, since global warming is expected to show effects in this region by causing changes in species composition, biomass, and production.
The fauna off the western and northern coast of Svalbard was dominated by sponges (>60% biomass) (mostly Geodia sp., Phakellia sp., and Haliclona sp.) which are filtering the productive Atlantic Water flowing northwards. A dominant filtrating fauna was also found at the shallow Spitsbergen Bank in the west, and on the slopes of the Kolguyev Island and Novaya Zembya, Goose Bank, and Kap Kanin Bank in the east. This fauna comprises sponges, the bivalve Chlamys islandica, and the sea cucumber Cucumaria frondosa contributing up to 73% of the total biomass. The Atlantic Water current at the Spitsbergen Bank, which follows the troughs between banks and slopes in the southeastern region (Matishov et al., 2009) seems to provide habitat for filter feeding species.
In some locations of the southeastern Barents Sea, 41% of the biomass at the Goose Bank was made up by the predatory snow crab (Chionoecetes opilio), and with lesser biomass dominance (15%) on the shallow bank areas inside the Pechora Sea and on Kanin Bank. The snow crab is a cold water species, living at water depths from 20 to 700 m, where water temperatures is below 5°C (Elner and Beninger, 1992). It is a non-native species, which most likely has spread into the Barents Sea, and now established a reproducing population (Alvsvåg et al., 2009). This crab is expected to increase and spread over the most of the Barents Sea. It was first detected in 1996 (Kuzmin, 2000) and was recorded close to northern Goose Bank in 2007 and also north and south of the Central Bank. The expanding snow crab population in the Barents Sea is spreading westward from Russian to the Norwegian zone (barentsobserver.com/2013, as of 24 June 2013). The snow crabs have mostly been recorded in waters with temperatures below 2°C, and in 2010 smaller crabs were exclusively found at the Goose Bank, indicating that this is a recruitment area (Agnalt et al., 2011). Warming might push the snow crab further north, and cause an establishment in the waters of Svalbard and Franz Josef Land. The snow crab is a predator on polychaetes, Pandalus borealis, brittlestars, and other accessible epibenhic preys, like gastropods, crabs, and sea urchins (Squires and Dawe, 2003). The snow crab, like the king crab, may have considerable impact on the population of their prey species. It is recommended to analyse possible changes in the composition of benthos by the use of quantitative grab and small trawls in selected areas. This should be done in addition to the groundfish survey adapted to observe possible changes in the distribution and composition of species assemblages, biomass, abundance, and functional traits, based on temporally repeated sampling of a regular station grid. It would also be of value to record the body size of species that are vulnerable to size-selective predation by the introduced snow and king crabs as well as other predators including commercial fish species (Dolgov et al., 2011).
The NW region lies close to, or to the north of the Polar Front in the western part of the Barents Sea. Though Arctic water masses dominate this region, Atlantic Water enters the deeper troughs both from the south (e.g. Loeng, 1991) and from the north (Loeng et al., 1997; Schauer et al., 2002; Gammelsrød et al., 2009; Lind and Ingvaldsen, 2012) but ice formation and brine excretion over banks (as the Great Bank, Central Bank, and Spitsbergen Bank) may lead to formation of dense cold water in winter, which subsequently flows off the banks and into depressions and deeper basins (Midttun, 1985; Schauer and Fahrbach, 1999; Årthun et al., 2011). This cold NW region had the highest number of benthic taxa. The dominant species (∼50% of the total benthic biomass) were the sea star C. crispatus, the sea urchin Strongylocentrotus sp., the brittlestar Gorgonacephalus arcticus, and the crangonid crustacean Sabinea septemcarinata. Sabinea septemcarinata is known to be a macrophagous predator that feeds on organisms on or close to the seabed (Jewett and Feder, 1982). Ctenodiscus crispatus is a detritivore, and might indicate a habitat in weak current regime, allowing small particles to settle on the seabed. Strongylocentrotus spp. is grazer in shallow waters and deposit feeders in the deeper waters.
In the southern part of the NW region (the Hopen Deep, Storfjord Trench and banks south of the Central Bank), the detrivores C. crispatus and M. borealis were among the dominant species, representing an extension of the SW region (the deeper Bear Island Channel) fauna described above. Further north on the shallow and cold Central Bank, on the slopes of the Great Bank and Kong Karls Land, and in areas east of Svalbard, the detrivore Strongylocentrotus spp. dominated in terms of biomass, along with plankton-feeding Gorgonocephalus eucnemis and the sea lily Heliometra glacialis. Associated fauna in these localities were the predatory sea stars Icasterias panopla (Hopen Deep), Urasterias linkii (banks and slopes), and the crustacean Sabinea septemcarinata.
Recent ocean warming has driven some commercial fish species further north. This includes the Atlantic cod (Gadus morhua), which has recently been recorded north to 82°N on the edge of the Barents Sea shelf to the Arctic Ocean (Johansen et al., 2013; Kjesbu et al., 2014). Consequently, the commercial trawling fleet might follow the Atlantic cod to more northern parts of the Barents Sea, e.g. from the Hopen Deep, to formerly ice-covered areas previously too cold for predominantly boreal fish species such as Atlantic cod. Bottom trawling can affect the benthic megafauna, particularly the erect sessile forms that are fragile and are easily damaged or killed by bottom trawl (Kaiser et al., 2002; Hiddink et al., 2006). Deep-water coral reefs and coral gardens are examples of well documented cases of severe damage caused by bottom trawls to biotic habitat structures (Fosså and Skjoldal, 2010). The Hopen Deep is an area extensively exploited by commercial prawn fisheries, and could explain why the large, erect, and fragile basket star Gorgonacephalus eucnemis and the sea lily Heliometra glacialis are rare in this area. Further to the north, on the Great Bank and in the Olga Deep, are areas with a minor commercial fishery, and there, the basket stars G. eucnemis and G. articus are dominating. These two species have overlapping distributions on the shelves and the slopes of the Barents Sea to the East Siberian Sea (Anisimova, 1989; Smirnov, 1994). Basket stars are specialized suspension feeders and favour rocky grounds with strong bottom currents (Piepenburg, 2000). They are easily caught by trawls, and are most likely vulnerable because the arms are easily fragmented by bottom trawls. The capability of the basket stars to survive after being released back into the water is unknown, and might influence whether fluctuations within the basket star population is a good candidate as indicator for trawling activity.
Earlier investigations from the NW area reported Ophiacantha bidentata to dominate the brittlestar fauna in deeper areas and Ophiocten sericeum in shallower waters (Piepenburg and Schmid, 1996a; Piepenburg, 2000). In our study, O. bidentata and O. sericeum were recorded much further north and east on the slopes of Franz-Victoria and St Anna Trough, and on the shallower eastern banks, respectively. It is therefore questioned whether a northeast displacement might have happened due to a possible increase in bottom temperatures. Monitoring the distribution of O. bidentata and O. sericeum might therefore give important information on changing environment integrated over several years, and indicating possible lasting displacement of species.
Along the coasts and inside fjords and sounds of western and northern Svalbard a diverse echinoderm fauna occurred, including the detrivores C. crispatus, Ophiura sarsi, and Strongylocentrotus spp., the predator Icasterias panopla, and the plankton-feeding Gorgonocephalus arcticus. Together these species contributed up to 61% of the biomass and represented one of the largest biomasses recorded within the 19 local areas.
The NE region is separated from the NW area by the elevation of the Skolten Bank in the south, Central Bank, and the Great Bank in the north (the boundary between NE and NW showed as dotted line in Figure 5). These Banks most likely separate the eastern Arctic water, and consequently the Arctic biogeographic region, from the warmer water in the west.
The northern part of the NE region had almost year-round ice coverage (293 d year−1), and had the highest mean biomass and abundances recorded in this region. Sponges (several species) dominated the biomass together with mass occurrences of the endemic-Arctic brittlestar Ophiopleura borealis and the boreal-Arctic species Ophiacantha bidentata. Brittlestars have been found in several studies to be a dominant group of benthos on high Arctic shelves with a biomass of up to 2–3 g C m−2 (equivalent to about 100–150 g wet weight m−2; Mayer and Piepenburg and Schmid, 1996b; Piepenburg, 2000; Ambrose et al., 2001), which is in the lower range of values for biomass of benthos on Arctic and sub-Arctic shelves (e.g. Denisenko et al., 2003; Grebmeier et al., 2006). Ophiacantha bidentata is found either coiled around gorgonians feeding on planktonic prey (e.g. crustaceans) or unattached living on the sediment surface where it feeds on benthic prey (e.g. polychaetes, foraminiferans; Pearson and Gage, 1984), but it can also change to a suspension feeding mode where currents carry a load of suspended particles (Warner, 1982; Piepenburg, 2000). Ophiopleura borealis is a mobile deposit feeder and an opportunistic scavenger (Hobson et al., 1995) and may not be as directly dependent on sedimentary biogenic material as O. bidentata (Gallagher et al., 1998).
The other dominating brittlestar was the basket star Gorgonocephalus arcticus. Emson et al., (1991) reported this euryhaline basket star as a predatory filter-feeder with a preference for the krill species Meganyctiphanes norvegica and adapted for life in strong currents. The high abundance of Gorgonocephalus indicates strong current flow and high abundance of zooplankton in the near-bottom layer and a possible strong bentho-pelagic coupling in the northern Barents Sea. Meganyctiphanes norvegica has been observed feeding on benthic particulate organic matter on the seabed and being consumed by benthic and epibenthic predators by Hirai and Jones (2012). The dominant large calanoid copepod in the Arctic waters of the northern Barents Sea, Calanus glacialis, can have a 2-year life cycle and is therefore commonly present with an accumulated biomass from two generations (Melle and Skjoldal, 1998) and, together with M. norvegica available as prey during the whole year. Plankton feeders among the benthos have also previously been recorded on the slope of the shelf, on-banks, and along other bottom topography that generates strong currents (Sokolova, 1956; Neyman, 1963; Kuznetsov, 1980). Zenkevitch and Brotzky (1939) reported mass occurrences of O. borealis and O. bidentata in the northern Barents Sea, and much of the benthic biomass and carbon demand (up to 80% of the total benthic oxygen uptake) can be attributed to the large standing stock of echinoderms at certain depths (Piepenburg et al., 1995). The echinoderms in the Barents Sea most likely play an important role in the redistribution and remineralization of the organic carbon reaching the seabed (Piepenburg, 2000; Bluhm et al., 2009; Blicher and Sejr, 2011).
The Southeast Basin, and areas next to the Goose Bank in the SEW region, was dominated by snow crab in terms of biomass. On the shallowest areas the snow crab dominated together with Strongylocentrotus sp. and Sabinea septemcarinata, but in the deeper areas the snow crab was found together with the polychaete Spiochaetopterus typicus and the sea cucumber M. borealis. Further north in the NE region, the cold (0.6°C) and deep St Anna and Franz-Victoria Troughs were dominated in biomass by the sea-pen Umbellula encrinus. This species has tentacles that can be spread out to maximize particle capture from the water column. It is uncertain whether they feed on zooplankton or suspended detritus, but they are found in cold water where detritus and plankton are rich (Barnes, 1987). This species, with its fragile body, is expected to be sensitive to bottom trawling, and its dominance verifies, that no trawling activities happens in these remote, pristine and fragile areas (Figs. 14.6.3 in Lyubin et al., 2011)
Concluding remarks
Benthic megafauna is important and relevant in the context of EA as it serves ecologically important trophic functions, by providing habitat, food and shelter for many species.
Several perturbations are acting simultaneously in the Barents Sea: (a) ocean warming is likely to push northward the boundaries between the Arctic and Boreal biogeographic regions in the Barents Sea; (b) commercial bottom trawling might follow a northward expansion of commercial marine resources, and enter areas with low or no previous trawling activity; (c) expanding snow crab and king crab populations will disperse from the east to the west into the open sea (snow crab) or along the coast (king crab).
The most important actions identified are: (i) intensify monitoring by adding more stations across the benthic Polar Front to track climate change effects; (ii) additional video monitoring of the Tromsø Plateau, along the shelves, bank-slopes and in the vast pristine areas of the northern Barents Sea; (iii) population monitoring of king and snow crab and, by the use of quantitative traditional benthic sampling gears, monitoring possible effects on prey species and displacement of native species.
The anticipated changes in the Barents Sea ecosystem, and globally in general, underline the importance of continued long-term monitoring of the benthic megafauna. Such long-term monitoring is both time- and cost-effective as an integral part of the annual fish stock assessment. When changes are detected in community structure and trophic traits by this type of monitoring, additional research including combinations of various sampling techniques, may be required to identify the possible causes of the putative changes. This might then act as a basis for possible adaptive and mitigating management actions, emphasizing the long-term well-being and sustainable use of the entire ecosystem and all its components, including the benthos.
It is encouraged that National agencies, responsible for fisheries and environmental management, should analyse the invertebrate megafauna as an integral part of annual trawl surveys. The results, as exemplified here from the Barents Sea, can reveal ecologically important geographic regions, and possible vulnerable or endangered species in a changing environment.
Supplementary data
Supplementary material is available at the ICESJMS online version of the manuscript.
Acknowledgements
Thanks to the Institute of Marine Research and PINRO this project was realized. The crew and the Russian and Norwegian benthic taxonomists on-board the Russian and Norwegian research vessels is acknowledged for the collection, identification, and quantification of the benthic fauna. We acknowledge the Norwegian Ministry of Foreign Affairs for economic support of exchange of Russian and Norwegian scientists between the research vessels. We thank the “BarEcoRe project”, financed by the Norwegian Scientific Council, for the investment in this project. We are grateful to Edda Johannesen and Henning Riis for reading and commenting on the text. We also thank Paul Renaud for editing the language and for given constructive comments. We thank two anonymous referees for valuable and constructive comments and corrections.
References
Author notes
Handling editor: Michel Kaiser