-
PDF
- Split View
-
Views
-
Cite
Cite
Inga Odenholt, Otto Cars, Elisabeth Löwdin, Pharmacodynamic studies of amoxicillin against Streptococcus pneumoniae: comparison of a new pharmacokinetically enhanced formulation (2000 mg twice daily) with standard dosage regimens, Journal of Antimicrobial Chemotherapy, Volume 54, Issue 6, December 2004, Pages 1062–1066, https://doi.org/10.1093/jac/dkh484
Close - Share Icon Share
Abstract
Objectives: To compare the pharmacodynamic effects of a pharmacokinetically enhanced formulation of amoxicillin 2000 mg twice daily, with amoxicillin 875 mg twice daily, 875 mg three times daily and 500 mg three times daily against Streptococcus pneumoniae with different susceptibility to amoxicillin in an in vitro kinetic model.
Methods: Strains of S. pneumoniae with amoxicillin MICs of 1, 2, 4 and 8 mg/L at an initial inoculum of approximately 105 cfu/mL were exposed to amoxicillin in an in vitro kinetic model simulating the human serum concentration–time profile of the pharmacokinetically enhanced formulation twice daily (Cmax 17 mg/L after 1.5 h). All isolates were also exposed to amoxicillin with concentration–time profiles correlating to the human dosage of 875 mg twice daily (Cmax 15 mg/L after 1 h), 875 mg three times daily and 500 mg (Cmax 8 mg/L after 1 h) three times daily with simulated half-life of 1 h. Repeated samples were taken regularly during 24 h and viable counts were carried out.
Results: Overall, the pharmacokinetically enhanced formulation was more effective at reducing bacterial counts than any of the other formulations evaluated. Eradication was achieved with the enhanced formulation for strains with a MIC of ≤2 mg/L, however, regrowth occurred with the other dosing regimens. In the experiments with the strain with a MIC of 4 mg/L, the enhanced formulation kept the bacterial counts ≤102 cfu/mL for at least 14 out of 24 h tested. In contrast, none of the other formulations reduced the bacterial counts down to ≤102 cfu/mL at any point. None of the regimens was able to eradicate the strain with an MIC of 8 mg/L, even though an initial substantial kill was noted with the enhanced formulation after both doses. The least effective dosage regimen for all strains was 875 mg twice daily.
Introduction
Amoxicillin/clavulanate is a broad-spectrum antibiotic for the treatment of a wide range of bacterial infections. Different dosage regimens of amoxicillin and clavulanic acid are currently used in clinical practice.1,2 A pharmacokinetically enhanced formulation of amoxicillin/clavulanate (2000/125 mg) has recently been introduced.2 The rationale behind this development is to optimize pharmacokinetic/pharmacodynamic (PK/PD) parameters to combat the increasing resistance reported in Streptococcus pneumoniae.3–5
In general, β-lactam antibiotics are known to exhibit time-dependent killing and their efficacy is mainly dependent on the time that the free (non-protein-bound) concentration stays above the MIC (t > MIC). The exact fraction of the dosage interval during which this concentration should be exceeded for optimal clinical efficacy is, however, not known and may vary according to the type of β-lactam antibiotic and the site of infection.6–12 In a recent study published in 2001, using an in vitro kinetic model, we showed that the t > MIC needed to obtain maximal efficacy of amoxicillin against susceptible S. pneumoniae was approximately 60% for a strain with an MIC of 2 mg/L.13 The aim of the present investigation was to compare, in an in vitro kinetic model, the pharmacodynamic effects of a pharmacokinetically enhanced formulation of amoxicillin 2000 mg twice daily with amoxicillin 875 mg twice daily, 875 mg three times daily and 500 mg three times daily against S. pneumoniae with different susceptibility to amoxicillin.
Materials and methods
Antibiotics
Amoxicillin and clavulanic acid were provided by GlaxoSmithKline (Collegeville, PA, USA). The antibiotic and clavulanic acid were obtained as reference powders with known potency. Amoxicillin was dissolved in 0.5 mL of 0.1 M sodium hydroxide and 0.5 mL of phosphate-buffered saline (pH 7.4). The clavulanate was dissolved in 0.1 M phosphate buffer (pH 6.0). Fresh solutions were made before each experiment.
Bacterial strains and medium
The strains used in this study included S. pneumoniae 1855, 40932, A13/96 and 542-2003 with different susceptibilities to amoxicillin. The strain 1855 was obtained from the Swedish Institute of Infectious Disease Control, Solna, Sweden and the other three from the Department of Microbiology, Uppsala, Sweden. Before each experiment, the strains were grown for 6–7 h at 37°C in air with 5% CO2 in Todd–Hewitt broth (Difco Laboratories, Detroit, MI, USA), yielding an inoculum of approximately 108 cfu/mL.
Determination of minimum inhibitory concentrations (MICs)
The MICs of the strains were determined in fluid media by a macro-dilution technique in duplicate on different occasions according to the National Committee for Clinical Laboratory Standards.14 Two-fold serial dilutions of amoxicillin were added to Todd–Hewitt broth and inoculated with a final inoculum of 105 cfu of the test strain per mL and incubated at 37°C for 22 h. The MIC was defined as the lowest concentration of the antibiotic allowing no visible growth. Determination of MIC was carried out in duplicate on different occasions.
Determination of the concentrations of amoxicillin
The concentrations of amoxicillin were determined by microbiological agar diffusion methods, using Bacillus stearothermophilus ATCC 3032 as the test organism. A standardized inoculum of spore suspension was mixed with nutrient agar, adjusted to pH 7.4 and plates poured. After drying the plates, 0.01 or 0.03 mL volumes of all samples and standards diluted in Todd–Hewitt broth were placed in agar wells and three parallel determinations were made.15 The limit of detection was 0.1 mg/L for amoxicillin. The correlation coefficient for the standard curves was always >0.99 and the coefficient of variation on samples analysed on different days was 9%.
In vitro kinetic model
A previously described in vitro kinetic model was used in these experiments.13,16–18 It consists of a spinner flask with a 0.45 μm filter membrane and a pre-filter fitted in between the upper and the bottom part. A magnetic stirrer ensures homogeneous mixing of the culture and prevents membrane pore blockage. In one of the sidearms of the culture vessel, a silicon membrane is inserted to enable repeated sampling. In the other arm, a thin plastic tube is inserted, which is connected to a vessel containing fresh medium. The medium is removed from the culture flask, through the filter, at a constant rate with a pump. Fresh sterile medium is sucked into the flask at the same rate by the negative pressure built up inside the culture vessel. The dosing system consists of a computer-controlled infusion pump, which is connected to a personal computer. The infusion pump is controlled by newly developed computer software (ARUDose 2, Snowfall Communications, Uppsala, Sweden). The software is programmed to infuse the drug at an exponentially decreasing rate C = (V0 × ka) × e–kat where C is the infusion rate, V0 is the initial volume in the syringe, ka the constant of the absorption rate and t is the time elapsing since the addition of the antibiotic.
In the software, the volume of the antibiotic, the rate of absorption (Ka), the diameter of the syringe and the infusion rates at which the infusion pump should terminate is entered. The apparatus was placed in a thermostatically controlled room at 37°C during the experiments.
Experiments
To determine the possible role of clavulanic acid in the killing of S. pneumoniae,10 an initial experiment with strain A13/96 (MIC of 4 mg/L) was carried out. The strain was exposed to a constant concentration of 15 mg/L of amoxicillin ± 3 mg/L clavulanate and killing curves during 24 h were performed. No difference in the rate or extent of killing between the two regimens was seen and therefore clavulanate was left out in the following experiments (data not shown). The strains of S. pneumoniae at an initial inoculum of approximately 105 cfu/mL were exposed to amoxicillin with a concentration–time profile simulating human serum levels of the pharmacokinetically enhanced amoxicillin/clavulanate formulation (obtained from GlaxoSmithKline) 2000/125 mg twice daily (amoxicillin Cmax 17 mg/L after 1.5 h).19 Experiments were carried out three times with each strain. All isolates were also exposed to amoxicillin with concentration–time profiles correlating to the human dosage of 875 mg twice daily (Cmax 15 mg/L after 1 h), 875 mg three times daily and 500 mg (Cmax 8 mg/L after 1 h) three times daily with simulated half-life of 1 h. These experiments were carried out in duplicate with each strain.
During the experiments simulating 875 mg three times daily, 500 mg three times daily and 2000 mg twice daily (enhanced formulation), repeated samples for viable counts were taken at the start of the experiments and every second hour up to 18 h and at 24 h. For the enhanced formulation, samples were also taken after 1 and 13 h. For the 875 mg twice-daily dosage regimen, samples were taken at the start of the experiments, every second hour up to 14 h and at 24 h. The samples were, if necessary, diluted in phosphate-buffered saline and three dilutions of each sample were spread on blood agar plates (Colombia agar base with 5% horse blood) incubated at 37°C in 5% CO2 in air and counted after 24 h. The limit of detection of the viable counts was 5 × 101 cfu/mL.
Results
Minimum inhibitory concentrations
The MICs of amoxicillin for the different strains were 1 mg/L for S. pneumoniae 1855, 2 mg/L for 40932, 4 mg/L for A13/96 and 8 mg/L for 542-2003.
Concentrations of amoxicillin
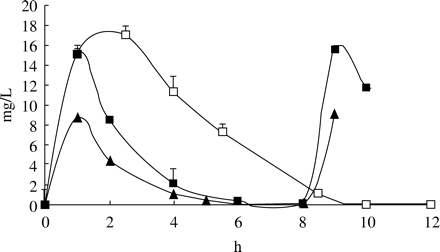
The concentrations of amoxicillin at standard dosage regimens were as expected with very little variation. The simulated concentrations of the new pharmacokinetically enhanced formulation, 875 and 500 mg of amoxicillin are shown in Figure 1.
Experiments
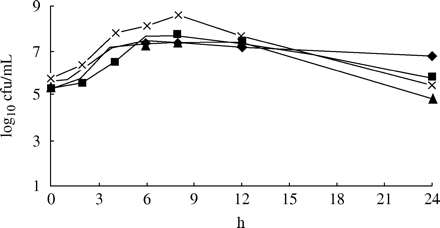
All strains grown in media without antibiotic reached approximately 107–108 cfu/mL at 6–8 h. As a result of spontaneous autolysis, there was a decline in cfu at 24 h (Figure 2).
The approximate t > MICs for the different dosage regimens and different strains according to their MIC are listed in Table 1.
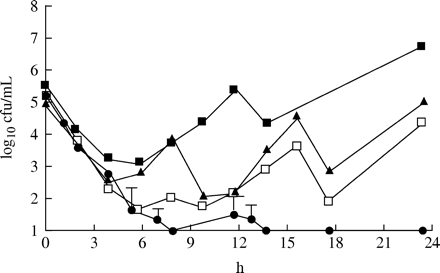
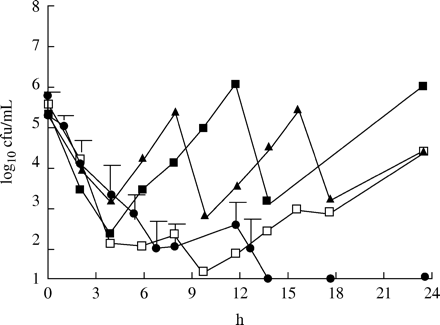
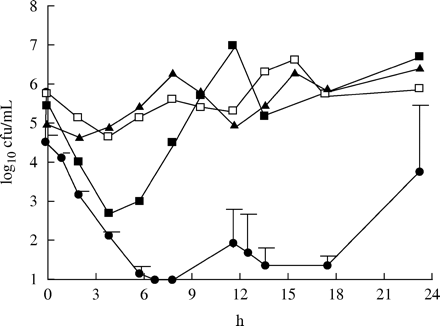
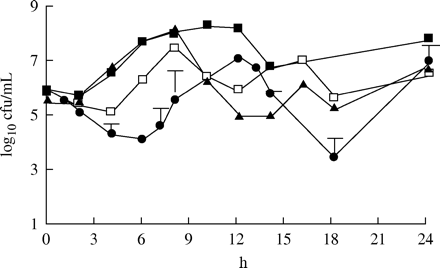
Figure 3 shows the effect of the different dosage regimens against S. pneumoniae with an MIC of 1 mg/L. Only the dosing regimen with the enhanced formulation given twice daily was able to eradicate the strain. For the 875 mg three times daily and 500 mg three times daily regimens, the amounts of cfu at 24 h were similar to those of the initial inoculum. With the 875 mg twice daily regimen, there was an approximately 1 log10 increase in cfu at 24 h in comparison to the initial inoculum. Figure 4 shows the effect against the strain with an MIC of 2 mg/L. Once again it was only the enhanced formulation that eradicated the strain. For the 875 mg three times daily and 500 mg three times daily regimen, there was a 1 log10 decrease in cfu at 24 h in comparison to the initial inoculum. The 875 mg twice daily regimen gave a static effect. Figure 5 displays the effect against the strain with an MIC of 4 mg/L. Once again the enhanced formulation exerted the best effect. The number of bacteria was under the detection limit after 6 h. However, regrowth was noted in all three experiments after 24 h. A slight increase in comparison to the initial inoculum or a static effect was noted for the other dosage regimens at 24 h. For the strain with the highest MIC of 8 mg/L, regrowth was seen for all four dosage regimens at 24 h. However, a substantial decrease in cfu was noted after both doses of the enhanced formulation in comparison to the other three regimens (Figure 6).
Discussion
Bacterial eradication is important to maximize clinical cure and minimize resistance development and spread of resistant clones.12,20,21 The prevalence of resistant strains of common respiratory tract pathogens is increasing. The frequency of penicillin resistance in S. pneumoniae varies between countries and is as high as 70% in southeast Asia.22 This escalating resistance seen in clinical isolates of S. pneumoniae has reduced the efficacy of many oral drugs and has stressed the need for new dosage regimens, since the efficacy of standard dosage regimens may be reduced.20,23,24 A pharmacokinetically enhanced formulation of co-amoxiclav has been developed to provide a more effective therapy against bacteria with increasing resistance to amoxicillin, notably S. pneumoniae. The drug was developed to obtain a t > MIC of ≥40% over a 12 h dosing interval for strains with a MIC of 4 mg/L.19 The efficacy of the new pharmacokinetically enhanced formulation of amoxicillin/clavulanic acid 2000 mg/125 mg given twice daily has been evaluated in several clinical studies in patients with respiratory tract infections predominantly caused by S. pneumoniae including strains resistant to penicillin. The outcome was successful for 98.2% (55/56) of the patients with infections caused by penicillin-resistant S. pneumoniae including two bacteraemic patients. Twelve of these resistant isolates had MICs of 4 mg/L and nine had MICs of 8 mg/L against amoxicillin.3–5
In this study, the strains with an MIC of 1 or 2 mg/L were eradicated at 24 h when the kinetics of the enhanced formulation were simulated. All the other regimens showed a static effect or a slight regrowth (875 mg twice daily) against these strains. Also for the strain with an MIC of 4 mg/L, the enhanced formulation was more effective than the other regimens and resulted in no detectable bacteria after 7 h although regrowth occurred at 24 h. For the strain with the highest MIC (8 mg/L), regrowth was noted for all regimens at that time. However, even for this strain, a substantial initial kill was obtained after both doses of the enhanced formulation. These findings are in accordance with an earlier study with the enhanced formulation of amoxicillin in the same in vitro kinetic model, where standard dosage regimens of amoxicillin gave inferior results in comparison with the enhanced formulation against Haemophilus influenzae.18
Many studies indicate that the magnitude of the PK/PD parameter required for efficacy is similar in animals and in humans.6–12,25 In thigh and lung infection models in neutropenic mice, Craig et al. have shown that the t > MIC for β-lactam antibiotics against S. pneumoniae needed to be approximately 40–50% of the dosage interval in order to achieve a 90–100% survival of the mice after 4 days of treatment. When the t > MIC was ≤20%, the mortality was almost 100%.11 In another study by Andes & Craig, renal impairment was induced in neutropenic mice to simulate the human pharmacokinetics of amoxicillin. Strains of S. pneumoniae with different amoxicillin MICs were studied. With a simulated dose of 500 mg (even though t > MIC was 1–1.5 h less than in humans) they found killing of strains with MICs up to 2 mg/L. Above an MIC of 2 mg/L, regrowth occurred.10 In this study, they also found that the differences between the extent of killing for amoxicillin and co-amoxiclav at the end of 24 h of therapy were insignificant.10 In another study, Woodnutt & Berry used a rat pneumonia model. They infected rats with S. pneumoniae with different amoxicillin MICs (2, 4 and 8 mg/L) and could show a significant reduction in bacterial counts in the lungs when concentrations of co-amoxiclav in plasma were above the MIC for at least 34% of a 24 h dosing interval.26
Concerning clinical trials, Craig & Andes compiled data retrospectively that included patients with otitis media caused by S. pneumoniae and H. influenzae, where the microbiological efficacy was followed by repeated tympanocenteses. They found that an increased time that free serum levels were above the MIC (calculated from published serum pharmacokinetic data in paediatric populations) correlated with an increased bacteriological eradication of the infecting pathogens. To achieve an 80–85% bacteriological cure rate of both cephalosporins and penicillins against the two pathogens, a time above the MIC of 50% and 40%, respectively was required.7 Recently, Dagan et al. have published data on bacterial eradication in the treatment of sinusitis, and found the same figures for t > MIC as with otitis media.12
This in vitro kinetic model provides a valuable tool to compare the antibacterial effects of different antibiotics, however, like other in vitro PK/PD models, the model more closely mimics the effects in an immunocompromised host as the synergic effects of the host are not taken into account. The pharmacokinetically enhanced amoxicillin 2000 mg twice daily formulation was more effective than any of the other formulations tested, achieving substantially better eradication rates against S. pneumoniae isolates with MICs commonly encountered in the clinic (amoxicillin MICs of 1 and 2 mg/L). Against less-common resistant isolates (amoxicillin MICs of 4 and 8 mg/L), the enhanced formulation reduced the bacterial counts more than the other formulations, but did not achieve eradication. However, with the interplay of the host's immune system, this reduction could provide some advantage over standard co-amoxiclav formulations in the clinic.
The concentrations of amoxicillin. Open squares=pharmacokinetically enhanced formulation of amoxicillin; filled squares=875 mg × 2; filled triangles=500 mg × 3 (mean of six experiments ± s.d.).
Growth controls of S. pneumoniae 1855, 40932, A13/96 and 542-2003.
The killing effect of amoxicillin at different dosage regimens against S. pneumoniae 1855 (MIC=1 mg/L). Filled squares=875 mg twice daily; filled triangles=500 mg three times daily; open squares=875 mg three times daily (mean of two experiments); filled circles=enhanced formulation twice daily (mean of three experiments ± s.d.).
The killing effect of amoxicillin at different dosage regimens against S. pneumoniae 40932 (MIC=2 mg/L). Filled squares=875 mg twice daily; filled triangles=500 mg three times daily; open squares=875 mg three times daily (mean of two experiments); filled circles=enhanced formulation twice daily (mean of three experiments ± s.d.).
The killing effect of amoxicillin at different dosage regimens against S. pneumoniae A13/96 (MIC=4 mg/L). Filled squares=875 mg twice daily; filled triangles=500 mg three times daily; open squares=875 mg three times daily (mean of two experiments); filled circles=enhanced formulation twice daily (mean of three experiments ± s.d.).
The killing effect of amoxicillin at different dosage regimens against S. pneumoniae 542-2003 (MIC=8 mg/L). Filled squares=875 mg twice daily; filled triangles=500 mg three times daily; open squares=875 mg three times daily (mean of two experiments); filled circles=enhanced formulation twice daily (mean of three experiments ± s.d.).
The approximate t > MIC of the different dosage regimens for the different strains according to their MIC
| MIC (mg/L) . | 875 mg twice daily . | 500 mg three times daily . | 875 mg three times daily . | Enhanced formulation twice daily . |
|---|---|---|---|---|
| 1 | 43% | 47% | 65% | 73% |
| 2 | 35% | 35% | 53% | 60% |
| 4 | 26% | 23% | 39% | 47% |
| 8 | 10% | 0% | 26% | 35% |
| MIC (mg/L) . | 875 mg twice daily . | 500 mg three times daily . | 875 mg three times daily . | Enhanced formulation twice daily . |
|---|---|---|---|---|
| 1 | 43% | 47% | 65% | 73% |
| 2 | 35% | 35% | 53% | 60% |
| 4 | 26% | 23% | 39% | 47% |
| 8 | 10% | 0% | 26% | 35% |
The approximate t > MIC of the different dosage regimens for the different strains according to their MIC
| MIC (mg/L) . | 875 mg twice daily . | 500 mg three times daily . | 875 mg three times daily . | Enhanced formulation twice daily . |
|---|---|---|---|---|
| 1 | 43% | 47% | 65% | 73% |
| 2 | 35% | 35% | 53% | 60% |
| 4 | 26% | 23% | 39% | 47% |
| 8 | 10% | 0% | 26% | 35% |
| MIC (mg/L) . | 875 mg twice daily . | 500 mg three times daily . | 875 mg three times daily . | Enhanced formulation twice daily . |
|---|---|---|---|---|
| 1 | 43% | 47% | 65% | 73% |
| 2 | 35% | 35% | 53% | 60% |
| 4 | 26% | 23% | 39% | 47% |
| 8 | 10% | 0% | 26% | 35% |
This study was supported by a grant from GlaxoSmithKline, Collegeville, PA, USA.
References
Todd, P. A. & Benfield, P. (
White, A. R., Kaye, C., Poupard, J. et al. (
File, T. M., Jr, Jacobs, M. R., Poole, M. D. et al. (
Petitpretz, P., Chidiac, C., Soriano, F. et al. (
Garau, J., Twynholm, M., Garcia-Mendez, E. et al. (
Vogelman, B., Gudmundsson, S., Turnidge, J. et al. (
Craig, W. A. & Andes, D. (
Cars, O. (
Drusano, G. I. & Craig, W. A. (
Andes, D. & Craig, W. A. (
Craig, W. A. (
Dagan, R., Klugman, K. P., Craig, W. A. et al. (
Gustafsson, I., Löwdin, E., Odenholt, I. et al. (
National Committee for Clinical Laboratory Standards. (
Cars, O., Henning, C. & Holm, S. E. (
Löwdin, E., Odenholt, I., Bengtsson, S. et al. (
Löwdin, E., Odenholt, I. & Cars, O. (
Löwdin, E., Odenholt, I. & Cars, O. (
Kaye, C., Allen, A., Perry, S. et al. (
Ball, P., Baquero, F., Cars, O. et al. (
Dagan, R. (
Jacobs, M. R., Felmingham, D., Appelbaum, P. C. et al. (
Aguilar, L., Giménez, M. J., Garcia-Rey, C. et al. (
Jacobs, M. R. (
Andes, D. & Craig, W. A. (