-
PDF
- Split View
-
Views
-
Cite
Cite
Valentin Fuhrmann, Peter Schenk, Walter Jaeger, Salwa Ahmed, Florian Thalhammer, Pharmacokinetics of moxifloxacin in patients undergoing continuous venovenous haemodiafiltration, Journal of Antimicrobial Chemotherapy, Volume 54, Issue 4, October 2004, Pages 780–784, https://doi.org/10.1093/jac/dkh421
Close - Share Icon Share
Abstract
Objective: Moxifloxacin is an 8-methoxy quinolone with a broad range of activity against clinically important pathogens. Therefore it is frequently administered in severe respiratory tract infections. Continuous venovenous haemodiafiltration (CVVHDF) is an important extracorporeal renal replacement therapy for intensive care patients suffering from sepsis and multiple organ failure. The aim of this study was to investigate the pharmacokinetics of intravenous moxifloxacin in anuric critically ill patients undergoing CVVHDF.
Patients and methods: Pharmacokinetic analysis was performed in nine intensive care patients with acute renal failure and suspected or proven infection sensitive to moxifloxacin, who received moxifloxacin 400 mg intravenously once daily. The concentration of moxifloxacin in serum and ultradiafiltrate was determined by HPLC.
Results: Peak and trough serum concentrations were 3.76±2.02 mg/L and 0.24±0.14 mg/L, respectively, at the arterial port after the first dose. The mean elimination half-life was 9.87±3.26 h, the volume of distribution 270±133 L and the calculated AUC0–24 18.41±8.46 mg·h/L. Total clearance was 19.09±8.22 L/h and the clearance of haemodiafiltration 1.63±0.33 L/h.
Conclusions: The pharmacokinetics of moxifloxacin in critically ill patients with acute renal failure undergoing CVVHDF was comparable to healthy subjects and patients without renal impairment. We recommend 400 mg of intravenous moxifloxacin once per day in anuric patients during CVVHDF.
Introduction
Moxifloxacin (Avelox; Bayer Corporation, Leverkusen, Germany) is an 8-methoxy quinolone with a broad range of activity against clinically important pathogens. It has extended antimicrobial activity and is increasingly administered to critically ill patients with respiratory tract infections.1,2 The bactericidal activity is achieved by inhibition of DNA gyrase and topoisomerase IV, which are required for bacterial DNA relocation, transcription, repair and recombination.3 Intravenous (iv) administration of a standard dose of 400 mg of moxifloxacin results in peak plasma concentrations in the range 3.62–5.09 mg/L.4,5 The volume of distribution is 3.3 L/kg and the protein binding is ∼47%.2 Total clearance and renal clearance of iv moxifloxacin are in the range of 9.1–11.6 L/h and 1.3–2.6 L/h, respectively.4,5 Its mean terminal half-life is about 12 h.2 In patients with renal impairment, no dosage adjustment is required.6
In intensive care patients suffering from sepsis and multiple organ failure, continuous venovenous haemodiafiltration (CVVHDF) is an important supportive extracorporeal renal replacement therapy. The drug elimination of renal replacement therapy is determined by physicochemical properties of the drug (protein-binding, volume of distribution, molecular charge, molecular weight) and characteristics of the renal replacement technique used (type of filter, blood flow rate, usage of counter-current dialysis, ultrafiltration rate, adsorption of the drug onto the filter).7,8
No dosage recommendation for iv moxifloxacin is available for patients undergoing renal replacement therapy. Taking into account the pharmacokinetic properties of moxifloxacin, we suggested that no dosage adaptation will be necessary during CVVHDF. However, as renal clearance data indicate that the mechanism of moxifloxacin excretion is glomerular filtration with partial tubular reabsorption,9 and as tubular reabsorption does not occur in anuric patients undergoing CVVHDF, the drug clearance might probably be higher than in physiological renal function, as reported, for example, in the case of fluconazole.10 The aim of our study was to investigate the pharmacokinetics of iv moxifloxacin in anuric critically ill patients undergoing CVVHDF.
Patients and methods
Patients
Nine intensive care patients (seven males, two females) with acute renal failure and suspected or proven infection sensitive to moxifloxacin were included in the study. Demographic characteristics are presented in Table 1. Mean age, body height and body weight were 56±17 years, 173±7 cm and 83±13 kg, respectively. Mean serum creatinine level was 382±223 μmol/L prior to CVVHDF. All patients were anuric. Haemodialysis was not employed during this study. Concomitant drug therapy consisted mainly of iv catecholamines (n=6), anticoagulation with heparin (n=8) or danaparoid sodium (n=1), morphine derivatives (n=4), midazolam (n=4) and ketamine (n=1). None of the patients received albumin substitution. All drugs were administered as clinically indicated by the attending physician. None of the patients had a known hypersensitivity or other intolerance to moxifloxacin or other fluoroquinolones. Patients with a history of convulsions, documented arthropathia or cartilage damage due to previous quinolone therapy, requirement for conventional haemodialysis rather than CVVHDF and QT-prolongation were excluded. The study was performed in accordance with local ethic committee requirements.
CVVHDF
CVVHDF was performed using an AN 69 HF hollow fibre haemofilter/dialyser (Prisma M100 Pre Set; Hospal Industrie, Meyzieu, France). Filters and lines were steam sterilized. No filter change was performed during the study period. The standard blood flow rate was 9 L/h. Pre-dilution fluid was infused at a rate of 1 L/h and dialysate flow was 1 L/h. Net fluid balance was adjusted according to clinical requirements (0–100 mL/h). Mean actual ultrafiltration rate was 1.01±0.07 L/h.
Drug administration and sampling
All patients received three doses of 400 mg moxifloxacin once daily injected over a period of 40 min into a central venous catheter, different from the venous catheter used for CVVHDF. Blood samples were drawn from the arterial (input) and venous (output) line of the extracorporeal circuit before and immediately after the end of the infusion, as well as 30, 140, 320, 680 and 1400 min after the end of the infusion; further blood samples were drawn immediately prior to, and 40 and 70 min after, the start of consecutive moxifloxacin infusions. Ultradiafiltration samples, collected from the outlet of the ultradiafiltrate compartment of the haemodiafilter, were taken at corresponding times. All samples were centrifuged immediately and stored at −70°C until analysis.
Drug assay
The concentration of moxifloxacin in serum and ultradiafiltrate was determined by HPLC. Briefly, after the addition of 750 μL of acetonitrile to 250 μL of serum or ultradiafiltrate, the samples were centrifuged (5000g for 5 min at 4°C) and 20 μL of the supernatant was injected onto the HPLC column. The chromatographic assay included a Merck ‘La Chrom’ system (Merck, Darmstadt, Germany), equipped with an L-7250 injector, an L-7100 pump, an L-7300 column oven (set at 35°C to keep the retention times constant), a D-7000 interface and an L-7480 fluorescence detector (excitation 296 nm, emission 504 nm). Separation of moxifloxacin was carried out using a Hypersil BDS-C18 column (5 μm, 250 × 4.6 mm I.D.; Thermo Hypersil–Keystone, Astmoor, UK) preceded by a Hypersil BDS-C18 pre-column (5 μm, 10 × 4.6 mm I.D.) at a flow rate of 1 mL/min. The mobile phase A consisted of potassium phosphate (50 mM, pH 4.0 with phosphoric acid) and heptanesulphonic acid (5 mM) and the mobile phase B consisted of methanol. The mobile phase was filtered through a 0.45 μM filter (HVLP04700; Millipore, Vienna, Austria). The gradient ranged from 30% methanol (0 min) to 80% B at 20 min and decreased linearly to 30% again at 22 min. The columns were allowed to re-equilibrate for 8 min between runs. Linear calibration curves were performed from the peak areas of moxifloxacin to the external standard by spiking drug-free human serum and ultradiafiltrate with standard solutions of moxifloxacin (final concentration in the range 5 ng–25 μg/mL).11 Detection limits, defined as a signal-to-noise-ratio of 3, ranged from 473 pg/mL for serum and 421 pg/mL for ultradiafiltrate. Intra-day variability was in the range 1.8%–4.5% and inter-day variability 2.8%–5.3% using moxifloxacin concentrations of 10, 100 and 1000 ng/mL serum.
Pharmacokinetic analysis
The serum concentration–time curves of moxifloxacin in plasma were adjusted to the data sets via non-linear iterative least-square regression analysis. Curve modelling was performed using the two-compartment open pharmacokinetic model with the program WinNonlin (version 1.5; Scientific Consulting, USA). The following parameters were calculated: area under the concentration curve from 0 to infinity (AUC) and area under the concentration curve from 0 to 24 h (AUC0–24) using the linear trapezoidal rule, total clearance (CLtot), volume of distribution (V), distribution half-life (t1/2α) and elimination half-life (t1/2β). The sieving coefficient (S) was calculated as S = CUDF/Ca, where CUDF is the concentration of moxifloxacin in the ultradiafiltrate and Ca is the concentration of moxifloxacin in the arterial (input) line of the extracorporeal circuit. The clearance of haemodiafiltration (CLCVVHDF) was determined according to the formula CLCVVHDF=(QUF + QD)×(CUDF/Ca)=(QUF + QD)×S, where QUF is the ultrafiltration rate and QD is the dialysation rate. The different values obtained in a patient were aggregated by arithmetic averaging. The rate of elimination was calculated as (QUF + QD)×CUDF, which represents the recovery in the filtrate–dialysate, and as (Ca–Cv)×QB, where QB is the blood flow rate, which represents the elimination of drug from blood circulation. Total removal (Retot) of the drug was calculated as Retot=[(Ca(0)–Camin)/Ca(0)]×100, where Ca(0) refers to the arterial serum concentration after the back-extrapolation from the terminal log-linear segment of the concentration curve up to the mid-infusion time, and Camin refers to the arterial serum concentration prior to the second infusion of moxifloxacin, respectively. Removal of moxifloxacin via haemodiafiltration (ReCVVHDF) was calculated as ReCVVHDF = (CLCVVHDF/CLtot)×100.
Results
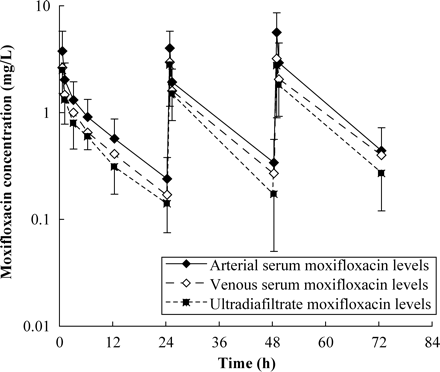
Moxifloxacin was well tolerated by all patients. Peak serum concentrations after the first dose were 3.76±2.02 mg/L at the arterial port and 2.64±0.68 mg/L at the venous port, after the second dose 4.01±1.78 mg/L at the arterial port and 2.97±1.51 mg/L at the venous port and after the third dose 5.65±2.96 mg/L at the arterial port and 3.20±0.90 mg/L at the venous port. Trough serum levels were 0.24±0.14 mg/L at the arterial port and 0.17±0.10 mg/dL at the venous port prior to the second dose, 0.34±0.22 mg/L at the arterial port and 0.27±0.15 mg/L at the venous port prior to the third infusion and 0.43±0.28 mg/L at the arterial port and 0.40±0.15 mg/L at the venous port after 72 h of the study period. Mean t1/2α was 0.53±0.39 h and mean t1/2β was 9.87±3.26 h; mean V of moxifloxacin was 270±133 L. The AUC and the AUC0–24 were 24.95±11.25 mg·h/L and 18.41±8.46 mg·h/L, respectively. Ca(0) was 1.46±0.85 mg/L and Retot 82.02%±9.47%. The sieving coefficient was 0.84±0.16. CLtot was 19.09±8.22 L/h and CLCVVHDF 1.63±0.33 L/h. Mean ReCVVHDF during the study period was 9.99%±4.25%. The mean difference in moxifloxacin concentration between the arterial and venous line was 21.55%±9.46%. The mean rate of elimination calculated via (QUF+QD)×CUDF was 2.23 ± 0.75 mg/h and the mean rate of elimination calculated via (Ca–Cv)×QB was 2.39±2.01 mg/h.
The pharmacokinetics of moxifloxacin during CVVHDF are summarized in Table 2. The serum concentration versus time profile is illustrated in Figure 1.
Discussion
The pharmacokinetics of orally administrated moxifloxacin is influenced only slightly by renal impairment.6 Although iv moxifloxacin is constantly used in the treatment of serious infections, no study investigating its pharmacokinetics in patients with renal insufficiency with or without renal replacement therapy has been published previously. The aim of this study was to investigate the pharmacokinetics of moxifloxacin in critically ill patients with acute renal failure undergoing CVVHDF.
Peak and trough moxifloxacin serum concentrations in our study were consistent with previous reports in healthy volunteers and patients with renal dysfunction.2,4–6,12,13t1/2 in our patients was in reasonable concurrence with published data in healthy volunteers.12,13 We observed a higher V compared with previous studies.4,6,12 Our patients' AUC was smaller and the CLtot higher than in patients not critically ill.2,4–6,12,13 This may be explained by physiological changes in critically ill patients (fluid-overloaded state, low albumin levels, capillary leakage). According to this hypothesis, Simon et al.14 reported a comparably high CLtot of moxifloxacin in mechanically ventilated intensive care patients, as did we in our patients.
The CLCVVHDF of moxifloxacin in our study is comparable with its renal clearance in healthy volunteers.12 The haemodialysis clearance of 400 mg moxifloxacin administered orally once per day is reported to be 5.7 L/h.15 In contrast, only 0.24 L/h of 400 mg moxifloxacin per os is eliminated via continuous ambulatory peritoneal dialysis,16 indicating varying moxifloxacin clearances via different renal replacement modalities. However, as the renal and extracorporeal clearance only count for about 10%–20% of the CLtot,6,15 the overall pharmacokinetics of moxifloxacin is barely affected by different renal replacement therapies.
AUC0–24/MIC90 and Cmax/MIC90 are helpful for dosing of fluoroquinolones. Cmax/MIC > 10 is reached at least for pathogens with MIC90<0.38 mg/L in our patients. In regard to AUC0–24/MIC90>30 h, which is required for fluoroquinolone bactericidal activity against Streptococcus pneumoniae,17 pneumococcal infections (reported MIC90 0.25 mg/dL)18 seem to be sufficiently covered by 400 mg iv moxifloxacin once daily in patients undergoing CVVHDF.
Reports on the behaviour of fluoroquinolones during continuous renal replacement therapy are controversial. Dosage recommendations for levofloxacin that is excreted primarily unchanged via the kidneys, vary between 250 and 1000 mg/day.19,20 For ciprofloxacin, another antimicrobial agent frequently used in critically ill patients with severe infections, dosage recommendations range from one third of the regular dose to no dosage reduction in selected patients.19,21
In conclusion, this study provides the first pharmacokinetic data of iv moxifloxacin during renal replacement therapy. Its pharmacokinetics in critically ill patients with acute renal failure undergoing CVVHDF is comparable to healthy subjects and patients without renal impairment. As 400 mg moxifloxacin iv once daily provides sufficient bactericidal activity, we recommend no dosage adaptation in anuric critically ill patients undergoing CVVDHF.
The study was performed at the Department of Internal Medicine 4, Intensive Care Unit, University Hospital Vienna, Austria
Moxifloxacin levels drawn from the arterial (input) (filled diamonds) (arithmetic mean values ± S.D.) and venous (output) (open diamonds) line of the extracorporal circuit and from the ultradiafiltrate fluid (filled squares) (arithmetic mean values ± S.D.) in anuric critically ill patients undergoing continuous venovenous haemodiafiltration.
Patient characteristics
| Patient number . | Sex . | Age (years) . | Ht (cm) . | Wt (kg) . | APACHE III score . | Diagnosis . | Clinical outcome . | Microbiological finding . |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 60 | 172 | 82 | 75 | CAP, septic shock | survived | Haemophilus influenzae |
| 2 | M | 73 | 173 | 69 | 34 | CAP | died | |
| 3 | F | 74 | 160 | 70 | 43 | CAP, cardiac failure | died | |
| 4 | M | 54 | 173 | 80 | 126 | COPD, CAP | survived | Klebsiella pneumoniae |
| 5 | M | 36 | 182 | 110 | 88 | CAP, liver cirrhosis | died | |
| 6 | M | 40 | 180 | 89 | 62 | CAP, septic shock | died | Streptococcus pneumoniae |
| 7 | M | 59 | 181 | 87 | 42 | CAP | survived | Streptococcus pneumoniae |
| 8 | F | 73 | 165 | 95 | 66 | cardiomyopathia, CAP | survived | |
| 9 | M | 51 | 172 | 80 | 58 | CAP | survived |
| Patient number . | Sex . | Age (years) . | Ht (cm) . | Wt (kg) . | APACHE III score . | Diagnosis . | Clinical outcome . | Microbiological finding . |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 60 | 172 | 82 | 75 | CAP, septic shock | survived | Haemophilus influenzae |
| 2 | M | 73 | 173 | 69 | 34 | CAP | died | |
| 3 | F | 74 | 160 | 70 | 43 | CAP, cardiac failure | died | |
| 4 | M | 54 | 173 | 80 | 126 | COPD, CAP | survived | Klebsiella pneumoniae |
| 5 | M | 36 | 182 | 110 | 88 | CAP, liver cirrhosis | died | |
| 6 | M | 40 | 180 | 89 | 62 | CAP, septic shock | died | Streptococcus pneumoniae |
| 7 | M | 59 | 181 | 87 | 42 | CAP | survived | Streptococcus pneumoniae |
| 8 | F | 73 | 165 | 95 | 66 | cardiomyopathia, CAP | survived | |
| 9 | M | 51 | 172 | 80 | 58 | CAP | survived |
Ht, height; Wt, weight; M, male; F, female; CAP, community-aquired pneumonia; COPD, chronic obstructive pulmonary disease.
Patient characteristics
| Patient number . | Sex . | Age (years) . | Ht (cm) . | Wt (kg) . | APACHE III score . | Diagnosis . | Clinical outcome . | Microbiological finding . |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 60 | 172 | 82 | 75 | CAP, septic shock | survived | Haemophilus influenzae |
| 2 | M | 73 | 173 | 69 | 34 | CAP | died | |
| 3 | F | 74 | 160 | 70 | 43 | CAP, cardiac failure | died | |
| 4 | M | 54 | 173 | 80 | 126 | COPD, CAP | survived | Klebsiella pneumoniae |
| 5 | M | 36 | 182 | 110 | 88 | CAP, liver cirrhosis | died | |
| 6 | M | 40 | 180 | 89 | 62 | CAP, septic shock | died | Streptococcus pneumoniae |
| 7 | M | 59 | 181 | 87 | 42 | CAP | survived | Streptococcus pneumoniae |
| 8 | F | 73 | 165 | 95 | 66 | cardiomyopathia, CAP | survived | |
| 9 | M | 51 | 172 | 80 | 58 | CAP | survived |
| Patient number . | Sex . | Age (years) . | Ht (cm) . | Wt (kg) . | APACHE III score . | Diagnosis . | Clinical outcome . | Microbiological finding . |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 60 | 172 | 82 | 75 | CAP, septic shock | survived | Haemophilus influenzae |
| 2 | M | 73 | 173 | 69 | 34 | CAP | died | |
| 3 | F | 74 | 160 | 70 | 43 | CAP, cardiac failure | died | |
| 4 | M | 54 | 173 | 80 | 126 | COPD, CAP | survived | Klebsiella pneumoniae |
| 5 | M | 36 | 182 | 110 | 88 | CAP, liver cirrhosis | died | |
| 6 | M | 40 | 180 | 89 | 62 | CAP, septic shock | died | Streptococcus pneumoniae |
| 7 | M | 59 | 181 | 87 | 42 | CAP | survived | Streptococcus pneumoniae |
| 8 | F | 73 | 165 | 95 | 66 | cardiomyopathia, CAP | survived | |
| 9 | M | 51 | 172 | 80 | 58 | CAP | survived |
Ht, height; Wt, weight; M, male; F, female; CAP, community-aquired pneumonia; COPD, chronic obstructive pulmonary disease.
Pharmacokinetics of moxifloxacin in nine patients undergoing CVVHDF
| Parameter . | Units . | Measurement . |
|---|---|---|
| Camax | mg/L | 3.76±2.02 |
| Camin | mg/L | 0.24±0.14 |
| Ca(0) | mg/L | 1.46±0.85 |
| AUC0–24 | mg·h/L | 18.41±8.46 |
| CLtot | L/h | 19.09±8.22 |
| CLCVVHDF | L/h | 1.63±0.33 |
| S | 0.84±0.16 | |
| V | L | 270±133 |
| t1/2β | h | 9.87±3.26 |
| Retot | % | 82.02±9.47 |
| ReCVVHDF | % | 9.99±4.25 |
| Parameter . | Units . | Measurement . |
|---|---|---|
| Camax | mg/L | 3.76±2.02 |
| Camin | mg/L | 0.24±0.14 |
| Ca(0) | mg/L | 1.46±0.85 |
| AUC0–24 | mg·h/L | 18.41±8.46 |
| CLtot | L/h | 19.09±8.22 |
| CLCVVHDF | L/h | 1.63±0.33 |
| S | 0.84±0.16 | |
| V | L | 270±133 |
| t1/2β | h | 9.87±3.26 |
| Retot | % | 82.02±9.47 |
| ReCVVHDF | % | 9.99±4.25 |
Camax, arterial peak serum concentration after the first dose; Camin, arterial trough serum concentration after the first dose; Ca(0), arterial serum concentration after the back-extrapolation from the terminal log-linear segment of the concentration curve up to the mid-infusion time; AUC0–24, area under the serum concentration time curve from 0–24 h; CLtot, total clearance; CLCVVHDF, CVVHDF clearance; S, sieving coefficient; V, volume of distribution; t1/2β, elimination half-life; Retot, total removal; ReCVVHDF, removal via CVVHDF.
Values are given as arithmetic means±S.D.
Pharmacokinetics of moxifloxacin in nine patients undergoing CVVHDF
| Parameter . | Units . | Measurement . |
|---|---|---|
| Camax | mg/L | 3.76±2.02 |
| Camin | mg/L | 0.24±0.14 |
| Ca(0) | mg/L | 1.46±0.85 |
| AUC0–24 | mg·h/L | 18.41±8.46 |
| CLtot | L/h | 19.09±8.22 |
| CLCVVHDF | L/h | 1.63±0.33 |
| S | 0.84±0.16 | |
| V | L | 270±133 |
| t1/2β | h | 9.87±3.26 |
| Retot | % | 82.02±9.47 |
| ReCVVHDF | % | 9.99±4.25 |
| Parameter . | Units . | Measurement . |
|---|---|---|
| Camax | mg/L | 3.76±2.02 |
| Camin | mg/L | 0.24±0.14 |
| Ca(0) | mg/L | 1.46±0.85 |
| AUC0–24 | mg·h/L | 18.41±8.46 |
| CLtot | L/h | 19.09±8.22 |
| CLCVVHDF | L/h | 1.63±0.33 |
| S | 0.84±0.16 | |
| V | L | 270±133 |
| t1/2β | h | 9.87±3.26 |
| Retot | % | 82.02±9.47 |
| ReCVVHDF | % | 9.99±4.25 |
Camax, arterial peak serum concentration after the first dose; Camin, arterial trough serum concentration after the first dose; Ca(0), arterial serum concentration after the back-extrapolation from the terminal log-linear segment of the concentration curve up to the mid-infusion time; AUC0–24, area under the serum concentration time curve from 0–24 h; CLtot, total clearance; CLCVVHDF, CVVHDF clearance; S, sieving coefficient; V, volume of distribution; t1/2β, elimination half-life; Retot, total removal; ReCVVHDF, removal via CVVHDF.
Values are given as arithmetic means±S.D.
The study was supported by the European Commission within the IST Craft-2001–52107 project PharmDIS.
References
Dalhoff, A., Peterson, U. & Endermann, R. (
Zhanel, G. G., Ennis, K., Vercaugne, L. et al. (
Pestova, E., Millichap, J. J., Noskin, G. A. et al. (
Stass, H. & Kubitza, D. (
Wise, R., Andrews, J. M., Marshall, G. et al. (
Stass, H., Kubitza, D., Halabi, A. et al. (
Cotterill, S. (
Golper, T. A. & Marx, M. A. (
Stass, H., Dalhoff, A., Kubitza, D. et al. (
Muhl, E., Martens, T., Iven, H. et al. (
Ba, B. B., Etienne, R., Ducint, D. et al. (
Lubasch, A., Keller, I., Corner, K. et al. (
Sullivan, J. T., Woodruff, M., Lettieri, J. et al. (
Simon, N., Sampol, E., Albanese, J. et al. (
Stass, H., Dammer, S., Kubitza, D. et al. (
Stass, H., Dammer, S., Kubitza, D. et al. (
Lister, P. D. & Sanders, C. C. (
Buxbaum, A., Straschil, U., Moser, C. et al. (
Malone, R., Fish, D., Abraham, E. et al. (
Traunmüller, F., Thalhammer-Scherrer, R., Locker, G. J. et al. (
Author notes
1Department of Internal Medicine 4, Intensive Care Unit, University Hospital Vienna, Waehringer Guertel 18–20, A-1090 Vienna; 2Pharmacy Centre, Institute of Pharmaceutical Chemistry, University of Vienna; 3Department of Internal Medicine 1, Division of Infectious Diseases, University Hospital Vienna, Vienna, Austria