-
PDF
- Split View
-
Views
-
Cite
Cite
Norman Mangner, David Holzhey, Martin Misfeld, Axel Linke, Treatment of a degenerated sutureless Sorin Perceval® valve using an Edwards SAPIEN 3, Interactive CardioVascular and Thoracic Surgery, Volume 26, Issue 2, February 2018, Pages 364–366, https://doi.org/10.1093/icvts/ivx338
Close - Share Icon Share
Abstract
Valve-in-valve implantation is an alternative to reoperation for patients with degenerated bioprostheses. This case report presents a 75-year-old woman presenting with worsening dyspnoea according New York Heart Association (NYHA) Class III after she had received a Sorin Perceval® S sutureless valve due to severe aortic valve stenosis 5 years ago. Echocardiography revealed a degenerated Perceval valve with severe valvular aortic regurgitation and stenosis. After exclusion of acute infective endocarditis, an Edwards SAPIEN 3 was implanted leading to an immediate haemodynamic improvement. A cerebral protection device caught a big embolized piece of material in the left carotid artery filter. The case demonstrates that not only suture-based stented and stentless bioprostheses can be treated by a valve-in-valve strategy, but it is also feasible to treat a failed sutureless Sorin Perceval using a balloon-expandable SAPIEN 3.
INTRODUCTION
Implantation of the sutureless Sorin Perceval® valve is a surgical tool for the treatment of aortic valve stenosis combining the potential benefit of native valve removal with a reduced cross-clamping time [1]. Only little is known about the long-term durability of this new prosthesis and whether it is possible to treat a degenerated Sorin Perceval with a valve-in-valve strategy comparable to suture-based stented and stentless bioprostheses [2].
CASE REPORT
A 75-year-old woman presented with worsening dyspnoea [New York Heart Association (NYHA) Class III] after she had received a Sorin Perceval S (21 mm) due to severe aortic valve stenosis 5 years ago. Immediate postoperative echocardiography documented gradients of 32/18 mmHg, an effective orifice area of 1.5 cm2 (0.8 cm2/m2) and a trace valvular but no paravalvular aortic regurgitation.
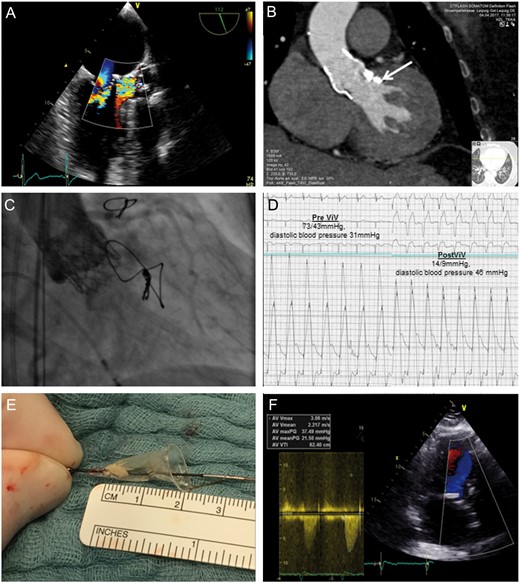
Currently, echocardiography revealed a degenerated prosthesis with severe valvular aortic regurgitation (pressure half time (PHT) 230 ms) and stenosis (maximum/mean gradient 69/44 mmHg). There was no evidence of paravalvular regurgitation (Fig. 1A). Computed tomography showed a well-adapted prosthesis with an effective annulus diameter of 21.1 mm and residual left ventricular outflow tract calcification (Fig. 1B, arrow). Coronary height was 11 mm for both the left and right coronary arteries. Active infective endocarditis was excluded by negative blood cultures and transoesophageal echocardiography.
Diagnostic findings prior to valve-in-valve procedure (A and B), implantation of the SAPIEN 3 into the Sorin Perceval leading to improvement of aortic regurgitation and stenosis (C and D), embolized material caught by the cerebral protection device (E) and postoperative findings (F).
The patient’s Society of Thoracic Surgery (STS) score for reoperation was 8.1% combined with a marked frailty (5-m gait speed 11 s, handgrip strength 14.3 kg and Katz score 4), which is known to be a strong predictor of mortality and disability [3], leading to the heart team decision for valve-in-valve transcatheter aortic valve implantation using a 23-mm Edwards SAPIEN 3. Size selection was based on both computed tomography scan and the available information within the Valve-in-Valve Aortic App (version 2.0).
The procedure was performed using a transfemoral approach under local anaesthesia and a cerebral neuroprotection device (Claret Sentinel®) due to the severely degenerated valve morphology. Implantation was performed without predilatation, and the Edwards SAPIEN 3 was placed in line with the lowest visible margin of the Perceval valve stent. This led to an immediate improvement of aortic regurgitation (Grade 3 to trace) and stenosis (invasive mean gradient from 43 to 9 mmHg) (Fig. 1C and D and Videos 1 and 2). Examination of the cerebral protection device showed a 5-mm × 4-mm embolized piece of material in the left carotid artery filter (Fig. 1E). Echocardiography prior to discharge revealed no aortic regurgitation, a mean gradient of 21 mmHg and an aortic valve area of 1.3 cm2 (0.7 cm2/m2), indicating a moderate patient prosthesis mismatch already existing directly after first operation (Fig. 1F).
Deployment of the Edwards SAPIEN 3 within the Sorin Perceval under rapid ventricular pacing.
Aortography demonstrating the final result with a trivial aortic regurgitation and patent flow to the coronary arteries.
DISCUSSION
The valve-in-valve approach may offer an effective and less invasive treatment for patients with failed surgical bioprostheses who are at a high risk for surgical reoperation. This case demonstrates that not only suture-based stented and stentless bioprostheses can be treated by a valve-in-valve strategy, but it is also feasible to treat a failed sutureless Sorin Perceval using a balloon-expandable SAPIEN 3, which led in our case to an acute haemodynamic improvement. However, several possible complications may occur in such a scenario. First, the risk of coronary obstruction is about 4-fold higher in valve-in-valve procedures compared with treatment of native valves [4]. The annular leaflet design of the Sorin Perceval in combination with a coronary height of 11 mm made us confident to prevent this devastating complication. Second, the elastic stent body of the Sorin Perceval may predispose to inadequate sealing and regurgitation or even migration/embolization of the transcatheter prosthesis. Therefore, we decided to use a balloon-expandable device, despite the possible disadvantage of higher gradients due to the annular design of the Edwards SAPIEN 3 [2]. In the end, we were able to reach a result comparable to first postoperative findings which may be attributed to some oversizing of the elastic stent. Third, the potential risk of cerebral embolism remains an important concern. Cerebral protection devices, e.g. the Claret Sentinel in our case, may have the potential to reduce this complication [5]. Finally, there is the personal observation that sutureless valves may have the tendency to induce relative severe calcifications of the aortic root arguing the concept of valve-in-valve approach. However, data about the long-term durability of the Sorin Perceval are still missing underlining the need to follow-up the patients in the multicentre study [1] to prove this observation and gain information about the durability of the Sorin Perceval.
Conflict of interest: David Holzhey reports other from Symetis and Medtronic, outside the submitted work. Axel Linke reports grants and personal fees from Medtronic, St. Jude, Boston Scientific and BARD, grants from Claret Medical, personal fees and other from Claret Medical, outside the submitted work. All other authors declared no conflict of interest.