-
PDF
- Split View
-
Views
-
Cite
Cite
Devyani Deshpande, Shashikant Srivastava, Jotam G. Pasipanodya, Pooi S. Lee, Tawanda Gumbo, A novel ceftazidime/avibactam, rifabutin, tedizolid and moxifloxacin (CARTM) regimen for pulmonary Mycobacterium avium disease, Journal of Antimicrobial Chemotherapy, Volume 72, Issue suppl_2, September 2017, Pages i48–i53, https://doi.org/10.1093/jac/dkx307
Close - Share Icon Share
Abstract
To compare the efficacy of ceftazidime/avibactam plus tedizolid-based combination regimens with the standard therapy of azithromycin, ethambutol and rifabutin for the treatment of pulmonary Mycobacterium avium complex (MAC) disease.
We mimicked the human pulmonary concentration–time profiles of ceftazidime/avibactam and tedizolid in combination, ceftazidime/avibactam, rifabutin, tedizolid and moxifloxacin (CARTM), and the standard regimen and examined microbial kill in triplicate hollow-fibre system model of intracellular pulmonary MAC (HFS-MAC) units. The tedizolid and moxifloxacin doses used were non-optimized; the tedizolid dose was that associated with bacteriostasis. Drugs were administered daily for 28 days. Each HFS-MAC was sampled in the central and peripheral compartment to ascertain that the intended drug exposures had been achieved. The peripheral compartments were sampled at regular intervals over the 28 days to quantify the burden of MAC.
MAC-infected macrophages in the HFS-MAC achieved multi-fold higher intracellular versus extracellular concentrations of rifabutin, moxifloxacin, ceftazidime/avibactam. The non-optimized ceftazidime/avibactam plus tedizolid dual therapy held the bacterial burden at the same level as day 0 (stasis) throughout the 28 days. The standard therapy reduced the bacterial load 2 log10 cfu/mL below stasis on day 14 but started failing after that. The CARTM regimen achieved 3.2 log10 cfu/mL kill below stasis on day 21, but had started to fail by day 28.
The CARTM regimen promises to have kill rates better than standard therapy. Experiments to identify exposures of each of the four drugs associated with optimal effect in the CARTM combination are needed in order to design a short-course chemotherapy regimen.
Introduction
The currently recommended treatment for pulmonary Mycobacterium avium complex (MAC) disease involves using a combination of macrolides, ethambutol and perhaps a rifamycin.1 Of these, macrolides are the only agents for which a correlation exists between in vitro susceptibility and clinical response.2–6 Macrolides are considered the cornerstone of the regimen but administration as monotherapy leads to development of resistance.3,5,7 Even with combination therapy the treatment course commonly lasts more than a year, but despite this long duration, response rates are frequently modest.8,9 In a meta-analysis of prospective clinical studies, sustained sputum conversion (defined as at least two consecutive negative smears) was achieved in only 54% of patients at 6 months treated with the macrolide-containing combination therapy regimen, and when therapy duration was prolonged beyond 12 months (which is the norm) the sustained sputum conversion rates were a meagre 22%.10 These findings argue for exploration of an alternative antimicrobial regimen for pulmonary MAC. Since the clinical performance of the standard or recommended regimen is known, we can use it to benchmark the expected performance of any new regimens that may improve response rates. An ambitious goal would be to design a 6 month regimen, associated with >90% response rates.
There are several basic pharmacological aspects that could guide design of a new combination regimen.11 One important consideration would be to use drugs without overlapping mechanisms of action. As an example, oxazolidinones inhibit bacterial protein synthesis by competitive binding to the 23S rRNA, the catalytic site of the bacterial 50S ribosomes.12,13 In the hollow-fibre model of intracellular pulmonary MAC (HFS-MAC), the oxazolidinone linezolid killed at least 1.0 log10 cfu/mL below the starting bacterial burden, whereas tedizolid achieved an even greater kill of 2.0 log10 cfu/mL.14,15 In the same model, the combination of ceftazidime, a third-generation cephalosporin, and avibactam, a non-β-lactam β-lactamase inhibitor, killed better than the drugs used in standard therapy regimens at more clinically achievable doses.16 β-Lactams kill by binding penicillin-binding proteins and inhibiting cell wall synthesis. A potential novel regimen that exploits the different mechanisms of action would be to combine tedizolid and ceftazidime/avibactam. In terms of other targets, fluoroquinolones (such as the 8-methoxy quinolone moxifloxacin) kill bacteria by inhibiting DNA gyrase and have been demonstrated to be efficacious against MAC in the HFS.17 This could make moxifloxacin a good companion agent to the other two drugs. Finally, rifamycins such as rifabutin and rifampicin kill mycobacteria by inhibiting the β-subunit of bacterial RNA polymerase (rpoB), a different mechanism of protein synthesis inhibition to that of oxazolidinones. As a first approach, we wanted to examine the effect of a combination of these drugs at the doses at which they are currently licensed (i.e. a low-hanging fruit approach), and determine whether the degree of bactericidal activity would exceed that of standard therapy, and thus potentially improve on current response rates. This also marked the first time we have examined rifabutin, a drug that sticks to the surface of a lot of laboratory apparatus, including the HFS material.
Materials and methods
Bacterial strain, chemicals and hollow-fibre cartridges
Mycobacterium avium subsp. hominissuis (ATCC 700898) was purchased from the ATCC (Manassas, VA, USA). Stock was prepared and propagation of cultures was as described previously.17–19 THP-1 monocytes (TIB-202) were purchased from the ATCC. The active moiety of tedizolid was synthesized for us by BOC Sciences (NY, USA). Ceftazidime/avibactam, moxifloxacin, azithromycin, ethambutol and rifabutin were purchased from the Baylor University Medical Center pharmacy. Hollow-fibre cartridges were purchased from FiberCell (Frederick, MD, USA).
Determination of MIC
The rifabutin MIC was determined using two different methods. First, the broth macrodilution method was as described previously for linezolid.14 The second method was the broth microdilution method described previously for ceftazidime/avibactam.16 The rifabutin concentrations used were 0, 0.0625, 0.125, 0.25, 0.5, 1.0, 2.0 and 4.0 mg/L in triplicate. The MICs for the MAC isolates were 1.25 mg/L tedizolid, 16 mg/L ceftazidime/avibactam, 8 mg/L ethambutol, 32 mg/L azithromycin and 1 mg/L moxifloxacin, as reported elsewhere.15–18,20
Combination studies in the HFS model of pulmonary MAC
The details of the HFS-MAC model and its construction have been described previously.14–16,17–19 THP-1 cells cultured in pre-warmed RPMI 1640 with 10% FBS were infected with MAC at a bacterium-to-monocyte ratio of 1:1, then washed, as described previously.14 We performed a pilot study in which we inoculated 20 mL of MAC-infected THP-1 cells into the external compartment of each of six HFS-MAC. Three of the HFS-MAC were treated with tedizolid and ceftazidime/avibactam dual therapy whilst the remaining three HFS-MAC served as non-drug-treated controls. The HFS-MAC were preconditioned with RPMI 1640 and 2% FBS (RPMI/FBS) and maintained in incubators at 37 °C for at least 72 h prior to start of drug treatment. Tedizolid was administered via computer-programmed syringe pumps into the infusion port to the central compartment over 1 h, followed by supplementation over 23 h to mimic a half-life of 12 h and the concentration–time profiles encountered in lungs of adult patients.21,22 For ceftazidime/avibactam we mimicked a half-life of 3 h and simulated the concentration–time profiles observed in human lungs, taking into consideration a penetration rate of 33% into epithelial lining fluid.23 The dosing frequency of ceftazidime/avibactam was every 8 h. Both drugs were administered for a duration of 28 days. The central compartments of each HFS-MAC were sampled at 0.5, 2, 5, 8, 10, 13, 16, 18 and 23 h following the first dose in order to validate the concentration–time profiles. In addition, the peripheral compartment of each of the HFS-MAC was sampled on days 0, 4, 7, 14, 21 and 28 of treatment to quantify the number of THP-1 cells and the bacterial burden. THP-1 cells in the samples were ruptured and contents cultured on agar at 37 °C under 5% CO2 for 14 days, after which colonies were counted.
We then performed a second experiment comprising of 12 HFS. The objectives of the study were: (i) to examine the effect of dual therapy with ceftazidime/avibactam and tedizolid at concentrations achieved with clinical doses; (ii) to examine the effect of quadruple therapy with moxifloxacin at high concentration (800 mg/day human equivalent) along with ceftazidime/avibactam, tedizolid and rifabutin at concentrations attained with clinical doses; (iii) to examine the microbial kill of the currently recommended regimen of azithromycin, ethambutol and rifabutin; and (iv) to determine the penetration of drugs into macrophages in the different treatment regimens. The treatment duration was 28 days, and sampling of peripheral compartments was done on days 0, 7, 9, 14, 21 and 28 for quantification of MAC burden. The central compartments of each HFS-MAC, as well as infected macrophages in the peripheral compartment, were sampled at 1, 4, 8, 12 and 24 h after the first dose for validation of the concentration–time profiles in each HFS-MAC.
Measurement of drug concentrations
Azithromycin, ceftazidime, ethambutol, rifabutin and moxifloxacin were purchased from Sigma (St Louis, MO, USA). Avibactam was from Advanced ChemBlocks Inc. (Burlingame, CA, USA). Tedizolid, ceftazidime-d5 [internal standard (IS) for ceftazidime] and clarithromycin-N-methyl-13Cd3 (IS for azithromycin) were from Toronto Research Chemicals (Toronto, Canada). Tazobactam (IS for avibactam), rifampicin-d3 (IS for rifabutin) and moxifloxacin-13Cd3 (IS for moxifloxacin) were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Ethambutol-d10 (IS for ethambutol) and linezolid-d3 (IS for tedizolid) were from CDN Isotopes (Quebec, Canada). Samples were analysed using LC-MS/MS. Calibrator, controls and internal standards were included in each analytical run for quantification. Stock solutions of the standards and internal standards were prepared in 80:20 methanol:water at a concentration of 1 mg/mL and stored at − 20 °C. An eight-point calibration curve was prepared by diluting the stock solution in drug-free RPMI 1640/2% FBS for extracellular samples and in drug-free saline for intracellular samples. Quality control samples were prepared by spiking drug-free media with stock standards for two levels of controls. Samples were prepared in 96-well microtitre plates by the addition of 10 μL of calibrator, quality controls or sample to 190 μL 0.1% formic acid in water containing 1 μg/mL of each of the IS followed by vortexing, then centrifuged at 4000 rpm for 5 min to remove cellular debris. Chromatographic separation was achieved by injecting 2 μL on a Waters Acquity UPLC HSS T3 column (50 × 2.1 mm; 1.8 μm) maintained at 30 °C at a flow of 0.2 mL/min with a binary gradient with a total run time of 6 min. Solvents for UPLC were: (A) water containing 0.1% formic acid, and (B) methanol containing 0.1% formic acid. The initial gradient condition was 5% B for the first 1.5 min and was increased in a linear fashion to 100% B at 1.8 min and held constant for 2.2 min. At 4.5 min the flow was reset to the initial conditions for 1.5 min. The flow from the column was delivered to the Z-spray source for the period of 0–3.25 min; the compounds were detected by MS/MS using positive electrospray ionization (ESI) for all compounds, except avibactam which used negative ESI. The mass transitions and assay characteristics are shown in Tables 1 and 2. Sample injection and separation was performed by an Acquity UPLC interfaced with a Xevo TQ mass spectrometer (Waters). All data were collected using MassLynx version 4.1 SCN810.
Characteristics of the extracellular drug concentration assay
| Compounds . | Transition (m/z) . | Calibration range (μg/mL) . | Correlation coefficient (r2) . | Quality control (μg/mL)a . | Interday %CVa . | Intraday %CVa . | LLOQ (μg/mL) . |
|---|---|---|---|---|---|---|---|
| Ethambutol | 205>116 | 0.02–20 | >0.99 | 0.4, 8.0 | 3, 3 | 8, 1 | 0.02 |
| Ethambutol-d10 | 215>123 | — | — | — | — | — | — |
| Linezolid-d3 | 341>297 | — | — | — | — | — | — |
| Tedizolid | 371>343 | 0.04–40 | >0.99 | 0.8, 16.0 | 24, 11 | 9, 6 | 0.04 |
| Moxifloxacin | 402>384 | 0.01–10 | >0.99 | 0.2, 4.0 | 9, 4 | 10, 3 | 0.01 |
| Moxifloxacin-13Cd3 | 406>388 | — | — | — | — | — | — |
| Ceftazidime | 547>468 | 0.2–200 | >0.99 | 4.0, 80 | 17, 12 | 17, 7 | 0.2 |
| Ceftazidime-d5 | 552>468 | — | — | — | — | — | — |
| Azithromycin | 750<158 | 0.01–10 | >0.990939 | 0.2, 4.0 | 21, 9 | 27, 7 | 0.05 |
| Clarithromycin-13Cd3 | 752>162 | — | — | — | — | — | — |
| Rifampicin-d3 | 826>794 | — | — | — | — | — | — |
| Rifabutin | 848>816 | 0.05, 2.0 | 27, 9 | 41, 5 | 0.05 | ||
| Avibactam | 264>96 | 0.04–40 | >0.99 | 0.8, 16.0 | 7, 2 | 5, 2 | 0.04 |
| Tazobactam | 299>138 | — | — | — | — | — | — |
| Compounds . | Transition (m/z) . | Calibration range (μg/mL) . | Correlation coefficient (r2) . | Quality control (μg/mL)a . | Interday %CVa . | Intraday %CVa . | LLOQ (μg/mL) . |
|---|---|---|---|---|---|---|---|
| Ethambutol | 205>116 | 0.02–20 | >0.99 | 0.4, 8.0 | 3, 3 | 8, 1 | 0.02 |
| Ethambutol-d10 | 215>123 | — | — | — | — | — | — |
| Linezolid-d3 | 341>297 | — | — | — | — | — | — |
| Tedizolid | 371>343 | 0.04–40 | >0.99 | 0.8, 16.0 | 24, 11 | 9, 6 | 0.04 |
| Moxifloxacin | 402>384 | 0.01–10 | >0.99 | 0.2, 4.0 | 9, 4 | 10, 3 | 0.01 |
| Moxifloxacin-13Cd3 | 406>388 | — | — | — | — | — | — |
| Ceftazidime | 547>468 | 0.2–200 | >0.99 | 4.0, 80 | 17, 12 | 17, 7 | 0.2 |
| Ceftazidime-d5 | 552>468 | — | — | — | — | — | — |
| Azithromycin | 750<158 | 0.01–10 | >0.990939 | 0.2, 4.0 | 21, 9 | 27, 7 | 0.05 |
| Clarithromycin-13Cd3 | 752>162 | — | — | — | — | — | — |
| Rifampicin-d3 | 826>794 | — | — | — | — | — | — |
| Rifabutin | 848>816 | 0.05, 2.0 | 27, 9 | 41, 5 | 0.05 | ||
| Avibactam | 264>96 | 0.04–40 | >0.99 | 0.8, 16.0 | 7, 2 | 5, 2 | 0.04 |
| Tazobactam | 299>138 | — | — | — | — | — | — |
%CV, percentage coefficient of variation; LLOQ, lower limit of quantification.
Values shown are the low quality control and the high quality control.
Characteristics of the extracellular drug concentration assay
| Compounds . | Transition (m/z) . | Calibration range (μg/mL) . | Correlation coefficient (r2) . | Quality control (μg/mL)a . | Interday %CVa . | Intraday %CVa . | LLOQ (μg/mL) . |
|---|---|---|---|---|---|---|---|
| Ethambutol | 205>116 | 0.02–20 | >0.99 | 0.4, 8.0 | 3, 3 | 8, 1 | 0.02 |
| Ethambutol-d10 | 215>123 | — | — | — | — | — | — |
| Linezolid-d3 | 341>297 | — | — | — | — | — | — |
| Tedizolid | 371>343 | 0.04–40 | >0.99 | 0.8, 16.0 | 24, 11 | 9, 6 | 0.04 |
| Moxifloxacin | 402>384 | 0.01–10 | >0.99 | 0.2, 4.0 | 9, 4 | 10, 3 | 0.01 |
| Moxifloxacin-13Cd3 | 406>388 | — | — | — | — | — | — |
| Ceftazidime | 547>468 | 0.2–200 | >0.99 | 4.0, 80 | 17, 12 | 17, 7 | 0.2 |
| Ceftazidime-d5 | 552>468 | — | — | — | — | — | — |
| Azithromycin | 750<158 | 0.01–10 | >0.990939 | 0.2, 4.0 | 21, 9 | 27, 7 | 0.05 |
| Clarithromycin-13Cd3 | 752>162 | — | — | — | — | — | — |
| Rifampicin-d3 | 826>794 | — | — | — | — | — | — |
| Rifabutin | 848>816 | 0.05, 2.0 | 27, 9 | 41, 5 | 0.05 | ||
| Avibactam | 264>96 | 0.04–40 | >0.99 | 0.8, 16.0 | 7, 2 | 5, 2 | 0.04 |
| Tazobactam | 299>138 | — | — | — | — | — | — |
| Compounds . | Transition (m/z) . | Calibration range (μg/mL) . | Correlation coefficient (r2) . | Quality control (μg/mL)a . | Interday %CVa . | Intraday %CVa . | LLOQ (μg/mL) . |
|---|---|---|---|---|---|---|---|
| Ethambutol | 205>116 | 0.02–20 | >0.99 | 0.4, 8.0 | 3, 3 | 8, 1 | 0.02 |
| Ethambutol-d10 | 215>123 | — | — | — | — | — | — |
| Linezolid-d3 | 341>297 | — | — | — | — | — | — |
| Tedizolid | 371>343 | 0.04–40 | >0.99 | 0.8, 16.0 | 24, 11 | 9, 6 | 0.04 |
| Moxifloxacin | 402>384 | 0.01–10 | >0.99 | 0.2, 4.0 | 9, 4 | 10, 3 | 0.01 |
| Moxifloxacin-13Cd3 | 406>388 | — | — | — | — | — | — |
| Ceftazidime | 547>468 | 0.2–200 | >0.99 | 4.0, 80 | 17, 12 | 17, 7 | 0.2 |
| Ceftazidime-d5 | 552>468 | — | — | — | — | — | — |
| Azithromycin | 750<158 | 0.01–10 | >0.990939 | 0.2, 4.0 | 21, 9 | 27, 7 | 0.05 |
| Clarithromycin-13Cd3 | 752>162 | — | — | — | — | — | — |
| Rifampicin-d3 | 826>794 | — | — | — | — | — | — |
| Rifabutin | 848>816 | 0.05, 2.0 | 27, 9 | 41, 5 | 0.05 | ||
| Avibactam | 264>96 | 0.04–40 | >0.99 | 0.8, 16.0 | 7, 2 | 5, 2 | 0.04 |
| Tazobactam | 299>138 | — | — | — | — | — | — |
%CV, percentage coefficient of variation; LLOQ, lower limit of quantification.
Values shown are the low quality control and the high quality control.
Characteristics of the intracellular drug concentration assay
| Compounds . | Transition (m/z) . | Calibration range (μg/mL) . | Correlation coefficient (r2) . | Quality control (μg/mL)a . | Interday %CVa . | Intraday %CVa . | LLOQ (μg/mL) . |
|---|---|---|---|---|---|---|---|
| Ethambutol | 205>116 | 0.02–20 | >0.99 | 0.4, 8.0 | 7, 5 | 13, 5 | 0.02 |
| Ethambutol-d10 | 215>123 | — | — | — | — | — | — |
| Linezolid-d3 | 341>297 | — | — | — | — | — | — |
| Tedizolid | 371>343 | 0.04–40 | >0.99 | 0.8, 16.0 | 27, 16 | 6, 14 | 0.2 |
| Moxifloxacin | 402>384 | 0.01–10 | >0.99 | 0.2, 4.0 | 12, 2 | 11, 3 | 0.01 |
| Moxifloxacin-13Cd3 | 406>388 | — | — | — | — | — | — |
| Ceftazidime | 547>468 | 0.2–200 | >0.99 | 4.0, 80 | 14, 4 | 14, 4 | 0.2 |
| Ceftazidime-d5 | 552>468 | — | — | — | — | — | — |
| Azithromycin | 750<158 | 0.01–10 | >0.99 | 0.2, 4.0 | 18, 17 | 12, 12 | 0.01 |
| Clarithromycin-13Cd3 | 752>162 | — | — | — | — | — | — |
| Rifampicin-d3 | 826>794 | — | — | — | — | — | — |
| Rifabutin | 848>816 | 0.002–2 | >0.99 | 0.05, 2.0 | 26, 5 | 16, 6 | 0.002 |
| Avibactam | 264>96 | 0.04–40 | >0.99 | 0.8, 16.0 | 3, 2 | 5, 3 | 0.04 |
| Tazobactam | 299>138 | — | — | — | — | — | — |
| Compounds . | Transition (m/z) . | Calibration range (μg/mL) . | Correlation coefficient (r2) . | Quality control (μg/mL)a . | Interday %CVa . | Intraday %CVa . | LLOQ (μg/mL) . |
|---|---|---|---|---|---|---|---|
| Ethambutol | 205>116 | 0.02–20 | >0.99 | 0.4, 8.0 | 7, 5 | 13, 5 | 0.02 |
| Ethambutol-d10 | 215>123 | — | — | — | — | — | — |
| Linezolid-d3 | 341>297 | — | — | — | — | — | — |
| Tedizolid | 371>343 | 0.04–40 | >0.99 | 0.8, 16.0 | 27, 16 | 6, 14 | 0.2 |
| Moxifloxacin | 402>384 | 0.01–10 | >0.99 | 0.2, 4.0 | 12, 2 | 11, 3 | 0.01 |
| Moxifloxacin-13Cd3 | 406>388 | — | — | — | — | — | — |
| Ceftazidime | 547>468 | 0.2–200 | >0.99 | 4.0, 80 | 14, 4 | 14, 4 | 0.2 |
| Ceftazidime-d5 | 552>468 | — | — | — | — | — | — |
| Azithromycin | 750<158 | 0.01–10 | >0.99 | 0.2, 4.0 | 18, 17 | 12, 12 | 0.01 |
| Clarithromycin-13Cd3 | 752>162 | — | — | — | — | — | — |
| Rifampicin-d3 | 826>794 | — | — | — | — | — | — |
| Rifabutin | 848>816 | 0.002–2 | >0.99 | 0.05, 2.0 | 26, 5 | 16, 6 | 0.002 |
| Avibactam | 264>96 | 0.04–40 | >0.99 | 0.8, 16.0 | 3, 2 | 5, 3 | 0.04 |
| Tazobactam | 299>138 | — | — | — | — | — | — |
%CV, percentage coefficient of variation; LLOQ, lower limit of quantification.
Values shown are the low quality control and the high quality control.
Characteristics of the intracellular drug concentration assay
| Compounds . | Transition (m/z) . | Calibration range (μg/mL) . | Correlation coefficient (r2) . | Quality control (μg/mL)a . | Interday %CVa . | Intraday %CVa . | LLOQ (μg/mL) . |
|---|---|---|---|---|---|---|---|
| Ethambutol | 205>116 | 0.02–20 | >0.99 | 0.4, 8.0 | 7, 5 | 13, 5 | 0.02 |
| Ethambutol-d10 | 215>123 | — | — | — | — | — | — |
| Linezolid-d3 | 341>297 | — | — | — | — | — | — |
| Tedizolid | 371>343 | 0.04–40 | >0.99 | 0.8, 16.0 | 27, 16 | 6, 14 | 0.2 |
| Moxifloxacin | 402>384 | 0.01–10 | >0.99 | 0.2, 4.0 | 12, 2 | 11, 3 | 0.01 |
| Moxifloxacin-13Cd3 | 406>388 | — | — | — | — | — | — |
| Ceftazidime | 547>468 | 0.2–200 | >0.99 | 4.0, 80 | 14, 4 | 14, 4 | 0.2 |
| Ceftazidime-d5 | 552>468 | — | — | — | — | — | — |
| Azithromycin | 750<158 | 0.01–10 | >0.99 | 0.2, 4.0 | 18, 17 | 12, 12 | 0.01 |
| Clarithromycin-13Cd3 | 752>162 | — | — | — | — | — | — |
| Rifampicin-d3 | 826>794 | — | — | — | — | — | — |
| Rifabutin | 848>816 | 0.002–2 | >0.99 | 0.05, 2.0 | 26, 5 | 16, 6 | 0.002 |
| Avibactam | 264>96 | 0.04–40 | >0.99 | 0.8, 16.0 | 3, 2 | 5, 3 | 0.04 |
| Tazobactam | 299>138 | — | — | — | — | — | — |
| Compounds . | Transition (m/z) . | Calibration range (μg/mL) . | Correlation coefficient (r2) . | Quality control (μg/mL)a . | Interday %CVa . | Intraday %CVa . | LLOQ (μg/mL) . |
|---|---|---|---|---|---|---|---|
| Ethambutol | 205>116 | 0.02–20 | >0.99 | 0.4, 8.0 | 7, 5 | 13, 5 | 0.02 |
| Ethambutol-d10 | 215>123 | — | — | — | — | — | — |
| Linezolid-d3 | 341>297 | — | — | — | — | — | — |
| Tedizolid | 371>343 | 0.04–40 | >0.99 | 0.8, 16.0 | 27, 16 | 6, 14 | 0.2 |
| Moxifloxacin | 402>384 | 0.01–10 | >0.99 | 0.2, 4.0 | 12, 2 | 11, 3 | 0.01 |
| Moxifloxacin-13Cd3 | 406>388 | — | — | — | — | — | — |
| Ceftazidime | 547>468 | 0.2–200 | >0.99 | 4.0, 80 | 14, 4 | 14, 4 | 0.2 |
| Ceftazidime-d5 | 552>468 | — | — | — | — | — | — |
| Azithromycin | 750<158 | 0.01–10 | >0.99 | 0.2, 4.0 | 18, 17 | 12, 12 | 0.01 |
| Clarithromycin-13Cd3 | 752>162 | — | — | — | — | — | — |
| Rifampicin-d3 | 826>794 | — | — | — | — | — | — |
| Rifabutin | 848>816 | 0.002–2 | >0.99 | 0.05, 2.0 | 26, 5 | 16, 6 | 0.002 |
| Avibactam | 264>96 | 0.04–40 | >0.99 | 0.8, 16.0 | 3, 2 | 5, 3 | 0.04 |
| Tazobactam | 299>138 | — | — | — | — | — | — |
%CV, percentage coefficient of variation; LLOQ, lower limit of quantification.
Values shown are the low quality control and the high quality control.
Results
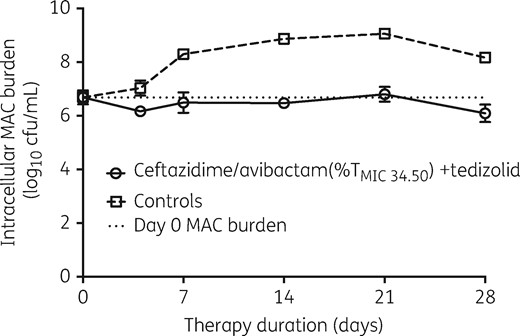
The rifabutin MIC for the MAC strain was 0.0625 mg/L, based on both broth macrodilution and broth microdilution methods. The time–kill plots for the pilot HFS-MAC study are shown in Figure 1. The figure shows that the combination of non-optimized ceftazidime/avibactam and tedizolid was effective in holding the bacterial burden at the same level as the starting inoculum for 28 days.
Time–kill curves of the combination of ceftazidime/avibactam and tedizolid in the HFS-MAC. The combination of ceftazidime/avibactam and tedizolid at exposures associated with bacteriostasis successfully prevented bacterial growth for the 28 day duration of the study.
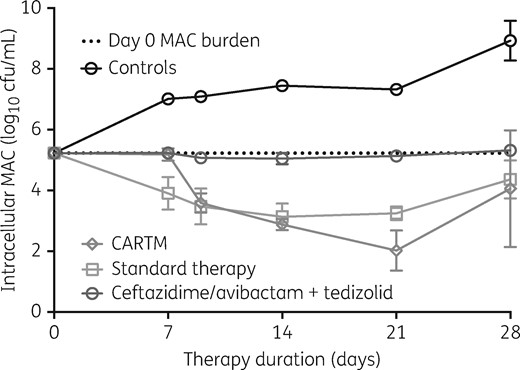
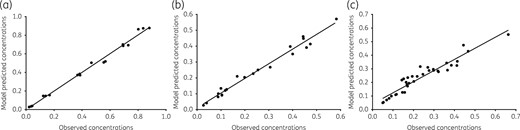
The time–kill plots of the second and more comprehensive HFS-MAC study are shown in Figure 2. The figure shows that the standard therapy regimen achieved a maximal microbial kill of 2.1 log10 cfu/mL on day 14, after which it failed. The ethambutol, azithromycin and rifabutin concentrations achieved in the HFS-MAC units treated with the standard regimen were measured in both the central compartment and inside the MAC-infected macrophages: the pharmacokinetic model predicted versus observed concentrations for these are shown in Figure 3. The intracellular concentrations of azithromycin and ethambutol were below limits of detection of our assays. In the central compartment, the mean ± SD ethambutol AUC0–24 achieved was 11.78 ± 0.18 mg·h/L, similar to that achieved by the recommended dose of 15 mg/kg in adult patients.24 The central compartment azithromycin AUC0–24 was 2.93 mg·h/L, slightly higher than that achieved with the 500 mg/day dose in adult humans.25 We found that rifabutin intracellular concentrations were 315.4 ± 269.5-fold higher than in the extracellular central compartment, which is a dramatic difference. Since the rifabutin extracellular versus intracellular elimination rate constants (kel) were 0.047 ± 0.006 h−1 versus 0.025 ± 0.034 h−1, it means that the higher intracellular rifabutin concentrations were due to increased penetration into cells instead of reduced clearance from infected macrophages.
Comparison of microbial kill of the CARTM regimen compared with standard therapy. The four-drug combination of ceftazidime/avibactam, rifabutin, tedizolid and moxifloxacin (CARTM), and non-optimized doses for the combination had higher kill rates that lasted for longer compared with the standard regimen in the hollow-fibre system model. The microbial burden versus time slopes (i.e. kill or growth slopes) for each regimen were calculated two ways, either taking the entire data series, or allowing automatic outlier elimination. Only in the CARTM regimen was there a difference, with a slope of −0.150 ± 0.012 log10 cfu/mL/day without elimination of the day 7 ‘outlier’ and 0.160 ± 0.006 log10 cfu/mL/day with outlier elimination, so that the two values were not statistically different. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Pharmacokinetic model predicted versus observed concentrations in the standard regimen. Concentrations from all replicate HFS-MAC were combined in one analysis, and run as a population pharmacokinetic model. (a) Ethambutol model predicted versus observed concentrations had a slope of 1.01 ± 0.02 (r2 > 0.99), indicating no bias. (b) The slope for azithromycin was 0.93 ± 0.03 (r2 = 0.98), which indicates minimal bias. (c) For rifabutin, the concentrations from the experimental regimen and those for standard regimen were co-analysed. The slope was 0.82 ± 0.04 (r2 = 0.91).
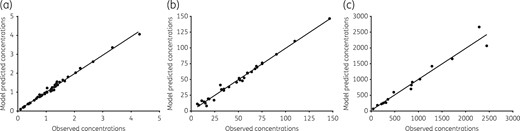
Figure 2 shows that the four-drug combination regimen of ceftazidime/avibactam, rifabutin, tedizolid and moxifloxacin (CARTM) achieved a maximum kill of 3.2 log10 cfu/mL below the day 0 burden on day 21. This extent of microbial kill was higher than the standard regimen. Furthermore, calculation of the slopes, with P values for significance of deviation of the slope from zero (flat-line), gave values of 0.136 ± 0.024 log10 cfu/mL/day for non-treated controls (P < 0.001) compared with −0.125 ± 0.019 log10 cfu/mL/day for standard therapy (P < 0.001) versus −0.160 ± 0.006 log10 cfu/mL/day for CARTM (P < 0.001), and −0.002 ± 0.003 log10 cfu/mL/day for ceftazidime/avibactam plus tedizolid dual therapy (P = 0.482). Thus, bacterial levels in non-treated controls grew, ceftazidime/avibactam plus tedizolid held the bacterial burden constant (flat), while standard therapy killed but the rate was exceeded by CARTM by > 30%. We also measured the drug concentrations that achieved this extent of bactericidal activity: the pharmacokinetic model predicted versus observed concentrations achieved are shown in Figure 4. The rifabutin concentrations in the CARTM regimen were identical to those in the standard therapy regimen. The tedizolid AUC0–24/MIC achieved in the central compartment was 13.99 ± 1.30, which is lower than the optimal AUC0–24/MIC exposure of 37.5 but virtually the same as that associated with bacteriostasis.15 The intracellular tedizolid concentrations were below the limits of detection of our assay. On the other hand, the observed ceftazidime central compartment concentrations persisted above the MIC of 16 mg/L in all systems for the entire 24 h dosing interval. Uniquely, we found that the intracellular ceftazidime concentrations were 148.0 ± 134.6 times higher than the central compartment concentrations, while avibactam concentrations were 164.3 ± 109.2 times higher. Thus, intracellular ceftazidime/avibactam concentrations were also above the MIC for the entire dosing interval, and avibactam concentrations were >1 mg/L for the same period. Finally, the moxifloxacin extracellular AUC0–24/MIC achieved was 121.0 ± 1.00, below the optimal exposures identified in the past.17 The moxifloxacin intracellular-to-central compartment penetration ratio was 49.49 ± 16.32. However, since the moxifloxacin kel in the extracellular compartment was 0.06 ± 0.00 h−1 compared with 0.04 ± 0.07 h−1 in the intracellular compartment, this means that the high intracellular moxifloxacin concentrations were mainly driven by high drug penetration and not reduced clearance from the infected cells. Thus, the CARTM regimen contains at least three drugs that achieve high intracellular penetration: moxifloxacin, ceftazidime/avibactam and rifabutin.
Pharmacokinetic model predicted versus observed concentrations in the experimental regimen. Concentrations from all replicate HFS-MAC for each drug were combined in one population pharmacokinetic analysis. (a) The tedizolid model predicted versus observed concentrations had a slope of 0.98 ± 0.01 (r2 = 0.99), which is close to unity, indicating minimal bias. (b) For ceftazidime, the slope was 0.99 ± 0.02 (r2 = 0.99), which also indicates minimal to no bias. (c) For moxifloxacin, the pharmacokinetic model predicted versus observed concentrations are given for the two-compartment model based on intracellular concentrations. The slope was 0.98 ± 0.06 (r2 = 0.96), again close to unity.
Discussion
This study demonstrates, firstly, the greater efficacy of the combination regimen of CARTM against MAC compared with the current standard regimen. The maximal microbial kill by the combination regimen of CARTM in the HFS-MAC exceeded that of the standard regimen. This was achieved with non-optimized doses of tedizolid, moxifloxacin and rifabutin in this experimental regimen. Thus, the CARTM regimen provides an opportunity to iteratively identify optimal exposures for each of the companion drugs in the regimen that would be at least additive or even synergistic. Elsewhere, we have shown in the case of intracellular Mycobacterium tuberculosis, in prospective clinical studies as well as in the HFS, that the same combination of antibiotics could be antagonistic, or additive or synergistic, depending on the concentrations tested.26,27 Thus, there is a possibility of extending the kill rates below 3.2 log10 cfu/mL after further optimization of this regimen, with the objective of completely eliminating the bacteria. There is also room to replace some of the component drugs with other antibiotics more bactericidal than those in the CARTM regimen. Regardless, we report a regimen that kills better than the standard regimen, with the potential to be further improved and developed into short-course chemotherapy for pulmonary MAC.
Secondly, MAC, as well as several other mycobacteria, are intracellular pathogens. Effective intracellular concentrations of antimicrobial agents are a prerequisite for effective treatments of such infections. Good intracellular penetration does not mean the drug will be effective, but the drug will not be effective against intracellular MAC if it does not penetrate into infected cells well. Here we show that rifabutin, ceftazidime/avibactam and moxifloxacin achieved high intracellular concentrations compared with extracellular concentrations. All three drugs likely achieved this by virtue of high penetration into the cells and not by reduced clearance by the infected monocytes. In the case of rifabutin and ceftazidime/avibactam this is, to our knowledge, the first time this has been directly quantified.
Thirdly, this study further benchmarks the effect of the current three-drug standard regimen in the HFS-MAC. The kill rates of the standard regimen in the HFS-MAC are important because the clinical performance of this regimen is well known and allows us to benchmark the extent and speed of microbial kill of experimental regimens so that those that exceed the standard regimen’s performance would have a greater chance of shortening the duration of therapy.10 We have shown in an accompanying paper that the dual combination of azithromycin and ethambutol in the HFS-MAC effectively killed 1.21 ± 0.74 log10 cfu/mL below stasis but only up to day 7.28 Addition of rifabutin to this dual regimen extended the depth of kill by 40% by day 14. Thus, it would be prudent always to add rifabutin to azithromycin and ethambutol in the treatment of pulmonary MAC.
Our study has some limitations. First, we examined the effect of two- and four-drug combination therapy. We examined sub-optimal exposures for tedizolid, moxifloxacin and rifabutin in the experimental regimen. This is crucial since tedizolid alone at optimal dose kills better than standard combination therapy. It will be necessary in the future to examine optimized doses in combination based on pharmacokinetic/pharmacodynamic design of concentrations identified as additive or synergistic. However, the intravenous nature of ceftazidime/avibactam may make the four-drug regimen more expensive. Second, once an optimal regimen has been chosen using a laboratory strain of MAC in the HFS model, it will be necessary to examine the performance of the optimized regimen in several different clinical strains in the HFS-MAC. That step would allow for generalization of the results.
In summary, we have identified a novel four-drug combination regimen, free of macrolides, that is likely to have improved kill rates compared with the current standard regimen. However, future studies identifying optimal exposures need to be performed for this combination.
Funding
This study was supported by the Baylor Research Institute, Baylor University Medical Center.
Transparency declarations
None to declare.
This article forms part of a supplement sponsored by the Baylor Research Institute.