-
PDF
- Split View
-
Views
-
Cite
Cite
Claus Klingenberg, Arnfinn Sundsfjord, Arild Rønnestad, Jarle Mikalsen, Peter Gaustad, Trond Flægstad, Phenotypic and genotypic aminoglycoside resistance in blood culture isolates of coagulase-negative staphylococci from a single neonatal intensive care unit, 1989–2000, Journal of Antimicrobial Chemotherapy, Volume 54, Issue 5, November 2004, Pages 889–896, https://doi.org/10.1093/jac/dkh453
Close - Share Icon Share
Abstract
Objectives: To investigate the prevalence of aminoglycoside resistance and genes encoding aminoglycoside-modifying enzymes (AME) in blood culture isolates of coagulase-negative staphylococci (CoNS) from neonates.
Materials and methods: A total of 180 isolates from 148 patients collected in a single neonatal unit over a 12 year period were examined for susceptibility to gentamicin, tobramycin, netilmicin, amikacin and arbekacin by Etest and/or disc diffusion. AME genes were detected by PCR.
Results: The overall non-susceptibility rates to gentamicin, tobramycin, netilmicin, amikacin and arbekacin were 66%, 68%, 52%, 38% and 1%, respectively. Gentamicin non-susceptibility rates were 4% and 91% in methicillin-susceptible and -resistant isolates, respectively. aac(6′)-Ie-aph(2″)-Ia, aph(3′)-IIIa and/or ant(4′)-Ia were encountered in 125 (69%), 1 (0.5%) and 30 (16.6%) isolates, respectively. Forty-six (26%) isolates negative for AME genes were susceptible to all aminoglycosides. In contrast, 115 (92%), 91 (73%) and 66 (53%) of aac(6′)-Ie-aph(2″)-Ia positive isolates were non-susceptible to gentamicin, netilmicin and amikacin, respectively. Only one isolate showed arbekacin resistance. However, aac(6′)-Ie-aph(2″)-Ia positive isolates and isolates with gentamicin MIC ≥128 mg/L displayed a significant reduction in arbekacin inhibition zones.
Conclusions: A high prevalence of aminoglycoside resistance was detected and associated with methicillin resistance. Discrepancies between phenotypic and genetic detection of aminoglycoside resistance were discerned. Gentamicin was the preferred substrate for phenotypic detection of aac(6′)-Ie-aph(2″)-Ia. Arbekacin showed favourable antibacterial activity even in aac(6′)-Ie-aph(2″)-Ia-positive isolates. We suggest including arbekacin in future clinical trials of empirical treatment of late onset neonatal sepsis.
Introduction
Coagulase-negative staphylococci (CoNS) are the most prevalent pathogens causing late onset neonatal sepsis, occurring after 72 h of age. These infections are rarely fatal, but cause significant morbidity in this patient population.1–3 Aminoglycosides are often used along with a β-lactamase-stable penicillin for empirical treatment of late onset neonatal sepsis.4 The anti-staphylococcal activity of aminoglycosides is considered important3,4 as methicillin resistance is frequently observed among CoNS blood isolates in neonates.5,6 Empirical use of vancomycin is an alternative treatment of suspected late onset neonatal sepsis. However, a large proportion of these suspected episodes are not confirmed by blood culture. As a result of the emergence of vancomycin resistance among enterococci and staphylococci, it is thus recommended to restrict vancomycin usage to blood culture-confirmed episodes of methicillin-resistant staphylococci or clinical failure of the initial regimen.4,7,8
In many countries, the prevalence of aminoglycoside resistance among CoNS has increased.9–11 The main mechanism of aminoglycoside resistance is drug inactivation by aminoglycoside-modifying enzymes (AMEs) encoded within mobile genetic elements.12–14 The following three AMEs are of particular significance among staphylococci since they modify and thereby inactivate the traditional aminoglycosides of therapeutic importance (Table 1): aminoglycoside-6′-N-acetyltransferase/2″-O-phosphoryltransferase [AAC(6′)/APH(2″)], aminoglycoside-4′-O-nucleotidyltransferase I [ANT(4′)-I] and aminoglycoside-3′-O-phosphoryltransferase III [APH(3′)-III].15–17 Variations in AME substrate specificity (Table 1) explain differences in antibacterial activity among the aminoglycosides.16 The bifunctional enzyme, AAC(6′)/APH(2″), is the most frequently encountered AME in staphylococcal isolates and modifies to a different degree essentially all clinically available aminoglycosides, except streptomycin.16–18 Arbekacin, currently used for neonates only in Japan,19,20 is stable against most AMEs and shows minimal inactivation by the bifunctional enzyme compared with other aminoglycosides.21,22
To our knowledge, no studies have focused on aminoglycoside resistance mechanisms among CoNS isolates from neonates. The aims of this study were to determine the susceptibility pattern of neonatal CoNS blood culture isolates to different aminoglycosides from a single neonatal unit over a 12 year period, and to correlate the presence of genes encoding AMEs to the aminoglycoside susceptibility pattern and methicillin resistance. We further discuss the clinical relevance of these data.
Materials and methods
Setting
All data were collected from the tertiary care neonatal intensive care unit (NICU) at the National Hospital, Oslo. This NICU has an annual admission rate of approximately 800 patients. Baseline clinical and laboratory data including birth weight, gestational age and C-reactive protein (CRP) levels, were registered for all patients. Netilmicin was the only aminoglycoside used in the unit during the entire study period. The regional committee for medical research ethics approved the collection and analysis of patient data.
Bacterial strains
Between 1989 and 2000, a total of 180 CoNS blood culture isolates were collected from 148 neonates admitted to the unit. Blood cultures were collected from a peripheral vein after skin cleansing with a chlorhexidine–ethanol solution or from an intravascular catheter at the time of insertion. The cultures were obtained only when indicated by clinical signs of infection. Duplicate cultures were not routinely obtained. There are difficulties in the diagnosis of CoNS sepsis in neonates as usual practice in most neonatal units is to obtain only a single blood culture and because clinical signs of neonatal sepsis often are subtle. No uniformly accepted criteria exist to differentiate between invasive blood culture isolates and contaminants.23 In this study, we have used the criteria proposed by Stoll et al. and defined an invasive CoNS infection as a positive monomicrobial blood culture in a child older than 72 h combined with elevated CRP >10 mg/L within 2 days of blood culture and no other obvious alternative explanation for the clinical picture. All other CoNS blood isolates analysed, including cultures growing more than one organism, were considered to be contaminants.1,23 According to this classification, 85 isolates from 81 patients (four patients suffered from two separate episodes of CoNS sepsis) were considered as invasive and 95 isolates from 77 patients were considered as contaminants. Ten patients included in the study experienced both episodes classified as septicaemia as well as episodes where growth in blood culture was considered as a contaminant.
Identification and susceptibility testing
We identified all isolates to the species level by ID 32 Staph (bioMérieux, Marcy l'Étoile, France) according to the manufacturer's instructions. Gentamicin, tobramycin, netilmicin and amikacin MIC values were determined by Etest (AB Biodisk, Solna, Sweden). Isolates were categorized as susceptible (S), intermediate susceptible (I) and resistant (R) according to the clinical MIC breakpoints of the European Committee on Antimicrobial Susceptibility Testing (EUCAST); gentamicin, tobramycin, netilmicin S≤2 mg/L and R > 4 mg/L, and amikacin S≤8 mg/L and R > 16 mg/L.24 Moreover, for statistical analyses and group comparisons, we termed intermediate susceptible and resistant isolates as non-susceptible. The susceptibility to arbekacin (30 μg, Eiken Chemical Ltd, Japan) was determined according to the NCCLS standards for antimicrobial disc susceptibility tests.25 For comparison, a gentamicin disc (30 μg, AB Biodisk) was always used in the same run. No NCCLS or EUCAST breakpoints exist for arbekacin, but in Japan, using the NCCLS standard disc diffusion method, the following zone diameters are used for interpretation; R ≤13 mm; I 14–17 mm; and S ≥ 18 mm (T. Ichiyanagi, Eiken Chemical Ltd, personal communication). In order to study co-resistance, we carried out Etests for oxacillin, fusidic acid and erythromycin. Phenotypic methicillin resistance was verified by detection of the mecA gene.26 The gentamicin- and methicillin-susceptible Staphylococcus epidermidis ATCC 12228 and the methicillin-resistant S. epidermidis ATCC 700586 were used as control strains.
PCR amplification of genes encoding aminoglycoside-modifying enzymes
Oligonucleotide primers for the three aminoglycoside resistance genes27 are listed in Table 1. Template DNA was prepared by dissolving a 1 μL loop of bacteria in 1 mL of TE buffer, centrifuged at 5000 rpm for 5 min, and the pellet resuspended in 100 μL of TE buffer. The suspension was boiled for 10 min before centrifugation at 5000 rpm for 5 min. The supernatant served as PCR template and PCRs were carried out as described previously28 with the following modification: thermal cycling consisted of an initial denaturing step of 1 min at 94°C; 30 cycles of 30 s at 95°C, 10 s at 58°C, and 30 s at 72°C; and a final extension step of 3 min at 72°C. Enterococcus faecium ATCC 51559 [possessing aac(6′)-Ie-aph(2″)-Ia], Staphylococcus aureus N315 [possessing ant(4′)-Ia] and Escherichia coli JM83 pUC18WaphA-3 [possessing aph(3′)-IIIa] served as positive controls. S. aureus NCTC 8325 served as negative control. Primers targeting the 16S RNA gene were used as an internal control for each amplification reaction to identify potentially false-negative results.28 In addition, all PCRs were repeated when there was discordance between genotype and expected phenotype.16
Statistics
The Mann–Whitney U-test was used for comparison between groups of non-parametric data. Categorical analyses of non-susceptibility rates between invasive isolates and contaminants were carried out using the χ2 test or Fisher's exact test, as appropriate. Categorical analyses of non-susceptibility rates between staphylococcal species were carried out using the χ2 test with Bonferroni's correction for multiple adjustments. Trend analyses for aminoglycoside MIC values were assessed in a univariate linear model with year of obtained specimen as covariate. Trend analyses for genes encoding AMEs and the mecA gene were assessed in a binary logistic regression model. SPSS (11.0 for Windows) was used for all data analyses and a P value <0.05 was considered significant.
Results
Species identification and phenotypic susceptibility to gentamicin, tobramycin, netilmicin and amikacin
In total, 130 S. epidermidis and 50 other CoNS isolates were included. The distribution of species defined as invasive (I) isolates or contaminants (C) was as follows: S. epidermidis (I = 63, C = 67), Staphylococcus haemolyticus (I = 12, C = 7), Staphylococcus warneri (I = 4, C = 13), Staphylococcus hominis (I = 4, C = 6) and Staphylococcus capitis (I = 2, C = 2).
Table 2 shows aminoglycoside non-susceptibility rates in: (1) all isolates, (2) among different CoNS species and (3) among invasive isolates versus contaminants. We found significant (P < 0.02) differences in gentamicin, tobramycin, netilmicin and amikacin non-susceptibility rates between S. epidermidis, S. haemolyticus and the three other CoNS species (Table 2). In addition, invasive isolates showed significantly higher rates of non-susceptibility (P < 0.01) to gentamicin, tobramycin and netilmicin. The differences between invasive isolates and contaminants remained significant when analysing the 130 S. epidermidis isolates separately.
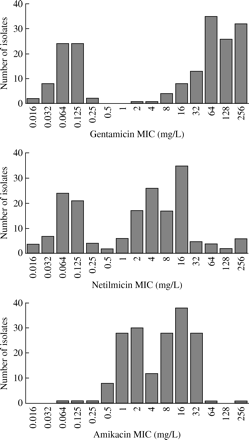
Figure 1 displays the distribution of MIC values for gentamicin, netilmicin and amikacin for all 180 isolates. A typical bimodal distribution pattern was found for gentamicin and netilmicin, but not for amikacin. Sixty of 62 gentamicin-susceptible isolates had MIC values between 0.016 and 0.25 mg/L and only two isolates showed a gentamicin MIC value of 2 and 4 mg/L, respectively. Looking only at gentamicin MIC values for the 130 S. epidermidis isolates, we have shown that 40 susceptible (wild-type) isolates showed MIC ≤0.25 mg/L, the other 90 isolates showed MIC ≥8 mg/L and no isolates displayed MICs in the range 0.5–4 mg/L. The majority of tobramycin MIC values were in the same range as for gentamicin (data not shown).
High level of gentamicin resistance, methicillin resistance and multidrug resistance
We specifically analysed isolates expressing high-level gentamicin resistance (HLGR), methicillin resistance or multidrug resistance. Fifty-seven isolates, 30 invasive and 27 contaminants, were defined as HLGR (MIC ≥ 128 mg/L). Among these 57 isolates, 15 and 33 isolates remained susceptible or intermediate susceptible to netilmicin and amikacin, respectively. Only 12 of 129 (9.3%) methicillin-resistant (MR) CoNS isolates were susceptible to gentamicin compared to 49 of 51 (96.1%) methicillin-susceptible (MS) CoNS isolates. However, a significant proportion of MR-CoNS was still susceptible to amikacin (47.3%) and netilmicin (27.9%). Fifty-four isolates, 29 invasive and 25 contaminants, were characterized as multidrug-resistant; oxacillin MIC ≥ 0.5 mg/L, erythromycin MIC ≥ 8 mg/L and fusidic acid MIC ≥ 1 mg/L. Five, 11 and 18 of these 54 isolates were susceptible to gentamicin, netilmicin and amikacin, respectively.
Phenotypic susceptibility to arbekacin
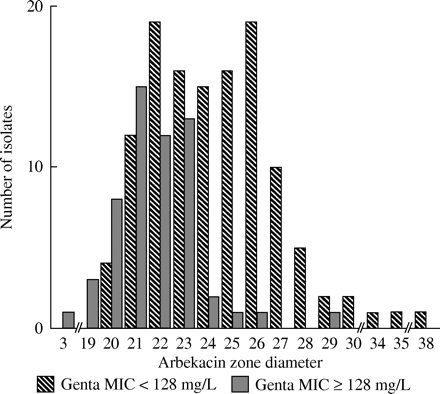
Only one isolate was resistant to arbekacin. This methicillin-resistant S. epidermidis was in addition highly resistant to all other aminoglycosides (MIC ≥ 256 mg/L), erythromycin (MIC ≥ 256 mg/L), clindamycin (MIC ≥ 256 mg/L), oxacillin (MIC ≥ 512 mg/L), rifampicin (MIC ≥ 256 mg/L) and fusidic acid (MIC ≥ 256 mg/L). The 179 other isolates showed an arbekacin (30 μg disc) zone diameter ≥ 19 mm (median 23 mm, range 19–39 mm). Conversely, only 59 isolates revealed a gentamicin (30 μg disc) zone diameter ≥19 mm corresponding to gentamicin MIC values ≤0.25 mg/L. Reduced arbekacin inhibition zone diameters were more prevalent in invasive isolates compared with contaminants (P=0.026). S. epidermidis and S. haemolyticus also revealed reduced arbekacin inhibition zones compared with other CoNS (P < 0.001). In addition, as shown in Figure 2, HLGR isolates had reduced arbekacin inhibition zones compared with all other isolates (median 22 mm versus 24 mm, P < 0.001).
Correlation between the presence of AME genes and susceptibility to aminoglycosides and other antibiotics
Table 3 shows the correlation between non-susceptibility to aminoglycosides and the presence of AME genes for all isolates. aac(6′)-Ie-aph(2″)-Ia was encountered in 125 isolates (69.4%). All 19 S. haemolyticus isolates carried this gene. We found both aac(6′)-Ie-aph(2″)-Ia and ant(4′)-Ia in 22 isolates (12.2%). Eight isolates (4.4%) carried ant(4′)-Ia only. No major difference in susceptibility pattern was found between gentamicin and tobramycin for aac(6′)-Ie-aph(2″)-Ia positive isolates. ant(4′)-Ia positive isolates were, as expected, more resistant to tobramycin than gentamicin (Tables 1 and 3). Only two isolates showed gentamicin ‘borderline’ MIC values (MIC 2–4 mg/L), both carried aac(6′)-Ie-aph(2″)-Ia. Seven out of nine tobramycin ‘borderline’ isolates carried either aac(6′)-Ie-aph(2″)-Ia (n=6) or ant(4′)-Ia (n=1).
Ten of 125 isolates carrying the aac(6′)-Ie-aph(2″)-Ia gene were gentamicin-susceptible. Conversely, 124 of these 125 isolates remained fully susceptible to arbekacin. However, the median arbekacin zone diameter was significantly reduced in aac(6′)-Ie-aph(2″)-Ia-positive isolates compared with aac(6′)-Ie-aph(2″)-Ia-negative isolates (median 22 mm versus 26 mm, P < 0.001). The one isolate resistant to arbekacin was aac(6′)-Ie-aph(2″)-Ia positive. The aph(3′)-IIIa gene was only found in one S. epidermidis isolate. This isolate showed an MIC value of 2 mg/L for netilmicin, 8 mg/L for tobramycin and amikacin, and 16 mg/L for gentamicin.
Forty-six isolates negative for all three AME genes were uniformly susceptible to all aminoglycosides tested (Table 3). All 46 isolates had gentamicin MIC values ≤0.25 mg/L. In addition, they displayed a similar susceptibility pattern for gentamicin and arbekacin using the disc diffusion method. The median zone diameter was 26 mm for both substrates; range arbekacin 22–34 mm and gentamicin 22–32 mm.
We found a close correlation between aminoglycoside non-susceptibility, detection of AME genes and methicillin resistance (Table 3). A similar correlation was seen among 54 multidrug-resistant isolates; 44 carried aac(6′)-Ie-aph(2″)-Ia, 12 carried both ant(4′)-Ia and aac(6′)-Ie-aph(2″)-Ia and one isolate carried only ant(4′)-Ia.
Trends in development of antibiotic resistance and resistance determinants
A significant trend towards lower MIC values for gentamicin (P=0.039) and netilmicin (P=0.041) was found during the study period. Analyses of invasive isolates revealed higher netilmicin MIC levels in the first 6 year period (n=47) compared with the second 6 year period (n=38); median MIC 16 mg/L and 4 mg/L, respectively (P < 0.001). For all other aminoglycosides, there were no significant trends in MIC levels during the study period. No significant trends were identified either in the prevalence of genes encoding AME or mecA. The proportion of mecA-positive isolates was 67.9%, 75.0%, 72.0% and 67.9% in the four consecutive 3 year periods of the study.
Discussion
In this study, we found a high overall prevalence of aminoglycoside non-susceptibility in 180 CoNS blood culture isolates from neonates. The higher rates of non-susceptibility in S. epidermidis and especially S. haemolyticus isolates than in other CoNS were consistent with other studies.12,29 In agreement with previous reports from Europe12,15aac(6′)-Ie-aph(2″)-Ia was the most prevalent AME gene, encountered in more than two-thirds of all isolates.
All isolates with gentamicin MIC > 0.25 mg/L carried either aac(6′)-Ie-aph(2″)-Ia, ant(4′)-Ia, the combination of these two genes or aph(3′)-IIIa. The concordances between gentamicin, tobramycin, netilmicin and amikacin non-susceptibility and the presence of aac(6′)-Ie-aph(2″)-Ia gene were 92%, 91%, 73% and 52%, respectively.
However, a substantial proportion of isolates carrying aac(6′)-Ie-aph(2″)-Ia, but still classified as susceptible to amikacin (28 out of 59) and netilmicin (17 out of 34), displayed MIC values of 8 and 2 mg/L, respectively. The relatively high MIC values in these strains are consistent with the expression of the aac(6′)-Ie-aph(2″)-Ia gene, as aac(6′)-Ie-aph(2″)-Ia-negative isolates showed much lower MIC values. Nevertheless, the distribution of MIC values (Figure 1) indicates, in agreement with other in vitro6,12,18,30 studies, that netilmicin and amikacin are more resistant to the substrate-inactivating effect of the AAC(6′)/APH(2″) enzyme than gentamicin.
Aminoglycosides exhibit a concentration-dependent bactericidal effect in vivo whereas disc diffusion methods only reflect their bacteriostatic effect.31,32 A therapeutic peak-to-MIC ratio of 8–10 is considered optimal, but even lower ratios may inhibit bacterial growth.33,34 Recent neonatal high-dosage, extended-interval regimens report peak concentrations in the following range: gentamicin 6–10 mg/L,35,36 netilmicin 8–12 mg/L37 and amikacin 22–32 mg/L.38,39 In our study, 65.5% of all isolates revealed gentamicin MIC values >4 mg/L, levels that most likely are limiting therapeutic efficacy. The interpretation of MIC data for netilmicin and amikacin is more controversial. A substantial proportion of aac(6′)-Ie-aph(2″)-Ia-positive isolates revealed netilmicin and amikacin MIC values in a range where one might expect therapeutic efficacy, provided optimal peak concentration. According to Livermore et al.,16 gentamicin-resistant CoNS carrying the bifunctional enzyme are likely to remain susceptible to amikacin and netilmicin at British Society for Antimicrobial Chemotherapy (BSAC) breakpoints. In contrast, Courvalin claims that the bactericidal effect of netilmicin and amikacin is always abolished in Gram-positive cocci carrying the bifunctional enzyme and that these drugs should thus be considered inactive from a therapeutic point of view in spite of phenotypic susceptibility.32 Furthermore, in vitro studies show that a peak-to-MIC ratio of less than 4:1 may lead to bacterial regrowth and selection of resistant isolates33 and that gentamicin-susceptible S. epidermidis isolates carrying the aac(6′)-Ie-aph(2″)-Ia gene change phenotype and turn resistant after sequential culturing.40 We believe that whether aminoglycoside treatment of phenotypically susceptible isolates carrying AME genes ultimately leads to therapeutic failure or not, warrants future studies. In the lack of these data, we still prefer interpretative reading with gentamicin as group representative for netilmicin and amikacin in clinical microbiological testing.32
Arbekacin is not modified by APH(3′)-III or ANT(4′)-I due to pharmacochemical modifications on the aminocyclitol ring.21 In addition, the bifunctional enzyme has a very low arbekacin inactivation rate compared with gentamicin.22 Traditional aminoglycosides are usually inactivated by AMEs. However, in vitro studies have shown that arbekacin may retain antibacterial activity despite being modified by AMEs.41,42 Arbekacin is thus considered to represent a new generation of aminoglycosides possessing a ‘double-stage activity’.42 In our study, arbekacin showed excellent in vitro activity, and the arbekacin inhibition zone diameters found in 179 of 180 isolates corresponded to gentamicin MIC values ≤0.25 mg/L. Despite this high prevalence of arbekacin susceptibility, aac(6′)-Ie-aph(2″)-Ia-positive isolates and isolates with HLGR showed significantly reduced inhibition zones. This indicates, as previously described,14 that the bifunctional enzyme still has a certain inactivating effect on arbekacin. Only a few, small clinical studies have evaluated the clinical effect of arbekacin on gentamicin-resistant MRSA despite being extensively used for treatment of MRSA infections in Japan since 1990.20,42–44 The clinical effect of arbekacin on invasive CoNS infections has not been evaluated in larger studies.
We detected ant(4′)-Ia in 30 (17%) of our isolates; of which 28 and 22 in addition carried the mecA gene and the aac(6′)-Ie-aph(2″)-Ia gene, respectively. In a recent Japanese study, a high prevalence (84.5%) of ant(4′)-Ia in MRSA was presumed to be due to the adjacent locations of ant(4′)-Ia and mecA.14 In a large European study, 32% of all CoNS carried ant(4′)-Ia.15 A Korean study also found a high prevalence (27%) of ant(4′)-Ia carriage among CoNS. In that study, however, ant(4′)-Ia was not strongly associated with mecA, but frequently combined with aac(6’)-Ie-aph(2″)-Ia carriage.13 The impact of ant(4′)-Ia on the total aminoglycoside resistance level in this study was limited to higher rates of tobramycin resistance. However, it must be emphasized that these strains can easily be misclassified as susceptible when using gentamicin as a group representative for all aminoglycosides in antimicrobial testing of staphylococci.
Changes in the prevalence of aminoglycoside resistance occur slowly after long-term changes in the aminoglycoside selective pressure and the resistance mechanisms are related to the type of aminoglycoside used.17 As an example, the prevalence of arbekacin-resistant MRSA in Japan did not increase in the first 7 years after introduction of arbekacin.45 It was reassuring to observe a stable or even, for gentamicin and netilmicin, gradually declining aminoglycoside resistance rate during the 12 year study period. However, netilmicin was the only aminoglycoside used in this NICU since the latter half of the 1970s and the level of genotypic resistance due to aac(6′)-Ie-aph(2″)-Ia was already high at the beginning of the study period.
NCCLS breakpoints25 for all aminoglycosides are higher compared with EUCAST.24 The chosen NCCLS breakpoint for susceptibility to netilmicin, 12 mg/L, is questionable as peak therapeutic concentrations in neonates infrequently exceed this value.25,37 From a pharmacokinetic point of view, EUCAST breakpoints for aminoglycosides seem more appropriate than NCCLS guidelines with regard to achievable peak concentrations in neonates. Moreover, even lower gentamicin and tobramycin breakpoints for CoNS (MIC ≤ 0.5 mg/L) would reflect the underlying genetic resistance mechanisms more precisely.
The mec complex, conferring methicillin resistance, is conveyed by a mobile chromosomal genetic element designated staphylococcal cassette chromosome mec (SCCmec). Plasmid pUB110, carrying ant(4′)-Ia, is integrated in the SCCmec type II and integrated plasmids carrying transposon Tn4001, encoding aac(6′)-Ie-aph(2″)-Ia,15 have been reported in SCCmec type IVc.46 These observations may explain the close correlation between aminoglycoside resistance and methicillin resistance found both in this and other studies.5,47
We conclude that 74% (134/180) of all blood culture isolates in this study carried an AME gene either alone or in combination. Gentamicin was the preferred substrate for phenotypic detection of aac(6′)-Ie-aph(2″)-Ia. Among traditional aminoglycosides, netilmicin and especially amikacin showed the highest in vitro susceptibility rates. However, to our knowledge, no clinical trials have yet shown that this higher in vitro susceptibility actually translates into greater clinical efficacy in the treatment of CoNS infections. There is a paucity of data on arbekacin and CoNS infections. In this study, arbekacin showed excellent in vitro antibacterial activity in 99.4% of all isolates. Recent studies on arbekacin treatment of neonates show safety profiles similar to those of other aminoglycosides.20 We suggest including arbekacin in future clinical trials of empirical treatment of late onset neonatal sepsis to assess clinical efficacy and the subsequent need for vancomycin therapy.
Distribution of MIC values of gentamicin, netilmicin and amikacin for 180 CoNS isolates.
Arbekacin zone diameter (mm) in 57 high level gentamicin-resistant CoNS isolates (MIC≥128 mg/L) compared to 123 CoNS isolates with gentamicin MIC < 128 mg/L. R ≤13 mm; I 14–17 mm; S ≥18 mm.
Genes encoding aminoglycoside-modifying enzymes, primer sequences and expected corresponding phenotype
| Aminoglycoside resistance gene . | Amplicon size (bp) . | Primer sequence (5′–3′)27 . | AME . | Expected phenotype16 . |
|---|---|---|---|---|
| aac(6′)-Ie-aph(2″)-Ia | 348 | CAGAGCCTTGGGAAGATGAAG CCTCGTGTAATTCATGTTCTGGC | AAC(6′)/APH(2″) | GEN-R, NET-r, TOB-R, AMK-r, KAN-R |
| ant(4′)-Ia | 294 | CAAACTGCTAAATCGGTAGAAGCC GGAAAGTTGACCAGACATTACGAACT | ANT(4′)-I | GEN-S, NET-S, TOB-R, AMK-R, KAN-R |
| aph(3′)-IIIa | 523 | GGCTAAAATGAGAATATCACCGG CTTTAAAAAATCATACAGCTCGCG | APH(3′)-III | GEN-S, NET-S, TOB-S, AMK-R, KAN-R |
| Aminoglycoside resistance gene . | Amplicon size (bp) . | Primer sequence (5′–3′)27 . | AME . | Expected phenotype16 . |
|---|---|---|---|---|
| aac(6′)-Ie-aph(2″)-Ia | 348 | CAGAGCCTTGGGAAGATGAAG CCTCGTGTAATTCATGTTCTGGC | AAC(6′)/APH(2″) | GEN-R, NET-r, TOB-R, AMK-r, KAN-R |
| ant(4′)-Ia | 294 | CAAACTGCTAAATCGGTAGAAGCC GGAAAGTTGACCAGACATTACGAACT | ANT(4′)-I | GEN-S, NET-S, TOB-R, AMK-R, KAN-R |
| aph(3′)-IIIa | 523 | GGCTAAAATGAGAATATCACCGG CTTTAAAAAATCATACAGCTCGCG | APH(3′)-III | GEN-S, NET-S, TOB-S, AMK-R, KAN-R |
GEN, gentamicin; TOB, tobramycin; NET, netilmicin; AMK, amikacin; KAN, kanamycin; S, susceptible; R, resistant; r, reduced zones but likely to remain susceptible at BSAC breakpoints.
Genes encoding aminoglycoside-modifying enzymes, primer sequences and expected corresponding phenotype
| Aminoglycoside resistance gene . | Amplicon size (bp) . | Primer sequence (5′–3′)27 . | AME . | Expected phenotype16 . |
|---|---|---|---|---|
| aac(6′)-Ie-aph(2″)-Ia | 348 | CAGAGCCTTGGGAAGATGAAG CCTCGTGTAATTCATGTTCTGGC | AAC(6′)/APH(2″) | GEN-R, NET-r, TOB-R, AMK-r, KAN-R |
| ant(4′)-Ia | 294 | CAAACTGCTAAATCGGTAGAAGCC GGAAAGTTGACCAGACATTACGAACT | ANT(4′)-I | GEN-S, NET-S, TOB-R, AMK-R, KAN-R |
| aph(3′)-IIIa | 523 | GGCTAAAATGAGAATATCACCGG CTTTAAAAAATCATACAGCTCGCG | APH(3′)-III | GEN-S, NET-S, TOB-S, AMK-R, KAN-R |
| Aminoglycoside resistance gene . | Amplicon size (bp) . | Primer sequence (5′–3′)27 . | AME . | Expected phenotype16 . |
|---|---|---|---|---|
| aac(6′)-Ie-aph(2″)-Ia | 348 | CAGAGCCTTGGGAAGATGAAG CCTCGTGTAATTCATGTTCTGGC | AAC(6′)/APH(2″) | GEN-R, NET-r, TOB-R, AMK-r, KAN-R |
| ant(4′)-Ia | 294 | CAAACTGCTAAATCGGTAGAAGCC GGAAAGTTGACCAGACATTACGAACT | ANT(4′)-I | GEN-S, NET-S, TOB-R, AMK-R, KAN-R |
| aph(3′)-IIIa | 523 | GGCTAAAATGAGAATATCACCGG CTTTAAAAAATCATACAGCTCGCG | APH(3′)-III | GEN-S, NET-S, TOB-S, AMK-R, KAN-R |
GEN, gentamicin; TOB, tobramycin; NET, netilmicin; AMK, amikacin; KAN, kanamycin; S, susceptible; R, resistant; r, reduced zones but likely to remain susceptible at BSAC breakpoints.
Percentage of aminoglycoside non-susceptible isolates in different CoNS species and among invasive isolates versus contaminants
| Aminoglycoside . | Total (n=180) . | S. epidermidis (n=130) . | S. haemolyticus (n=19) . | Other CoNS (n=31) . | Invasive (n=85) . | Contaminant (n=95) . |
|---|---|---|---|---|---|---|
| Gentamicin MIC >2 mg/L | 66.1% | 69.2% | 100% | 32.3% | 76.5% | 56.8% |
| Tobramycin MIC >2 mg/L | 67.6% | 70.8% | 100% | 35.5% | 77.4% | 58.9% |
| Netilmicin MIC >2 mg/L | 52.2% | 56.9% | 94.7% | 6.5% | 64.7% | 41.1% |
| Amikacin MIC >8 mg/L | 38.0% | 38.5% | 88.9% | 6.5% | 42.9% | 33.7% |
| Arbekacin ZD <18 mm | 0.6% | 0.8% | 0% | 0% | 0% | 1.1% |
| Aminoglycoside . | Total (n=180) . | S. epidermidis (n=130) . | S. haemolyticus (n=19) . | Other CoNS (n=31) . | Invasive (n=85) . | Contaminant (n=95) . |
|---|---|---|---|---|---|---|
| Gentamicin MIC >2 mg/L | 66.1% | 69.2% | 100% | 32.3% | 76.5% | 56.8% |
| Tobramycin MIC >2 mg/L | 67.6% | 70.8% | 100% | 35.5% | 77.4% | 58.9% |
| Netilmicin MIC >2 mg/L | 52.2% | 56.9% | 94.7% | 6.5% | 64.7% | 41.1% |
| Amikacin MIC >8 mg/L | 38.0% | 38.5% | 88.9% | 6.5% | 42.9% | 33.7% |
| Arbekacin ZD <18 mm | 0.6% | 0.8% | 0% | 0% | 0% | 1.1% |
ZD, zone diameter.
Percentage of aminoglycoside non-susceptible isolates in different CoNS species and among invasive isolates versus contaminants
| Aminoglycoside . | Total (n=180) . | S. epidermidis (n=130) . | S. haemolyticus (n=19) . | Other CoNS (n=31) . | Invasive (n=85) . | Contaminant (n=95) . |
|---|---|---|---|---|---|---|
| Gentamicin MIC >2 mg/L | 66.1% | 69.2% | 100% | 32.3% | 76.5% | 56.8% |
| Tobramycin MIC >2 mg/L | 67.6% | 70.8% | 100% | 35.5% | 77.4% | 58.9% |
| Netilmicin MIC >2 mg/L | 52.2% | 56.9% | 94.7% | 6.5% | 64.7% | 41.1% |
| Amikacin MIC >8 mg/L | 38.0% | 38.5% | 88.9% | 6.5% | 42.9% | 33.7% |
| Arbekacin ZD <18 mm | 0.6% | 0.8% | 0% | 0% | 0% | 1.1% |
| Aminoglycoside . | Total (n=180) . | S. epidermidis (n=130) . | S. haemolyticus (n=19) . | Other CoNS (n=31) . | Invasive (n=85) . | Contaminant (n=95) . |
|---|---|---|---|---|---|---|
| Gentamicin MIC >2 mg/L | 66.1% | 69.2% | 100% | 32.3% | 76.5% | 56.8% |
| Tobramycin MIC >2 mg/L | 67.6% | 70.8% | 100% | 35.5% | 77.4% | 58.9% |
| Netilmicin MIC >2 mg/L | 52.2% | 56.9% | 94.7% | 6.5% | 64.7% | 41.1% |
| Amikacin MIC >8 mg/L | 38.0% | 38.5% | 88.9% | 6.5% | 42.9% | 33.7% |
| Arbekacin ZD <18 mm | 0.6% | 0.8% | 0% | 0% | 0% | 1.1% |
ZD, zone diameter.
Correlation between AME genes, mecA and aminoglycoside non-susceptibility
| AME genes detected by PCR . | . | . | Total . | mecA PCR positive . | Gentamicin MIC >2 mg/L . | Tobramycin MIC >2 mg/L . | Netilmicin MIC >2 mg/L . | Amikacin MIC >8 mg/L . | Arbekacin ZD <18 mm . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| aac(6′)-Ie-aph(2″)-Ia | ant(4′)-Ia | aph(3′)-IIIa | |||||||||
| + | − | − | 103 | 95 (92%) | 93 (90%) | 93 (90%) | 75 (73%) | 49 (48%) | 1 (1%) | ||
| + | + | − | 22 | 22 (100%) | 22 (100%) | 21 (95%) | 16 (73%) | 17 (77%) | 0 (0%) | ||
| − | + | − | 8 | 6 (75%) | 3 (38%) | 7 (88%) | 3 (38%) | 3 (38%) | 0 (0%) | ||
| − | − | + | 1 | 1 (100%) | 0 (0%) | 1 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| − | − | − | 46 | 5 (11%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Total | 180 | 129 (72%) | 119 (66%) | 123 (68%) | 95 (53%) | 71 (39%) | 1 (1%) | ||||
| AME genes detected by PCR . | . | . | Total . | mecA PCR positive . | Gentamicin MIC >2 mg/L . | Tobramycin MIC >2 mg/L . | Netilmicin MIC >2 mg/L . | Amikacin MIC >8 mg/L . | Arbekacin ZD <18 mm . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| aac(6′)-Ie-aph(2″)-Ia | ant(4′)-Ia | aph(3′)-IIIa | |||||||||
| + | − | − | 103 | 95 (92%) | 93 (90%) | 93 (90%) | 75 (73%) | 49 (48%) | 1 (1%) | ||
| + | + | − | 22 | 22 (100%) | 22 (100%) | 21 (95%) | 16 (73%) | 17 (77%) | 0 (0%) | ||
| − | + | − | 8 | 6 (75%) | 3 (38%) | 7 (88%) | 3 (38%) | 3 (38%) | 0 (0%) | ||
| − | − | + | 1 | 1 (100%) | 0 (0%) | 1 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| − | − | − | 46 | 5 (11%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Total | 180 | 129 (72%) | 119 (66%) | 123 (68%) | 95 (53%) | 71 (39%) | 1 (1%) | ||||
ZD, zone diameter.
Correlation between AME genes, mecA and aminoglycoside non-susceptibility
| AME genes detected by PCR . | . | . | Total . | mecA PCR positive . | Gentamicin MIC >2 mg/L . | Tobramycin MIC >2 mg/L . | Netilmicin MIC >2 mg/L . | Amikacin MIC >8 mg/L . | Arbekacin ZD <18 mm . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| aac(6′)-Ie-aph(2″)-Ia | ant(4′)-Ia | aph(3′)-IIIa | |||||||||
| + | − | − | 103 | 95 (92%) | 93 (90%) | 93 (90%) | 75 (73%) | 49 (48%) | 1 (1%) | ||
| + | + | − | 22 | 22 (100%) | 22 (100%) | 21 (95%) | 16 (73%) | 17 (77%) | 0 (0%) | ||
| − | + | − | 8 | 6 (75%) | 3 (38%) | 7 (88%) | 3 (38%) | 3 (38%) | 0 (0%) | ||
| − | − | + | 1 | 1 (100%) | 0 (0%) | 1 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| − | − | − | 46 | 5 (11%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Total | 180 | 129 (72%) | 119 (66%) | 123 (68%) | 95 (53%) | 71 (39%) | 1 (1%) | ||||
| AME genes detected by PCR . | . | . | Total . | mecA PCR positive . | Gentamicin MIC >2 mg/L . | Tobramycin MIC >2 mg/L . | Netilmicin MIC >2 mg/L . | Amikacin MIC >8 mg/L . | Arbekacin ZD <18 mm . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| aac(6′)-Ie-aph(2″)-Ia | ant(4′)-Ia | aph(3′)-IIIa | |||||||||
| + | − | − | 103 | 95 (92%) | 93 (90%) | 93 (90%) | 75 (73%) | 49 (48%) | 1 (1%) | ||
| + | + | − | 22 | 22 (100%) | 22 (100%) | 21 (95%) | 16 (73%) | 17 (77%) | 0 (0%) | ||
| − | + | − | 8 | 6 (75%) | 3 (38%) | 7 (88%) | 3 (38%) | 3 (38%) | 0 (0%) | ||
| − | − | + | 1 | 1 (100%) | 0 (0%) | 1 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| − | − | − | 46 | 5 (11%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Total | 180 | 129 (72%) | 119 (66%) | 123 (68%) | 95 (53%) | 71 (39%) | 1 (1%) | ||||
ZD, zone diameter.
We thank Gry Kjeldsen and Anne Kristin Natland for excellent technical assistance and the Department of Microbiology, University Hospital of North Norway for kindly providing control strains. We also thank Gunnar Skov Simonsen, Anne-Merethe Hanssen and Johanna U. Ericson Sollid for critical reading of the manuscript. This study was supported by grants from Northern Norway Regional Health Authority and Fredriksens legat.
References
Stoll, B. J., Hansen, N., Fanaroff, A. A. et al. (
Maayan-Metzger, A., Linder, N., Marom, D. et al. (
Isaacs, D. (
Isaacs, D. (
De Giusti, M., Pacifico, L., Tufi, D. et al. (
Ronnestad, A., Abrahamsen, T. G., Gaustad, P. et al. (
Karlowicz, M. G., Buescher, E. S. & Surka, A. E. (
Krediet, T. G., Jones, M. E., Gerards, L. J. et al. (
Schmitz, F. J., Theis, S., Fluit, A. C. et al. (
Archer, G. L. & Climo, M. W. (
Bannerman, T. L., Rhoden, D. L., McAllister, S. K. et al. (
Busch-Sorensen, C., Frimodt-Moller, N., Miller, G. H. et al. (
Choi, S. M., Kim, S. H., Kim, H. J. et al. (
Ida, T., Okamoto, R., Shimauchi, C. et al. (
Schmitz, F. J., Fluit, A. C., Gondolf, M. et al. (
Livermore, D. M., Winstanley, T. G. & Shannon, K. P. (
Miller, G. H., Sabatelli, F. J., Hare, R. S. et al. (
Udo, E. E. & Dashti, A. A. (
Suzuki, K., Tanikawa, K. & Matsuzaki, T. (
Suzuki, K. (
Mingeot-Leclercq, M. P., Glupczynski, Y. & Tulkens, P. M. (
Inoue, M., Nonoyama, M., Okamoto, R. et al. (
Craft, A. & Finer, N. (
EUCAST clinical MIC breakpoints. [Online.] http://www.srga.org/eucastwt/MICTAB/MICaminoglycosides.html (8 September 2004, date last accessed).
National Committee for Clinical Laboratory Standards. (
Predari, S. C., Ligozzi, M. & Fontana, R. (
Vakulenko, S. B., Donabedian, S. M., Voskresenskiy, A. M. et al. (
Hanssen, A. M., Kjeldsen, G. & Sollid, J. U. (
Tabe, Y., Nakamura, A., Oguri, T. et al. (
Shore, K. & Morris, A. (
Miron, D. (
Courvalin, P. (
Blaser, J., Stone, B. B., Groner, M. C. et al. (
Moore, R. D., Lietman, P. S. & Smith, C. R. (
Lundergan, F. S., Glasscock, G. F., Kim, E. H. et al. (
Rastogi, A., Agarwal, G., Pyati, S. et al. (
Klingenberg, C., Smabrekke, L., Lier, T. et al. (
Langhendries, J. P., Battisti, O., Bertrand, J. M. et al. (
Berger, A., Kretzer, V., Gludovatz, P. et al. (
Martineau, F., Picard, F. J., Grenier, L. et al. (
Hotta, K., Zhu, C. B., Ogata, T. et al. (
Kondo, S. & Hotta, K. (
Hayashi, I., Inoue, M. & Hashimoto, H. (
Fujii, R., Fujita, K., Sakata, Y. et al. (
Deguchi, K., Suzuki, Y., Ishihara, R. et al. (
Ito, T., Okuma, K., Ma, X. X. et al. (
Diekema, D. J., Pfaller, M. A., Schmitz, F. J. et al. (
Author notes
1Department of Paediatrics, University Hospital of North-Norway, N-9038 Tromsø; 2Department of Paediatrics, Institute of Clinical Medicine, University of Tromsø, Tromsø; 3Department of Microbiology and Virology, Institute of Medical Biology, University of Tromsø, Tromsø; 4Division of Infectious Disease Control, Norwegian Institute of Public Health, Oslo; 5Department of Paediatrics, National Hospital, Oslo; 6Department of Microbiology, National Hospital, Oslo, Norway