-
PDF
- Split View
-
Views
-
Cite
Cite
J. May, C. H. Chan, A. King, L. Williams, G. L. French, Time–kill studies of tea tree oils on clinical isolates, Journal of Antimicrobial Chemotherapy, Volume 45, Issue 5, May 2000, Pages 639–643, https://doi.org/10.1093/jac/45.5.639
Close - Share Icon Share
Abstract
Tea tree oil has recently emerged as an effective topical antimicrobial agent active against a wide range of organisms. Tea tree oil may have a clinical application in both the hospital and community, especially for clearance of methicillin-resistant Staphylococcus aureus (MRSA) carriage or as a hand disinfectant to prevent cross-infection with Gram-positive and Gramnegative epidemic organisms. Our study, based on the time–kill approach, determined the kill rate of tea tree oil against several multidrug-resistant organisms, including MRSA, glycopeptide-resistant enterococci, aminoglycoside-resistant klebsiellae, Pseudomonas aeruginosa and Stenotrophomonas maltophilia, and also against sensitive microorganisms. The study was performed with two chemically different tea tree oils. One was a standard oil and the other was Clone 88 extracted from a specially bred tree, which has been selected and bred for increased activity and decreased skin irritation. Our results confirm that the cloned oil had increased antimicrobial activity when compared with the standard oil. Most results indicated that the susceptibility pattern and Gram reaction of the organism did not influence the kill rate. A rapid killing time (less than 60 min) was achieved with both tea tree oils with most isolates, but MRSA was killed more slowly than other organisms.
Introduction
The emergence of resistant microorganisms in hospitals and the community is causing problems for both the treatment of patients and infection control. Organisms of particular concern include methicillin-resistant Staphylococcus aureus (MRSA), glycopeptide-resistant enterococci (GRE), gentamicin-resistant and extended-spectrum β- lactamase-producing klebsiellae, and multi-resistant pseudomonads.1–3 All these organisms are commonly transferred from patient to patient on staff hands.
A recent major review of antibiotic resistance emphasized the importance of hospital infection control, and the control of these organisms,4 and many authorities have reiterated the key role of hand-washing with appropriate disinfectants in this process.5,6
Mupirocin nasal ointment (for staphylococci) and hand disinfectants are widely used to control the carriage and spread of these organisms, but resistance is increasing and eradication with current agents is not always successful.7–12 Alternative strategies are required and more effective agents are needed.
Tea tree oil is obtained by steam distillation of the leaves of Melaleuca alternifolia, a tree native to Australia, and is reported to have antibacterial, antifungal, antiviral, anti-inflammatory and analgesic properties.13 Currently, tea tree oil is used in cosmetics and healthcare products and has recently re-emerged as an effective antiseptic.14 A limited number of published controlled clinical trials support this latter use.15–17
The concentrations of tea tree oil found in commercially available products range from 2 to 5%.18 Terpinen-4-ol is the main antimicrobial component but other components, such as α-terpineol, also have antimicrobial activities similar to those of terpinen-4-ol.19,20 Previous studies of the in vitro activity of tea tree oil have employed broth dilution and disc diffusion techniques.14,18,21 We have performed time–kill studies with a standard tea tree oil and an oil extracted from the superior tree (Clone 88) with increased concentrations of terpinen-4-ol,22 to determine the killing effect of the oils against clinically significant bacterial isolates.
Materials and methods
Microorganisms
We examined clinical isolates from Guy's and St Thomas' Hospitals, chosen for their varying degrees of sensitivity and resistance to a range of antibiotics. Isolates of MRSA, GRE and multidrug-resistant Klebsiella species, isolated from outbreaks of hospital infection, were included. The isolates included were S. aureus (four isolates: two methicillin sensitive, two methicillin resistant/vancomycin tolerant), Enterococcus faecium (four: one vancomycin sensitive, three vancomycin resistant), Enterococcus faecalis (three: two vancomycin sensitive, one vancomycin resistant), Pseudomonas aeruginosa (four: two gentamicin sensitive, two gentamicin resistant), Stenotrophomonas maltophilia (two) and Klebsiella pneumoniae (three: one gentamicin sensitive, two gentamicin resistant). Organisms were stored at –70°C in glycerol broth. Fresh subcultures were used for each experiment.
Tea tree oils
We tested two types of tea tree oil supplied by The Oil Fields (Sydney, NSW, Australia). Standard oil had a 34.8% terpinen-4-ol and 5.5% cineole content, whereas the Clone 88 oil had a 43.1% terpinen-4-ol and a 1% cineole content.
Preparation of inocula
Inocula for the time–kill determinations were prepared following NCCLS guidelines.23 Organisms were grown overnight at 37°C in Brain–Heart Infusion broth (BHI; Oxoid, Basingstoke, UK). The overnight broth was adjusted to a 0.5 McFarland standard in IsoSensitest broth (ISB; Oxoid, Basingstoke, UK) and a further dilution was made by inoculating 200 μL in glass flasks containing 20 mL of ISB. The final bacterial concentration in the flasks was (1–5) × 105 cfu/mL. The flasks for all isolates were shaken at 150 rpm (Certomat, B. Braun Biotech, Aylesbury, UK) for 90 min at 37°C (120 min for the pseudomonads) to ensure that the organisms were out of their lag phases and into their logarithmic phases.
Time–kill studies
The time–kill studies were performed according to NCCLS guidelines.23 Each isolate was inoculated into three flasks, one as a growth control and one for each oil type. After the 90 min (or 120 min) initial incubation period, an aliquot of the broth was taken from each flask to determine the initial inoculum. The tea tree oil and Tween 80 (Sigma–Aldrich Ltd, Poole, UK) were pre-mixed and added to each designated flask; Tween 80 only was added to the control flask. The final concentrations of the oil and Tween 80 were 5% and 0.5%, respectively. All flasks were shaken at 150 rpm at 37°C, and samples subcultured at 0, 0.5, 1, 1.5, 2, 4 and 6 h for all isolates except the enterococci. The enterococci were subcultured at 0, 10, 20, 30, 60, 90 and 120 min. Colony counts were performed by making appropriate dilutions in physiological saline, plating 100 μL of each dilution on to pre-warmed blood agar (Columbia base agar, Oxoid) supplemented with 7% sterile defibrinated horse blood (TCS Botolph, Clayton, UK) and incubated for 48 h at 37°C. Viable counts were calculated to give cfu/mL, and kill curves were plotted with time against the logarithm of the viable count. Each experiment was performed twice on separate occasions.
Determining time–kill endpoints
A bactericidal effect is defined as a 3 log decrease in the cfu/mL or a 99.9% kill over a specified time.23 The definition of kill for this study has been described by May et al.,24 together with modifications based on a suggestion by Handwerger & Tomasz that a kill can be determined at 6 h.25 A constant logarithmic rate of kill has been assumed during a time–kill. A 90% kill at 6 h is equivalent to a 99.9% kill at 24 h. In this study the kill measurement was determined by the actual reduction in viable counts at 6 h for each isolate.
Results
Measurements of time of killing by the two oils were made for each isolate and the activity of the standard oil was compared with that of the cloned oil. Repeat experiments gave similar results for all isolates and the growth controls showed at least a 103 increase in bacterial numbers by 6 h. The oil of Clone 88 showed greater or the same activity against all isolates in comparison with the standard oil.
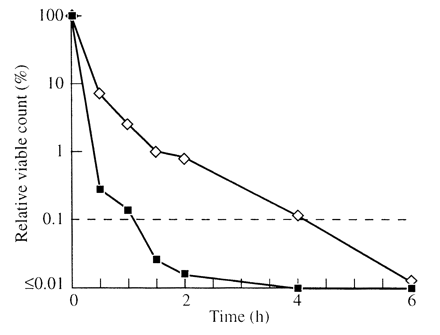
The two tea tree oils were more active against methicillin-sensitive S. aureus (MSSA) than against MRSA. Both isolates of MSSA were killed within 30 min by both oils. The standard oil killed the MRSA by 6 h (Figure 1). The cloned oil killed one of the two isolates of MRSA by 1.5 h but the other isolate was not killed until 3.5 h.
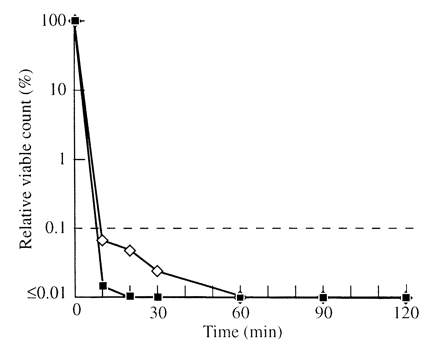
All enterococci were killed within 60 min by the standard oil. The decrease in viable count was much more rapid with the standard oil for E. faecium than for E. faecalis, requiring 10 and 20 min, respectively, to achieve a 99% decrease. All of the enterococci were killed within 10 min by the cloned oil (Figure 2).
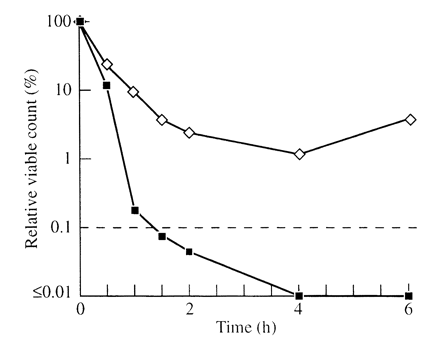
The standard tea tree oil did not kill any of the isolates of P. aeruginosa: after an initial decrease in viable count during the first 2 h there was a subsequent regrowth over the next 4 h. The cloned oil killed three of the four isolates within 1.5 h and the fourth isolate showed a 99% decrease in viable count after 1 h and the count remained at this level for the remaining 5 h (Figure 3).
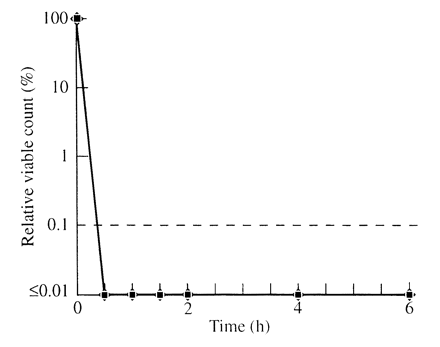
The isolates of K. pneumoniae (Figure 4) were all killed by 30 min by both oils and there was no difference between the antibiotic-sensitive and -resistant isolates of this species. The isolates of S. maltophilia were also all killed by 30 min and the kill curves were indistinguishable from those of K. pneumoniae.
Discussion
The incidence of multi-resistant organisms is increasing and is a problem of global concern. As resistance to antibiotics increases, so too does the importance of infection control. The application of various nasal disinfectants has proved useful in eliminating carriage of MRSA, and hand disinfectants are important for the prevention of transmission of many organisms on staff hands. However, resistance to these disinfectants is increasing and has prompted a search for new topical disinfectants. Our in vitro results suggest that tea tree oil of Clone 88 might be useful as a topical agent for the control of these organisms.
Many claims have been made about the wonders of tea tree oil but few clinical studies have been published. However, in vitro susceptibility studies of its activity against a variety of organisms suggest that tea tree oil may have a place in modern medicine.
Tea tree oil is a complex mixture of terpenes and related alcohols with over 100 components.26 However, up to 90% of the whole oil content is made up by the following components: terpinen-4-ol, 1,8-cineole, α-terpineol, terpinolene and α- and γ-terpinene. International standards of tea tree oil have been most recently defined by ISO 4730.27 The main antimicrobial component is terpinen-4-ol. Recent studies indicate that the component 1,8-cineole, previously held responsible for undesirable side effects (mucus and skin irritation), is now thought not to be the agent responsible for hypersensitivity reactions.28,29 Cytotoxicity through the use of high concentrations of tea tree oil has been reported; however, limited data are available, suggesting that more in vivo work is required to confirm this.30,31 In this study an oil concentration of 5% in broth was chosen to reflect the normal concentration found in some commercially available products.18
The two oils provided were chemically different. The standard oil provided a concentration of terpinen-4-ol and 1,8-cineole found in commercial preparations of tea tree oil. The oil of Clone 88 was selected for its higher content of the active ingredient terpinen-4-ol and its lower content of 1,8-cineole.14 Oils with increased concentrations of terpinen-4-ol have displayed enhanced antimicrobial activity20 and our results support this.
Most reported studies on tea tree oil are based on the measurement of MICs and MBCs. One limitation of these procedures is the inability of the method to determine how quickly an agent acts on the organisms. In our studies, we assessed the viability of the organisms against tea tree oil with time–kill determinations. This method determines the viability of the organisms after contact with the oil for a specified time period.
The results of the time–kill studies show the oil of Clone 88 to be more or equally effective compared with the standard oil. Some organisms such as Klebsiella spp. and S. maltophilia were rapidly killed by both oils but organisms that were killed more slowly showed a greater susceptibility to the cloned oil than to the standard oil.
There were differences in the activity of the oils against the different genera of bacteria and even within some genera, notably the enterococci. The standard oil produced a significantly more rapid decrease in viable count during the first 10 min for E. faecium than for E. faecalis but the cloned oil produced a rapid kill within 10 min for both species. MRSA isolates were killed more slowly than MSSA, and the standard oil was less effective than the cloned oil. Although we have not tested S. epidermidis, previous MIC studies found S. aureus to be more susceptible to tea tree oil than was S. epidermidis.21
The antibiotic susceptibility pattern for the organisms did not appear to predict the activity of the oil against most of the organisms. For enterococci and klebsiellae there was no difference in kill rate if the organism was multi-resistant. However, the oils were less active against antibioticresistant isolates of staphylococci and P. aeruginosa and, against these isolates, the cloned oil was more active than the standard oil.
Previous studies have reported MICs and MBCs of tea tree oil against the same species of organisms that we have tested by the time–kill approach.13,21 These report tea tree oil MICs of 0.12–0.5% for S. aureus (including MRSA) and K. pneumoniae, 0.5–1% for vancomycin-resistant enterococci and 2–5% for P. aeruginosa, and MBCs of 0.12–0.5% for K. pneumoniae, 0.25–2% for S. aureus and 2–5% for P. aeruginosa. These differences in MIC and MBC results for tea tree oil against the species are reflected in our time–kill studies.
There were problems with time–kill studies of P. aeruginosa. Despite the extra time provided for the initial inoculum to achieve exponential phase, some isolates of P. aeruginosa could not be used in our tests because they had not reached their logarithmic phase of growth in that time. Results of isolates exhibiting regrowth were repeated to ensure reproducibility. It has been demonstrated previously that P. aeruginosa required a higher concentration of oil to produce an effective kill.21,32
There are also inherent problems to performing MIC, MBC and time–kill determinations with tea tree oil. The oil is insoluble in water and must be solublized by the use of detergents or emulsifiers.33 In this study, Tween 80 was used, and it is important to mix the Tween 80 with the oil before the addition of the oil into the shaking flask. Unfortunately, the oil–Tween 80 mixture forms a turbid suspension. This prevents visual determination of growth in the flasks and hinders the reading of endpoints in broth MICs.34
The mode of action of tea tree oil is unclear. A recent study has shown that tea tree oil stimulates autolysis in exponential and stationary phase cells of Escherichia coli.35 This study also showed that exponentially growing cells were more susceptible to autolysis by tea tree oil than are stationary cells.
Multi-resistant organisms are difficult to eradicate from skin, and staphylococci, enterococci and klebsiellae are transmitted by direct contact.36 Adherence to infection control protocol (e.g. hand-washing) is critical to reduce transmission but effective hand disinfectants are also required. One study has shown that tea tree oil is more active against the organisms associated with transient carriage than against commensal skin flora and thus may be useful in eliminating the transient flora while suppressing but maintaining commensal flora.21 The rapid kill rate of Clone 88 compared with standard tea tree oil shown for most of the organisms tested in the present study is encouraging and suggests that further clinical studies should be carried out. Tea tree oil in a topical formulation might eliminate organisms from carriage sites such as the hairline, axilla, nares, groin and perineum, and incorporation of tea tree oil in hand-washing formulations may reduce the transmission of many multi-resistant organisms associated with nosocomial infections.
Killing of methicillin-resistant Staphylococcus aureus by tea tree oils. ⋄, standard tea tree oil; ▪, oil of Clone 88; dashed line, 99.9% kill.
Corresponding author. Tel: +44-171-928-9292, ext. 2456; Fax: +44-171-928-0730; E-mail: anna.king@kcl.ac.uk
We would like to thank Kevin Shannon and Connie Lynn for their valuable assistance in this study, The Oil Fields Pty Ltd for providing the funding for this study, and Yan Wang and Justine Stockley for providing the gas chromatographic analysis of the oil samples.
References
French, G. L. & Phillips, I. (1996). Antimicrobial resistance in hospital flora and nosocomial infections. In Hospital Epidemiology and Infection Control, (Mayhall, C., Ed.), pp. 980–999. Williams and Wilkins, Baltimore, MD.
French, G. L. & Phillips, I. (1997). Resistance. In Antibiotic and Chemotherapy: Anti-infective Agents and their Use in Therapy, 7th Edn, (O'Grady, F., Lambert, H. P., Finch, R. G. & Greenwood, D., Eds), pp. 23–43. Churchill Livingstone, New York.
French G. L. (1998). The emergence of multiresistant Grampositive infections. In Treatment of Gram-positive Infections: the Role of Quinupristin/Dalfopristin, (Moellering, R., Ed.) pp. 115–31. ParmaLibri, Montreal.
House of Lords Select Committee on Science and Technology. (1998). Resistance to Antibiotics and other Antimicrobial Agents. Stationery Office, London.
Larson, E. L. (
Handwashing Liaison Group. (
Irish, D., Eltringham, I., Teall, A., Pickett, H., Farelly, H., Reith, S. et al. (
Kampf, G., Jarosch, R. & Rüden, H. (
Wade, J. J., Desai, N. & Casewell, M. W. (
Kjolen, H. & Andersen, B. M. (
Bamber, A. I. & Neal, T. J. (
Irizarry, L., Merlin, T., Rupp, J. & Griffith, J. (
Carson, C. F., Riley, T. V. & Cookson, B. D. (
Williams, L. R., Stockley, J. K., Home, V. N. & Yan, W. (
Buck, D. S., Nidorf, D. M. & Addino, J. G. (
Jandourek, A., Vaishampayan, J. K. & Vazquez, J. A. (
Bassett, I. B., Pannowitz, D. L. & Barnetson, R. S. (
Carson, C. F., Cookson, B. D., Farrelly, H. D. & Riley, T. V. (
Carson, C. F. & Riley, T. V. (
Williams, L. R., Home, V. N. & Lusunzi, I. (
Hammer, K. A., Carson, C. F. & Riley, T. V. (
Williams, L. R. & Wang, Y. (1996). Selection of superior trees of Melaleuca species to increase the commercial potential for the production of tea tree oil. In Program and Abstracts of the First Australian New Crops Conference, University of Queensland, Gatton College, 1996, 2. pp. 299–306.
National Committee for Clinical Laboratory Standards. (1992). Methods for Determining Bactericidal Activity of Antimicrobial Agents: Tentative Guideline M26-T. NCCLS, Villanova, PA.
May, J., Shannon, K., King, A. & French, G. (
Handwerger, S. & Tomasz, A. (
Brophy, J. J., Davies, N. W., Southwell, I. A., Stiff, I. A. & Williams, L. R. (
International Organization for Standardization. (1996). International Standard ISO 4730 Oil of Melaleuca, terpinen-4-ol type (Tea tree oil). ISO, Geneva.
de Groot, A. C. & Weyland, J. W. (
Knight, T. E. & Hausen, B. M. (
Faoagali, J., George, N. & Leditschke, J. F. (
Markham, J. (1996). Antimicrobial effectiveness of tea tree oil. AusTTeam Conference Proceedings, pp. 30–7.
Beyleir, M. F. & Givaudan, S. A. (
Carson, C. F., Hammer, K. A. & Riley, T. V. (
Gustafson, J. E., Liew, Y. C., Chew, S., Markham, J., Bell, H. C., Wyllie, S. G. et al. (