-
PDF
- Split View
-
Views
-
Cite
Cite
Alexander K Rowe, Samantha Y Rowe, Robert W Snow, Eline L Korenromp, Joanna RM Armstrong Schellenberg, Claudia Stein, Bernard L Nahlen, Jennifer Bryce, Robert E Black, Richard W Steketee, The burden of malaria mortality among African children in the year 2000, International Journal of Epidemiology, Volume 35, Issue 3, June 2006, Pages 691–704, https://doi.org/10.1093/ije/dyl027
Close - Share Icon Share
Abstract
Background Although malaria is a leading cause of child deaths, few well-documented estimates of its direct and indirect burden exist. Our objective was to estimate the number of deaths directly attributable to malaria among children <5 years old in sub-Saharan Africa for the year 2000.
Methods We divided the population into six sub-populations and, using results of studies identified in a literature review, estimated a malaria mortality rate for each sub-population. Malaria deaths were estimated by multiplying each sub-population by its corresponding rate. Sensitivity analyses were performed to assess the impact of varying key assumptions.
Results The literature review identified 31 studies from 14 countries in middle Africa and 17 studies and reports from four countries in southern Africa. In 2000, we estimated that ∼100 million children lived in areas where malaria transmission occurs and that 803 620 (precision estimate: 705 821–901 418) children died from the direct effects of malaria. For all of sub-Saharan Africa, including populations not exposed to malaria, malaria accounted for 18.0% (precision estimate: 15.8–20.2%) of child deaths. These estimates were sensitive to extreme assumptions about the causes of deaths with no known cause.
Conclusions These estimates, based on the best available data and methods, clearly demonstrate malaria's enormous mortality burden. We emphasize that these estimates are an approximation with many limitations and that the estimates do not account for malaria's large indirect burden. We describe information needs that, if filled, might improve the validity of future estimates.
Introduction
Malaria is a leading cause of mortality among children in sub-Saharan Africa. Valid quantitative estimates of malaria mortality are useful for monitoring the impact of prevention and control activities, targeting interventions, and advocacy. Unfortunately, vital registration systems in most countries in sub-Saharan Africa have low coverage and do not produce dependable estimates.1–3
To fill this gap, a variety of estimates have been made, but most are simplistic or lacked documentation of the methods and data.2,4–7 (For details, see the complete report, which is available on the Internet at: http://rbm.who.int/partnership/wg/wg_monitoring/docs/CHERG_final_report.pdf) One approach,8 which the World Health Organization has used to produce annual estimates, identified populations at risk for malaria with a model that predicts where the climate is suitable for Plasmodium falciparum transmission.9 The median malaria mortality rate, from studies identified in a literature review, was applied to these malaria-risk populations to produce an estimate of ∼766 000 deaths among children <5 years old in sub-Saharan Africa for 1995. This model was recently revised to account for variations in malaria transmission intensity10 and urbanization,11 resulting in estimates of 742 000 and 680 000 child malaria deaths, respectively, for the year 2000. Although these latter models were superior to previous ones, not all contemporary data were used, and use of the median to estimate the malaria mortality rate assumed all studies had equal precision and did not permit an investigation of factors that might have led to a prediction model that could better account for inter-country differences and time trends.
Our objective was to estimate the number of ‘direct’ malaria deaths for children <5 years old in sub-Saharan Africa for the year 2000 by refining the models by Snow et al.8,10,11 and using all available contemporary mortality data. By direct malaria death, we mean that malaria was the underlying cause (i.e. ‘the disease or injury which initiated the train of morbid events leading directly to death’12). Our analysis does not attempt to quantify ‘indirect’ malaria deaths, in which malaria was a contributing cause (e.g. a child with malaria-associated anaemia dies after developing pneumonia, but neither the anaemia nor pneumonia would have been fatal in isolation); although we acknowledge that the burden of indirect malaria mortality is probably substantial.13 Nor does our analysis quantify ‘consequential’ malaria deaths, in which consequences of clinical management (e.g. exposure to HIV during a blood transfusion) or malaria sequelae (e.g. epilepsy after cerebral malaria) lead to death.10 The analysis was limited to Africa because this is where most malaria deaths (perhaps 80–90%) are thought to occur.2,14,15 Additionally, we sought to identify research areas or information that might improve future estimates.
Methods
Overview
First, we conducted a literature review to identify studies of childhood malaria mortality. Second, for each country, we estimated sub-populations of children with different risks of dying from malaria for the year 2000. Third, we estimated a malaria mortality rate for each sub-population using studies from the literature review. To estimate rates, we developed a prediction model with covariates that were available from studies used in the prediction model and from countries to which the predicted rates would be applied. As there were almost no mortality studies with results for the year 2000, we used studies from the past two decades, included a covariate for study year in the prediction model, and estimated rates by giving the study year covariate a value to indicate the year 2000. Fourth, for each malaria risk sub-population in each country, we estimated malaria deaths by multiplying the sub-population by its corresponding rate. Finally, we summed malaria deaths for all sub-populations from all countries.
Literature review
To identify studies of the causes of child deaths in Africa where malaria transmission occurs, we reviewed references used by Snow et al.,8 conducted an independent search of the PubMed database, consulted experts, and asked researchers for unpublished studies. For the PubMed search, we searched for all ‘malaria/mortality’ Medical Subject Headings, and within these references, searched for articles on infants (birth to 23 months) and preschool children (2–5 years). The inclusion criteria were that studies: (i) were community-based, (ii) had results that permitted a malaria mortality rate to be estimated, (iii) had a duration of a multiple of 12 months (malaria is highly seasonal), (iv) included children exactly 0–59 months old (malaria mortality is unevenly distributed in this age range), (v) had a proportion of deaths with no known cause <30%, (vi) began in 1980 or later, and (vii) did not overlap with another study in the analysis in time and place. All studies used verbal autopsies (interviews with the deceased child's relatives are interpreted by clinicians or an algorithm) to determine causes of death,16,17 and we note that International Classification of Diseases12 rules might not have been used to determine causes of death. For studies that were intervention trials, only results from control groups were included. Some studies met nearly all inclusion criteria but had a minor limitation (e.g. duration = 11 months). Therefore, we created two categories: Group A (studies meeting all inclusion criteria) and Group B (studies with minor limitations) (Table 1). For southern Africa, we included 16 estimates of the malaria mortality rate from disease surveillance reports, plus one community-based study of children 0–4 years (Table 2). To examine time trends, for multi-year studies, results were abstracted for individual years whenever possible; and, with one exception, results for all years were included. The exception was a 9-year study from Morogoro, Tanzania,18,19 which had some data completeness concerns;20,21 for this study, data for one recent year (2001) that were of good quality were included.
Studies from countries in middle Africa that were included in the analysis
| Country and site . | Time period . | Deaths attributed to malaria . | Deaths with no known cause . | Total deaths (all causes combined) . | Person-time (person-years) . | Malaria mortality ratea (per 1000 per year) . | Parasite prevalence (%) . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group A studies from rural settings with high-intensity malaria transmission (n = 46 estimates from 15 studies) | ||||||||||||||
| Burundi (Nyanza-Lac District)75 | 1990–91 | 55 | 4 | 160 | 3815 | 14.8 | 75.075 | |||||||
| Democratic Republic of Congo (Kivu)76 | 1986–87 | 44 | 0 | 358 | 5187 | 8.5 | 34.477 | |||||||
| Gambia (Farafenni, South Bank)72 | 1988–90 | 75 | 0 | 296 | 5393 | 13.9 | 60.978 | |||||||
| Gambia (Farafenni, North Bank)79 | 1982–83 | 24 | 0 | 171 | 2505 | 9.6 | 32.479 | |||||||
| Gambia (Upper River Division)80 | 1989–93 | 891 | 953 | 3776 | 113 334 | 10.5 | 71.281 | |||||||
| Ghana (Central Region)82 | 1987 | 6 | 0 | 15 | 832 | 7.2 | 30.583 | |||||||
| Guinea Bissau (Bandim)84 | 1987–90 | 22 | 22 | 153 | 2753 | 9.3 | 32.685 | |||||||
| Kenya (Saradidi)86 | 1981–83 | 15 | 0 | 348 | 8107 | 1.9 | 83.587 | |||||||
| Kenya (Asembo Bay)88 | 1997–98 | 96 | 43 | 359 | 4537 | 24.0 | 83.089 | |||||||
| Senegal (Bandafassi)55 | 1984–95 | 99 | 0 | 1167 | 16 973 | 5.8 | 95.055 | |||||||
| Senegal (Niakhar)55 | 1984–95 | 495 | 0 | 3242 | 58 985 | 8.4 | 50.055 | |||||||
| Senegal (Mlomp)55 | 1992–95 | 19 | 0 | 86 | 3145 | 6.0 | 46.090 51.091 b | |||||||
| Tanzania (Bagamoyo District)92 | 1983–84 | 67 | 0 | 325 | 8098 | 8.3 | 83.693 | |||||||
| Tanzania (Tanga)94 | 1992–93 | 30 | 7 | 90 | 2051 | 15.9 | 69.595 | |||||||
| Tanzania (Morogoro)18,19 | 2001 | 241 | 2 | 430 | 15 711 | 15.4 | 51.696 | |||||||
| Group B studies from rural settings with high-intensity malaria transmission (n = 11 estimates from 10 studies) | ||||||||||||||
| Burkina Faso (Kongodjan area)97 | 1982–86 | 2 | 11 | 43 | 1186 | 2.3 | 53.597 | |||||||
| Gambia (5 areas along Gambia River)98 | 1992–93 | 84 | 218 | 424 | 13 469 | 12.8 | 38.798 | |||||||
| Ghana (Kassena-Nankana District)99 | 1989–91 | 116 | 161 | 685 | 10 140 | 15.0 | 87.1100 | |||||||
| Ghana (Kassena-Nankana District)101 | 1993–95 | 189 | 84 | 618 | 15 895 | 13.8 | 64.0102 | |||||||
| Guinea (Mandiana)40 | 1998–99 | 104 | 30 | 353 | 16 202 | 7.0 | NAc | |||||||
| Kenya (Kilifi North)103,104 | 1991–93 | 155 | 0 | 455 | 20 679 | 7.5 | 49.0105 | |||||||
| Nigeria (Akoko North)41 | 1987 | 23 | 0 | 120 | 1929 | 11.9 | NAc | |||||||
| Sierra Leone (Bo)106 | 1990 | 9 | 2 | 35 | 776 | 12.3 | 61.0107 | |||||||
| Sierra Leone (Western Area and Porto Loko)42 | 1988–93 | 80 | 0 | 251 | 8468 | 9.4 | NAc | |||||||
| Tanzania (Yombo Division, Bagamoyo District)108 | 1992–94 | 54 | 74 | 192 | 5850 | 15.0 | 82.0109 | |||||||
| Group A studies from rural settings with low-intensity malaria transmission (n = 19 estimates from 5 studies) | ||||||||||||||
| Kenya (Brookebond Tea Estate)54 | 1997–98 | 58 | 0 | 325 | 30 623 | 1.9 | 11.7110 | |||||||
| Senegal (Mlomp)55 | 1985–91 | 4 | 0 | 108 | 5527 | 0.7 | 3.0,111 24.5b | |||||||
| Sudan (refugee camps in eastern Sudan)56 | 1997 | 27 | 0 | 47 | 4269 | 6.3 | 0.856 | |||||||
| Tanzania (Hai District)18,19 | 1993–2001 | 430 | 73 | 2449 | 191 980 | 2.3 | 4.0112 | |||||||
| Uganda (Mbarara District)57 | 1988–89 | 14 | 0 | 104 | 4320 | 3.2 | 18.3113 | |||||||
| Group B study from a rural setting with low-intensity malaria transmission (n = 1 estimate from 1 study) | ||||||||||||||
| Kenya (Muranga District)114 | 1985–88 | 13 | 16 | 232 | 37 000 | 0.4 | 24.0115 | |||||||
| Group A study from an urban setting (n = 9 estimates from 1 study) | ||||||||||||||
| Tanzania (Dar es Salaam)18,19 | 1993–2001 | 498 | 68 | 1809 | 88 528 | 5.8 | 27.8116 | |||||||
| Country and site . | Time period . | Deaths attributed to malaria . | Deaths with no known cause . | Total deaths (all causes combined) . | Person-time (person-years) . | Malaria mortality ratea (per 1000 per year) . | Parasite prevalence (%) . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group A studies from rural settings with high-intensity malaria transmission (n = 46 estimates from 15 studies) | ||||||||||||||
| Burundi (Nyanza-Lac District)75 | 1990–91 | 55 | 4 | 160 | 3815 | 14.8 | 75.075 | |||||||
| Democratic Republic of Congo (Kivu)76 | 1986–87 | 44 | 0 | 358 | 5187 | 8.5 | 34.477 | |||||||
| Gambia (Farafenni, South Bank)72 | 1988–90 | 75 | 0 | 296 | 5393 | 13.9 | 60.978 | |||||||
| Gambia (Farafenni, North Bank)79 | 1982–83 | 24 | 0 | 171 | 2505 | 9.6 | 32.479 | |||||||
| Gambia (Upper River Division)80 | 1989–93 | 891 | 953 | 3776 | 113 334 | 10.5 | 71.281 | |||||||
| Ghana (Central Region)82 | 1987 | 6 | 0 | 15 | 832 | 7.2 | 30.583 | |||||||
| Guinea Bissau (Bandim)84 | 1987–90 | 22 | 22 | 153 | 2753 | 9.3 | 32.685 | |||||||
| Kenya (Saradidi)86 | 1981–83 | 15 | 0 | 348 | 8107 | 1.9 | 83.587 | |||||||
| Kenya (Asembo Bay)88 | 1997–98 | 96 | 43 | 359 | 4537 | 24.0 | 83.089 | |||||||
| Senegal (Bandafassi)55 | 1984–95 | 99 | 0 | 1167 | 16 973 | 5.8 | 95.055 | |||||||
| Senegal (Niakhar)55 | 1984–95 | 495 | 0 | 3242 | 58 985 | 8.4 | 50.055 | |||||||
| Senegal (Mlomp)55 | 1992–95 | 19 | 0 | 86 | 3145 | 6.0 | 46.090 51.091 b | |||||||
| Tanzania (Bagamoyo District)92 | 1983–84 | 67 | 0 | 325 | 8098 | 8.3 | 83.693 | |||||||
| Tanzania (Tanga)94 | 1992–93 | 30 | 7 | 90 | 2051 | 15.9 | 69.595 | |||||||
| Tanzania (Morogoro)18,19 | 2001 | 241 | 2 | 430 | 15 711 | 15.4 | 51.696 | |||||||
| Group B studies from rural settings with high-intensity malaria transmission (n = 11 estimates from 10 studies) | ||||||||||||||
| Burkina Faso (Kongodjan area)97 | 1982–86 | 2 | 11 | 43 | 1186 | 2.3 | 53.597 | |||||||
| Gambia (5 areas along Gambia River)98 | 1992–93 | 84 | 218 | 424 | 13 469 | 12.8 | 38.798 | |||||||
| Ghana (Kassena-Nankana District)99 | 1989–91 | 116 | 161 | 685 | 10 140 | 15.0 | 87.1100 | |||||||
| Ghana (Kassena-Nankana District)101 | 1993–95 | 189 | 84 | 618 | 15 895 | 13.8 | 64.0102 | |||||||
| Guinea (Mandiana)40 | 1998–99 | 104 | 30 | 353 | 16 202 | 7.0 | NAc | |||||||
| Kenya (Kilifi North)103,104 | 1991–93 | 155 | 0 | 455 | 20 679 | 7.5 | 49.0105 | |||||||
| Nigeria (Akoko North)41 | 1987 | 23 | 0 | 120 | 1929 | 11.9 | NAc | |||||||
| Sierra Leone (Bo)106 | 1990 | 9 | 2 | 35 | 776 | 12.3 | 61.0107 | |||||||
| Sierra Leone (Western Area and Porto Loko)42 | 1988–93 | 80 | 0 | 251 | 8468 | 9.4 | NAc | |||||||
| Tanzania (Yombo Division, Bagamoyo District)108 | 1992–94 | 54 | 74 | 192 | 5850 | 15.0 | 82.0109 | |||||||
| Group A studies from rural settings with low-intensity malaria transmission (n = 19 estimates from 5 studies) | ||||||||||||||
| Kenya (Brookebond Tea Estate)54 | 1997–98 | 58 | 0 | 325 | 30 623 | 1.9 | 11.7110 | |||||||
| Senegal (Mlomp)55 | 1985–91 | 4 | 0 | 108 | 5527 | 0.7 | 3.0,111 24.5b | |||||||
| Sudan (refugee camps in eastern Sudan)56 | 1997 | 27 | 0 | 47 | 4269 | 6.3 | 0.856 | |||||||
| Tanzania (Hai District)18,19 | 1993–2001 | 430 | 73 | 2449 | 191 980 | 2.3 | 4.0112 | |||||||
| Uganda (Mbarara District)57 | 1988–89 | 14 | 0 | 104 | 4320 | 3.2 | 18.3113 | |||||||
| Group B study from a rural setting with low-intensity malaria transmission (n = 1 estimate from 1 study) | ||||||||||||||
| Kenya (Muranga District)114 | 1985–88 | 13 | 16 | 232 | 37 000 | 0.4 | 24.0115 | |||||||
| Group A study from an urban setting (n = 9 estimates from 1 study) | ||||||||||||||
| Tanzania (Dar es Salaam)18,19 | 1993–2001 | 498 | 68 | 1809 | 88 528 | 5.8 | 27.8116 | |||||||
a Malaria mortality rate = (Malaria deaths/{person-time × [1 − (deaths with no known cause/total deaths)]}) × 1000, where malaria deaths are shown in column 3 of the table, person-time is in column 6, deaths with no known cause are in column 4, and total deaths are in column 5. Note that the results in this column are the only results that do not come directly from the individual malaria studies (i.e. results in all other columns in the table come directly from the individual study reports).
b Over the long course of this study, four values for parasite prevalence were used: 3.0% (for 1985–89), 24.5% (for 1990–91), 46.0% (for 1992–93), and 51.0% (for 1994–95). For 1990–91, no value was available; so the values for 1985–89 (3.0%) and 1992–93 (46.0%) were averaged [24.5% = (3.0% + 46.0%)/2].
c NA = Not available. Study population was assumed to have a parasite prevalence in the ‘≥25%’ category based on a prediction model [see Methods and Ref. (43) for details].
Studies from countries in middle Africa that were included in the analysis
| Country and site . | Time period . | Deaths attributed to malaria . | Deaths with no known cause . | Total deaths (all causes combined) . | Person-time (person-years) . | Malaria mortality ratea (per 1000 per year) . | Parasite prevalence (%) . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group A studies from rural settings with high-intensity malaria transmission (n = 46 estimates from 15 studies) | ||||||||||||||
| Burundi (Nyanza-Lac District)75 | 1990–91 | 55 | 4 | 160 | 3815 | 14.8 | 75.075 | |||||||
| Democratic Republic of Congo (Kivu)76 | 1986–87 | 44 | 0 | 358 | 5187 | 8.5 | 34.477 | |||||||
| Gambia (Farafenni, South Bank)72 | 1988–90 | 75 | 0 | 296 | 5393 | 13.9 | 60.978 | |||||||
| Gambia (Farafenni, North Bank)79 | 1982–83 | 24 | 0 | 171 | 2505 | 9.6 | 32.479 | |||||||
| Gambia (Upper River Division)80 | 1989–93 | 891 | 953 | 3776 | 113 334 | 10.5 | 71.281 | |||||||
| Ghana (Central Region)82 | 1987 | 6 | 0 | 15 | 832 | 7.2 | 30.583 | |||||||
| Guinea Bissau (Bandim)84 | 1987–90 | 22 | 22 | 153 | 2753 | 9.3 | 32.685 | |||||||
| Kenya (Saradidi)86 | 1981–83 | 15 | 0 | 348 | 8107 | 1.9 | 83.587 | |||||||
| Kenya (Asembo Bay)88 | 1997–98 | 96 | 43 | 359 | 4537 | 24.0 | 83.089 | |||||||
| Senegal (Bandafassi)55 | 1984–95 | 99 | 0 | 1167 | 16 973 | 5.8 | 95.055 | |||||||
| Senegal (Niakhar)55 | 1984–95 | 495 | 0 | 3242 | 58 985 | 8.4 | 50.055 | |||||||
| Senegal (Mlomp)55 | 1992–95 | 19 | 0 | 86 | 3145 | 6.0 | 46.090 51.091 b | |||||||
| Tanzania (Bagamoyo District)92 | 1983–84 | 67 | 0 | 325 | 8098 | 8.3 | 83.693 | |||||||
| Tanzania (Tanga)94 | 1992–93 | 30 | 7 | 90 | 2051 | 15.9 | 69.595 | |||||||
| Tanzania (Morogoro)18,19 | 2001 | 241 | 2 | 430 | 15 711 | 15.4 | 51.696 | |||||||
| Group B studies from rural settings with high-intensity malaria transmission (n = 11 estimates from 10 studies) | ||||||||||||||
| Burkina Faso (Kongodjan area)97 | 1982–86 | 2 | 11 | 43 | 1186 | 2.3 | 53.597 | |||||||
| Gambia (5 areas along Gambia River)98 | 1992–93 | 84 | 218 | 424 | 13 469 | 12.8 | 38.798 | |||||||
| Ghana (Kassena-Nankana District)99 | 1989–91 | 116 | 161 | 685 | 10 140 | 15.0 | 87.1100 | |||||||
| Ghana (Kassena-Nankana District)101 | 1993–95 | 189 | 84 | 618 | 15 895 | 13.8 | 64.0102 | |||||||
| Guinea (Mandiana)40 | 1998–99 | 104 | 30 | 353 | 16 202 | 7.0 | NAc | |||||||
| Kenya (Kilifi North)103,104 | 1991–93 | 155 | 0 | 455 | 20 679 | 7.5 | 49.0105 | |||||||
| Nigeria (Akoko North)41 | 1987 | 23 | 0 | 120 | 1929 | 11.9 | NAc | |||||||
| Sierra Leone (Bo)106 | 1990 | 9 | 2 | 35 | 776 | 12.3 | 61.0107 | |||||||
| Sierra Leone (Western Area and Porto Loko)42 | 1988–93 | 80 | 0 | 251 | 8468 | 9.4 | NAc | |||||||
| Tanzania (Yombo Division, Bagamoyo District)108 | 1992–94 | 54 | 74 | 192 | 5850 | 15.0 | 82.0109 | |||||||
| Group A studies from rural settings with low-intensity malaria transmission (n = 19 estimates from 5 studies) | ||||||||||||||
| Kenya (Brookebond Tea Estate)54 | 1997–98 | 58 | 0 | 325 | 30 623 | 1.9 | 11.7110 | |||||||
| Senegal (Mlomp)55 | 1985–91 | 4 | 0 | 108 | 5527 | 0.7 | 3.0,111 24.5b | |||||||
| Sudan (refugee camps in eastern Sudan)56 | 1997 | 27 | 0 | 47 | 4269 | 6.3 | 0.856 | |||||||
| Tanzania (Hai District)18,19 | 1993–2001 | 430 | 73 | 2449 | 191 980 | 2.3 | 4.0112 | |||||||
| Uganda (Mbarara District)57 | 1988–89 | 14 | 0 | 104 | 4320 | 3.2 | 18.3113 | |||||||
| Group B study from a rural setting with low-intensity malaria transmission (n = 1 estimate from 1 study) | ||||||||||||||
| Kenya (Muranga District)114 | 1985–88 | 13 | 16 | 232 | 37 000 | 0.4 | 24.0115 | |||||||
| Group A study from an urban setting (n = 9 estimates from 1 study) | ||||||||||||||
| Tanzania (Dar es Salaam)18,19 | 1993–2001 | 498 | 68 | 1809 | 88 528 | 5.8 | 27.8116 | |||||||
| Country and site . | Time period . | Deaths attributed to malaria . | Deaths with no known cause . | Total deaths (all causes combined) . | Person-time (person-years) . | Malaria mortality ratea (per 1000 per year) . | Parasite prevalence (%) . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group A studies from rural settings with high-intensity malaria transmission (n = 46 estimates from 15 studies) | ||||||||||||||
| Burundi (Nyanza-Lac District)75 | 1990–91 | 55 | 4 | 160 | 3815 | 14.8 | 75.075 | |||||||
| Democratic Republic of Congo (Kivu)76 | 1986–87 | 44 | 0 | 358 | 5187 | 8.5 | 34.477 | |||||||
| Gambia (Farafenni, South Bank)72 | 1988–90 | 75 | 0 | 296 | 5393 | 13.9 | 60.978 | |||||||
| Gambia (Farafenni, North Bank)79 | 1982–83 | 24 | 0 | 171 | 2505 | 9.6 | 32.479 | |||||||
| Gambia (Upper River Division)80 | 1989–93 | 891 | 953 | 3776 | 113 334 | 10.5 | 71.281 | |||||||
| Ghana (Central Region)82 | 1987 | 6 | 0 | 15 | 832 | 7.2 | 30.583 | |||||||
| Guinea Bissau (Bandim)84 | 1987–90 | 22 | 22 | 153 | 2753 | 9.3 | 32.685 | |||||||
| Kenya (Saradidi)86 | 1981–83 | 15 | 0 | 348 | 8107 | 1.9 | 83.587 | |||||||
| Kenya (Asembo Bay)88 | 1997–98 | 96 | 43 | 359 | 4537 | 24.0 | 83.089 | |||||||
| Senegal (Bandafassi)55 | 1984–95 | 99 | 0 | 1167 | 16 973 | 5.8 | 95.055 | |||||||
| Senegal (Niakhar)55 | 1984–95 | 495 | 0 | 3242 | 58 985 | 8.4 | 50.055 | |||||||
| Senegal (Mlomp)55 | 1992–95 | 19 | 0 | 86 | 3145 | 6.0 | 46.090 51.091 b | |||||||
| Tanzania (Bagamoyo District)92 | 1983–84 | 67 | 0 | 325 | 8098 | 8.3 | 83.693 | |||||||
| Tanzania (Tanga)94 | 1992–93 | 30 | 7 | 90 | 2051 | 15.9 | 69.595 | |||||||
| Tanzania (Morogoro)18,19 | 2001 | 241 | 2 | 430 | 15 711 | 15.4 | 51.696 | |||||||
| Group B studies from rural settings with high-intensity malaria transmission (n = 11 estimates from 10 studies) | ||||||||||||||
| Burkina Faso (Kongodjan area)97 | 1982–86 | 2 | 11 | 43 | 1186 | 2.3 | 53.597 | |||||||
| Gambia (5 areas along Gambia River)98 | 1992–93 | 84 | 218 | 424 | 13 469 | 12.8 | 38.798 | |||||||
| Ghana (Kassena-Nankana District)99 | 1989–91 | 116 | 161 | 685 | 10 140 | 15.0 | 87.1100 | |||||||
| Ghana (Kassena-Nankana District)101 | 1993–95 | 189 | 84 | 618 | 15 895 | 13.8 | 64.0102 | |||||||
| Guinea (Mandiana)40 | 1998–99 | 104 | 30 | 353 | 16 202 | 7.0 | NAc | |||||||
| Kenya (Kilifi North)103,104 | 1991–93 | 155 | 0 | 455 | 20 679 | 7.5 | 49.0105 | |||||||
| Nigeria (Akoko North)41 | 1987 | 23 | 0 | 120 | 1929 | 11.9 | NAc | |||||||
| Sierra Leone (Bo)106 | 1990 | 9 | 2 | 35 | 776 | 12.3 | 61.0107 | |||||||
| Sierra Leone (Western Area and Porto Loko)42 | 1988–93 | 80 | 0 | 251 | 8468 | 9.4 | NAc | |||||||
| Tanzania (Yombo Division, Bagamoyo District)108 | 1992–94 | 54 | 74 | 192 | 5850 | 15.0 | 82.0109 | |||||||
| Group A studies from rural settings with low-intensity malaria transmission (n = 19 estimates from 5 studies) | ||||||||||||||
| Kenya (Brookebond Tea Estate)54 | 1997–98 | 58 | 0 | 325 | 30 623 | 1.9 | 11.7110 | |||||||
| Senegal (Mlomp)55 | 1985–91 | 4 | 0 | 108 | 5527 | 0.7 | 3.0,111 24.5b | |||||||
| Sudan (refugee camps in eastern Sudan)56 | 1997 | 27 | 0 | 47 | 4269 | 6.3 | 0.856 | |||||||
| Tanzania (Hai District)18,19 | 1993–2001 | 430 | 73 | 2449 | 191 980 | 2.3 | 4.0112 | |||||||
| Uganda (Mbarara District)57 | 1988–89 | 14 | 0 | 104 | 4320 | 3.2 | 18.3113 | |||||||
| Group B study from a rural setting with low-intensity malaria transmission (n = 1 estimate from 1 study) | ||||||||||||||
| Kenya (Muranga District)114 | 1985–88 | 13 | 16 | 232 | 37 000 | 0.4 | 24.0115 | |||||||
| Group A study from an urban setting (n = 9 estimates from 1 study) | ||||||||||||||
| Tanzania (Dar es Salaam)18,19 | 1993–2001 | 498 | 68 | 1809 | 88 528 | 5.8 | 27.8116 | |||||||
a Malaria mortality rate = (Malaria deaths/{person-time × [1 − (deaths with no known cause/total deaths)]}) × 1000, where malaria deaths are shown in column 3 of the table, person-time is in column 6, deaths with no known cause are in column 4, and total deaths are in column 5. Note that the results in this column are the only results that do not come directly from the individual malaria studies (i.e. results in all other columns in the table come directly from the individual study reports).
b Over the long course of this study, four values for parasite prevalence were used: 3.0% (for 1985–89), 24.5% (for 1990–91), 46.0% (for 1992–93), and 51.0% (for 1994–95). For 1990–91, no value was available; so the values for 1985–89 (3.0%) and 1992–93 (46.0%) were averaged [24.5% = (3.0% + 46.0%)/2].
c NA = Not available. Study population was assumed to have a parasite prevalence in the ‘≥25%’ category based on a prediction model [see Methods and Ref. (43) for details].
Studies and surveillance reports included in the analysis that were from areas in southern Africa categorized as having high-intensity malaria transmission
| Country and sitea . | Time period . | Age group . | Malaria deaths . | Person-time (person-years) . | Malaria mortality rates (deaths per 1000 per year) . |
|---|---|---|---|---|---|
| Hlabisa, South Africa | 1987–90 | All ages | 9 | 150 218 | 0.0599 |
| Ubombo, South Africa | 1987–90 | All ages | 17 | 164 274 | 0.1035 |
| Ingwavuma, South Africa | 1987–90 | All ages | 25 | 211 161 | 0.1184 |
| 7 malarious districts, Botswana | 1988–94 | All ages | 405 | 2 496 172 | 0.1622 |
| Southern District, Namibia | 1992–96 | All ages | 79 | 1 896 789 | 0.0416 |
| Central District, Namibia | 1992–96 | All ages | 115 | 1 214 125 | 0.0947 |
| North Eastern District, Namibia | 1992–96 | All ages | 526 | 1 133 340 | 0.4641 |
| North Western District, Namibia | 1992–96 | All ages | 690 | 3 462 498 | 0.1993 |
| Manicaland Province, Zimbabwe | 1997 | All ages | 409 | 1 819 338 | 0.2248 |
| Mashona Central Province, Zimbabwe | 1997 | All ages | 153 | 1 021 976 | 0.1497 |
| Mashona East Province, Zimbabwe | 1997 | All ages | 274 | 1 208 733 | 0.2267 |
| Mashona West Province, Zimbabwe | 1997 | All ages | 108 | 1 314 909 | 0.0821 |
| Matabele North Province, Zimbabwe | 1997 | All ages | 251 | 759 032 | 0.3307 |
| Matabele South Province, Zimbabwe | 1997 | All ages | 32 | 694 907 | 0.0460 |
| Midlands Province, Zimbabwe | 1997 | All ages | 70 | 1 552 593 | 0.0451 |
| Masvingo Province, Zimbabwe | 1997 | All ages | 196 | 1 453 557 | 0.1348 |
| Agincourt, South Africa | 1992–95 | 0–4 years | 2 | 20 800 | 0.0962 |
| Total | 3361 | 20 574 422 | 0.163 (95% CI 0.113–0.214) |
| Country and sitea . | Time period . | Age group . | Malaria deaths . | Person-time (person-years) . | Malaria mortality rates (deaths per 1000 per year) . |
|---|---|---|---|---|---|
| Hlabisa, South Africa | 1987–90 | All ages | 9 | 150 218 | 0.0599 |
| Ubombo, South Africa | 1987–90 | All ages | 17 | 164 274 | 0.1035 |
| Ingwavuma, South Africa | 1987–90 | All ages | 25 | 211 161 | 0.1184 |
| 7 malarious districts, Botswana | 1988–94 | All ages | 405 | 2 496 172 | 0.1622 |
| Southern District, Namibia | 1992–96 | All ages | 79 | 1 896 789 | 0.0416 |
| Central District, Namibia | 1992–96 | All ages | 115 | 1 214 125 | 0.0947 |
| North Eastern District, Namibia | 1992–96 | All ages | 526 | 1 133 340 | 0.4641 |
| North Western District, Namibia | 1992–96 | All ages | 690 | 3 462 498 | 0.1993 |
| Manicaland Province, Zimbabwe | 1997 | All ages | 409 | 1 819 338 | 0.2248 |
| Mashona Central Province, Zimbabwe | 1997 | All ages | 153 | 1 021 976 | 0.1497 |
| Mashona East Province, Zimbabwe | 1997 | All ages | 274 | 1 208 733 | 0.2267 |
| Mashona West Province, Zimbabwe | 1997 | All ages | 108 | 1 314 909 | 0.0821 |
| Matabele North Province, Zimbabwe | 1997 | All ages | 251 | 759 032 | 0.3307 |
| Matabele South Province, Zimbabwe | 1997 | All ages | 32 | 694 907 | 0.0460 |
| Midlands Province, Zimbabwe | 1997 | All ages | 70 | 1 552 593 | 0.0451 |
| Masvingo Province, Zimbabwe | 1997 | All ages | 196 | 1 453 557 | 0.1348 |
| Agincourt, South Africa | 1992–95 | 0–4 years | 2 | 20 800 | 0.0962 |
| Total | 3361 | 20 574 422 | 0.163 (95% CI 0.113–0.214) |
a The reference for the study in Agincourt, South Africa is Kahn et al.58 All other results were from reports that were summarized on a Malaria Burden of Disease in Africa data collection pro forma (RW Snow, personal communication, March 31, 2004).
Studies and surveillance reports included in the analysis that were from areas in southern Africa categorized as having high-intensity malaria transmission
| Country and sitea . | Time period . | Age group . | Malaria deaths . | Person-time (person-years) . | Malaria mortality rates (deaths per 1000 per year) . |
|---|---|---|---|---|---|
| Hlabisa, South Africa | 1987–90 | All ages | 9 | 150 218 | 0.0599 |
| Ubombo, South Africa | 1987–90 | All ages | 17 | 164 274 | 0.1035 |
| Ingwavuma, South Africa | 1987–90 | All ages | 25 | 211 161 | 0.1184 |
| 7 malarious districts, Botswana | 1988–94 | All ages | 405 | 2 496 172 | 0.1622 |
| Southern District, Namibia | 1992–96 | All ages | 79 | 1 896 789 | 0.0416 |
| Central District, Namibia | 1992–96 | All ages | 115 | 1 214 125 | 0.0947 |
| North Eastern District, Namibia | 1992–96 | All ages | 526 | 1 133 340 | 0.4641 |
| North Western District, Namibia | 1992–96 | All ages | 690 | 3 462 498 | 0.1993 |
| Manicaland Province, Zimbabwe | 1997 | All ages | 409 | 1 819 338 | 0.2248 |
| Mashona Central Province, Zimbabwe | 1997 | All ages | 153 | 1 021 976 | 0.1497 |
| Mashona East Province, Zimbabwe | 1997 | All ages | 274 | 1 208 733 | 0.2267 |
| Mashona West Province, Zimbabwe | 1997 | All ages | 108 | 1 314 909 | 0.0821 |
| Matabele North Province, Zimbabwe | 1997 | All ages | 251 | 759 032 | 0.3307 |
| Matabele South Province, Zimbabwe | 1997 | All ages | 32 | 694 907 | 0.0460 |
| Midlands Province, Zimbabwe | 1997 | All ages | 70 | 1 552 593 | 0.0451 |
| Masvingo Province, Zimbabwe | 1997 | All ages | 196 | 1 453 557 | 0.1348 |
| Agincourt, South Africa | 1992–95 | 0–4 years | 2 | 20 800 | 0.0962 |
| Total | 3361 | 20 574 422 | 0.163 (95% CI 0.113–0.214) |
| Country and sitea . | Time period . | Age group . | Malaria deaths . | Person-time (person-years) . | Malaria mortality rates (deaths per 1000 per year) . |
|---|---|---|---|---|---|
| Hlabisa, South Africa | 1987–90 | All ages | 9 | 150 218 | 0.0599 |
| Ubombo, South Africa | 1987–90 | All ages | 17 | 164 274 | 0.1035 |
| Ingwavuma, South Africa | 1987–90 | All ages | 25 | 211 161 | 0.1184 |
| 7 malarious districts, Botswana | 1988–94 | All ages | 405 | 2 496 172 | 0.1622 |
| Southern District, Namibia | 1992–96 | All ages | 79 | 1 896 789 | 0.0416 |
| Central District, Namibia | 1992–96 | All ages | 115 | 1 214 125 | 0.0947 |
| North Eastern District, Namibia | 1992–96 | All ages | 526 | 1 133 340 | 0.4641 |
| North Western District, Namibia | 1992–96 | All ages | 690 | 3 462 498 | 0.1993 |
| Manicaland Province, Zimbabwe | 1997 | All ages | 409 | 1 819 338 | 0.2248 |
| Mashona Central Province, Zimbabwe | 1997 | All ages | 153 | 1 021 976 | 0.1497 |
| Mashona East Province, Zimbabwe | 1997 | All ages | 274 | 1 208 733 | 0.2267 |
| Mashona West Province, Zimbabwe | 1997 | All ages | 108 | 1 314 909 | 0.0821 |
| Matabele North Province, Zimbabwe | 1997 | All ages | 251 | 759 032 | 0.3307 |
| Matabele South Province, Zimbabwe | 1997 | All ages | 32 | 694 907 | 0.0460 |
| Midlands Province, Zimbabwe | 1997 | All ages | 70 | 1 552 593 | 0.0451 |
| Masvingo Province, Zimbabwe | 1997 | All ages | 196 | 1 453 557 | 0.1348 |
| Agincourt, South Africa | 1992–95 | 0–4 years | 2 | 20 800 | 0.0962 |
| Total | 3361 | 20 574 422 | 0.163 (95% CI 0.113–0.214) |
a The reference for the study in Agincourt, South Africa is Kahn et al.58 All other results were from reports that were summarized on a Malaria Burden of Disease in Africa data collection pro forma (RW Snow, personal communication, March 31, 2004).
Data from studies were abstracted onto standardized paper forms, double-entered into an electronic database, and validated. Key data elements (e.g. number of malaria deaths) were double-checked by two investigators. Data from disease surveillance reports from southern Africa were abstracted onto standardized paper forms and entered into an electronic database by one investigator.
Estimating populations at risk for malaria
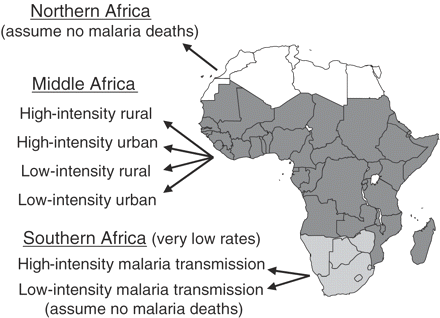
We divided Africa into seven sub-populations (Figure 1). As done by Snow et al.,8,10 we first divided Africa into three regions: northern Africa (Algeria, Egypt, Libya, Morocco, and Tunisia; assumed to have no malaria deaths because the climate is generally unsuitable for P. falciparum transmission), southern Africa (Botswana, Lesotho, Namibia, South Africa, Swaziland, and Zimbabwe; very low malaria mortality rates assumed because of a less suitable climate and intense efforts to control the mosquito vector22), and middle Africa (African countries in neither northern nor southern Africa; variable, including high, malaria mortality rates).
Seven sub-populations in Africa with different risks of dying from malaria
For middle and southern Africa, we used results from the Mapping Malaria Risk in Africa (MARA) project to estimate populations at risk for malaria in 2000.8,9,23 Briefly, a grid of 5 × 5 km2 was superimposed on a map of sub-Saharan Africa. For each square, MARA estimated the 1990 population24 and a ‘climate suitability index’ based on a fuzzy logic model of temperature and rainfall determinants of the parasite's sporogenic cycle and mosquito's survival.25 The index describes the probability that the climate is suitable for P. falciparum transmission, and it varies continuously from 0 (probably unsuitable) to 1 (probably suitable). MARA results were applied to United Nations country population estimates for the year 2000.26 Of the five middle Africa countries excluded from the MARA model, two were assumed to have no malaria deaths (Mauritius15,27 and Seychelles28) and three had populations at risk estimated from other sources (Cape Verde,15,28,29 Comoros,15,28 and São Tomé and Príncipe15,28,30).
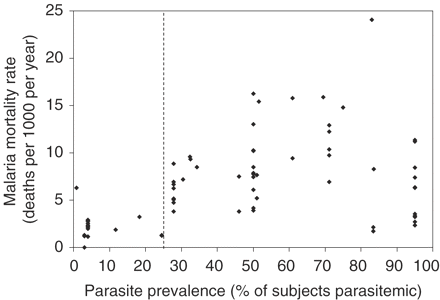
We categorized populations as being exposed to one of three levels of malaria transmission intensity: zero (populations with a MARA index = 0), low intensity (index >0 and <0.75), or high intensity (index ≥0.75). This categorization (developed by Snow et al.10) was based on the observation that a MARA index <0.75 generally corresponded to a parasite prevalence <25% and an index ≥0.75 generally corresponded to a parasite prevalence ≥25%.31 Parasite prevalence (percentage of a community-based sample of children who were parasitaemic with P. falciparum) was considered an approximate indicator for malaria transmission intensity and the malaria mortality rate.32,33 (For details, see the complete report, which is available on the Internet at: http://rbm.who.int/partnership/wg/wg_monitoring/docs/CHERG_final_report.pdf) Results from our dataset suggested malaria mortality rates were lower in populations with low (<25%) parasite prevalence and generally higher in populations with high parasite prevalence (Figure 2).
Distribution of malaria mortality rates as a function of parasite prevalence among studies of childhood mortality from middle Africa, 1980–2001 (74 estimates from 20 Group A studies; all studies except one were conducted in rural areas). The dashed vertical line indicates the chosen threshold between ‘low-intensity’ (i.e. parasite prevalence <25%) and ‘high-intensity’ (i.e. parasite prevalence ≥25%) malaria transmission
This method does not explicitly include a category for epidemics. We assumed that much of what is called ‘epidemic’ malaria occurs in populations with low levels of endemic malaria transmission that occasionally have spikes of malaria deaths that appear to be epidemics. ‘True’ epidemic deaths (i.e. malaria deaths where no ongoing transmission occurs) were considered rare for a typical year (i.e. zero, for our estimates; although a sensitivity analysis explored the potential impact of epidemics—see model ‘E’ of Table 4).
Middle Africa populations were further subdivided into those living in urban and rural areas. Urban populations have relatively low malaria mortality rates,18,19,34,35 less malaria transmission,11,36,37 and better access to life-saving malaria treatment.11,38
To estimate urban and rural populations for each malaria transmission category in each country, we used United Nations estimates of the proportion of all residents living in urban areas for 2000.39 Because this source only provides national estimates, we assumed the proportion of a country's population living in urban areas was the same for populations exposed to zero, low-intensity, and high-intensity transmission. For the 40 middle African countries in the analysis, the median urban residence proportion was 33.4% (range: 6.2–84.0%). Urban residence proportions were based on census data and assumed to have no uncertainty (T. Buettner, personal communication, UN Population Division, October 29, 2003).
Estimating malaria mortality rates for each at-risk sub-population
For middle Africa, results from mortality studies in middle African countries were analysed. To categorize studies as being from a low-intensity or high-intensity transmission area, we identified parasite prevalence values for all but six studies (Table 1). Parasite prevalence ≥25% indicated high-intensity transmission, and prevalence <25% indicated low-intensity transmission. Regarding the six mortality studies without a parasite prevalence value, three were from West Africa;40–42 with a model of parasite prevalence for West Africa,23,43 we estimated that all three studies were in high-intensity transmission areas. The remaining studies (two from Ethiopia44,45 and one from Somalia46) were excluded because the study sites were in areas with highly variable transmission levels and no conclusive determination could be made.
We calculated malaria mortality rates (expressed as deaths per 1000 children per year) with the formula: rate = malaria deaths/{person-time × [1 − (deaths with unknown causes/total deaths)]}. This expression ensures that the proportion of deaths attributable to malaria (PDAM) equals the malaria mortality rate divided by the all-cause mortality rate, and it assumes the PDAM for deaths with unknown causes equals the PDAM for deaths with known causes.47 The calculation of mortality rates and all other analyses were performed with SAS version 8.0 (SAS Institute, Cary, NC).
We did not adjust results for the potential bias introduced by verbal autopsy's imperfect sensitivity and specificity for identifying malaria deaths and that false-negatives probably do not equal false-positives.17,48 Although it is theoretically possible to correct this bias with sensitivity and specificity estimates from validation studies,49 we did not believe that estimates of adequate quality and representativeness were available.50
For middle Africa populations in rural areas with high-intensity transmission, we ran a series of univariate Poisson regression models to identify covariates (obtainable for studies in our dataset and countries in middle Africa) that explained the large variation observed in malaria mortality rates and that could be used to predict rates for individual countries. Modelling was performed with the SAS GENMOD procedure, which uses generalized estimating equations (GEE) to account for over-dispersion of the rates and the potential correlation of mortality rates for individual years from the same study.51,52 For precision estimates, we used results from the empirical covariance matrix.
We examined the following covariates: study year (mid-point of data collection; analysed separately as a series of dichotomous and trichotomous variables); proportion of births attended by a health professional, as an indicator of health care access and infrastructure [source = surveys near study sites, e.g. province-level Demographic and Health Survey (DHS)38 results]; study location (East vs West Africa, longitude, and distance from coast); and malaria season duration [<8 months vs ≥8 months, and as a continuous variable (source = MARA maps)].
Five other covariates were excluded: female literacy rate, access to clean water, study year as a continuous variable, coverage of three diphtheria-pertussis-tetanus vaccine doses (DPT3), and all-cause child mortality. Data on female literacy and access to water were not available for many studies. Study year (continuous variable) was excluded because it reflects both time and place (studies conducted in different years were often from different places) and, when study year is assumed to indicate time, outliers have a greater influence on model results. DPT3 coverage was excluded because, in our dataset, it increased from 1980 to 2001; therefore, we were concerned that study year and DPT3 coverage were collinear. All-cause child mortality, used in other disease burden models as an indicator of socioeconomic conditions,53 was excluded because it ‘contains’ the malaria mortality rate and is, therefore, not very informative. In other words, a model with all-cause mortality models the malaria mortality rate with a ‘noisy’ version of itself. Additionally, in our dataset, all-cause child mortality was not significantly associated with malaria mortality.
Significant (P-value < 0.05) covariates in the univariate analysis were entered into multivariate models; selected interactions were tested.
For middle Africa populations in rural areas with low-intensity transmission, the five Group A studies from this population18,19,54–57 were analysed. The malaria mortality rate was estimated by a Poisson regression model (using the GEE approach described above) that only contained an intercept, which essentially pooled the five studies. This method was used to ensure the 95% confidence interval (95% CI) accounted for over-dispersion and correlation of the rates.
To estimate urban rates for populations exposed to high-intensity or low-intensity transmission, we first considered using studies of urban populations; however, too few studies were available. As an alternative, we multiplied the rural malaria mortality rate for a population in a particular country by an estimate of the urban–rural malaria mortality rate ratio (i.e. urban malaria mortality rate/rural malaria mortality rate) for that country. We estimated country-specific urban–rural malaria mortality rate ratios with the urban–rural 5q0 ratio (UR5q0R) (i.e. the country's 5q0 for urban populations/the country's 5q0 for rural populations, where 5q0 is the probability a live-born child dies before the age of 5 years). This method assumes the urban–rural mortality rate ratio is similar for malaria deaths and all non-malaria deaths. Although a rough assumption, we reasoned that if a main justification for adjusting for urban residence was that access to life-saving medical care was better in urban areas, such a benefit might exist for all major causes of child deaths. UR5q0R values were estimated with data from DHSs and Multiple Indicator Cluster Surveys (E. Loaiza, personal communication, UNICEF, May 19, 2004). (For details, see the complete report, which is available on the Internet at: http://rbm.who.int/partnership/wg/wg_monitoring/docs/CHERG_final_report.pdf)
For southern Africa, we assumed no malaria deaths for populations exposed to low-intensity transmission.8 For populations exposed to high-intensity transmission, we analysed data from 16 government disease surveillance reports from southern Africa, and one prospective, community-based study from South Africa of children 0–4 years that used verbal autopsies58 (Table 2). As with studies from low-intensity transmission areas, the malaria mortality rate was estimated by an intercept-only Poisson regression model using the GEE approach. The use of surveillance reports was justified because the quality of surveillance data for malaria was generally good in southern Africa in the 1990s.8 The use of all-age malaria mortality rates was justified because these populations have little acquired immunity against malaria, and malaria mortality rates are thought to be similar for all age groups. In fact, the malaria mortality rate of the one prospective study58 of children (0.096 deaths per 1000 per year) was similar to the unweighted mean of the 16 estimates from the surveillance reports (0.155 deaths per 1000 per year).
Estimating precision
To estimate the precision of the number of malaria deaths, we derived a 95% CI formula using the delta method,59 which estimates the variance of a function. We used the term ‘precision estimate’ instead of ‘95% CI’ to remind readers that the interpretation of our precision estimate as a 95% CI rests on the assumption that the data were from a probability sample. Clearly, the data were not from a probability sample; but they are the best data we could find after an intensive search, and they are at least representative of some groups of children in malarious parts of Africa. Sources of uncertainty included were the precision of the malaria mortality rates in rural areas with high-intensity and low-intensity transmission (from the two Poisson models) and the urban adjustment factor, UR5q0R (from DHSs). (For details, see the complete report, which is available on the Internet at: http://rbm.who.int/partnership/wg/wg_monitoring/docs/CHERG_final_report.pdf) Uncertainty in population size and urban residence was not included because they were based on census data and precision estimates were not available. Uncertainty resulting from the non-representativeness of study sites and use of verbal autopsies was not included because the major type of uncertainty they could introduce is bias, not random error; and the delta method is designed only to combine multiple sources of random error. As the magnitude and direction of these potential biases were difficult to specify, we thought that attempts to make adjustments for them might introduce additional biases. Therefore, as mentioned above for verbal autopsies, no adjustments were made.
Sensitivity analyses
To examine the robustness of our results, we performed seven sensitivity analyses (brief descriptions in Table 4). (For details, see the complete report, which is available on the Internet at: http://rbm.who.int/partnership/wg/wg_monitoring/docs/CHERG_final_report.pdf)
Results
Literature review
We identified 31 studies (20 in Group A and 11 in Group B; 16 in West Africa and 15 in East Africa) from 14 countries in middle Africa (Table 1) and 17 reports from four countries in southern Africa (Table 2). Essentially no studies were identified for central Africa, and nearly all were from rural areas. Altogether, Group A and B studies from middle Africa included 4039 malaria deaths during 708 267 person-years of follow-up.
Populations at risk and malaria mortality rates
In 2000, among 111 million children in sub-Saharan Africa, 100 million lived in areas where malaria transmission occurred (Table 3). About half (48 million) of these 100 million children lived in areas with the highest risk of dying from malaria—rural areas in middle Africa with high-intensity transmission.
Final results of the ‘best’ model to estimate direct malaria mortality among children <5 years old in sub-Saharan Africa for the year 2000: populations at risk, malaria mortality rates, and malaria death counts (Model A)
| Description of sub-populations . | Estimated population . | Estimated malaria mortality rate (deaths per 1000 per year) (95% CI) . | Estimated no. of malaria deathsa (precision estimateb) . |
|---|---|---|---|
| Middle Africa, rural areas, high-intensity malaria transmission | 47 869 554 | 11.36 (9.80–12.92)c | 543 883 (469 111–618 656) |
| Middle Africa, urban areas, high-intensity malaria transmission | 25 495 836 | Varies by country; rate = (above rural rate of 11.36) × (urban 5q0/rural 5q0); average rate = 8.26 | 210 603 (174 734–246 472) |
| Middle Africa, rural areas, low-intensity malaria transmission | 16 586 307 | 2.31 (2.11–2.51) | 38 281 (34 985–41 578) |
| Middle Africa, urban areas, low-intensity malaria transmission | 5 833 184 | Varies by country; rate = (above rural rate of 2.31) × (urban 5q0/rural 5q0); average rate = 1.80 | 10 521 (9458–11 584) |
| Southern Africa, high-intensity malaria transmission | 2 025 102 | 0.163 (0.113–0.214) | 331 (229–433) |
| Southern Africa, low-intensity malaria transmission | 2 624 145 | 0 | 0 |
| Total at risk for malaria | 100 434 128 | Varies by country; average rate = 8.00 | 803 620 (705 821–901 418) |
| Total (including populations not at risk for malaria) | 111 253 237 | Varies by country; average rate = 7.22 | 803 620 (705 821–901 418) |
| Description of sub-populations . | Estimated population . | Estimated malaria mortality rate (deaths per 1000 per year) (95% CI) . | Estimated no. of malaria deathsa (precision estimateb) . |
|---|---|---|---|
| Middle Africa, rural areas, high-intensity malaria transmission | 47 869 554 | 11.36 (9.80–12.92)c | 543 883 (469 111–618 656) |
| Middle Africa, urban areas, high-intensity malaria transmission | 25 495 836 | Varies by country; rate = (above rural rate of 11.36) × (urban 5q0/rural 5q0); average rate = 8.26 | 210 603 (174 734–246 472) |
| Middle Africa, rural areas, low-intensity malaria transmission | 16 586 307 | 2.31 (2.11–2.51) | 38 281 (34 985–41 578) |
| Middle Africa, urban areas, low-intensity malaria transmission | 5 833 184 | Varies by country; rate = (above rural rate of 2.31) × (urban 5q0/rural 5q0); average rate = 1.80 | 10 521 (9458–11 584) |
| Southern Africa, high-intensity malaria transmission | 2 025 102 | 0.163 (0.113–0.214) | 331 (229–433) |
| Southern Africa, low-intensity malaria transmission | 2 624 145 | 0 | 0 |
| Total at risk for malaria | 100 434 128 | Varies by country; average rate = 8.00 | 803 620 (705 821–901 418) |
| Total (including populations not at risk for malaria) | 111 253 237 | Varies by country; average rate = 7.22 | 803 620 (705 821–901 418) |
CI = Confidence interval; 5q0 = probability a live-born child will die before his or her fifth birthday.
a Number of deaths may not exactly equal the product of a malaria mortality rate and its corresponding population at risk because of rounding.
b See the ‘Estimating precision’ sub-section of the Methods for an explanation.
c Regression model: Loge(malaria mortality rate) = β0 + (β1 × YEAR2), where YEAR2 = 0 for 1980s and 1 for 1990 and later, β0 = – 4.9019 (standard error = 0.1229), β1 = 0.4244 (standard error = 0.1388; P = 0.0022).
Final results of the ‘best’ model to estimate direct malaria mortality among children <5 years old in sub-Saharan Africa for the year 2000: populations at risk, malaria mortality rates, and malaria death counts (Model A)
| Description of sub-populations . | Estimated population . | Estimated malaria mortality rate (deaths per 1000 per year) (95% CI) . | Estimated no. of malaria deathsa (precision estimateb) . |
|---|---|---|---|
| Middle Africa, rural areas, high-intensity malaria transmission | 47 869 554 | 11.36 (9.80–12.92)c | 543 883 (469 111–618 656) |
| Middle Africa, urban areas, high-intensity malaria transmission | 25 495 836 | Varies by country; rate = (above rural rate of 11.36) × (urban 5q0/rural 5q0); average rate = 8.26 | 210 603 (174 734–246 472) |
| Middle Africa, rural areas, low-intensity malaria transmission | 16 586 307 | 2.31 (2.11–2.51) | 38 281 (34 985–41 578) |
| Middle Africa, urban areas, low-intensity malaria transmission | 5 833 184 | Varies by country; rate = (above rural rate of 2.31) × (urban 5q0/rural 5q0); average rate = 1.80 | 10 521 (9458–11 584) |
| Southern Africa, high-intensity malaria transmission | 2 025 102 | 0.163 (0.113–0.214) | 331 (229–433) |
| Southern Africa, low-intensity malaria transmission | 2 624 145 | 0 | 0 |
| Total at risk for malaria | 100 434 128 | Varies by country; average rate = 8.00 | 803 620 (705 821–901 418) |
| Total (including populations not at risk for malaria) | 111 253 237 | Varies by country; average rate = 7.22 | 803 620 (705 821–901 418) |
| Description of sub-populations . | Estimated population . | Estimated malaria mortality rate (deaths per 1000 per year) (95% CI) . | Estimated no. of malaria deathsa (precision estimateb) . |
|---|---|---|---|
| Middle Africa, rural areas, high-intensity malaria transmission | 47 869 554 | 11.36 (9.80–12.92)c | 543 883 (469 111–618 656) |
| Middle Africa, urban areas, high-intensity malaria transmission | 25 495 836 | Varies by country; rate = (above rural rate of 11.36) × (urban 5q0/rural 5q0); average rate = 8.26 | 210 603 (174 734–246 472) |
| Middle Africa, rural areas, low-intensity malaria transmission | 16 586 307 | 2.31 (2.11–2.51) | 38 281 (34 985–41 578) |
| Middle Africa, urban areas, low-intensity malaria transmission | 5 833 184 | Varies by country; rate = (above rural rate of 2.31) × (urban 5q0/rural 5q0); average rate = 1.80 | 10 521 (9458–11 584) |
| Southern Africa, high-intensity malaria transmission | 2 025 102 | 0.163 (0.113–0.214) | 331 (229–433) |
| Southern Africa, low-intensity malaria transmission | 2 624 145 | 0 | 0 |
| Total at risk for malaria | 100 434 128 | Varies by country; average rate = 8.00 | 803 620 (705 821–901 418) |
| Total (including populations not at risk for malaria) | 111 253 237 | Varies by country; average rate = 7.22 | 803 620 (705 821–901 418) |
CI = Confidence interval; 5q0 = probability a live-born child will die before his or her fifth birthday.
a Number of deaths may not exactly equal the product of a malaria mortality rate and its corresponding population at risk because of rounding.
b See the ‘Estimating precision’ sub-section of the Methods for an explanation.
c Regression model: Loge(malaria mortality rate) = β0 + (β1 × YEAR2), where YEAR2 = 0 for 1980s and 1 for 1990 and later, β0 = – 4.9019 (standard error = 0.1229), β1 = 0.4244 (standard error = 0.1388; P = 0.0022).
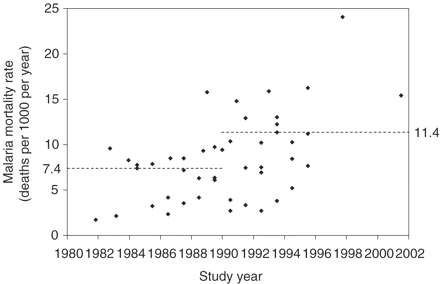
In the analysis to estimate a malaria mortality rate for rural populations in middle Africa with high-intensity transmission, the only significant univariate associations were several dichotomous variables for study year. Although results for these variables were similar, the one with the best model fit coded study year as 1980–89 vs 1990 and later (rate of the 1990s+ period was 1.529 times the rate in the 1980s; 95% CI 1.165–2.007) (Figure 3). Thus, the final prediction model included only study year (1980s vs 1990s+), and the rate for rural areas in middle Africa with high-intensity transmission was predicted to be constant from 1990–2001 for all countries: 11.36 deaths per 1000 children per year (95% CI 9.80–12.92) (Figure 3 and Table 3, first row). For urban populations in middle Africa with high-intensity transmission, the rate was different for each country but generally about three-quarters of the rural rate (Table 3, second row) (median UR5q0R = 0.72, range: 0.54–0.94).
Distribution of malaria mortality rates as a function of study year (46 rates from 15 Group A studies; all studies of rural populations in middle Africa exposed to high-intensity malaria transmission) The dashed horizontal lines indicate the predicted malaria mortality rate from the final Poisson regression model for rural populations in middle Africa exposed to high-intensity malaria transmission: the predicted rate is constant over study years 1980–89 (7.43 per 1000 per year) and constant over study years 1990–2001 (11.36 per 1000 per year)
The estimated malaria mortality rate for rural populations in middle Africa with low-intensity transmission was 2.31 deaths per 1000 per year (95% CI 2.11–2.51) (Table 3, third row). As above, the corresponding urban rate was about three-quarters of the rural rate (Table 3, fourth row). For southern Africa, we estimated a rate of 0.163 deaths per 1000 per year (95% CI 0.113–0.214) for areas with high-intensity transmission and assumed a rate of zero for areas with low-intensity transmission (Table 3, rows 5 and 6).
Africa-wide mortality estimates
By summing deaths for the six sub-populations in Table 3, we estimated that 803 620 (precision estimate: 705 821–901 418) child malaria deaths occurred in 2000. Nearly all (754 486/803 620, or 93.9%) malaria deaths occurred in areas with high-intensity transmission in middle Africa. Two-thirds (543 883/803 620, or 67.7%) of malaria deaths occurred in rural areas with high-intensity transmission in middle Africa, and one-quarter (210 603/803 620, or 26.2%) occurred in urban areas with high-intensity transmission in middle Africa. Only 6.1% (48 802/803 620) occurred in areas with low-intensity transmission in middle Africa, and 0.04% (331/803 620) occurred in southern Africa. For all of sub-Saharan Africa, including areas without malaria transmission, 18.0% (803 620/4 462 437; precision estimate: 15.8–20.2%) of all deaths among children <5 years old were directly attributable to malaria (Table 4, first row).
Summary of sensitivity analyses of the estimates of direct malaria mortality among children <5 years old in sub-Saharan Africa for the year 2000
| Model description . | Malaria deaths (precision estimatea) . | Proportion of deaths attributable to malariab (precision estimatea) . | ||
|---|---|---|---|---|
| ‘Best’ model | ||||
| Model A: Model for the malaria mortality rate in rural areas with high-intensity transmission included only study yearc; analysis only included data from Group A studies (see Methods) | 803 620 (705 821–901 418) | 18.0% (15.8–20.2%) | ||
| Other models | ||||
| Model B: Same as model A, except estimates are based on a dataset that includes a combination of Group A studies and Group B studies | 774 572 (688 079–861 065) | 17.4% (15.4–19.3%) | ||
| Model C: Same as Model A, except the urban malaria mortality rate for all malarious parts of Middle Africa is estimated by the rate from the one study in Dar es Salaam, Tanzania18,19 | 765 618 (688 971–842 266) | 17.2% (15.4–18.9%) | ||
| Model D: Same as Model A, except 50% of the urban population of Middle Africa is re-classified as rural population to allow for possible overestimation of urban populations | 844 628 (740 865–948 391) | 18.9% (16.6–21.3%) | ||
| Model E: Same as Model A, except epidemic deaths are added to low-intensity transmission areasd | 818 632 (720 831–916 434) | 18.3% (16.2–20.5%) | ||
| Model F: Same as Model A, except the malaria mortality rate = malaria deaths/person-time (i.e. assume deaths with no known cause were never malaria deaths) | 687 609 (546 369–828 849) | 15.4% (12.2–18.6%) | ||
| Model G: Same as Model A, except the malaria mortality rate = (malaria deaths + deaths with no known cause)/person-time (i.e. assume deaths with no known cause were always malaria deaths) | 1 028 000 (858 236–1 197 764) | 23.0% (19.2–26.8%) | ||
| Model H: Estimates based on the proportion of deaths attributable to malaria (instead of the rate)d | 1 094 444 (892 516–1 297 165) | 24.5% (20.0–29.1%) | ||
| Model description . | Malaria deaths (precision estimatea) . | Proportion of deaths attributable to malariab (precision estimatea) . | ||
|---|---|---|---|---|
| ‘Best’ model | ||||
| Model A: Model for the malaria mortality rate in rural areas with high-intensity transmission included only study yearc; analysis only included data from Group A studies (see Methods) | 803 620 (705 821–901 418) | 18.0% (15.8–20.2%) | ||
| Other models | ||||
| Model B: Same as model A, except estimates are based on a dataset that includes a combination of Group A studies and Group B studies | 774 572 (688 079–861 065) | 17.4% (15.4–19.3%) | ||
| Model C: Same as Model A, except the urban malaria mortality rate for all malarious parts of Middle Africa is estimated by the rate from the one study in Dar es Salaam, Tanzania18,19 | 765 618 (688 971–842 266) | 17.2% (15.4–18.9%) | ||
| Model D: Same as Model A, except 50% of the urban population of Middle Africa is re-classified as rural population to allow for possible overestimation of urban populations | 844 628 (740 865–948 391) | 18.9% (16.6–21.3%) | ||
| Model E: Same as Model A, except epidemic deaths are added to low-intensity transmission areasd | 818 632 (720 831–916 434) | 18.3% (16.2–20.5%) | ||
| Model F: Same as Model A, except the malaria mortality rate = malaria deaths/person-time (i.e. assume deaths with no known cause were never malaria deaths) | 687 609 (546 369–828 849) | 15.4% (12.2–18.6%) | ||
| Model G: Same as Model A, except the malaria mortality rate = (malaria deaths + deaths with no known cause)/person-time (i.e. assume deaths with no known cause were always malaria deaths) | 1 028 000 (858 236–1 197 764) | 23.0% (19.2–26.8%) | ||
| Model H: Estimates based on the proportion of deaths attributable to malaria (instead of the rate)d | 1 094 444 (892 516–1 297 165) | 24.5% (20.0–29.1%) | ||
a See the ‘Estimating precision’ sub-section of the Methods for an explanation.
b Total number of deaths (all causes combined) for children <5 years old from 48 countries in sub-Saharan Africa was estimated as 4 462 437.117
c Dichotomous variable for the mid-point of when data for a malaria mortality estimate were collected: 1980–89 vs 1990 and later.
d For details, see the Methods section of the complete report, which is available on the Internet at: http://rbm.who.int/partnership/wg/wg_monitoring/docs/CHERG_final_report.pdf
Summary of sensitivity analyses of the estimates of direct malaria mortality among children <5 years old in sub-Saharan Africa for the year 2000
| Model description . | Malaria deaths (precision estimatea) . | Proportion of deaths attributable to malariab (precision estimatea) . | ||
|---|---|---|---|---|
| ‘Best’ model | ||||
| Model A: Model for the malaria mortality rate in rural areas with high-intensity transmission included only study yearc; analysis only included data from Group A studies (see Methods) | 803 620 (705 821–901 418) | 18.0% (15.8–20.2%) | ||
| Other models | ||||
| Model B: Same as model A, except estimates are based on a dataset that includes a combination of Group A studies and Group B studies | 774 572 (688 079–861 065) | 17.4% (15.4–19.3%) | ||
| Model C: Same as Model A, except the urban malaria mortality rate for all malarious parts of Middle Africa is estimated by the rate from the one study in Dar es Salaam, Tanzania18,19 | 765 618 (688 971–842 266) | 17.2% (15.4–18.9%) | ||
| Model D: Same as Model A, except 50% of the urban population of Middle Africa is re-classified as rural population to allow for possible overestimation of urban populations | 844 628 (740 865–948 391) | 18.9% (16.6–21.3%) | ||
| Model E: Same as Model A, except epidemic deaths are added to low-intensity transmission areasd | 818 632 (720 831–916 434) | 18.3% (16.2–20.5%) | ||
| Model F: Same as Model A, except the malaria mortality rate = malaria deaths/person-time (i.e. assume deaths with no known cause were never malaria deaths) | 687 609 (546 369–828 849) | 15.4% (12.2–18.6%) | ||
| Model G: Same as Model A, except the malaria mortality rate = (malaria deaths + deaths with no known cause)/person-time (i.e. assume deaths with no known cause were always malaria deaths) | 1 028 000 (858 236–1 197 764) | 23.0% (19.2–26.8%) | ||
| Model H: Estimates based on the proportion of deaths attributable to malaria (instead of the rate)d | 1 094 444 (892 516–1 297 165) | 24.5% (20.0–29.1%) | ||
| Model description . | Malaria deaths (precision estimatea) . | Proportion of deaths attributable to malariab (precision estimatea) . | ||
|---|---|---|---|---|
| ‘Best’ model | ||||
| Model A: Model for the malaria mortality rate in rural areas with high-intensity transmission included only study yearc; analysis only included data from Group A studies (see Methods) | 803 620 (705 821–901 418) | 18.0% (15.8–20.2%) | ||
| Other models | ||||
| Model B: Same as model A, except estimates are based on a dataset that includes a combination of Group A studies and Group B studies | 774 572 (688 079–861 065) | 17.4% (15.4–19.3%) | ||
| Model C: Same as Model A, except the urban malaria mortality rate for all malarious parts of Middle Africa is estimated by the rate from the one study in Dar es Salaam, Tanzania18,19 | 765 618 (688 971–842 266) | 17.2% (15.4–18.9%) | ||
| Model D: Same as Model A, except 50% of the urban population of Middle Africa is re-classified as rural population to allow for possible overestimation of urban populations | 844 628 (740 865–948 391) | 18.9% (16.6–21.3%) | ||
| Model E: Same as Model A, except epidemic deaths are added to low-intensity transmission areasd | 818 632 (720 831–916 434) | 18.3% (16.2–20.5%) | ||
| Model F: Same as Model A, except the malaria mortality rate = malaria deaths/person-time (i.e. assume deaths with no known cause were never malaria deaths) | 687 609 (546 369–828 849) | 15.4% (12.2–18.6%) | ||
| Model G: Same as Model A, except the malaria mortality rate = (malaria deaths + deaths with no known cause)/person-time (i.e. assume deaths with no known cause were always malaria deaths) | 1 028 000 (858 236–1 197 764) | 23.0% (19.2–26.8%) | ||
| Model H: Estimates based on the proportion of deaths attributable to malaria (instead of the rate)d | 1 094 444 (892 516–1 297 165) | 24.5% (20.0–29.1%) | ||
a See the ‘Estimating precision’ sub-section of the Methods for an explanation.
b Total number of deaths (all causes combined) for children <5 years old from 48 countries in sub-Saharan Africa was estimated as 4 462 437.117
c Dichotomous variable for the mid-point of when data for a malaria mortality estimate were collected: 1980–89 vs 1990 and later.
d For details, see the Methods section of the complete report, which is available on the Internet at: http://rbm.who.int/partnership/wg/wg_monitoring/docs/CHERG_final_report.pdf
Sensitivity analyses
The first five sensitivity analyses for middle Africa (Models B–E, Table 4) produced results that were remarkably similar to the ‘best’ model (Model A, Table 4). As expected, extreme assumptions led to estimates that were either considerably lower (Model F) or greater (Model G) than the ‘best’ estimate.
The last sensitivity analysis for middle Africa (Model H, Table 4), based on the PDAM (conceptually, the malaria mortality rate/all-cause mortality rate), produced estimates that were considerably (36%) higher than the ‘best’ estimate. Model H probably overestimated malaria deaths because the PDAM was probably overestimated. The PDAM was probably overestimated because: (i) studies in the Model H analysis had all-cause mortality rates (the PDAM denominator) that were much (28.2%) lower than rates in the general population;26 (For details, see the complete report, which is available on the Internet at: http://rbm.who.int/partnership/wg/wg_monitoring/docs/CHERG_final_report.pdf) and (ii) as two-thirds (10/15) of the studies had a malaria focus, we suspected that the malaria mortality rates (the PDAM numerator) from these studies probably did not greatly underestimate the rate in the general population (e.g. malaria-focused studies might have been more likely to be conducted in areas with high malaria mortality, or to classify deaths as malaria deaths).
Discussion
Our result of 803 620 (precision estimate: 705 821–901 418) child malaria deaths in 2000 is generally consistent with three recent estimates of the direct burden of malaria mortality among children in sub-Saharan Africa for the year 2000, although the studies used different methods and data. Snow et al.10 estimated 742 318 malaria deaths (interquartile range: 541 491–1 069 153); Hay et al.,11 who used the method by Snow et al. adjusted for urbanization, estimated 679 658 malaria deaths (interquartile range: 478 118–944 024); and Morris et al.60 estimated a PDAM of 23.7% (95% CI 17.0–37.0%), which corresponds to ∼1 058 000 malaria deaths (95% CI 759 000–1 651 000). Remarkably, when our malaria mortality rates were applied to the populations at risk estimated by Hay et al.,11 the result (802 373 deaths; precision estimate: 701 059–903 687) was nearly identical to our estimates.
Our estimates have several important limitations. First, data from research sites are clearly not a probability sample of African children. If research sites were chosen because malaria was especially common, results (when generalized to the entire African population) might overestimate the true burden. In contrast, burden might be underestimated if study populations had above-average socioeconomic status because they were located near a city or main roads or if the presence of the research project improved the population's health.
A second limitation is the use of verbal autopsies. Although non-specific and insensitive, verbal autopsies do not introduce bias if false-positives (e.g. bacterial sepsis death incorrectly classified as malaria) equal false-negatives (e.g. death from malaria-related anaemia incorrectly classified as pneumonia). Unfortunately, verbal autopsy validation studies do not reveal a clear trend in the way verbal autopsies misclassify deaths. Depending on the study and diagnostic algorithm (some studies examined several algorithms), false-negatives can outnumber false-positives or vice versa.61–65
Additionally, the finding that malaria mortality rates may decrease at high parasite prevalence levels66–68 (For details, see the complete report, which is available on the Internet at: http://rbm.who.int/partnership/wg/wg_monitoring/docs/CHERG_final_report.pdf) suggests that verbal autopsies may lose sensitivity in settings with high-intensity transmission (i.e. more false-negatives occur because more malaria deaths result from severe anaemia, which verbal autopsies may misclassify as pneumonia deaths). However, by defining transmission intensity with broad parasite prevalence categories, this potential problem probably was not an important source of bias for our estimates.
A third limitation is that our methods require numerous assumptions, some with unknown validity. Fourth, some data had unknown validity and precision, e.g. population estimates, MARA results, parasite prevalence estimates, and covariate data used in the models. Although our assumptions and data almost certainly introduced biases, it is difficult to estimate their directions and magnitudes.
To address the preceding two limitations, at least partially, we conducted a variety of sensitivity analyses. We found the estimates were relatively insensitive to varying assumptions about the urban malaria mortality rate, urban population size, and deaths from epidemic malaria. In addition, the estimates changed little when we included studies with minor deviations from our ideal inclusion criteria. The estimates were, however, sensitive to extreme assumptions about deaths with unknown causes. The analysis based on the PDAM gave results that were much higher than our best estimates, but differences may be explained by the fact that all-cause mortality rates in study sites are not representative of the general population.
Finally, we caution readers that our precision estimates can only be interpreted as 95% CIs if the data are assumed to be a probability sample. Also, the precision estimates are probably too narrow, because they do not account for uncertainty in population size and urban residence.
For these reasons, our estimates should be considered an approximation of the true burden of direct malaria mortality. Information and data that might improve future estimates are listed in Box 1. Some issues are already being addressed. For example, the Global Rural Urban Mapping Programme, which distinguishes urban and rural areas with satellite-measured night-time light levels, is being used to provide more precise estimates of urban and rural populations.11
Better understanding of the relationship between verbal autopsy results and true malaria deaths (e.g. a verbal autopsy validation study conducted in a community setting)
Better understanding of how malaria mortality rates from research sites differ from rates in the general population
Community-based, longitudinal data on the malaria mortality rate in central Africa
Better definitions of and data on:
– Malaria transmission intensity (and a better understanding of how transmission intensity affects malaria mortality)
– Urban populations (and a better understanding of how urban residence, including different types of urban residence, affects malaria mortality)
– Malaria's indirect impact on mortality
– The impact of malaria epidemics on malaria mortality (e.g. community-based, longitudinal data on malaria mortality during epidemics)
Better estimates of the size of populations at risk for malaria, especially sub-populations that potentially have different levels of mortality risk, such as:
– Populations living in areas with higher vs lower levels of malaria transmission intensity
– Urban and rural populations
– Populations exposed to malaria interventions (e.g. insecticide-treated bednets)
How should our results be used? First, it is probably safe to say that in 2000, a rough approximation of the burden of direct malaria mortality among children <5 years old in sub-Saharan Africa was between 700 000 and 900 000 deaths. Second, the validity of estimates for specific countries is even more questionable than for continental estimates. Third, our results cannot be reliably extrapolated to years outside those in the dataset (e.g. if the child population in 2005 is 5% higher than in 2000, increasing our estimates by 5% may not provide a valid estimate for 2005). Fourth, our results should not be used to monitor trends in malaria mortality over time by comparing our estimates with other estimates based on different methods and data. For example, it would be inappropriate to conclude that malaria mortality had increased because our estimate for the year 2000 (803 620 deaths) is higher than the estimate by Snow et al.8 for 1995 (765 775 deaths).
Fifth, our results should not be used to estimate how many deaths would be prevented if malaria transmission were reduced or eliminated. Such estimates would probably be a gross underestimate because they do not include the large burden of indirect malaria mortality. Studies in Kenya, Nigeria, and Tanzania from the 1950s and 1970s found that after malaria transmission was drastically reduced with insecticide spraying, which would have had little or no effect on other causes of child mortality, all-cause mortality rates for infants and children 1–4 years old decreased by as much as 40–50%.69–71 Similarly, when malaria chemoprophylaxis and insecticide-treated nets were used to prevent malaria in The Gambia, the all-cause child mortality rate decreased by 42%.72
Finally, caution should be exercised in using our results to evaluate malaria prevention and control efforts, such as those of the Roll Back Malaria partnership. Such an evaluation would presumably involve repeating our estimation method using similar data after interventions were implemented with reasonably high coverage. However, if the interpretation of verbal autopsies remains unchanged, even if malaria were eradicated, verbal autopsy's high false-positive rate will show erroneously that malaria is present. In 2000, when coverage of most interventions was very low, false-positives were ‘helpful’ because they partially counter-balanced false-negatives; but as coverage increases and the malaria burden is reduced and false-negatives disappear, one is left with ‘unpreventable’ malaria-like deaths (false-positives due to bacterial infections, etc., that could not be prevented with malaria-specific interventions). Such results would underestimate the impact of malaria prevention and control efforts.
Our results suggest that malaria mortality rates have increased between the 1980s and 1990s, although we were unable to find a difference between West and East Africa [results not shown (For details, see the complete report, which is available on the Internet at: http://rbm.who.int/partnership/wg/wg_monitoring/docs/CHERG_final_report.pdf)]. It is important to remember, however, that ‘study year’ may also represent study place. In other words, increasing mortality rates may simply reflect a trend in which areas with higher malaria mortality rates were more often selected as study sites in the 1990s relative to the 1980s. However, some studies lasting many years found increasing trends,55 with increasing resistance to the commonly-used antimalarial, chloroquine, often cited as the cause.35,55,68,73
In conclusion, we do not know how many direct malaria deaths occurred among children in sub-Saharan Africa in 2000. However, our study has several strengths: we conducted an intensive literature search, selected studies with rigorous inclusion criteria, introduced stringent quality controls on the data abstraction process, incorporated a novel approach to account for urbanization, utilized a statistically-based method for estimating uncertainty, and made extensive use of sensitivity analyses to evaluate assumptions. Although the results are clearly imperfect, we hope that they are less imperfect, and methods more transparent, than earlier efforts.
One important byproduct of this work is a better understanding of the inadequacy of current data as a basis for developing estimates and measuring the impact of malaria interventions. Relatively few data of adequate quality could be identified, and no data were found for vast geographical areas with some of the world's poorest populations.74 Sound epidemiological estimates are an essential part of public health decision-making. More and better studies are needed to support prevention and control programmes addressing malaria, which is directly responsible for ∼18% of all child deaths in sub-Saharan Africa.
The burden of malaria mortality among children in countries without adequate vital registration systems can be estimated by extrapolating mortality rates observed in small-scale community studies, stratified by level of malaria transmission intensity, to continent-wide populations at risk using maps of malaria risk and population distribution.
In sub-Saharan Africa, where most malaria deaths occur, a rough approximation of the burden of direct malaria mortality for the year 2000 among children <5 years old is between 700 000 and 900 000 deaths.
Current models of malaria mortality estimated direct malaria deaths and do not account for malaria's probably large indirect burden (where malaria is one of several diseases leading to death, but the death is attributed to another primary cause).
The small number of community studies, their inherent problems in identifying malaria deaths, and other knowledge gaps limit the validity of epidemiological models and estimates; more community-based mortality studies with greater standardization, and updates and refinements of malaria risk maps are needed.
This study was part of a larger effort by the Child Health Epidemiology Reference Group (CHERG) to describe the burden of disease among children in developing countries, and we thank all CHERG members for their comments, collaboration, and encouragement. The CHERG received financial support from The Bill & Melinda Gates Foundation and was coordinated by the Department of Child and Adolescent Health and Development of the World Health Organization. We also thank Melissa Thumm, Karen Edmond, and Simon Cousens for planning and supervising the data abstraction process; and, the Adult Morbidity and Mortality Project (AMMP) for contributing data from three sites in Tanzania. AMMP is a project of the Tanzanian Ministry of Health and local councils, implemented in partnership with the University of Newcastle upon Tyne, and funded by the Department for International Development (UK). Some data used in this study include those produced through funding from the Wellcome Trust, UK (#058992) and The Bill & Melinda Gates Foundation (#17408) as support to Professor Robert Snow for the Burden of Malaria in Africa (BOMA) project. We thank F. Pelletier, UN Population Division, for providing all-cause mortality data for children under the age of 5 years for African countries; these estimates were derived from the 2002 Revision of the World Population Prospects.
References
Murray CJL, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study.
Murray CJL, Lopez AD, Mathers CD, Stein C. The Global Burden of Disease 2000 project: aims, methods and data sources. Global Programme on Evidence for Health Policy Discussion Paper No. 36. Geneva: World Health Organization, November
Mathers CD, Ma Fat D, Inoue M, Rao C, Lopez AD. Counting the dead and what they died from: an assessment of the global status of cause of death data.
Breman J, Campbell C. Combating severe malaria in African children.
Murray CJL, Lopez AD. Global Health Statistics: A Compendium of Incidence, Prevalence and Mortality Estimates for Over 200 Conditions. Cambridge: Harvard University Press,
Snow RW, Craig M, Deichmann U, Marsh K. Estimating mortality, morbidity, and disability due to malaria among Africa's non-pregnant population.
Snow RW, Craig M, Deichmann U, le Sueur D. A preliminary continental risk map for malaria mortality among African children.
Snow RW, Craig MH, Newton CRJC, Steketee RW. The public health burden of Plasmodium falciparum malaria in Africa: Deriving the numbers. Working Paper No. 11, Disease Control Priorities Project. Bethesda, MD: Fogarty International Center, National Institutes of Health, August 2003. Available at: http://www.fic.nih.gov/dcpp/wps/wp11.pdf (Accessed January 21, 2005).
Hay SI, Guerra CA, Tatem AJ, Atkinson PM, Snow RW. Urbanization, malaria transmission and disease burden in Africa.
WHO. International Statistical Classification of Diseases and Related Health Problems. Tenth Revision. Volume 2: Instruction Manual. Geneva: World Health Organization,
Snow RW, Korenromp EL, Gouws E. Pediatric mortality in Africa: Plasmodium falciparum malaria as a cause or risk?
World Health Organization and UNICEF. World Malaria Report 2005. Report no. WHO/HTM/MAL/2005.1102. Geneva: World Health Organization,
Garenne M, Fontaine O. Assessing probable causes of deaths using a standardized questionnaire: a study in rural Senegal. In: Vallin J, D'Souza S, Palloni A (eds). Measurement and Analysis of Mortality—New Approaches. Oxford: Clarendon Press,
Anker M. The effect of misclassification error on reported cause-specific mortality fractions from verbal autopsy.
Adult Morbidity and Mortality Project. Unpublished data from Morogoro Rural District, Dar es Salaam, and Hai District, Tanzania, January 1993–December 2001.
Adult Morbidity and Mortality Project. The Policy Implications of Adult Morbidity and Mortality End of Phase 1 Report. August 1997. Available at: http://www.ncl.ac.uk/ammp/site_files/public_html/ammp_rep/ammp_rpt.pdf (Accessed August 10,
INDEPTH Monograph Series: Demographic Surveillance Systems for Assessing Populations and their Health in Developing countries, Volume 1: Population, Health and Survival in INDEPTH Sites. Ottawa: International Development Research Centre,
Armstrong Schellenberg JRM, Adam T, Mshinda H, Masanja H, Kabadi G, Mukasa O et al. Effectiveness and cost of facility-based Integrated Management of Childhood Illness (IMCI) in Tanzania.
Mabaso MLH, Sharp B, Lengeler C. Historical review of malarial control in southern African with emphasis on the use of indoor residual house-spraying.
Mapping Malaria Risk in Africa (MARA) Collaboration. Towards an atlas of malaria risk in Africa. First technical report of the MARA/ARMA Collaboration. Albany Print: Durban, South Africa, December
Deichmann U. African Population Database. Digital Database and Documentation. Santa Barbara, CA: National Center for Geographic Information and Analysis. Available at: http://grid2.cr.usgs.gov/globalpop/africa/.
Craig MH, Snow RW, le Sueur D. A climate-based distribution model of malaria transmission in sub-Saharan Africa.
United Nations. World Population Prospects—The 2002 Revision. New York: United Nations Population Division,
Ravavoodoo C. Situation du paludisme à Maurice. [Malaria situation in Mauritius].
Centers for Disease Control and Prevention. Health Information for International Travel 2003–2004. Atlanta: US Department of Health and Human Services, Public Health Service,
Statoids website (http://www.statoids.com/ucv.html, accessed November 15, 2005). Based on: Gwillim Law. Administrative Divisions of Countries. McFarland & Company, Jefferson, North Carolina, October 1999.
Hagmann R, Charlwood JD, Gil V, Ferreira C, do Rosário V, Smith TA. Malaria and its possible control on the island of Príncipe.
Omumbo JA, Hay SI, Guerra CA, Snow RW. The relationship between the Plasmodium falciparum ratio in childhood and climate estimates of malaria transmission in Kenya.
Beier JC, Killeen GF, Githure JI. Entomologic inoculation rates and Plasmodium falciparum prevalence in Africa.
Smith TA, Leuenberger R, Lengeler C. Child mortality and malaria transmission intensity in Africa.
Trape JF, Quinet MC, Nzingoula S, Senga P, Tchichelle F, Carme B et al. Malaria and urbanization in Central Africa: the example of Brazzaville. Part V. Pernicious attacks and mortality.
Greenberg AE, Ntumbanzondo M, Ntula N, Mawa L, Howell J, Davachi F. Hospital-based surveillance of malaria-related paediatric morbidity and mortality in Kinshasa, Zaire.
Hay SI, Rogers DJ, Toomer JF, Snow RW. Annual Plasmodium falciparum entomological inoculation rates (EIR) across Africa: literature survey, internet access and review.
Robert V, Macintyre K, Keating J, Trape JF, Duchemin JB, Warren M et al. Malaria transmission in urban sub-Saharan Africa.
ORC Macro. MEASURE DHS+ STATcompiler. Available at: http://www.measuredhs.com (Accessed October 6,
United Nations. World Urbanization Prospects: The 2001 Revision. Department of Economic and Social Affairs, Population Division, United Nations. Document ST/ESA/SER.A/216. New York: United Nations,
Schumacher R, Swedberg E, Diallo MO, Keita DR, Kalter H, Pasha O. Mortality Study in Guinea: Investigating the Causes of Death in Children Under 5. Arlington, Virginia: Save the Children and BASICS II,
Jinadu M, Olusi S, Agun J, Fabiyi A. Childhood diarrhoea in rural Nigeria. I. Studies on prevalence, mortality and socio-environmental factors.
Amin R. Immunization coverage and child mortality in two rural districts of Sierra Leone.
Kleinschmidt I, Omumbo J, Briet O, van de Giesen N, Sogoba N, Mensah NK et al. An empirical malaria distribution map for West Africa.
Kidane G, Morrow RH. Teaching mothers to provide home treatment of malaria in Tigray, Ethiopia: a randomized trial.
Alemayehu T, Ghebreyesus TA, Witten KH, Bosman A, Teklehaimanot A. Community-based malaria control programme in Tigray Region, Northern Ethiopia: results of a mortality survey of rural under-five children.
Ibrahim MM, Omar HM, Persson LÅ, Wall S. Child mortality in a collapsing African society.
Rowe AK. Analysis of deaths with an unknown cause in epidemiologic analyses of mortality burden.
Rowe AK. Should verbal autopsy results for malaria be adjusted to improve validity?
Chandramohan D, Setel P, Quigley M. Effect of misclassification of causes of death in verbal autopsy: can it be adjusted?
Stokes ME, Davis CS, Koch GG. Categorical Data Analysis Using the SAS® System. 2nd edn. Cary, NC: SAS Institute Inc.,
Koch GG, Stokes ME. Some statistical strategies for analyzing confirmatory studies involving one or more occurrences of primary events. Paper 212–29. SAS Users Group International Conference (SUGI 29), Montreal, Canada,
Williams BG, Gouws E, Boschi-Pinto C, Bryce J, Dye C. Estimates of world-wide distribution of child deaths from acute respiratory infections.
Snow RW, Biomonde K, Shanks GD. Unpublished data from Brookebond Tea Estate, Kericho, Kenya, January 1997–December 1998.
Trape J-F, Pison G, Preziosi MP, et al. Impact of chloroquine resistance on malaria mortality.
Charlwood J, Qassim M, Elnsur E, Donnelly M, Petrarca V, Billingsley PF et al. The impact of indoor residual spraying with malathion on malaria in refugee camps in eastern Sudan.
Vella V, Tomkins A, Nidku J, Marshall T. Determinants of child mortality in south-west Uganda.
Kahn K, Tollman SM, Garenne M, Gear JS. Who dies from what? Determining cause of death in South Africa's rural north-east.
Rice JA. Mathematical Statistics and Data Analysis. Belmont, CA: Wadsworth & Brooks/Cole Publishers,
Morris SS, Black RE, Tomaskovic L. Predicting the distribution of under-five deaths by cause in countries without vital registration systems.
Todd J, De Francisco A, O'Dempsey T, Greenwood B. The limitations of verbal autopsy in a malaria-endemic region.
Mobley CC, Boerma JT, Titus S, Lohrke B, Shangula K, Black RE. Validation study of a verbal autopsy method for causes of childhood mortality in Namibia.
Anker M, Black RE, Coldham C et al. A Standard Verbal Autopsy Method for Investigating Causes of Death in Infants and Children. Geneva: World Health Organization,
Snow RW, Armstrong JRM, Forster D, Winstanley MT, Marsh VM, Newton CR et al. Childhood deaths in Africa: uses and limitations of verbal autopsies.
Quigley MA, Armstrong Schellenberg JR, Snow RW. Algorithms for verbal autopsies: a validation study in Kenyan children.
Snow RW, Marsh K. Will reducing Plasmodium falciparum transmission alter mortality among African children?
Snow RW, Marsh K. The consequences of reducing Plasmodium falciparum transmission in Africa.
Korenromp EL, Williams BG, Gouws E, Dye C, Snow RW. Measuring trends in childhood malaria mortality in Africa: a new assessment of progress toward targets based on verbal autopsy.
Bradley DJ. Morbidity and mortality at Pare-Taveta, Kenya and Tanzania, 1954–66: the effects of a period of malaria control. In: Feachem RG, Jamison DT (eds). Disease and Mortality in Sub-Saharan Africa. The World Bank. Oxford: Oxford University Press,
Payne D, Grab B, Fontain RE, Hempel JHG. Impact of control measures on malaria transmission and general mortality.
Molineaux L, Gramiccia G. The Garki Project: Research on the Epidemiology and Control of Malaria in the Sudan Savanna of West Africa. Geneva: World Health Organization,
Alonso P, Lindsay S, Armstrong J, Conteh M, Hill AG, David PH et al. The effect of insecticide-treated bednets on mortality of Gambian children.
Snow RW, Trape JF, Marsh K. The past, present and future of childhood malaria mortality in Africa.
Rudan I, Lawn J, Cousens S, Rowe AK, Boschi-Pinto C, Tomašković L et al. Gaps in policy-relevant information on burden of disease in children: a systematic review.
Delacollette C, Barutwanayo M. Mortalite et morbidite aux jeunes ages dans une region a paludisme hyperendemique stable, commune de Nyanza-Lac, Imbo Sud, Burundi.
Delacollette C, van der Stuyft P, Molima K, Delacollette-Lebrun C, Wery M. Study of the global mortality and the malaria associated mortality in the Kivu mountains, Zaire.
Delacollette C, Van der Stuyft P, Molima K, Hendrix L, Wery M. Indices paludometriques selon l'age et selon les saisons dans la zone de sante de Katana, au Kivu Montagneux, Zaire [Malaria index according to age and seasons in the health region of Katana, in mountainous Kivu, Zaire]
Alonso PL, Lindsay SW, Armstrong Schellenberg JRM, Gomez P, Hill AG, David PH et al. A malaria control trial using insecticide-treated bednets and targeted chemoprophylaxis in a rural area of The Gambia, West Africa. 2. Mortality and morbidity from malaria in the study area.
Greenwood B, Bradley A, Greenwood A, Byass P, Jammeh K, Marsh K et al. Mortality and morbidity from malaria among children in a rural area of The Gambia, West Africa.
Jaffar S, Leach A, Greenwood A, Jepson A, Muller O, Ota MO et al. Changes in the pattern of infant and childhood mortality in Upper River Division, The Gambia, from 1989 to 1993.
Thomson MC, D'Alessandro U, Bennett S, Connor SJ, Langerock P, Jawara M et al. Malaria prevalence is inversely related to vector density in The Gambia, West Africa.
Afari E, Nkrumah F, Nakana T, Sakatoku H, Hori H, Binka F. Impact of primary health care on childhood and mortality in rural Ghana: the Gomoa experience.
Afari EA, Nakano T, Binka F, Owusu-Agyei S, Asigbee J. Seasonal characteristics of malaria infection in under-five children of a rural community in southern Ghana.
Molbak K, Aaby P, Ingholt L, Hojlyng N, Gottschau A, Andersen H et al. Persistent and acute diarrhea as the leading causes of child mortality in urban Guinea-Bissau.
Unpublished study by Lars Smedman and the CESAS/SAREC Project Team. Available at: http://ukwangela.ki.se/parasit.htm (Accessed January 14,
Spencer HC, Kaseje DC, Mosley WH, Sempebwa EK, Huong AY, Roberts JM. Impact on mortality and fertility of a community-based malaria control programme in Saradidi, Kenya.
Spencer HC, Kaseje DCO, Collins WE, Shehata MG et al. Community-based malaria control in Saradidi, Kenya: description of the programme and impact on parasitaemia rates and antimalarial antibodies.
Phillips-Howard PA, Nahlen BL, Kolczak MS, Hightower AW, ter Kuile FO, Alaii JA et al. Efficacy of permethrin-treated bednets in the prevention of mortality in young children in an area of high perennial malaria transmission in western Kenya.
Bloland PB, Boriga DA, Ruebush TK, McCormick JB, Roberts JM, Oloo AJ et al. Longitudinal cohort study of the epidemiology of malaria infections in an area of intense malaria transmission. II. Descriptive epidemiology of malaria infection and disease among children.
Sokhna CS, Molez JF, Ndiaye P, Sane B, Trape J-F. Tests in vivo de chimiosensibilité de Plasmodium falciparum à la chloroquine au Sénégal: evolution de la résistance et estimation de l'efficacité thérapeutique. [In vivo sensitivity of Plasmodium falciparum to chloroquine in Senegal: evolution of resistance and assessment of therapeutic efficacy].
Sane B. Etude de l'impact de la chloroquinorésistance sur l'épidémiologie du paludisme à Mlomp (Casamance), Sénégal. Mémoire de DEA de biologie animale. Dakar: Université de Dakar,
Mtango F, Neuvians D. Acute respiratory infections in children under five years. Control project in Bagamoyo District, Tanzania.
Shiff CJ, Minjas JN, Hall T, Hunt RH, Lyimo S, Davis JR. Malaria infection potential of anopheline mosquitoes sampled by light trapping indoors in coastal Tanzanian villages.
Salum F, Wilkes T, Kivumbi K, Curtis C. Mortality of under-fives in a rural area of holoendemic malaria transmission.
Lyimo EO, Msuya FHM, Rwegoshora RT, Nicholson EA, Mnzava AE, Lines JD et al. Trial of pyrethroid impregnated bednets in an area of Tanzania holoendemic for malaria. Part 3. Effects on the prevalence of malaria parasitemia and fever.
Van den Homberg J. Malarial anaemia in infants and young children from Morogoro region in Tanzania. Epidemiology and clinical aspects. Masters of Science in Communicable Diseases thesis, London School of Hygiene and Tropical Medicine, University of London,
Gazin de Raucourt P. Le paludisme au Burkino Faso: Etude épidémiologique de la transmission, des indices parasitologiques, de la morbidité, de la létalité. Parasitologie, Ecologie, et Pathologie. Montpellier: Université de Montpellier I, Unité de Formation et de Recherche de Médecine,
D'Alessandro U, Olaleye B, McGuire W, Langerock P, Bennett S, Aikins MK et al. Mortality and morbidity from malaria in Gambian children after introduction of an impregnated bednet programme.
Ghana VAST. Vitamin A supplementation in northern Ghana: effects on clinic attendances, hospital admissions, and child mortality.
Binka FN, Morris SS, Ross DA, Arthur P, Aryeetey ME. Patterns of malaria morbidity and mortality in children in northern Ghana.
Binka F, Kubaje A, Adjuik M, Williams LA, Lengeler C, Maude GH et al. Impact of permethrin impregnated bednets on child mortality in Kassena-Nankana district, Ghana: a randomized controlled trial.
Unpublished report to TDR/WHO by F. Binka,
Snow RW, Mung'ala VO, Foster D, Marsh K. The role of the district hospital in child survival at the Kenyan Coast.
Snow RW, Omumbo JA, Lowe B, Molyneux CS, Obiero JO, Palmer A et al. Relation between severe malaria morbidity in children and level of Plasmodium falciparum transmission in Africa.
Barnish G, Maude G, Bockaire M, Eggelte T, Greenwood B, Ceesay S. Malaria in a rural area of Sierra Leone. I. Initial results.
Barnish G, Maude GH, Bockarie MJ, Erunkulu OA, Dumbuya MS, Greenwood BM. Malaria in a rural area of Sierra Leone. II. Parasitological and related results from pre- and post-rains clinical surveys.
Premji Z, Ndayanga P, Shiff C, Minjas J, Lubega P, MacLeod J. Community based studies on childhood mortality in a malaria holoendemic area on the Tanzanian coast.
Premji Z, Hamisi Y, Shiff C, Minjas J, Lubega P, Makwaya C. Anaemia and Plasmodium falciparum infections among young children in an holoendemic area, Bagamoyo, Tanzania.
Malaria Burden of Disease in Africa data collection proforma (provided by R.W. Snow) for the reference: "Unpublished data from Brookebond Tea Estate, Kenya, January 1997–December 1998 by Shanks GD, Biomonde K, Snow RW; 1999" Section 6.2 of this proforma cited the following as the source of the parasite prevalence data: Dennis Shanks, personal communication,
Pison G, Trape JF, Lefebvre M, Enel C. Rapid decline in child mortality in a rural area of Senegal.
Omumbo JA, Snow RW. Plasmodium falciparum prevalence in East Africa: a review.
Jelliffe EFP, Jelliffe DB. Plasmodium malariae in Ugandan children. I. Prevalence in young children in rural communities.
Mirza N, Macharia W, Wafula E, Agwanda R, Onyango F. Mortality patterns in a rural Kenyan community.
Njoroge, EP. Community parasite survey monthly report. Kenya: Division of Vector Borne Diseases, MOH,
Yamagata Y. Malaria control in the United Republic of Tanzania (1993–1996). Report by Japanese International Cooperation Agency to Ministry of Health, Tanzania, October