-
PDF
- Split View
-
Views
-
Cite
Cite
Kayoko Natsume, Norihiko Shiiya, Katsushi Yamashita, Naoki Washiyama, Spinal cord ischaemia after endovascular aneurysm repair, Interactive CardioVascular and Thoracic Surgery, Volume 25, Issue 5, November 2017, Pages 827–829, https://doi.org/10.1093/icvts/ivx121
Close - Share Icon Share
Abstract
We report the case of a patient who developed paraparesis 2 days after endovascular aneurysm repair for a right common iliac aneurysm. The patient had undergone thoracic endovascular aortic repair. The left subclavian artery was occluded, but the left internal iliac artery was preserved. The patient fully recovered from the paralysis within 3 months. This case illustrates the importance of collateral blood supply to the spinal cord from the lumbosacral region, especially when other sources are occluded.
INTRODUCTION
Spinal cord ischaemia (SCI) is rare after endovascular aneurysm repair (EVAR). We report a case of SCI after EVAR for a right common iliac artery aneurysm (CIAA) in a patient with preceding thoracic endovascular aortic repair (TEVAR), in which the left internal iliac artery was preserved, but the great radicular artery and the left subclavian artery were occluded.
CASE REPORT
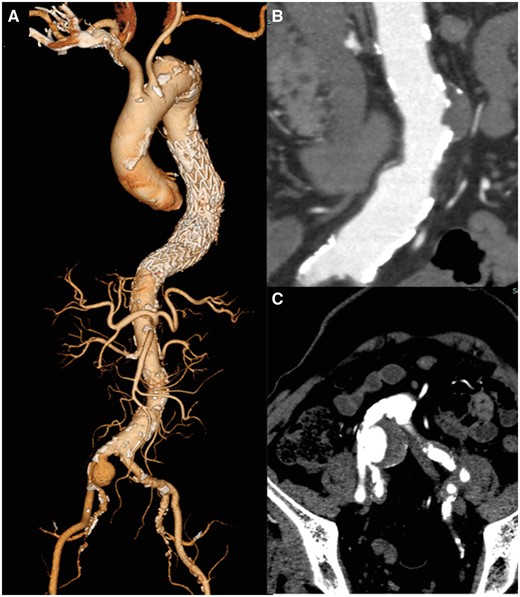
An 88-year-old man presented with a descending aortic aneurysm causing severe back pain and a concomitant right CIAA. The left subclavian artery was occluded with thrombus. He underwent emergent TEVAR through the left external iliac artery (cTAG 34-34-150 + 40-40-150, W. L. Gore, Flagstaff, AZ, USA), which covered the descending thoracic aorta from the T6 to the T11 level, with no SCI. Postoperatively, he started taking apixaban for paroxysmal atrial fibrillation, and a pacemaker was implanted for sick sinus syndrome. Postoperative contrast-enhanced computed tomography (CT) showed no endoleak. The right CIAA was 40 mm in diameter, and a 29-mm saccular protrusion of the infrarenal abdominal aorta was also noted (Fig. 1). EVAR for the right CIAA was scheduled 3 months later.
(A) 3D computed tomography reconstruction before endovascular aneurysm repair. (B) Small saccular protrusion of the abdominal aorta. (C) Right common iliac artery aneurysm.
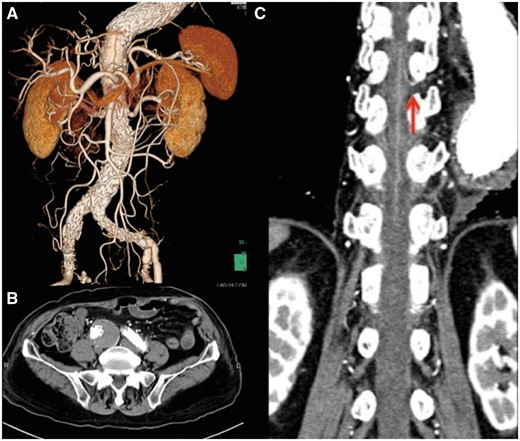
First, we performed coil embolization of the right internal iliac artery. Then, the principal devices (Endurant II 25-16-145 + 16-20-124 + 16-10-93, Medtronic Vascular, Santa Rosa, CA, USA) were deployed from the lower renal artery to the right external iliac artery and left common iliac artery. The left internal iliac artery was preserved. No paralysis was noted immediately after surgery. Apixaban was started on the first postoperative day. On the second postoperative day, weakness of the lower extremities became apparent, and the manual muscle test of the quadriceps femoris muscles was 2/5 for both sides. There was no episode of hypotension throughout the postoperative course. Brain CT revealed no cerebral infarction. Magnetic resonance imaging could not be performed because of pacemaker implantation. From these findings, a neurologist made a diagnosis of SCI. The patient received naloxone, edaravone and norepinephrine to keep the mean blood pressure over 90 mmHg. Cerebrospinal fluid drainage was not performed at the onset because of the use of apixaban and because the paralysis already showed some improvement the next day. Postoperative CT showed no endoleak. The great radicular artery was determined to arise from the left T9 segmental artery, the origin of which was covered by the previous stent graft (Fig. 2). After physical therapy, the patient fully recovered from the paraparesis within 3 months.
(A) 3D computed tomography reconstruction after endovascular aneurysm repair. (B) The right common iliac aneurysm after endovascular aneurysm repair. There is no endoleak. (C) The great radicular artery arising from Th9 intercostal artery (red arrow).
DISCUSSION
The incidence of SCI after abdominal aortic surgery has been reported to be 0.3% [1]. Rosenthal reported that prolonged aortic cross-clamping, intraoperative hypotension, aortic embolization and interruption of internal iliac artery circulation, all of which have been suggested as possible causes of SCI, have not proved to be significant as the sole causes of SCI [2]. The incidence of SCI after EVAR is much less frequent. Yet SCI continues to be a real threat because of its multifactorial nature. We therefore need to pursue a better understanding of the mechanism of injury.
The anterior spinal artery is fed by the radicular arteries that originate from the subclavian, intercostal and lumbar arteries. Usually the radicular arteries have a rich collateral network. The blood supply to the distal spinal cord is derived from the branches of the internal iliac artery. In our case, the intercostal artery supplying the great radicular artery had been covered by TEVAR, and the left subclavian artery was occluded. Therefore, the blood supply to the thoracolumbar spinal cord was presumably derived from the collateral network through the upper intercostal, lumbar or iliac arteries. Although the thoracodorsal arteries, internal thoracic arteries and inferior epigastric arteries have been reported to be important sources of the collateral network [1, 3], we could not visualize such pathways in the present case, and therefore, we suspect that the lumbar arteries or the right internal iliac artery were important for the collateral blood supply. The preceding left carotid–subclavian bypass may thus have prevented SCI.
Sacrifice of the bilateral internal iliac arteries may be associated with an elevated risk of SCI after EVAR, especially after TEVAR with coverage of the great radicular artery. In the present case, SCI occurred although the left internal iliac artery was patent and preserved. In performing internal iliac artery embolization, it is important to maintain communication between the patent distal branches of the internal iliac artery to avoid bowel complications. Experience with the present case suggests that this principle may also be important to avoid spinal cord complications. The use of an iliac branch device may be advantageous in such cases. Alternatively, patent lumbar arteries that were occluded by EVAR may have been important collateral sources. We chose to cover the entire infrarenal abdominal aorta because of the presence of saccular protrusion. SCI could have been avoided if we had performed a simple stent graft to the right iliac artery instead of EVAR.
Preceding TEVAR with great radicular artery and left subclavian occlusion may be associated with an elevated risk of SCI after EVAR. In this situation, even unilateral internal iliac artery sacrifice may compromise the blood supply of the spinal cord.
Conflict of interest: none declared.