-
PDF
- Split View
-
Views
-
Cite
Cite
Vicky Katsidoni, Andreas Kastellakis, George Panagis, Biphasic effects of Δ9-tetrahydrocannabinol on brain stimulation reward and motor activity, International Journal of Neuropsychopharmacology, Volume 16, Issue 10, November 2013, Pages 2273–2284, https://doi.org/10.1017/S1461145713000709
Close - Share Icon Share
Abstract
Δ9-tetrahydrocannabinol (Δ9-THC), the main psychoactive ingredient of marijuana, has led to equivocal results when tested with the intracranial self-stimulation (ICSS) procedure or the open-field test for motor activity, two behavioural models for evaluating the reward-facilitating and locomotor stimulating effects of drugs of abuse, respectively. Therefore, in the present study, the effects of high and low doses of Δ9-THC were compared in the ICSS procedure and the open-field test. Moreover, the involvement of CB1 receptors in tentative Δ9-THC-induced effects was investigated by pre-treating the animals with the CB1 receptor antagonist SR141716A (rimonabant). The results obtained show that low doses of Δ9-THC induce opposite effects from high doses of Δ9-THC. Specifically, 0.1 mg/kg Δ9-THC decreased ICSS thresholds and produced hyperactivity, whereas 1 mg/kg increased ICSS thresholds and produced hypoactivity. Both effects were reversed by pre-treatment with SR141716A, indicating the involvement of CB1 receptors on these actions. Altogether, our results indicate that Δ9-THC can produce acute activating effects in locomotion that coincide with its reward-facilitating effects in the ICSS paradigm. The present findings provide further support that Δ9-THC induces behaviours typical of abuse and substantiate the notion that marijuana resembles other drugs of abuse.
Introduction
Cannabis products are the most widely abused drugs among illicit compounds currently available for recreational use (Ramo et al., 2012). The main psychoactive ingredient of these preparations is the alkaloid Δ9-tetrahydrocannabinol (Δ9-THC), which is well known to produce feelings of euphoria and relaxation in human users (Haney et al., 1997; Justinova et al., 2005). These feelings may play a central role in the reinforcement of repeated use and abuse of cannabis preparations and, in some cases, the development of dependence. However, the popularity of marijuana and other cannabis products may also stem from the fact that they are more socially tolerated and often perceived as harmless and non-habit-forming drugs. Thus, assessment of cannabis abuse potential is an important issue addressed by researchers and may have implications for public policy and health.
Despite the clear evidence for rewarding effects of cannabis preparations and Δ9-THC in humans (Hart et al., 2005), rewarding effects of Δ9-THC or other cannabinoids in animal models of drug abuse and dependence have been controversial and appear to be very much dependent on the experimental conditions (Parolaro et al., 2005; Solinas et al., 2007; Panagis et al., 2008). Additionally, the lack of a pronounced withdrawal syndrome following abrupt cessation of cannabis has reinforced the notion that cannabinoids are only mildly addictive (Smith, 2002). However, it is very likely that such findings reflect the chemistry and pharmacokinetics of these compounds (i.e. high lipophilicity and long duration of action) rather than their low abuse potential (Maldonado, 2002). Moreover, studies examining the rewarding effects of Δ9-THC may be further confounded by the drug's tendency to produce aversive feelings at higher doses (Lepore et al., 1995; Sanudo-Pena et al., 1997; Hutcheson et al., 1998; Mallet and Beninger, 1998; Robinson et al., 2003). Indeed, several studies using lower doses have found that Δ9-THC is self-administered by experimental animals (Justinova et al., 2003; Braida et al., 2004; Le Foll et al., 2006) and produces conditioned place preference (Lepore et al., 1995; Valjent and Maldonado, 2000; Braida et al., 2004). Interestingly, microinjections of Δ9-THC into the posterior ventral tegmental area and the posterior shell of the nucleus accumbens also produce conditioned place preference (Zangen et al., 2006).
An animal behavioural model commonly used to determine the effects of psychotropic drugs in reward processes is the intracranial self-stimulation (ICSS) paradigm (Wise, 1996; Carlezon and Chartoff, 2007; Vlachou and Markou, 2011). Most drugs of abuse are able to lower ICSS thresholds, an effect that supports the notion that they activate the same substrate with electrical stimulation in a synergistic manner (Wise, 1996). Only a few studies have been conducted on the effects of Δ9-THC in the ICSS paradigm. According to Gardner and colleagues, 1 and 1.5 mg/kg Δ9-THC decrease the ICSS threshold in Lewis rats but not in Fisher 344 rats, whereas in Sprague–Dawley rats the effect was only marginal (Gardner et al., 1988; Lepore et al., 1996). In contrast, studies from our group failed to show an enhancement of brain stimulation reward with Δ9-THC in the dose range from 0.5 to 2 mg/kg (Vlachou et al., 2007; Fokos and Panagis, 2010). Thus, a major problem regarding the robustness of Δ9-THC in ICSS is the lack of agreement between different studies. This is quite contrary to the consistency of the findings with other abused substances, such as psychostimulants, nicotine and opioids (Wise, 1996).
The measurement of motor activity is another behavioural test commonly used in the study of drugs of abuse (Geyer and Paulus, 1992). Most drugs of abuse tend to stimulate motor activity, an effect that may become sensitized and contribute to drug addiction (Wise, 1988). Several studies have shown that cannabinoids, including Δ9-THC, suppress ambulation and rearing in higher doses (Jarbe et al., 2002; Wiley and Martin, 2003; Smirnov and Kiyatkin, 2008; Polissidis et al., 2010); whereas in lower doses, increases in such measurements have been reported (Sanudo-Pena et al., 2000; Polissidis et al., 2010). The degree by which locomotor stimulating effects of Δ9-THC can be detected relates to experimental design (i.e. strain of animal, rat phenotype, habituation and reaction to novelty, influence of the light/dark cycle) and dose regimen. Thus, although a biphasic stimulatory/inhibitory effect of Δ9-THC on motor activity has been reported, the literature is lacking a detailed time resolution of this effect.
In the present study, effects of high and low doses of Δ9-THC were compared in the ICSS procedure and the open-field test for motor activity. Finally, the involvement of CB1 receptors in tentative Δ9-THC-induced effects was investigated by pre-treating the animals with the CB1 receptor antagonist SR141716A (rimonabant).
Method
Animals and surgery
Male Sprague–Dawley rats (n = 46) weighing 300–350 g were used. Animals were housed two or three per cage under a 12 h light–12 h dark cycle (lights on 08:00 hours) with free access to food and water. Surgery for self-stimulation followed previously described procedures (Katsidoni et al., 2011). Experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize animal suffering and to reduce the number of animals used.
Apparatus and procedures for ICSS
After 1 wk recovery, the rats were tested for self-stimulation in an operant chamber made of transparent Plexiglas (25-cm wide, 25-cm deep and 30-cm high). A stainless steel rodent lever protruded 2 cm from the left wall at a height of 4 cm from the floor. Each bar-press triggered a constant current stimulator (Med Associates, USA) that delivered a 0.4-s train of rectangular cathodal pulses of constant duration (0.1 ms) and intensity (250 mA) and variable frequency (15–100 Hz, i.e. 6–40 number of pulses/0.4 s). The pulse frequency, i.e. the number of pulses within a train, was progressively increased up to 40 per stimulation train until the animal showed vigorous self-stimulation. During the acquisition phase, the animals were trained to self-stimulate for at least 3 consecutive days (1 h daily), using stimulation parameters that maintained near maximal bar-pressing rates. After self-stimulation was acquired and stabilized for a given pulse frequency, rats were trained to self-stimulate using four alternating series of ascending and descending pulse frequencies. The pulse frequency was varied by steps of approximately 0.1 log units. Each frequency was tested within trials of 60 s in duration, followed by an extinction period of 30 s. For each trial, there was an initial ‘priming’ phase, during which the animals received three trains of stimulation at the frequency that was available for the specific trial. A rate–frequency determination session lasted approximately 45 min. One rate–frequency curve was established daily, for 10–14 d, depending on the period when the self-stimulation indices (i.e. curve shift and threshold measure) were stable. The stimulation parameters, ICSS sessions and data collection were controlled by a computer.
Drug testing began for each animal when the rate–frequency function was stable for at least 3 consecutive days.
Data analysis and statistics for ICSS studies
Assessment of locomotor activity
Spontaneous motor activity was measured using an activity recording system (Model 7445; Ugo Basile, Italy). Each system consists of an animal cage and an electronic unit incorporating a counter and a printer. The rectangular animal cage (56 × 56 × 30 cm) has transparent sides and lid to allow observation. The cage floor has horizontal and vertical infrared sensors. The counter sums up the photocell disruptions and a printer displays the results at preset intervals. In our studies, a summation of photocell disruptions of ambulatory distance and rearing, for each 10-min interval period, during the 3 h observation period was registered. The behavioural testing was performed between 08:00 and 16:00 hours. One day before the drug testing, each rat was gently handled for 15 min and habituated to the experimental room and the open-field for 1 h.
Data analysis and statistics for locomotor activity studies
In the motor activity experiments, total ambulatory distance and rearing counts over the 3 h observation period were evaluated. In the first experiment, the significance of the drug effect and time was statistically evaluated initially using two-way (ANOVA) with repeated measures. In the second experiment, three-way ANOVA with repeated measures was performed to evaluate statistically significant interactions and the main effects. In both cases, when the interaction in the two-way ANOVA was significant we considered Bonferroni's inequality approach or paired sample t test, dependent on the case.
Drugs and drug administration
Δ9-THC (Sigma-Aldrich, USA) and SR141716A (Cayman, USA) were dissolved into a vehicle solution that consisted of 5% dimethylsulfoxide, 5% cremophor EL and 90% of 0.9% NaCl and injected i.p. at a volume of 3 ml/kg body weight. Control animals received, i.p., the corresponding vehicle solutions in the same injection volume. Based on the reports of behavioural studies that effects of Δ9-THC and other cannabinoid agonists follow a biphasic mode, a low dose of 0.1 mg/kg and a high dose of 1 mg/kg were selected based on their possible stimulatory and inhibitory effects, respectively.
All animals took part in only one experiment and received all drug treatments of the experiment. The order of testing for various doses of each drug treatment was counterbalanced according to a Latin-square design and a 3-d period was allowed between injections.
Behavioural studies: ICSS studies
Expt 1: effects of systematically administrated Δ9-THC on brain stimulation reward
In the first experiment, a group of animals (n = 8) was used to evaluate the effects of the acute administration of Δ9-THC (0, 0.1 and 1 mg/kg i.p.) on brain stimulation reward. Each drug or vehicle self-stimulation test consisted of a pre-drug and two post-drug rate–frequency function determinations (for 45 min each). The injection of the compound was given immediately following the pre-drug rate–frequency function determination. The first session began 20 min post-injection, while the second session started 80 min after Δ9-THC injection.
Expt 2: effects of the CB1 receptor antagonist SR141716A on Δ9-THC-induced changes on brain stimulation reward
The purpose of the second experiment was to examine whether the CB1 receptor antagonist SR141716A could reverse the reward-facilitating effect of Δ9-THC. Thus, a group of animals (n = 8) received SR141716A (0.02 mg/kg i.p.) or vehicle followed 5 min later by Δ9-THC (0.1 mg/kg i.p.) or vehicle. Each drug or vehicle self-stimulation test consisted of a pre-drug and two post-drug rate–frequency function determinations (for 45 min each). The first session began 20 min post-injection, while the second session started 80 min after Δ9-THC injection.
Behavioural studies: locomotor activity studies
Expt 1: effects of systematically administrated Δ9-THC on locomotor activity
A group of animals (n = 10) was used to examine the effects of Δ9-THC (0, 0.1 and 1 mg/kg i.p.) on locomotor activity. Animals were injected with Δ9-THC or its vehicle and placed immediately in the centre of the activity box. Locomotor activity was recorded for 3 h.
Expt 2: reversal of Δ9-THC-induced changes in locomotion with the CB1 receptor antagonist SR141716A
Two groups of animals (n = 20) were injected with SR141716A (0.02 mg/kg) or its vehicle, and 5 min later the first group (n = 10) received 0.1 mg/kg of Δ9-THC or its vehicle and the second group (n = 10) received 1 mg/kg of Δ9-THC or its vehicle, and placed immediately in the centre of the activity box. Locomotor activity was recorded for 3 h.
Results
Behavioural studies: ICSS studies
Expt 1: effects of systematically administrated Δ9-THC on brain stimulation reward
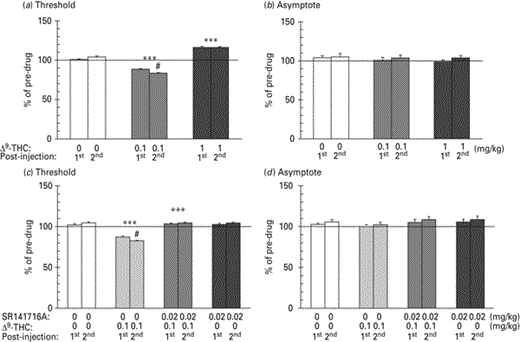
The changes in ICSS threshold and asymptotic rate of responding after acute administration of Δ9-THC (0, 0.1 and 1 mg/kg i.p.) are presented in Fig. 1a, b, respectively. Two-way ANOVA with repeated measures showed a statistically significant Δ9-THC × time interaction (F2,14 = 13.279, p < 0.001) on the ICSS threshold. Repeated measures on the simple effect of the first post-injection showed a statistical significant effect of Δ9-THC (F2,14 = 282.828, p < 0.001). Paired-sample t test using Bonferroni's adjustment for multiple comparisons revealed that Δ9-THC at the dose of 0.1 mg/kg significantly decreased (p < 0.001), while at the dose of 1 mg/kg significantly increased (p < 0.001) the ICSS threshold. Repeated measures on the simple effect of the second post-injection showed a statistically significant effect of Δ9-THC (F2,14 = 471.248, p < 0.001). Paired-sample t test using Bonferroni's adjustment for multiple comparisons revealed that Δ9-THC at the dose of 0.1 mg/kg significantly decreased (p < 0.001) while at the dose of 1 mg/kg significantly increased (p < 0.001) the ICSS threshold. Repeated measures on the simple effect of the dose of 0.1 mg/kg Δ9-THC showed a statistically significant effect of time (F1,7 = 11.436, p < 0.05). Paired sample t test using Bonferroni's adjustment for multiple comparisons revealed that the dose 0.1 mg/kg significantly decreased the ICSS threshold during the first post-injection (p < 0.001), while the decrease was more pronounced during the second post-injection (p < 0.05). Repeated measures on the simple effect of the doses of 0 and 1 mg/kg Δ9-THC did not reveal any statistically significant effect of time.
Changes in intracranial self-stimulation (ICSS) threshold (a, c) and asymptotic rate of responding (b, d) expressed as percentage of pre-drug values, following acute Δ9-tetrahydrocannabinol (Δ9-THC; 0, 0.1 and 1 mg/kg i.p.) administration. Vertical bars represent the means ± s.e.m. * Signifies an ICSS threshold significantly different from the respective control group (vehicle): *** p < 0.001. # Signifies a statistically significant effect compared to the first post-injection effect of the same dose: # p < 0.05. + Signifies a statistically significant effect compared to the SR141716A 0 mg/kg – Δ9-THC 0.1 mg/kg group: +++ p < 0.001. The dose of 0.1 mg/kg decreased, whereas the dose of 1 mg/kg increased ICSS thresholds. The effects of Δ9-THC on ICSS thresholds remained for 2 h post-injection. SR141716A antagonized the reward-facilitating effect of Δ9-THC.
Two-way ANOVA with repeated measures showed no statistically significant effect of Δ9-THC (F2,14 = 0.429, p > 0.05), time (F2,14 = 1.772, p > 0.05) or their interaction (F2,14 = 1.214, p > 0.05) on the asymptotic rate of responding.
Expt 2: effects of the CB1 receptor antagonist SR141716A on Δ9-THC-induced changes on brain stimulation reward
The changes in ICSS threshold and asymptotic rate of responding after acute administration of SR141716A (0, 0.02 mg/kg i.p.) and Δ9-THC (0 and 0.1 mg/kg i.p.) are presented in Fig. 1c, d respectively. Three-way ANOVA with repeated measures showed a statistically significant SR141716A × Δ9-THC × time interaction (F1,7 = 49.075, p < 0.01) on the ICSS threshold. Two-way ANOVA with repeated measures for the first post-injection showed a statistically significant SR141716A × Δ9-THC interaction (F1,7 = 96.108, p < 0.001). Paired sample t test using Bonferroni's adjustment for multiple comparisons revealed that SR141716A blocked the reward-facilitating effect of Δ9-THC at the dose of 0.1 mg/kg (p < 0.001). Similarly, two-way ANOVA with repeated measures for the second post-injection showed a statistically significant SR141716A × Δ9-THC interaction (F2,14 = 187.699, p < 0.001). Paired sample t test using Bonferroni's adjustment for multiple comparisons revealed that SR141716A blocked the reward-facilitating effect of Δ9-THC at the dose of 0.1 mg/kg (p < 0.001).
Three-way ANOVA with repeated measures did not reveal any statistically significant effect of SR141716A (F1,7 = 1.745, p > 0.05), Δ9-THC (F1,7 = 5.623, p > 0.05), time (F1,7 = 1.419, p > 0.05) or their interaction (F2,14 = 1.214, p > 0.05) on the asymptotic rate of responding.
Behavioural studies: locomotor activity studies
Expt 1: effects of systematically administrated Δ9-THC on locomotor activity
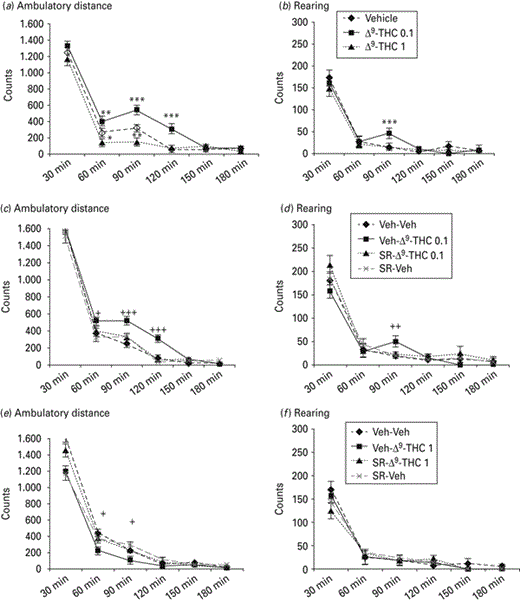
The changes on ambulatory distance and rearing after acute administration of Δ9-THC (0, 0.1 and 1 mg/kg i.p.) are presented in Fig. 2a, b, respectively. Two-way ANOVA with repeated measures showed a statistically significant Δ9-THC × time interaction (F1,6 = 31.452, p < 0.001) on ambulatory distance. Repeated measures on the simple effect of the dose 0.1 mg/kg of Δ9-THC showed a statistically significant effect of time (F1,7 = 99.311, p < 0.001). Paired sample t test using Bonferroni's adjustment for multiple comparisons revealed that the dose of 0.1 mg/kg significantly increased the ambulatory distance at 60 min (p < 0.01), 90 min (p < 0.001) and 120 min (p < 0.001). Repeated measures on the simple effect of the dose of 1 mg/kg of Δ9-THC showed a statistically significant effect of time (F1,7 = 85.513, p < 0.001). Paired sample t test using Bonferroni's adjustment for multiple comparisons revealed that the dose of 1 mg/kg significantly decreased the ambulatory distance at 60 min (p < 0.05) and 90 min (p < 0.01).
Effects of Δ9-tetrahydrocannabinol (Δ9-THC; 0, 0.1 and 1 mg/kg i.p.) on locomotor activity and effect of SR141716A (0.02 mg/kg) on Δ9-THC 0.1 mg/kg-induced hyperactivity and Δ9-THC 1 mg/kg-induced hypoactivity. Histograms represent the photocell disruptions caused by the animals’ ambulatory distance travelled (a, c, e) and rearing (b, d, f) (mean±s.e.m.). * Signifies a statistically significant effect compared to the vehicle (Veh) group: * p < 0.05, ** p < 0.01, *** p < 0.001. + Signifies a statistically significant effect compared to the Veh – Δ9-THC 0.1 mg/kg group (c, d) and the Veh – Δ9-THC 1 mg/kg group (e, f): + p < 0.05, ++ p < 0.01, +++ p < 0.001. The dose of 0.1 mg/kg increased, whereas the dose of 1 mg/kg decreased spontaneous motor activity. These effects were reversed by pre-treatment with SR141716A.
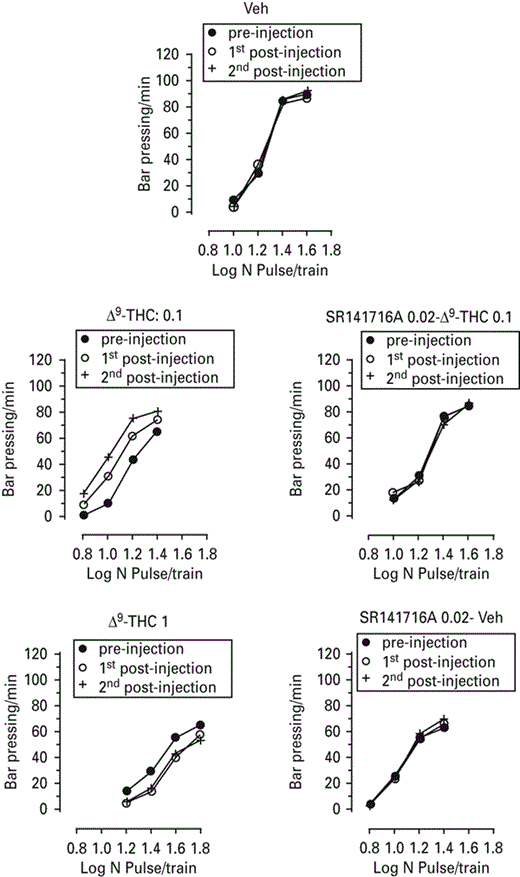
Rate–frequency functions (rate of lever pressing as a function of stimulation frequency) taken from representative animals for each drug treatment. Each plot represents data from a single animal under pre-drug and drug conditions. Rate frequency functions were obtained by logarithmically decreasing the frequency of the stimulation pulses from a value that sustained maximal lever pressing to one that failed to sustain lever pressing. The dose of 0.1 mg/kg caused parallel leftward shifts in the rate–frequency function, whereas the dose of 1 mg/kg caused rightward shifts. Veh, Vehicle; Δ9-tetrahydrocannabinol (Δ9-THC).
Two-way ANOVA with repeated measures showed a statistically significant Δ9-THC × time interaction on rearing (F1,6 = 25.397, p < 0.01). Repeated measures on the simple effect of the dose of 0.1 mg/kg Δ9-THC showed a statistically significant effect of time (F1,7 = 73.134, p < 0.01). Paired sample t test using Bonferroni's adjustment for multiple comparisons revealed that the dose of 0.1 mg/kg significantly increased rearing at 90 min (p < 0.001). Repeated measures on the simple effect of the dose of 1 mg/kg Δ9-THC showed no statistically significant effect of time. Repeated measures on the simple effect of all time-points did not reveal any statistically significant effect for the dose of 1 mg/kg Δ9-THC.
Expt 2: reversal of Δ9-THC-induced changes in locomotion with the CB1 receptor antagonist SR141716A
The changes on ambulatory distance and rearing after acute administration of SR141716A (0, 0.02 mg/kg i.p.) and Δ9-THC (0 and 0.1 mg/kg i.p.) are presented in Fig. 2c, d, respectively. Three-way ANOVA with repeated measures showed a statistically significant SR141716A × Δ9-THC × time interaction (F1,6 = 61.287, p < 0.001) on ambulatory distance. Two-way ANOVA with repeated measures for the dose of 0.1 mg/kg Δ9-THC showed a statistically significant SR141716A × time interaction (F1,7 = 102.586, p < 0.001). Repeated measures on the simple effect of the dose of 0.02 mg/kg SR141716A showed a statistically significant effect of time (F1,7 = 97.138, p < 0.001). Paired sample t test using Bonferroni's adjustment for multiple comparisons revealed that the dose of 0.02 mg/kg SR141716A blocked the hyperlocomotion induced by 0.1 mg/kg Δ9-THC at the time-points of 60 min (p < 0.05), 90 min (p < 0.001) and 120 min (p < 0.001). Two-way ANOVA with repeated measures for the dose of 0 mg/kg Δ9-THC did not reveal any statistically significant SR141716A × time interaction (F1,7 = 0.297, p > 0.05).
Three-way ANOVA with repeated measures showed a statistically significant SR141716A × Δ9-THC (0 and 0.1 mg/kg) × time interaction (F1,6 = 50.716 p < 0.01) on rearing. Two-way ANOVA with repeated measures for the dose of 0.1 mg/kg Δ9-THC showed a statistically significant SR141716A × time interaction (F1,7 = 89.134, p < 0.01). Repeated measures on the simple effect of the dose of 0.02 mg/kg SR141716A showed a statistically significant effect of time (F1,7 = 71.003, p < 0.01). Paired sample t test using Bonferroni's adjustment for multiple comparisons revealed that the dose 0.02 mg/kg SR141716A blocked the increased rearing induced by 0.1 mg/kg Δ9-THC at the time-point of 90 min (p < 0.05).
The changes on ambulatory distance and rearing after acute administration of SR141716A (0, 0.02 mg/kg i.p.) and Δ9-THC (0 and 1 mg/kg i.p.) are presented in Fig. 2e, f, respectively. Three-way ANOVA with repeated measures showed a statistically significant SR141716A × Δ9-THC × time interaction (F1,6 = 122.336, p < 0.001) on ambulatory distance. Two-way ANOVA with repeated measures for the dose of 1 mg/kg Δ9-THC showed a statistically significant SR141716A × time interaction (F1,7 = 89.378, p < 0.01). Repeated measures on the simple effect of the dose of 0.02 mg/kg SR141716A showed a statistically significant effect of time (F1,7 = 92.587, p < 0.001). Paired sample t test using Bonferroni's adjustment for multiple comparisons revealed that SR141716 blocked the hypolocomotion induced by 1 mg/kg Δ9-THC at the time-points of 60 min (p < 0.05) and 90 min (p < 0.05). Two-way ANOVA with repeated measures for the dose of 0 mg/kg Δ9-THC did not reveal any statistically significant SR141716A × time interaction (F1,7 = 1.897, p > 0.05).
Three-way ANOVA with repeated measures did not reveal any statistically significant effect of SR141716A (F1,7 = 0.998, p > 0.05), Δ9-THC (F1,7 = 2.545, p > 0.05), time (F1,7 = 2.785, p > 0.05) or their interaction (F1,6 = 2.001, p > 0.05) on rearing.
Discussion
The first finding of the present study is that Δ9-THC is able to induce both rewarding and anhedonic effects in the ICSS paradigm in Sprague–Dawley rats, depending on the dose used. Indeed, a low dose of 0.1 mg/kg decreased ICSS thresholds and caused parallel leftward shifts in the rate–frequency function, whereas a higher dose of 1 mg/kg increased ICSS thresholds, producing rightward shifts (see Fig. 3). In other words, the low dose of Δ9-THC reduced the amount of stimulation necessary to sustain responding at a given criterion level (Miliaressis et al., 1986), increasing the rewarding efficacy of the stimulation. The observed effects of Δ9-THC on ICSS thresholds were relatively long-lasting, since they remained for 2 h post-injection.
The reward-facilitating effect observed after 0.1 mg/kg Δ9-THC was more pronounced in the second post-injection trial and was nearly equivalent to that produced by low doses of cocaine (5 mg/kg) (Vlachou et al., 2003, 2008; Katsidoni et al., 2011). Moreover, this study replicated our previous findings that 1 mg/kg Δ9-THC increases ICSS thresholds. Δ9-THC did not significantly affect the maximal rates of responding at any of the doses tested. There is strong evidence that the presently used ICSS paradigm provides ICSS threshold estimates that are unaffected by performance effects (Miliaressis and Rompre, 1987). This is also evident in the present study, in which the increases in ICSS thresholds produced by Δ9-THC were not accompanied by significant changes in asymptotic rates of responding.
SR141716A administered in a dose that by itself was ineffective in altering ICSS thresholds (0.02 mg/kg) significantly antagonized the reward-facilitating effect of Δ9-THC, indicating that the rewarding effects observed herein are specifically mediated by cannabinoid CB1 receptors. Remarkably, the anhedonic effects of Δ9-THC are also mediated via CB1 receptor stimulation, since they have been blocked by pre-treatment with SR141716A (0.02 mg/kg) in a previous study from our group (Vlachou et al., 2007).
It has been suggested that cannabinoids exhibit rewarding and hedonic-like properties in experimental animals mostly under particular experimental conditions. However, in the present study, a low dose of Δ9-THC induced clear and dose-dependent reward-facilitating effects in ICSS, as already reported for other recreational and abused drugs (Wise, 1996). This substantiates previous findings in the literature. Indeed, according to Gardner and colleagues, Δ9-THC in a dose range of 1 and 1.5 mg/kg lowered ICSS thresholds in Lewis rats but not in Fisher 344 rats, whereas in Sprague–Dawley the effect was very limited and significant only when in the analysis of the data the Θ0 criterion and not the M50 criterion for threshold measure was used (Gardner et al., 1988; Lepore et al., 1996). It can thus be suggested that Lewis rats may have a differential sensitivity to Δ9-THC compared to Sprague–Dawley and Fisher 344 rats. Nevertheless, in our study 1 mg/kg Δ9-THC produced a clear anhedonic effect in Sprague–Dawley rats. Although this finding is in contradiction with previous ICSS results reported in the literature, it could be due to differences in methodology and the experimental design, as has been detailed by Vlachou et al. (2007). Apart from this slight discordance, our results confirm data obtained from conditioned place preference studies in rats, in which 0.1 mg/kg Δ9-THC produces preference (Le Foll et al., 2006), whereas 1 mg/kg produces aversion (Lepore et al., 1995; Parker and Gillies, 1995; Mallet and Beninger, 1998).
It is fundamental to note that other behavioural models of drug reward, such as the self-administration and the conditioned place preference paradigm, have provided inconsistent results with Δ9-THC (Panagis et al., 2008). Indeed, many of the studies have shown Δ9-THC self-administration in rodents only under a limited set of conditions, such as previous drug exposure, food and water deprivation (Deneau and Kaymakcalan, 1971; Takahashi and Singer, 1979; Tanda et al., 2000). However, Justinova and colleagues showed beyond any doubt that low doses of Δ9-THC can initiate and sustain high rates of i.v. self-administration in drug-naive squirrel monkeys (Justinova et al., 2003). The self-administration of Δ9-THC in the latter study has been attributed to the rapid rate at which Δ9-THC was infused and the range of the doses tested. In a more recent study, the self-administration of Δ9-THC was antagonized by a systemic injection of SR141716A, indicating that it was mediated by the CB1 receptor (Justinova et al., 2008). Importantly, the pattern of self-administration with other cannabinoids also reveals a biphasic effect, showing both positive reinforcing and aversive effects, depending on the dose used (Martellotta et al., 1998; Braida et al., 2001b).
As previously mentioned, several studies have shown that Δ9-THC and other cannabinoids produce dose-dependent conditioned effects in the conditioned place preference paradigm. Thus, at high doses, both Δ9-THC and synthetic cannabinoid agonists produce conditioned place aversion (Lepore et al., 1995; McGregor et al., 1996; Sanudo-Pena et al., 1997; Chaperon et al., 1998; Hutcheson et al., 1998; Mallet and Beninger, 1998; Cheer et al., 2000; Valjent and Maldonado, 2000; Robinson et al., 2003), whereas lower doses have been shown to produce conditioned place preference (Lepore et al., 1995; Valjent and Maldonado, 2000; Braida et al., 2001a, 2004). Both the conditioned place preference observed at low doses and the conditioned place aversion observed at high doses have been blocked by the CB1 receptor antagonist SR141716A (Chaperon et al., 1998; Braida et al., 2001a, 2004). This bidirectional effect in reward is also reported in the present study. Overall, these findings indicate that the motivational responses of Δ9-THC are dose-dependent and directly mediated by the CB1 receptor.
Interestingly, biphasic effects have been also described with Δ9-THC and other cannabinoid agonists in other emotional-related behaviours. For instance, several studies that have been carried out using various animal models of anxiety in rodents report that Δ9-THC and other cannabinoid agonists display a dose-dependent biphasic profile, with low doses producing anxiolytic-like responses, whereas higher doses produce anxiogenic-like and aversive responses (Onaivi et al., 1990; Berrendero and Maldonado, 2002; Valjent et al., 2002; Patel and Hillard, 2006; Braida et al., 2007). Similarly, in human users, cannabis derivatives can produce opposite effects, varying from euphoria (high) to dysphoria and from relaxation to anxiety or even panic (Hart et al., 2002; Wachtel et al., 2002).
Importantly, biphasic effects of cannabinoids have been also reported on other aspects of brain function, such as neurotransmitter release (Tzavara et al., 2003). Moreover, a biphasic effect of Δ9-THC has been reported on cerebral metabolism using the 2-deoxyglucose autoradiographic imaging technique, with a low dose of 0.2 mg/kg causing an increase of metabolism in cortical and limbic structures, whereas higher doses of 2 mg/kg cause a reduction of metabolism in these regions (Margulies and Hammer, 1991). Thus, we hypothesize that the reward-facilitating effect of the low dose of Δ9-THC could be related to the reported increase in metabolism in limbic structures (Margulies and Hammer, 1991) and the increased dopamine release in the shell of the nucleus accumbens (Tanda et al., 1997).
According to the results of the second study, Δ9-THC influenced locomotion in a dose-dependent biphasic manner. The low dose of 0.1 mg/kg increased, whereas the higher dose of 1 mg/kg decreased, spontaneous motor activity. The hyperactivity produced by the low dose of Δ9-THC was accompanied by a profound increase in investigatory responses, as indicated by the increased rearing counts. In contrast, the high dose of Δ9-THC, although it produced hypoactivity, did not influence investigatory behaviour, since the rearing counts were not further suppressed. Interestingly, dose inducing hyperactivity coincides with the dose that decreased ICSS thresholds (present study) and produces place preference (Le Foll et al., 2006). Thus, the relationship between these effects induced by the low dose of Δ9-THC is explicit.
Biphasic dose-dependent effects of Δ9-THC and other cannabinoid agonists on spontaneous motor activity have been reported by several studies (Sanudo-Pena et al., 2000; Jarbe et al., 2002; Wiley and Martin, 2003; Le Foll et al., 2006; Smirnov and Kiyatkin, 2008; Polissidis et al., 2010). Thus, typically low doses of cannabinoids increase and higher doses decrease motor activity and produce catalepsy, although several pharmacological (i.e. dose and route of administration) and non-pharmacological (i.e. rat phenotype, habituation and reaction to novelty, influence of the light/dark cycle) factors significantly influence these effects. In most studies demonstrating effects of Δ9-THC on motor activity, the behavioural responses were examined for 1 h after drug administration. McMahon and Koek report that the hypoactivity induced by Δ9-THC was maximal from 1 to 2 h after drug administration and was not detected after 4 h (McMahon and Koek, 2007). In line with this finding, we observed that the effects of Δ9-THC on spontaneous motor activity were maximal from 1 to 2 h after drug administration. Moreover, we observed that hyperlocomotion induced by the low dose of Δ9-THC lasts longer than the hypolocomotion induced by the higher dose.
The hyperlocomotion and the hypolocomotion observed after low and high doses of Δ9-THC, respectively, were reversed by pre-treatment with SR141716A. Importantly, there were no statistically significant changes in motor activity when SR141716A (0.02 mg/kg) was administered alone. Similar results have been obtained in a previous study by our group with the CB1 receptor agonist WIN55,212-2 (Vlachou et al., 2008). These findings indicate that Δ9-THC exhibited its actions through CB1 receptor stimulation.
The neuroanatomical substrate that mediates the rewarding and psychomotor stimulant effects of cannabinoids has been identified by intracranial microinjections in rats. Microinjections of Δ9-THC into the posterior ventral tegmental area and the posterior shell of the nucleus accumbens increase locomotion and produce conditioned place preference, an effect that is blocked by SR141716A (Zangen et al., 2006). Moreover, Δ9-THC is directly self-administered into the posterior ventral tegmental area and the shell of the nucleus accumbens of rats (Zangen et al., 2006). Noteworthy, in the present study, the stimulating electrodes for ICSS have been implanted in the medial forebrain bundle, which connects these structures.
In conclusion, we have demonstrated in two separate experimental paradigms that low doses of Δ9-THC induce opposite effects from high doses of Δ9-THC. Specifically, 0.1 mg/kg Δ9-THC decreased ICSS thresholds and produced hyperactivity, whereas 1 mg/kg increased ICSS thresholds and produced hypoactivity. Both effects were reversed by pre-treatment with SR141716A, indicating the involvement of CB1 receptors on these actions. For several years, brain stimulation reward has remained the most ambiguous aspect of cannabinoid-related reward. Our experiments revealed that Δ9-THC can produce acute activating effects in locomotion that coincide with its reward-facilitating effects in the ICSS paradigm. The present data are part of a growing body of evidence indicating that Δ9-THC induce behaviours typical of abuse and substantiate the notion that marijuana resembles other drugs of abuse.
Acknowledgements
This study was supported by the Department of Psychology of the University of Crete. Vicky Katsidoni was supported by a scholarship from the Research Committee of the University of Crete (C. Spyraki scholarship).
Statement of Interest
None.
References