-
PDF
- Split View
-
Views
-
Cite
Cite
Mirjam I Bakker, Mochammad Hatta, Agnes Kwenang, William R Faber, Stella M van Beers, Paul R Klatser, Linda Oskam, Population survey to determine risk factors for Mycobacterium leprae transmission and infection, International Journal of Epidemiology, Volume 33, Issue 6, December 2004, Pages 1329–1336, https://doi.org/10.1093/ije/dyh266
Close - Share Icon Share
Abstract
Background Not every leprosy patient is equally effective in transmitting Mycobacterium leprae. We studied the spatial distribution of infection (using seropositivity as a marker) in the population to identify which disease characteristics of leprosy patients are important in transmission.
Methods Clinical data and blood samples for anti-M. leprae ELISA were collected during a cross-sectional survey on five Indonesian islands highly endemic for leprosy. A geographic information system (GIS) was used to define contacts of patients. We investigated spatial clustering of patients and seropositive people and used logistic regression to determine risk factors for seropositivity.
Results Of the 3986 people examined for leprosy, 3271 gave blood. Seroprevalence varied between islands (1.7–8.7%) and correlated significantly with leprosy prevalence. Five clusters of patients and two clusters of seropositives were detected. In multivariate analysis, seropositivity significantly differed by leprosy status, age, sex, and island. Serological status of patients appeared to be the best discriminator of contact groups with higher seroprevalence: contacts of seropositive patients had an adjusted odds ratio (aOR) of 1.75 (95% CI 0.922–3.31). This increased seroprevalence was strongest for contact groups living ≤75 m of two seropositive patients (aOR = 3.07; 95% CI 1.74–5.42).
Conclusions In this highly endemic area for leprosy, not only household contacts of seropositive patients, but also people living in the vicinity of a seropositive patient were more likely to harbour antibodies against M. leprae. Through measuring the serological status of patients and using a broader definition of contacts, higher risk groups can be more specifically identified.
Leprosy is an infectious disease caused by Mycobacterium leprae and is endemic in many developing countries. The World Health Organization (WHO) has adopted the goal of eliminating leprosy as a public health problem by the year 2005, defined as reducing the national prevalence below 1/10 000.1 Until now, the prevalence decreased mainly due to the introduction and subsequent shortening of multidrug treatment (MDT). Leprosy control strategies are designed to stop transmission through early case detection and treatment with MDT, but do not seem to have the desired effect. The number of new cases—719 330 in 20002—did not decline over the last 15 years,3 indicating that transmission is continuing at the same level.
Leprosy manifests itself as a disease spectrum, which for treatment purposes has been divided into two forms: multibacillary (MB) and paucibacillary (PB) leprosy. Not every leprosy patient is equally effective in transmitting the disease;4 it is generally accepted that untreated MB patients are the most important source of transmission,4–6 but patient characteristics other than classification, such as bacterial index (BI) or seropositivity, may be important as well. There is a weakly positive correlation between BI and antibody levels to M. leprae.7 Once it is known which patients are most efficiently transmitting leprosy and which contact groups are most at risk of becoming infected, intervention strategies like prophylactic treatment can be better targeted at specific high-risk groups.
For control strategies it is necessary to have a clear definition of contacts. The 'stone-in-the-pond' concept, originally developed for tuberculosis, was used for leprosy describing transmission in concentric circles around a patient. This study indicated that not only household contacts but also neighbours and social contacts have an increased risk of developing leprosy.4
In endemic populations infection can be detected by the presence of elevated titres of IgM antibodies against M. leprae-specific phenolic glycolipid-I (PGL-I).8–10 To assess the transmission potential of different types of patients, we compared the seroprevalence in contacts of these patients with non-contacts. Studies investigating the seroprevalence among contacts of leprosy patients have shown variable results. Some studies did find an increased seroprevalence among contacts9,11,12 and others did not.8,13,14
Here we studied clustering of seropositives and leprosy patients and identified risk factors for infection using seropositivity as a marker. We also investigated which disease characteristics of leprosy patients (classification, BI or serology) determine transmission most. We used a geographic information system (GIS) to combine spatial, clinical, and demographic data. GIS is a powerful tool for examining spatial patterns15 and, apart from surveillance studies, has never been used in leprosy research before.
Methods
Study population and sample collection
Five isolated islands in the Flores Sea in Indonesia were selected for this study, namely Tampaang, Pelokang, Kembanglemari, Sailus besar, and Sapuka besar. The description of these islands was published previously.16 We received ethical clearances from the Ethical Research Committee of the Hasanuddin University and the Ministry of Health of the Republic of Indonesia.
During a cross-sectional study in June/July 2000 the population was clinically examined for leprosy. The diagnosis was based on the WHO classification.16,17 Patients with one lesion were classified as PB1 and with 2–5 lesions as PB2–5. Patients with >5 five lesions and/or with a positive BI in at least one of three skin smears were classified as MB. Simultaneously, we collected venous blood of the population aged >5 years. Serum was separated by centrifugation on the same day and kept frozen until use.
ELISA
The presence of IgM antibodies to M. leprae PGL-I was measured using an enzyme-linked immunosorbent assay (ELISA) as described previously18 using the natural trisaccharide moiety of PGL-I linked to bovine serum albumin (NT-P-BSA). Pre-coated plates were used. Serum was diluted 1:500 and tested in duplo. The optical density at 450 nm (OD) of each serum was calculated by subtracting the OD value of BSA coated wells from that of NT-P-BSA-coated wells. A positive reference serum on each plate was used to minimize plate-to-plate variation. When this serum reached an OD value of 0.6, the colour reactions of the entire plate were stopped. The cut-off value for seropositivity was set at 0.200.
For quality control 10% of the samples were randomly chosen and re-tested with the same protocol in a different laboratory. These results did not differ significantly from the main results.
Preparation of maps
Longitudes and latitudes of approximately every fifth house were measured using a hand-held Global Positioning System (GPS, Garmin, Kansas USA). In Arcview 3.2 (Esri, California USA) the remaining houses were situated between the geo-referenced houses using detailed hand-drawn maps.
Contact definition
People were only classified as 'contact' when the contact had lasted for a minimum of 6 months and had ended not longer than 6 months prior to the survey as determined through a registration survey about 6 months prior to the examination.
Types of contacts were determined by two factors, namely (1) the type of index patient based on his/her classification, serological status and BI, and (2) the distance between the houses of the contact and the index patient. The distance to an index patient was determined with buffers: circles with a radius of 0, 25, 50, 75, 100, 125, or 150 m around the patient. Household contacts (circle radius = 0) were defined as people who shared a house with a patient. Buffer 1 contacts were defined as people who lived within a radius of 25 metres around a patient, buffer 2 contacts between 26–50 m, and so forth up to buffer 6 contacts (126–150 m).
Data analyses
The leprosy prevalence was defined as the proportion of leprosy patients registered for therapy at the end of the survey (July 2000) over the examined population at that time. The seroprevalence was defined as the proportion of seropositives over the population screened for antibodies (excluding patients).
Logistic regression was used to determine independent factors associated with a positive ELISA result. Factors associated with a positive ELISA result in univariate analyses (P < 0.15) were selected for multivariate analyses. For the final model we tested for statistically significant (P < 0.05) interactions between factors and for confounding.
To investigate clustering of patients and of seropositive people the Kulldorff spatial scan statistic was used in Satscan version 2.1.19 Clustering occurs when the probability of having leprosy/being seropositive is not randomly distributed, but concentrated on certain parts of the islands. Houses were used as census areas. We used purely spatial analyses. The P-value was obtained from a likelihood ratio test based on Monte Carlo simulation with 9999 replicates. The Satscan was performed per island using the patients as cases and the examined people without leprosy as controls. Satscan was once performed using all patients and once using only PB2–5 and MB. For the detection of clusters of seropositive people we used the seropositives as cases and the seronegatives as controls. This was done once including and once excluding seropositive patients.
Results
In July 2000, we clinically examined 3986/4748 registered inhabitants for leprosy (coverage: 84%). Ninety-one active patients were found (80 new and 11 patients who had received leprosy treatment before, but still had active lesions) which gave a prevalence of 228/10 000 (see Table 1). Fifteen people were found who were released from leprosy treatment (RFT).
Leprosy prevalence and seroprevalence (July 2000) per island
| Islands . | Screened n (cov %a) . | Leprosy patients (n) . | Prevalence/10000 (95% CI) . | Serum (n) . | Seroprevalence (95%CI)b . |
|---|---|---|---|---|---|
| Sapuka | 1695 (82%) | 26 | 153 (128–178) | 1411 | 1.7% (1.3–2.1) |
| Sailus | 1217 (84%) | 26 | 214 (182–246) | 978 | 2.2% (1.7–2.7) |
| Tampaang | 161 (74%) | 2 | 124 (36–212) | 141 | 2.9% (1.2–4.6) |
| Pelokang | 353 (91%) | 9 | 255 (205–305) | 309 | 4.0% (2.9–5.1) |
| Kembanglemari | 560 (88%) | 28 | 500 (438–562) | 432 | 8.7% (7.0–10.3) |
| TOTAL | 3986 (84%) | 91 | 228 (209–247) | 3271 | 3.0% (2.7–3.4) |
| Islands . | Screened n (cov %a) . | Leprosy patients (n) . | Prevalence/10000 (95% CI) . | Serum (n) . | Seroprevalence (95%CI)b . |
|---|---|---|---|---|---|
| Sapuka | 1695 (82%) | 26 | 153 (128–178) | 1411 | 1.7% (1.3–2.1) |
| Sailus | 1217 (84%) | 26 | 214 (182–246) | 978 | 2.2% (1.7–2.7) |
| Tampaang | 161 (74%) | 2 | 124 (36–212) | 141 | 2.9% (1.2–4.6) |
| Pelokang | 353 (91%) | 9 | 255 (205–305) | 309 | 4.0% (2.9–5.1) |
| Kembanglemari | 560 (88%) | 28 | 500 (438–562) | 432 | 8.7% (7.0–10.3) |
| TOTAL | 3986 (84%) | 91 | 228 (209–247) | 3271 | 3.0% (2.7–3.4) |
Coverage.
Seroprevalence among non-patient population.
Leprosy prevalence and seroprevalence (July 2000) per island
| Islands . | Screened n (cov %a) . | Leprosy patients (n) . | Prevalence/10000 (95% CI) . | Serum (n) . | Seroprevalence (95%CI)b . |
|---|---|---|---|---|---|
| Sapuka | 1695 (82%) | 26 | 153 (128–178) | 1411 | 1.7% (1.3–2.1) |
| Sailus | 1217 (84%) | 26 | 214 (182–246) | 978 | 2.2% (1.7–2.7) |
| Tampaang | 161 (74%) | 2 | 124 (36–212) | 141 | 2.9% (1.2–4.6) |
| Pelokang | 353 (91%) | 9 | 255 (205–305) | 309 | 4.0% (2.9–5.1) |
| Kembanglemari | 560 (88%) | 28 | 500 (438–562) | 432 | 8.7% (7.0–10.3) |
| TOTAL | 3986 (84%) | 91 | 228 (209–247) | 3271 | 3.0% (2.7–3.4) |
| Islands . | Screened n (cov %a) . | Leprosy patients (n) . | Prevalence/10000 (95% CI) . | Serum (n) . | Seroprevalence (95%CI)b . |
|---|---|---|---|---|---|
| Sapuka | 1695 (82%) | 26 | 153 (128–178) | 1411 | 1.7% (1.3–2.1) |
| Sailus | 1217 (84%) | 26 | 214 (182–246) | 978 | 2.2% (1.7–2.7) |
| Tampaang | 161 (74%) | 2 | 124 (36–212) | 141 | 2.9% (1.2–4.6) |
| Pelokang | 353 (91%) | 9 | 255 (205–305) | 309 | 4.0% (2.9–5.1) |
| Kembanglemari | 560 (88%) | 28 | 500 (438–562) | 432 | 8.7% (7.0–10.3) |
| TOTAL | 3986 (84%) | 91 | 228 (209–247) | 3271 | 3.0% (2.7–3.4) |
Coverage.
Seroprevalence among non-patient population.
A total of 3271 people (80% of the population >5 years) gave blood for antibody testing. Of these 3271, 112 were seropositive, 16 of which were leprosy patients. The overall seroprevalence was 3.0%. A weak, but positive correlation was found between BI and antibody levels among 86 patients for whom both results were available (Spearman's r = 0.312, P = 0.003). The leprosy prevalence correlated significantly with the seroprevalence among the 'non-leprosy' population on each island (Pearson's r = 0.946, P < 0.02). Serum was collected from at least one household member in 1054/1104 houses. Ninety of 1054 (8.5%) houses accommodated one seropositive person (including patients), 8/1054 (0.8%) houses two seropositives and 2/1054 (0.2%) houses three seropositives.
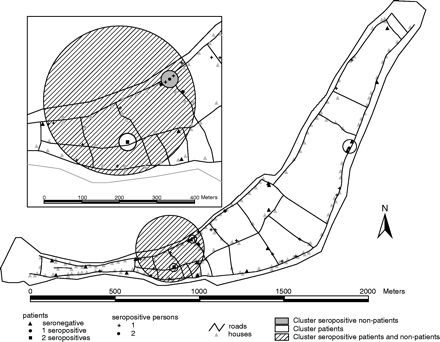
Table 2 shows all the detected clusters and Figure 1 shows the clusters on Sapuka as an example. The Satscan analyses identified three significant clusters of leprosy patients varying in size from one to five houses: two on Sapuka (both P = 0.014) and one on Kembanglemari (P = 0.016). Furthermore, two clusters were found on Sailus with a P < 0.10. These five clusters included 20 patients, of which 13 were MB patients. Repeating the same analysis, but now excluding the PB1 patients, did not change the overall result, but the two clusters on Sailus became significant.
Map of Sapuka and detailed map showing the houses of the patients, seropositive persons, and significant clusters (only one-third of the other houses is shown)
Clusters of leprosy patients/seropositives detected using a spatial scan
| Radius cluster (m)a . | Population . | Cases observed . | Cases expected . | Observed/Expected . | P-value . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Clusters of leprosy patients | ||||||||||
| 0b | 12 | 4 | 0.18 | 21.7 | 0.014 | |||||
| 0c | 2 | 2 | 0.04 | 50.5 | 0.062 | |||||
| 10d | 11 | 5 | 0.55 | 9.1 | 0.016 | |||||
| 40b | 23 | 5 | 0.35 | 14.2 | 0.014 | |||||
| 50c | 14 | 4 | 0.28 | 14.4 | 0.072 | |||||
| Clusters of seropositives (excluding patients) | ||||||||||
| 10b | 11 | 4 | 0.19 | 21.0 | 0.017 | |||||
| 10d | 14 | 6 | 1.21 | 4.9 | 0.088 | |||||
| Clusters of seropositives and seropositive patients | ||||||||||
| 200b | 259 | 16 | 5.69 | 2.8 | 0.026 | |||||
| Radius cluster (m)a . | Population . | Cases observed . | Cases expected . | Observed/Expected . | P-value . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Clusters of leprosy patients | ||||||||||
| 0b | 12 | 4 | 0.18 | 21.7 | 0.014 | |||||
| 0c | 2 | 2 | 0.04 | 50.5 | 0.062 | |||||
| 10d | 11 | 5 | 0.55 | 9.1 | 0.016 | |||||
| 40b | 23 | 5 | 0.35 | 14.2 | 0.014 | |||||
| 50c | 14 | 4 | 0.28 | 14.4 | 0.072 | |||||
| Clusters of seropositives (excluding patients) | ||||||||||
| 10b | 11 | 4 | 0.19 | 21.0 | 0.017 | |||||
| 10d | 14 | 6 | 1.21 | 4.9 | 0.088 | |||||
| Clusters of seropositives and seropositive patients | ||||||||||
| 200b | 259 | 16 | 5.69 | 2.8 | 0.026 | |||||
Radius cluster indicates the size of the cluster in metres. When the radius of the cluster is 0 only one house is involved.
From Sapuka;
from Sailus;
from Kembanglemari.
Clusters of leprosy patients/seropositives detected using a spatial scan
| Radius cluster (m)a . | Population . | Cases observed . | Cases expected . | Observed/Expected . | P-value . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Clusters of leprosy patients | ||||||||||
| 0b | 12 | 4 | 0.18 | 21.7 | 0.014 | |||||
| 0c | 2 | 2 | 0.04 | 50.5 | 0.062 | |||||
| 10d | 11 | 5 | 0.55 | 9.1 | 0.016 | |||||
| 40b | 23 | 5 | 0.35 | 14.2 | 0.014 | |||||
| 50c | 14 | 4 | 0.28 | 14.4 | 0.072 | |||||
| Clusters of seropositives (excluding patients) | ||||||||||
| 10b | 11 | 4 | 0.19 | 21.0 | 0.017 | |||||
| 10d | 14 | 6 | 1.21 | 4.9 | 0.088 | |||||
| Clusters of seropositives and seropositive patients | ||||||||||
| 200b | 259 | 16 | 5.69 | 2.8 | 0.026 | |||||
| Radius cluster (m)a . | Population . | Cases observed . | Cases expected . | Observed/Expected . | P-value . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Clusters of leprosy patients | ||||||||||
| 0b | 12 | 4 | 0.18 | 21.7 | 0.014 | |||||
| 0c | 2 | 2 | 0.04 | 50.5 | 0.062 | |||||
| 10d | 11 | 5 | 0.55 | 9.1 | 0.016 | |||||
| 40b | 23 | 5 | 0.35 | 14.2 | 0.014 | |||||
| 50c | 14 | 4 | 0.28 | 14.4 | 0.072 | |||||
| Clusters of seropositives (excluding patients) | ||||||||||
| 10b | 11 | 4 | 0.19 | 21.0 | 0.017 | |||||
| 10d | 14 | 6 | 1.21 | 4.9 | 0.088 | |||||
| Clusters of seropositives and seropositive patients | ||||||||||
| 200b | 259 | 16 | 5.69 | 2.8 | 0.026 | |||||
Radius cluster indicates the size of the cluster in metres. When the radius of the cluster is 0 only one house is involved.
From Sapuka;
from Sailus;
from Kembanglemari.
Satscan also identified one significant cluster of seropositives on Sapuka (P = 0.017). This cluster included four seropositives in three houses situated <75 m from the houses of two seropositive patients. Furthermore, one cluster (P = 0.088) was found on Kembanglemari with six seropositives in three houses situated <100 m from the houses of two seropositive patients. When the analysis was performed including the seropositive patients, one significant cluster (P = 0.026) on Sapuka was found including 80 houses, 12 seropositive non-patients, four seropositive, and three seronegative patients.
Table 3 shows risk factors for leprosy seropositivity among patients and non-patients. In univariate analyses the variables status, age, island, and household size (seroprevalence higher for houses with more than nine inhabitants) were significantly related with seropositivity. All, except household size, remained statistically significant in the multivariate analysis. The variable sex, after adjustment for the other factors, appeared to relate significantly with seropositivity. Women were more likely to be seropositive than men (adjusted odds ratio [aOR] = 1.59, 95% CI 1.06–2.40). The prevalence of seropositivity decreased with age. Those aged 4–14 and 15–29 years had an aOR of 2.55 (95% CI 1.04–6.25) and 2.80 (95% CI 1.17–6.73), respectively, compared with people aged 45–59 years. Seroprevalence was significantly higher on Kembanglemari (aOR = 3.13, 95% CI 1.75–5.60) and Pelokang (aOR: 2.17, 95% CI 1.14–4.13) compared with the island Sapuka. No significant interaction existed between the variables.
Logistic regression to identify risk factors for seropositivity of leprosy on the five islands
| . | . | . | Unadjusted . | . | Adjusted . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | n . | % seropositive . | ORa . | 95% CI . | OR . | 95% CI . | ||||||
| Status | ||||||||||||
| MBb | 40 | 32.5% | 21.4 | 10.3–44.3 | 17.1 | 7.73–37.8 | ||||||
| PB2-5b | 13 | 15.4% | 8.08 | 1.74–37.6 | 5.85 | 1.16–29.4 | ||||||
| PB1b | 34 | 2.9% | 1.35 | 0.18–10.1 | 0.79 | 0.10–6.08 | ||||||
| Household contacts | 215 | 5.1% | 2.40 | 1.22–4.72 | 1.38 | 0.65–2.94 | ||||||
| Buffer 1 contacts | 522 | 4.6% | 2.14 | 1.29–3.56 | 1.18 | 0.63–2.20 | ||||||
| Buffer 2 contacts | 491 | 3.7% | 1.69 | 0.97–2.96 | 1.43 | 0.80–2.56 | ||||||
| Non-contacts | 1956 | 2.2% | 1.0 | 1.0 | ||||||||
| P < 0.001 | P < 0.001 | |||||||||||
| Sex | ||||||||||||
| Male | 1428 | 2.7% | 1.0 | |||||||||
| Female | 1843 | 4.0% | 1.47 | 0.99–2.18 | 1.59 | 1.06–2.40 | ||||||
| P = 0.057 | P = 0.026 | |||||||||||
| Age (years) | ||||||||||||
| 4–14 | 917 | 3.7% | 2.40 | 0.999–5.77 | 2.57 | 1.05–6.30 | ||||||
| 15–29 | 1057 | 4.6% | 3.03 | 1.29–7.13 | 2.81 | 1.17–6.74 | ||||||
| 30–44 | 682 | 2.9% | 1.88 | 0.75–4.73 | 1.74 | 0.68–4.47 | ||||||
| 45–59 | 380 | 1.6% | 1.0 | 1.0 | ||||||||
| ≥60 | 235 | 1.3% | 0.81 | 0.20–3.25 | 0.88 | 0.22–3.62 | ||||||
| P = 0.020 | P = 0.039 | |||||||||||
| Island | ||||||||||||
| Kembanglemari | 432 | 8.6% | 4.17 | 2.55–6.80 | 3.30 | 1.79–6.09 | ||||||
| Pelokang | 309 | 4.9% | 2.27 | 1.21–4.26 | 2.17 | 1.14–4.14 | ||||||
| Tampaang | 141 | 2.8% | 1.30 | 0.45–3.73 | 1.42 | 0.49–4.10 | ||||||
| Sailus | 978 | 2.6% | 1.17 | 0.69–1.99 | 1.12 | 0.65–1.94 | ||||||
| Sapuka | 1411 | 2.2% | 1.0 | 1.0 | ||||||||
| P < 0.001 | P = 0.001 | |||||||||||
| . | . | . | Unadjusted . | . | Adjusted . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | n . | % seropositive . | ORa . | 95% CI . | OR . | 95% CI . | ||||||
| Status | ||||||||||||
| MBb | 40 | 32.5% | 21.4 | 10.3–44.3 | 17.1 | 7.73–37.8 | ||||||
| PB2-5b | 13 | 15.4% | 8.08 | 1.74–37.6 | 5.85 | 1.16–29.4 | ||||||
| PB1b | 34 | 2.9% | 1.35 | 0.18–10.1 | 0.79 | 0.10–6.08 | ||||||
| Household contacts | 215 | 5.1% | 2.40 | 1.22–4.72 | 1.38 | 0.65–2.94 | ||||||
| Buffer 1 contacts | 522 | 4.6% | 2.14 | 1.29–3.56 | 1.18 | 0.63–2.20 | ||||||
| Buffer 2 contacts | 491 | 3.7% | 1.69 | 0.97–2.96 | 1.43 | 0.80–2.56 | ||||||
| Non-contacts | 1956 | 2.2% | 1.0 | 1.0 | ||||||||
| P < 0.001 | P < 0.001 | |||||||||||
| Sex | ||||||||||||
| Male | 1428 | 2.7% | 1.0 | |||||||||
| Female | 1843 | 4.0% | 1.47 | 0.99–2.18 | 1.59 | 1.06–2.40 | ||||||
| P = 0.057 | P = 0.026 | |||||||||||
| Age (years) | ||||||||||||
| 4–14 | 917 | 3.7% | 2.40 | 0.999–5.77 | 2.57 | 1.05–6.30 | ||||||
| 15–29 | 1057 | 4.6% | 3.03 | 1.29–7.13 | 2.81 | 1.17–6.74 | ||||||
| 30–44 | 682 | 2.9% | 1.88 | 0.75–4.73 | 1.74 | 0.68–4.47 | ||||||
| 45–59 | 380 | 1.6% | 1.0 | 1.0 | ||||||||
| ≥60 | 235 | 1.3% | 0.81 | 0.20–3.25 | 0.88 | 0.22–3.62 | ||||||
| P = 0.020 | P = 0.039 | |||||||||||
| Island | ||||||||||||
| Kembanglemari | 432 | 8.6% | 4.17 | 2.55–6.80 | 3.30 | 1.79–6.09 | ||||||
| Pelokang | 309 | 4.9% | 2.27 | 1.21–4.26 | 2.17 | 1.14–4.14 | ||||||
| Tampaang | 141 | 2.8% | 1.30 | 0.45–3.73 | 1.42 | 0.49–4.10 | ||||||
| Sailus | 978 | 2.6% | 1.17 | 0.69–1.99 | 1.12 | 0.65–1.94 | ||||||
| Sapuka | 1411 | 2.2% | 1.0 | 1.0 | ||||||||
| P < 0.001 | P = 0.001 | |||||||||||
Odds ratio.
Multibacillary leprosy; PB2-5 = paucibacillary leprosy with 2–5 lesions; PB1 = single lesion paucibacillary leprosy.
Logistic regression to identify risk factors for seropositivity of leprosy on the five islands
| . | . | . | Unadjusted . | . | Adjusted . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | n . | % seropositive . | ORa . | 95% CI . | OR . | 95% CI . | ||||||
| Status | ||||||||||||
| MBb | 40 | 32.5% | 21.4 | 10.3–44.3 | 17.1 | 7.73–37.8 | ||||||
| PB2-5b | 13 | 15.4% | 8.08 | 1.74–37.6 | 5.85 | 1.16–29.4 | ||||||
| PB1b | 34 | 2.9% | 1.35 | 0.18–10.1 | 0.79 | 0.10–6.08 | ||||||
| Household contacts | 215 | 5.1% | 2.40 | 1.22–4.72 | 1.38 | 0.65–2.94 | ||||||
| Buffer 1 contacts | 522 | 4.6% | 2.14 | 1.29–3.56 | 1.18 | 0.63–2.20 | ||||||
| Buffer 2 contacts | 491 | 3.7% | 1.69 | 0.97–2.96 | 1.43 | 0.80–2.56 | ||||||
| Non-contacts | 1956 | 2.2% | 1.0 | 1.0 | ||||||||
| P < 0.001 | P < 0.001 | |||||||||||
| Sex | ||||||||||||
| Male | 1428 | 2.7% | 1.0 | |||||||||
| Female | 1843 | 4.0% | 1.47 | 0.99–2.18 | 1.59 | 1.06–2.40 | ||||||
| P = 0.057 | P = 0.026 | |||||||||||
| Age (years) | ||||||||||||
| 4–14 | 917 | 3.7% | 2.40 | 0.999–5.77 | 2.57 | 1.05–6.30 | ||||||
| 15–29 | 1057 | 4.6% | 3.03 | 1.29–7.13 | 2.81 | 1.17–6.74 | ||||||
| 30–44 | 682 | 2.9% | 1.88 | 0.75–4.73 | 1.74 | 0.68–4.47 | ||||||
| 45–59 | 380 | 1.6% | 1.0 | 1.0 | ||||||||
| ≥60 | 235 | 1.3% | 0.81 | 0.20–3.25 | 0.88 | 0.22–3.62 | ||||||
| P = 0.020 | P = 0.039 | |||||||||||
| Island | ||||||||||||
| Kembanglemari | 432 | 8.6% | 4.17 | 2.55–6.80 | 3.30 | 1.79–6.09 | ||||||
| Pelokang | 309 | 4.9% | 2.27 | 1.21–4.26 | 2.17 | 1.14–4.14 | ||||||
| Tampaang | 141 | 2.8% | 1.30 | 0.45–3.73 | 1.42 | 0.49–4.10 | ||||||
| Sailus | 978 | 2.6% | 1.17 | 0.69–1.99 | 1.12 | 0.65–1.94 | ||||||
| Sapuka | 1411 | 2.2% | 1.0 | 1.0 | ||||||||
| P < 0.001 | P = 0.001 | |||||||||||
| . | . | . | Unadjusted . | . | Adjusted . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | n . | % seropositive . | ORa . | 95% CI . | OR . | 95% CI . | ||||||
| Status | ||||||||||||
| MBb | 40 | 32.5% | 21.4 | 10.3–44.3 | 17.1 | 7.73–37.8 | ||||||
| PB2-5b | 13 | 15.4% | 8.08 | 1.74–37.6 | 5.85 | 1.16–29.4 | ||||||
| PB1b | 34 | 2.9% | 1.35 | 0.18–10.1 | 0.79 | 0.10–6.08 | ||||||
| Household contacts | 215 | 5.1% | 2.40 | 1.22–4.72 | 1.38 | 0.65–2.94 | ||||||
| Buffer 1 contacts | 522 | 4.6% | 2.14 | 1.29–3.56 | 1.18 | 0.63–2.20 | ||||||
| Buffer 2 contacts | 491 | 3.7% | 1.69 | 0.97–2.96 | 1.43 | 0.80–2.56 | ||||||
| Non-contacts | 1956 | 2.2% | 1.0 | 1.0 | ||||||||
| P < 0.001 | P < 0.001 | |||||||||||
| Sex | ||||||||||||
| Male | 1428 | 2.7% | 1.0 | |||||||||
| Female | 1843 | 4.0% | 1.47 | 0.99–2.18 | 1.59 | 1.06–2.40 | ||||||
| P = 0.057 | P = 0.026 | |||||||||||
| Age (years) | ||||||||||||
| 4–14 | 917 | 3.7% | 2.40 | 0.999–5.77 | 2.57 | 1.05–6.30 | ||||||
| 15–29 | 1057 | 4.6% | 3.03 | 1.29–7.13 | 2.81 | 1.17–6.74 | ||||||
| 30–44 | 682 | 2.9% | 1.88 | 0.75–4.73 | 1.74 | 0.68–4.47 | ||||||
| 45–59 | 380 | 1.6% | 1.0 | 1.0 | ||||||||
| ≥60 | 235 | 1.3% | 0.81 | 0.20–3.25 | 0.88 | 0.22–3.62 | ||||||
| P = 0.020 | P = 0.039 | |||||||||||
| Island | ||||||||||||
| Kembanglemari | 432 | 8.6% | 4.17 | 2.55–6.80 | 3.30 | 1.79–6.09 | ||||||
| Pelokang | 309 | 4.9% | 2.27 | 1.21–4.26 | 2.17 | 1.14–4.14 | ||||||
| Tampaang | 141 | 2.8% | 1.30 | 0.45–3.73 | 1.42 | 0.49–4.10 | ||||||
| Sailus | 978 | 2.6% | 1.17 | 0.69–1.99 | 1.12 | 0.65–1.94 | ||||||
| Sapuka | 1411 | 2.2% | 1.0 | 1.0 | ||||||||
| P < 0.001 | P = 0.001 | |||||||||||
Odds ratio.
Multibacillary leprosy; PB2-5 = paucibacillary leprosy with 2–5 lesions; PB1 = single lesion paucibacillary leprosy.
Table 3 shows that household contacts had an aOR of 1.38 (95%CI 0.65–2.94) for being seropositive compared with non-contacts. In Table 4 contacts living 0–50 m from patients were grouped on the basis of patient characteristics (serological status, BI, and MB/PB classification) to identify associations between contact status and seropositivity. Serological status of patients appeared to be the best discriminator of contact groups with higher seroprevalence: contacts of seropositive patients had an aOR of 1.75 (95% CI 0.92–3.31) compared with non-contacts. Contacts of patients with a positive BI had an aOR of 1.41 (95% CI 0.73–2.74) and contacts of MB patients had an aOR of 1.32 (95% CI 0.73–2.38). This did not differ substantially between household and buffer contacts (results not shown). There was no interaction between the variables defining the different contact groups and the co-variables (age, sex, and island).
Logistic regression to identify the association between contact status and seropositivity of leprosy using several definitions of contacts among non-patients (n = 3184)
| Contactsaof patients grouped into different categories . | . | . | Adjustedb . | . | |
|---|---|---|---|---|---|
. | n . | % seropositive . | ORc . | 95% CI . | |
| Contacts of seropositive patients | 287 | 6.6% | 1.75 | 0.92–3.31 | |
| Contacts of only seronegative patients | 941 | 3.6% | 0.84 | 0.47–1.49 | |
| Non-contacts | 1956 | 1.0 | |||
| P = 0.05 | |||||
| Contacts of BId-positive patients | 345 | 6.1% | 1.41 | 0.73–2.74 | |
| Contacts of only BI-negative patients | 883 | 3.6% | 0.95 | 0.54–1.66 | |
| Non-contacts | 1956 | 1.0 | |||
| P = 0.39 | |||||
| Contacts of MBd-patients | 615 | 5.7% | 1.32 | 0.73–2.38 | |
| Contacts of only PBd-patients | 613 | 2.9% | 0.85 | 0.46–1.58 | |
| Non-contacts | 1956 | 1.0 | P = 0.34 | ||
| Contactsaof patients grouped into different categories . | . | . | Adjustedb . | . | |
|---|---|---|---|---|---|
. | n . | % seropositive . | ORc . | 95% CI . | |
| Contacts of seropositive patients | 287 | 6.6% | 1.75 | 0.92–3.31 | |
| Contacts of only seronegative patients | 941 | 3.6% | 0.84 | 0.47–1.49 | |
| Non-contacts | 1956 | 1.0 | |||
| P = 0.05 | |||||
| Contacts of BId-positive patients | 345 | 6.1% | 1.41 | 0.73–2.74 | |
| Contacts of only BI-negative patients | 883 | 3.6% | 0.95 | 0.54–1.66 | |
| Non-contacts | 1956 | 1.0 | |||
| P = 0.39 | |||||
| Contacts of MBd-patients | 615 | 5.7% | 1.32 | 0.73–2.38 | |
| Contacts of only PBd-patients | 613 | 2.9% | 0.85 | 0.46–1.58 | |
| Non-contacts | 1956 | 1.0 | P = 0.34 | ||
Contacts = household, buffer 1, and buffer 2 contacts (0–50 m).
Adjusted for age, sex, and island.
Odds ratio.
BI = bacterial index; MB = multibacillary leprosy; PB = paucibacillary leprosy.
Logistic regression to identify the association between contact status and seropositivity of leprosy using several definitions of contacts among non-patients (n = 3184)
| Contactsaof patients grouped into different categories . | . | . | Adjustedb . | . | |
|---|---|---|---|---|---|
. | n . | % seropositive . | ORc . | 95% CI . | |
| Contacts of seropositive patients | 287 | 6.6% | 1.75 | 0.92–3.31 | |
| Contacts of only seronegative patients | 941 | 3.6% | 0.84 | 0.47–1.49 | |
| Non-contacts | 1956 | 1.0 | |||
| P = 0.05 | |||||
| Contacts of BId-positive patients | 345 | 6.1% | 1.41 | 0.73–2.74 | |
| Contacts of only BI-negative patients | 883 | 3.6% | 0.95 | 0.54–1.66 | |
| Non-contacts | 1956 | 1.0 | |||
| P = 0.39 | |||||
| Contacts of MBd-patients | 615 | 5.7% | 1.32 | 0.73–2.38 | |
| Contacts of only PBd-patients | 613 | 2.9% | 0.85 | 0.46–1.58 | |
| Non-contacts | 1956 | 1.0 | P = 0.34 | ||
| Contactsaof patients grouped into different categories . | . | . | Adjustedb . | . | |
|---|---|---|---|---|---|
. | n . | % seropositive . | ORc . | 95% CI . | |
| Contacts of seropositive patients | 287 | 6.6% | 1.75 | 0.92–3.31 | |
| Contacts of only seronegative patients | 941 | 3.6% | 0.84 | 0.47–1.49 | |
| Non-contacts | 1956 | 1.0 | |||
| P = 0.05 | |||||
| Contacts of BId-positive patients | 345 | 6.1% | 1.41 | 0.73–2.74 | |
| Contacts of only BI-negative patients | 883 | 3.6% | 0.95 | 0.54–1.66 | |
| Non-contacts | 1956 | 1.0 | |||
| P = 0.39 | |||||
| Contacts of MBd-patients | 615 | 5.7% | 1.32 | 0.73–2.38 | |
| Contacts of only PBd-patients | 613 | 2.9% | 0.85 | 0.46–1.58 | |
| Non-contacts | 1956 | 1.0 | P = 0.34 | ||
Contacts = household, buffer 1, and buffer 2 contacts (0–50 m).
Adjusted for age, sex, and island.
Odds ratio.
BI = bacterial index; MB = multibacillary leprosy; PB = paucibacillary leprosy.
The results of a logistic regression for all the different contact buffers of seropositive patients revealed that those living in close proximity to one or more seropositive patients (≤75 m/buffer 3) were more likely to be seropositive compared with 'non-contacts' (>150 m from a seropositive patient). It appeared that this increased seroprevalence mainly counted for those contact groups living in the vicinity of two seropositive patients. People living 0–75 metres from one seropositive patient had an aOR of 1.24 (95% CI 0.48–3.23). Persons living 0–75 m from two seropositive patients had an aOR of 3.07 (95% CI 1.74–5.42) compared with non contacts (data not shown).
Performing the analysis on household level with seropositivity defined as at least one person in the house being seropositive did not change the results.
Discussion
The general serology results presented here are in line with findings of others: seroprevalence was higher among females, children, and young adults. The highest seroprevalence was found among MB patients, but it was much lower (32.5%) than findings in other studies where it varied between 75–100%.20–22 This is in all probability due to different classification criteria used. The seroprevalence among the general population on the islands (3.0%) is in accordance with other studies that found seroprevalences varying from 1.7% to 5.9%.8–10 The seroprevalence on the islands correlated strongly with the leprosy prevalence. This was also seen in the study of Van Beers et al.23 where the seroprevalence among school children correlated with the leprosy detection rate. We found the same weak, but positive correlation between BI and antibody levels as Roche et al.7
We detected five significant clusters of leprosy patients (especially PB2-5 and MB patients) which included 22% of all patients and 32% of the MB patients. Of the RFT patients, 27% were living in a cluster of leprosy patients. All detected clusters of leprosy patients were small in size, varying from one to five houses, indicating that close contact is important in the transmission of leprosy. Clustering can also be caused by a tendency of family members with high genetic susceptibility to live together24 or a common underlying factor like low socio-economic status in certain neighbourhoods.25 However, there are no specific neighbourhoods for rich or poor people in our study area.
We used seroprevalence in the community as a marker for prevalence of infection.26 Follow-up studies showed that seropositive contacts run an increased risk of developing leprosy, especially MB leprosy.8 It is well known that mainly MB patients produce antibodies against M. leprae. Based on this one can argue that seropositivity in contacts is a marker for incubating multibacillary infection and not infection with M. leprae in general. Since there is no 'gold standard' for measuring M. leprae infection, it is difficult to indicate sensitivity and specificity of the assay used. Speculations have been made in other studies about the possibility of cross-reactivity due to environmental mycobacteria, which would lower the specificity of the test,27 but this hypothesis has never been substantiated.
Several studies investigated the seroprevalence among contacts of leprosy patients, but with different and often contradicting outcomes which can be attributed to different endemicities, methodologies, and classification criteria. However, none of the studies took other characteristics of the patients, such as the serological status of the patient, into consideration.
Some studies in high endemic areas with widespread infection found an increased seroprevalence among household contacts compared with non-contacts,9,12 whereas other studies could not detect this increase.8,13,14
When making a distinction between household contacts of MB patients and of PB patients, several studies could not find an increased seroprevalence among MB contacts,9,10,21,27 whereas others did find an increased seroprevalence among household contacts of MB patients.26,28,29
Only one study made a comparison between household contacts of active lepromatous patients (BI+) and inactive lepromatous patients (BI−). It could not detect a significant difference in seroprevalence.26
In one study the seroprevalence among household contacts of MB patients plus their four nearest neighbours was significantly higher compared with non-contacts.11
In this study we related seropositivity among contacts with the type of index patient based on their classification, serological status and BI. Seropositivity among contacts was most closely related to the serological status of the index patient. Thus, the serological status of a patient seems to be a better indicator for the transmission potential than the BI. This supports earlier theories that antibodies to PGL-I may be a better reflection of total bacterial load in the body than the BI of a local skin smear.30,31 It also indicates that knowledge of the serological status of a patient is important in order to assess the transmission potential.
Based on earlier findings by Van Beers et al.4 we expanded the concept of contact from household contacts to people living in neighbouring houses. Social contacts (in mosques, schools or other meeting places) were not investigated. Since the definition of neighbours can be very subjective, especially when houses are not ordered in straight lines, we standardized this by using a buffer concept prepared in a GIS, consisting of circles with a fixed radius. We studied the significance of the distance between the houses of the contact and the seropositive patient(s) for the seropositivity of the contact.
We found that seroprevalence is higher among people living in close proximity to seropositive patients (≤75 m). It appeared that this increased seroprevalence mainly counted for those contacts living in the vicinity of two seropositive patients, maybe due to increased opportunities to acquire infection. There were five pairs of seropositive patients living on the islands; contact groups of only two of these five pairs showed this increased seroprevalence (data not shown). The fact that not all the pairs of seropositive patients were equally efficient at transmitting infection suggests that other factors apart from distance (such as social behaviour and/or duration of disease) may be important. In our study area we found that this increased seroprevalence seemed to be limited to a buffer of 0–75 m around two seropositive patients. However, this maximum distance of 75 m may depend on the average distance between houses and could be different for different epidemiological and/or sociological settings. Also, GPS, map preparation and the choice of radius of the buffers may influence the outcome and can cause imprecision.
Seropositive non-contacts could also be a contact of an undetected leprosy patient. Even though the coverage of our study was high (84%), among the 16% not screened one would still expect another 17 patients.
In conclusion, we showed that living in the vicinity of two seropositive patients increases the risk of harbouring antibodies to M. leprae. Thus it may be important for a more accurate estimation of transmission potential to measure the serological status of all patients with newly developed simple tools such as the ML-Flow test32 and to include a broader definition of contacts than only household contacts in the contact survey of each new patient. People at risk should be followed carefully or be the subject of intervention strategies such as prophylactic treatment to prevent new leprosy patients arising.
Leprosy prevalence correlated strongly with seroprevalence on the five islands in the study area.
Clusters of seropositives existed in this highly endemic area.
Serological status appeared to be the best indicator for transmission potential of patients.
This study is part of the project 'Development of strategies for pro-active case finding in leprosy control' financed by The Netherlands Leprosy Relief. We thank Dr HM Noor, Head of the Health District Pangkep for permission and active support. We thank H Hasan Basri and H Baso Datu of the P2M Province, H Azis Nur of the P2M district Pangkep, I Nengah Sudja of the P2M District Maros, Hj Asriah of the laboratory of Maros, Mr Saeni of the laboratory of Pangkep, and Mr Syafri of the Hasanuddin University (UNHAS) who were involved in the supervision of the clinical and/or laboratory examinations.
We are grateful to Hj Rabiah, Head of the Health Centre Liukang Tangaya Pangkep for her enthusiastic support before and during the fieldwork. Furthermore we want to thank Amril, Arief, Sufrianti, and Syahuni of the local health centre and Mr Mus Jebaru of UNHAS for their participation in the fieldwork. We thank Mr Romi Usman and Mr Marwani of UNHAS and Lara Siebeling and Sanne Wouterse (medical students of AMC/University of Amsterdam) for their participation in the fieldwork and testing the serum samples. We thank all the inhabitants of the five islands for their co-operation.
References
World Health Organization. The Final Push Towards Elimination of Leprosy, Strategic Plan 2000–2005. Geneva: World Health Organization,
Van Beers SM, Hatta M, Klatser PR. Patient contact is the major determinant in incident leprosy: implication for future control.
Anonymous. Report of the International Leprosy Association Technical Forum. Paris, France, 22–28 February 2002.
Fine PEM, Sterne JAC, Pönnighaus JM et al. Household and dwelling contact as risk factors for leprosy in Northern Malawi.
Roche PW, Britton WJ, Failbus SS, Williams D, Pradhan HM, Theuvenet WJ. Operational value of serological measurements in multibacillary leprosy patients: clinical and bacteriological correlates on antibody responses.
Cunanan A, Chan GP, Douglas JT. Risk of development of leprosy among culion contacts.
Cellona RV, Walsh GP, Fajardo TT et al. Cross-sectional assessment of ELISA reactivitity in leprosy patients, contacts and normal population using the semisynthetic antigen natural disaccharide octyl bovine serum albumin (ND-O-BSA) in Cebu, The Philippines.
Gonzalez-Abreu E, Mora N, Perez M, Pereira M, Perez J, Gonzalez AB. Serodiagnosis of leprosy in patients' contacts by enzyme-linked immunosorbent assay.
Hatta M, van Beers SM, Madjid B, Djumadi A, de Wit MYL, Klatser PR. Distribution and persistence of Mycobacterium leprae nasal carriage among a population in which leprosy is endemic in Indonesia.
Chanteau S, Cartel JL, Roux J, Plichart R, Bach MA. Comparison of synthetic antigens for detecting antibodies to phenolic glycolipid I in patients with leprosy and their household contacts.
Van Beers SM, Izumi S, Madjid B, Maeda Y, Day R, Klatser PR. An epidemiological study of leprosy infection by serology and polymerase chain reaction.
Bagshawe AF, Garsia RJ, Baumgart K, Astbury L. IgM serum antibodies to phenolic glycolipid-I and clinical leprosy: two years' observation in a community with hyperendemic leprosy.
Clarke KC, McLafferty SL, Tempalski BJ. On epidemiology and geographic information systems: a review and discussion of future directions.
Bakker MI, Hatta M, Kwenang A, Klatser PR, Oskam L. Epidemiology of leprosy of five isolated islands in the Flores Sea, Indonesia.
World Health Organization. WHO Expert Committee on leprosy.
Bührer-Sekula S, Sarno EN, Oskam L et al. Use of ML dipstick as a tool to classify leprosy patients.
Kulldorff M, Nagarwalla N. Spatial disease clusters: detection and inference.
Bührer SS, Smits HL, Gussenhoven GC, van Ingen CW, Klatser PR. A simple dipstick assay for the detection of antibodies to phenolic glycolipid-I of
Hussain R, Jamil S, Kifayet A et al. Quantitation of IgM antibodies to the M. leprae synthetic dissacharide can predict early bacterial multiplication in leprosy.
Saad MHF, Medeiros MA, Gallo MEN, Gontijo PP, Fonseca LS. IgM immunoglobulins reacting with the phenolic glycolipid-1 antigen from mycobacterium leprae in sera of leprosy patients and their contacts.
Van Beers S, Hatta M and Klatser PR. Seroprevalence rates of antibodies to phenolic glycolipid-I among school children as an indicator of leprosy endemicity.
Ponnighaus JM, Fine PEM, Sterne JAC, Malema SS, Bliss L, Wilson RJ. Extended schooling and good housing conditions are associated with reduced risk of leprosy in rural Malawi.
Menzel S, Harboe M, Bergsvik H, Brennan PJ. Antibodies to a synthetic analog of phenolic glycolipid-I of Mycobacterium leprae in healthy household contacts of patients with leprosy.
Fine PEM, Ponnighaus JM, Burgess P, Clarkson JA, Draper CC. Seroepidemiological studies of leprosy in Northern Malawi based on an Enzyme-linked Immunosorbent Assay using synthetic glycoconjugate antigen.
Soebono H, Klatser PR. A seroepidemiological study of leprosy in high- and low-endemic Indonesian villages.
Agis F, Schlich P, Cartel JL, Guidi C, Bach MA. Use of anti-M leprae phenolic glycolipid-I antibody detection for early diagnosis and prognosis of leprosy.
Douglas JT, Hirsch DS, Fajardo TT et al. Evaluation of Mycobacterium leprae antigens in the serological monitoring of a clofazimine-based chemotherapy study of dapsone resistant lepromatous leprosy patients in Cebu, Philippines.
Klatser PR, de Wit MYL, Fajardo TT et al. Evaluation of Mycobacterium leprae antigens in the monitoring of a dapsone-based chemotherapy of previously untreated lepromatous patients in Cebu, Philippines.