-
PDF
- Split View
-
Views
-
Cite
Cite
Julien Guihaire, Simon Dang Van, Simon Rouze, Sébastien Rosier, Antoine Roisne, Thierry Langanay, Hervé Corbineau, Jean-Philippe Verhoye, Erwan Flécher, Clinical outcomes in patients after extracorporeal membrane oxygenation support for post-cardiotomy cardiogenic shock: a single-centre experience of 92 cases, Interactive CardioVascular and Thoracic Surgery, Volume 25, Issue 3, September 2017, Pages 363–369, https://doi.org/10.1093/icvts/ivx155
Close - Share Icon Share
Abstract
Post-cardiotomy cardiogenic shock is a major concern in cardiac surgery. We reviewed our experience of extracorporeal membrane oxygenation (ECMO) as temporary circulatory support in post-cardiotomy cardiogenic shock.
Between January 2005 and December 2014, adult patients implanted with ECMO after cardiac surgical procedures were included. Indications for ECMO were failure to be withdrawn from cardiopulmonary bypass or refractory cardiogenic shock occurring during postoperative Days 1 and 2. Patients’ characteristics and outcomes were prospectively collected in a local ECMO database.
Ninety-two patients, median age of 63 years (17–83 years), were supported by ECMO following valvular surgery (66%), acute aortic dissection (10%) and coronary artery bypass grafting (9%). A total of 37% were combined surgical procedures, 24% were redo procedures and 33% were emergent procedures. The median duration of ECMO support was 6 days (1–28 days). The weaning rate from mechanical support was 48%. Overall 1-month and 6-month survival rates were, respectively, 42% and 39%. Survivors were younger (57 vs 63 years old, P = 0.02) and had a higher preoperative left ventricular ejection fraction (52.5 vs 44.1%, P = 0.017). There was a trend for lower serum creatinine levels and total bilirubin rates in the survivors’ group 24 h after initiation of ECMO (respectively, 162 vs 212 µmol/l, P = 0.06; 25.3 vs 54.2 mg/dl, P = 0.08). Valvular surgery and peak lactic acid serum level were associated with poor outcomes. The mean health-related quality of life EuroQoL scale was 68 ± 16/100 at 2 years.
Refractory cardiogenic shock requiring ECMO was most frequently observed after redo valvular surgery in the present study. The overall 6-month survival rate was 39% after ECMO support for post-cardiotomy cardiogenic shock with acceptable health-related quality of life. Improved kidney and liver functions after 24 h of support were associated with favourable outcomes.
INTRODUCTION
The incidence of cardiogenic shock after cardiac surgery ranges from 3% to 5% with fewer than 25% of those patients surviving to hospital discharge [1, 2]. Myocardial stunning as a result of ischaemia-reperfusion injury is the main cause of post-cardiotomy cardiogenic shock (PCCS).
Several technologies have been used to promote cardiac recovery after PCCS including extracorporeal membrane oxygenation (ECMO). ECMO is a life-saving therapy for patients with unstable haemodynamics despite optimal loading and maximal dose of an inotrope medication. Technical innovations and improving management of patients with mechanical circulatory support (MCS) has led to the increased use of ECMO over the last 2 decades. Venoarterial ECMO is currently the treatment of choice for patients with refractory cardiogenic shock and impaired oxygenation thanks to the availability of full cardiopulmonary support [3]. Cardiogenic shock related to acute myocarditis, acute myocardial infarction, cardiac allograft dysfunction or refractory arrhythmias is recognized as an indication for temporary MCS. In the setting of PCCS, ECMO has been associated with less favourable outcomes with 1-month survival rates lower than 40% in most studies [4–7]. Moreover, the prognosis of patients weaned off ECMO support after complicated cardiac surgery remains poor [6–9].
We report a monocentric retrospective series of ECMO implants in patients with refractory PCCS. We sought to investigate patients’ outcomes and quality of life after their ECMO support. We analysed risk factors that may impact myocardial recovery including patients’ characteristics, surgical procedures and postoperative events. The aim of this study was therefore to identify predictors of poor hospital survival rates and to analyse mid-term outcomes in this critically ill population.
METHODS
Study design
Between January 2005 and December 2014, we prospectively collected patients’ characteristics and outcomes in our institutional ECMO registry. The study protocol was approved by the institutional review board, who waived the need for informed consent. Baseline preoperative clinical data were recorded, and operative risk was predicted for each patient according to the European System for Cardiac Operative Risk Evaluation II [10]. A vasoactive-inotropic score was calculated as follows: 100 × (epinephrine dose [µg/kg/min] + norepinephrine dose [µg/kg/min]) + dobutamine dose (µg/kg/min) [11]. Operative characteristics [cardiopulmonary bypass (CPB) and aortic cross-clamping times] and postimplantation outcomes were also analysed. Serum creatinine, bilirubin and lactate levels were collected after 24 h of ECMO support. Our database was regularly updated and completed by dedicated research nurses. Survivors, defined as any patient weaned from ECMO support and successfully discharged, were regularly contacted by the clinical research team to collect functional status and any medical event that occurred since they were discharged alive from the hospital.
Inclusion/exclusion criteria
All consecutive adult patients implanted with venoarterial ECMO for PCCS during the 48 h following cardiac operative procedures were included in this retrospective analysis. Indication for ECMO therapy included either failure to wean off CPB in the setting of maximum inotropic support (epinephrine >0.2 µg/kg/min or dobutamine >15 µg/kg/min with or without norepinephrine >0.2 µg/kg/min) with or without an intra-aortic balloon pump (IABP) or postoperative refractory cardiogenic shock when satisfactory systemic perfusion (systolic artery pressure >80 mmHg, left atrial pressure <20 mmHg, cardiac index higher than 1.8 l/min/m2) could not be achieved despite optimal loading conditions, high-dose inotropic medication and/or IABP. The decision to initiate ECMO was made by the surgeon and the anaesthesiologist or attending staff in the intensive care unit. Transoesophageal echocardiography helped when to determine biventricular function and loading conditions. Patients supported with ECMO for primary graft failure following heart transplant or for postoperative acute respiratory distress syndrome were excluded.
Materials
We previously reported our technique and materials for ECMO support [12, 13]. Briefly, peripheral cannulation was most commonly deployed (88%) through the groin. A modified Seldinger technique was used to safely cannulate the femoral vessels (17–21 Fr for the inflow cannula and 18–32 Fr for the drainage cannula, according to the patient’s body surface area) (Edwards Lifesciences, Inc., Irvine, CA, USA). ECMO support was conducted under normothermia using a magnetic centrifugal pump (Rotaflow®, Maquet Inc., Hirrlingen, Germany) and a Quadrox® oxygenator (Maquet). The entire ECMO circuit was coated with heparin. After an initial bolus of 5000 IU, anticoagulation was not maintained during the first 4 h following the operation with the condition that the pump would provide full blood flow. In absence of haemorrhagic complication in this post-cardiotomy setting, unfractionated heparin was thereafter given in a continuous infusion to maintain an activated clotting time between 150 and 180 s. Pump speed was adjusted to obtain a cardiac index within the 2.2–2.8-ml/min/m2 range, a mean arterial pressure >65 mmHg, a left atrial pressure <20 mmHg and a central venous pressure <10 mmHg. Please refer to the Supplementary Material for details.
Management
Bedside transthoracic or transoesophageal echocardiography was performed daily by experienced practitioners to assess biventricular function, aortic valve opening, left ventricular (LV) distension and right ventricular unloading. The non-pulsatile pump flow was adjusted according to the mean artery pressure value, pulmonary capillary wedge pressure, mixed venous oxygen saturation, end-organ perfusion and serial lactate measures. Norepinephrine was frequently added after optimization of loading conditions to get a mean artery pressure higher than 65 mmHg. In the absence of aortic valve opening, dobutamine was introduced at a dose of 5 µg/kg/min to enhance LV contractility. Pulmonary oedema under ECMO support, manifest as radiographic ‘whiteout’ of the lung field resulting from increased left atrial pressure (>20 mmHg), was managed by diuretics, adjustment of inotropic medications and/or initiation of IABP to reduce LV afterload. In the most severe cases, a vent was placed in the left atrium after iterative sternotomy. In cases of major bleeding requiring interruption of heparin infusion, the pump speed was maintained at a high level to avoid the development of blood clots in the ECMO circuit. Blood transfusions were performed when necessary to achieve a haematocrit of 28%-30%. Limb ischaemia related to peripheral cannulation was managed by surgical revision of the reperfusion catheter associated with arteriography and aponeurotomies when necessary.
Removal
The protocol for weaning from ECMO is detailed in the Supplementary Material. Briefly, criteria for ECMO removal included haemodynamic stability during 24–48 h despite reduction of pump flow from full flow to 1.0 l/min, stability of renal and liver functions and a low level of inotropic medication that could be transiently increased during explantation. In patients without cardiac recovery despite 7 days of support, the patient was bridged to a heart transplant or to long-term MCS. Heart transplant was considered for patients younger than 66 years, without a history of malignant disease over the past 5 years and without irreversible pulmonary hypertension according to preoperative pulmonary haemodynamic analysis. Patients supported by ECMO who were eligible for a heart transplant had a national urgent high priority for any compatible cardiac allograft in France for a 48-h period, renewable once. In the absence of an available organ or in case of temporary contraindication for a heart transplant, patients were considered for implantation of a ventricular assist device.
Health-related quality of life
Patient-reported health-related quality of life was assessed among survivors 2 years after ECMO support using the EQ-5 D survey (Rabin, 2001) [14]. The following dimensions were investigated: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Each dimension was measured for each patient in terms of 3 levels: no problems, some problems and extreme problems. The patient’s self-rated health condition was moreover scored using the EQ visual analogue scale, from 0 (worst imaginable health state) to 100 (best imaginable health state).
Statistical analysis
Patients who died before discharge were defined as the no survivors group including patients weaned from ECMO but who died afterwards and patients who died while under ECMO support. The survivors group included patients successfully weaned from ECMO support and discharged home. All statistical analyses were performed with the GRAPHPAD PRISM software (GraphPad, Inc., La Jolla, USA). Continuous variables were reported as mean standard deviation and compared between groups (survivors and no survivors) using the unpaired 2-tailed t-test. Categorical variables were expressed as proportion (%) and were compared using the chi-square test. A P-value lower than 5% was considered significant. The overall survival was studied using the Kaplan–Meier approach; all graphs are shown with 95% confidence intervals (CIs). Comparative survival rates were analysed using the log-rank test considering timing of ECMO initiation (failure of CPB weaning, <24 h or <48 h following CPB), combined versus isolated surgery, type of surgery [valvular, coronary bypass grafting (CABG), aortic dissection, others], de novo versus iterative surgery. To identify independent predictors for the in-hospital mortality rate, Cox multivariable proportional hazards regression method was used. All variables with P <0.20 by univariable analysis were included in the multivariable model.
RESULTS
Patients’ characteristics
A total of 13 131 patients underwent a cardiac surgical procedure in our institution between January 2005 and December 2014. Among them, 92 required ECMO support for PCCS, 59% were men and the median age was 64.5 years (18–83). Valvular surgery was the main aetiology of PCCS requiring ECMO in the present study (69%) followed by coronary artery bypass grafting (CABG, 13%), acute aortic dissection (13%), LV assist device implantation (2%) and others (2%). Combined surgical procedures associating either valvular surgery and CABG or multiple valvular procedures were performed in 37%. One-third of the patients were operated on emergently and 24% of cases were redo procedures. Compared to non-survivors (n = 58), survivors (n = 34) were younger (56.8 ± 15.5 vs 63.5 ± 11.4 years, P = 0.019) and more frequently men (76.5% vs 45.6%, P = 0.008). Survivors shared a higher preoperative LV ejection fraction (52.5 ± 13.9% vs 44.1 ± 18.5%, P = 0.017). The mean European System for Cardiac Operative Risk Evaluation II was 6.2 ± 1.8% in survivors versus 9.4 ± 1.5% in patients who died (P = 0.191). There were no differences between groups in preoperative New York Heart Association functional class, systolic pulmonary artery pressure and creatinine serum dosage. The aortic cross-clamping time was 107 ± 12 min in survivors versus 111 ± 9 min in patients who died (P = 0.955). Similarly, the mean CPB time was not found significantly different between the groups (P = 0.955). The mean vasoactive inotropic score after aortic clamp release was higher among non-survivors compared to survivors but did not reach statistical significance (respectively, 71.3 ± 8.5 vs 48.8 ± 9.3, P = 0.091). Patients’ characteristics and operative features of the 2 groups are summarized in Table 1.
Demographic and pre-implant variables of patients supported by extracorporeal membrane oxygenation for post-cardiotomy cardiogenic shock
| . | Survivors (n = 34) . | Non-survivors (n = 58) . | P-value . |
|---|---|---|---|
| Age (years) | 56.8 ± 15.5 | 63.5 ± 11.4 | 0.019 |
| BMI (kg/m2) | 24.7 ± 3.6 | 26.4 ± 5.4 | 0.519 |
| Male gender (M) | 26 (76.5%) | 27 (45.6%) | 0.008 |
| NYHA class | 0.959 | ||
| I | 1 (2.9%) | 1 (1.7%) | |
| II | 13 (38.2%) | 28 (48.3%) | |
| III | 10 (29.4%) | 15 (25.9%) | |
| IV | 10 (29.4%) | 14 (24.1%) | |
| LVEF (%) | 52.5 ± 13.9 | 44.1 ± 18.5 | 0.017 |
| sPAP (mmHg) | 42.1 ± 12.3 | 44.7 ± 15.2 | 0.451 |
| Creatinine (µmol/l) | 103.2 ± 36.3 | 102.5 ± 58.5 | 0.959 |
| Reoperation | 5 (14.7%) | 18 (31%) | 0.134 |
| Emergency | 14 (41.2%) | 19 (32.7%) | 0.501 |
| Surgical procedure | 0.372 | ||
| Valve | 21 (61.7%) | 43 (74.1%) | |
| CABG | 7 (20.6%) | 5 (8.6%) | |
| Acute aortic dissection | 5 (14.7%) | 7 (12.1%) | |
| LVAD | 0 (0%) | 2 (3.4%) | |
| Miscellaneous | 1 (3%) | 1 (1.7%) | |
| Combined surgerya | 11 (32.3%) | 20 (34.5%) | 0.978 |
| EuroSCORE II (%) | 6.2 ± 1.8 | 9.4 ± 1.5 | 0.191 |
| Aortic cross-clamp time (min) | 107 ± 12 | 111 ± 9 | 0.840 |
| CPB time (min) | 172 ± 24 | 174 ± 15 | 0.955 |
| Vasoactive inotropic score | 48.8 ± 9.3 | 71.3 ± 8.5 | 0.091 |
| IABP | 11 (32.3%) | 14 (24.1%) | 0.469 |
| . | Survivors (n = 34) . | Non-survivors (n = 58) . | P-value . |
|---|---|---|---|
| Age (years) | 56.8 ± 15.5 | 63.5 ± 11.4 | 0.019 |
| BMI (kg/m2) | 24.7 ± 3.6 | 26.4 ± 5.4 | 0.519 |
| Male gender (M) | 26 (76.5%) | 27 (45.6%) | 0.008 |
| NYHA class | 0.959 | ||
| I | 1 (2.9%) | 1 (1.7%) | |
| II | 13 (38.2%) | 28 (48.3%) | |
| III | 10 (29.4%) | 15 (25.9%) | |
| IV | 10 (29.4%) | 14 (24.1%) | |
| LVEF (%) | 52.5 ± 13.9 | 44.1 ± 18.5 | 0.017 |
| sPAP (mmHg) | 42.1 ± 12.3 | 44.7 ± 15.2 | 0.451 |
| Creatinine (µmol/l) | 103.2 ± 36.3 | 102.5 ± 58.5 | 0.959 |
| Reoperation | 5 (14.7%) | 18 (31%) | 0.134 |
| Emergency | 14 (41.2%) | 19 (32.7%) | 0.501 |
| Surgical procedure | 0.372 | ||
| Valve | 21 (61.7%) | 43 (74.1%) | |
| CABG | 7 (20.6%) | 5 (8.6%) | |
| Acute aortic dissection | 5 (14.7%) | 7 (12.1%) | |
| LVAD | 0 (0%) | 2 (3.4%) | |
| Miscellaneous | 1 (3%) | 1 (1.7%) | |
| Combined surgerya | 11 (32.3%) | 20 (34.5%) | 0.978 |
| EuroSCORE II (%) | 6.2 ± 1.8 | 9.4 ± 1.5 | 0.191 |
| Aortic cross-clamp time (min) | 107 ± 12 | 111 ± 9 | 0.840 |
| CPB time (min) | 172 ± 24 | 174 ± 15 | 0.955 |
| Vasoactive inotropic score | 48.8 ± 9.3 | 71.3 ± 8.5 | 0.091 |
| IABP | 11 (32.3%) | 14 (24.1%) | 0.469 |
Multiple valve repair or valvular surgery combined with coronary artery bypass grafting.
BMI: body mass index; NYHA: New York Heart Association; LVEF: left ventricular ejection fraction; sPAP: systolic pulmonary artery pressure; CABG: coronary artery bypass grafting; LVAD: left ventricular assist device; CPB: cardiopulmonary bypass; EuroSCORE: European System for Cardiac Operative Risk Evaluation; IABP: intra-aortic balloon pump.
Demographic and pre-implant variables of patients supported by extracorporeal membrane oxygenation for post-cardiotomy cardiogenic shock
| . | Survivors (n = 34) . | Non-survivors (n = 58) . | P-value . |
|---|---|---|---|
| Age (years) | 56.8 ± 15.5 | 63.5 ± 11.4 | 0.019 |
| BMI (kg/m2) | 24.7 ± 3.6 | 26.4 ± 5.4 | 0.519 |
| Male gender (M) | 26 (76.5%) | 27 (45.6%) | 0.008 |
| NYHA class | 0.959 | ||
| I | 1 (2.9%) | 1 (1.7%) | |
| II | 13 (38.2%) | 28 (48.3%) | |
| III | 10 (29.4%) | 15 (25.9%) | |
| IV | 10 (29.4%) | 14 (24.1%) | |
| LVEF (%) | 52.5 ± 13.9 | 44.1 ± 18.5 | 0.017 |
| sPAP (mmHg) | 42.1 ± 12.3 | 44.7 ± 15.2 | 0.451 |
| Creatinine (µmol/l) | 103.2 ± 36.3 | 102.5 ± 58.5 | 0.959 |
| Reoperation | 5 (14.7%) | 18 (31%) | 0.134 |
| Emergency | 14 (41.2%) | 19 (32.7%) | 0.501 |
| Surgical procedure | 0.372 | ||
| Valve | 21 (61.7%) | 43 (74.1%) | |
| CABG | 7 (20.6%) | 5 (8.6%) | |
| Acute aortic dissection | 5 (14.7%) | 7 (12.1%) | |
| LVAD | 0 (0%) | 2 (3.4%) | |
| Miscellaneous | 1 (3%) | 1 (1.7%) | |
| Combined surgerya | 11 (32.3%) | 20 (34.5%) | 0.978 |
| EuroSCORE II (%) | 6.2 ± 1.8 | 9.4 ± 1.5 | 0.191 |
| Aortic cross-clamp time (min) | 107 ± 12 | 111 ± 9 | 0.840 |
| CPB time (min) | 172 ± 24 | 174 ± 15 | 0.955 |
| Vasoactive inotropic score | 48.8 ± 9.3 | 71.3 ± 8.5 | 0.091 |
| IABP | 11 (32.3%) | 14 (24.1%) | 0.469 |
| . | Survivors (n = 34) . | Non-survivors (n = 58) . | P-value . |
|---|---|---|---|
| Age (years) | 56.8 ± 15.5 | 63.5 ± 11.4 | 0.019 |
| BMI (kg/m2) | 24.7 ± 3.6 | 26.4 ± 5.4 | 0.519 |
| Male gender (M) | 26 (76.5%) | 27 (45.6%) | 0.008 |
| NYHA class | 0.959 | ||
| I | 1 (2.9%) | 1 (1.7%) | |
| II | 13 (38.2%) | 28 (48.3%) | |
| III | 10 (29.4%) | 15 (25.9%) | |
| IV | 10 (29.4%) | 14 (24.1%) | |
| LVEF (%) | 52.5 ± 13.9 | 44.1 ± 18.5 | 0.017 |
| sPAP (mmHg) | 42.1 ± 12.3 | 44.7 ± 15.2 | 0.451 |
| Creatinine (µmol/l) | 103.2 ± 36.3 | 102.5 ± 58.5 | 0.959 |
| Reoperation | 5 (14.7%) | 18 (31%) | 0.134 |
| Emergency | 14 (41.2%) | 19 (32.7%) | 0.501 |
| Surgical procedure | 0.372 | ||
| Valve | 21 (61.7%) | 43 (74.1%) | |
| CABG | 7 (20.6%) | 5 (8.6%) | |
| Acute aortic dissection | 5 (14.7%) | 7 (12.1%) | |
| LVAD | 0 (0%) | 2 (3.4%) | |
| Miscellaneous | 1 (3%) | 1 (1.7%) | |
| Combined surgerya | 11 (32.3%) | 20 (34.5%) | 0.978 |
| EuroSCORE II (%) | 6.2 ± 1.8 | 9.4 ± 1.5 | 0.191 |
| Aortic cross-clamp time (min) | 107 ± 12 | 111 ± 9 | 0.840 |
| CPB time (min) | 172 ± 24 | 174 ± 15 | 0.955 |
| Vasoactive inotropic score | 48.8 ± 9.3 | 71.3 ± 8.5 | 0.091 |
| IABP | 11 (32.3%) | 14 (24.1%) | 0.469 |
Multiple valve repair or valvular surgery combined with coronary artery bypass grafting.
BMI: body mass index; NYHA: New York Heart Association; LVEF: left ventricular ejection fraction; sPAP: systolic pulmonary artery pressure; CABG: coronary artery bypass grafting; LVAD: left ventricular assist device; CPB: cardiopulmonary bypass; EuroSCORE: European System for Cardiac Operative Risk Evaluation; IABP: intra-aortic balloon pump.
Extracorporeal membrane oxygenation support parameters
The main characteristics of ECMO support are listed in Table 2. The primary cause of PCCS was LV failure in both groups followed by right ventricular dysfunction. ECMO support was initiated most commonly in the operating room (86.9%) and less frequently in the intensive care unit (13.1%). Timing for implantation after surgery was as follows: immediately (CPB weaning was impossible) for 47% of patients in the survivors group versus 46.5% in the non-survivor group, during the first 24 h after the operation for 26.5% vs 22.4%, respectively, and between 24 and 48 h after the operation for 26.5% vs 31.0%, respectively (P = 0.860). Peripheral cannulation was the most frequent approach for ECMO support in both groups (85.3% in survivors vs 84.5% in non-survivors, P = 0.524) and the mean ECMO blood flow was similar 6 h after MCS initiation (respectively, 3.9 ± 0.2 l/min vs 3.7 ± 0.2 l/min, P = 0.401). IABP was initiated before ECMO in 25 patients (11 in survivors and 14 in non-survivors, P = 0.469). Seven patients in the survivors group (20.5%) and 6 patients in the non-survivors group (10.3%) had an additional LV vent during ECMO support (P = 0.359). The serum lactic acid level after 24 h of ECMO support was lower in survivors compared to non-survivors (2.3 ± 0.4 vs 4.1 ± 0.6 mmol/l, P = 0.029). Similarly, the serum creatinine level and the total bilirubin rate were higher in non-survivors after 24 h, but without statistical significance (respectively, 163 ± 13 vs 207 ± 19 µmol/l, P = 0.087; 25.3 ± 5.4 vs 59.8 ± 9.3 mg/l, P = 0.064). There was a trend for a longer duration of ECMO support in survivors compared to non-survivors (7.9 ± 0.9 days vs 5.9 ± 0.6 days, P = 0.078).
Characteristics of venoarterial extracorporeal membrane oxygenation support
| . | Survivors (n = 34) . | Non-survivors (n = 58) . | P-value . |
|---|---|---|---|
| Cause of PCCS | 0.897 | ||
| LV failure | 23 (67.6%) | 39 (67.2%) | |
| RV failure | 6 (17.6%) | 12 (20.7%) | |
| Unknown | 5 (14.7%) | 7 (12.1%) | |
| Delay for ECMO initiation | 0.860 | ||
| Failure to wean from CPB | 16 (47%) | 27 (46.5%) | |
| <24 h | 9 (26.5%) | 13 (22.4%) | |
| <48 h | 9 (26.5%) | 18 (31.0%) | |
| Route of cannulation | 0.524 | ||
| Peripheral | 29 (85.3%) | 49 (84.5%) | |
| Central | 5 (14.7%) | 9 (15.5%) | |
| Mean ECMO blood flow (l/min/m2)a | 1.7 ± 0.1 | 1.6 ± 0.1 | 0.876 |
| LV vent | 7 (20.6%) | 6 (10.3%) | 0.219 |
| Lactic acid at 24 h (mmol/l) | 2.3 ± 0.4 | 4.1 ± 0.6 | 0.029 |
| Troponin at 24 h (ng/ml) | 1791 ± 600 | 2135 ± 719 | 0.741 |
| Creatinine at 24 h (µmol/l) | 163 ± 13 | 207 ± 19 | 0.087 |
| Bilirubin at 24 h (mg/l) | 25.3 ± 5.4 | 59.8 ± 9.3 | 0.064 |
| Duration of support (days) | 7.9 ± 0.9 | 5.9 ± 0.6 | 0.078 |
| . | Survivors (n = 34) . | Non-survivors (n = 58) . | P-value . |
|---|---|---|---|
| Cause of PCCS | 0.897 | ||
| LV failure | 23 (67.6%) | 39 (67.2%) | |
| RV failure | 6 (17.6%) | 12 (20.7%) | |
| Unknown | 5 (14.7%) | 7 (12.1%) | |
| Delay for ECMO initiation | 0.860 | ||
| Failure to wean from CPB | 16 (47%) | 27 (46.5%) | |
| <24 h | 9 (26.5%) | 13 (22.4%) | |
| <48 h | 9 (26.5%) | 18 (31.0%) | |
| Route of cannulation | 0.524 | ||
| Peripheral | 29 (85.3%) | 49 (84.5%) | |
| Central | 5 (14.7%) | 9 (15.5%) | |
| Mean ECMO blood flow (l/min/m2)a | 1.7 ± 0.1 | 1.6 ± 0.1 | 0.876 |
| LV vent | 7 (20.6%) | 6 (10.3%) | 0.219 |
| Lactic acid at 24 h (mmol/l) | 2.3 ± 0.4 | 4.1 ± 0.6 | 0.029 |
| Troponin at 24 h (ng/ml) | 1791 ± 600 | 2135 ± 719 | 0.741 |
| Creatinine at 24 h (µmol/l) | 163 ± 13 | 207 ± 19 | 0.087 |
| Bilirubin at 24 h (mg/l) | 25.3 ± 5.4 | 59.8 ± 9.3 | 0.064 |
| Duration of support (days) | 7.9 ± 0.9 | 5.9 ± 0.6 | 0.078 |
6 h after ECMO initiation.
PCCS: post-cardiotomy cardiogenic shock; LV: left ventricle; RV: right ventricle; ECMO: extracorporeal membrane oxygenation; CPB: cardiopulmonary bypass.
Characteristics of venoarterial extracorporeal membrane oxygenation support
| . | Survivors (n = 34) . | Non-survivors (n = 58) . | P-value . |
|---|---|---|---|
| Cause of PCCS | 0.897 | ||
| LV failure | 23 (67.6%) | 39 (67.2%) | |
| RV failure | 6 (17.6%) | 12 (20.7%) | |
| Unknown | 5 (14.7%) | 7 (12.1%) | |
| Delay for ECMO initiation | 0.860 | ||
| Failure to wean from CPB | 16 (47%) | 27 (46.5%) | |
| <24 h | 9 (26.5%) | 13 (22.4%) | |
| <48 h | 9 (26.5%) | 18 (31.0%) | |
| Route of cannulation | 0.524 | ||
| Peripheral | 29 (85.3%) | 49 (84.5%) | |
| Central | 5 (14.7%) | 9 (15.5%) | |
| Mean ECMO blood flow (l/min/m2)a | 1.7 ± 0.1 | 1.6 ± 0.1 | 0.876 |
| LV vent | 7 (20.6%) | 6 (10.3%) | 0.219 |
| Lactic acid at 24 h (mmol/l) | 2.3 ± 0.4 | 4.1 ± 0.6 | 0.029 |
| Troponin at 24 h (ng/ml) | 1791 ± 600 | 2135 ± 719 | 0.741 |
| Creatinine at 24 h (µmol/l) | 163 ± 13 | 207 ± 19 | 0.087 |
| Bilirubin at 24 h (mg/l) | 25.3 ± 5.4 | 59.8 ± 9.3 | 0.064 |
| Duration of support (days) | 7.9 ± 0.9 | 5.9 ± 0.6 | 0.078 |
| . | Survivors (n = 34) . | Non-survivors (n = 58) . | P-value . |
|---|---|---|---|
| Cause of PCCS | 0.897 | ||
| LV failure | 23 (67.6%) | 39 (67.2%) | |
| RV failure | 6 (17.6%) | 12 (20.7%) | |
| Unknown | 5 (14.7%) | 7 (12.1%) | |
| Delay for ECMO initiation | 0.860 | ||
| Failure to wean from CPB | 16 (47%) | 27 (46.5%) | |
| <24 h | 9 (26.5%) | 13 (22.4%) | |
| <48 h | 9 (26.5%) | 18 (31.0%) | |
| Route of cannulation | 0.524 | ||
| Peripheral | 29 (85.3%) | 49 (84.5%) | |
| Central | 5 (14.7%) | 9 (15.5%) | |
| Mean ECMO blood flow (l/min/m2)a | 1.7 ± 0.1 | 1.6 ± 0.1 | 0.876 |
| LV vent | 7 (20.6%) | 6 (10.3%) | 0.219 |
| Lactic acid at 24 h (mmol/l) | 2.3 ± 0.4 | 4.1 ± 0.6 | 0.029 |
| Troponin at 24 h (ng/ml) | 1791 ± 600 | 2135 ± 719 | 0.741 |
| Creatinine at 24 h (µmol/l) | 163 ± 13 | 207 ± 19 | 0.087 |
| Bilirubin at 24 h (mg/l) | 25.3 ± 5.4 | 59.8 ± 9.3 | 0.064 |
| Duration of support (days) | 7.9 ± 0.9 | 5.9 ± 0.6 | 0.078 |
6 h after ECMO initiation.
PCCS: post-cardiotomy cardiogenic shock; LV: left ventricle; RV: right ventricle; ECMO: extracorporeal membrane oxygenation; CPB: cardiopulmonary bypass.
Postoperative outcomes
Pneumonia was the most common complication in patients under ECMO support, affecting 35% of the survivors and 62% of the non-survivors (P = 0.011). Pulmonary oedema as a result of left atrial pressure overload was more frequently observed in patients who died (55%) compared to survivors (29%, P = 0.018). Other complications occurring during MCS are listed in Table 3. The mean stay in the intensive care unit was 24.8 ± 14.3 days in survivors vs 20.0 ± 9.9 days in non-survivors (P = 0.297). Two patients were placed on the high-emergency heart transplant list and were successfully bridged to heart transplant. The first one received the transplant under ECMO after 21 days of support for PCCS following emergent CABG surgery. The second patient was initially supported for 8 days following iterative polyvalvular surgery and received a transplant 6 weeks later for refractory biventricular failure. In the present series, a third patient was bridged to heart transplant under ECMO support 6 days after pericardectomy. He died on the 12th post-transplant day of multiorgan failure. Two other patients survived after successful bridge to a LV assist device after 21 and 16 days of ECMO support, respectively. New York Heart Association functional Class at 1 year after hospital discharge was Grade 1 in 45% and Grade 2 in 55% of patients.
Comparison of outcomes with venoarterial extracorporeal membrane oxygenation between survivors and non-survivors
| . | Survivors (n = 34) . | Non-survivors (n = 58) . | P-value . |
|---|---|---|---|
| Complications under ECMO support | |||
| Pulmonary oedema | 10 (29%) | 32 (55%) | 0.018 |
| Pneumonia | 12 (35%) | 36 (62%) | 0.011 |
| Bleedinga | 6 (18%) | 12 (21%) | 0.791 |
| Limb ischaemia | 4 (12%) | 5 (9%) | 0.721 |
| Stroke | 1 (3%) | 2 (3%) | 1.000 |
| Retroperitoneal haematoma | 0 (0%) | 2 (3.4%) | 0.529 |
| Red cell concentrates per patient | 12.5 ± 1.5 | 11.3 ± 1.2 | 0.514 |
| ICU stay (days) | 24.8 ± 14.3 | 20.0 ± 9.9 | 0.297 |
| Bridge to heart transplant | 1 (2.9%) | 1 (1.7%) | |
| Bridge to LVAD | 2 (5.9%) | 0 | |
| Cause of death | |||
| Multiorgan failure | 41 (70.7%) | ||
| Septic shock | 12 (20.7%) | ||
| Haemorrhagic shock | 4 (6.9%) | ||
| Prosthesis thrombosis | 1 (1.7%) | ||
| . | Survivors (n = 34) . | Non-survivors (n = 58) . | P-value . |
|---|---|---|---|
| Complications under ECMO support | |||
| Pulmonary oedema | 10 (29%) | 32 (55%) | 0.018 |
| Pneumonia | 12 (35%) | 36 (62%) | 0.011 |
| Bleedinga | 6 (18%) | 12 (21%) | 0.791 |
| Limb ischaemia | 4 (12%) | 5 (9%) | 0.721 |
| Stroke | 1 (3%) | 2 (3%) | 1.000 |
| Retroperitoneal haematoma | 0 (0%) | 2 (3.4%) | 0.529 |
| Red cell concentrates per patient | 12.5 ± 1.5 | 11.3 ± 1.2 | 0.514 |
| ICU stay (days) | 24.8 ± 14.3 | 20.0 ± 9.9 | 0.297 |
| Bridge to heart transplant | 1 (2.9%) | 1 (1.7%) | |
| Bridge to LVAD | 2 (5.9%) | 0 | |
| Cause of death | |||
| Multiorgan failure | 41 (70.7%) | ||
| Septic shock | 12 (20.7%) | ||
| Haemorrhagic shock | 4 (6.9%) | ||
| Prosthesis thrombosis | 1 (1.7%) | ||
Defined as any bleeding event requiring surgical revision of either the mediastinum or the groin (cannulation route).
ECMO: extracorporeal membrane oxygenation; ICU: intensive care unit; LVAD: left ventricular assist device.
Comparison of outcomes with venoarterial extracorporeal membrane oxygenation between survivors and non-survivors
| . | Survivors (n = 34) . | Non-survivors (n = 58) . | P-value . |
|---|---|---|---|
| Complications under ECMO support | |||
| Pulmonary oedema | 10 (29%) | 32 (55%) | 0.018 |
| Pneumonia | 12 (35%) | 36 (62%) | 0.011 |
| Bleedinga | 6 (18%) | 12 (21%) | 0.791 |
| Limb ischaemia | 4 (12%) | 5 (9%) | 0.721 |
| Stroke | 1 (3%) | 2 (3%) | 1.000 |
| Retroperitoneal haematoma | 0 (0%) | 2 (3.4%) | 0.529 |
| Red cell concentrates per patient | 12.5 ± 1.5 | 11.3 ± 1.2 | 0.514 |
| ICU stay (days) | 24.8 ± 14.3 | 20.0 ± 9.9 | 0.297 |
| Bridge to heart transplant | 1 (2.9%) | 1 (1.7%) | |
| Bridge to LVAD | 2 (5.9%) | 0 | |
| Cause of death | |||
| Multiorgan failure | 41 (70.7%) | ||
| Septic shock | 12 (20.7%) | ||
| Haemorrhagic shock | 4 (6.9%) | ||
| Prosthesis thrombosis | 1 (1.7%) | ||
| . | Survivors (n = 34) . | Non-survivors (n = 58) . | P-value . |
|---|---|---|---|
| Complications under ECMO support | |||
| Pulmonary oedema | 10 (29%) | 32 (55%) | 0.018 |
| Pneumonia | 12 (35%) | 36 (62%) | 0.011 |
| Bleedinga | 6 (18%) | 12 (21%) | 0.791 |
| Limb ischaemia | 4 (12%) | 5 (9%) | 0.721 |
| Stroke | 1 (3%) | 2 (3%) | 1.000 |
| Retroperitoneal haematoma | 0 (0%) | 2 (3.4%) | 0.529 |
| Red cell concentrates per patient | 12.5 ± 1.5 | 11.3 ± 1.2 | 0.514 |
| ICU stay (days) | 24.8 ± 14.3 | 20.0 ± 9.9 | 0.297 |
| Bridge to heart transplant | 1 (2.9%) | 1 (1.7%) | |
| Bridge to LVAD | 2 (5.9%) | 0 | |
| Cause of death | |||
| Multiorgan failure | 41 (70.7%) | ||
| Septic shock | 12 (20.7%) | ||
| Haemorrhagic shock | 4 (6.9%) | ||
| Prosthesis thrombosis | 1 (1.7%) | ||
Defined as any bleeding event requiring surgical revision of either the mediastinum or the groin (cannulation route).
ECMO: extracorporeal membrane oxygenation; ICU: intensive care unit; LVAD: left ventricular assist device.
Survival analysis and health-related quality of life
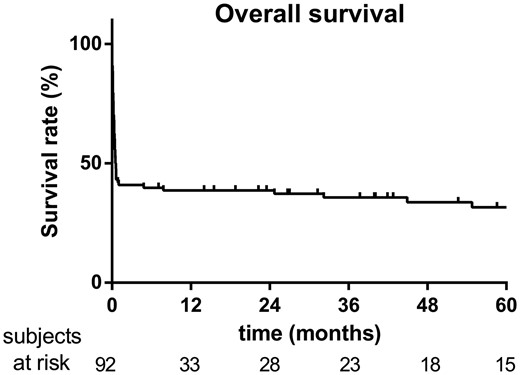
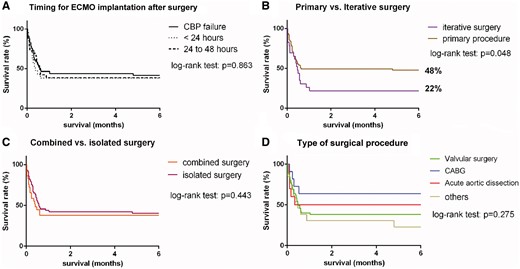
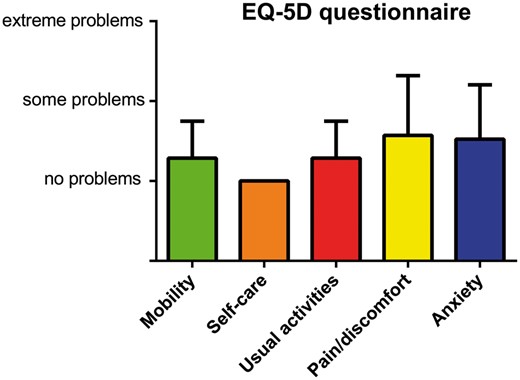
One- and 6-month, 2- and 5-year overall survival rates were 42%, 39%, 37% and 32% respectively (Fig. 1). The main cause of early death in the present series was multiorgan failure (70.7%) followed by septic shock (20.7%) and haemorrhagic shock (6.9%). The average follow-up period was 20.9 ± 32.8 months. No patients died during the 6 postoperative months after being successfully withdrawn from ECMO. Seven late deaths occurred among the 1-year survivors with a mean delay of 49 ± 33 months after hospital discharge. Among these patients, only 1 died of a major cardiac event. Comparative survival curves are shown in Fig. 2. There was no significant difference in survival according to the timing of EMCO implantation (CPB failure, <24 h or between 24 and 48 h). Similarly combined surgical procedures were not associated with a higher mortality rate compared to isolated procedures. There was a trend for a higher mortality rate after valvular surgery followed by acute aortic dissection and CABG, but without reaching statistical significance (P = 0.275). Redo surgical procedures complicated by PCCS requiring ECMO support were associated with a lower survival rate at 6 months compared to de novo surgeries (48% vs 22%, P = 0.048). Univariable analysis identified patient’s age (P = 0.045), body mass index (P = 0.038), preoperative LV ejection fraction (P = 0.014), valvular surgery (P = 0.029), duration of ECMO support (P = 0.002), peak creatinine serum level (P = 0.024) and peak lactic acid serum level after 24 h of ECMO (P = 0.001) as risk factors for in-hospital death in patients with refractory PCCS requiring MCS. The patient’s age (95% CI 1.017–1.0.87, P = 0.003), valvular surgery (95% CI 1.2–5.491, P = 0.015) and peak lactic acid serum level (95% CI 1.145–9.561, P = 0.027) were predictors of death by multivariable analysis (Table 4). The mean self-rated health score was 68 ± 16 (min = 0, max = 100) among survivors at 2 years after ECMO support. The quality of life for these patients is illustrated in Fig. 3 showing no limitation for self-care in all cases and minor problems considering other dimensions (mobility, usual activities, pain and anxiety).
Univariable and multivariable analyses for in-hospital mortality rates
| Variables . | Univariable . | Multivariable . | RR . | CI . |
|---|---|---|---|---|
| Age | 0.045 | 0.003 | 3.127 | 1.123–8.784 |
| BMI | 0.038 | 0.716 | ||
| Redo surgery | 0.150 | 0.073 | ||
| Preoperative LVEF | 0.014 | 0.342 | ||
| Valvular surgery | 0.195 | 0.015 | 2.567 | 1.201–5.491 |
| Peak lactate H24 | 0.027 | 0.003 | 3.308 | 1.145–9.561 |
| Peak creatinine H24 | 0.024 | 0.149 | ||
| Duration of ECMO support | 0.003 | 0.157 |
| Variables . | Univariable . | Multivariable . | RR . | CI . |
|---|---|---|---|---|
| Age | 0.045 | 0.003 | 3.127 | 1.123–8.784 |
| BMI | 0.038 | 0.716 | ||
| Redo surgery | 0.150 | 0.073 | ||
| Preoperative LVEF | 0.014 | 0.342 | ||
| Valvular surgery | 0.195 | 0.015 | 2.567 | 1.201–5.491 |
| Peak lactate H24 | 0.027 | 0.003 | 3.308 | 1.145–9.561 |
| Peak creatinine H24 | 0.024 | 0.149 | ||
| Duration of ECMO support | 0.003 | 0.157 |
All factors with P <0.20 by univariable analysis were included in the multivariable model.
BMI: body mass index; LVEF: left ventricular ejection fraction; ECMO: extracorporeal membrane oxygenation; H24: 24 h after ECMO implantation; RR: relative risk; CI: confidence index.
Univariable and multivariable analyses for in-hospital mortality rates
| Variables . | Univariable . | Multivariable . | RR . | CI . |
|---|---|---|---|---|
| Age | 0.045 | 0.003 | 3.127 | 1.123–8.784 |
| BMI | 0.038 | 0.716 | ||
| Redo surgery | 0.150 | 0.073 | ||
| Preoperative LVEF | 0.014 | 0.342 | ||
| Valvular surgery | 0.195 | 0.015 | 2.567 | 1.201–5.491 |
| Peak lactate H24 | 0.027 | 0.003 | 3.308 | 1.145–9.561 |
| Peak creatinine H24 | 0.024 | 0.149 | ||
| Duration of ECMO support | 0.003 | 0.157 |
| Variables . | Univariable . | Multivariable . | RR . | CI . |
|---|---|---|---|---|
| Age | 0.045 | 0.003 | 3.127 | 1.123–8.784 |
| BMI | 0.038 | 0.716 | ||
| Redo surgery | 0.150 | 0.073 | ||
| Preoperative LVEF | 0.014 | 0.342 | ||
| Valvular surgery | 0.195 | 0.015 | 2.567 | 1.201–5.491 |
| Peak lactate H24 | 0.027 | 0.003 | 3.308 | 1.145–9.561 |
| Peak creatinine H24 | 0.024 | 0.149 | ||
| Duration of ECMO support | 0.003 | 0.157 |
All factors with P <0.20 by univariable analysis were included in the multivariable model.
BMI: body mass index; LVEF: left ventricular ejection fraction; ECMO: extracorporeal membrane oxygenation; H24: 24 h after ECMO implantation; RR: relative risk; CI: confidence index.
Kaplan–Meier analysis of overall survival in patients supported by venoarterial extracorporeal membrane oxygenation for refractory post-cardiotomy cardiogenic shock (n = 92, from 2005 to 2014).
Comparative survival in patients after extracorporeal membrane oxygenation support for refractory post-cardiotomy cardiogenic shock considering timing for extracorporeal membrane oxygenation implantation (A), primary or iterative surgery (B), isolated or combined surgery (C) and type of surgical procedure (D). CPB: cardiopulmonary bypass; CABG: coronary artery bypass grafting.
Patient-reported health-related quality-of-life outcomes using the EQ-5 D health questionnaire. Quality of life was assessed at 2 years (n = 28) using the following dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Each dimension was measured for each patient at 3 different levels: no problems, some problems and extreme problems.
DISCUSSION
The main indication for ECMO was failure to withdraw from CPB despite maximum pharmacological support. Survival to hospital discharge was similar to that in previous reports [2, 4–6, 9, 15]. Among variables associated with poor prognosis, advanced age, valvular surgery and peak serum lactate level at 24 h after ECMO initiation were found to be associated with a higher mortality rate. In accordance with the largest series, preoperative pulmonary hypertension was not associated with a higher mortality rate [6]. The hospital mortality rate was influenced by the complexity of the cardiac procedure, as illustrated by the lower survival rate associated with iterative procedures. Moreover, a persistent, elevated lactate level under ECMO support was associated with a higher mortality rate, as previously reported [5, 6]. The higher vasoactive inotropic score in non-survivors at the time of ECMO implantation suggests more severe cardiac failure in these patients and a potentially deleterious delay before initiation of ECMO.
Multivariable analysis found that valvular surgery, preoperative LV systolic dysfunction and advanced age were associated with poor survival rates in patients with PCCS. The upper age limit to implant an ECMO for PCCS should be determined individually according to the potential for cardiac recovery. The median age of the patients was 64.5 years, which was comparable with that in previous reports [2, 4–7]. The main findings reported in recent publications are summarized in Supplementary Material, Table S1 [2, 4–9, 15–17].
The hospital mortality rate associated with ECMO for PCCS was considerable, up to 60% in the present series. Recent publications showed 1-month survival rates ranging from 29% to 47% (Supplementary Material, Table S1). No remarkable change was observed over the last decade despite major improvements in ECMO materials including a newly developed heparin-bound membrane oxygenator, better cannula design, magnetically levitated centrifugal pumps and a biocompatible bypass circuit. The primary aim of ECMO for refractory PCCS is to emergently provide haemodynamic support. Second, the potential benefits of ECMO for myocardial recovery remain a matter of debate. The increasing ECMO blood flow may adversely impact LV performance due to a marked increase in LV stroke work (Ostadal, 2015) [18]. Venoarterial ECMO induces an elevation in LV wall stress due to a slight increase in afterload. In the absence of residual contractility, this LV pressure overload state enhances LV failure and may in part explain the limited survival of patients supported for refractory cardiogenic shock. In our series, the duration of mechanical ventilation was longer among survivors because of the early death of non-survivors. Indeed, the rates of pulmonary oedema and pneumonia were almost 2 times higher among the patients who died. These findings suggest impaired LV unloading under ECMO support. In this situation, addition of IABP has been shown to reduce LV overload and blood pulmonary pressure, but without improving microcirculation blood flow [19]. The rate of IABP was extremely low in our series (lower than one-third of patients). We do not believe that it could durably decrease LV pressure overload, but it might prevent pulmonary oedema at the early stage of ECMO initiation [19]. We recently used the small heart pump Impella CP (Abiomed, Danvers, MA, USA) in this situation to directly unload the LV without incurring structural damage [20]. We reversed pulmonary oedema within a few hours with this approach. However, these patients were not included in the present series, and this strategy needs further investigation. Moreover, antegrade flow of intrathoracic aortic cannulation has been suggested to improve myocardial recovery compared to peripheral cannulation, even if no reliable evidence supports this hypothesis. Recently, Schiller et al. [21] showed experimentally in healthy pigs that central and peripheral venoarterial ECMO are both associated with a marked increase in LV volumes and a constant decrease in LV contractile performance. We used the central approach at the beginning of our experience, but we had to deal with major bleeding events in the post-cardiotomy setting. The large number of patients who had peripheral ECMO in this series (88%) may in part explain the relatively low rate of bleeding requiring surgical revision. Central cannulation also required resternotomy to remove the cannula and was associated with a higher risk of mediastinitis compared to peripheral cannulation through the femoral vessels. Consistent with this experience and in the absence of a demonstrated haemodynamic benefit for the central approach, peripheral cannulation is the preferred approach in our group for PCCS (88% of cases in the present study).
Another important finding of the present study is that all patients who survived the early postoperative period were alive at 1 year. Consistent with these favourable outcomes, we observed an acceptable health-related quality of life among survivors at 2 years. These data support aggressive treatment for patients with refractory PCCS as confirmed by recent publications [2, 5, 6]. ECMO is characterized by ease of peripheral placement and remains the gold standard of full cardiopulmonary support techniques. Moreover, major vascular complications related to peripheral cannulation were uncommon and were observed mainly at the beginning of our experience.
Limitations
We performed a retrospective analysis but the variables were collected prospectively by clinical research nurses. The statistical power of the present study is low, mainly due to the small sample size of the cohort. Percutaneous coronary intervention was preferred in patients with severe coronary disease and poor LV function. This approach explains the relatively small number of patients with coronary issues. The 10-year study period may have a low impact on patients’ outcomes since materials and the surgical team did not change during this period. However, as mentioned previously, the surgical approach for ECMO support moved from central to peripheral cannulation over time. Moreover, at the beginning of the ECMO program, patients older than 70 years were not considered for mechanical circulatory support in case of PCCS. Surprisingly, the mean preoperative LVEF was not markedly decreased in either group, whereas patients with low ejection fractions were considered for ECMO in the present study. We hypothesize that LVEF may have been overestimated in patients operated on for valvular disease (69% of the cohort), especially for those with severe mitral regurgitation.
CONCLUSION
Refractory cardiogenic shock requiring ECMO support was most frequently observed after redo valvular operations in the present study. In patients surviving to hospital discharge, no late deaths were observed. The overall 6-month survival rate was 39% after ECMO implantation with an acceptable quality of life. Improved kidney and liver functions after 24 h of support were associated with favourable outcomes. The approaches for LV unloading, as well as the results of the bridge to heart transplant or to long-term MCS in the absence of myocardial recovery, have to be further investigated.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online. Additional details related to materials used for ECMO as well as Table S1 are reported in Supplementary Material.
ACKNOWLEDGEMENTS
The authors thank Pascale Rouault, Veronique Desriac and Leo Bunel for collecting data. PR and VD also followed the patients after discharge. The authors thank Anne Ingels for statistical assistance.
Conflict of interest: none declared.
REFERENCES
Author notes
Presented at the 30th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Barcelona, Spain, 1–5 October 2016.